Abstract
IMPORTANCE
More than half of youth with autism spectrum disorders (ASDs) have sensory overresponsivity (SOR), an extreme negative reaction to sensory stimuli. However, little is known about the neurobiological basis of SOR, and there are few effective treatments. Understanding whether SOR is due to an initial heightened sensory response or to deficits in regulating emotional reactions to stimuli has important implications for intervention.
OBJECTIVE
To determine differences in brain responses, habituation, and connectivity during exposure to mildly aversive sensory stimuli in youth with ASDs and SOR compared with youth with ASDs without SOR and compared with typically developing control subjects.
DESIGN, SETTING, AND PARTICIPANTS
Functional magnetic resonance imaging was used to examine brain responses and habituation to mildly aversive auditory and tactile stimuli in 19 high-functioning youths with ASDs and 19 age- and IQ-matched, typically developing youths (age range, 9-17 years). Brain activity was related to parents’ ratings of children's SOR symptoms. Functional connectivity between the amygdala and orbitofrontal cortex was compared between ASDs subgroups with and without SOR and typically developing controls without SOR. The study dates were March 2012 through February 2014.
MAIN OUTCOMES AND MEASURES
Relative increases in blood oxygen level–dependent signal response across the whole brain and within the amygdala during exposure to sensory stimuli compared with fixation, as well as correlation between blood oxygen level–dependent signal change in the amygdala and orbitofrontal cortex.
RESULTS
The mean age in both groups was 14 years and the majority in both groups (16 of 19 each) were male. Compared with neurotypical control participants, participants with ASDs displayed stronger activation in primary sensory cortices and the amygdala (P < .05, corrected). This activity was positively correlated with SOR symptoms after controlling for anxiety. The ASDs with SOR subgroup had decreased neural habituation to stimuli in sensory cortices and the amygdala compared with groups without SOR. Youth with ASDs without SOR showed a pattern of amygdala downregulation, with negative connectivity between the amygdala and orbitofrontal cortex (thresholded at z > 1.70, P < .05).
CONCLUSIONS AND RELEVANCE
Results demonstrate that youth with ASDs and SOR show sensorilimbic hyperresponsivity to mildly aversive tactile and auditory stimuli, particularly to multiple modalities presented simultaneously, and show that this hyperresponsivity is due to failure to habituate. In addition, findings suggest that a subset of youth with ASDs can regulate their responses through prefrontal downregulation of amygdala activity. Implications for intervention include minimizing exposure to multiple sensory modalities and building coping strategies for regulating emotional response to stimuli.
Overresponsivity to sensory stimuli is a common symptom of autism spectrum disorders (ASDs) that is understudied, likely because it was only recently added to DSM-5 diagnostic criteria.1 At least 56% to 70% of youth with ASDs meet criteria for sensory overresponsivity (SOR),2,3 which includes severe negative responses to stimuli (eg, noisy environments, scratchy clothing, and being touched) that do not elicit such responses in individuals without SOR.4 Sensory overresponsivity is associated with greater functional impairment in individuals with ASDs, deficits in social and adaptive skills, and anxiety.4-6 Little is known about the neurobiological basis of SOR. However, electroencephalography studies7,8 have demonstrated deficits in sensory gating and selective attention of sensory input, suggesting that individuals with ASDs may become easily overwhelmed by irrelevant or multiple stimuli. Most important, ASDs represent a heterogeneous disorder, and only some diagnosed individuals have SOR. An electrodermal study9 found that high-functioning youth with ASDs showed high arousal and slow habituation or low arousal and fast habituation.
While research on the neurological basis of SOR is new, results of a recent functional magnetic resonance imaging (fMRI) study10 suggest that SOR is related to hyperactivity in brain areas involved in primary sensory processing, emotion regulation,and response to threat. The authors found that youth with ASDs had overactivation in limbic areas, primary sensory cortices, and orbitofrontal cortex (OFC) compared with typically developing (TD) control subjects in response to mildly aversive visual and auditory stimuli. Furthermore, activity in these regions correlated with parents’ reports of SOR. Limbic overactivation is consistent with the co-occurrence of SOR and anxiety11 as well as with amygdala hyperactivity in response to faces in children with ASDs.12,13 Notably, overreactivity observed in individuals with ASDs was most evident when auditory and visual stimuli occurred simultaneously, consistent with the sensory gating hypothesis and with neurophysiological multisensory integration investigations.14
In the present study, we examined responses to tactile and auditory stimuli. Overresponsivity to these stimuli has been found to best distinguish individuals with and without SOR.15-17 These stimuli are also among those most often reported as aversive for children with ASDs.17,18 Evidence from a 2008 fMRI study19 suggests that adolescents with ASDs have heightened neural responses to novel sounds in higher-level processing areas, including prefrontal and inferior parietal areas. Individuals with ASDs may also have overreactive brain responses to unpleasant touch but a diminished response to pleasant touch,20 highlighting the importance of using aversive sensory stimuli when examining patients with SOR.
To our knowledge, other than the study by Green et al,10 no studies have examined fMRI responses to multiple sensory stimuli simultaneously, which more closely resembles real-world environments. We aimed to follow up on whether SOR is related to reduced habituation rather than to a higher initial response to sensory stimuli. In a tactile discrimination study,21 adaptation stimuli were found to have a reduced effect on youth with ASDs, suggesting deficits in habituation and inhibition. Youth with ASDs have also shown decreased amygdala habituation to other arousing stimuli (eg, faces).12,22 Accordingly, we hypothesized that SOR would be related to reduced habituation to sensory stimuli, particularly in the amygdala and primary sensory cortices.
Finally, Green et al10 found that SOR symptoms correlated with hyperactivity in the amygdala and OFC. The OFC receives inputs from all sensory modalities, has strong connectivity with the amygdala,23 and is associated with top-down emotion regulation.24 Amygdala and prefrontal (including the OFC) activity is typically negatively coupled such that increased prefrontal activation is associated with decreased amygdala activation in response to threat-relevant stimuli.25-27 Simultaneous overactivity in the amygdala and OFC could indicate an ineffective regulatory system, whereby the OFC activates but fails to sufficiently downregulate the amygdala, such as in social anxiety disorder.28,29 Alternatively, this pattern could indicate an immature emotion regulation system. Neurotypical youth display positive connectivity between the amygdala and prefrontal cortex,30 and there is evidence of reduced structural connectivity between the amygdala and OFC in ASDs.31 Herein, we examined functional amygdala-OFC connectivity during exposure to sensory stimuli to determine how it might relate to SOR in youth with ASDs.
Methods
Participants
Participants were 19 youths with ASDs and 19 TD matched controls 9 to 17 years old (mean [SD] age, 13.66 [2.11] years). The groups did not differ significantly in age, IQ, or motion during fMRI (Table 1 and eMethods in the Supplement). Participants with ASDs had a prior diagnosis of ASDs, confirmed using the Autism Diagnostic Interview–Revised32 and the Autism Diagnostic Observation Schedule, Second Edition.33 All study procedures were approved by the University of California, Los Angeles, Institutional Review Board. Written informed consent was obtained from parents and participants 13 years or older. Written assent was obtained from participants younger than 13 years. The study was conducted between March 2012 and February 2014.
Table 1.
Descriptive Statistics
| Variable | ASDs (n = 19) | TD (n = 19) | t Statistic or χ2 Statistic |
|---|---|---|---|
| Age, mean (SD), y | 13.71 (1.60) | 13.61 (2.57) | 0.13 |
| Male sex, No. (%) | 16 (84) | 16 (84) | 0.00 |
| Right-handedness, No. (%) | 18 (95) | 19 (100) | 0.31 |
| IQ, mean (SD) | |||
| Full-scale | 104.63 (13.22) | 107.37 (15.06) | –0.59 |
| Verbal | 103.74 (13.49) | 107.63 (13.17) | –0.90 |
| Performance | 103.70 (14.47) | 105.76 (16.00) | –0.42 |
| Absolute motion, mean (SD), mm | |||
| Mean | 0.33 (0.17) | 0.31 (0.23) | 0.21 |
| Maximum | 0.94 (0.64) | 0.87 (0.97) | 0.29 |
| Relative motion, mean (SD), mm | |||
| Mean | 0.09 (0.04) | 0.13 (0.20) | –0.90 |
| Maximum | 0.80 (0.63) | 0.61 (1.15) | 0.62 |
| Sensory Over-Responsivity Scales score, mean (SD) | (n = 17) | ||
| Tactile count | 4.79 (5.57) | 2.76 (4.12) | 1.22 |
| Auditory count | 6.89 (7.06) | 1.56 (3.90) | 2.87a |
| Short Sensory Profile score, mean (SD)b | (n = 18) | ||
| Auditory and visual | 19.32 (5.10) | 24.28 (2.11) | –3.90a |
| Auditory filtering | 17.42 (6.00) | 26.11 (4.01) | –5.20c |
| Tactile sensitivity | 27.32 (6.19) | 32.89 (3.64) | –3.31a |
| Sensory overresponsivity composite score, mean (SD) | 0.45 (0.93) | –0.45 (0.51) | 3.71a |
| Screen for Child Anxiety Related Emotional Disorders anxiety total score, mean (SD) | 13.84 (9.44) | 5.47 (5.88) | 3.28a |
Abbreviations: ASDs, autism spectrum disorders; TD, typically developing.
P < .01.
Lower scores indicate higher symptom severity.
P < .001.
fMRI Sensory Paradigm
Participants were exposed to 3 stimulus conditions in a counterbalanced block design paradigm (eFigure 1 in the Supplement). These stimuli included an auditory condition, a tactile condition, and a joint condition in which the auditory and tactile stimuli were presented simultaneously. Auditory stimuli consisted of traffic noises. The tactile stimulus was a scratchy wool fabric rubbed on participants’ inner arms at the rate of one stroke per second. Stimuli were chosen that best differentiated the ASDs vs TD groups based on pilot testing with the Sensory Over-Responsivity Scales.34 Participants were instructed to focus on a central fixation cross throughout the task. Each condition was presented 4 times, lasting 15 seconds (each defined as one block), with 12.5 seconds of fixation between trials. Total scan length was 5 minutes and 42.5 seconds, including 12.5-second initial and final fixations. The eMethods in the Supplement contains information on functional magnetic resonance imaging data acquisition.
Behavioral Measures
Diagnostic and cognitive measures were administered at a clinical assessment visit. Child anxiety and sensory responsivity questionnaires were completed by parents (Table 1 and eMethods in the Supplement). An SOR composite score was created by standardizing and averaging relevant subscales of the SOR measures (Short Sensory Profile17 auditory and visual sensitivity, tactile sensitivity, and auditory filtering scales and Sensory Over-Responsivity Scales34 auditory and tactile scores) across all participants. Children in the top 25th percentile of this composite (9 with ASDs and 1 TD) were categorized as having elevated SOR. For analyses comparing SOR subgroups, participants were divided into the following 3 groups: ASDs with SOR, ASDs without SOR, and TD without SOR. For analyses comparing diagnostic groups (TD vs ASDs), subgroups were collapsed so that all TD participants were compared with all participants with ASDs.
fMRI Data Analysis
Within-group activation maps for each condition (vs fixation) were thresholded at z > 2.30 (P < .01) and whole-brain cluster corrected at P < .05 using the FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl). Between-group comparisons were thresholded at z > 1.70 (P < .05) and whole-brain cluster corrected at P < .05, and only clusters with peaks of z > 2.30 are reported as significant. For all analyses except habituation analyses, activation was averaged across the 4 blocks in each condition. For habituation analyses, activation parameter estimates (PEs) were extracted separately from each block in the joint condition. Age was covaried in group-level analyses. Because of a priori interest in the amygdala (defined by the Harvard-Oxford Probabilistic Atlas [http://fsl.fmrib.ox.ac.uk/fsl/fsl4.0/fslview/atlas-descriptions.html#ho], thresholded at 75%), small-volume correction was used to correct for multiple comparisons within this region of interest using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html).
Correlation With SOR Scores
To determine whether SOR predicted blood oxygen level–dependent response over and above anxiety, regression analyses were performed with the SOR composite score as the independent variable and the Screen for Child Anxiety Related Emotional Disorders35 total anxiety scores as covariates. Parameter estimates for significant clusters in functionally defined regions of interest (primary somatosensory and auditory cortices and the amygdala) were extracted from each participant and plotted.
Neural Habituation
Habituation was assessed by examining the pattern of activation across time in the amygdala and somatosensory and auditory cortices. Region-of-interest masks were created by drawing a sphere around the peak coordinate in each region of interest for each group and then adding the spheres together. Sphere sizes (before adding) were 4 mm for the amygdala, 6 mm for the somatosensory cortex, and 10 mm for the auditory cortex (Table 2 gives the coordinates). For each participant, PEs from the 4 blocks of the joint condition (vs rest) were extracted from the masks. Repeated-measures analyses of variance were used to examine group differences in PEs between diagnostic groups (ASDs vs TD) and across SOR subgroups (ASDs without SOR vs ASDs with SOR vs TD). We report results for the joint condition because this condition elicited the greatest between-group differences.
Table 2.
Montreal Neurologic Institute (MNI) Coordinates for the Joint Auditory and Tactile Condition Compared With Baselinea
| TD |
ASDs |
ASDs > TD |
Regress With Sensory Overresponsivity |
|||||
|---|---|---|---|---|---|---|---|---|
| MNI Peak, mm |
Maximum |
MNI Peak, mm |
Maximum |
MNI Peak, mm |
Maximum |
MNI Peak, mm |
Maximum |
|
| Variable | x, y, z | z Score | x, y, z | z Score | x, y, z | z Score | x, y, z | z Score |
| Postcentral gyrus | ||||||||
| Right | 26, –38, 66 | 4.98 | 24, –38, 68 | 6.58 | 50, –16, 36 | 4.63 | 30, –38, 58 | 3.64 |
| Left | –52, –22, 24 | 3.64 | –22, –42, 68 | 4.49 | –60, –10, 40 | 3.47 | –62, –26, 30 | 2.47 |
| Precentral gyrus | ||||||||
| Right | 28, –16, 66 | 3.30 | 26, –14, 64 | 4.01 | 58, 8, 36 | 3.42 | 34, –10, 70 | 4.30 |
| Left | NA | NA | –62, 6, 26 | 4.41 | NA | NA | –56, 4, 24 | 3.70 |
| Right supplementary motor cortex | NA | NA | 6, –10, 56 | 2.82 | 6, 6, 50 | 3.31 | 6, –12, 58 | 2.74 |
| Inferior frontal gyrus | ||||||||
| Right | NA | NA | 58, 16, –4 | 4.79 | NA | NA | NA | NA |
| Left | NA | NA | –48, 10, 16 | 3.50 | –48, 12, 16 | 2.50 | NA | NA |
| Frontal gyrus | ||||||||
| Right superior | NA | NA | NA | NA | 4, 22, 46 | 2.45 | NA | NA |
| Right middle | NA | NA | NA | NA | 54, 20, 38 | 3.58 | NA | NA |
| Right frontal pole | NA | NA | 12, 50, 46 | 4.11 | 38, 50, 16 | 4.43 | NA | NA |
| Frontal orbital cortex | ||||||||
| Right | NA | NA | 42, 22, –14 | 4.29 | 42, 22, –14 | 2.72 | NA | NA |
| Left | NA | NA | 48, 14, 46 | 3.15 | NA | NA | NA | NA |
| Heschl gyrus | ||||||||
| Right | 52, –20, 10 | 6.62 | 48, –20, 12 | 7.10 | NA | NA | NA | NA |
| Left | –46, –14, 4 | 5.79 | –42, –22, 0 | 5.34 | NA | NA | NA | NA |
| Superior temporal gyrus | ||||||||
| Right | 62, –34, 18 | 6.15 | 62, –20, 12 | 6.61 | NA | NA | NA | NA |
| Left | –40, –32, 14 | 5.90 | –54, –8, 4 | 5.99 | –52, 10, –16 | 3.45 | –66, –32, 18 | 4.92 |
| Left temporal pole | NA | NA | NA | NA | NA | NA | –48, 10, –8 | 3.31 |
| Middle temporal gyrus | ||||||||
| Right | NA | NA | 54, 14, –16 | 3.15 | 62, –20, –14 | 3.90 | NA | NA |
| Left | NA | NA | NA | NA | NA | NA | –52, –56, 8 | 2.36 |
| Operculum | ||||||||
| Right | 48, 10, 0 | 3.45 | 40, –26, 22 | 6.18 | –54, –6, 4 | 3.07 | 58, –28, 26 | 4.31 |
| Left | NA | NA | –40, –32, 18 | 7.15 | NA | NA | –40, –4, 16 | 3.46 |
| Insula | ||||||||
| Right | 38, –20, 0 | 4.91 | 38, –20, 0 | 5.43 | NA | NA | NA | NA |
| Left | NA | NA | –38, –4, –12 | 4.60 | NA | NA | –34, –16, 8 | 2.80 |
| Left supramarginal gyrus | NA | NA | –64, –30, 20 | 5.53 | –64, –36, 32 | 3.12 | –62, –52, 18 | 3.27 |
| Posterior cingulate | NA | NA | NA | NA | –2, –36, 24 | 4.91 | 0, –26, 26 | 3.44 |
| Superior parietal lobule | ||||||||
| Right | NA | NA | 18, –50, 74 | 5.28 | 18, –52, 74 | 3.36 | 20, –50, 70 | 2.65 |
| Left | NA | NA | –34, –46, 64 | 4.04 | NA | NA | NA | NA |
| Left fusiform | NA | NA | NA | NA | –28, –74, –18 | 3.81 | NA | NA |
| Caudate | ||||||||
| Right | NA | NA | 14, –2, 18 | 3.19 | 16, 18, 10 | 3.15 | NA | NA |
| Left | NA | NA | –12, 12, 12 | 3.22 | –18, 26, 4 | 2.69 | NA | NA |
| Putamen | ||||||||
| Right | NA | NA | 28, 10, 2 | 4.87 | 28, 10, 0 | 3.06 | NA | NA |
| Left | NA | NA | –26, 6, –6 | 3.97 | –24, 12, 2 | 3.17 | NA | NA |
| Right pallidum | NA | NA | NA | NA | 20, –8, 0 | 3.00 | NA | NA |
| Right thalamus | ||||||||
| Ventral nucleus | NA | NA | 14, –16, 12 | 3.78 | –10, –12, 4 | 2.31 | NA | NA |
| Pulvinar | NA | NA | 16, –26, 2 | 3.64 | 14, –28, 14 | 3.13 | NA | NA |
| Left hippocampus and parahippocampal gyrus | NA | NA | NA | NA | –28, –12, –22 | 3.84 | NA | NA |
| Cerebellum | NA | NA | 34, –78, –42 | 3.63 | –42, –60, –46 | 4.29 | –10, –70, –38 | 3.47 |
Abbreviations: ASD, autism spectrum disorder group; NA, not applicable; TD, typically developing.
The x, y, and z refer to left-right, anterior-posterior, and inferior-superior dimensions, respectively, and the z score refers to the score at those coordinates (local maximum or submaximum). Within-group analyses are cluster corrected for multiple comparisons at z >2.30 (P < .05), and between-group and regression analyses are thresholded at z > 1.70 (corrected). Between-group analyses are masked by regions of significant activation in either within-group analysis at the liberal threshold of z > 1.70 (uncorrected). Regression results show clusters with activation significantly correlated with the sensory overresponsivity composite score within the ASDs group over and above age and anxiety symptons.
Functional Connectivity
A psychophysiological interaction analysis was used to examine functional connectivity between the amygdala and OFC during the joint condition. This analysis examines interaction between a task and the time series of seed region (here the amygdala) to identify brain areas (here the OFC as the a priori region of interest) where activity is more correlated with the seed region during the task vs baseline. Both positive connectivity and negative connectivity (areas showing increased and decreased activity as a function of increased activity in the amygdala) were examined.
Results
Behavioral Findings
Independent-sample t tests showed that the ASDs group was rated as having significantly more severe anxiety and SOR symptoms than the TD group on all measures except the Sensory Over-Responsivity Scales tactile count (Table 1). Correlation between the Screen for Child Anxiety Related Emotional Disorders anxiety total score and the SOR composite score was significant in both groups (r = 0.56, P < .05 for the TD group and r = 0.69, P < .01 for the ASDs group). The eResults in the Supplement contains additional group comparisons.
fMRI Findings
Within-Group and Between-Group Findings
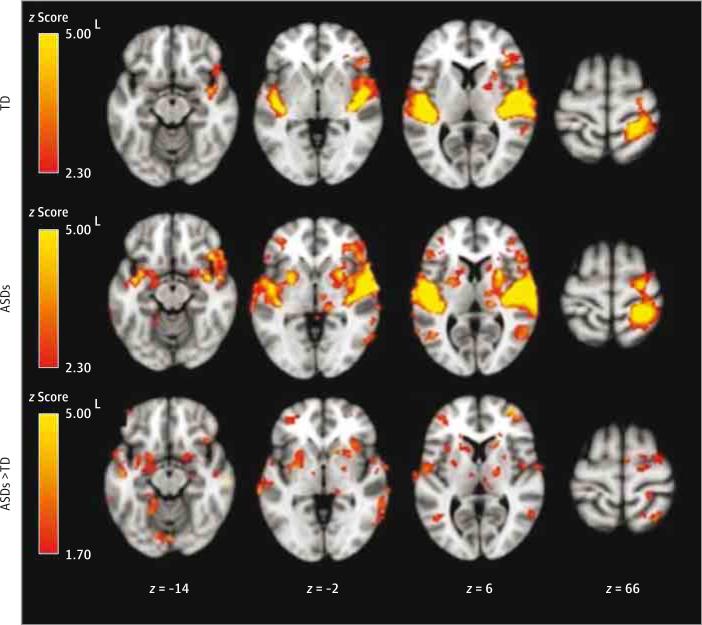
Within-group and additional between-group results are summarized in Table 2 and Figure 1 and eTables 1, 2, and 3 and eFigures 2 and 3 in the Supplement. There were no significant TD vs ASDs group differences in the auditory condition. During the tactile condition, the ASDs group had greater activation in the bilateral somatosensory cortex. In the joint condition, the ASDs group had greater activation in the bilateral somatosensory cortex, left superior temporal gyrus, right OFC, and left amygdala as well as additional subcortical areas. There were no clusters with significantly greater activation in the TD group compared to the ASDs group.
Figure 1.
Within-Group and Between-Group Results for the Joint Auditory and Tactile Condition
Within-group contrasts are thresholded at z > 2.30 (corrected at P < .05). Between-group contrasts are thresholded at z > 1.70 (corrected). Between-group maps are masked by regions active in either within-group condition at z > 1.70 (uncorrected). ASDs indicates autism spectrum disorders; L, left; and TD, typically developing.
Correlation With SOR Severity
In youth with ASDs, SOR scores were positively correlated with signal increases in bilateral somatosensory cortices and the right amygdala as well as additional parietal and temporal areas. These results are summarized in Table 2 and eTable 3 and eFigure 4 in the Supplement.
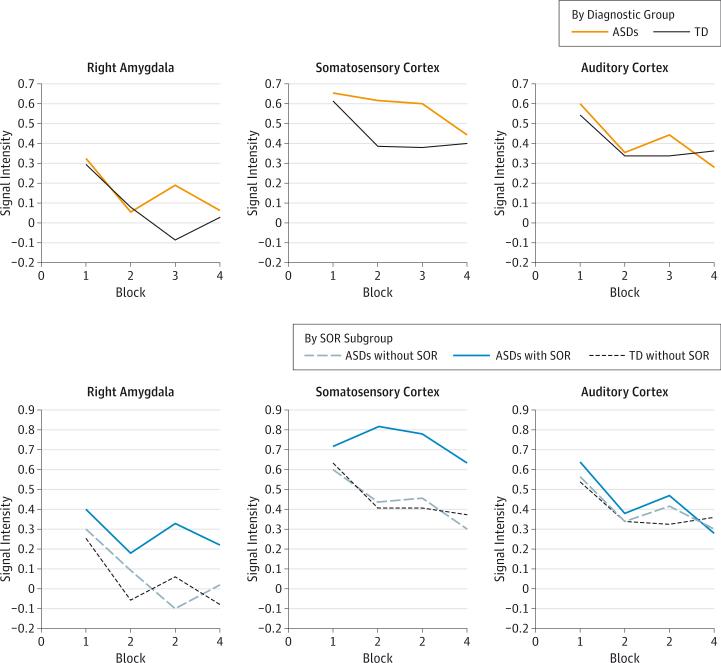
Neural Habituation During the Joint Condition
In primary auditory cortex, there were significant linear and quadratic decreases in activation across the scan, indicating that auditory neural responses decreased quickly but then leveled off. No significant differences were observed among diagnostic groups or SOR subgroups (Figure 2 and eTable 4 in the Supplement).
Figure 2.
Amygdala and Sensory Cortex Habituation by Diagnostic Group and by Sensory Overresponsivity (SOR) Subgroup
The vertical axis represents region-of-interest parameter estimates during the tactile or joint condition compared with baseline. The horizontal axis represents each block of the condition. ASDs indicates autism spectrum disorders; TD, typically developing.
Activation in the somatosensory cortex decreased linearly across the scan, and no main effect of diagnostic group was observed. There was a trend-level (P = .06) diagnosis × time interaction for the quadratic slope term, indicating that somatosensory cortex activation decreased more slowly across the scan in the ASDs group than in the TD group. There was no significant main effect of SOR subgroup × time interaction. However, a post hoc least significant difference test indicated that activation in the ASDs with SOR subgroup was marginally higher than activation in the ASDs without SOR subgroup (P = .07) and the TD subgroup (P = .05).
In the amygdala, there was a main effect of time such that activation decreased significantly, and the rate of decrease slowed across the 4 joint condition blocks. No main effect of diagnostic group was observed, but there was a significant diagnosis × time interaction for the cubic slope parameter, reflecting that, for the ASDs group, amygdala activation began to rise again toward the second half of the scan, whereas it continued to decrease for the TD group. There was a trend-level main effect of SOR subgroup (P = .08) and a significant SOR subgroup × time interaction for the cubic slope parameter. A post hoc least significant difference test showed a significant difference between the ASDs with SOR subgroup and the ASDs without SOR subgroup (P = .04) and the TD subgroup (P = .047). There was no significant difference between the ASDs without SOR subgroup and the TD subgroup.
Taken together, these results show SOR subgroup differences in habituation in the amygdala and somatosensory cortices but not in the auditory cortex. All groups had similar initial activity, but it quickly decreased in the TD subgroup and the ASDs without SOR subgroup, whereas it decreased more slowly or inconsistently in the ASDs with SOR subgroup.
Functional Connectivity
Within the TD group only, the right amygdala had positive functional connectivity with the bilateral OFC during the joint condition. Between-group analyses showed significant group differences in connectivity between the right amygdala and left OFC (peak coordinates, −42, 36, −6). Extraction of PEs from the left OFC showed that the ASDs group had negative connectivity with the left OFC, whereas the TD group had positive connectivity.
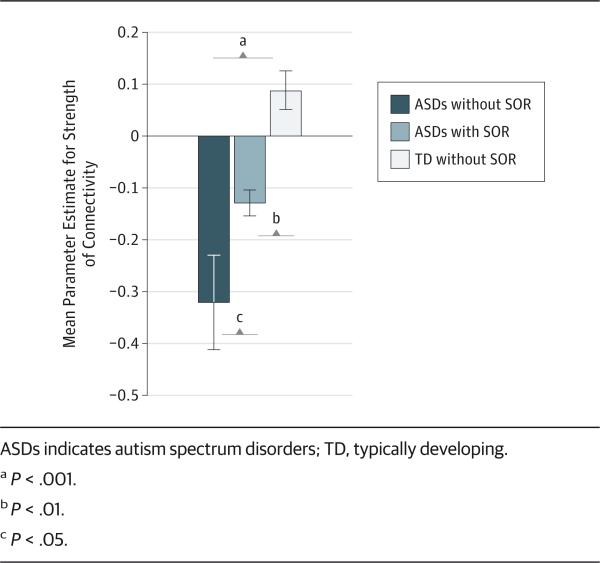
To further examine differences in amygdala-prefrontal connectivity among the 3 SOR subgroups, we conducted a 1-way analysis of variance using PEs of connectivity between the amygdala and OFC (Figure 3). There were significant differences between all 3 subgroups (F2 = 16.96, P < .001). A post hoc least significant difference test indicated that the ASDs without SOR subgroup (mean [SD], −0.32 [0.29]) showed significantly greater negative connectivity than the ASDs with SOR subgroup (mean [SD], −0.13 [0.07]; P = .03) and the TD sub-group group (mean [SD], 0.09 [0.14]; P < .001). The TD subgroup was also significantly different from the ASDs with SOR subgroup (P = .006). In summary, the ASDs without SOR subgroup showed the most strongly negative connectivity, the ASDs with SOR subgroup showed less negative connectivity, and the TD subgroup showed slightly positive connectivity between the right amygdala and OFC.
Figure 3.
Amygdala–Orbitofrontal Cortex Connectivity by Sensory Overresponsivity (SOR) Subgroup
Discussion
This study investigated the neurobiological basis of SOR by comparing brain responses to aversive sensory stimuli in TD youth and youth with ASDs with and without SOR. In addition, we explored whether SOR is related to abnormalities in initial sensory processing or to regulation of emotional response to sensory information by examining neural habituation in sensory cortices and the amygdala as well as amygdala-OFC functional connectivity during exposure to mildly aversive stimuli.
Results indicated that youth with ASDs have greater neural responses to mildly aversive sensory stimuli compared with TD youth. There were no group differences in response to auditory stimuli alone. Lack of aversiveness or familiarity with these traffic sounds might have reduced differential group responses in this condition. Conversely, the tactile condition elicited group differences in the primary somatosensory cortex, and the extent of activation in this area correlated with parent-reported SOR symptoms. There were no overall ASDs vs TD group differences in emotional processing regions in response to the tactile stimulus, but SOR symptoms within the ASDs group were correlated with increased response in the insula and amygdala. The insula is involved in interoception and emotional processing of sensory stimuli and receives inputs from the amygdala based on the perceived saliency of touch.36,37 Furthermore, overreactive insula response during emotional processing is associated with anxiety,38,39 consistent with the common co-occurrence of SOR and anxiety.
The greatest differences between youth with and without ASDs occurred in response to simultaneous auditory and tactile stimuli. Here, the ASDs group showed stronger neural responses in sensory processing regions, including auditory and tactile sensory cortices and the thalamus, and in emotional processing regions, including the amygdala and OFC. Consistent with previous findings,11 the extent of activation in sensory cortices, the amygdala, and the insula was correlated with SOR severity within the ASDs group.
Habituation analyses in the amygdala and somatosensory cortex showed that both diagnostic groups began with similar activation, which decreased over time, but that the TD group habituated more quickly. When the ASDs group was divided into 2 subgroups with and without SOR, it became clear that the ASDs with SOR subgroup not only habituated more slowly but also ended the scan with higher activity levels than the other subgroups, whereas the ASDs without SOR subgroup had habituation more similar to that of the TD subgroup.
To further understand how emotion regulation might relate to SOR, we examined functional connectivity between the amygdala and OFC during the joint condition. The ASDs without SOR subgroup had the most significant negative amygdala-OFC connectivity, the ASDs with SOR subgroup had slightly negative connectivity, and the TD subgroup had slightly positive connectivity. This finding is in contrast to the study by Green et al,10 who found that higher SOR was related to higher OFC activation. However, medial OFC in their study was related to SOR as opposed to the more lateral areas seen in the present study. Medial OFC has more connectivity with the hippocampus,40 which showed more activation in the study by Green et al,10 perhaps due to the participants’ associating sounds with the visual context.40 Lateral OFC has greater connectivity with the amygdala and insula and is involved in inhibiting instinctive responses.40 Such inhibition could help youth with ASDs without SOR avoid the behavioral responses to sensory stimuli seen in youth with ASDs with SOR, which is consistent with the more typical habituation pattern found in the ASDs without SOR subgroup. Conversely, the TD subgroup may not perceive the stimuli as aversive, thus not having an overreactive amygdala response and not requiring prefrontal downregulation.
Taken together, these results confirm previous evidence of overreactive brain responses to sensory stimuli in youth with ASDs.10 Our findings further show that these overresponses are specific to youth with ASDs and elevated SOR, who show decreased habituation in the amygdala and sensory cortex and absence of amygdala-prefrontal negative connectivity. These results are consistent with previous studies22,41 of amygdala overreactivity and reduced habituation in ASDs in response to faces but also suggest that amygdala abnormalities in ASDs are not limited to social contexts. Rather, youth with ASDs may have overall difficulty determining the saliency and threat relevance of stimuli. Reduced top-down regulation in youth with ASDs and SOR could contribute to deficits in using context to assess the saliency of stimuli31 as well as to failures of selective inhibition and attention, which is consistent with sensory gating hypotheses.7,8
Strengths of this study include examining multiple modalities of sensory stimuli, accounting for within-group heterogeneity in SOR, and investigating brain overreactivity from multiple perspectives. We had limited power to examine within-group differences, but the pattern of results was consistent in showing greater overreactivity and reduced habituation in the ASDs with SOR subgroup. Additional research should examine this subgroup with larger samples. Future studies should also examine youth with ASDs without SOR in more detail because this subgroup may have developed unique coping strategies to inhibit sensory responses. In addition, we were limited to parental reports of SOR, and future studies might benefit from including examiner-administered physiological measures to help identify the role of state anxiety in SOR.
Conclusions
These findings have implications for intervention. First, the greatest overresponsiveness occurred in response to multiple simultaneous stimuli, suggesting that minimizing exposure to multiple sensory modalities could help youth with ASDs cope with SOR (eg, a child might be more tolerant of being touched in a quiet house than in a noisy movie theater). Second, youth with ASDs without SOR appear to have more ability to downregulate their response to sensory stimuli. This finding may indicate that intervention for SOR should focus on building coping strategies rather than on normalizing sensory processing. Successful interventions for teaching coping strategies to reduce anxiety in ASDs already exist.42,43 Given the high co-occurrence of anxiety and SOR in ASDs,11 it may be possible to adapt these interventions to target SOR.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grant P50 HD055786 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, by grant 1 R01 HD065280-01 from the National Institute of Mental Health, by National Research Service Award Individual Predoctoral Fellowship F31 MH093999-01A1 (Dr Green), and by grants RR12169, RR13642, and RR00865 from the National Center for Research Resources.
Footnotes
Author Contributions: Dr Green had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Green, Tottenham, Dapretto.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Green, Krasileva.
Critical revision of the manuscript for important intellectual content: Green, Hernandez, Tottenham, Bookheimer, Dapretto.
Statistical analysis: Green, Tottenham, Krasileva. Obtained funding: Dapretto.
Administrative, technical, or material support: Hernandez.
Study supervision: Tottenham, Bookheimer, Dapretto.
Conflict of Interest Disclosures: None reported.
Role of the Funder/Sponsor: The funding sources and organizations listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Additional Contributions: For generous support, we thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones Simon Foundation, The Capital Group Companies Charitable Foundation, and Robson Family and Northstar Fund.
Supplemental content at jamapsychiatry.com
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association Publishing; Arlington, VA: 2013. [Google Scholar]
- 2.Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Sasson A, Cermak SA, Orsmond GI, et al. Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. Am J Occup Ther. 2007;61(5):584–592. doi: 10.5014/ajot.61.5.584. [DOI] [PubMed] [Google Scholar]
- 4.Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer B, Kinnealey M, Reed C, Herzberg G. Sensory modulation and affective disorders in children and adolescents with Asperger's disorder. Am J Occup Ther. 2005;59(3):335–345. doi: 10.5014/ajot.59.3.335. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, Carter AS. Sensory clusters of toddlers with autism spectrum disorders: differences in affective symptoms. J Child Psychol Psychiatry. 2008;49(8):817–825. doi: 10.1111/j.1469-7610.2008.01899.x. [DOI] [PubMed] [Google Scholar]
- 7.Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69(5, pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuzaki J, Kagitani-Shimono K, Sugata H, et al. Progressively increased M50 responses to repeated sounds in autism spectrum disorder with auditory hypersensitivity: a magnetoencephalographic study. PLoS One. 2014;9(7):e102599. doi: 10.1371/journal.pone.0102599. doi:10.1371/journal.pone.0102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoen SA, Miller LJ, Brett-Green B, Hepburn SL. Psychophysiology of children with autism spectrum disorder. Res Autism Spectr Disord. 2008;2(3):417–429. doi:10.1016/j.rasd.2007.09.002. [Google Scholar]
- 10.Green SA, Rudie JD, Colich NL, et al. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(11):1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green SA, Ben-Sasson A. Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: is there a causal relationship? J Autism Dev Disord. 2010;40(12):1495–1504. doi: 10.1007/s10803-010-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinhans NM, Johnson LC, Richards T, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 13.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2014;9(1):106–117. doi: 10.1093/scan/nst050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo N, Foxe JJ, Brandwein AB, Altschuler T, Gomes H, Molholm S. Multisensory processing in children with autism: high-density electrical mapping of auditory-somatosensory integration. Autism Res. 2010;3(5):253–267. doi: 10.1002/aur.152. [DOI] [PubMed] [Google Scholar]
- 15.Kern JK, Trivedi MH, Garver CR, et al. The pattern of sensory processing abnormalities in autism. Autism. 2006;10(5):480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- 16.Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord. 2007;37(5):894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- 17.Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the Short Sensory Profile. Am J Occup Ther. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- 18.Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- 19.Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131(pt 9):2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- 20.Cascio CJ, Moana-Filho EJ, Guest S, et al. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Res. 2012;5(4):231–244. doi: 10.1002/aur.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puts NA, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RA. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol. 2014;111(9):1803–1811. doi: 10.1152/jn.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Wright P, Albarracin D, Brown RD, Li H, He G, Liu Y. Dissociated responses in the amygdala and orbitofrontal cortex to bottom-up and top-down components of emotional evaluation. Neuroimage. 2008;39(2):894–902. doi: 10.1016/j.neuroimage.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 26.Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28(5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sladky R, Höflich A, Küblböck M, et al. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI. Cereb Cortex. 2015;25(4):895–903. doi: 10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 30.Gee DG, Humphreys KL, Flannery J, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zalla T, Sperduti M. The amygdala and the relevance detection theory of autism: an evolutionary perspective. Front Hum Neurosci. 2013;7:894. doi: 10.3389/fnhum.2013.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 33.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule. Second Edition (ADOS-2) Western Psychological Services; Torrance, CA: 2012. [Google Scholar]
- 34.Schoen SA, Miller LJ, Green KE. Pilot study of the Sensory Over-Responsivity Scales: assessment and inventory. Am J Occup Ther. 2008;62(4):393–406. doi: 10.5014/ajot.62.4.393. [DOI] [PubMed] [Google Scholar]
- 35.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 37.Wei P, Bao R. The role of insula-associated brain network in touch. Biomed Res Int. 2013;2013:734326. doi: 10.1155/2013/734326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60(4):402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 39.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 40.Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 41.Kleinhans NM, Richards T, Sterling L, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 42.Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety. 2013;30(8):697–708. doi: 10.1002/da.22053. [DOI] [PubMed] [Google Scholar]
- 43.Wood JJ, Drahota A, Sze K, Har K, Chiu A, Langer DA. Cognitive behavioral therapy for anxiety in children with autism spectrum disorders: a randomized, controlled trial. J Child Psychol Psychiatry. 2009;50(3):224–234. doi: 10.1111/j.1469-7610.2008.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.