Abstract
Background. Fomites are objects that can become colonized and serve as vectors in the transmission of pathogenic microorganisms. Literature examining the knowledge of healthcare personnel about this method of spread of infection is lacking. We conducted a study to assess the knowledge, attitude, and practices of healthcare personnel across different areas of patient care regarding the spread of infections at a tertiary care hospital in Karachi, Pakistan.
Methods. A descriptive, cross-sectional study was conducted among healthcare personnel using a self-administered questionnaire. The questionnaire contained sections pertaining to demographic details and knowledge, attitude, and practices regarding fomites and their role in the transmission of pathogens.
Results. Three hundred and fifty-three participants completed the questionnaire: 168 were male and 185 were female. Laboratory coats, stethoscopes, and bedside curtains were most frequently identified as fomites by the participants. Medical students had significantly lower mean scores in the knowledge and attitude sections than consultant physicians, resident physicians, and nurses. Nurses scored higher than consultant physicians, resident physicians, and medical students regarding practices that minimize fomite-borne spread of infections. 95% of the participants scored above 50% on the knowledge component of the questionnaire, but only 32.3% scored above 50% in the practices section.
Conclusions. Our results show a large gap between the knowledge about fomites acting as vectors in the spread of pathogens and practices done to minimize this spread. Possessing adequate knowledge is ineffectual until and unless it is translated into the proper application of infection control practices. Incorporating awareness sessions and exercises into curricula are a reasonable way to raise awareness regarding this subject.
Keywords: contamination, fomites, hospital-acquired infections, resistant pathogens, transmission
INTRODUCTION
Fomites are objects that can become colonized with pathogenic microorganisms and serve as vectors in their transmission. During and after the course of infectious diseases, pathogens are shed from various bodily secretions. Fomites become contaminated by direct contact with bodily secretions or soiled hands. Once contaminated, the transmission of pathogens from one fomite to another occurs easily, between animate and inanimate objects and vice versa [1]. Variations in survival of pathogens make this spread a more complicated issue [2]. Highly virulent microorganisms, particularly those known to cause nosocomial infections in admitted patients, such as Enterococcus species, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Klebsiella species, Pseudomonas aeruginosa, and Acinetobacter species, are capable of surviving for several days on hospital surfaces [3]. Scientific literature contains abundant studies [4–9] proving the colonization of our everyday hospital objects with highly resistant and pathogenic microorganisms. They are not only found on hospital objects but also on items that form the very basic elements of a physician's attire (i.e., white coats, stethoscopes, identity badges, etc.). In addition, other instruments, such as cell phones, thermometers, and particularly bronchoscopes, have all shown evidence of harboring disease-causing pathogens [7, 10–13]. These studies have demonstrated a strong association between colonization of these objects, transmission of pathogens via handling by healthcare personnel, and consequent hospital-acquired (or nosocomial) infections [1, 3, 6, 7, 14]. Nosocomial infections are a historically proven cause of patient mortality and morbidity. Previous studies have proven that environmental colonization by nosocomial pathogens, their survival, and subsequent transmission through direct contact is the underlying cause perpetuating nosocomial infections in hospitals [8, 15, 16]. Nosocomial infections have been shown to be directly and indirectly responsible for an alarming number of patient deaths annually, which are estimated to be more than 19 000 and 80 000, respectively [17, 18]. Furthermore, these infections are the source of an immense financial burden on the healthcare system, with an estimated $4.5 billion spent in the treatment of nosocomial infections in 1992 alone [19]. A study by Weber et al [14] showed that up to 40% of inpatient infections are attributable to cross-infection via healthcare personnels' hands having initially come into contact with contaminated fomites.
There is strong scientific evidence documenting the various pathogens found colonizing hospital surfaces and fluids. The most common organisms implicated in nosocomial infections are Gram-negative rods, mainly E. coli, Pseudomonas species, Enterobacter species, and Gram-positive cocci such as Enterococci and S. aureus [15, 20, 21]. Methicillin-resistant S. aureus and vancomycin-resistant Enterococci (VRE) are common contaminants on various hospital surfaces, whereas Norovirus, Clostridium difficile, and multidrug-resistant (MDR) Acinetobacter species have been added to this list in light of recent evidence [14, 15]. However, literature examining the awareness of healthcare personnel and hospital workers to this particular mode of infection transmission is lacking. Common medical and nonmedical tools, such as thermometers [22], stethoscopes [23], bronchoscopes [24], pens [25], bedside rails, and tables [22, 26] have all been shown to harbor microbes. A study by Cohen et al [27] reported that stethoscopes and otoscopes possessed a bacterial colonization rate of 100% and 90%, respectively. In the same study, MRSA was found on 7.3% and 9.5% of stethoscopes and otoscopes, respectively. Likewise, resistant microorganisms such as VRE, MRSA, and C. difficile have been proven to survive on equipment such as stethoscopes, changing gowns, and bed rails [28]. Although the concentration of pathogens on the surface of fomites is significantly low compared with the skin of an ill patient, the high virulence and infectivity of these nosocomial pathogens means that a much lower infectious dose is required for these pathogens to cause significant disease [29]. The durability of pathogens in terms of survival time on fomites is another valid concern. Highly resistant bacteria, such as Enterococcus faecalis and Enterococcus faecium, are capable of surviving on countertops, telephone receivers, stethoscopes, and gloved and ungloved fingers for up to 5 and 7 days, respectively, whereas MRSA can potentially survive up to 9 days [30]. An association has also been established between the ease and frequency of cleaning hospital surfaces and decreased survival of pathogens [3, 30].
We conducted a cross-sectional study to assess the knowledge, attitude, and practices of healthcare personnel across different areas of patient care regarding the spread of infectious microorganisms at a tertiary care hospital in Karachi, Pakistan.
MATERIALS AND METHODS
A descriptive, cross-sectional study was conducted among healthcare personnel at the Aga Khan University Hospital (AKUH), Karachi, Pakistan using a self-administered questionnaire. Healthcare personnel with direct contact with hospitalized patients were recruited to participate in the study. This included consultant physicians, fellows, resident physicians, nurses, and medical students in the 3rd, 4th, and 5th clinical year. Participants were selected via convenient sampling. Care was taken to obtain questionnaires from a well stratified study population. If a subject refused to participate in the study, personnel of the same profession in the closest proximity were approached. No background information regarding how fomites relate to infection transmission was provided to any of the approached participants.
Data were collected using a predesigned, self-administered questionnaire (Supplementary Appendix 1), from March 2, 2015 to March 13, 2015. The questionnaire consisted of 2 sections: section 1 contained questions regarding demographic details of participants such as age, gender, area of profession, and department of employment; and section 2 consisted of items testing the knowledge, attitude, and practices of healthcare personnel regarding fomite-mediated spread of infections. Items with sequential options were graded using the Likert scale method. The cumulative score for each category was calculated by adding up the score for responses to each item in that category. A cutoff score of 50% of the maximum of each category was used as criterion for the knowledge, attitude, and practices of the participants who were considered to be adequate or appropriate. The details of scoring and analysis for each item in each category are mentioned in Supplementary Appendix 2.
The study was overseen and evaluated by the Ethics Review Committee at AKUH before initiation. Verbal and written informed consent was obtained, and the nature of the study was explained to each participant before administration of the questionnaire. The informed consent forms were separated from questionnaires to maintain anonymity of the participants. Participants were approached during regular workdays at the institution. Medical students, residents, and nurses were approached at the end of large class format sessions, which are regularly held during the week according to their respective curricula. Fellows and consultants were approached when available at their respective office locations.
The data were entered into Epidata separately by 2 investigators to maintain consistency. The data were then transferred to Statistical Package for Social Sciences 20.0 (SPSS, Inc., Chicago, IL). Data analysis was carried out in SPSS 20.0 using descriptive statistics, χ2 test for assessing significance between qualitative data, and t test and analysis of variance (ANOVA) for assessing significance of quantitative data. An alpha value of 0.05 with a 95% confidence interval was used to measure significance for all statistical tests.
RESULTS
Four hundred participants were approached to take part in this study. Of these, 353 (88.25%) participants completed and returned the self-administered questionnaire. Of the 353 who returned the survey, 168 were male and 185 were female. The basic demographic characteristics of the 353 participants and their mean scores in the study parameters, according to designation, are shown in Tables 1 and 2, respectively. Three hundred twenty-three participants (91.5%) knew the meaning of ‘fomites’. Of the 30 (8.5%) participants who did not know the meaning of ‘fomites’, 20 were medical students, 5 were nurses, 3 were resident physicians, 1 was a fellow, and 1 was a consultant physician. Laboratory coats, stethoscopes, and bedside curtains were most frequently identified as fomites by the participants. Prescription papers were identified by the least number of participants (Table 3).
Table 1.
Demographic Details of the 353 Participants
| Study Parameters | Healthcare Personnel, n (%) |
|||||
|---|---|---|---|---|---|---|
| Consultant Physicians | Fellows | Resident Physicians | Nurses | Medical Students | All | |
| Total number | 35 | 14 | 73 | 77 | 154 | 353 |
| M/F | 19/16 | 8/6 | 40/33 | 31/46 | 70/84 | 168/185 |
| Mean age ± SD, years | 44.8 ± 9.3 | 32.7 ± 3.7 | 28.2 ± 2.6 | 25.8 ± 4.3 | 22.5 ± 2.0 | 27 ± 7.7 |
Abbreviation: SD, standard deviation.
Table 2.
Knowledge, Attitude, and Practices of Healthcare Personnel Regarding the Spread of Infections via Fomites
| Study Parameters |
Healthcare Personnel, n (%) |
||||||
|---|---|---|---|---|---|---|---|
| Consultant Physicians | Fellows | Resident Physicians | Nurses | Medical Students | All | ||
| Total number | 35 | 14 | 73 | 77 | 154 | 353 | |
| Knowledge | Score, mean ± SD | 13.6 ± 2.1 | 13.1 ± 2.0 | 13.1 ± 2.0 | 13.5 ± 2.2 | 12.1 ± 2.5 | 12.8 ± 2.4 |
| ≥50% | 34 (97) | 14 (100) | 72 (98.6) | 75 (97) | 143 (93) | 338 (95.8) | |
| <50% | 1 (3) | 0 | 1 (1.4) | 2 (3) | 11 (7) | 15 (4.2) | |
| Attitude | Score, mean ± SD | 11.4 ± 2.3 | 10.6 ± 2.6 | 12.1 ± 2.2 | 12.4 ± 2.9 | 11.4 ± 2.2 | 11.7 ± 2.4 |
| ≥50% | 28 (80) | 10 (71) | 59 (81) | 65 (84) | 120 (78) | 282 (79.9) | |
| <50% | 7 (20) | 4 (29) | 14 (19) | 12 (16) | 34 (22) | 71 (20.1) | |
| Practices | Score, mean ± SD | 2.8 ± 1.6 | 3.1 ± 1.5 | 2.9 ± 1.6 | 3.9 ± 1.2 | 2.2 ± 1.3 | 2.8 ± 1.5 |
| ≥50% | 9 (25.7) | 6 (42.9) | 22 (30.1) | 51 (66.2) | 26 (16.9) | 114 (32.3) | |
| <50% | 26 (74.3) | 8 (57.1) | 51 (69.9) | 26 (33.8) | 128 (83.1) | 239 (67.7) | |
Abbreviation: SD, standard deviation.
Table 3.
Objects Identified as Fomites by Participants (With Frequency)
| Object | Participants Identifying Object as Fomite, n (%) |
|---|---|
| All participants | 353 |
| Laboratory coats | 271 (76.8) |
| Stethoscopes | 257 (72.8) |
| Curtains | 246 (69.7) |
| Patient files | 235 (66.6) |
| Doorknobs | 219 (62) |
| ID badges/ID cards | 199 (56.4) |
| Cell phones | 191 (54.1) |
| Shoes | 187 (53) |
| Pens | 184 (52.1) |
| Computer keyboards/interfaces | 176 (49.9) |
| Ties | 175 (49.6) |
| Keys | 160 (45.3) |
| Taps/faucets | 159 (45) |
| Light switches | 155 (43.9) |
| Prescription papers | 129 (36.5) |
Abbreviation: ID, identification.
One-way ANOVA was conducted for the mean scores regarding knowledge, attitude, and practices about the spread of infections by fomites. The mean scores for each component according to type of healthcare personnel are given in Table 2. The analysis produced a statistically significant ANOVA for the mean scores regarding knowledge (F [4, 348] = 6.46, P < .001), attitude (F [4, 348] = 3.81, P = .005), and practices (F [4, 347] = 18.50, P < .001) among different types of healthcare personnel. This indicated that there were differences among these means. Multiple comparisons with the Tukey post hoc test indicated that medical students had a significantly lower mean score in knowledge compared with consultants, residents, and nurses (mean knowledge scores: 22.5 vs. 44.8, 28.2, and 25.8, respectively). Overall, the mean score in attitude was significantly lower for medical students compared with residents and nurses (mean scores: 11.4 vs. 12.1 and 12.4, respectively). Nurses scored a significantly higher mean score than consultants, resident physicians, and medical students in regards to practices that could reduce the spread of infections via fomites (mean scores: 3.9 vs. 2.8, 2.9, 2.2, respectively). Eta squared (η2) for knowledge, attitude, and practices were 0.069, 0.042, and 0.176, respectively, indicating a moderate effect size.
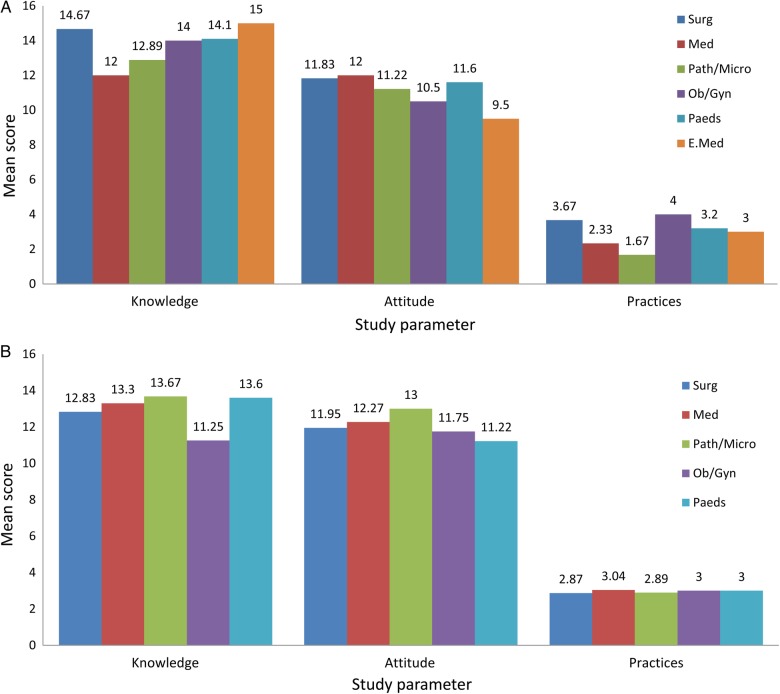
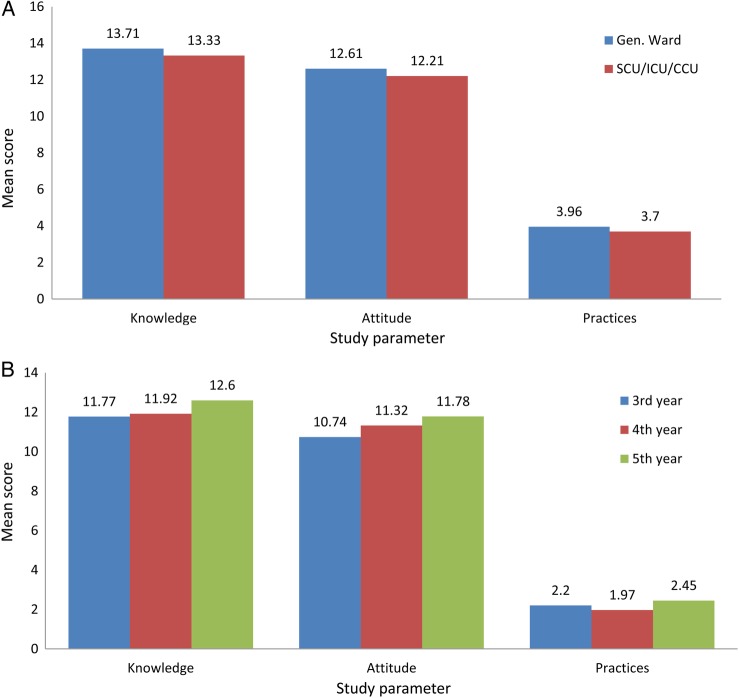
The trends in the means scores of consultants and residents on the basis of knowledge, attitude, and practices regarding the spread of infections by fomites are displayed in Figure 1. For consultants and residents, these trends are according to discipline of practice and training, respectively. One-way ANOVA showed no significant differences in the mean scores of the knowledge, attitude, and practices of consultants or resident physicians according to discipline of practice and training, respectively (P > .1). Figure 2 shows the trend in mean scores of knowledge, attitude, and practices for nurses according to their designated area of patient care and for medical students according to their year of study. One-way ANOVA showed no significant differences in the mean scores of the knowledge, attitude, and practices of medical students according to year of study (P > .1). The t test showed no significant differences in the mean scores of the knowledge, attitude, and practices of nurses according to area of patient care (P > .1). The calculated internal reliability for our study questionnaire in this study was 0.69.
Figure 1.
Mean scores of consultants (A) and residents (B), according to discipline of practice and training, respectively.
Figure 2.
Mean scores of nurses, according to area of designation (A), and medical students, according to year of study (B).
Two hundred fifty-seven (72.8%) participants identified stethoscopes as fomites, and 60.6% of these participants took measures to sanitize them. One hundred ninety-nine (56.4%) and 191 (54.1%) study participants identified identification (ID) badges and cell phones as fomites, respectively, yet only a few admitted to have regularly sanitized their ID badges and cell phones (15.9% and 19.8%, respectively) to prevent the spread of infections from these objects. Alcohol swabs and hand sanitizer gel were the methods most often used to sanitize stethoscopes, whereas alcohol swabs alone were most often used to sanitized ID badges and cell phones.
The participants were asked whether they had come across or heard of a patient with hospital-acquired, resistant infections in their time in the medical field. Two hundred fifty (70.8%) participants reported to have come across hospital-acquired pathogens very often, whereas 83 (23.5%) participants said to have occasionally come across the pathogens. The most frequently encountered microorganism was MRSA, followed by P. aeruginosa and Acinetobacter baumannii. Other frequently encountered hospital-acquired bacteria were VRE, K. pneumoniae, carbapenem-resistant Enterobacteriaceae, and extended-spectrum β-lactamase (ESBL)-producing E. coli (Table 4). Only 23 (29.9%) nurses cleaned patients’ folders after discharge.
Table 4.
Hospital-Acquired Microorganisms Encountered by Healthcare Personnel (With Frequency)
| Microorganism | Participants Encountering the Organism, n (%) |
|---|---|
| All participants | 353 |
| Methicillin-resistant Staphylococcus aureus | 206 (58.4) |
| Pseudomonas aeruginosa | 135 (38.2) |
| Acinetobacter baumannii | 101 (28.6) |
| Vancomycin-resistant Enterococci | 60 (17.0) |
| Klebsiella pneumoniae | 48 (13.6) |
| Carbapenem-resistant Enterobacteriaceae | 47 (13.3) |
| Extended-spectrum-β-lactamase producing Escherichia coli | 46 (13.0) |
Two hundred twenty-six participants had previously come across information on infection spread and fomites. The majority of these participants got this information from the internet and text books; 36.3% and 23.9%, respectively. One hundred twenty-seven (36%) of the 353 participants had not come across any information about fomites or their potential role in the spread of infections. Of these participants, 61.4% (n = 78) were medical students. The maximum number of participants who had prior information about infection spread and fomites were nurses; 83% (n = 64).
Two hundred sixty-nine (76%) subjects believed they had acquired new information about what fomites are via our study questionnaire, and 337 (94.9%) subjects identified the need for awareness classes to inform healthcare personnel about fomites and their role in the spread of infectious organisms. A majority of the participants, 298 (83.9%), believed that fomites can potentially act as carriers for resistant hospital-acquired pathogens into the community.
DISCUSSION
In our study, a majority (70.8%) of the participants came across hospital-acquired infections. Methicillin-resistant S. aureus and P. aeruginosa were the most commonly encountered microorganisms in hospital-acquired infections. VRE, ESBL-producing E. coli, carbapenem-resistant Enterobacteriaceae, and K. pneumonia were also among commonly encountered hospital-acquired pathogens. The participants showed reasonable knowledge regarding the aforementioned microorganisms' etiological role in hospital-acquired infections. Of the participants in our study, 95.8% scored above 50% in the knowledge category of the questionnaire, indicating adequate knowledge about spread of infections via fomites.
Apart from bacteria, several viruses have also been implicated in nosocomial infection transmission, especially respiratory and enteric viruses. Enteric viruses have been found to spread from aerosolized vomit or by the transfer of vomit and fecal matter from hands to hospital surfaces [31]. Likewise, rotavirus, the major etiologic agent of life-threatening diarrhea in infants and young children, has been isolated from telephone receivers, drinking fountains, and toilet handles at day care centers, with a 19% contamination rate [32].
Computer technology has become an essential part of modern medicine, often positioned in close proximity to the patient's bedside for quick and convenient access of patient records and clinical information by healthcare personnel. Concerns have risen regarding the potential spread of pathogens via contact with contaminated computer hardware due to this practice [8]. In a study examining the contamination of hospital computer interfaces in an intensive care unit (ICU) of a tertiary care hospital, the highest rate of contamination was found on keyboards (with 5.4% Enterococcus species) and computer mice (with 5.9% S. aureus) [33]. In a similar study by Bures et al [4], up to 26% of computer keyboards in an ICU of another tertiary care hospital were found to be contaminated with pathogenic bacteria, including MRSA, Enterococcus species, and Enterobacter species. In the same study, concurrence was observed between pathogens isolated from patient samples and those found on the equipment. In our study, 176 (49.9%) of the participants identified computer interfaces as potential fomites capable of spreading infectious pathogens in the hospital setting. Colonization rates for keyboards and faucet handles is higher than other well studied fomites in the rooms of patients suffering from MRSA infection, because frequent handling is involved by both the healthcare provider and the patient[4]. This evidence strongly suggests that these fomites can act as reservoirs, enabling persistence and perpetuation of nosocomial infections with virulent and highly resistant microorganisms.
As with computers, several features of cell phones, including quick and efficient access to information, rapid communication, and improved organization in patient care teams, have increased the use of these devices by healthcare personnel severalfold [34]. Unlike the computer interfaces, which remain stationary, a cell phone's mobility makes it more difficult to keep the transmission of pathogens under control. A study by Borer et al [35] revealed a significant percentage of cell phones and healthcare worker's hands to be contaminated with MDR Acinetobacter species. The same study also documented cell phones, healthcare workers' hands, and patients' skin to be cross-contaminated by identical Acinetobacter strains, again implicating a potential chain of pathogen transmission due to these fomites. In the present study, 191 (54.1%) of the participants identified cell phones as fomites and acknowledged their role in the transmission of pathogens. A large study by Goldblatt et al [36] in 2007 showed that 20% of cell phones sampled harbored potentially pathogenic microorganisms, with Acinetobacter lwoffii being the most common isolate, followed by MRSA and P. aeruginosa. Intuitively, the cell phones that were found to be free of microbial contamination had never been taken inside hospital premises. This observation signifies a strong association between the use of cell phones within the hospital boundaries and their contamination with nosocomial pathogens, and it also indicates that decreasing the number and frequency of such objects in hospital premises will lead to fewer nosocomial transmission rates via fomites. However, due to their integral role in the everyday lives of individuals, it remains difficult to restrict the carrying of cell phones into hospitals. In situations such as this, proper sterilization and antiseptic measures have also shown to lower the rates of transmission. Cleaning common fomites with just alcohol swabs is an effective way of sterilizing these objects, reducing microbial colonization by up to 96.3% [27].
Interrupting the chain of transmission of pathogens via fomites can lead to a significant reduction in the spread and frequency of nosocomial infections. In a study in 1999, it was found that the rate of infections in a burn unit rose with the introduction of computer stations, and this rate fell to its original level after disinfection measures were implemented while handling computer interfaces [37]. Regular surface disinfection may be an effective way to combat nosocomial pathogens, but one should note that certain pathogens, such as Norovirus and C. difficile, are relatively resistant to commonly used disinfectant solutions and antiseptics [20]. Hence, infection control guidelines and disinfection protocols need to be implemented and modified accordingly, keeping these resistant pathogens in mind.
Despite the abundant scientific evidence on the role of fomites in the spread of nosocomial infections and the rising incidence of resistant hospital infections, such as MRSA and VRE infections [20, 38, 39], it is startling that only a minority of healthcare workers takes appropriate steps to counter this transmissibility. Approximately half of our study participants acknowledged that items such as stethoscopes, cell phones, and ID badges were fomites, yet a striking majority admittedly did not take appropriate disinfection measures to sterilize these items after each patient contact. In the present study, cell phones were identified as fomites by 191 (54.1%) participants, but only 70 (19.8%) of these participants regularly sanitized their cell phones. Likewise, ID badges were identified as fomites by 199 (56.4%) participants, but only 56 (15.9%) of these participants regularly sanitized their badges. Ulger et al [40] reported that as many as 89.5% of healthcare professionals never cleaned their cell phones despite routine use in hospital setting. In our study, a comparatively larger proportion of the participants identifying stethoscopes as fomites practiced regularly sanitizing their stethoscopes (214–60.6%), although this number is not ideally as high as should be expected. Our study results also demonstrate the vast gap between the knowledge and practices of healthcare personnel in this particular matter. Possessing adequate knowledge is ineffectual until and unless it is translated into the proper application of infection control practices by all tiers of the healthcare system and medical professionals. Of the participants in the present study, 94.9% identified the need to conduct awareness sessions to make personnel aware of this mode of spread of infectious microorganisms.
The 2008 Healthcare Infection Control Practice Advisory Committee of the Centers for Disease Control and Prevention (Atlanta, GA) guidelines [41] require the use of detergent-disinfectant to clean surfaces on a regular basis as well as terminal cleaning after patient discharge to reduce the risk of fomite-mediated microbial transmission.
Along with this recommendation, there is a great need to educate healthcare workers about this mode of transmission and to put emphasis on appropriate decontamination measures in an attempt to prevent further spread. One way of achieving this goal would be to incorporate educational sessions and exercises into core healthcare curricula or conduct regular awareness courses that highlight the significance and risk of fomites and teach proper practices to avoid the consequences [8, 40].
CONCLUSIONS
In conclusion, proper disinfection and cleaning correlates with lower incidence of hospital- and healthcare-acquired infections as outlined in the Discussion. Our results demonstrate the vast gap between the knowledge and practices of healthcare personnel in this particular matter. Possessing adequate knowledge is ineffectual until and unless it is translated into the proper application of infection control practices by all tiers of the healthcare system and medical professionals. This is especially important in the education and training of medical students, who represent the majority subset of healthcare professionals who do not follow appropriate practices relating to the spread of pathogens via fomites. We recommend proper measures to interrupt fomite-mediated transmission of pathogens by ensuring optimal hand hygiene among healthcare workers and frequent environment disinfection, especially the surfaces that are frequently in contact with patient and patients' surroundings. Today, with the increasing use of computer equipment and cell phones, we also encourage regular cleaning of these devices as well because they are frequently overlooked. The first step we will take with the results of this study is to present them at core curriculum sessions for each group of participants. We have proposed the incorporation of these sessions in the academic curricula of all trainees to the academic council at our institution, including large class format sessions and small group tutorials, for example, at the beginning of ward rotations for medical students. We have also proposed holding workshops and continuing medical education sessions at the university and invite trainees from other hospitals to spread awareness and provide strategies to keep infection transmission via fomites to a minimum.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Dr. Afia Zafar (Professor and Consultant, Section of Microbiology, Department of Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan) for help in developing and validating the questionnaire used in this study.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Boone SA, Gerba CP. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol 2007; 73:1687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattar SA. Survival of microorganisms on animate and inanimate surfaces and their disinfection. In: Disinfection, Sterilization and Antisepsis: Principles and Practices in Healthcare Facilities. Association for Professionals in Infection Control and Epidemiology, Inc; Washington, DC; 2001: pp 195–205. [Google Scholar]
- 3.Kramer A, Schwebke I, Kampf GN. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006; 6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bures S, Fishbain JT, Uyehara CF et al. Computer keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. Am J Infect Control 2000; 28:465–71. [DOI] [PubMed] [Google Scholar]
- 5.Garvin KW, Lipira L, Neradileck M et al. Attitudes regarding the safety of health care provider attire. Am J Infect Control 2014; 42:1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehmood Z, Mubeen SM, Afzal MS, Hussain Z. Potential risk of cross-infection by tourniquets: a need for effective control practices in Pakistan. Int J Prev Med 2014; 5:1119–24. [PMC free article] [PubMed] [Google Scholar]
- 7.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008; 46:752–60. [DOI] [PubMed] [Google Scholar]
- 8.Neely AN, Sittig DF. Basic microbiologic and infection control information to reduce the potential transmission of pathogens to patients via computer hardware. J Am Med Inform Assoc 2002; 9:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanessa dos Santos da Silva J, Henrique de Mello M, Staggemeier R et al. Adenovirus presence in surfaces and equipment from ambulatories, internship units, and operating rooms in a Brazilian hospital. Am J Infect Control 2014; 42:693–4. [DOI] [PubMed] [Google Scholar]
- 10.Bhoonderowa A, Gookool S, Biranjia-Hurdoyal SD. The importance of mobile phones in the possible transmission of bacterial infections in the community. J Community Health 2014; 39:965–7. [DOI] [PubMed] [Google Scholar]
- 11.Khan A, Rao A, Reyes-Sacin C et al. Use of portable electronic devices in a hospital setting and their potential for bacterial colonization. Am J Infect Control 2015; 43:286–8. [DOI] [PubMed] [Google Scholar]
- 12.Kumar BV, Hobani YH, Abdulhaq A et al. Prevalence of antibacterial resistant bacterial contaminants from mobile phones of hospital inpatients. Libyan J Med 2014; 9:25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamimi AH, Carlino S, Gerba CP. Long-term efficacy of a self-disinfecting coating in an intensive care unit. Am J Infect Control 2014; 42:1178–81. [DOI] [PubMed] [Google Scholar]
- 14.Weber DJ, Rutala WA, Miller MB et al. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control 2010; 38:25–33. [DOI] [PubMed] [Google Scholar]
- 15.Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol 2011; 32:687–99. [DOI] [PubMed] [Google Scholar]
- 16.Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis 2004; 39:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control (CDC). Public health focus: surveillance, prevention, and control of nosocomial infections. MMWR Morb Mortal Wkly Rep 1992; 41:783–7. [PubMed] [Google Scholar]
- 18.Maki D. Nosocomial infection in the intensive care unit. In: Parrillo JE, Bone RC, ed. Critical Care Medicine: Principles of Diagnosis and Management. St. Louis, MO: Mosby; 1995: pp 893–954. [Google Scholar]
- 19.Martone WJ, Jarvis WR, Culver DH, Haley RW. Incidence and nature of endemic and epidemic nosocomial infections. In Bennett JV, Brachman PS, ed. In: Hospital Infections. Boston, MA: Little, Brown; 1992: pp 577–97. [Google Scholar]
- 20.Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control 1995; 16:577–81. [DOI] [PubMed] [Google Scholar]
- 21.Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med 1991; 91:72S–5S. [DOI] [PubMed] [Google Scholar]
- 22.Porwancher R, Sheth A, Rhemphery S et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemol 1997; 18:771–3. [DOI] [PubMed] [Google Scholar]
- 23.Boyce JM, Opal SM, Chow JW et al. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol 1994; 32:1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorin M, Segal-Maurer S, Mariano N et al. Nosocomial transmission of imipenem-resistant Pseudomonas aeruginosa following bronchoscopy associated with improper connection to the Steris System 1 processor. Infect Control Hosp Epidemiol 2001; 22:409–13. [DOI] [PubMed] [Google Scholar]
- 25.Datz C, Jungwirth A, Dusch H et al. What's on doctors’ ball point pens? Lancet 1997; 350:1824. [DOI] [PubMed] [Google Scholar]
- 26.Slaughter S, Hayden MK, Nathan C et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996; 125:448–56. [DOI] [PubMed] [Google Scholar]
- 27.Cohen HA, Amir J, Matalon A et al. Stethoscopes and otoscopes--a potential vector of infection? Fam Pract 1997; 14:446–9. [DOI] [PubMed] [Google Scholar]
- 28.Weber DJ, Rutala WA. Role of environmental contamination in the transmission of vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 1997; 18:306–9. [DOI] [PubMed] [Google Scholar]
- 29.Duckro AN, Blom DW, Lyle EA et al. Transfer of vancomycin-resistant enterococci via health care worker hands. Arch Intern Med 2005; 165:302–7. [DOI] [PubMed] [Google Scholar]
- 30.Huang R, Mehta S, Weed D et al. Methicillin-resistant Staphylococcus aureus survival on hospital fomites. Infect Control Hosp Epidemiol 2006; 27:1267–9. [DOI] [PubMed] [Google Scholar]
- 31.Barker J, Vipond IB, Bloomfield SF. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J Hosp Infect 2004; 58:42–9. [DOI] [PubMed] [Google Scholar]
- 32.Butz AM, Fosarelli P, Dick J et al. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics 1993; 92:202–5. [PubMed] [Google Scholar]
- 33.Hartmann B, Benson M, Junger A et al. Computer keyboard and mouse as a reservoir of pathogens in an intensive care unit. J Clin Monit Comput 2004; 18:7–12. [DOI] [PubMed] [Google Scholar]
- 34.Robinson T, Cronin T, Ibrahim H et al. Smartphone use and acceptability among clinical medical students: a questionnaire-based study. J Med Syst 2013; 37:9936. [DOI] [PubMed] [Google Scholar]
- 35.Borer A, Gilad J, Smolyakov R et al. Cell phones and Acinetobacter transmission. Emerg Infect Dis 2005; 11:1160–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldblatt JG, Kreif I, Kolonsky T et al. Use of cellular telephones and transmission of pathogens by medical staff in New York and Israel. Infect Control Hosp Epidemiol 2007; 28:500–3. [DOI] [PubMed] [Google Scholar]
- 37.Neely AN, Maley MP, Warden GD. Computer keyboards as reservoirs for Acinetobacter baumannii in a burn hospital. Clin Infect Dis 1999; 29:1358–60. [DOI] [PubMed] [Google Scholar]
- 38.Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997:622–7. [PubMed] [Google Scholar]
- 39.Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982; 97:309–17. [DOI] [PubMed] [Google Scholar]
- 40.Ulger F, Esen S, Dilek A et al. Are we aware how contaminated our mobile phones with nosocomial pathogens? Ann Clin Microbiol Antimicrob 2009; 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutala WA, Weber DJ, C. Centers for Disease. Guideline for Disinfection and Sterilization in Healthcare Facilities 2008. Centers for Disease Control and Prevention, 2008. Available at: http://stacks.cdc.gov/view/cdc/11560/#main-content. Accessed XX XXXX XXXX. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.