Abstract
Associative learning and timing are clearly inter-related, but are they separate processes or is timing a core part of the associative structure? Emerging research suggests that temporal information is acquired rapidly and that CR's are timed correctly from the start of associative learning. Moreover, specific temporal knowledge can be disclosed even in cases where CR's were not emitted. Timing is not only critical for CR timing, but also contributes to CR expression through the comparison of reinforcer rates, and through the formation of temporal maps. A conceptual framework is proposed in which timing is a core part of the content of associative learning.
Introduction
The idea that time is involved in associative learning has been an intrinsic part of thinking since temporal contiguity was suggested as essential for association formation by Aristotle. This idea was developed by the British associationist philosophers of the 19th century and became foundational for modern experimental psychology. The idea that learning depends on temporal contiguity is often accepted as axiomatic [1-3]. In this view, time is important for the establishment of associations but is not part of what is encoded in the association. More recently, there has been considerable work showing that times are encoded in associative learning paradigms and determine the type, vigor and temporal patterns of behavior. We review this work and discuss how these findings open new theoretical possibilities for understanding the nature of learning.
Time affects acquisition speed and asymptote
There has been considerable research showing that temporal factors alter the speed of conditioned response (CR) emergence as well as the asymptotic level of responding. In general, the greater the temporal contiguity between the conditioned stimulus (CS) and unconditioned stimulus (US) the greater the conditioning, provided that CS onset occurs prior to US occurrence. This pattern is observed in autoshaping [4,5], goal tracking [6-9], eyeblink conditioning [10-12], conditioned suppression [13], salivary conditioning [14], paw flexion [15], and heart rate conditioning [16] paradigms. However, if CSs are too brief, conditioning is diminished, perhaps because the CS is not attended to on some trials, or that a very brief cue is not sufficient to recruit the specific CR [17].
In addition to affecting the likelihood or strength of CR's, the CS-US interval also affects the form of CR's. Holland [18] found that when a short duration auditory CS was paired with food, rats startled and jerked their heads to the CS. However, when the CSs were longer the dominant response was approach to the magazine. Thus, the probability of a specific response cannot be unambiguously taken as an index of the strength of learning, as different responses may be predominantly expressed to different duration CSs. Similar observations have been made about the impact of temporal variables on the form of CR's in fear conditioning [19], eyeblink conditioning [20], sexual conditioning [21], and other appetitive conditioning paradigms [22,23]. Thus, one should not conclude that changing contiguity of the CS and US necessarily changes the underlying learning.
Another temporal interval that affects conditioning is the duration of the intertrial interval (ITI). In appetitive conditioning in pigeons [4,5] or rodents [6-9] and in fear conditioning [13,24], longer ITIs result in stronger CR's than shorter ones. The ITI, though, does not seem to be the determining factor. When the time between trials (CS-US pairings) is held constant but additional USs are presented during the intertrial interval, conditioning is weakened [25] suggesting that the key variable is the US-US interval rather than the ITI. In standard conditioning protocols longer ITIs are associated with longer US-US intervals, and the latter interval seems to be the key feature determining the effect of trial spacing [26,27]. It is worth noting, however, that even in cases where the average rate of reinforcement is the same, or even is lower during the CS than during the ITI, anticipatory timing is still observed [28,29]. Thus, timing is apparent even in situations that are not advantageous for CR expression.
That the CS-US and US-US interval durations affect conditioning seems indisputable. It has been further claimed that the processes that underlie the learning and expression of CR's are determined by the ratio of these two intervals [27,30,31]. In other words, conditioning is determined by the degree to which the CS signals a reduction in the wait between USs. Such a view implies that these intervals are encoded and serve as the basis for the emergence of anticipatory CR's. The absolute duration of these intervals may also contribute to conditioning [6-8], providing yet another route for the CS-US and US-US intervals to impact on anticipatory CR's.
Learning time versus temporal modulation of conditioning
As described above there is no controversy over whether time affects conditioning. The controversy arises over how to interpret these effects. Does time modulate conditioning processes because contiguity affects the formation of associative bonds (that include temporal information) or is time the foundational content of the learning that determines the behavioral output [30-33]?
From the time of Pavlov it was known that CR's often coincide with the expected time of US presentation when a predictable CS-US interval is delivered. In eyeblink conditioning, conditioned lid closure slightly precedes the expected time of a shock or airpuff [e.g., 10,20]. In appetitive conditioning, mice and rats become more and more likely to put their head into a feeder as the expected time of reward approaches [6-9,23,34]. Likewise, when animals are trained to fear cues, behavioral (e.g., freezing, startle potentiation) and physiological (e.g., respiration) responses peak at the time that a particular cue signals the arrival of shock [35,36]. Further evidence that time is encoded during the initial learning comes from studies that look at CR timing when CR's first emerge. While good timing is not universally observed at the start of conditioning [34] it is often the case [7,37,38] that when CR's first emerge they are timed to anticipate the US. It has even been shown that times are encoded accurately before the first CR's emerge [39]. In addition, the fact that CR's are not well timed does not mean that times have not been encoded. Diaz-Mataix et al. [40] found that after fear conditioning with a CS-US interval freezing responses were poorly timed. However, a single reminder trial with a different CS-US interval triggered a reconsolidation process. If the CS-US interval remained the same on the reminder trial, reconsolidation was not triggered. This indicates that the reconsolidation was triggered by the mismatch between the remembered CS-US interval and the new one, indicating that the CS-US interval was encoded.
Another way to examine temporal expectations when CR's are not temporally graded is to use a transfer test and see whether behavioral effects in the transfer test are maximal at the previously trained times of US presentation. In a blocking procedure, for example, a CS1 is paired with a US during the first phase of training and then a novel CS2 is compounded with CS1 and pairings continue. Typically, the learned expectancy of the US during the first phase interferes with the CS2's capacity to evoke CR's. It has further been found in blocking procedures that changing the time at which the US is presented during the second phase attenuates the blocking effect [41,42], although this effect is not always obtained [43-45].
Similar results have been reported for transfer tests of overshadowing [two cues are presented simultaneously from the start of training; 46,47] and conditioned inhibition [conditioned inhibition is maximal at the time at which the US had been expected but was omitted during inhibitory training; 48-51]. Thus, it appears the CS-US temporal relationship is an important element of the learning that occurs in cue competition paradigms.
A related piece of evidence that times are encoded comes from demonstrations that animals can integrate information about time across separate experiences [52-54]. In second-order conditioning, a CS1 is followed by the US, and then CS1 is later paired with a novel CS2 in the absence of the US (Figure 1A). CS2 is now able to evoke CR's, even though it was never directly paired with the US. Associative theory proposes that CS2 inherits associative strength through mediated associations. Miller and colleagues provided an alternative account, the temporal coding hypothesis [55,56], which proposes that a temporal map is formed and connected together across the two learning episodes, similar to processes that occur with spatial maps. The connector is CS1, which is common between the two stages of training. The temporal map would encode the temporal relationships between the CS1, CS2, and US, as shown in Map A, in which the expected time of the US is simultaneous with CS2 onset. This leads to the prediction that second-order conditioning should be comparatively weak given that simultaneous conditioning generally results in weak anticipatory CR's (see above). Indeed, Cole et al. [57] confirmed that this is the case [see also 32,54 for related evidence in appetitive conditioning] by comparing standard second-order conditioning with trace second-order conditioning (Figure 1B). Trace conditioning resulted in weaker initial conditioning, and thus should support weaker mediated association transfer to CS2. Instead, CS2 resulted in more robust CR's in the trace group, consistent with temporal map formation. In Map B, CS2 occurs in a stronger predictive arrangement with the US in comparison to Map A. Evidence for temporal map formation has been found in a range of conditioning paradigms including overshadowing, blocking, and conditioned inhibition paradigms [e.g., 41,46,48,57,58,59]. In addition, it appears that temporal maps may be flexible in their scaling similar to spatial maps [60] and contain bidirectional representations [55].
Figure 1.
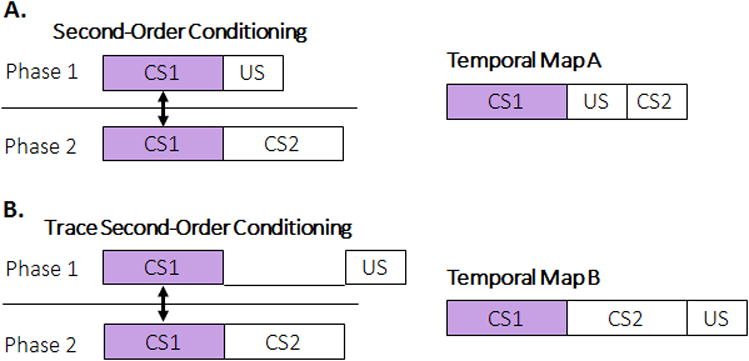
The formation of temporal maps in second-order conditioning. A. A standard second-order conditioning task in which first-order conditioning is delivered in a delay conditioning arrangement with CS1 followed directly by the US. In phase 2, the original CS1 is now followed by CS2, in the absence of any US presentations. The resulting Temporal Map A contains a layout of the CSs and the US in terms of their timing and order of occurrence. B. A trace-second-order conditioning task and the resulting temporal map. In trace conditioning, the CS1 and US in Phase 1 are separated by a gap.
Conclusions
All associative learning procedures involve CS and US events that unfold in time, and thus can be specified by the events (CSs and USs), their coincidence (pairing) and order of occurrence, the timing of events, and the probability of occurrence of each event. The key question raised by the evidence reviewed above is how to understand the relationship between timing and associative learning. Timing models such as scalar timing theory [61,62] account for specific interval learning, whereas rate expectancy theory [27,30] and information theoretic models [31,32,63-65] account for interval comparisons, and the temporal encoding hypothesis [55,56] for temporal maps. Each of the theories does a reasonable job of accounting for effects within its domain, albeit with some weaknesses. However, there are many gaps in the current state of the knowledge in the field, and many of the gaps occur at the interface of the different processes [66]. For example, are temporal maps formed through a separate process from associative learning? And, if so, are they formed in parallel or in series with associative processing? We describe two very different conceptualizations of these questions, shown in Figure 2.
Figure 2.
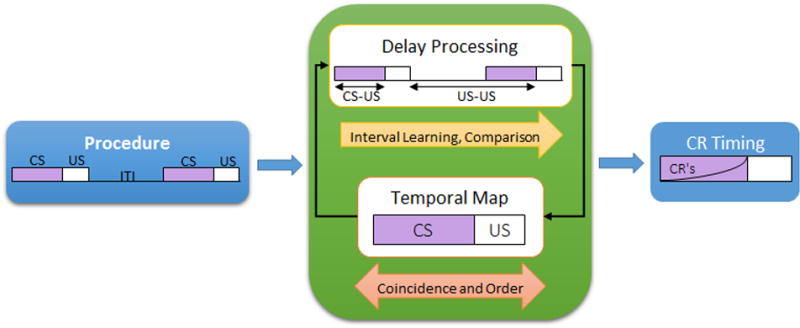
A conceptualization of the timing processes in associative learning. The pairing of CS and US in a contiguous forward relationship results in two potential processes. An associative learning process involves the learning of specific associations and temporal intervals that make up the conditioning paradigm. The combination of information informs CR expression and timing, providing the appearance of a temporal map. The second potential process involves the rapid formation of a rich temporal map that encodes the nature (location, modality, and intensity) of events, their durations, and their timing of occurrence. CR's are expressed when sufficient evidence suggests that effort should be allocated to responding.
One possibility is that information about time (coincidence, order, and timing of events) is an integral part of content of associative learning [e.g., 56]. In the associative view, very early in training (perhaps even during/after the first trial), CS-US presentations result in coincidence detection and determination of the order of events. This process is an essential determinant for conditioning as the contiguity and order of events has a major impact on learning. A second critical process is temporal processing, wherein the specific intervals between events is learned. This involves learning the CS-US and US-US intervals, both individually for guiding CR timing, and also their comparison for guiding CR expression. These specific associations contain the knowledge that makes it appear as though there is a temporal map. Future research to assess these processes should target these systems to determine their inter-relationship. And, future theories need to deal more explicitly with the interface of these processes.
Alternatively, temporal maps may be rapidly apprehended in the same way that spatial, auditory and visual properties of objects are apprehended and encoded as they are dynamically encountered. Specific information about events (location, modality, intensity, duration, and timing of events) can be extracted from those integrated map-like representations of the world. In this view, representations are not built up from the association of elements, rather information about elements is extracted from holistic representations of experiences that are anchored in space and time. Thus, the slow emergence of CR's is not because of a slow buildup of underlying learning, but because the reliability of the information needs to be assessed before action is guided by that information. Future research guided by this view might well focus on how representations of the stream of events changes over the course of exposure to conditioning protocols and how these changing representations translate into specific responses.
The study of timing and associative learning deals with fundamental principles of learning which broadly impact on human and animal behavior. We have learned much about these principles, but it is also clear that we have much yet to learn. Further examinations of the different timing processes in conditioning, both individually and in concert is critical for future research in the field. The evidence reviewed here suggests that rich temporal maps may be formed well before CR's are expressed, indicating the need for more sensitive measurements to disclose the nature of information encoding within associative learning paradigms.
Highlights.
Temporal information is acquired rapidly in associative learning
CR's are timed correctly from the start of associative learning
Specific temporal knowledge can be disclosed even in when CR's are not emitted
Rich temporal maps are formed during associative learning
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) Grant 5R01MH068073 (P.D.B) and by NIMH Grant 5R01MH085739 (K.K).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kimberly Kirkpatrick, Kansas State University.
Peter D Balsam, Barnard College, Columbia University.
References
- 1.Delamater AR, Lattal KM. The study of associative learning: mapping from psychological to neural levels of analysis. Neurobiol Learn Mem. 2014;108:1–4. doi: 10.1016/j.nlm.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boakes RA. Temporal contiguity in associative learning: Interference and decay from an historical perspective. J Exp Psychol Anim Learn Cog. 2014;40:381–400. doi: 10.1037/xan0000040. [DOI] [PubMed] [Google Scholar]
- 3•.Miller RR, Witnauer JE. Retrospective revaluation: The phenomenon and its theoretical implications. Behav Process. 2016 doi: 10.1016/j.beproc.2015.09.001. Demonstrates that retrospective revaluation is a critical benchmark for evaluating traditional and newer models of conditioning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbon J, Baldock C, Locurto CM, Gold L, Terrace HS. Trial and intertrial durations in autoshaping. J Exp Psychol Anim Behav Process. 1977;3:264–284. [Google Scholar]
- 5.Terrace HS, Gibbon J, Farrell L, Baldock MD. Temporal factors influencing the acquisition and maintenance of an autoshaped keypeck. Anim Learn Behav. 1975;3:53–62. [Google Scholar]
- 6.Holland PC. Trial and intertrial durations in appetitive conditioning in rats. Anim Learn Behav. 2000;28:121–135. [Google Scholar]
- 7.Kirkpatrick K, Church RM. Independent effects of stimulus and cycle duration in conditioning: the role of timing processes. Anim Learn Behav. 2000;28:373–388. [Google Scholar]
- 8.Lattal KM. Trial and intertrial durations in Pavlovian conditioning: issues of learning and performance. J Exp Psychol Anim Behav Process. 1999;25:433–450. doi: 10.1037/0097-7403.25.4.433. [DOI] [PubMed] [Google Scholar]
- 9.Ward RD, Gallistel CR, Jensen G, Richards VL, Fairhurst S, Balsam PD. CS Informativeness Governs CS-US Associability. J Exp Psychol Anim Behav Process. 2012;38:217–232. doi: 10.1037/a0027621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MC. CS-US interval and US intensity in classical conditioning of the rabbit's nictitating membrane response. J Comp Physiol Psychol. 1968;66:679–687. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds B. The acquisition of a trace conditioned response as a function of the magnitude of the stimulus trace. J Exp Psychol Anim Behav Process. 1945;35:15–30. [Google Scholar]
- 12.Gormezano I, Kehoe EJ. Classical conditioning and the law of contiguity. Adv Anal Behav. 1981;2:1–45. [Google Scholar]
- 13.Stein L, Sidman M, Brady JV. Some effects of Two Temporal Variables on Conditioned Suppression. J Exp Anal Behav. 1958;1:153–162. doi: 10.1901/jeab.1958.1-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ost J, Lauer D. Classical Conditioning: A Symposium. New York: Appleton-Century-Crofts; 1965. Some investigations of classical salivary conditioning in the dog; pp. 192–207. [Google Scholar]
- 15.Wickens DDM, Patricia M, Sullivan Shirley N. Classical GSR conditioning, conditioned discrimination, and interstimulus intervals in cats. J Comp Physiol Psychol. 1961;54:572–576. doi: 10.1037/h0042131. [DOI] [PubMed] [Google Scholar]
- 16.Vandercar DH, Schneiderman N. Interstimulus interval functions in different response systems during classical discrimination conditioning of rabbits. Psych Sci. 1967;9:9–10. [Google Scholar]
- 17.Timberlake W. Motivational modes in behavior systems. In: Mowren RR, Klein SB, editors. Handbook of contemporary learning theories. Lawrence Erlbaum Associates Publishers; 2001. pp. 155–209. [Google Scholar]
- 18•.Holland PC. CS-US interval as a determinant of the form of Pavlovian appetitive conditioned responses. J Exp Psychol Anim Behav Process. 1980;6:155–174. Examining multiple behaviors as potential CR's is critical as shorter CSs were shown to engender different responses than longer CSs. [PubMed] [Google Scholar]
- 19.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and learning. Lawrence Erlbaum Associates; 1988. pp. 185–212. [Google Scholar]
- 20.Johansson FF. Activation of a Temporal Memory in Purkinje Cells by the mGluR7 Receptor. Cell Reports. 2015;13:1741–1746. doi: 10.1016/j.celrep.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 21.Akins CK, Domjan M, Gutiérrez G. Topography of sexually conditioned behavior in male Japanese quail (Coturnix japonica) depends on the CS-US interval. J Exp Psychol Anim Behav Process. 1994;20:199–209. [PubMed] [Google Scholar]
- 22.Silva KM, Timberlake W. The organization and temporal properties of appetitive behavior in rats. Anim Learn Behav. 1998;26:182–195. [Google Scholar]
- 23•.Escobar MM. Do long delay conditioned stimuli develop inhibitory properties? Front Psychol. 2015;6:1606. doi: 10.3389/fpsyg.2015.01606. Demonstrates that the initial segment of a long delay CS produces latent inhibition rather than conditioned inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman DA, Hemmes NS, Brown BL. Relative Durations of Conditioned-Stimulus and Intertrial Interval in Conditioned Suppression. J Exp Anal Behav. 1986;46:51–66. doi: 10.1901/jeab.1986.46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins HM, Barnes RA, Barrera FJ. Why autoshaping depends on trial spacing. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and conditioning theory. Academic Press; 1981. pp. 255–284. [Google Scholar]
- 27.Gibbon J, Balsam PD. Spreading association in time. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and conditioning theory. Academic Press; 1981. pp. 219–253. [Google Scholar]
- 28.Kirkpatrick K, Church RM. Temporal learning in random control procedures. J Exp Psychol Anim Behav Process. 2004;30:213–228. doi: 10.1037/0097-7403.30.3.213. [DOI] [PubMed] [Google Scholar]
- 29•.Williams DA, Lawson C, Cook R, Mather AA, Johns KW. Timed excitatory conditioning under zero and negative contingencies. J Exp Psychol Anim Behav Process. 2008;34:94–105. doi: 10.1037/0097-7403.34.1.94. Timing of conditioned responding occurs even when conditions do not promote excitatory learning. [DOI] [PubMed] [Google Scholar]
- 30.Gallistel CR, Gibbon J. Time, rate, and conditioning. Psych Rev. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- 31.Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends Neurosci. 2009;32:73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Balsam PD, Drew MR, Gallistel CR. Time and Associative Learning. Comp Cogn Behav Rev. 2010;5:1–22. doi: 10.3819/ccbr.2010.50001. This article provides evidence for the idea that temporal information is the foundation of associative learning in detail. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallistel CR, Balsam PD. Time to rethink the neural mechanisms of learning and memory. Neurobiol Learn Mem. 2014;108:136–144. doi: 10.1016/j.nlm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delamater AR, Holland PC. The influence of CS-US interval on several different indices of learning in appetitive conditioning. J Exp Psychol Anim Behav Process. 2008;34:202–222. doi: 10.1037/0097-7403.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis M, Schlesinger LS, Sorenson CA. Temporal specificity of fear conditioning: effects of different conditioned stimulus-unconditioned stimulus intervals on the fear-potentiated startle effect. J Exp Psychol Anim Behav Process. 1989;15:295–310. [PubMed] [Google Scholar]
- 36.Shionoya K, Hegoburu C, Brown BL, Sullivan RM, Doyère V, Mouly AM. It's time to fear! Interval timing in odor fear conditioning in rats. Front Behav Neuro. 2013;7:128. doi: 10.3389/fnbeh.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Balsam PD, Drew MR, Yang C. Timing at the start of associative learning. Learn Motiv. 2002;33:141–155. CR's are timed accurately from the onset of their expression, lending evidence to the critical importance of timing processes early in associative learning. [Google Scholar]
- 38.Mauk MD, Ruiz BP. Learning-dependent timing of Pavlovian eyelid responses: Differential conditioning using multiple interstimulus intervals. Behav Neurosci. 1992;106:666–681. doi: 10.1037//0735-7044.106.4.666. [DOI] [PubMed] [Google Scholar]
- 39••.Ohyama T, Mauk MD. Latent acquisition of timed responses in cerebellar cortex. J Neurosci. 2001;21:682–690. doi: 10.1523/JNEUROSCI.21-02-00682.2001. CR timing is acquired even though CR's may not be expressed, indicating that timing is acquired very early in conditioning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Díaz-Mataix L, Tallot L, Doyère V. The amygdala: A potential player in timing CS–US intervals. Behav Process. 2014;101:112–122. doi: 10.1016/j.beproc.2013.08.007. CS-US intervals are encoded accurately even in situations where CR timing may be poor. [DOI] [PubMed] [Google Scholar]
- 41.Barnet RC, Grahame NJ, Miller RR. Temporal encoding as a determinant of blocking. J Exp Psychol Anim Behav Process. 1993;19:327–341. doi: 10.1037//0097-7403.19.4.327. [DOI] [PubMed] [Google Scholar]
- 42.Schreurs BG, Westbrook RF. The effects of changes in the CS-US interval during compound conditioning upon an otherwise blocked element. Q J Exp Psychol. 1982;34:19–30. doi: 10.1080/14640748208400887. [DOI] [PubMed] [Google Scholar]
- 43.Kohler EA, Ayres JJB. The Kamin blocking effect with variable-duration CSs. Anim Learn Behav. 1979;7:347–350. [Google Scholar]
- 44.Kohler EA, Ayres JJB. Blocking with serial and simultaneous compounds in a trace conditioning procedure. Anim Learn Behav. 1982;10:277–287. [Google Scholar]
- 45.Maleske RT, Frey PW. Blocking in eyelid conditioning: effect of changing the CS-US interval and introducing an intertrial stimulus. Anim Learn Behav. 1979;7:452–456. [Google Scholar]
- 46.Blaisdell AP, Denniston JC, Miller RR. Temporal encoding as a determinant of overshadowing. J Exp Psychol Anim Behav Process. 1998;24:72–83. doi: 10.1037//0097-7403.24.1.72. [DOI] [PubMed] [Google Scholar]
- 47.Blaisdell AP, Savastano HI, Miller RR. Overshadowing of explicitly unpaired conditioned inhibition is disrupted by preexposure to the overshadowed inhibitor. Anim Learn Behav. 1999;27:346–357. [Google Scholar]
- 48.Barnet RC, Miller RR. Temporal encoding as a determinant of inhibitory control. Learn Motiv. 1996;27:73–91. [Google Scholar]
- 49.Burger DC, Denniston JC, Miller RR. Temporal coding in conditioned inhibition: Retardation tests. Anim Learn Behav. 2001;29:281–290. [Google Scholar]
- 50.Denniston JC, Blaisdell AP, Miller RR. Temporal coding in conditioned inhibition: Analysis of associative structure of inhibition. J Exp Psychol Anim Behav Process. 2004;30:190–202. doi: 10.1037/0097-7403.30.3.190. [DOI] [PubMed] [Google Scholar]
- 51.Denniston JC, Cole RP, Miller RR. The role of temporal relationships in the transfer of conditioned inhibition. J Exp Psychol Anim Behav Process. 1998;24:200–214. doi: 10.1037//0097-7403.24.2.200. [DOI] [PubMed] [Google Scholar]
- 52•.Polack CW. Associative structure of integrated temporal relationships. Learn Behav. 2013;41:443–454. doi: 10.3758/s13420-013-0119-5. Temporal integration occurs at the moment of testing and results in the establishment of a new direct association with the US. This paper provides new insights into the mechanisms of temporal map formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thrailkill EA. Temporal integration and instrumental conditioned reinforcement. Learn Behav. 2014;42:201–208. doi: 10.3758/s13420-014-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Taylor KM, Joseph V, Zhao AS, Balsam PD. Temporal maps in appetitive Pavlovian conditioning. Behav Process. 2014;101:15–22. doi: 10.1016/j.beproc.2013.08.015. Provides evidence in favor of the operation of temporal maps in coding of event order in appetitive conditioning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Arcediano F, Miller RR. Some constraints for models of timing: a temporal coding hypothesis perspective. Learn Motiv. 2002;33:105–123. This article reviews evidence in favor of temporal map formation, and shows that temporal maps contain bi-directional encoding of information, suggesting that the maps contain rich temporal information. [Google Scholar]
- 56.Savastano HI, Miller RR. Time as content in Pavlovian conditioning. Behav Process. 1998;44:147–162. doi: 10.1016/s0376-6357(98)00046-1. [DOI] [PubMed] [Google Scholar]
- 57•.Cole RP, Barnet RC, Miller RR. Temporal encoding in trace conditioning. Anim Learn Behav. 1995;23:144–153. Predictions of associative learning theory are directly contrasted against the temporal encoding hypothesis, reporting evidence in favor of temporal map formation in second-order conditioning paradigms. Information is integrated across learning experiences to induce a temporal map that encodes the order and duration of events. [Google Scholar]
- 58.Barnet RC, Arnold HM, Miller RR. Simultaneous conditioning demonstrated in second-order conditioning: evidence for similar associative structure in forward and simultaneous conditioning. Learn Motiv. 1991;22 [Google Scholar]
- 59.Barnet RC, Cole RP, Miller RR. Temporal integration in second-order conditioning and sensory preconditioning. Anim Learn Behav. 1997;25:221–233. [Google Scholar]
- 60•.Wan M, Djourthe M, Taylor KM, Balsam PD. Relative temporal representations in Pavlovian conditioning. Behav Process. 2010;83:154–161. doi: 10.1016/j.beproc.2009.11.012. Demonstrates that temporal maps display relative scalability similar to spatial maps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gibbon J, Church RM. Sources of variance in an information processing theory of timing. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal cognition. Elrbaum: 1984. pp. 465–488. [Google Scholar]
- 62.Gibbon J, Church RM, Meck WH. Scalar timing in memory. In: Gibbon J, Allan L, editors. Timing and time perception (Annals of the New York Academy of Sciences) Vol. 423. New York Academy of Sciences; 1984. pp. 52–77. [DOI] [PubMed] [Google Scholar]
- 63.Gallistel CR. Conditioning from an information processing perspective. Behav Process. 2003;62:89–101. doi: 10.1016/s0376-6357(03)00019-6. [DOI] [PubMed] [Google Scholar]
- 64•.Gallistel CR. Temporal contingency. Behav Process. 2014;101:89–96. doi: 10.1016/j.beproc.2013.08.012. This article demonstrates the application of information theory to explain temporal contingency effects through time-based processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Ward RD, Gallistel CR, Balsam PD. It's the information! Behav Process. 2013;95:3–7. doi: 10.1016/j.beproc.2013.01.005. Describes evidence in favor of the view that CSs will support conditioned responding if they reduce uncertainty about the timing of the next US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Marshall AT, Kirkpatrick K. Everywhere and everything: The power and ubiquity of time. Int J Comp Psychol. 2015;28 A review of the relationship between timing and other cognitive processes, including discussion of behavioral, cognitive, and neurobiological evidence. [PMC free article] [PubMed] [Google Scholar]


