SUMMARY
Patients with head and neck squamous cell carcinoma (HNSCC) demonstrate poor survival and significant treatment morbidity with standard therapy. The immune profile in HNSCC, whether caused by carcinogen exposure or human papillomavirus (HPV), is notably immunosuppressive. Early clinical trials of immunotherapy in HNSCC were troubled by systemic toxicity or difficulties in local administration. Now, interest in immunotherapy has been revitalized by mechanistic insights into immune evasion by HNSCC, coupled to ongoing development of novel immunotherapies. This review will summarize immune escape mechanisms in HNSCC, namely downregulation of tumor antigen (TA) presentation, aberrant regulation of the signal transducer and activator of transcription (STAT) family, the immunosuppressive cytokine milieu, and dysregulation of immune effector cells. Therapeutic strategies hypothesized to specifically counter HNSCC immunosuppression will then be discussed. We will survey TA-targeted monoclonal antibodies (mAb), including the prototype cetuximab, as well as adjunctive strategies to enhance antibody-dependent cell-mediated cytotoxicity. We will review immunomodulation to restore STAT1/STAT3 activation balance. Examples of mAb therapy to block immunosuppressive cytokines, such as interleukin-6 or VEGF, will be provided. mAbs which release co-inhibitory T cell receptors such as CTLA-4 and PD-1, overexpressed in HNSCC, also hold therapeutic promise. Finally, we will describe principles for therapeutic vaccination in HPV-associated HNSCC, where non-host TAs such as viral oncoproteins represent ideal targets, and HPV-negative HNSCC, where p53 is a promising target. Insights into immunosuppression in HNSCC have elucidated mechanistic targets for immunotherapy. Rational clinical investigation may lead to effective stand alone or combinatorial treatment approaches.
Keywords: Head and neck cancer, Immunotherapy, Monoclonal antibody, STAT, CTLA-4, PD-1, HPV, p53, Cytokine
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth leading incident cancer worldwide [1]. In the United States, the 2012 disease burden included 52,600 new cases and 11,500 deaths [2]. Despite diagnostic and therapeutic advances, 5-year overall survival (OS) is 40–60%, with only a modest improvement over the past two decades [3]. Improved prognosis is largely attributable to the emerging epidemic of oral human papillomavirus infection (HPV). An increasing proportion of oropharyngeal HNC is driven by oncogenic HPV, rather than the classic risk factors of tobacco and alcohol; HPV etiology is associated with improved survival after conventional treatments [4,5]. Although two distinct causes of HNSCC exist, environmental carcinogenesis or transformation by HPV oncogenes, in both cases HNSCC is associated with a fundamental failure of immune surveillance, where tumor cells have escaped recognition and lysis by the cytotoxic T lymphocytes (CTLs) of adaptive immunity.
Classically, HNSCC is understood as an immunosuppressive disease. Patients demonstrate lower absolute lymphocyte counts than healthy subjects [6], spontaneous apoptosis of CTLs [7], impaired natural killer (NK) cell activity [8,9], and poor antigen-presenting function [10,11]. Early recognition of immunosuppression in HNSCC patients resulted in clinical trials testing available immunostimulatory strategies including interleukin (IL)-2 and interferon (IFN)-α2a. While local IL-2 based therapies were associated with favorable local and systemic immune activation [12,13], as well as a positive randomized phase III study in operable HNSCC [14], the feasibility of tumoral injection has hampered development outside of select academic centers. The systemic combination of IL-2 and IFN-α2a demonstrated modest clinical activity with excess toxicity in recurrent/metastatic disease, and was abandoned [15]. However, interest in the paradigm of systemic immunotherapy has been reinvigorated by progressive mechanistic insights into immune evasion by HNSCC, coupled to recent successful development of novel immunotherapies in other solid tumors. This review will survey promising systemic immunotherapies currently in human clinical trials, which exploit established mechanisms of immune escape by HNSCC.
HNSCC: mechanisms of immune escape
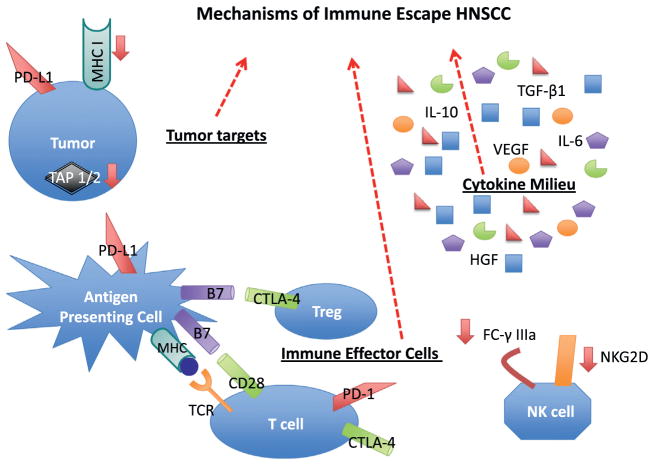
Fundamentally, successful immune eradication of HNSCC requires tumor recognition and lysis by the tumor antigen (TA)-specific CTL – the effector cell of adaptive immunity. Various strategies are employed by HNSCC cells to evade immune recognition and destruction. Conceptually, these strategies can be categorized with reference to the tumor target, the cytokine milieu, or the immune effector cells as graphically depicted in Fig. 1.
Figure 1.
Mechanisms of Immune Escape in HNSCC. A mechanistic understanding of HNSCC immune escape reveals potential targets for immunotherapy: (1) HNSCC tumors down-regulate antigen processing (TAP 1/2) and presentation (MHC 1). Tumors also commonly express PD-L1, the ligand for the immune checkpoint receptor PD-1. (2) The HNSCC microenvironment is characterized by an excess of immunosuppressive cytokines (e.g. TGF-β1, VEGF, IL-6 and IL-10) and a deficiency of immunostimulatory cytokines (e.g. IFN-γ). The cytokine milieu is influenced by imbalanced STAT1/STAT3 signaling. (3) Effector T cells receive negative feedback from immune checkpoints (CTLA-4, PD-1), co-inhibitory receptors which promote pathologic immune tolerance in the setting of HNSCC. Regulatory T cells also promote immune tolerance through CTLA-4 signaling. Down-regulation of NK cell receptors such as NKG2D and Fc-γ IIIa inhibits innate immune responses, including ADCC.
Primary target: tumor antigen presentation
Immunogenic TA exist in HNSCC, as confirmed by peripheral detection of CTLs specific for wild type p53, epidermal growth factor receptor (EGFR), and in the case of HPV-related cancer, the E7 HPV oncoprotein [16–18]. Nonetheless, TA-specific CTLs fail to recognize and kill HNSCC tumor cells. HNSCC subverts TA recognition by selective loss of human leukocyte antigen (HLA) I, the major histocompatibility complex (MHC) required for interaction between TA and TA-specific CTLs [19]. More important, HNSCC tumors are functionally deficient in antigen processing machinery (APM) [11,19]. In preclinical models, non-recognition of HNSCC by functional HLA class I antigen-restricted, TA-specific CTLs is associated with downregulation of the IFN-γ inducible APM components including the transporter associated with antigen processing (TAP)-1/2 heterodimer [11]. Deficits in TA processing and presentation occur in both HNSCC tumor cells and in dendritic cells (DC), the most potent antigen-presenting cell (APC); aberrant regulation of the signal transducer and activator of transcription (STAT) family drives these deficits. In tumor cells, reduced expression of HLA I APM components results from basal deficiency in activated (phosphorylated) STAT1, and is restored in vitro by IFN-γ [20]. Failed activation of TA-specific CTLs is compounded by excessive tumoral STAT3 signaling, which impairs TA presentation by DC [21]. While these data reinforce that targeting TA is feasible in HNSCC, strategies to restore the balance of STAT1/STAT3 signaling may enhance antigen presentation.
The microenvironment: immunosuppressive cytokine milieu
The cytokine family of proteins, comprised of interleukins, interferons, tumor necrosis factors, growth factors and chemokines regulates cellular growth, proliferation, migration and signaling in both the tumoral and immune compartments. The HNSCC microenvironment is characterized by an imbalanced cytokine profile, favoring immunosuppressive over stimulatory cytokines. Systemic therapies to reverse the immunosuppressive cytokine balance could be particularly relevant in HNSCC, where the NFκB repertoire of inflammatory cytokines, including IL-6, VEGF, and HGF, is detected in high concentration in the sera of patients and longitudinally correlates with relapse [22]. Major contributors to the cytokine milieu include imbalanced STAT1/STAT3 signaling within tumor cells, hepatocyte growth factor (HGF) production by tumor-associated fibroblasts (TAF), and production of multiple proproliferative, immunosuppressive cytokines by tumor-associated macrophages (TAM). Deficiency of tumoral pSTAT1 signaling results in low production of CCL5 and CXCL10, chemokines which recruit effector T cells to the microenvironment [23]. Excessive pSTAT3 signaling increases production of TGF-β1, VEGF, IL-6 and IL-10, cytokines that negatively regulate pro-inflammatory danger signals, DC maturation, and cytolysis by natural killer (NK) cells and CTLs [8,21,24,25]. IL-10 also induces regulatory T cells (Tregs) [26]. In a paracrine loop, HNSCC stimulates HGF production by TAFs, in turn a mediator of HNSCC proliferation and metastasis [27]; HGF also inhibits DC maturation [28]. Through secretion of colony stimulating factor (CSF)-1 and other chemokines, HNSCC tumors recruit TAMs to the microenvironment. TAMs create a favorable milieu for tumor survival and immune escape by secreting TGF-β1, IL-6, and prostaglandin-E2 among other immunosuppressive cytokines [29].
Immune effector cells
The critical effector cell of adaptive antitumor immunity is the activated CD8(+) CTL. Activation of the naïve, antigen-restricted CD8(+) CTL first requires binding of the T cell receptor (TCR) to its cognate TA in complex with HLA I. Although TA-TCR receptor engagement is necessary, it is not sufficient for CTL activation and tumor cytolysis. Initial activation also depends upon the balance of co-stimulatory vs. co-inhibitory signaling by DCs and CD4(+) helper T cells, as well as freedom from suppression by CD4(+) regulatory T cells (Treg). HNSCC elicits T cell anergy in both peripheral and tumor-infiltrating lymphocytes (TIL). Functional defects in TILs include low production and response to IL-2 [30,31]; vulnerability to spontaneous apoptosis, mediated by the Fas/Fas-ligand pathway [32]; low expression of CD3-ζ, OX40, and 4-1BB, co-stimulatory molecules required for signaling by the TCR [31,33]; and high expression of co-inhibitory receptors, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed-death 1 (PD-1) [31,34]. Of note, the PD-1 ligand, PD-L1, is expressed in the majority of HNSCC [35]; moreover, the interaction between PD-L1 and PD-1 has been linked to the immune-privileged evolution of HPV-associated HNSCC [36]. CTLs are also inhibited by disproportionate recruitment of Tregs to the HNSCC tumor microenvironment. Tregs promote tolerance by signaling through the inhibitory CTLA-4 receptor [37].
The NK cell is a major effector of innate immunity. NK cells are large granular lymphocytes that participate in immune responses to pathogens and tumor cells. In particular, theNKcell executes antibody-dependent, cell-mediated cytotoxicity (ADCC). The primary cytotoxicity receptor, NKG2D, is downregulated by excessive TGF-β1 in HNSCC [8]. Similarly, CD16 or Fcγ-Receptor (Fcγ-R) IIIa, which mediates ADCC, is downregulated in HNSCC. The activation status of NK cells is also important to the cytokine milieu, as activated NK cells secrete the immunostimulatory cytokines IFN-γ and TNF-α.
Promising immunotherapies
Although discussed above compartmentally, immune evasion in HNSCC is characterized by extensive and redundant interactions among tumor cells, cytokines, and effector cells. For purposes of academic review, we have categorized novel systemic immunotherapies by impact on TA recognition and presentation, ADCC, cytokine balance, or CTL activation (Table 1), recognizing that immunoregulatory cross-talk makes such categorical distinctions artificial.
Table 1.
Promising Immunotherapies in HNSCC
| Drug | Mechanism | Phase of development in human cancer | HNSCC development |
|---|---|---|---|
| TA-specific monoclonal antibodies | |||
| Cetuximab | Anti-EGFR chimeric IgG1 mAb | FDA approved in HNSCC, colon cancer | FDA approved |
| Panitumumab | Anti-EGFR IgG2 humanized mAb | II/III | II/III |
| Nimotuzumab | Anti-EGFR IgG1 humanized mAb | II | II |
| Onartuzumab | Anti-cMet IgG1 single-arm humanized mAb | II/III | NA |
| AV-203 | Anti-HER3 IgG1 humanized mAb | I | I |
| MM-121 | Anti-HER3 IgG2 humanized mAb | I/II | NA |
| Cixutumumab | Anti-IGFR IgG1 humanized mAb | II | II |
| Enhancing ADCC to TA-specific mAb | |||
| IL-12 | Cytokine agonist of NK cell activation | II | II |
| VTX-2337 | TLR 8 agonist; enhanced DC activation and IL-12 secretion | II | I/II |
| Restoring STAT1/STAT3 signaling balance | |||
| Ruxolitinib | JAK 1 and JAK 2 inhibitor | I/II/III | NA |
| SAR302503 | Selective JAK 2 inhibitor | II | NA |
| BMS911543 | Selective JAK 2 inhibitor | I/II | NA |
| Pegylated IFN-γ | Upregulation of pSTAT1 | NA; phase III in chronic hepatitis C | NA |
| Targeting immunosuppressive cytokines | |||
| Bevacizumab | Anti-VEGF IgG1 humanized mAb | FDA approved in lung, colon cancers | III |
| Ficlatuzumab | Anti-HGF IgG1 humanized mAb | I/II | I |
| AMG 102 | Anti-HGF IgG2 humanized mAb | I/II | NA |
| Siltuximab | Anti-IL-6 IgG1 humanized mAb | I/II | NA |
| T cell checkpoint inhibitors | |||
| Ipilimumab | Anti-CTLA4 IgG1 humanized mAb | FDA approved in melanoma | I |
| Tremelimumab | Anti-CTLA4 IgG2 humanized mAb | III | NA |
| BMS-936558 | Anti-PD-1 IgG4 humanized mAb | I–III | NA |
| BMS-936559 | Anti-PD-L1 IgG4 humanized mAb | I | NA |
| Therapeutic cancer vaccines | |||
| HPV 16 E6 and E7 peptide vaccine | Long peptide vaccine with incomplete freund’s adjuvant | I | NA |
| MAGE-3 and HPV-16 vaccine | Trojan construct peptide vaccine | I | I |
| HPV pNGVL4a-CRT/E7 (Detox) DNA vaccine | Detoxified E7 DNA in a pNGVL4a plasmid backbone | I | I |
| TG4001 vaccine | Modified vaccinia virus expressing E6 and E7 oncoproteins | I | NA |
| Lm-LLO-E7 vaccine | Live-attenuated Listeria monocytogenes bacterium vaccine which secretes HPV-16 E7 antigen fused to listeriolysin O | I | I |
| Multi-epitope p53 vaccine | Wild type peptide/autologous dendritic cell vaccine | I | I |
Targeting tumor antigens: the TA-targeted monoclonal antibody
The most active area of immunotherapy in HNSCC in the last decade has been the development of monoclonal antibodies (mAbs) targeting TA on the cell surface. The majority of inquiry has focused on EGFR. From a non-immunologic standpoint, mAbs in solid tumor oncology including cetuximab (anti-EGFR) and trastuzumab (anti-human epidermal growth factor receptor 2 [HER2]) were developed to block pro-proliferative, anti-apoptotic signaling transmitted by respective transmembrane growth factor receptors. Clinical response to TA-targeted mAbs, while limited to a minority, was often superior to non-immunogenic small molecule inhibitors of the same receptors. This conundrum prompted recognition of additional, immune-based mechanisms of mAb efficacy. Cetuximab, the first FDA-approved molecularly targeted drug in HNSCC, is a prototype mAb with dual signaling and immunologic mechanisms.
EGFR is over-expressed in HNSCC; high expression correlates with increased stage at presentation and poor prognosis [38]. Activation of EGFR by its ligands, amphiregulin and TGF-α, triggers oncogenic signaling along the Ras/MAPK and PI3K/Akt pathways. Although EGFR overexpression correlates with HPV-negative status, EGFR activation and trafficking are deregulated by HPV oncoproteins independent of EGFR expression level [39–42]. Cetuximab is a chimeric IgG1-isotype mAb, which binds to the extracellular domain of EGFR, competitively inhibiting activation. Cetuximab improves response and survival when added to radiation in locally advanced HNSCC, or to chemotherapy in recurrent/metastatic disease [43,44]. Cetuximab is also indicated as monotherapy following failure of platinum in recurrent/metastatic disease, where response rate is 10–13% [45]. In addition to blockade of downstream EGFR signaling, cetuximab has a number of clinically important immune mechanisms: (1) activation of the FcγR IIIa on NK cells, triggering antibody-dependent cellular cytotoxicity (ADCC); (2) IFN-γ secretion by activated NK cells, resulting in upregulation of APM in DCs and enhanced antigen presentation; (3) cross-priming of NK and DCs, stimulating TA-specific CTLs [46,47]. The efficacy of cetuximab may differ in HPV-associated vs. HPV-negative disease. While the phase III survival benefit of cetuximab-radiotherapy appeared driven by the oropharyngeal subgroup, tissue was not available to confirm whether HPV mediated this differential benefit. Subsequent single-institution series have noted inferiority of cetuximab as compared to cisplatin-radiotherapy in locally advanced HNSCC [48,49], irrespective of HPV status, however were flawed by retrospective design and contradicted by others [50,51]. This question will be addressed definitively by RTOG 1016, the head-to-head phase III trial evaluating the noninferiority of cetuximab vs. cisplatin-radiotherapy in HPV-associated HNSCC [52].
Two other anti-EGFR mAbs have been clinically evaluated in HNSCC. Panitumumab, a fully human IgG2 mAb, is theoretically less immunogenic than IgG1 isotype mAbs due to poor FcγRIIIa binding, although binding through an alternative FcγR (IIa) has been proposed [53]. Clinical activity appears inferior to cetuximab. Panitumumab failed to improve survival when added to platinum-based chemotherapy in recurrent/metastatic HNSCC, although a secondary analysis noted improvement in patients with p-16 negative tumors, a surrogate for HPV-negative status [54]. In a phase II trial comparing cisplatin-radiotherapy (CRT) to panitumumab-radiotherapy in locally advanced disease, the primary endpoint of 2-year locoregional control favored CRT [55]. Nimotuzumab is a fully humanized IgG1 mAb specific to the extracellular domain of EGFR. Preliminary data from a phase II trial in locally advanced HNSCC, evaluating (chemo) radiation with or without nimotuzumab, suggested a correlation between EGFR expression and survival benefit [56]. In a biomarker-modulation study, 9 of 10 patients with unresectable HNSCC achieved objective responses to nimotuzumabradiotherapy [57]. Immunologic correlates were not reported.
A well-established signaling-based resistance mechanism to cetuximab is alternate activation of parallel growth factor receptors including HER2, HER3, cMet, and insulin growth factor receptor (IGFR) [58]. mAbs targeting these receptors are in clinical development; representative class members include trastuzumab and pertuzumab (anti-HER2), AV-203 (anti-HER3), onartuzumab (anti-cMet), and cixutumumab (anti-IGFR). In HER2 expressing xenograft models treated with trastuzumab, pertuzumab enhanced ADCC [59], raising the hypothesis that co-targeting TA with mAb may co-stimulate immune response. AV-203 is undergoing phase I development in combination with cetuximab, including a HNSCC cohort (NCT01603979). Cixutumumab, with or without cetuximab, was of disappointing efficacy in a randomized phase II study in recurrent/metastatic HNSCC; neither arm demonstrated improved PFS relative to the historical control arm of cetuximab monotherapy [60]. Cixutumumab is currently being evaluated in a neoadjuvant biomarker-modulation study in operable HNSCC (NCT00957853).
Enhancing ADCC-related response to TA-specific mAb
Insights into the immunologic mechanisms of cetuximab point toward clinical strategies to amplify immune response, in particular ADCC. Such strategies include patient selection for favorable FcγR polymorphisms, co-administration of exogenous cytokines, or agonism of the toll like receptor (TLR) family of pathogen-recognition receptors key to innate immunity.
Among innate immune effectors, the NK cell is uniquely able to execute ADCC. NK cells express the activating FcγR IIIa, which binds the Fc component of IgG antibodies coating their target. Due to in vitro correlation of ADCC activity with FcγR polymorphisms, related to differential binding of the Fc component of the mAb to the FcγR, patient selection theoretically could enrich for responders to cetuximab. However, the association of cetuximab clinical activity with the favorable VV FcγR genotype, first demonstrated in colorectal cancer [61], was not confirmed in HNSCC patients [62].
Cytolysis triggered by binding of the NK cell FcγR to antibody is dramatically magnified by the stimulatory cytokines IFN-α and IL-12, endogenously released by activated DCs. Exogenous IL-12 is now in clinical development, due to preclinical models demonstrating enhanced activation of NK cells when costimulated by IL-12 and FcγR binding [63]. A phase I study of paclitaxel, trastuzumab and intravenous IL-12 in HER2 expressing malignancies demonstrated an acceptable toxicity profile; clinical benefit correlated with immune response, characterized by increased IFN-γ secretion [64]. Local injections of IL-12 have been evaluated in 10 previously untreated patients with HNSCC. Tissue correlates of NK cell activation, including upregulation of IFN-γ and relocation to the primary tumor and draining lymph nodes, encouraged systemic investigation [65]; a phase II trial of cetuximab and subcutaneous IL-12 in recurrent/metastatic disease is ongoing (NCT01468896).
Hematopoietic cells express multiple members of the TLR family. TLR signaling is the sentinel danger response of innate immunity, and is triggered by recognition of fixed pathogen-associated molecular patterns. TLR 3, 7, 8 and 9 are located within the endolysosome, and recognize foreign nucleic acid motifs from intracellular pathogens. In particular, activation of TLR7 or TLR8 signaling within DCs enhances phagocytosis of apoptotic or necrotic cells; theoretically, administration as an immune adjunct in cancer could increase TA uptake and presentation [66,67]. Moreover, concomitant increase in secretion of TNFα and IL-12 by activated DCs could augment ADCC [68]. Currently, the TLR7 agonist imiquimod is under study as a topical immune adjuvant in melanoma [69]. VTX-2337 is a selective, subcutaneously-administered TLR8 agonist in phase I development in combination with cetuximab in recurrent/metastatic HNSCC (NCT01334177). A randomized phase II trial of first line platinum-doublet chemotherapy and cetuximab, with or without VTX-2337, is also underway (NCT01836029).
Restoring STAT1/STAT3 signaling balance
IFN-γ upregulates HLA and antigen presentation, in both dendritic and tumor cells; however it is deficient in the HNSCC microenvironment. This deficiency is associated with suppressed IFN-γ induced pSTAT1 signaling in tumor cells. Conversely, DC maturation is suppressed by tumoral secretion of STAT3-induced cytokines, in particular IL-6. Balance in STAT1/STAT3 signaling could theoretically be restored via upregulation of pSTAT1 or downregulation of pSTAT3.
While recombinant human IFN-γ (rhIFN-γ) has been clinically available since the 1980s, early development was impaired by short half-life and need for continuous intravenous infusion. When evaluated in 8 patients with advanced HNSCC, weekly 24-h infusions of rhIFN-γ resulted in measurable disease regression in 3 patients, and stable disease in 4. There has been little work in HNSCC since this study [70]. However, pegylated forms of IFN-γ with prolonged half-lives are currently under study for the treatment of chronic Hepatitis C [71]. Investigation of pegylated IFN-γ as an immune adjunct in HNSCC, to increase transcription of pSTAT1 target genes, appears warranted.
In vitro STAT3 inhibition reverses the immunosuppressive phenotype of HNSCC [72]. However, as with other transcription factors, STAT3 has proven challenging to target directly in humans. In an intra-operative phase 0 trial conducted in HNSCC, a STAT3 oligonucleotide decoy reduced target gene expression; however the requirement for local injection impedes broader clinical development [73]. Although STAT3 is constitutively phosphorylated, activating mutations are not described in HNSCC [74]. However, STAT3 is phosphorylated by upstream growth factor receptors including Janus kinases (JAK) and Src. Specifically, IL-6 stimulates phosphorylation of STAT3 through JAK 1 and 2. The recent availability of JAK receptor antagonists raises the possibility of blocking this autocrine loop. Ruxolitinib is a selective ATP-competitive inhibitor of JAK1 and JAK2 kinases; it is the first JAK family inhibitor approved by the FDA, with an indication in myelofibrosis [75]. A similar preclinical JAK 1/2 inhibitor, AZD1480, abrogated IL-6 induced STAT3 phosphorylation and suppressed the growth of human solid tumor xenografts with constitutive STAT3 activity [76]. Another developing drug, BMS-911543, is a JAK2 selective inhibitor with antiproliferative activity in JAK2 mutated myeloproliferative neoplasms [77]. Although JAK inhibitors have not yet been investigated in HNSCC clinical trials, they have intriguing potential to block both oncogenic and immunosuppressive STAT3 signaling.
Targeting immunosuppressive cytokines
The counterpoint to clinical administration of immunostimulatory cytokines is the blockade of immunosuppressive cytokines. Specifically relevant to HNSCC are mAbs against the growth receptor ligands VEGF, HGF, and IL-6. Anti-cytokine mAbs bind their respective ligands, preventing them from engaging and activating growth receptors.
Bevacizumab is specific therapeutic monoclonal antibody against the pro-angiogenic, immunosuppressive VEGF ligand, secreted by tumors and TAMs. VEGF tumor levels in HNSCC correlate with shortened disease free and overall survival [78]. Tumor microvascular density is also a negative prognostic indicator; therefore, agents that target tumor angiogenesis are attractive [79]. While ineffective as monotherapy, bevacizumab increased overall survival when added to standard carboplatin and paclitaxel in advanced non-small cell lung cancer [80]. In a single-arm phase II trial in recurrent/metastatic HNSCC, bevacizumab failed to improve response rate or disease control when added to cetuximab [81]. However, an ongoing randomized phase III trial is evaluating platinum doublet chemotherapy with or without bevacizumab, in the front line management of patients with recurrent/metastatic HNSCC (NCT00588770).
The HGF/cMet oncogenic signaling pathway is under development as a clinical target in HNSCC due to evidence that it mediates resistance to EGFR blockade, platinum chemotherapy, and radiation [82–84]. In addition to stimulating oncogenic tumor signaling, stromal HGF suppresses maturation of DCs. Co-investigation of immune correlates will be important in the clinical development of two anti-HGF mAbs, AV-299 and AMG-102. Trials in HNSCC are being planned with both of these compounds.
Tumors and TAMs secrete IL-6, a cytokine which blocks DC maturation and activation. Siltuximab, an anti-IL-6 mAb, is in phase II development in prostate cancer and multiple myeloma (NCT00385827; NCT01484275). Given the purported immune mechanism, siltuximab would be an attractive immune adjunct to evaluate in HNSCC.
Targeting immune effector cells: releasing coinhibitory receptors
Successful activation of the CTL following binding of TA-TCR binding depends upon predominance of co-stimulatory vs. coinhibitory signaling by accessory receptors. CTLA-4 and PD-1 are so-called “immune checkpoints,” co-inhibitory receptors which downmodulate CTL response in the setting of chronic antigen stimulation – a useful adaptation for resolving the inflammatory response following infection and in preventing auto-immunity. However in the setting of cancer, these receptors induce pathologic tolerance. A new therapeutic paradigm is the design of mAbs to block co-inhibitory signaling, releasing the CTL from anergy. The first in class is ipilimumab, an IgG1 mAb against the CTLA-4 co-inhibitory receptor expressed on activated CTLs and Tregs. CTLA-4 and the major co-stimulatory receptor CD28 compete for the same ligand on APCs, B7. Blockade of CTLA-4 releases B7 to bind CD28, thus propagating the B7-CD28 co-signal required for TA-specific TCR activation. Ipilimumab has been FDA-approved in melanoma [85]. Release from CTLA-4 coinhibitory signaling appears to upregulate TA-specific CTLs and mediate therapeutic response. Blockade of constitutive CTLA-4 signaling in Tregs also potentiates response [86]. However, nonspecific CTL upregulation can lead to significant autoimmune adverse events including colitis, dermatitis, and panhypopituitarism [87]. A similar drug, the IgG2 mAb tremelimumab, is also under development [87]. CTLA-4 checkpoint inhibitors have striking potential to release T-cell immunosuppression in HNSCC, where the immune microenvironment is characterized by CTL anergy and Treg infiltration. A phase I trial of ipilimumab, cetuximab and radiotherapy in high risk, locally advanced disease is underway (UPCI 12-084).
PD-1 is a second inhibitory member of the CD28/CTLA-4 family of co-receptors. Expressed on CTL, NK cells, B cells and macrophages, PD-1 is thought to be a broader negative regulator of immune response than CTLA-4. PD-1 has two ligands, PD-L1 and PD-L2. PD-L1 is upregulated on DCs and macrophages in response to chronic antigen stimulation, as is the case in the tumor microenvironment; many tumors including HNSCC coopt expression of PD-L1 to induce CTL and NK anergy. Therapeutic mAbs against both PD-1 and PD-L1 are entering advanced stages of clinical development [37,88]. A phase I trial of BMS-936558, a humanized IgG4 anti-PD-1 mAb, was conducted in patients with advanced solid tumors including NSCLC, renal carcinoma and melanoma. There were 31 responses; 20 of these were durable, lasting greater than one year. Immune adverse events, including pneumonitis, vitiligo, colitis, hepatitis, did not limit treatment. Of note, objective responses correlated with PD-L1 expression on tumor. Similarly, a large phase I study of BMS-936559, a humanized IgG4 anti-PD-L1 mAb, documented durable objective responses in 6–17% of patients with advanced solid tumors. Given the expression of PD-L1 in the majority of HPV-negative and HPV-positive HNSCC, these therapeutic antibodies are of particular interest in HNSCC – as monotherapy, or as adjuncts to conventional therapies including cetuximab.
Therapeutic cancer vaccines: instigating and expanding CTL response
Enthusiasm for the development of head and neck tumor vaccines is motivated by the observation of nascent CTL responses against unique TAs; the existence of this thwarted immune response implies the potential to harness and amplify adaptive immunity. Fundamentally, the goal of therapeutic cancer vaccination is inculcation of a persistent, TA-specific T cell response which kills tumor cells – abating tumor progression or even resulting in cure. In general, an effective vaccine will require successful TA presentation by professional APCs and a consequent TA-specific CTL response. Vaccines may target two forms of TA: (1) tumor specific antigens (TSA), or (2) tumor associated antigens (TAA) [89]. TSA are oncoproteins unique to the tumor not occurring in normal host cells (e.g. mutated p53 protein or the E6/E7 HPV oncoproteins). Targeting TSA may be advantageous as these proteins are often central to tumorigenesis, and their specificity would avoid auto-immune sequelae for normal tissue. However, a TSA-targeting vaccine may be applicable to only a small minority, whose tumor bears the candidate somatic mutation. This can be particularly prohibitive when the target is a tumor suppressor gene inactivated by a variety of point mutations, frameshifts or deletions – as is the case for TP53 mutation, the most common genetic mutation in HNSCC [74]. TAA are proteins over-expressed in tumor cells however also are expressed in normal tissues (e.g. wild type EGFR). While TAA over-expression is prevalent in tumors with a common histology, making them a broadly applicable target, TAAs are limited by weak immunogenicity and self-tolerance.
Ultimately, cancer vaccines must deliver antigenic peptides to professional APCs for presentation in association with MHC to the cognate CTL. Various vaccination methods exist, each with their own particular advantages and drawbacks. Three methods are of interest in HNSCC: (1) protein-based or peptide vaccines, consisting of pre-assembled proteins; (2) DNA vaccines, consisting of recombinant, TA-encoding DNA in a plasmid backbone; (3) recombinant vector-based vaccines, where a viral, bacterial or yeast vector is loaded with recombinant DNA encoding the TA of interest. In peptide vaccines, for example HPV oncoprotein peptide vaccines, oncogenic activity must be inactivated while maintaining sufficient peptide length to stimulate CTL response. Advantages to this approach include ease of production and the ability to target TSA, whereas downsides include host proteolysis, weak immunogenicity, HLA restriction, and poor long-lasting immunity [90]. DNA vaccines are more stable than peptides, however DNA uptake by APC associated with effective antigen expression is limited. Delivery methods, such as by electroporation or gene gun, can enhance uptake and immunogenicity [91]. Vector-based vaccines may overcome the poor antigenicity of naked DNA vaccines, due to a cross-over effect from the robust inflammatory response against vector antigens.
HPV is an ideal vaccine target, due to expression of non-host TSAs and constitutive expression of these viral oncoproteins to maintain the transformed state. Proof-of-principle has been demonstrated by the successful development of HPV prevention vaccines, Cervarix® and Gardasil®. While these marketed vaccines prevent anogenital HPV infection, their impact on the natural history of oral HPV is as of yet unknown. Regardless, the capsid antibodies triggered by these L1 peptide vaccines are useful only for primary prevention; humoral blockade of the viral entry step is not relevant for established, HPV-transformed malignancies. Therapeutic vaccines for HPV-related cancers are of substantial interest in HNSCC. Five promising vaccination strategies have entered clinical development in HPV-induced neoplasia including two peptide vaccines, a detoxified E7 DNA vaccine, and two vector vaccines. (1) The HPV 16 E6 and E7 long peptide vaccine with incomplete freund’s adjuvant was studied in 20 women with HPV-16 associated vulvar intraepithelial neoplasia. All patients had vaccine-induced CTL responses; 15 out of 19 patients had clinical responses [92]. (2) In a phase I study of a Trojan peptide vaccine containing HLA-I and HLA-II restricted Melanoma Antigen E (MAGE-A3) and HPV-16 derived peptides, immunogenicity was documented in 4 out of 5 patients with advanced HNSCC, however none exhibited an objective response [93]. (3) The HPV pNGVL4a-CRT/E7 (Detox) DNA vaccine contains the HPV 16 E7 gene engineered to disrupt the retinoblastoma binding site, thereby abrogating oncogenicity, embedded in the pNGVL-4a plasmid backbone [94]. This vaccine is under phase I study in patients with HPV-associated HNSCC following definitive multimodality therapy (NCT01493154). 4) TG4001, a modified vaccinia virus expressing the HPV-16 oncoproteins E6 and E7 as well as human interleukin-2, has been studied in 21 patients with cervical intraepithelial neoplasia (CIN). HPV 16 clearance was associated with cytologic regression in 7 out of 10 clinical responders. Additionally, 7 of 8 patients cleared HPV infection without conization and had no residual suspicion of CIN2/3 [95]. (5) The Lm-LLO-E7 vaccine harnesses a live-attenuated Listeria monocytogenes bacterium engineered to secrete the HPV-16 E7 antigen fused to listeriolysin O, the virulence factor permitting cytosolic replication in APCs [96]. This vaccine was evaluated for safety in 15 patients with advanced cervical carcinoma [97]. Dose-limiting toxicities consisted of pyrexia and diastolic hypotension; assessment of CTL response was technically limited. This vaccine is current under phase I investigation in patients with HPV-associated HNSCC with no evidence of disease after completion of standard therapy (NCT 01598792).
In HPV-negative HNSCC, overexpressed wild type (WT) TAAs, such as p53, are potential vaccine targets. Although TP53 mutation is the most commonly identified mutation in HPV-negative HNSCC, most mutations result in the accumulation of p53; non-mutated portions of the protein are susceptible to degradation into WT peptide sequences appropriate for immune presentation. A phase I trial (NCT00404339) examining p53 multiple-epitope/dendritic cell vaccine in HNSCC patients was reported in 2009. Following definitive therapy, patients with locally advanced HNSCC were vaccinated with WT p53 sequences pre-loaded onto autologous dendritic cells. At 15-month follow up 11/16 patients were alive without disease. Analysis of immunogenicity indicated p53-specific CTLs in 5 of 16 patients [98]. This trial has since been completed and is soon to be in press.
Conclusion
HNSCC is an immunosuppressive disease. Tumor cells evade recognition and destruction through multiple reinforcing mechanisms, including downregulation of antigen-processing and presentation, imbalanced STAT1/STAT3 signaling, secretion of immunosuppressive cytokines, and expression of PD-L1. Recruitment of TAMs reinforces the immunosuppressive cytokine milieu, inhibiting the maturation of DCs and activation of effector cells while enriching for Tregs. A host of relevant immunotherapies are now in clinical development. In HNSCC, the degree of immunosuppressive cross-talk suggests that single immunotherapies may be inadequate to achieve therapeutic inroads. As synergy can be hypothesized among immunotherapies, multiple coordinate or sequential interventions may be required.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clini Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:3755–62. doi: 10.1158/1078-0432.CCR-04-0054. [DOI] [PubMed] [Google Scholar]
- 7.Whiteside TL. Immunobiology of head and neck cancer. Cancer Metast Rev. 2005;24:95–105. doi: 10.1007/s10555-005-5050-6. [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta S, Bhattacharya-Chatterjee M, O’Malley BW, Jr, Chatterjee SK. Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol. 2005;175:5541–50. doi: 10.4049/jimmunol.175.8.5541. [DOI] [PubMed] [Google Scholar]
- 9.Bauernhofer T, Kuss I, Henderson B, Baum AS, Whiteside TL. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol. 2003;33:119–24. doi: 10.1002/immu.200390014. [DOI] [PubMed] [Google Scholar]
- 10.Ferris R, Whiteside TL, Ferrone S. Clinical significance of downregulated antigen processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–5. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Albaitero A, Nayak JV, Ogino T, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–9. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 12.Whiteside TL, Letessier E, Hirabayashi H, et al. Evidence for local and systemic activation of immune cells by peritumoral injections of interleukin 2 in patients with advanced squamous cell carcinoma of the head and neck. Cancer Res. 1993;53:5654–62. [PubMed] [Google Scholar]
- 13.Hadden J, Verastegui E, Barrera JL, et al. A trial of IRX-2 in patients with squamous cell carcinomas of the head and neck. Int Immunopharmacol. 2003;3:1073–81. doi: 10.1016/S1567-5769(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 14.De Stefani A, Forni G, Ragona R, et al. Improved survival with perilymphatic interleukin 2 in patients with resectable squamous cell carcinoma of the oral cavity and oropharynx. Cancer. 2002;95:90–7. doi: 10.1002/cncr.10654. [DOI] [PubMed] [Google Scholar]
- 15.Urba SG, Forastiere AA, Wolf GT, Amrein PC. Intensive recombinant interleukin-2 and alpha-interferon therapy in patients with advanced head and neck squamous carcinoma. Cancer. 1993;71:2326–31. doi: 10.1002/1097-0142(19930401)71:7<2326::aid-cncr2820710725>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Albers AE, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer Immunol Immunother. 2005;54:1072–81. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuler PJ, Boeckers P, Engers R, et al. EGFR-specific T cell frequencies correlate with EGFR expression in head and neck squamous cell carcinoma. J Transl Med. 2011;9:168. doi: 10.1186/1479-5876-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:11146–55. doi: 10.1158/0008-5472.CAN-05-0772. [DOI] [PubMed] [Google Scholar]
- 19.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res. 2005;33:113–33. doi: 10.1385/IR:33:2:113. [DOI] [PubMed] [Google Scholar]
- 20.Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60:525–35. doi: 10.1007/s00262-010-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 22.Allen C, Duffy S, Teknos T, et al. Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:3182–90. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 23.Leibowitz MS, Srivastava RM, Andrade Filho PA, et al. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res. 2013;19:798–808. doi: 10.1158/1078-0432.CCR-12-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 25.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 26.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 27.Leef G, Thomas SM. Molecular communication between tumor-associated fibroblasts and head and neck squamous cell carcinoma. Oral Oncol. 2013;49:381–6. doi: 10.1016/j.oraloncology.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singhal E, Sen P. Hepatocyte growth factor-induced c-Src-phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway inhibits dendritic cell activation by blocking IkappaB kinase activity. Int J Biochem Cell Biol. 2011;43:1134–46. doi: 10.1016/j.biocel.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Young MR, Wright MA, Lozano Y, Matthews JP, Benefield J, Prechel MM. Mechanisms of immune suppression in patients with head and neck cancer: influence on the immune infiltrate of the cancer. Int J Cancer. 1996;67:333–8. doi: 10.1002/(SICI)1097-0215(19960729)67:3<333::AID-IJC5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Baruah P, Lee M, Odutoye T, et al. Decreased levels of alternative co-stimulatory receptors OX40 and 4-1BB characterise T cells from head and neck cancer patients. Immunobiology. 2012;217:669–75. doi: 10.1016/j.imbio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–62. [PubMed] [Google Scholar]
- 33.Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–47. [PubMed] [Google Scholar]
- 34.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 35.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47:1148–53. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–84. [PubMed] [Google Scholar]
- 39.Spangle JM, Munger K. The HPV16 E6 oncoprotein causes prolonged receptor protein tyrosine kinase signaling and enhances internalization of phosphorylated receptor species. PLoS Pathog. 2013;9:e1003237. doi: 10.1371/journal.ppat.1003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troy JD, Weissfeld JL, Youk AO, Thomas S, Wang L, Grandis JR. Expression of EGFR, VEGF, and NOTCH1 suggest differences in tumor angiogenesis in HPV-positive and HPV-negative head and neck squamous cell carcinoma. Head Neck Pathol. 2013 doi: 10.1007/s12105-013-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pim D, Collins M, Banks L. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992;7:27–32. [PubMed] [Google Scholar]
- 42.Crusius K, Auvinen E, Steuer B, Gaissert H, Alonso A. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp Cell Res. 1998;241:76–83. doi: 10.1006/excr.1998.4024. [DOI] [PubMed] [Google Scholar]
- 43.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 44.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 45.Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–9. doi: 10.1002/cncr.23442. [DOI] [PubMed] [Google Scholar]
- 46.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–9. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50:248–54. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koutcher L, Sherman E, Fury M, et al. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:915–22. doi: 10.1016/j.ijrobp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Riaz N, Sherman EJ, Fury M, Lee N. Should cetuximab replace Cisplatin for definitive chemoradiotherapy in locally advanced head and neck cancer? J Clin Oncol. 2013;31:287–8. doi: 10.1200/JCO.2012.46.9049. [DOI] [PubMed] [Google Scholar]
- 50.Caudell JJ, Sawrie SM, Spencer SA, et al. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys. 2008;71:676–81. doi: 10.1016/j.ijrobp.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 51.Levy AR, Johnston KM, Sambrook J, et al. Indirect comparison of the efficacy of cetuximab and cisplatin in squamous cell carcinoma of the head and neck. Curr Med Res Opin. 2011;27:2253–9. doi: 10.1185/03007995.2011.633989. [DOI] [PubMed] [Google Scholar]
- 52.RTOG. RTOG 1016 Phase III Trial of radiotherapy plus cetuximab versus chemoradiotherapy in HPV-associated oropharynx cancer. 2013 [Google Scholar]
- 53.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–20. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 54.Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 55.Giralt J, Trigo J, Nuyts S, et al. Phase 2, randomized trial (CONCERT-2) of panitumumab plus radiotherapy compared with chemoradiotherapy in patients with unresected, locally advanced squamous cell carcinoma of the head and neck. Ann Oncol. 2012;23(Supplement 9):ix334–47. doi: 10.1016/S1470-2045(14)71200-8. [DOI] [PubMed] [Google Scholar]
- 56.Basavaraj C, Sierra P, Shivu J, Melarkode R, Montero E, Nair P. Nimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFR. Cancer Biol Ther. 2010;10:673–81. doi: 10.4161/cbt.10.7.12793. [DOI] [PubMed] [Google Scholar]
- 57.Rojo F, Gracias E, Villena N, et al. Pharmacodynamic trial of nimotuzumab in unresectable squamous cell carcinoma of the head and neck: a SENDO Foundation study. Clin Cancer Res. 2010;16:2474–82. doi: 10.1158/1078-0432.CCR-09-3042. [DOI] [PubMed] [Google Scholar]
- 58.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–56. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–6. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 60.Glisson BS, Tseng JE, Marur S, et al. Randomized phase II trial of cixutumumab alone or with cetuximab for refractory recurrent/metastatic squamous cancer of head and neck. J Clin Oncol. 2013:29. [Google Scholar]
- 61.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–8. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava RM, Lee SC, Andrade Filho PA, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kondadasula SV, Roda JM, Parihar R, et al. Colocalization of the IL-12 receptor and FcgammaRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-gamma. Blood. 2008;111:4173–83. doi: 10.1182/blood-2007-01-068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bekaii-Saab TS, Roda JM, Guenterberg KD, et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8:2983–91. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Herpen CM, Looman M, Zonneveld M, et al. Intratumoral administration of recombinant human interleukin 12 in head and neck squamous cell carcinoma patients elicits a T-helper 1 profile in the locoregional lymph nodes. Clin Cancer Res. 2004;10:2626–35. doi: 10.1158/1078-0432.ccr-03-0304. [DOI] [PubMed] [Google Scholar]
- 66.Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–68. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 67.Schnurr M, Chen Q, Shin A, et al. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–72. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 68.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–42. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 69.Adams S, O’Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richtsmeier WJ, Koch WM, McGuire WP, Poole ME, Chang EH. Phase I–II study of advanced head and neck squamous cell carcinoma patients treated with recombinant human interferon gamma. Arch Otolaryngol Head Neck Surg. 1990;116:1271–7. doi: 10.1001/archotol.1990.01870110043004. [DOI] [PubMed] [Google Scholar]
- 71.George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacol Ther. 2012;135:44–53. doi: 10.1016/j.pharmthera.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Albesiano E, Davis M, See AP, et al. Immunologic consequences of signal transducers and activators of transcription 3 activation in human squamous cell carcinoma. Cancer Res. 2010;70:6467–76. doi: 10.1158/0008-5472.CAN-09-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sen M, Thomas SM, Kim S, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaddi K, Sarlis NJ, Gupta V. Ruxolitinib, an oral JAK1 and JAK2 inhibitor, in myelofibrosis. Expert Opin Pharmacother. 2012;13:2397–407. doi: 10.1517/14656566.2012.732998. [DOI] [PubMed] [Google Scholar]
- 76.Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–97. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Purandare AV, McDevitt TM, Wan H, et al. Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia. 2012;26:280–8. doi: 10.1038/leu.2011.292. [DOI] [PubMed] [Google Scholar]
- 78.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:624–30. doi: 10.1007/s00432-005-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lionello M, Staffieri A, Marioni G. Potential prognostic and therapeutic role for angiogenesis markers in laryngeal carcinoma. Acta Otolaryngol. 2012;132:574–82. doi: 10.3109/00016489.2011.652308. [DOI] [PubMed] [Google Scholar]
- 80.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 81.Argiris A, Kotsakis AP, Hoang T, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2012 doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun S, Wang Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129:2337–48. doi: 10.1002/ijc.25927. [DOI] [PubMed] [Google Scholar]
- 83.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–50. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103:645–61. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 85.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dillman RO. Cancer immunotherapy. Cancer Biother Radiopharm. 2011;26:1–64. doi: 10.1089/cbr.2010.0902. [DOI] [PubMed] [Google Scholar]
- 88.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. J Biomed Biotechnol 2010. 2010 doi: 10.1155/2010/596432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Best SR, Peng S, Juang CM, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–9. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 93.Voskens CJ, Sewell D, Hertzano R, et al. Induction of mage-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck. 2012 doi: 10.1002/hed.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng S, Lyford-Pike S, Akpeng B, et al. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer Immunol Immunother. 2013;62:171–82. doi: 10.1007/s00262-012-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brun JL, Dalstein V, Leveque J, et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol. 2011;204(169):e161–168. doi: 10.1016/j.ajog.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 96.Wallecha A, French C, Petit R, Singh R, Amin A, Rothman J. Lm-LLO-based immunotherapies and HPV-associated disease. J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–83. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 98.Andrade P, Deleo A, Visus C, Butterfield L, Argiris A, Ferris RL, et al. Phase I adjuvant trial of multi-epitope p53 vaccine for patients with squamous cell carcinoma of the head and neck (SCCHN): a preliminary report. J Clin Oncol. 2009;27(15s):1–2. suppl; abstr 3012. [Google Scholar]