Abstract
The present research examined the effect of the 5-HTTLPR polymorphism in the serotonin transporter gene on objectively coded positive emotional expressions (i.e., laughing and smiling behavior objectively coded using the Facial Action Coding System). Three studies with independent samples of participants were conducted. Study 1 examined young adults watching still cartoons. Study 2 examined young, middle-aged, and older adults watching a thematically ambiguous yet subtly amusing film clip. Study 3 examined middle-aged and older spouses discussing an area of marital conflict (which typically produces both positive and negative emotion). Aggregating data across studies, results showed that the short allele of 5-HTTLPR predicted heightened positive emotional expressions. Results remained stable when controlling for age, gender, ethnicity, and depressive symptoms. These findings are consistent with the notion that the short allele of 5-HTTLPR functions as an emotion amplifier, which may confer heightened susceptibility to environmental conditions.
Keywords: Genetic polymorphisms, 5-HTTLPR, positive emotional expressions, differential susceptibility
The short allele of the 5-HTTLPR polymorphism in the promoter region of the serotonin transporter gene (SLC6A4) was initially regarded as a risk, vulnerability, or diathesis factor (Caspi et al., 2003; Lesch et al., 1996). Numerous studies showed that individuals with the short allele were at heightened risk for distal negative outcomes including depression (Caspi et al., 2003), anxiety (Lesch et al., 1996), substance use (Covault, Tennen, Armeli, Conner, Herman, Cillessen, & Kranzler, 2007), and suicide (Roy, Hu, Janal, & Goldman, 2007), especially when they were exposed to early adversity and life stress. Initial enthusiasm for these findings was dampened by replication issues and the appearance of two meta-analyses that concluded that the interaction effect of 5-HTTLPR x stress did not predict depression (Munafò, Durrant, Lewis, & Flint, 2009; Risch et al., 2009). However, a more recent meta-analysis (Karg, Burmeister, Shedden, & Sen, 2011) that included all available studies (the earlier meta-analyses were more selective) found support for the effect.
Moving away from earlier views of the short allele of 5-HTTLPR as a risk factor, researchers began to conceive of the short allele as a susceptibility or plasticity factor that predicts negative outcomes in negative contexts and positive outcomes in positive contexts (Belsky & Pluess, 2009; Boyce & Ellis, 2005; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011). Supporting this view, short-allele carriers were shown to both suffer most from negative conditions and to benefit most from positive conditions (for a meta-analysis see van IJzendoorn, Belsky, & Bakermans-Kranenburg, 2012) including family environments (Taylor, Way, Welch, Hilmert, Lehman, & Eisenberger, 2006), parenting (Hankin et al., 2011), life events (Kuepper, Wielpuetz, Alexander, Mueller, Grant, & Hennig, 2012; Pluess, Belsky, Way, & Taylor, 2010), and the emotional climate in marriages (Haase et al., 2013).
Complementing these studies that focus on effects of 5-HTTLPR on distal outcomes such as psychopathology and marital satisfaction, laboratory studies using well-controlled experimental paradigms have probed more proximal effects of 5-HTTLPR, particularly on stress and negative emotional reactivity. Reflecting earlier views of the short allele as a risk factor, numerous studies have documented a link between the short allele and heightened negative emotional reactivity including heightened amygdala reactivity (Hariri et al., 2002), heightened cortisol reactivity (Miller, Wankerl, Stalder, Kirschbaum, & Alexander, 2013), heightened startle reactivity (Brocke et al., 2006), and heightened subjective, behavioral, and physiological reactivity (Gyurak et al., 2013; Study 1) to different kinds of negative emotional stimuli. Moreover, we (Gyurak et al., 2013; Study 2) also found evidence that the short allele is linked to heightened self-conscious emotional reactivity (i.e., heightened embarrassment-related emotional reactions when watching oneself singing in a Karaoke task).
Research examining the proximal effects of 5-HTTLPR on positive emotional reactivity has been relatively rare. Yet, the available studies suggest that the short allele may not only be linked to heightened negative but also to heightened positive emotional reactivity. Specifically, laboratory-based studies have shown that the short allele is linked to heightened attention to positive face stimuli (Beevers, Marti, Lee, Stote, Ferrell, Hariri, & Telch, 2011), heightened positive attentional bias after training (Fox, Zougkou, Ridgewell, & Garner, 2011), and heightened self-reported positive affect in response to positive spousal affect (Schoebi, Way, Karney, & Bradbury, 2012). In our own work, we have found that short-allele carriers reported greater amusement in an embarrassing situation, perhaps reflecting a greater focus on the humorous aspects of the situation (Gyurak et al., 2013). In addition to these studies with adults, several studies have examined the link between 5-HTTLPR and positive emotions in infants and children (Auerbach, Faroy, Ebstein, Kahana, & Levine, 2001; Grossmann, Johnson, Vaish, Hughes, Quinque, Stoneking, & Friederici, 2011; Hayden et al., 2010), although these studies have yielded less consistent findings.
In sum, support for the plasticity model of the short allele has been growing. There is now ample evidence showing that the short allele predicts greater negative distal outcomes in negative contexts and greater positive distal outcomes in positive contexts. Heightened emotional reactivity (Carver, Johnson, & Joormann, 2008,2009) seems to be a prime candidate for a proximal mechanism that contributes to these distal outcomes. Although many studies have documented heightened negative emotional reactivity in short-allele carriers, research examining effects of 5-HTTLPR on positive emotional reactivity has been rare. In particular, there have been no studies with adults that examined objectively coded positive emotional behavior across multiple contexts.
Studying effects of 5-HTTLPR on emotional expressions avoids some of the problems typically associated with self-report measures of emotions including those that focus on remembered or hypothetical emotional functioning (cf. Robinson & Clore, 2002). Although self-report measures of emotions have many virtues (e.g., Feldman Barrett, Mesquita, Ochsner, & Gross, 2007), they do not always track emotional expressions well (e.g., Reisenzein, Bördgen, Holtbernd, & Matz, 2006). In addition, individuals differ greatly in how sensitive they are to their emotional states (e.g., Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005; Sze, Gyurak, Yuan, & Levenson, 2010), which can also alter self-report measures. For these reasons, the present study focused on objectively measured smiling and laughing behaviors, which are commonly considered to be behavioral indicators of positive emotion (Ekman & Friesen, 1982).
The Present Research
The present research sought to examine the effect of 5-HTTLPR on positive emotional expressions measured by objectively coded smiling and laughing behavior. We hypothesized that the short allele of 5-HTTLPR would be linked to higher levels of positive emotional expressions.
The design of this research reflects issues raised in the replication debates that have surrounded 5-HTTLPR research in particular (Karg et al., 2011; Kaufman, Gelernter, Kaffman, Caspi, & Moffitt, 2010; Munafò et al., 2009; Risch et al., 2009) and psychological research in general (Pashler & Wagenmakers, 2012). First, following recommended procedures (e.g., Hewitt, 2012), we tested our hypothesis drawing from three independent studies. The three studies assessed 5-HTTLPR using similar procedures and assessed positive emotional expressions using the same facial coding system, but each study used different participant samples and different emotion-eliciting procedures. Specifically, across the three studies, positive emotional expressions were coded from video recordings by trained coders who were blind to participants’ genotype. Coding was based on the Facial Action Coding System (FACS; Ekman, Friesen, & Hager, 2002), which enables reliable specification of the particular muscular contractions that produce observable changes in facial expression. Importantly, FACS allows for identifying expressions of positive emotion that are most likely to be genuine rather than being produced for other reasons (i.e., social convention; Ekman & Friesen, 1982). Study 1 examined young adults viewing still cartoons. Study 2 examined young, middle-aged, and older adults watching a film that was thematically ambiguous yet subtly amusing. Study 3 examined middle-aged and older spouses discussing an area of disagreement in their marriage. Our choice to probe positive emotional expressions in a marital conflict discussion was based on a large body of research showing that (genuine) positive emotions do occur in the midst of negative experiences (Folkman & Moskowitz, 2000; Papa & Bonanno, 2008) including marital conflict discussions (Carstensen, Gottman, & Levenson, 1995; Haase et al., 2013). Positive emotions serve important functions in negative contexts, including physiological soothing (Yuan, McCarthy, Holley, & Levenson, 2010) and “undoing” the cardiovascular effects of negative emotions (Fredrickson & Levenson, 1998; Fredrickson, Mancuso, Branigan, & Tugade, 2000).
The present studies utilize data from larger research projects from which other findings have been reported previously (e.g., Haase, Seider, Shiota, & Levenson, 2012; Levenson, Carstensen, & Gottman, 1993; Rodrigues, Saslow, Garcia, John, & Keltner, 2009; Shiota & Levenson, 2009). Genetic effects on positive emotional expressions have not been analyzed previously in any of these research projects.
Statistical Analyses
A number of different strategies have been recommended for analyzing data from multiple studies. Whereas the “traditional” approach has been to analyze data from multiple studies separately, a recent paper (Schimmack, 2012) recommends aggregation across studies, arguing that “the number of studies is irrelevant because the strength of the empirical evidence is a function of the total sample size rather than the number of studies” (p. 553). In the present article, we chose to combine the traditional approach with the approach recommended by Schimmack (2012)1. Thus, we first present the three studies and their results separately and then present results aggregating data across the three studies to test our hypothesis.
To prepare the statistical analyses, the coding of all variables was harmonized across studies. Thus, across studies, 5-HTTLPR genotype was coded using an additive coding scheme (1 = l/l; 2 = s/l; 3 = s/s) as in many previous studies (e.g., Caspi et al., 2003). In Study 1, a few participants carried a rare extra-long (xl) allele and were included in the group coded as 1 (l/l).2 Coding for all other categorical variables was likewise harmonized across studies (i.e., gender: 0 = male, 1 = female; ethnicity: 1 = African American, 2 = Asian, 3 = Caucasian, 4 = Latina/o, 5 = Other). Categorical variables were subsequently dummy coded to allow for inclusion in the regression analyses. All continuous variables (including positive emotional expressions) were z-standardized. We then conducted analyses for each of the studies separately. This was followed by an analysis that aggregated data across studies following Schimmack (2012).
Data were analyzed using a series of hierarchical regression analyses.3 In the first step, 5-HTTLPR was entered as a predictor variable of positive emotional expressions. In the second step, covariates (i.e., age, gender, ethnicity dummy variables, depressive symptoms) were entered as additional predictors to determine robustness of the findings. When analyzing the aggregate sample, two dummy variables indicating study sample were included as additional covariates. In addition, when analyzing the aggregate sample, moderation by age, gender, and ethnicity was examined by including the interaction terms between 5-HTTLPR and each of these factors in a third step. These moderation analyses were only conducted for the aggregate sample in order to maximize statistical power. Finally, we repeated analyses for Study 3 and for the aggregate sample accounting for the partly clustered (i.e., nonindependent) nature of the data (as a proportion of Study 3 data was nested within couples for 49 couples). Specifically, we used Stata’s cluster tool (StataCorp., 2007), which corrects results for nonindependence between husbands and wives as in previous studies (Haase et al., 2013).
Study 1
Method
Participants
Participants in Study 1 (N = 128) represented a subsample from a larger research project (N = 298 undergraduate students) on individual differences in emotional functioning (Rodrigues et al., 2009). One hundred and forty-one participants from this research project were genotyped for 5-HTTLPR. Facial behavior could not be coded for 13 of these participants because they did not provide consent for videotaping, resulting in N = 128 for Study 1. Sociodemographic characteristics of the participants are shown in Table 1. Participants in the subsample did not differ from the other participants in the research project in terms of gender, age, and ethnicity, ps > .05.
Table 1.
Sociodemographic Characteristics of Individual and Aggregate Study Samples
| Study 1 | Study 2 | Study 3 | Aggregate | |
|---|---|---|---|---|
| N | 128 | 93 | 115 | 336 |
| 5-HTTLPR | ||||
| s/xl or l/xl | 8 | - | - | 8 |
| l/l | 25 | 32 | 33 | 90 |
| s/l | 60 | 47 | 64 | 171 |
| s/s | 35 | 14 | 18 | 67 |
| Age | 20.48 (3.13) | 47.89 (16.39) | 51.46 (9.59) | 38.89 (17.74) |
| Gender | ||||
| Males | 49 | 34 | 57 | 140 |
| Females | 79 | 59 | 58 | 196 |
| Ethnicity | ||||
| African American | 4 | 12 | 6 | 22 |
| Asian | 49 | 14 | - | 63 |
| Caucasian | 41 | 52 | 98 | 191 |
| Latina/o | 8 | 13 | 3 | 24 |
| Other | 21 | - | 8 | 29 |
| Missing | 5 | 2 | - | 7 |
| Depressive symptomsa | 1.12 (.64) | −.45 (.55) | −.78 (.32) | 0 (1) |
Note. Sample sizes (N/n) or Means (M; SD in parentheses) shown.
z-standardized variable.
Measures
5-HTTLPR. Study 1 participants were invited to participate in a DNA assessment during the laboratory session and those whose DNA samples allowed for genotyping 5-HTTLPR were included in the sample analyzed here. DNA was collected and extracted from saliva using Oragene kits (DNA Genotek, Kanata, Ontario, Canada) according to manufacturer’s protocol. Anonymized DNA samples were extracted and purified by Creative Genomics (Port Jefferson Station, NY). The extracted DNA was genotyped at the University of California, San Francisco Genomics Core using a procedure described previously (Rodrigues et al., 2009) with an Applied Biosystems genotyping assay designed for 5-HTTLPR. Data quality was assessed by duplicating a subset of random DNA samples; genotype data reproducibility was 100%. Genotype distribution (19.5% l/l; 46.9% s/l; 27.3% s/s) did not deviate from Hardy-Weinberg equilibrium, χ2(1) = 0.01, p = .92. In addition to the two commonly studied 5-HTTLPR alleles, the 14-repeat short and the 16-repeat long allele, a few subjects in Study 1 (n = 8, 6.3%) carried an extralong (xl) allele (l/xl = 4.7%; s/xl = 1.6%). The xl variants are very rare and have been predominantly found among individuals of African (Delbrück, Wendel, Grunewald, Sander, Morris-Rosendahl, Crocq, Berrettini, & Hoehe, 1997) and Asian (Goldman, Glei, Lin, & Weinstein, 2010) descent.
Positive emotional expressions. Participants’ positive emotional expressions were coded by a FACS-certified coder who was blind to participants’ 5-HTTLPR genotype and study hypothesis. Coding criteria were derived from FACS (Ekman et al., 2002). The facial behavior of interest was genuine (i.e., Duchenne) laughing behavior, which was defined by the combined and symmetric contraction of the Zygomatic Major (lifts lip corners, action unit [AU] 12) and Orbicularis Oculi, Pars Orbitalis (raises cheeks, AU 6), accompanied by either forced vocalized or unvocalized expiration that was not coughing, clearing the throat, or sighing (Keltner & Bonanno, 1997; Ruch, 1993). Combinations of AU 6 and 12 that were accompanied or followed by other AUs such as AU 4 (frowning), AU 15 (pulling the lip corners down) or headshakes as a response to the same cartoon were not considered Duchenne displays (Beermann & Ruch, 2011; Ruch, 1995). Exceptions to this rule were eyelid tightening (AU 7) and mouth opening (AU 25, 26), which were allowed because they often occur in combination with Duchenne laughter (Keltner & Bonanno, 1997; Ruch, 1995). A Duchenne laugh frequency score was computed as the number of cartoons participants responded to with a Duchenne laugh. Intensity of Duchenne laughter was coded at the apex of the response to each cartoon (1 = minimum, 5 = maximum). If a participant laughed at more than one cartoon, the intensity scores were averaged across cartoons. A Duchenne laughter composite score was created by normalizing the frequency and intensity scores (using the means and standard deviations for the entire sample) and then averaging the two z-scores (Keltner & Bonanno, 1997). To determine interrater reliability, a second FACS-certified coder examined a random subsample of 21 (10%) participants’ laughter responses to all 20 cartoons. Interrater reliability was fair to good (single-measures intraclass correlation [ICC] = .56; average-measures ICC = .72) following recommended guidelines (Cicchetti, 1994).
Covariates were gender, age, ethnicity (Caucasian, Asian, Latina/o, African American, Other), and depressive symptoms (measured using the Neuroticism-Depression subscale of the Big Five Inventory; John, Naumann, & Soto, 2008).
Procedure
Participants came to the laboratory and participated in a well-established procedure for studying individual differences in emotional functioning using self-report, behavioral, and physiological measures. The present study examined the behavioral data.
As part of this procedure, participants viewed 20 still, line-drawing cartoons chosen from a subsample of cartoons collected by previous researchers (Watson, Matthews, & Allman, 2007). Seven of the cartoons were from The Far Side by Gary Larson and 13 were from the New Yorker. Each cartoon was shown on a computer screen for 8 seconds each. Participants were videotaped while watching the cartoons.
Results
Hierarchical regression analyses showed that 5-HTTLPR genotype predicted positive emotional expressions in response to the cartoons, B = .30, SE(B) = .14, 95% CI [.03, .57], β = .21, p = .028. The effect was positive, indicating that the short allele predicted greater levels of positive emotional expressions. The effect remained stable when controlling for gender, age, ethnicity, and depressive symptoms, B = .31, SE(B) = .14, 95% CI [.02, .59], β = .21, p = .034. Results are shown in Table 2.
Table 2.
5-HTTLPR and Positive Emotional Expressions: Results from Individual Study Samples
| Study 1 | Study 2 | Study 3 | ||||
|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | |
| 5-HTTLPR | .21* | .21* | .28** | .26* | −.04 | −.02 |
| Age | - | −.01 | - | −.13 | - | −.16 |
| Gender | - | −.10 | - | −.10 | - | .13 |
| African American or Latina/o a | - | .02 | - | .23 | - | .01 |
| Asian | - | −.06 | - | .39 | - | - |
| Caucasian | - | −.10 | - | .28 | - | .19 |
| Depressive symptoms | - | .03 | - | −.07 | - | −.21* |
| R2 | .04 | .06 | .08 | .14 | .00 | .11 |
Note. Standardized regression coefficients (β) shown. Study 1: n = 112. Study 2: n = 87. Study 3: n = 115.
Combined into one category due to small cell sizes.
p < .05.
Study 2
Method
Participants
Participants in Study 2 (N = 93) represented a subsample from a larger research project (N = 222 young, middle-aged, and older adults) on individual differences in emotional reactivity and regulation (Shiota & Levenson, 2009). In 2009, participants from this research project were recontacted and 107 participants were successfully genotyped. Facial behavior could not be coded for 14 of these participants because of low video quality resulting in N = 93 for Study 2. Sociodemographic characteristics of the participants are shown in Table 1. Participants in the study sample did not differ from the other participants in the research project in terms of ethnicity, ps > .05, but were more likely to be older and female, ps < .05.
Measures
5-HTTLPR. Procedures for DNA assessment in Study 2 were similar to Study 1. DNA was collected and extracted from saliva using Oragene kits (DNA Genotek, Kanata, Ontario, Canada) according to manufacturer’s protocol. Anonymized DNA samples were extracted and purified at the Abbott-UCSF Viral Diagnostics and Discovery Center, San Francisco, CA. The extracted DNA was genotyped at the University of California, Los Angeles using the procedure described in Assal, Alarcon, Solomon, Masterman, Geschwind, and Cummings (2004) with slight modifications. A PCR product was amplified with primers (5′-GGCGTTGCCGCTCTGAATGC-3′ and 5′-GAGGGACTGAGCTGGACAACCA-3′) flanking the region containing the gene variation. The PCR conditions consisted of a 2-min denaturation step at 94°C, 35 cycles of 30-sec denaturation at 95°C, 30-sec annealing at 60°C, and 30-sec extension at 72°C, and a final 7-min extension step at 72°C. 5-HTTLPR genotyping using these methods has shown high reliability. Specifically, a blind re-analysis of 8 DNA samples from a larger genotyping project that included samples from all of the participants analyzed here yielded 100% genotype reproducibility. 5-HTTLPR genotype distribution (34.4% l/l; 50.5% s/l; 15.1% s/s) did not deviate from Hardy-Weinberg equilibrium, χ2(1) = .23, p = .632.
Positive emotional expressions. Participants’ positive emotional expressions were coded by three trained coders who were blind to study hypothesis and participants’ 5-HTTLPR genotype. Coding criteria were derived from FACS (Ekman et al., 2002) as well as its more economical variant, EMFACS. As in Study 1, AU 12 was considered a required action unit. However, in Study 2, AU 6 was considered an optional/enhancing action unit because the film used in this study elicited smiles of low intensity (under these conditions, genuine smiling behavior may result in low-intensity smiles where the human eye can detect AU 12 but not AU 6; Ruch, 1993, p. 606). AU 24 as a smile control was also considered acceptable as an optional action unit. As in Study 1, a composite was created based on standardized frequency and intensity (0 = absent, 1 = minimally present, 2 = moderately present, 3 = long and maximally present) scores of smiling behavior. Coding was segmented into 5-second bins, each of which received a score of 0, 1, 2, or 3 according to the scheme above. All three coders scored facial behavior for 14 participants from the original, total sample. Interrater reliability for smiling codes at the level of bin was good to excellent (single-measures ICC = .72; average-measures ICC = .89) following recommended guidelines (Cicchetti, 1994). The remaining participants were therefore scored by only one coder, and single-coder data are used in all analyses.
Covariates. Covariates were gender, age, education, ethnicity (Caucasian, Asian, Latina/o, African American; dummy coded in the regression analyses), and depressive symptoms (using the Depression subscale of the Symptom Checklist [SCL-90]; Derogatis, 1993).
Procedure
Participants came to the laboratory and participated in a well-established procedure for studying individual differences in emotional reactivity and regulation using self-report, behavioral, and physiological measures (Shiota & Levenson, 2009). The present study examined the behavioral data.
The procedure consisted of six trials in which participants viewed film clips. Within each trial, participants viewed: (1) a large “X” on a television monitor for 60 seconds, during which they were instructed to clear their minds of thoughts, feelings, and memories (pre-film period); (2) on-screen instructions for the trial displayed for 5 seconds; (3) a film clip, which lasted approximately three minutes; and (4) a blank screen that appeared for 60 seconds. Participants’ facial behavior was videotaped using a remotely-controlled camera that was partially concealed behind darkened glass in a bookcase. The present study examined the first trial, during which participants watched a thematically-ambiguous film clip with the instruction to “just watch the film clip as though you were watching television at home, or a movie in a movie theater”. This clip was an excerpt from the film Stranger than Paradise (Driver & Jarmusch, 1984) in which two men engage in a halting conversation that appears devoid of purpose, intent, or theme (Haase et al., 2012), but is funny in a subtle, absurdist way.4 Stranger than Paradise has been described as an absurdist-deadpan comedy film with “a loopy charm and subtle but potent humor” (Deming, 2010). The remaining trials consisted of a sadness reactivity, a disgust reactivity (Seider, Shiota, Whalen, & Levenson, 2011), and three sadness and disgust regulation trials (Shiota & Levenson, 2009). Data from these other trials were not utilized for the present study, consistent with our interest in the effect of 5-HTTLPR on positive emotional expressions.
Results
Hierarchical regression analyses showed that 5-HTTLPR genotype predicted positive emotional expressions in response to the thematically ambiguous film, B = .45, SE(B) = .17, 95% CI [.12, .79], β = .28, p = .009. The effect was positive, indicating that the short allele predicted greater levels of positive emotional expressions. The effect remained stable when controlling for gender, age, ethnicity, and depressive symptoms, B = .42, SE(B) = .18, 95% CI [.07, .78], β = .26, p = .021. Results are shown in Table 2.
Study 3
Method
Participants
Participants in Study 3 represented a subsample (N = 115) from a longitudinal study of long-term married couples (for full details see Levenson et al., 1993). The original sample (N = 156 middle-aged and older couples) was recruited in 1989–1990 to be representative of a random sample of marriages in the San Francisco Bay Area in terms of socioeconomic status, religion, ethnicity, and marital satisfaction. The sample was recruited so that older couples were between the ages of 60 and 70 and married for at least 35 years (M = 40.3, SD = 3.7); middle-aged couples were between the ages of 40 and 50 and married for at least 15 years (M = 21.1, SD = 3.4). As in Study 2, we examined a subsample of spouses who were recontacted in 2009 for genotyping. In 51 couples both spouses participated; in 23 couples only one spouse participated. Facial behavior could not be coded for 10 spouses because their original videos were handled by a different research group and could not be retrieved in time resulting in N = 115 for Study 3. Sociodemographic characteristics of the participants are shown in Table 1. Participants in the study sample did not differ from the other participants in the research project in terms of gender and ethnicity, ps > .05, but they were younger, p < .05.
Measures
5-HTTLPR. 5-HTTLPR was assessed using the same procedure as in Study 2. 5-HTTLPR genotype distribution (males: 35.1% l/l; 52.6% s/l; 12.3% s/s; females: 22.4% l/l; 58.6% s/l; 19.0% s/s; overall: 28.7% l/l; 55.7% s/l; 15.7% s/s) did not deviate from Hardy-Weinberg equilibrium, males: χ2(1) = .69, p = .405, females: χ2(1) = 1.75, p = .186, overall: χ2(1) = 2.01, p = .156.
Positive emotional expressions. Participants’ positive emotional expressions were coded by three FACS-certified coders who were blind to participants’ 5-HTTLPR genotype. Each coder scored the facial behavior of one third of the spouses (i.e., 37 to 38 spouses) during the first five minutes of the 15-minute conflict conversation. Coding criteria were derived from FACS and were similar to Study 1. A laugh was defined as a sharp exhale or a series of exhales that did not resemble a cough, sigh, or a clearing of the throat and laugh onsets and offsets were defined by increases and returns to normal levels in somatic activity. As in Study 1, the behavior of primary interest was genuine (i.e., Duchenne) laughing behavior, indicated by a combination of AU 6 and AU 12 and the absence of any other action unit that would disqualify the genuineness of laughs (Ruch, 1995). As in Study 1, eyelid tightening (AU 7) and mouth opening (AU 25, 26), were exceptions to this rule (Keltner & Bonanno, 1997; Ruch, 1995). Using this approach, it was possible to distinguish genuine laughter from laughter that was blended with displays of potentially negative emotions, such as nervous or angry laughter. Laughing behavior was coded in terms of frequency (number of laughs during the conflict conversation), intensity (1 = minimum, 5 = maximum), and duration (time in seconds from the onset to the offset of each laugh, averaged across all laughs within the 5-minute period of a conversation). Duration was coded due to its stronger impact in a longer interaction as compared to responses to short stimuli in Studies 1 and 2. A Duchenne laughter composite score was created based on standardized frequency, intensity, and duration scores of Duchenne laughter (see Bonanno & Keltner, 1997).
In order to determine inter-rater reliability, all three coders and an additional fourth FACS-certified coder scored an overlap of seven conversations (13 spouses, as one of the spouses did not consent to being recorded on video). For each pair of coders, we computed an agreement ratio by multiplying the number of AUs that two coders agreed on by two and then dividing the result by the total number of AUs scored by both coders (Ekman et al., 2002). The mean ratio among the four coders was .77.
Covariates included age, gender, ethnicity, and depressive symptoms (SCL-90 depression subscale; Derogatis, 1993).
Procedure
Over approximately a 20-year period, couples came to the laboratory every 5–6 years and participated in a well-established procedure for studying marital interaction (Levenson & Gottman, 1983; Levenson, Carstensen, & Gottman, 1994). At each assessment couples engaged in three 15-minute unrehearsed discussions of topics related to their marriage: (a) events of the day (at the first assessment, events of the past five years at subsequent assessments); (b) a topic of continuing disagreement in their marriage (conflict topic); and (c) something they enjoyed doing together (pleasant topic). Conversations were recorded on videotape for subsequent analysis of emotional behavior and a number of physiological measures were recorded continuously from each spouse. For the present study, only the behavioral data obtained during the conflict conversation at the first assessment was used.
Results
A hierarchical regression analysis showed that 5-HTTLPR genotype did not predict positive emotional expressions during marital conflict, B = −.06, SE(B) = .13, 95% CI [−.31, .20], β = −.04, p = .661. This result remained unchanged when controlling for gender, age, ethnicity, and depressive symptoms, B = −.03, SE(B) = .13, 95% CI [−.28, .23], β = −.02, p = .834. Results are shown in Table 2. When we repeated these analyses using Stata’s cluster tool, which corrects results for nonindependence between husbands and wives, the effect of 5-HTTLPR on positive emotional expressions was again not significant, B = −.06, SE(B) = .14, 95% CI [−.33, .22], p = .685.
Study 1, 2, and 3: Aggregate Results
Based on Schimmack (2012), we tested our hypothesis aggregating data across studies. Table 1 shows sociodemographic characteristics of the aggregate sample. 5-HTTLPR genotype distribution in the aggregate sample (26.8% l/l; 50.9% s/l; 19.9% s/s) did not deviate from Hardy-Weinberg equilibrium, χ2(1) = .75, p = .386. A univariate ANOVA with 5-HTTLPR genotype as the dependent variable, gender and ethnicity as random factors, and age as a covariate showed that neither age, p = .161, nor gender, p = .299, predicted 5-HTTLPR, but ethnicity did, F(4, 322) = 2.41, p = .049.
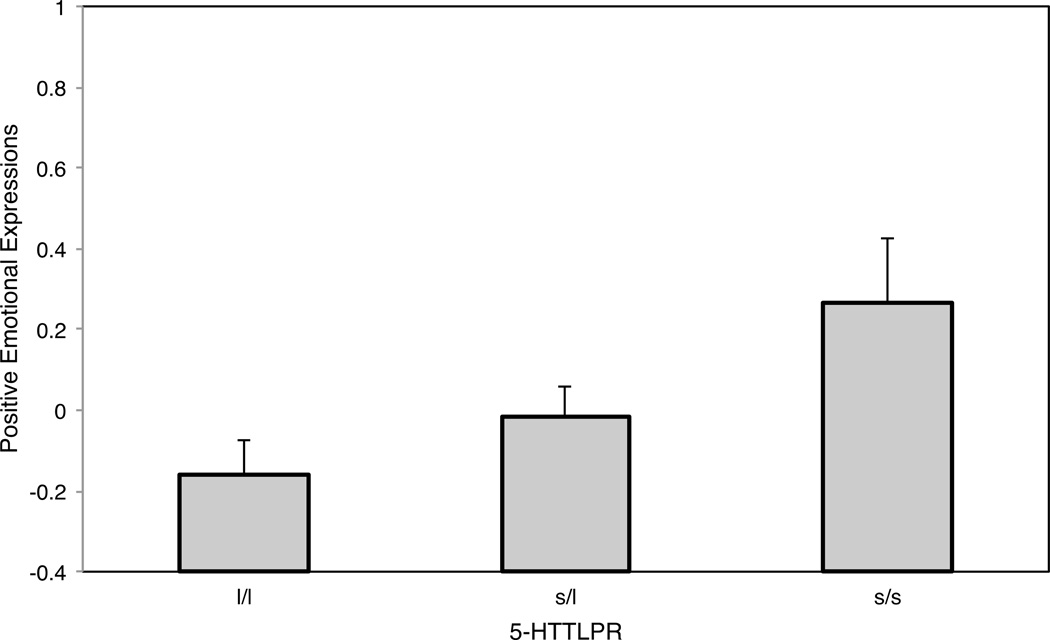
A hierarchical regression analysis showed that 5-HTTLPR genotype predicted positive emotional expressions aggregating data across studies, B = .22, SE(B) = .08, 95% CI [.06, .38], β = .15 p = .008. The effect was positive, indicating that the short allele predicted greater levels of positive emotional expressions. A post-hoc analysis of achieved power using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) indicated that the overall sample size provided reasonable statistical power to detect an effect of this size at an alpha level of .05 (power with two-tailed test = .76; power with one-tailed test = .85). Figure 1 shows the effect of 5-HTTLPR on positive emotional expressions. The effect remained stable when controlling for age, gender, ethnicity, depressive symptoms, and study, B = .21, SE(B) = .08, 95% CI [.04, .37], β = .14, p = .014. The effect was not moderated by age, gender, or ethnicity as indicated by nonsignificant interaction terms, ps ≥ .147. Results from the hierarchical regression analyses are shown in Table 3.
Figure 1.
5-HTTLPR and Positive Emotional Expressions: Results from Aggregate Study Sample
Note. Means (Ms) shown. Standard errors represent SE(M). The y-axis represents the z-standardized positive emotional expressions score. l/l = Individuals with two long alleles (n = 90) or at least one xl allele (n = 8) of 5-HTTLPR. s/l = Individuals with one short and one long allele of 5-HTTLPR (n = 171). s/s = Individuals with two short alleles of 5-HTTLPR (n = 67).
Table 3.
5-HTTLPR and Positive Emotional Expressions: Results from Aggregate Study Sample
| Step 1 | Step 2 | Step 3 | |
|---|---|---|---|
| 5-HTTLPR | .15** | .14* | .26 |
| Age | - | −.20* | .05 |
| Gender | - | −.05 | .19 |
| African American | - | −.12 | −.01 |
| Asian | - | .06 | −.04 |
| Caucasian | - | .02 | .10 |
| Latina/o | - | .06 | .04 |
| Depressive symptoms | - | −.10 | −.09 |
| Study 1 | - | −.11 | −.14 |
| Study 2 | - | .02 | −.01 |
| 5-HTTLPR x Age | - | - | −.27 |
| 5-HTTLPR x Gender | - | - | −.27 |
| 5-HTTLPR x African American | - | - | −.12 |
| 5-HTTLPR x Asian | - | - | .12 |
| 5-HTTLPR x Caucasian | - | - | −.08 |
| 5-HTTLPR x Latina/o | - | - | .03 |
| R2 | .02 | .06 | .09 |
Note. Standardized regression coefficients (β) shown. Sample: n = 314.
p < .05.
p < .01.
When we repeated these analyses excluding 21 multivariate outliers (i.e., Mahalanobis distance values derived from step 3, p < .001), the effect of 5-HTTLPR on positive emotional expressions remained stable when examined alone, B = .25, SE(B) = .09, 95% CI [.07, .42], β = .16, p = .006, and remained stable when controlling for age, gender, ethnicity, depressive symptoms, and study, B = .23, SE(B) = .09, 95% CI [.05, .41], β = .15, p = .015. The effect was not moderated by age, gender, or ethnicity as indicated by nonsignificant interaction terms, ps ≥ .123.
When we repeated these analyses using Stata’s cluster tool, which corrects results for nonindependence between husbands and wives, all results remained stable, effect of 5-HTTLPR on positive emotional expressions, B = .21, SE(B) = .09, 95% CI [.04, .38], p = .017 (step 1), B = .20, SE(B) = .10, 95% CI [.01, .39], p = .037 (step 2, controlled for covariates).
Discussion
The present findings from three independent samples of participants demonstrate that the short allele of 5-HTTLPR is linked to heightened objectively coded positive emotional expressions (i.e., FACS-coded laughing and smiling behavior). Analyzing the three studies separately, the short allele predicted higher levels of positive emotional expressions in Study 1 and 2 but not in Study 3. Aggregating data across the three studies, the short allele predicted higher levels of positive emotional expressions. The effect remained stable when controlling for covariates (i.e., age, gender, ethnicity, depressive symptoms, and study) and was not moderated by participants’ age, gender, or ethnicity.
The Short Allele of 5-HTTLPR as Emotion Amplifier
Serotonin is a neurotransmitter that is centrally involved in emotional functioning. A key regulator is the serotonin transporter (5-HTT), which removes serotonin released into the synaptic cleft. The serotonin transporter protein is encoded by a single gene (SLC6A4). Transcriptional activity of the SLC6A4 gene is modulated by several common variants, including variations in the serotonin transporter linked polymorphic region (5-HTTLPR). The different variants of 5-HTTLPR result in differential serotonin expression and functioning, with the short allele variant leading to lower serotonin transporter expression and thus lower levels of uptake of serotonin from the synaptic cleft (for a review see Canli & Lesch, 2007).
Numerous laboratory-based studies have shown that the short allele of 5-HTTLPR predicts heightened stress reactivity (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010), heightened negative emotional reactivity (e.g., Gyurak et al., 2013; Hariri et al., 2002) as well as heightened self-conscious emotional reactivity (Gyurak et al., 2013). More limited evidence has suggested a link between the short allele and heightened positive emotional reactivity (Beevers et al., 2011; Schoebi et al., 2012).
Building on and extending this research, the present findings show that the short allele of 5-HTTLPR predicts heightened positive emotional reactivity. We analyzed data from three independent studies, which were comparable in terms of how 5-HTTLPR and positive emotional expressions were assessed and different in terms of emotion-eliciting stimuli (i.e., still cartoons in Study 1, a film clip in Study 2, and a marital conflict discussion in Study 3), participants’ ages (young adults in Study 1; young, middle-aged and older adults in Study 2; middle-aged and older adults in Study 3), and participants’ ethnicities (ethnically diverse in Study 1 and 2; predominantly Caucasian in Study 3). Analyzing the three studies separately, the short allele predicted higher levels of positive emotional expressions in Study 1 and 2 but not in Study 3.
Based on Schimmack (2012), we proceeded to examine our hypothesis aggregating data across the three studies and achieved reasonable statistical power (cf. Button, Ioannidis, Mokrysz, Nosek, Flint, Robinson, & Munafo, 2013) to detect a small (Cohen, 1992) effect. Specifically, in this aggregate analysis, individuals’ 5-HTTLPR genotype explained about 2% of variance in positive emotional expressions. This is consistent with the idea that these kinds of genetic influences do not determine individuals’ emotional reactivity (Haase et al., 2013). Rather, candidate genes such as 5-HTTLPR represent one small source of individual differences in positive emotional expressions, alongside other sources such as age (Carstensen et al., 1995), gender (LaFrance, Hecht, & Paluck, 2003), culture (Ekman & Friesen, 1969), depressive symptomatology (Tremeau et al., 2005), and other genetic influences (Pelc, Cheron, & Dan, 2008). The effect of 5-HTTLPR of positive emotional expressions remained stable when controlling for a variety of covariates (i.e., age, gender, ethnicity, depressive symptoms, study), providing evidence of robustness. In addition, there was no evidence that the effect of 5-HTTLPR on positive emotional expressions was moderated by age, gender, or ethnicity. It is important to note, however, that these findings must be tempered by the lower statistical power to detect moderator effects (McClelland & Judd, 1993).
Taken together, findings from our own work (Gyurak et al., 2013; Haase et al., 2013) as well as from others (e.g., Beevers et al., 2011; Brocke et al., 2006; Hariri & Holmes, 2006; Schoebi et al., 2012; Way & Taylor, 2010) suggest that the short allele of 5-HTTLPR acts as an emotion amplifier, increasing the size of emotional reactions across emotion types and emotional situations. Compared to long-allele carriers, short-allele carriers may have higher highs and lower lows emotionally across a broad range of emotions (negative, self-conscious, positive) in response to a wide range of emotion-eliciting situations they encounter in daily life. We believe this heightened emotional reactivity represents a proximal mechanism for understanding the more distal effects of the short allele such as greater proneness for developing psychopathology in difficult environments (e.g., Caspi et al., 2003; Karg et al., 2011) and predicting positive changes in marital satisfaction in positive emotional climates and negative changes in marital satisfaction in negative emotional climates (Haase et al., 2013). In this model, the distal effects result from the small proximal effects of amplified emotional reactivity being aggregated over long periods of time.
Strengths and Limitations
The present studies have several strengths. Notably, the findings were based on objective coding of positive emotional expressions (i.e., FACS-coded laughing and smiling behavior) and were focused on behaviors that are typically associated with genuine enjoyment (Ekman & Friesen, 1982; Ruch, 1995). Moreover, findings were obtained in a way that enabled evaluation of generalizability (i.e., conducting three studies with independent samples of participants).
The studies also had several limitations. First, although our sample size was quite respectable for studies involving intensive behavioral coding and provided reasonable statistical power to detect small effect sizes of 5-HTTLPR on positive emotional expressions, they may seem small to researchers who conduct large-scale surveys to examine effects of 5-HTTLPR (sometimes including thousands of participants; e.g., Karg et al., 2011). Larger sample sizes would have given us greater power to detect 2-way interaction effects and thus to examine generalizability across age, gender, and ethnicity in greater depth. Second, related to this issue, when analyzed separately, Study 3 did not show a positive effect of 5-HTTLPR on positive emotional expressions. It remains to be seen whether the absence of an effect in Study 3 is a result of low statistical power (cf. Schimmack, 2012), reflects sample variation around the true effect, or hints towards context-dependency of the effect of 5-HTTLPR on positive emotional expressions. In terms of context, Studies 1 and 2 both used experimental manipulations to create a positive context (cartoons in Study 1, a film clip in Study 2). In Study 3, couples “created” their own contexts by discussing a marital problem, which produced a complex mix of both negative and positive emotions. It may be that this latter context is not optimal for revealing the effect of 5-HTTLPR on positive emotions.
Implications
The present findings have implications for future research. The notion that the short allele of 5-HTTLPR acts as a positive emotion amplifier merits further investigation examining other positive emotions (e.g., anticipatory enthusiasm, attachment love, nurturant love, amusement, awe; Shiota, Neufeld, Yeung, Moser, & Perea, 2011), other experimental paradigms (e.g., positive social feedback, gift giving, self-affirmation, marital discussions of neutral and positive topics), other samples (e.g., other age ranges including infancy to late life; other countries outside the US), and other measures (e.g., autonomic and central physiological measures, subjective experience).
Moreover, the present findings emphasize the need for further research that examines the neurobiological links between the influence that allelic variations in 5-HTTLPR have on serotonergic functioning and amplified emotional reactivity. For example, findings that the influence of 5-HTTLPR extends to positive emotional reactivity are consistent with the role that serotonin plays in reward processing (Kranz, Kasper, & Lanzenberger, 2009).
More generally, the present findings that the short allele of 5-HTTLPR predicted heightened positive emotional reactivity combine with our previous findings that the short allele predicted negative and self-conscious emotional reactivity (Gyurak et al., 2013), thus, supporting views of the short allele as a susceptibility or plasticity factor (Belsky & Pluess, 2009; Ellis et al., 2011) rather than a risk factor (Caspi et al., 2003). A growing body of work indicates that short-allele carriers suffer most in negative contexts but also obtain the best outcomes in positive contexts (for a meta-analysis see van IJzendoorn et al., 2012). Proximal effects of 5-HTTLPR in amplifying negative emotional reactivity (e.g., Gyurak et al., 2013; Hariri et al., 2002) provide a reasonable link (Rottenberg & Johnson, 2007) to more distal negative outcomes such as greater depression in negative contexts (Caspi et al., 2003). Similarly the proximal effects of 5-HTTLPR in amplifying positive emotional reactivity found in the present study provide reasonable links to more positive distal outcomes including greater relationship satisfaction (Haase et al., 2013), lower depressive symptoms (Taylor et al., 2006), lower neuroticism (Pluess et al., 2010), higher positive affect (Hankin et al., 2011), higher life satisfaction (Kuepper et al., 2012), and greater therapy success (Eley et al., 2012) in positive contexts.
The present studies were designed as tests of the proximal effects of 5-HTTLPR on a specific indicator of positive emotion (i.e., positive emotional expressions) in much the same way as we have previously examined proximal effects of 5-HTTLPR on negative and self-conscious emotions (Gyurak et al., 2012). Probing proximal effects of candidate genes using well-defined and carefully measured behaviors can help us better understand the precise nature of these gene-behavior relationships and how they are influenced by context. They can help elucidate whether candidate genes represent plasticity factors that enhance reactivity to both positive and negative contexts, whether they represent risk, vulnerability, or diathesis (Monroe & Simons, 1991) factors that enhance reactivity to negative contexts only or whether they represent vantage sensitivity (Pluess & Belsky, 2013) factors that enhance reactivity to positive contexts only.
Conclusion
Aggregating data from three independent studies, we found that the short allele of 5-HTTLPR predicts heightened positive emotional reactivity using objectively coded positive emotional expressions (i.e., FACS-coded laughing and smiling behavior). These findings extend previous work from our laboratory and others showing that short-allele carriers not only have heightened negative and self-conscious emotional reactivity (e.g., Gyurak et al., 2013; Hariri et al., 2002; Kuepper et al., 2012) but also heightened positive emotional reactivity. We suggest that these proximal effects of the short allele of 5-HTTLPR on emotional reactivity aggregated over long periods of time can be helpful in understanding effects of 5-HTTLPR on more distal outcomes such as the development of psychopathology (e.g., Caspi et al., 2003) and changes in relationship satisfaction (e.g., Haase et al., 2013) in ways that are consistent with the differential susceptibility model of 5-HTTLPR (Belsky & Pluess, 2009; Ellis et al., 2011).
Acknowledgments
This research was supported by a National Institute on Aging (American Recovery and Reinvestment Act) grant 3R37-AG017766-09S3 to Robert W. Levenson, a Career Support Grant (Nachwuchsförderungskredit) of the University of Zurich, Switzerland, to Ursula Beermann, and a National Center for Complementary and Alternative Medicine (NCCAM) grant T32AT003997 to Laura R. Saslow. We would like to thank Samantha Neufeld, Uriah Anderson, Maren True, and Wan Yeung for their work on coding and data management for Study 2.
Footnotes
We thank Ulrich Schimmack for this suggestion.
When we repeated the analyses using a different coding scheme for 5-HTTLPR (1 = l/xl; 2 = l/l; 3 = s/xl; 4 = s/l; 5 = s/s) based on Goldman and colleagues (2010), the effect of 5-HTTLPR on positive emotional expressions was stable, Study 1: B = .18, SE(B) = .08, 95% CI [.02, .34], β = .20, p = .032, R2 = .04; Aggregate sample: B = .13, SE(B) = .05, 95% CI [.03, .22], β = .14, p = .013, R2 = .02.
As some participants had missing values on some covariates, the hierarchical regression analyses were based on the following sample sizes, Study 1: n = 112, Study 2: n = 87, Study 3: n = 115, Aggregate sample: n = 314. When we examined the effect of 5-HTTLPR on emotional expressions without covariates, the results were largely similar, Study 1 (N = 128): B = .21, SE(B) = .13, 95% CI [.04, .54], β = .20, p = .024, R2 = .04; Study 2 (N = 93): B = .39, SE(B) = .16, 95% CI [.08, .70], β = .25, p = .016, R2 = .06; Study 3 (N = 115): B = −.06, SE(B) = .13, 95% CI [−.31, .20], β = −.04, p = .661, R2 = .00; Aggregate sample (N = 336): B = .20, SE(B) = .08, 95% CI [.05, .36], β = .14, p = .008, R2 = .02.
In previous publications using this film clip, we described it as thematically ambiguous and emotionally neutral (Haase et al., 2012; Seider et al., 2011). When we examined facial behavior for the present paper, participants on average showed positive emotional expressions during 13.4% of the time (i.e., number of bins during which positive expressions were coded out of a total of 28 bins: M = 2.09, SD = 4.08, range: 0–23). Thus, in the present paper, we refer to the film as thematically ambiguous yet subtly amusing.
References
- Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Archives of Neurology. 2004;61:1249–1253. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promotor gene (5-HTTLPR) with temperament in 12-month-old infants. Journal of Child Psychology and Psychiatry. 2001;42:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Beermann U, Ruch W. Can people really “laugh at themselves?”--experimental and correlational evidence. Emotion. 2011;11:492–501. doi: 10.1037/a0023444. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Marti CN, Lee H-J, Stote DL, Ferrell RE, Hariri AR, Telch MJ. Associations between serotonin transporter gene promoter region (5-HTTLPR) polymorphism and gaze bias for emotional information. Journal of Abnormal Psychology. 2011;120:187–197. doi: 10.1037/a0022125. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Keltner D. Facial expressions of emotion and the course of conjugal bereavement. Journal of Abnormal Psychology. 1997;106:126–137. doi: 10.1037//0021-843x.106.1.126. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Developmental Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. http://dx.doi.org/10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brocke B, Armbruster D, Müller J, Hensch T, Jacob CP, Lesch KP, Kirschbaum C, Strobel A. Serotonin transporter gene variation impacts innate fear processing: Acoustic startle response and emotional startle. Molecular Psychiatry. 2006;11:1106–1112. doi: 10.1038/sj.mp.4001908. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafo MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Review Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch K-P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Gottman JM, Levenson RW. Emotional behavior in long-term marriage. Psychology and Aging. 1995;10:140–149. doi: 10.1037//0882-7974.10.1.140. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders. Current Directions of Psychological Science. 2009;18:195–199. doi: 10.1111/j.1467-8721.2009.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6:284–290. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AHN, Kranzler HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biological Psychiatry. 2007;61:609–616. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Delbrück, Wendel, Grunewald, Sander, Morris-Rosendahl, Crocq, Berrettini, Hoehe 1997 doi: 10.1159/000134726. [DOI] [PubMed] [Google Scholar]
- Deming M. Stranger Than Paradise (1984) New York Times. 2010 Retrieved from http://www.nytimes.com/movies/movie/47216/Stranger-Than-Paradise/overview.
- Derogatis LR. BSI: Administration, scoring, and procedures for the Brief Symptom Inventory. 3rd. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Driver S, Jarmusch J. Stranger than paradise [Motion picture] USA/Germany: Cinesthesia Productions Inc; 1984. [Google Scholar]
- Ekman P, Friesen W. The repertoire of nonverbal behavior: Categories, origins, usage, and coding. Semiotica. 1969;1:49–98. [Google Scholar]
- Ekman P, Friesen WV. Felt, false and miserable smiles. Journal of Nonverbal Behavior. 1982;6:238–252. [Google Scholar]
- Ekman P, Friesen W, Hager J. The manual on CD ROM. Salt Lake City: Network Information Research Corporation; 2002. Facial Action Coding System. [Google Scholar]
- Eley TC, Hudson JL, Creswell C, Tropeano M, Lester KJ, Cooper P, Farmer A, Lewis CM, Lyneham HJ, Rapee RM, Uher R, Zavos HM, Collier DA. Therapygenetics: The 5HTTLPR and response to psychological therapy. Molecular Psychiatry. 2012;17:236–237. doi: 10.1038/mp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary--neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Feldman Barrett L, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55:647–654. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- Fox E, Zougkou K, Ridgewell A, Garner K. The serotonin transporter gene alters sensitivity to attention bias modification: Evidence for a plasticity gene. Biological Psychiatry. 2011;70:1049–1054. doi: 10.1016/j.biopsych.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion. 1998;12:191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motivation and Emotion. 2000;24:237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Lin YH, Weinstein M. The serotonin transporter polymorphism (5-HTTLPR): Allelic variation and links with depressive symptoms. Depression and Anxiety. 2010;27:260–269. doi: 10.1002/da.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, Vaish A, Hughes DA, Quinque D, Stoneking M, Friederici AD. Genetic and neural dissociation of individual responses to emotional expressions in human infants. Developmental Cognitive Neuroscience. 2011;1:57–66. doi: 10.1016/j.dcn.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Haase CM, Sze J, Goodkind MS, Coppola G, Lane J, Miller BL, Levenson RW. The effect of the serotonin transporter polymorphism (5-HTTLPR) on empathic and self-conscious emotional reactivity. Emotion. 2013;13:25–35. doi: 10.1037/a0029616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase CM, Seider BH, Shiota MN, Levenson RW. Anger and sadness in response to an emotionally neutral film: Evidence for age-specific associations with well-being. Psychology and Aging. 2012;27:305–317. doi: 10.1037/a0024959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase CM, Saslow LR, Bloch L, Saturn SR, Casey JJ, Seider BH, Lane J, Coppola G, Levenson RW. The 5-HTTLPR polymorphism in the serotonin transporter gene moderates the association between emotional behavior and changes in marital satisfaction over time. Emotion. 2013;13:1068–1079. doi: 10.1037/a0033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JR, Smolen A, Ormel J, Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect ‘for better and worse’. Translational Psychiatry. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Sheikh HI, Olino TM, Dougherty LR, Dyson MW, Durbin CE, Singh SM. The serotonin transporter promoter polymorphism and childhood positive and negative emotionality. Emotion. 2010;10:696–702. doi: 10.1037/a0019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt JK. Editorial policy on candidate gene association and candidate gene-by-environment interaction studies of complex traits. Behavior Genetics. 2012;42:1–2. doi: 10.1007/s10519-011-9504-z. [DOI] [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big-Five trait taxonomy: History, measurement, and conceptual issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. New York, NY: Guilford Press; 2008. pp. 114–158. [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Gelernter J, Kaffman A, Caspi A, Moffitt T. Arguable assumptions, debatable conclusions. Biological Psychiatry. 2010;67:e19–e20. doi: 10.1016/j.biopsych.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner D, Bonanno GA. A study of laughter and dissociation: Distinct correlates of laughter and smiling during bereavement. Journal of Personality and Social Psychology. 1997;73:687–702. doi: 10.1037//0022-3514.73.4.687. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2009;166(4):1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Kuepper Y, Wielpuetz C, Alexander N, Mueller E, Grant P, Hennig J. 5-HTTLPR S-allele: A genetic plasticity factor regarding the effects of life events on personality? Genes Brain, and Behavior. 2012;11:643–650. doi: 10.1111/j.1601-183X.2012.00783.x. [DOI] [PubMed] [Google Scholar]
- LaFrance M, Hecht MA, Paluck EL. The contingent smile: A meta-analysis of sex differences in smiling. Psychological Bulletin. 2003;129:305–334. doi: 10.1037/0033-2909.129.2.305. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. Long-term marriage: Age, gender, and satisfaction. Psychology and Aging. 1993;8:301–313. doi: 10.1037//0882-7974.8.2.301. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. The influence of age and gender on affect, physiology, and their interrelations: A study of long-term marriages. Journal of Personality and Social Psychology. 1994;67:56–68. doi: 10.1037//0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin. 1993;114:376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: A meta-analysis. Molecular Psychiatry. 2013;18:1018–1024. doi: 10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Papa A, Bonanno GA. Smiling in the face of adversity: The interpersonal and intrapersonal functions of smiling. Emotion. 2008;8:1–12. doi: 10.1037/1528-3542.8.1.1. [DOI] [PubMed] [Google Scholar]
- Pashler H, Wagenmakers E-J. Editors’ introduction to the Special Section on replicability in Psychological Science: A crisis of confidence? Perspectives on Psychological Science. 2012;7:528–530. doi: 10.1177/1745691612465253. [DOI] [PubMed] [Google Scholar]
- Pelc K, Cheron G, Dan B. Behavior and neuropsychiatric manifestations in Angelman syndrome. Neuropsychiatric Disease and Treatment. 2008;4:577–584. doi: 10.2147/ndt.s2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J, Way BM, Taylor SE. 5-HTTLPR moderates effects of current life events on neuroticism: Differential susceptibility to environmental influences. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:1070–1074. doi: 10.1016/j.pnpbp.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin. 2013;139:901–916. doi: 10.1037/a0030196. [DOI] [PubMed] [Google Scholar]
- Reisenzein R, Bördgen S, Holtbernd T, Matz D. Evidence for strong dissociation between emotion and facial displays: The case of surprise. Journal of Personality and Social Psychology. 2006;91:295–315. doi: 10.1037/0022-3514.91.2.295. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC: American Psychological Association; 2007. [Google Scholar]
- Roy A, Hu X-Z, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Ruch W. Exhilaration and humor. In: Lewis M, Haviland JM, editors. Handbook of emotions. New York, NY: Guilford Press; 1993. pp. 605–616. [Google Scholar]
- Ruch W. Will the real relationship between facial expression and affective experience please stand up: The case of exhilaration. Cognition and Emotion. 1995;9:33–58. [Google Scholar]
- Schimmack U. The ironic effect of significant results on the credibility of multiple-study articles. Psychological Methods. 2012;17:551–566. doi: 10.1037/a0029487. [DOI] [PubMed] [Google Scholar]
- Schoebi D, Way BM, Karney BR, Bradbury TN. Genetic moderation of sensitivity to positive and negative affect in marriage. Emotion. 2012;12:208–212. doi: 10.1037/a0026067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider BH, Shiota MN, Whalen P, Levenson RW. Greater sadness reactivity in late life. Social Cognitive and Affective Neuroscience. 2011;6:186–194. doi: 10.1093/scan/nsq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota MN, Neufeld SL, Yeung WH, Moser SE, Perea EF. Feeling good: Autonomic nervous system responding in five positive emotions. Emotion. 2011;11:1368–1378. doi: 10.1037/a0024278. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Sze JA, Gyurak A, Yuan JW, Levenson RW. Coherence between emotional experience and physiology: Does body awareness training have an impact? Emotion. 2010;10:803–814. doi: 10.1037/a0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tremeau F, Malaspina D, Duval F, Correa H, Hager-Budny M, Coin-Bariou L, Macher JP, Gorman JM. Facial expressiveness in patients with schizophrenia compared to depressed patients and nonpatient comparison subjects. American Journal of Psychiatry. 2005;162:92–101. doi: 10.1176/appi.ajp.162.1.92. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry. 2012;2:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Matthews BJ, Allman JM. Brain activation during sight gags and language-dependent humor. Cerebral Cortex. 2007;17:314–324. doi: 10.1093/cercor/bhj149. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JW, McCarthy M, Holley SR, Levenson RW. Physiological down-regulation and positive emotion in marital interaction. Emotion. 2010;10:467–474. doi: 10.1037/a0018699. [DOI] [PubMed] [Google Scholar]