Abstract
Objective
The effect of filler content in dental restorative composites on the polymerization shrinkage stress (PS) is not straightforward and has caused much debate in the literature. Our objective in this study was to clarify the PS/filler content relationship based on analytical and experimental approaches, so that guidelines for materials comparison in terms of PS and clinical selection of dental composites with various filler content can be provided.
Methods
Analytically, a simplified model based on linear elasticity was utilized to predict PS as a function of filler content under various compliances of the testing system, a cantilever beam-based instrument used in this study. The predictions were validated by measuring PS of composites synthesized using 50/50 mixtures of two common dimethacrylate resins with a variety of filler contents.
Results
Both experiments and predictions indicated that the influence of filler content on the PS highly depended on the compliance of the testing system. Within the clinic-relevant range of compliances and for the specific sample configuration tested, the PS increased with increasing filler content at low compliance of instrument, while increasing the compliance caused the effect of filler content on the PS to gradually diminish. Eventually, at high compliance, the PS inverted and decreased with increasing filler content.
Significance
This compliance-dependent effect of filler content on PS suggests: (1) for materials comparison in terms of PS, the specific compliance at which the comparison being done should always be reported and (2) clinically, composites with relatively lower filler content could be selected for such cavities with relatively lower compliance (e.g. a Class-I cavity with thick tooth walls or the basal part in a cavity) and vice versa in order to reduce the final PS.
Keywords: Dental composites, Polymerization shrinkage stress, Filler content, Curing kinetics, Compliance of constraint
1. Introduction
In recent years, photo-polymerized composites have been widely used in restorative dentistry mainly due to their esthetical appearance and environment-friendly nature [1]. During the clinical placement of the composite restorations, volumetric shrinkage of the material induced by polymerization densification of the resin matrix is constrained by the cavity walls, resulting in the generation of undesirable polymerization shrinkage stress (PS). The presence of this PS is considered to be one of the most important causes of tooth-restorative interfacial failure and subsequent detrimental consequences [2–4]. To mitigate the effect of polymerization shrinkage and also to accommodate the mechanical/physical performances of the restorative, various amount of inorganic fillers (from ca. 20 to 70 vol.%) has been utilized in modern dental composite formulations [5]. An increase in the filler content results in a decrease in the shrinkage and a simultaneous increase in the elastic modulus of the composite; therefore, the effect of filler content on PS (typically a result of the product of shrinkage and modulus) is not apparent and has induced much debate in the literature [6–13]. Also, the PS, as an engineering property, is dictated not only by the development of material shrinkage and modulus, but also the compliance of external constraint [14–18]. The objective of this study is to unravel the dependence of PS on filler content under various instrumental compliances using analytical and experimental methods, such that guidelines for future experimental comparison of PS and clinical selection of dental composites with various filler content can be provided.
Several instruments for in vitro PS measurement have been introduced in the literature and sold commercially [19–22]. These instruments typically have utilized a force transducer to record the uniaxial force induced from a disk/cylinder-shape specimen under axial constraint. Although each instrument works largely well for intra-laboratory research purpose, inter-laboratory comparison (both quantitative and qualitative) of the PS results obtained by different instruments is not achievable mainly due to the different instrumental compliances included [3,4]. For example, Musanje et al. and Gonçalves et al. compared PS magnitude of several commercial composites (with different filler content) using different instruments while keeping other testing conditions (i.e. irradiation and specimen dimensions) similar. They found that the ranking of the tested composites based on the PS was not consistent for the different instruments used [3,12,23]. Similar findings were also reported by Braga et al. using same instrument but with different compliances [24]. Moreover, the effect of composite filler content on PS was also found to be instrument-dependent. By using such instruments with very low compliance, the PS was reported to increase with increasing filler content, indicating that the increase in modulus played a more significant role than the decrease in shrinkage in determining the PS development [6–8]. In contrast, the opposite trend of PS vs. filler content (i.e. PS decreased with increasing filler content) was reported when the PS was measured using relatively compliant instruments [9–11].
While it would be beneficial if the in vitro PS tests could provide at least qualitative guidelines for the clinical use of dental composites, the inconsistent results appearing in the literature indicate limited and inexplicit information in this regard. To this end, combined analytical and experimental approaches were adopted in this study to systematically investigate the effects of composite filler content on PS under various clinically relevant instrumental compliances. Analytically, a simplified model based on linear elasticity and a uniaxial constraint was used to approximate the induced PS of composite, and effects of filler content on PS under various compliances were predicted. The analytical results were validated by experiments using a NIST-developed cantilever-beam based instrument [22,25]. The desired compliance of the testing instrument can be easily achieved by varying the sample position along the cantilever beam. Within the clinic-relevant range of compliances and for the specific sample configuration tested, the PS increased with increasing filler content when measured in low compliance region of the instrument. In an intermediate instrumental compliance range, the measured PS was essentially independent of the filler content, while at higher compliance, a reverse trend was observed where the PS decreased with increasing filler content. The presented results not only show that the effect of filler content on PS highly depends upon the instrumental compliance, but also provide a full picture of the PS/filler content correlation and explain the inconsistent trends reported in the literature. Since PS development is associated with the kinetics of curing and degree of monomer conversion (an important index for dental composites), the effect of filler content on the evolution of conversion was also simultaneously measured in this study. For the composites with micro-scale fillers tested in this study, the results indicate that the final conversion is not affected significantly as either the filler content or the instrumental compliance changed.
2. Materials and methods2
In this study, experimental composites comprised of typical dental resins filled with inorganic fillers were synthesized and tested. Bisphenol A-glycidyl methacrylate (Bis-GMA) and triethylene glycol dimethacrylate (TEGDMA) (both from Esstech Inc., Essington, PA) were mixed in a 50:50 mass ratio to act as the resin matrix for the composites. A visible light initiator system consisting of 0.2 wt.% (relative to the resins) camphorquinone (CQ) and 0.8 wt.% ethyl 4-dimethylaminobenzoate (EDAB) (both from Sigma–Aldrich, Milwaukee, WI) were incorporated. A commercial dental filler (TPH silanized MLD glass, supplied by Dentsply-Caulk, Milford, DE) with the mean particle size of 1 μm was used as the inorganic part of the composites. The filler was mixed with the resins in 15 wt.% increments to obtain filler contents of 0–75 wt.% (0–52.87 vol.%). Fillers and resins were blended in a centrifugal mixer (DAC 150FVZ, FlackTek Inc., Landrum, SC) to ensure sufficient mixing.
The polymerization stress (PS) of the prepared composites was measured using the NIST-developed cantilever-beam based instrument (tensometer) [25]. The tensometer setup is coupled with an in situ near-infrared (NIR) spectrometer, which allows simultaneous monitoring of the curing kinetics (real-time double-bond conversion) in transmission. A more detailed description about the testing mechanics and instrument setup has been reported in our previous studies [22,25]. Briefly, an uncured specimen in disk shape (2.5 mm diameter, 2 mm height) was placed between two flat quartz rods that had been treated with a methacrylate silane to promote adhesion between the specimen and rods. The upper rod was clamped to the cantilever beam and the lower one was fixed to the base. A non-tacky Teflon sleeve with an injection hole and a smaller air-venting hole was used to encase the rods and the specimen (i.e. no constraint on the specimen in the radial direction thus no stress developed in that direction). As the curing light (QHL75™ curing lamp, Dentsply-Caulk with 500 ± 10 mW/cm2 intensity at the top end of the lower rod where the specimen is attached and a 40 s irradiation duration for all the tests) was transmitted through the lower rod onto the specimen, polymerization shrinkage occurred and the resulting axial shrinkage stress caused a deflection in the beam. This deflection was recorded by a displacement sensor at the free end of the beam and used to deduce the axial PS based on a beam formula. The simultaneous measurement of the double-bond (6165 cm−1) conversion was realized by guiding the NIR signal through the sides of the specimen using optical fiber cables (1 mm diameter) [25,26]. The dynamic fractional conversion was calculated by taking the peak area of the sample prior to the start of irradiation (Areamonomer) and at each time point during the polymerization process (Areapolymer) based on the following formula: conversion = 1 – Areapolymer/Areamonomer [27]. The temperature rise during the exothermic polymerization, which correlates with the curing kinetics, was also measured in parallel using a T-type microprobe thermocouple (0.1 mm diameter, Physitemp Instruments, Clifton, NJ) inserted into the center of the specimen. The synchronized PS/conversion/temperature data were collected continuously for 30 min with a 10 Hz sampling temporal resolution.
To test the influence of instrumental compliance on the PS/filler content dependency, the PS measurement for each sample composition was performed under three different compliances (0.33, 2.65, and 12.10 μm/N) that were chosen comparable to the compliance of tooth cavities reported in the literature [28,29]. The various instrumental compliances were achieved by changing the specimen position along the cantilever beam or using a beam with different height or made of different materials. As reported in our previous study [30], by combining different beam materials and heights as well as different specimen positions, an instrumental compliance ranging from 0.33 to 2186.06 μm/N can be obtained with the cantilever-beam instrument.
Axial shrinkage and Young's modulus of the composite samples were measured to support the PS testing results. The axial shrinkage was measured using the tensometer instrument at the extreme instrumental compliance (essentially unconstrained) of 2186.06 μm/N (ε = Δh/h, where ε is the axial shrinkage, Δh is the beam deflection at the sample position, and h is the height of uncured sample). Given the sample configuration adopted (i.e. 2.5 mm diameter and 2 mm height), the measured axial shrinkage approximated the true linear shrinkage based on the results reported in the literature [31,32]. Shrinkage measurement in this manner has been justified in our previous study [30]. The Young's modulus of the cured samples was measured using Instron compression tests (model 5500R, Instron Corp., Canton, MA). Cylindrical specimens with 4 mm diameter and 4 mm height were prepared in a Teflon mold by applying a high intensity LED irradiation (2000 mW/cm2, LZ1-00DB00, LED Engin, Inc.) for 2 min. Immediately after the irradiation, the specimen was removed from the mold and another irradiation with the same amount of irradiance was applied to the reverse side of the specimen to ensure a thorough curing. The cured specimens were stored in ambient conditions for 48 h before the compression test to relieve any residual stress. The compression tests were performed using a 5 kN load cell with a crosshead speed of 1 mm/min. The Young's modulus of each sample was determined by the slope of the initial linear region in the stress–strain curve. All the measurements and sample preparations were performed under a yellow light environment to minimize premature photopolymerization and at room temperature. For each set of the experiments, at least three replicates were conducted.
3. Results and discussion
Among the existing instruments for PS measurement, the most universal method has been the use of a load cell (i.e. cantilever-beam for the tensometer instrument) to record uniaxial forces from a disk/cylinder shaped specimen [3,4]. During the evolution of polymerization shrinkage, the stress measured by the load cell to balance the sample uniaxial shrinkage is registered as the PS. Based on a ‘force-controlled shrink-pull’ concept (Supplementary Data (SD), Fig. S1 and Section S1), a simplified model (Eq. (1)) for the approximation of PS development (σ) has been derived in our previous study [30]:
| (1) |
where k is the spring constant of the load cell, which equals to the inverse of the instrumental compliance (k = 1/C, where C is the compliance); E is the elastic modulus of the sample; ε is the axial shrinkage; and ks = AE/h is the effective spring constant of the sample where A and h are the cross-sectional area and the height, respectively.
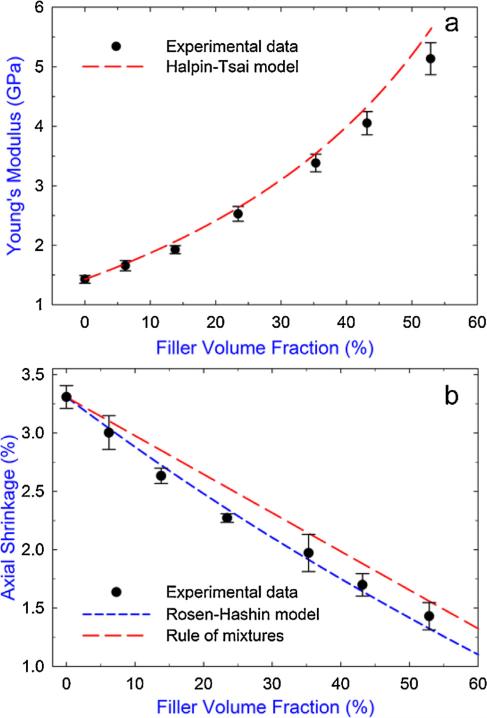
Eq. (1) can be used to predict the trend in the final PS as a function of filler content if the analytical expressions for the two dependent variables (i.e. E and ε) as a function of filler content are given. In the literature, several models (either semi-empirical or physical) have been introduced to predict the elastic modulus for composite systems based on the matrix and filler properties [33,34]. Among these models, the Halpin–Tsai model obtained through numerical modeling agrees well with experimental results for particulate-filled dental composites [33,35]. Also, the Rosen–Hashin model, originally presented for the prediction of the coefficient of thermal expansion, is capable of predicting the shrinkage of dental composites [33,35,36]. Specific expressions for these two models and the parameters involved in this study are given in the SD, Section S2. Fig. 1 shows the predicted results of E and ε as a function of filler content for the composite system tested in this study and the respective fit with experimental data. As a comparison, the rule of mixtures for the shrinkage prediction (i.e. composite shrinkage = resin shrinkage × (1 – filler volume fraction) since the fillers do not shrink) is also included in Fig. 1b. It can be seen from Fig. 1a that the Halpin–Tsai model showed good agreement with the measured Young's moduli except for small discrepancies for the composite with the higher filler contents. A possible reason for this discrepancy could be the fact that as filler content became higher, a uniform distribution of the fillers was harder to achieve in the matrix. Clusterization of the fillers could cause stress concentration during loading and affected the overall stiffness of the composite [37]. For the composite shrinkage (Fig. 1b), the non-linear Rosen–Hashin model showed better agreement with the experimental results than the linear rule of mixtures. This indicates that either the addition of fillers limited the overall monomer conversion (proportional to the shrinkage) of the composite or the resin-filler interactions (residual stresses) constrained the resin shrinkage to some extent [33]. The contribution from the former factor can be excluded based on the measured double-bond conversion as shown later.
Fig. 1.
(a) Experimental results of the Young's modulus of the composites as a function of filler content and the corresponding prediction based on the Halpin–Tsai model. (b) Axial shrinkage of the composites measured at 30 min after irradiation as a function of filler content and the corresponding predictions using the Rosen–Hashin model and rule of mixtures. Error bars indicate the standard deviation of the measurement. Data values for the experimental results are given in the SD, Table S2.
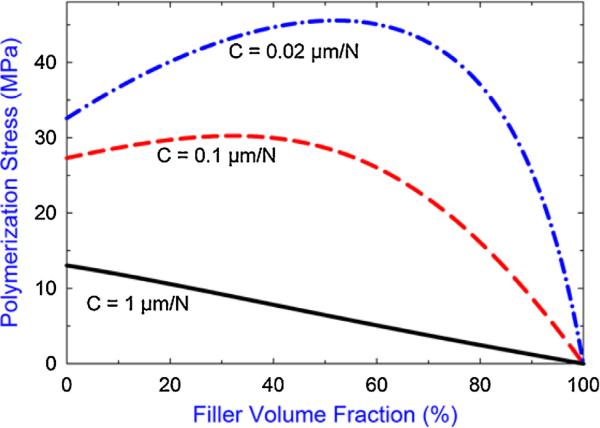
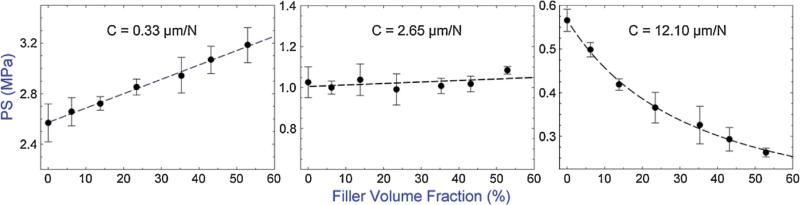
By incorporating the Halpin–Tsai and Rosen–Hashin models into Eq. (1), predicted PS as a function of filler content for the specific sample parameters shown in Table S1, SD were obtained for three selected instrumental compliances as shown in Fig. 2. Practically, filler contents greater than 70 vol.% are rarely seen in either commercial or experimental composites; therefore, not applicable to this study. It can be seen from Fig. 2 that under low compliance conditions (0.02 μm/N), the PS increases with increasing filler content until a peak value is reached when the filler content approximates 60 vol.%; after that, the final PS decreases with further increasing filler content. Increasing the instrumental compliance (0.1 μm/N) results in a less pronounced dependence between PS and filler content. Also, the filler content where the PS is at its maximum shifts to the lower volume fractions of filler content (i.e. becoming smaller). As the compliance is further increased (1 μm/N), the PS decreases with increasing filler content over the entire filler content range. These three distinct PS/filler content correlations under three different compliances are further validated by our experimental results shown in Fig. 3. As can be seen, for the specific sample configuration adopted in this study, the PS increased with increasing filler content for all of the composites tested (filler fraction from 0 to 53 vol.%) when the low instrumental compliance of 0.33 μm/N was applied. At the middle compliance of 2.65 μm/N, the PS was not influenced (i.e. the trend was completely flat) by the change of filler content, while at the high compliance of 12.10 μm/N, the PS decreased with increasing filler content over the entire filler content range. A recent analytical study based on a more comprehensive mathematical model also predicted a similar result of the combined effect of filler content and instrumental compliance on the PS [38]. However, only the first two distinct PS/filler content correlations were reported and no experiments were conducted to support the result in that study.
Fig. 2.
Predicted trends of polymerization stress as a function of filler content at three selected instrumental compliances (C). Parameters used for the predictions are given in the SD, Table S1.
Fig. 3.
Experimental polymerization stress (PS) measured at 30 min after irradiation as a function of filler content under three instrumental compliances tested. Error bars indicate the standard deviation of the measurement. Dashed lines connecting the data points are provided for visual assistance. Data values are given in the SD, Table S2.
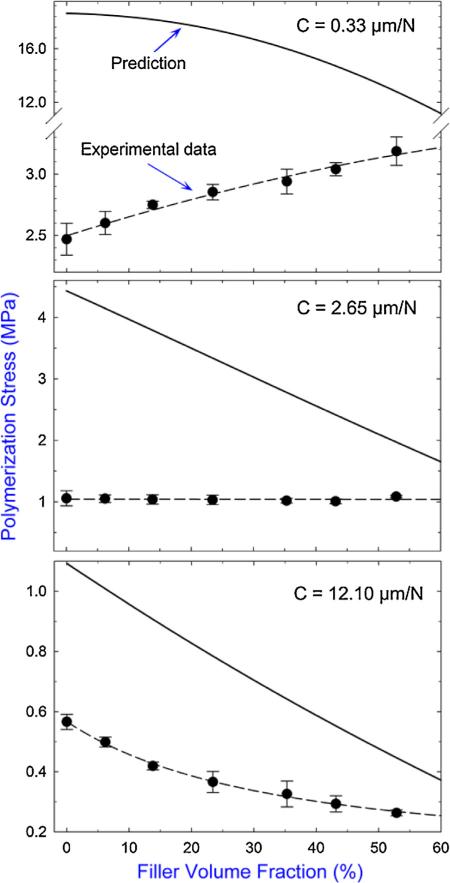
It is worthy to note that the model for the PS approximation (Eq. (1)) is based on purely elastic responses of the sample and does not take into account the incremental change of the sample modulus and shrinkage during the evolution of polymerization [3]; therefore, quantitative agreements between the prediction and experiment are not expected. However, the model still captures the main feature of the polymerization stress due to the compliance of testing system, thus agreements in the general trends are still observed. Fig. 4 compares the predicted and measured PS of the composites, and shows the models overestimate the PS magnitude for all the composites and instrumental compliances tested. In addition, the difference between the predicted and measured PS increases with decreasing filler content and decreasing instrumental compliance. This can be explained by the fact that (1) the lower the filler content the lower the viscosity (i.e. the more flowable) of the uncured specimen and (2) the lower the instrumental compliance the higher the stress applied on the specimen. Either of these facts facilitates a greater amount of stress relaxation caused by material flow (molecular rearrangement) or viscoelastic deformation [7,17,39–41] during polymerization that is not included in the predictions.
Fig. 4.
Comparison of the predicted (–) and measured (●) polymerization stress as a function of filler content under three different instrumental compliances tested.
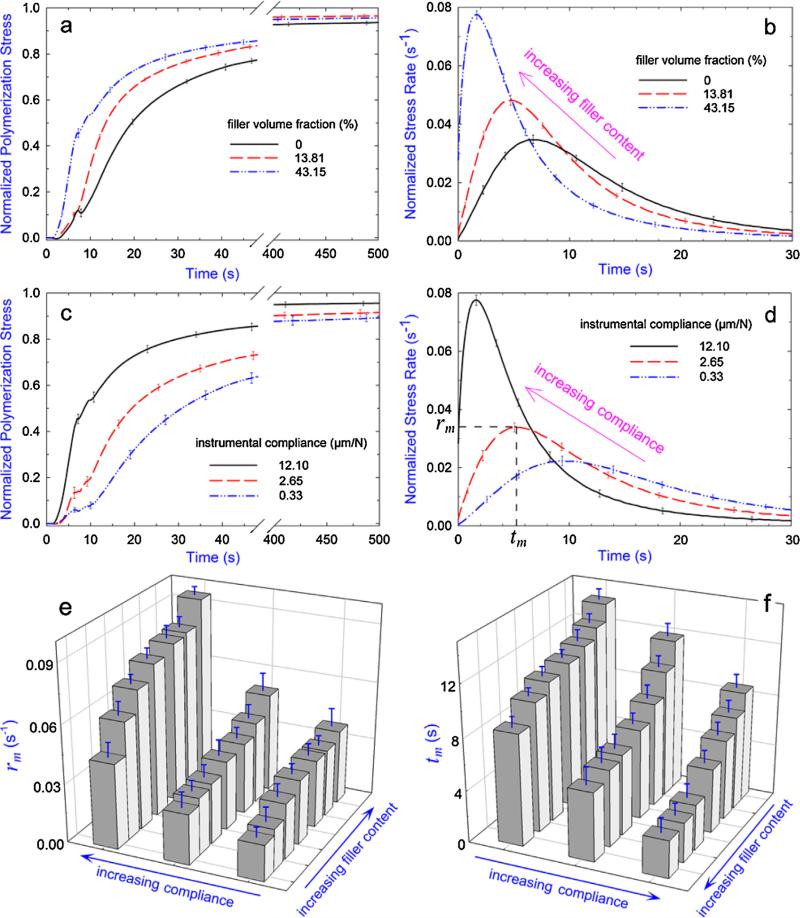
Since the stress relaxation occurs mostly during the early stage of polymerization where the material is still in liquid or rubbery state [17,40,41], the greater stress relaxation reduces the rate of PS buildup (i.e. the more the stress relaxation, the slower the PS evolution). Fig. 5 shows the effects of filler content and instrumental compliance on the measured real-time PS development and associated kinetics (PS rate) from the inception of photo-irradiation. It can be seen from Fig. 5a to d that the PS developed faster for composites with higher filler content and under higher instrumental compliance condition. By using the quantified parameters defined in Fig. 5d (i.e. rm, maximum of the relative PS rate and tm, time to reach the maximum), Fig. 5e and f show that for all the composites and instrumental compliances tested, rm increased while tm decreased with the increasing filler content and increasing instrumental compliance. A smaller tm while a higher rm corresponded to a faster developed PS (i.e. less relaxed stress). Therefore, results of PS rate agree with those of stress relaxation and further explain the differences between predictions and experiments shown in Fig. 4.
Fig. 5.
Effects of filler content and instrumental compliance on the rate of PS development: (a) the real-time PS evolution of selected composites under instrumental compliance of 12.10 μm/N, (b) the corresponding PS rate obtained from the first derivative of the real-time PS curve by applying Hill's 4-parameter non-linear regression; R2 > 0.99 for all the regressions, (c) the real-time PS evolution for the composite with 43.15 vol.% filler content under three different instrumental compliances and (d) the corresponding PS rates. For the purpose of comparison, all the PS developments are normalized by the final magnitude (i.e. 30 min value) in each case. Kinetic parameters of (e) rm (maximum PS rate) and (f) tm (time to reach rm) as defined in (d) as a function of filler content and instrumental compliance. Error bars indicate the standard deviation of the measurement.
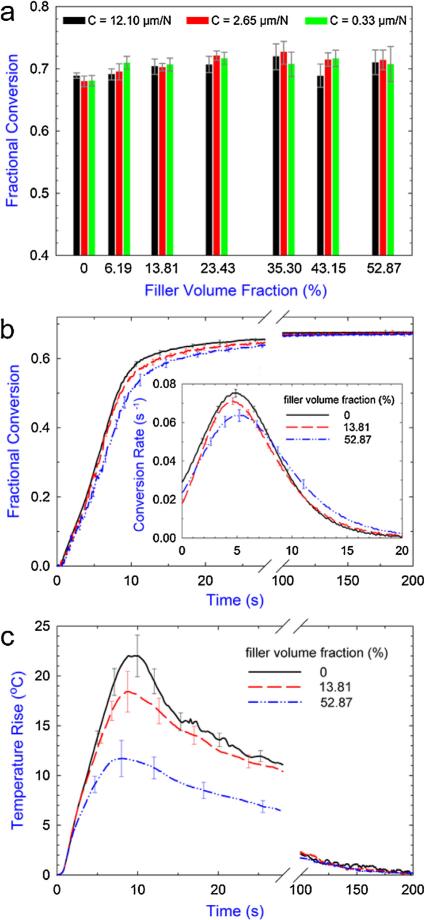
In addition to the PS development, the degree of conversion and the associated curing kinetics are also important indexes for the evaluation of dental composites because (1) the unconverted monomers have potential to leach out and cause harmful effects in clinic due to their cytotoxicity [42] and (2) the curing kinetics are correlated with the evolution of various material properties (e.g. shrinkage, modulus, viscosity, etc.) during polymerization [43]. By optimizing the instrument design and using a special sample configuration, our tensometer instrument coupled with in situ NIR spectrometer is capable of simultaneously capturing the PS development and curing kinetics for a wide range of materials (from pure resin to commercial opaque composites with high filler contents of ca. 80 wt.%) with unprecedented resolution and speed [25]. Fig. 6 shows the measured double-bond conversion and curing kinetics for composites with various filler contents and under various instrumental compliances. It can be seen that the final conversion did not change significantly as either the filler content or the instrumental compliance changed (Fig. 6a). For all the composites tested, the degree of conversion reached a similar level of 70 ± 2% at 30 min after photo-irradiation. This result agrees with certain studies in the literature, which also report the addition of inorganic fillers does not significantly influence the final conversion of the subject composites [9,44,45]. It should be noted that although the opposite observation where the final conversion progressively decreases with increasing filler content has also been reported [10,11,46,47], the composites tested in these studies usually include nanoscale fillers (typically less than 100 nm), which have much higher surface area than the fillers used in this study (mean size of 1 μm). The high surface area of the nanofillers results in an increased interface between the fillers and matrix, which could restrict the mobility of the reactants in the matrix and thus limit the final conversion [46–48].
Fig. 6.
(a) The fractional double-bond conversion measured at 30 min after irradiation as a function of filler content under various instrumental compliances. (b) The real-time evolution of the conversion for selected composites under a compliance of 2.65 μm/N and the corresponding conversion rate during the first 20 s after photo-activation (inset). (c) The real-time evolution of the temperature change (compared to the room temperature) for selected composites. Error bars indicate the standard deviation of the measurement. Data values of the final conversion and maximum temperature rise are given in the SD, Table S3.
Although the final conversions were almost the same, the paths to achieve the final value (i.e. curing kinetics) were not identical for the composites tested. As shown in Fig. 6b for the selected kinetics curves, the composites with lower filler content cured slightly faster than those with higher filler content during the early stages of polymerization. The maximum conversion rate differed by ca. 15% between the samples with lowest and highest filler content tested (inset of Fig. 6b). A few reasons can explain this trend. First, composites with lower filler content had a higher concentration of monomer units available for reaction at the beginning of the polymerization. Second, the filler particles could reflect, scatter, and absorb the curing light, which would slow down the rate of free-radical generation [49]. Last, the higher concentration of monomer caused a significantly higher reaction exotherm for the composites with lower filler content, as evidenced in Fig. 6c for a comparison of the real-time temperature evolution. The higher temperature could promote a higher mobility for the reactants, which in turn facilitated further the polymerization reaction until the polymer network became highly diffusion-limited [50].
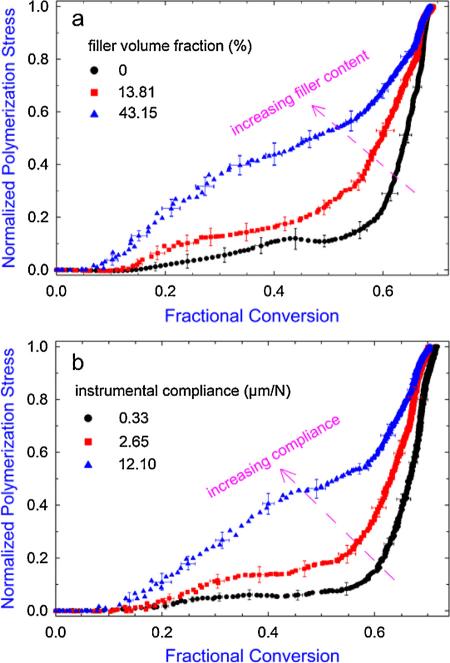
Since the PS and the double-bond conversion were collected synchronously during the experiment, a conversion-based PS evolution (compared to the time-based shown in Fig. 5a and c) can be obtained by plotting the real-time PS against the real-time conversion, as shown in Fig. 7. For the same instrumental compliance, increasing filler content resulted in a faster developed PS in response to the evolving conversion (Fig. 7a). For composites with the same filler content, increasing instrumental compliance also resulted in a very similar effect (Fig. 7b). These trends agree with those observed in the time-based PS development, which are mainly caused by the different amounts of stress relaxation in different filler content/compliance conditions. From Fig. 7, it can also be observed that all PS started to increase at a conversion close to or less than 10% (some of them developed very slowly), which reasonably approximated the gelation conversion for typical dimethacrylate system [51,52]. In addition, for all the experiment sets, a rapid increase in the PS beginning at about 50–55% conversion was observed, corresponding to the onset of macroscopic vitrification with significant amount of modulus being developed [11,15,52,53]. It should be noted that the vitrification occurred earlier for the composite with higher filler content (Fig. 6a), which could be a result of an earlier-developed modulus due to the covalent linkages formed at the filler-resin interface (providing additional reinforcement for the cross-linked network) and reduced cooperative movement between polymer chains, as has been recently demonstrated [11].
Fig. 7.
The conversion-based PS development for (a) selected composites under an instrumental compliance of 12.10 μm/N and (b) composite with 52.87 vol.% filler content under three different instrumental compliances. For the purpose of comparison, the PS developments are normalized by the final magnitude (i.e. 30 min value) in each case.
In summary, our experimental and analytical results show that the presence of a composite filler significantly influences the development of polymerization shrinkage stress; however, this influence is highly dependent on the compliance of the external constraint. Also, for the material compositions tested, our experimental results show that changing filler content has an insignificant effect on the final degree of conversion although the curing kinetics is influenced. The first finding that a compliance-dependent correlation exists between the final PS and the filler content can explain, at least in part, the inconsistent literature reports on comparisons of PS for certain commercial products (with different filler contents) measured using different instruments (with different compliances) [12,23,24]. This indicates that, the instrumental compliance should always be mentioned in in vitro studies of PS and the results obtained from such instruments whose compliances are not clinic-relevant might not be clinically-representative. Considering the approximate compliance of tooth cavities reported in the literature (e.g. around 3 μm/N for the remaining cusps of a Class-II cavity [28], and an average of 0.4 μm/N for typical Class-I cavity [29]), our findings suggest that composites with relatively lower filler content such as flowable composites should be more suitable for such cavities with relatively lower compliance (e.g. Class-I cavity or the basal part in a cavity). This argument validates the use of flowable composites as liner layers that has been adopted in current clinic procedures [54,55]. In contrast, for relatively compliant cavities such as the cusp regions of a Class-II cavity, composites with higher filler content (e.g. packable composites) should be more suitable. As indicated in this study, these composites have less significant difference in the PS and degree of conversion compared to those with lower filler content under the relatively compliant conditions, while usually exhibiting better mechanical performances and wear resistance.
4. Conclusion
Filler content effects on polymerization shrinkage stress (PS) of dental composites have been studied in a thorough, systematic and comprehensive manner. Within the clinic-relevant range of compliances and for the specific sample configuration tested, the dependence of PS on filler content is shown highly related to the compliance of surrounding constraint. PS increased with increasing filler content in low compliance systems, became independent of filler content at intermediate instrument compliances, and eventually decreased with increasing filler content with further elevated compliances. These varying trends are the combinative results of the composite modulus, shrinkage, and viscosity in response to different external constraint. The compliance-dependent PS/filler content correlation suggests that: (1) For experimental materials comparison in terms of PS, the specific instrumental compliance at which the comparison being done should always be mentioned; (2) the instrumental compliance for PS study should be clinic-relevant in order to provide useful information; (3) in clinical situations, restorative composites with different filler content can be adopted for cavities with different level of compliance in order for a better performance.
Supplementary Material
Acknowledgements
Financial support was provided through an Interagency Agreement between the National Institute of Dental and Craniofacial Research (NIDCR) and NIST (Y1-DE-7005-01). We thank Dr. Forrest A. Landis and Dr. Joseph M. Antonucci for their help in preparing the composites, Dr. Jae Hyun Kim and Mr. Anthony A.M. Giuseppetti for their help in the elastic modulus measurement.
Footnotes
Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States.
Certain commercial materials and equipment are identified in this manuscript in order to specify adequately the experimental and analysis procedures. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology (NIST) nor does it imply that they are necessarily the best available for the purpose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dental.2016.01.006.
REFERENCES
- 1.Ferracane JL. Resin composite – state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J Dent. 1997;25:435–40. doi: 10.1016/s0300-5712(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 3.Ferracane JL. Placing dental composites – a stressful experience. Oper Dent. 2008;33:247–57. doi: 10.2341/07-BL2. [DOI] [PubMed] [Google Scholar]
- 4.Schneider LFJ, Cavalcante LM, Silikas N. Shrinkage stresses generated during resin-composite applications: a review. J Dent Biomech. 2010:131630. doi: 10.4061/2010/131630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–18. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 6.Condon JR, Ferracane JL. Assessing the effect of composite formulation on polymerization stress. J Am Dent Assoc. 2000;131:497–503. doi: 10.14219/jada.archive.2000.0207. [DOI] [PubMed] [Google Scholar]
- 7.Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater. 2005;21:1150–7. doi: 10.1016/j.dental.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Boaro LC, Gonçalves F, Braga RR. Influence of the bonding substrate in dental composite polymerization stress testing. Acta Biomater. 2010;6:547–51. doi: 10.1016/j.actbio.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Gonçalves F, Kawano Y, Braga RR. Contraction stress related to composite inorganic content. Dent Mater. 2010;26:704–9. doi: 10.1016/j.dental.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Gonçalves F, Azevedo CLN, Ferracane JL, Braga RR. BisGMA/TEGDMA ratio and filler content effects on shrinkage stress. Dent Mater. 2011;27:520–6. doi: 10.1016/j.dental.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Shah PK, Stansbury JW. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent Mater. 2014;30:586–93. doi: 10.1016/j.dental.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonçalves F, Boaro LC, Ferracane JL, Braga RR. A comparative evaluation of polymerization stress data obtained with four different mechanical testing systems. Dent Mater. 2012;28:680–6. doi: 10.1016/j.dental.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Meira JBC, Braga RR, Ballester RY, Tanaka CB, Versluis A. Understanding contradictory data in contraction stress tests. J Dent Res. 2011;90:364–70. doi: 10.1177/0022034510388039. [DOI] [PubMed] [Google Scholar]
- 14.Gonçalves F, Pfeifer CS, Ferracane JL, Braga RR. Contraction stress determinants in dimethacrylate composites. J Dent Res. 2008;87:367–71. doi: 10.1177/154405910808700404. [DOI] [PubMed] [Google Scholar]
- 15.Stansbury JW, Trujillo-Lemon M, Lu H, Ding X, Lin Y, Ge J. Conversion-dependent shrinkage stress and strain in dental resins and composites. Dent Mater. 2005;21:56–67. doi: 10.1016/j.dental.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Pfeifer CS, Ferracane JL, Sakaguchi RL, Braga RR. Factors affecting photopolymerization stress in dental composites. J Dent Res. 2008;87:1043–7. doi: 10.1177/154405910808701114. [DOI] [PubMed] [Google Scholar]
- 17.Davidson CL, de Gee AJ. Relaxation of polymerization contraction stresses by flow in dental composites. J Dent Res. 1984;63:146–8. doi: 10.1177/00220345840630021001. [DOI] [PubMed] [Google Scholar]
- 18.Watts DC, Satterthwaite JD. Axial shrinkage-stress depends upon both C-factor and composite mass. Dent Mater. 2008;24:1–8. doi: 10.1016/j.dental.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Condon JR, Ferracane JL. Reduced polymerization stress through non-bonded nanofiller particles. Biomaterials. 2002;23:3807–15. doi: 10.1016/s0142-9612(02)00099-6. [DOI] [PubMed] [Google Scholar]
- 20.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 21.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin-composites: I. Shrinkage stress characterization technique. J Mater Sci Mater Med. 2004;15:1097–103. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]
- 22.Chiang MYM, Giuseppetti AAM, Qian J, Dunkers JP, Antonucci JM, Schumacher GE, et al. Analysis of a cantilever-beam based instrument for evaluating the development of polymerization stresses. Dent Mater. 2011;27:899–905. doi: 10.1016/j.dental.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musanje L, Sakaguchi RL, Ferracane JL, Murchison CF. Light-source, material and measuring-device effects on contraction stress in composites. J Dent Res. 2005:84. Abstract #294. [Google Scholar]
- 24.Gonçalves F, Pfeifer CS, Meira JBC, Ballester RY, Lima RG, Braga RR. Polymerization stress of resin composites as a function of system compliance. Dent Mater. 2008;24:645–52. doi: 10.1016/j.dental.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Landis FA, Giuseppetti AAM, Lin-Gibson S, Chiang MYM. Simultaneous measurement of polymerization stress and curing kinetics for photopolymerized composites with high filler content. Dent Mater. 2014;30:1316–24. doi: 10.1016/j.dental.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin composites. II. Novel method of simultaneous measurement of polymerization shrinkage stress and conversion. J Biomed Mater Res B. 2004;71:206–13. doi: 10.1002/jbm.b.30088. [DOI] [PubMed] [Google Scholar]
- 27.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–9. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Chang J, Ferracane J, Lee IB. Influence of instrument compliance and specimen thickness on the polymerization shrinkage stress measurement of light-cured composites. Dent Mater. 2007;23:1093–100. doi: 10.1016/j.dental.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues FP, Lima RG, Muench A, Watts DC, Ballester RY. A method for calculating the compliance of bonded-interfaces under shrinkage: validation for Class I cavities. Dent Mater. 2014;30:936–44. doi: 10.1016/j.dental.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Chiang MYM. Correlation between polymerization shrinkage stress and C-factor depends upon cavity compliance. Dent Mater. 2016;32:343–52. doi: 10.1016/j.dental.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Feilzer AJ, De Gee AJ, Davidson CL. Increased wall-to-wall curing contraction in thin bonded resin layers. J Dent Res. 1989;68:48–50. doi: 10.1177/00220345890680010701. [DOI] [PubMed] [Google Scholar]
- 32.Lee IB, Cho BH, Son HH, Um CM, Lim BS. The effect of consistency, specimen geometry and adhesion on the axial polymerization shrinkage measurement of light cured composites. Dent Mater. 2006;22:1071–9. doi: 10.1016/j.dental.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Lingois P, Berglund L. Modeling elastic properties and volume change in dental composites. J Mater Sci. 2002;37:4573–9. [Google Scholar]
- 34.Chantler PM, Hu X, Boyd NM. An extension of a phenomenological model for dental composites. Dent Mater. 1999;15:144–9. doi: 10.1016/s0109-5641(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Li H, Fox ASL, Watts DC. Numerical evaluation of bulk material properties of dental composites using two-phase finite element models. Dent Mater. 2012;28:996–1003. doi: 10.1016/j.dental.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Rosen BW, Hashin Z. Effective thermal expansion coefficient and specific heats of composite materials. Int J Eng Sci. 1970;8:157–73. [Google Scholar]
- 37.Fu SY, Feng XQ, Lauke B, Mai YW. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate-polymer composites. Composites B. 2008;39:933–61. [Google Scholar]
- 38.Fox ASL. Shrinkage stress development in dental composites – an analytical treatment. Dent Mater. 2013;29:1108–15. doi: 10.1016/j.dental.2013.08.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Stansbury JW, Bowman CN. Towards the elucidation of shrinkage stress development and relaxation in dental composites. Dent Mater. 2004;20:979–86. doi: 10.1016/j.dental.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Feilzer AJ, De Gee AJ, Davidson CL. Quantitative determination of stress reduction by flow in composite restorations. Dent Mater. 1990;6:167–71. doi: 10.1016/0109-5641(90)90023-8. [DOI] [PubMed] [Google Scholar]
- 41.Min SH, Ferracane J, Lee IB. Effect of shrinkage strain, modulus, and instrument compliance on polymerization shrinkage stress of light-cured composites during the initial curing stage. Dent Mater. 2010;26:1024–33. doi: 10.1016/j.dental.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Kawahara T, Nomura Y, Tanaka N, Teshima W, Okazaki M, Shintani H. Leachability of plasticizer and residual monomer from commercial temporary restorative resins. J Dent. 2004;32:277–83. doi: 10.1016/j.jdent.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Braga RR, Ferracane JL. Contraction stress related to degree of conversion and reaction kinetics. J Dent Res. 2002;81:114–8. [PubMed] [Google Scholar]
- 44.Atai M, Watts DC. A new kinetic model for the photopolymerization shrinkage-strain of dental composites and resin-monomers. Dent Mater. 2006;22:785–91. doi: 10.1016/j.dental.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Nie J, Rabek JF, Linden LA. Photopolymerization of poly(melamine-co-formaldehyde) acrylate for dental restorative resins. Polym Int. 1999;48:129–36. [Google Scholar]
- 46.Garoushi S, Vallittu PK, Watts DC, Lassila LV. Effect of nanofiller fractions and temperature on polymerization shrinkage on glass fiber reinforced filling material. Dent Mater. 2008;24:606–10. doi: 10.1016/j.dental.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Halvorson RH, Erickson RL, Davidson CL. The effect of filler and silane content on conversion of resin-based composite. Dent Mater. 2003;19:327–33. doi: 10.1016/s0109-5641(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 48.Satterthwaite JD, Maisuria A, Vogel K, Watts DC. Effect of resin-composite filler particle size and shape on shrinkage-stress. Dent Mater. 2012;28:609–14. doi: 10.1016/j.dental.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Emami N, Sjödahl M, Söderholm K-JM. How filler properties, filler fraction, sample thickness and light source affect light attenuation in particulate filled resin composites. Dent Mater. 2005;21:721–30. doi: 10.1016/j.dental.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Howard B, Wilson ND, Newman SM, Pfeifer CS, Stansbury JW. Relationships between conversion, temperature and optical properties during composite photopolymerization. Acta Biomater. 2010;6:2053–9. doi: 10.1016/j.actbio.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. Delayed gelation through chain-transfer reactions: mechanism for stress reduction in methacrylate networks. Polymer. 2011;52:3295–303. doi: 10.1016/j.polymer.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abu-elenain DA, Lewis SH, Stansbury JW. Property evolution during vitrification of dimethacrylate photopolymer networks. Dent Mater. 2013;29:1173–81. doi: 10.1016/j.dental.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi RL, Shah NC, Lim BS, Ferracane JL, Borgersen SE. Dynamic mechanical analysis of storage modulus development in light-activated polymer matrix composites. Dent Mater. 2002;18:197–202. doi: 10.1016/s0109-5641(01)00082-3. [DOI] [PubMed] [Google Scholar]
- 54.Leevailoj C, Cochran MA, Matis BA, Moore BK, Platt JA. Microleakage of posterior packable resin composites with and without flowable liners. Oper Dent. 2001;26:302–7. [PubMed] [Google Scholar]
- 55.Braga RR, Hilton TJ, Ferracane JL. Contraction stress of flowable composite materials and their efficacy as stress-relieving layers. J Am Dent Assoc. 2003;134:721–8. doi: 10.14219/jada.archive.2003.0258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.