Abstract
A unique method to affect intramolecular aminooxygenation and dioxygenation of allenols and allenylsulfonamides is described. These operationally simple reactions occur under neutral or basic conditions where copper(II) carboxylates serve as reaction promoter, oxidant, and carboxylate source. Moderate to high yields of heterocycle-functionalized vinyl carboxylate esters are formed with moderate to high levels of diastereoselectivity. Such vinyl carboxylate esters could serve as precursors to α-amino and α-oxy ketones and derivatives thereof.
Graphical Abstract
Transition-metal-catalyzed cyclization of alcohols and amine derivatives onto pendant allenes has emerged as a powerful approach to heterocycle synthesis.1–5 Both endo and exo cyclization modes have been explored, and hydrofunctionalization as well as difunctionalization transformations have been achieved. Hydrofunctionalization reactions have been catalyzed by complexes of [Ln], [Sm], [Y], [Pd], [Au], [Ag], and [Cu].1,3–5 Difunctionalization reactions such as net carboamination and carboetherification have been catalyzed by [Ru], [Pd], [Co], and [Cu].1,6–10 Haloamination has been achieved by use of [Pd] catalysts in the presence of [Cu] and halide salts,11,12 and more recently, use of chiral [Au] catalysts in the presence of electrophilic bromine sources has provided chiral heteroatom-functionalized vinyl bromides.13 Despite these advances, no methods for allene aminooxygenation and dioxygenation have been reported.
For the past decade, our group has developed copper-facilitated alkene addition reactions14 including oxyamination, aminooxygenation, diamination, and dioxygenation reactions.15–18 In the interest of expanding the scope, we investigated allene difunctionalizations (Scheme 1).
Scheme 1.
Copper-Promoted Allene Cyclization
Copper catalysis in this area can be further summarized. Halolactonization and halolactamization promoted by CuBr2 and CuCl2 endocyclization of 2,3-allenoate derivatives have been reported by Ma and co-workers.19–21 Gevorgyan and co-workers have investigated copper-facilitated endocyclization of in situ formed allenylpyridines en route to N-fused heterocycles.22 Both endo- and exo-hydroamination/cyclization of electron-rich allenylamines (e.g., N-benzyl) catalyzed by a variety of copper salts were reported by Okamoto (Scheme 1).23 Lee and co-workers recently reported an endo-selective hydroalkoxylation catalyzed by CuCl2 (Scheme 1).24 Kanai and co-workers have developed copper(I)-catalyzed endocyclizations of allenyl alcohols and amine derivatives.25–27
Surprisingly, reactions of allenols and allenylamine derivatives with copper(II) carboxylate salts alone have not been reported. In this paper, we report that copper(II) carboxylate promoted cyclizations of allenols and allenylamine derivatives can result in net dioxygenation and aminooxygenation. These new reactions provide useful heterocycle functionalized vinyl carboxylate esters,28,29 products that heretofore have not been obtained directly from allenes by other reaction protocols.
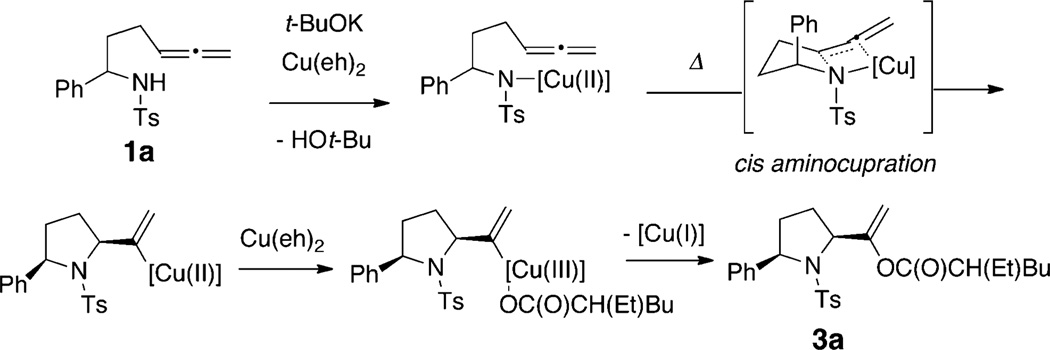
We initially investigated the cyclization of N-tosylallenyl amide 1 using catalytic copper(2-ethylhexanoate)2 [Cu(eh)2] and copper(trifluoroacetate)2 (Scheme 2). After a brief screen of reaction conditions, we found hydroamination to be optimal using 20 mol % Cu(OC(O)CF3)2 and 40 mol % N,N-diethylsalicylamide as ligand in toluene at 120 °C for 24 h. No base or oxidant is required in this reaction and the relative configuration of the major diastereomer 2 (dr = 15:1) was assigned to be cis.30 Under the same conditions but with Cu(eh)2, we obtained a mixture of the expected hydroamination product 2 along with a new aminooxygenation product, vinyl ester 3 (ratio 2:3 = 6:1).
Scheme 2.
Hydroamination and Aminooxygenation
Vinyl esters can undergo hydrolysis to give the corresponding ketones or they can undergo carbon–carbon bond formation with electrophiles.31–38 Reported methods to form vinyl esters include metal-catalyzed addition of acids to alkynes, O-acylation of silyl enol ethers, and Baeyer–Villiger oxidation of enones.39–45
We were intrigued with the formation of the pyrrolidine-functionalized vinyl ester 3 and set about to optimize for this more unique reaction. We reasoned that since vinyl ester 3 obtains its carboxylate from the copper(II) salt, a copper-promoted reaction would better facilitate its production. Furthermore, we hypothesized that while the hydroamination product 2 can be directly obtained from a vinylcopper intermediate via protonation, the vinyl ester product 3 likely arises from oxidation of the copper(II) intermediate to a copper(III) intermediate and subsequent reductive elimination (Scheme 3).
Scheme 3.
Proposed Aminooxygenation Mechanism
The aminooxygenation reaction of allenyl sulfonamide 4 was optimized as shown in Table 1 using 300 mol % of Cu(eh)2 (less [Cu] gave lower ratios of 5:6). In the event, reaction of sulfonamide 4 gave 59% combined yield of a 2:1 ratio of aminooxygenation and hydroamination adducts 5a and 6. We hypothesized that the ratio could be further shifted toward aminooxygenation either by disfavoring the hydroamination by removing sources of H+ or by increasing the oxidizing conditions to enable more facile access to Cu(III) intermediates. When the TEMPO radical was added to increase the oxidizing ability of the solution, the yield and ratio did increase to 63% and 4:1, respectively. Notably, no TEMPO adduct was observed, which may indicate that a vinyl radical is not a reaction intermediate. However, use of 1 equiv of the strong base KO-t-Bu was more effective, providing 78% yield of essentially only aminooxygenation product 5a (Table 1, entry 3). Reaction with Cu[OC(O)-i-Pr]2 gave 5b and 6 in a 1:1.7 ratio (Table 1, entry 4). Cu(OAc)2 gave hydroamination product 6 exclusively (not shown). These differences are likely due to the respective solubilities of these copper(II) carboxylates in toluene. When O2 (1 atm, balloon) was used with 100 mol % of Cu(eh)2, 31% of 5a, along with other minor products (but not 6), was formed.
Table 1.
Aminooxygenation Optimizationa
 | |||
|---|---|---|---|
| entry | additive | yield 5b (%) | ratio 5:6c |
| 1 | 59 | 2:1 | |
| 2d | TEMPO | 63 | 4:1 |
| 3e | KO-t-Bu | 78 | 20:1 |
| 4e,f | KO-t-Bu | 24 | 1:1.7 |
| 5e,g | KO-t-Bu, O2 | 31 | 20:1 |
Reaction conditions: allene 4 (39 mg, 0.10 mmol), Cu(eh)2 (3 equiv), 1.0 mL of PhCH3, and 4 Å molecular sieves were heated for 2 h at 105 °C in a sealed tube.
Isolated yield.
Ratio based on analysis of crude 1H NMR.
TEMPO (1 equiv) was added to the reaction mixture before heating.
Substrate 4 was treated with KO-t-Bu (1 equiv) for 0.5 h at rt before Cu(eh)2 was added.
Cu(OC(O)-i-Pr)2 was used.
Cu(eh)2 (1 equiv) and O2 (1 atm, balloon) were used; reaction in round-bottomed flask.
The analogous reaction with allenyl alcohol 7 was examined (Table 2). This substrate preferentially formed dioxygenation adduct 8 over the hydroetherification product 9 even in the absence of base or oxidant (Table 2, entry 1). At both reduced Cu(eh)2 loading (2.2 equiv) and reaction temperature (90 °C), the reaction was most efficient, producing vinyl ester 8 essentially exclusively in 90% yield (Table 2, entry 2). While toluene was the most effective (compare Table 2, entry 2, to entries 4 and 5) the reaction also worked in 1,2-dichloroethane and acetonitrile.
Table 2.
Dioxygenation Optimizationa
 | |||||
|---|---|---|---|---|---|
| entry | [Cu] (equiv) | solvent | temp (°C) | yieldb (%) | ratio 8:9c |
| 1 | 3 | PhCH3 | 105 | 71 | 20:1 |
| 2 | 2.2 | PhCH3 | 90 | 90 | 20:1 |
| 3 | 2.2 | PhCH3 | 75 | 64 | 15:1 |
| 4 | 2.2 | DCE | 90 | 66 | 20:1 |
| 5 | 2.2 | CH3CN | 90 | 70 | 20:1 |
Allenyl alcohol 7, Cu(eh)2 (2.2–3 equiv), solvent (0.2 M), and 4 Å molecular sieves (20 mg/mL) were heated in a sealed tube for 2 h.
Isolated yield.
Ratio determined by analysis of the 1H NMR of the crude reaction mixture.
The effects of backbone substituents and amine functionalization on the aminooxygenation reaction were explored (Table 3). In the diastereoselective reactions, use of Cu(2-ethylhexanoate)2 provides the possibility of four diastereomers since 2-ethyl hexanoate has a stereocenter and is racemic. The effect of this stereocenter on the 1H NMR spectra of these compounds is minimal, but to avoid ambiguity in relative stereochemistry configuration assignment, reactions were also run with Cu[OC-(O)-i-Pr]2.
Table 3.
Scope of the Aminooxygenation Reaction
| entry | substrate | product | yield (%)c |
drd |
|---|---|---|---|---|
| 1a |  |
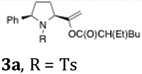 |
51 | 20:1 |
| 2a | 1b, R = SES | 3b, R = SES | 83 | 20:1 |
| 3b | 1a | 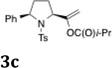 |
30 | 20:1 |
| 4a | 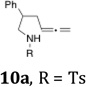 |
 |
55 | 4:1 |
| 5a | 10b, R = Cbz | 11b, R = Cbz | no rxn | |
| 6b | 10a | 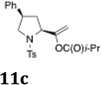 |
37 | 4:1 |
| 7a |  |
 |
73 | 20:1 |
| 8b | 12 |  |
35 | 20:1 |
| 9a |  |
 |
43 |
Table 1, entry 3, conditions were used unless otherwise noted.
Cu[OC(O)-i-Pr]2 was used instead of Cu(eh)2.
Isolated yield.
Diastereomeric ratio refers to relative stereochemistry about the pyrrolidine backbone and not to 2-ethyl hexanoate derived diastereomers. Ratio determined by analysis of the crude 1H NMR. SES = 2-trimethylsilylethane sulfonyl.
As indicated in Table 3, α-phenyl sulfonamides 1 gave high selectivity for the 2,5-cis-pyrrolidine diastereomers 3. Both aryl and alkyl sulfonyl N-substituents were reactive (Table 3, entries 1–3). The β-phenyl sulfonamide 10a provided moderate (4:1) selectivity for the cis-pyrrolidine diastereomers 11 (Table 3, entries 4 and 6). The N-CBz substrate 10b was unreactive (Table 3, entry 5). γ-Benzylsulfonamide 12 provided 2,3-trans-pyrrolidines 13 with excellent selectivity (Table 3, entries 7 and 8). Urea 14 gave cyclic urea 15 in moderate yield (Table 3, entry 9).
A number of allenyl alcohols were next explored in the copper-promoted allene dioxygenation reaction (Table 4). 2,3-trans-2-Vinylcarboxytetrahydrofurans formed efficiently from γ-benzyl allenyl alcohols 16, 18, 20, and 22 (Table 4, entries 1–5). The β-phenyl allenyl alcohol 24 gave the 2,4-cis-2-vinylcarboxytetrahydrofurans 25 (Table 4, entries 6 and 7). α-Phenyl alcohol 26 cyclized with modest diastereoselectivity (Table 4, entry 8). The 2-allenyl tertiary benzyl alcohol 28 cyclized readily to provide phthalan 29 (Table 4, entry 9).
Table 4.
Scope of the Dioxygenation Reaction
| entry | substrate | product | yield (%) | dr |
|---|---|---|---|---|
| 1a |  |
 |
90 | 20:1 |
| 2b | 16 |  |
50 | 20:1 |
| 3a |  |
 |
78 | 20:1 |
| 4a |  |
 |
77 | 20:1 |
| 5a |  |
 |
85 | 20:1 |
| 6a |  |
 |
87 | 19:1 |
| 7b | 24 |  |
33 | 20:1 |
| 8a |  |
 |
60 | 2:1 |
| 9a,c |  |
 |
56 |
Table 2, entry 5, conditions were used unless otherwise noted.
Cu[OC(O)-i-Pr]2 (3 equiv) was used.
Isolated yield.
Ratio determined by analysis of the 1H NMR of the crude reaction mixture. Ratio refers to relative configuration about the tetrahydrofuran ring backbone and not to 2-ethylhexanoate-derived diastereomers.
Reaction run for 8 h.
The proposed mechanism for the copper(II) carboxylate-promoted aminooxygenation of allenes is presented in Scheme 3. In this scenario, sulfonamide 1a engages the [Cu(II)] in a N–Cu(II) bond. Subsequent thermally promoted diastereoselective cis-aminocupration46 across the allene provides the nitrogen heterocycle with a pendant vinylcopper(II) moiety. Oxidation of the vinylcopper(II) to vinylcopper(III) with additional Cu-(eh)247,48 and reductive elimination involving a carboxylate ligand on the copper center provides the vinyl ester 3a.
The TMSOTf-catalyzed hydrolysis of vinyl ester 5a in CH2Cl2 provided ketone 30 (eq 1). Oxidation of 5a with m-CPBA gave
 |
(1) |
 |
(2) |
α-acetoxy ketone 31 (eq 2). Ketone 31 resembles JTP-4819, a prolyl endopeptidase inhibitor and cognition enhancer.49 Further investigation of copper-catalyzed allene difunctionalizations and application of products derived thereof are merited.
Supplementary Material
Acknowledgments
The financial support of the National Institutes of Health (NIGMS RO1 078383) is gratefully acknowledged. The Egyptian Government is gratefully acknowledged for the graduate school fellowship to Z.M.K.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Experimental procedures, characterization of new compounds, and copies of NMR spectra (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Bates RW, Satcharoen V. Chem. Soc. Rev. 2002;31:12. doi: 10.1039/b103904k. [DOI] [PubMed] [Google Scholar]
- 2.Ma S. Acc. Chem. Res. 2003;36:701. doi: 10.1021/ar020133k. [DOI] [PubMed] [Google Scholar]
- 3.Krause N, Winter C. Chem. Rev. 2011;111:1994. doi: 10.1021/cr1004088. [DOI] [PubMed] [Google Scholar]
- 4.Alcaide B, Almendros P. Adv. Synth. Catal. 2011;353:2561. [Google Scholar]
- 5.Barreiro EM, Adrio LA, Hii KK, Brazier JB. Eur. J. Org. Chem. 2013;2013:1027. [Google Scholar]
- 6.Trost BM, Pinkerton AB. J. Am. Chem. Soc. 1999;121:10842. [Google Scholar]
- 7.Trost BM, Pinkerton AB, Kremzow D. J. Am. Chem. Soc. 2000;122:12007. [Google Scholar]
- 8.Zimmer R, Dinesh CU, Nandanan E, Khan FA. Chem. Rev. 2000;100:3067. doi: 10.1021/cr9902796. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu Y, Kanai M. Tetrahedron Lett. 2014;55:3727. [Google Scholar]
- 10.Liu G, Lu X. Org. Lett. 2001;3:3879. doi: 10.1021/ol016727p. [DOI] [PubMed] [Google Scholar]
- 11.Jonasson C, Horvath A, Backvall J-E. J. Am. Chem. Soc. 2000;122:9600. [Google Scholar]
- 12.Jonasson C, Karstens WFJ, Hiemstra H, Backvall J-E. Tetrahedron Lett. 2000;41:1619. [Google Scholar]
- 13.Miles DH, Veguillas M, Toste FD. Chem. Sci. 2013;4:3427. [Google Scholar]
- 14.Chemler SR. J. Organomet. Chem. 2011;696:150. doi: 10.1016/j.jorganchem.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paderes MC, Chemler SR. Org. Lett. 2009;11:1915. doi: 10.1021/ol9003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequeira FC, Chemler SR. Org. Lett. 2012;14:4482. doi: 10.1021/ol301984b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequeira FC, Turnpenny BW, Chemler SR. Angew. Chem., Int. Ed. 2010;49:6365. doi: 10.1002/anie.201003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma S, Wu S. J. Org. Chem. 1999;64:9314. [Google Scholar]
- 20.Ma S, Wu S. Chem. Commun. 2001:441. [Google Scholar]
- 21.Ma S, Xie H. Org. Lett. 2000;2:3801. doi: 10.1021/ol006504j. [DOI] [PubMed] [Google Scholar]
- 22.Chernyak D, Gadamsetty SB, Gevorgyan V. Org. Lett. 2008;10:2307. doi: 10.1021/ol8008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuhako A, Oikawa D, Sakai K, Okamoto S. Tetrahedron Lett. 2008;49:6529. [Google Scholar]
- 24.Kim S, Lee PH. J. Org. Chem. 2012;77:215. doi: 10.1021/jo2018125. [DOI] [PubMed] [Google Scholar]
- 25.Kawai J, Chikkade PK, Shimizu Y, Kanai M. Angew. Chem., Int. Ed. 2013;52:7177. doi: 10.1002/anie.201302027. [DOI] [PubMed] [Google Scholar]
- 26.Chikkade PK, Shimizu Y, Kanai M. Chem. Sci. 2014;5:1585. [Google Scholar]
- 27.Itoh T, Shimizu Y, Kanai M. Org. Lett. 2014;16:2736. doi: 10.1021/ol501022d. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh AK, Liu C. J. Am. Chem. Soc. 2003;125:2374. doi: 10.1021/ja021385j. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal VK, Sheldon CG, Macdonald GJ, Martin WP. J. Am. Chem. Soc. 2002;124:10300. doi: 10.1021/ja027061c. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher T, Jones SW, Mahon MF, Molloy KC. J. Chem. Soc., Perkin Trans. 1991;1:2193. [Google Scholar]
- 31.Ogiwara Y, Tamura M, Kochi T, Matsuura Y, Chatani N, Kakiuchi F. Organometallics. 2014;33:402. [Google Scholar]
- 32.Webb NJ, Marsden SP, Raw SA. Org. Lett. 2014;16:4718. doi: 10.1021/ol502095z. [DOI] [PubMed] [Google Scholar]
- 33.Yanagisawa M, Shimamura T, Iida D, Matsuo J-i, Mukaiyama T. Chem. Pharm. Bull. 2000;48:1838. doi: 10.1248/cpb.48.1838. [DOI] [PubMed] [Google Scholar]
- 34.Mukaiyama T, Izawa T, Saigo K. Chem. Lett. 1974:323. [Google Scholar]
- 35.Yanagisawa A, Matsumoto Y, Asakawa K, Yamamoto H. J. Am. Chem. Soc. 1999;121:892. [Google Scholar]
- 36.Isambert N, Cruz M, Arevalo MJ, Gomez E, Lavilla R. Org. Lett. 2007;9:4199. doi: 10.1021/ol701717z. [DOI] [PubMed] [Google Scholar]
- 37.Panda N, Mothkuri R, Pal A, Paital AR. Adv. Synth. Catal. 2013;355:2809. [Google Scholar]
- 38.Zacharia JT, Tanaka T, Hayashi M. J. Org. Chem. 2010;75:7514. doi: 10.1021/jo101542y. [DOI] [PubMed] [Google Scholar]
- 39.Poladura B, Martinez-Castaneda A, Rodriguez-Solla H, Llavona R, Concellon C, del Amo V. Org. Lett. 2013;15:2810. doi: 10.1021/ol401143q. [DOI] [PubMed] [Google Scholar]
- 40.Hua R, Tian X. J. Org. Chem. 2004;69:5782. doi: 10.1021/jo049455n. [DOI] [PubMed] [Google Scholar]
- 41.Ito H, Ishizuka T, Tateiwa J-i, Hosomi A. Tetrahedron Lett. 1998;39:6295. [Google Scholar]
- 42.Melis K, Opstal T, Verpoort F. Eur. J. Org. Chem. 2002;2002:3779. [Google Scholar]
- 43.Nishiumi M, Miura H, Wada K, Hosokawa S, Inoue M. ACS Catal. 2012;2:1753. [Google Scholar]
- 44.Chary BC, Kim S. J. Org. Chem. 2010;75:7928. doi: 10.1021/jo101543q. [DOI] [PubMed] [Google Scholar]
- 45.Doucet H, Martin-Vaca B, Bruneau C, Dixneuf PH. J. Org. Chem. 1995;60:7247. [Google Scholar]
- 46.Paderes MC, Belding L, Fanovic B, Dudding T, Keister JB, Chemler SR. Chem. - Eur. J. 2012;18:1711. doi: 10.1002/chem.201101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King AE, Brunold TC, Stahl SS. J. Am. Chem. Soc. 2009;131:5044. doi: 10.1021/ja9006657. [DOI] [PubMed] [Google Scholar]
- 48.Shade RE, Hyde AM, Olsen J-C, Merlic CA. J. Am. Chem. Soc. 2010;132:1202. doi: 10.1021/ja907982w. [DOI] [PubMed] [Google Scholar]
- 49.Wroblewski T, Silvestre J, Castaner J. Drugs Future. 1998;23:384. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.