Abstract
Purpose
Functionally relevant alterations in resting state fMRI (rs-fMRI) connectivity have been identified in adults with traumatic brain injury (TBI). We evaluated rs-fMRI connectivity in children with TBI and explored the relationship between altered connectivity and measures of neurological function.
Methods
Rs-fMRI was obtained in 14 children after TBI and 14 controls matched for age, sex, and handedness. Whole-brain connectivity was evaluated separately for the default mode network (DMN) and dorsal attention network (DAN); Between-group contrasts identified regions with altered connectivity between TBI and control cohorts. In children with TBI, the relationships between regions of altered connectivity and performance on relevant functional measures were examined.
Results
Compared to controls, children with TBI showed significantly greater connectivity between DMN and right dorsal premotor cortex (RdPM) and between DAN and bilateral sensorimotor cortex (SM1). In children with TBI, greater DMN-RdPM connectivity was associated with worse motor performance whereas greater DAN-LSM1 connectivity was associated with better motor performance; furthermore, DMN-RdPM and DAN-LSM1 connectivity were negatively correlated.
Conclusion
Rs-fMRI reveals significant altered connectivity in children with TBI compared to controls. After TBI in children, patterns of altered connectivity appear divergent, with increased DMN-motor network connectivity associated with worse motor control whereas increased DAN-motor network connectivity appears compensatory.
Keywords: TBI, pediatric, resting-state networks, fMRI
Introduction
Traumatic brain injury (TBI), of all severity, is a primary cause of morbidity and mortality in children (1). While developmental consequences following more severe forms of childhood TBI have long been recognized, recently there is increased awareness of the frequency and potential long-term consequences from even mild brain injury (2, 3). Understanding the pathophysiology underlying recovery from milder severities of TBI is a critical next step to improving management and outcomes in this population.
Resting state fMRI (rs-fMRI) examines alterations in the intrinsic connectivity (IC) of brain networks and is a methodology which has shown promise for describing abnormalities after mild, moderate, and severe TBI in adults. Many rs-fMRI studies have focused on the default mode, or task negative, network (DMN), a well-characterized network which is active during self-reflective thought but suppressed during attentionally-demanding tasks (4). In adults with TBI, rs-fMRI has revealed altered DMN IC throughout recovery (5-7). Additionally, a recent study of adolescents symptomatic from sports-related concussion identified altered IC within the DMN in even this population (8). A second fundamental network, the dorsal attention network (DAN) consists of regions active during voluntary, externally-focused attention-based tasks (9-11). This network may be responsible for actively representing stimuli and selecting responses relevant during tasks and thus is critical for perceptual decision-making (9-10). The DAN is one of several task positive networks which are involved in cognitive control functions and have an antagonistic role with the DMN (12). Arguably, it is the DAN that is most strongly anti-correlated with the DMN, and the two networks form close inverse mirror images of one another (13). Therefore, DAN function may depend on DMN IC and vice versa. In adults with TBI of all severity, neural network changes, including in regions of the DMN and DAN, have been shown to correlate with functional deficits after injury (14-20).
From a developmental standpoint, the fundamental nodes of the DMN and DAN are apparent from early in life (21), and late-developing connections of both networks have been implicated in the maturation of cognitive control abilities (22, 23). Given the potential of TBI to significantly impact neurodevelopment, further evaluation of resting state network connectivity, and the relationship between network changes and function, is warranted in the pediatric population.
The purpose of this study was to evaluate whole-brain connectivity of the DMN and DAN in children with mild-complicated to moderate TBI in the sub-acute phase of recovery. We hypothesized that 1) children with TBI would show altered connectivity of the DMN and DAN compared to an age- and sex-matched cohort and 2) altered IC would be related to measures of neurological function in children with TBI.
Methods
Participants
Fourteen children aged 11-17 years with mild-complicated to moderate TBI participated in the parent project for this study, completing neuroimaging and a neuropsychological battery focused on response control including tasks examining motor and processing speed and inhibitory control. Subjects were recruited through fliers and advertisements accessible by the general population with additional recruiting efforts from children with TBI seen within our institution. Children with TBI were evaluated an average of 68 days after injury (range 29-102 days). Inclusion criteria included TBI with a GCS score ≤ 12 upon arrival to the first hospital emergency room, or loss of consciousness (LOC) longer than 15 minutes, or post-traumatic amnesia (PTA) longer than 1 hour, or presence of injury-related intracranial abnormality on clinical brain CT or MRI. Exclusion criteria were open TBI evidenced by a dural tear; inability to complete tasks; ongoing post-traumatic amnesia; premorbid intellectual disability, psychiatric or developmental disorder other than ADHD; pregnancy; or hardware that interfered with neuroimaging. Information was not kept for potential participants who failed screening or declined participation.
Neuroimaging data were available for 14 typically-developing control subjects matched for age, sex, and handedness. Controls had documented absence of history of TBI, ADHD, or learning disorders per parent report.
Standard protocol approvals and participant consent
The Johns Hopkins Medicine IRB approved this study. Written informed consent and assent were obtained from a parent or legal guardian and child participants, respectively.
Image Acquisition
Imaging was completed on a 3T Philips scanner. Images for clinical interpretation included T1, T2, and FLAIR. MPRAGE, a high resolution anatomical scan (8-channel head coil, TR=7.99 ms, TE=3.76 ms, Flip angle=8°), was attained for image co-registration, segmentation, and normalization processing. Rs-fMRI scan duration was 5 minutes 20 seconds (D-SENSE EPI, 8-channel head coil, TR=2500 ms, TE=30 ms, Flip angle = 70°); participants were instructed to fixate on a centrally located crosshair mark during scan acquisition.
Rs-fMRI Processing
Standard image data preparation, processing, and analysis were completed with Statistical Parametric Mapping (SPM8). Pre-processing of functional images was performed using SPM8 and Matlab scripts, including reorientation, slice time correction, motion correction, co-registration, segmentation, and normalization. Nuisance variables were removed from each voxel, including cerebrospinal fluid and white matter signals identified with the CompCor method, global mean signal, and six motion parameters. Functional images were spatially smoothed using a 6 mm full width at half maximum filter and then temporally filtered (bandpass 0.01-0.1 Hz). Two authors (SRR and ADB) confirmed image quality and alignment; no participant was excluded due to motion or other artifact.
Rs-fMRI connectivity analysis
IC was evaluated, separately for the DMN and DAN, by examining whole-brain connectivity generated from the mean time course for each of the three seeds per network and averaging the three -seed maps of each network to develop one DMN and one DAN map per subject. 6 mm-radius 3D seeds were centered at locations from prior rs-fMRI studies (13, 22). The 3 DMN seeds included the medial prefrontal cortex (MPFC, Talairach coordinates: −1, 47, −4), posterior cingulate cortex (PCC: −5, −49, 40), and the lateral parietal cortex (LP: −45, −67, 36). The 3 DAN seeds included the intra-parietal sulcus (IPS: −25, −57, 46), frontal eye field (FEF: 25, −13, 50), and the middle temporal region (MT: −45, −69, −2). The seed coordinates were converted to Montreal Neurological Institute (MNI) space using the Lancaster transformation (24).
Whole-brain connectivity maps for DMN and DAN were evaluated using SPM8 second-level analyses. The single subject maps for the TBI and control cohorts were entered into 2-sample t-tests to analyze between-group differences for the DMN and DAN with voxel-level threshold of p<0.01 and family-wise error correction for multiple comparisons at a cluster-level threshold of p<0.05 in accordance with random-field theory (25). Region labels were identified using the Automatic Anatomical Labeling Atlas.
Brain-behavior analyses
For each participant, the connectivity values were extracted, separately for DMN and DAN analyses, from each voxel in each region with significant between-group differences. Within regions, these values were averaged to create one connectivity value per region per participant, and this mean IC value was used in brain-behavior analyses. As the regions of altered IC identified during imaging analyses involved motor regions, items from the neuropsychological battery that examined motor control were selected for brain-behavior analyses.
Measures of Motor Control
The Physical and Neurological Examination of Subtle Signs (PANESS)(26) measures timed gross-motor movements and motor responses under subconscious control, specifically motor overflow and dysrhythmia. While overflow and dysrhythmia are normal in younger children, these subconscious movements are not typically present in children after 10 years of age(27). Mirror movements are an example of overflow movements that are scored throughout administration of the PANESS. A finger sequencing error during the finger sequence task is an example of dysrhythmia scored during timed motor tasks. PANESS scores used for brain-behavior analyses included 1) total gaits and stations (calculated based on errors during active and static movements, total overflow and involuntary movements), 2) total overflow (overflow observed across all tasks), 3) dysrhythmia (sequencing errors during timed tasks), and 4) timed total (calculated based on age and sex-based norms for speed of repetitive movements plus presence of overflow or dysrhythmia). In all cases, higher scores reflect worse performance.
The Conflicting and Contralateral Motor Response Tasks (MRT) (28, 29) require conscious effort to respond with a gesture opposite of the tester thus measure more direct motor control requiring deliberate inhibition of the incorrect motor response. The conflicting MRT was adapted from Luria-Christensen Battery(29). During this task, the participant is instructed to raise the finger of their dominant hand as quickly as possible when the examiner raises their fist and vice versa. The contralateral MRT was developed for analysis of motor neglect in monkeys(30) but has been subsequently applied to studies of motor response in children with ADHD and autism(28). During this task, the child is instructed to close their eyes and to raise the opposite hand of the one touched by the examiner. Scoring on both the conflicting and contralateral MRT is based on total number of correct responses (48 maximum) thus a lower score represents more errors during the task.
The Delis–Kaplan Executive Function System (D-KEFS) Trail Making Test (31) and the Lafayette Grooved Pegboard (LGP) evaluate visuomotor planning and motor speed. The D-KEFS was developed as a test of executive function. Within the D-KEFS Trail Making Test, the scaled scores of the motor speed (condition 5) and number sequencing (condition 2) tasks were used for brain-behavior analyses as both tasks require motor control and visuospatial planning. The Lafayette Grooved Pegboard requires the subject to place grooved pegs into a slotted board, requiring motor coordination and visuospatial planning. Scoring is based on the speed required to complete the task and Z-scores for speed of task completion for the dominant and non-dominant hands were used in brain-behavior analyses.
Clinical measures
For exploratory analyses, clinical variables were obtained from medical record review and parent report. Loss of consciousness (LOC) at the time of injury was categorized as present or absent. Duration of post-traumatic amnesia (PTA) was recorded as < or ≥ 24 hours. Descriptions of abnormalities on acute clinical head CT were obtained from available documentation, and an attending neuroradiologist provided a clinical read of study structural MRI acquisitions. Intracranial bleed or brain signal abnormality were considered positive neuroimaging findings. Time from injury to imaging was calculated as the number of days between injury and the study scan. The presence of TBI-related symptoms at the time of research imaging was based on child or parent report of new symptoms commonly associated with brain injury noted within documentation from the study visit or other clinical documentation in closest proximity (within one month) to the study visit.
Statistical analysis
Independent samples t-test and Chi Square were used to compare age and sex respectively between TBI and control cohorts. In participants with TBI, post-hoc independent samples t-tests and Pearson correlations were used to evaluate associations between connectivity values (CV) derived from the regions with between-group IC differences and 1) performance on motor control assessment, 2) clinical factors (age and sex), 3) injury-related variables (LOC, PTA ≥ 24 hours, positive acute head CT), and 4) study-related variables (time from injury to imaging, symptoms at time of imaging, positive MRI). Correlation analyses included all subjects with TBI with the exception of two data points, nearly 3 standard deviations from the mean, which were felt to be erroneous. One participant was excluded from D-KEFS motor speed analysis and another subject excluded from the LGP dominant-hand analysis due to incorrect administration of the task and/or findings which were inconsistent with the individual participant's performance on all other motor tasks. P-value < 0.05 was considered statistically significant for all analyses. Post-hoc Pearson correlation was used to compare connectivity values between regions of altered connectivity of TBI and control cohorts.
Results
Participant Characteristics
Mean age was 14.6 years in children with TBI (11-17.9 years) and 14.5 years (11-18.1 years) in the control group (p = 0.83); there were 9 males in each group. Thirteen of the 14 children in both cohorts were right handed. Within the TBI cohort, one child had a history of pre-injury inattention which resolved with more challenging coursework, and another participant was taking sertraline for pre-injury obsessive/compulsive symptoms; neither met criteria for any prior or current psychiatric diagnosis on parent interview with the Diagnostic Interview for Children and Adolescents (32). Imaging and brain-behavior analyses did not change with exclusion of these participants, so all presented results include the complete TBI cohort. Clinical, injury and study related measures of participants with TBI are presented in Table 1.
Table 1.
Demographic, injury-related, and study-related variables in children with TBI
| TBI | Cause of TBI | Any LOC | PTA > 24 hours | Head CT report at Injury* | Time to imaging (days) | Symptoms at imaging | Anatomical brain MRI report** |
|---|---|---|---|---|---|---|---|
| 1 | Non-sport | Y | Y | *2 punctate hemorrhages (L F WM) | 29 | Y | **Multiple small B/L F contusions |
| 2 | Non-sport | Y | N | *Cortical contusions (B/L F and L T); Fracture (L T to R F), underlying EDH | 73 | N | **L T contusion |
| 3 | Sport | Y | N | Normal | 79 | N | **Few areas of hypointensity (R F) |
| 4 | Non-sport | Y | N | Normal | 67 | N | Normal |
| 5 | Sport | N | Y | N/A | 60 | N/A | Normal |
| 6 | Sport | Y | N | Facial fractures | 87 | N | Normal |
| 7 | Sport | Y | N | Fracture (O) | 41 | Y | **Single punctate hyperintensity (R F) |
| 8 | Non-sport | Y | N | *Fracture (L T), underlying SDH | 65 | Y | Normal |
| 9 | Non-sport | N/A | N | N/A | 63 | N | Normal |
| 10 | Sport | Y | N | *Fracture (L parietal), underlying EDH | 102 | N | **Scattered subcortical punctate foci hyperintensity (B/L F, L P) |
| 11 | Non-sport | Y | N | Normal | 65 | Y | Normal |
| 12 | Non-sport | Y | N | Normal | 100 | Y | **Punctate focus (L F deep WM) |
| 13 | Sport | N | N | Normal | 66 | N | Normal |
| 14 | Sport | N | N | Normal | 59 | N | Normal |
considered positive Head CT for this study
considered positive MRI for this study
Abbreviations: TBI= traumatic brain injury; M = male; F= female; MVA = motor vehicle accident; N/A = not available; LOC = loss of consciousness; PTA = post traumatic amnesia; CT = computed tomography; L = left; F = frontal lobe; WM = white matter; B/L = bilateral; T = Temporal lobe; R= right; EDH = epidural hematoma; O= Occipital lobe; SDH = subdural hematoma; Y = Yes, N = No. MRI = magnetic resonance imaging; P=parietal.
Rs-fMRI between-group analyses of whole brain connectivity
The average connectivity values and peak Talairach coordinates from regions with significant between-group differences in whole-brain connectivity are presented in Table 2. Mean connectivity in the TBI cohort was in the opposite direction of mean connectivity in the control group for four of five regions with significant between-group differences.
Table 2.
Rs-fMRI whole-brain connectivity analyses: between-group differences
| Regions with significant between-group differences | TBI | CONTROLS | p-value** | Direction of connectivity differences between groups | Peak Talairach coordinates (x, y, z) |
|---|---|---|---|---|---|
| DMN-RdPM | −0.015* | −0.172* | 0.041 | TBI>CONTROLS | 22, 3, 55 31, 1, 55 34, 12, 55 |
| DMN-RSM1 | −0.120* | 0.037* | 0.055 | CONTROLS>TBI | 56, −3, 32 53, −6, 37 57, −2, 21 |
| DAN-LSM1 | 0.059* | −0.071* | 0.003 | TBI>CONTROLS | −43, −13,
36 −44, −12, 17 −58, −13, 32 |
| DAN-RSM1 | 0.087* | −0.063* | 0.001 | TBI>CONTROLS | 58, −1, 32 47, −10, 35 47, −20, 55 |
| DAN-LC | −0.075* | 0.051* | 0.044 | CONTROLS>TBI | −11, 10, 14 −21, −3, 20 −11, −5, 15 |
Mean Connectivity Values
p-value family-wise error corrected for multiple comparisons
Abbreviations: DMN = default mode network, RdPM = right dorsal premotor cortex, RSM1 = right primary sensorimotor cortex, LSM1 = left primary sensorimotor cortex, DAN = dorsal attention network, LC = left caudate
Altered DMN Connectivity
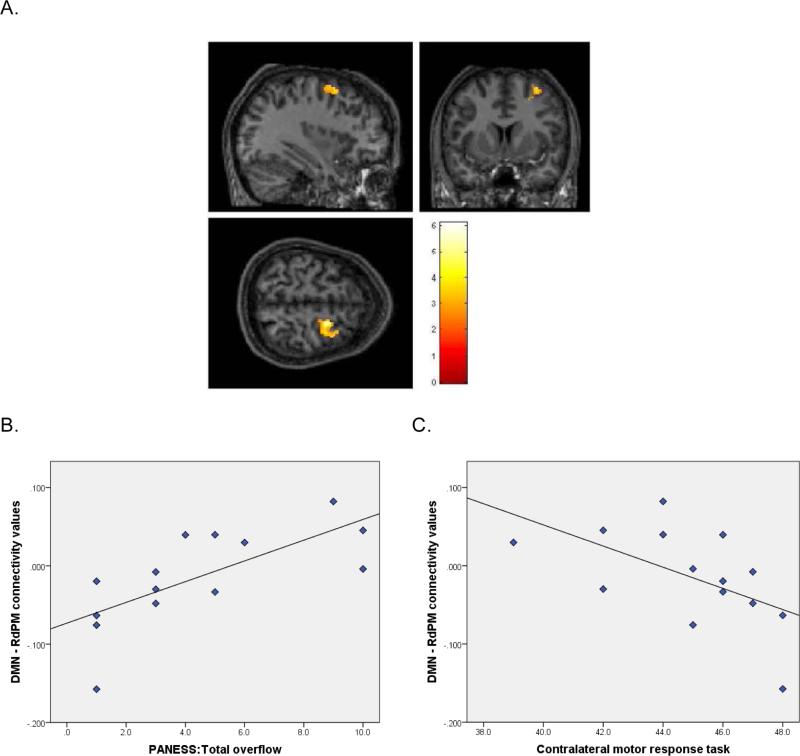
Compared to controls, children with TBI demonstrated increased connectivity between the DMN and the right dorsal premotor cortex (RdPM; p = 0.041; Figure 1) and reduced connectivity between the DMN and right sensorimotor cortex (RSM1; p = 0.055, trend level of significance).
Figure 1. DMN-RdPM Connectivity and Correlation with motor control.
A. Sectional maps showing the DMN-RdPM region where between-group rs-fMRI analyses indicated children with TBI had significantly greater IC than CONTROLS. B. Plot of the correlation between connectivity values derived from the DMN-RdPM region and PANESS total overflow scores in children with TBI. C. Plot of the correlation between connectivity values derived from the DMN-RdPM region and Contralateral MRT scores in children with TBI.
Altered DAN Connectivity
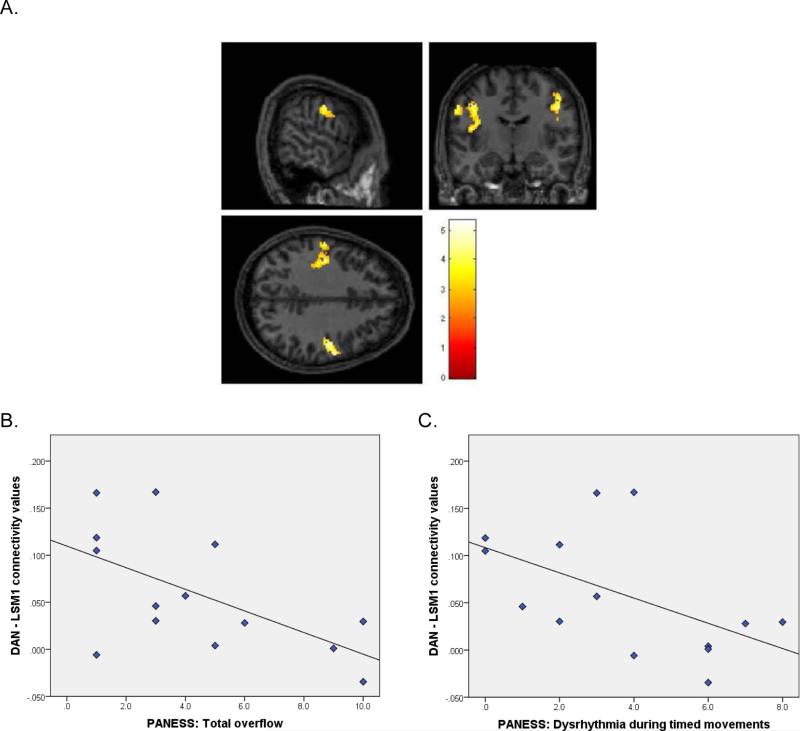
Compared to controls, children with TBI demonstrated increased IC between the DAN and left sensorimotor cortex (LSM1, p < 0.003; Figure 2) and RSM1 (p < 0.001) and reduced IC between the DAN and left caudate (LC) (p = 0.044).
Figure 2. DAN-LSM1 Connectivity and Correlation with motor control.
A. Sectional maps showing the DAN-LSM1 region where between-group rs-fMRI analyses indicated children with TBI had significantly greater IC than CONTROLS. B. Plot of the correlation between connectivity values derived from the DAN-LSM1region and PANESS total overflow scores in children with TBI. C. Plot of the correlation between connectivity values derived from the DAN-LSM1 region and PANESS dysrhythmia scores in children with TBI.
Correlations of connectivity with motor control
DMN–RdPM
Connectivity values between the DMN and RdPM correlated with higher (worse) PANESS total overflow (r= 0.71, p = 0.005; Figure 1) and higher dysrhythmia (r = 0.53, p = 0.05), indicating that increased DMN-RdPM connectivity was associated with worse motor coordination and control in children with TBI. Additionally, DMN-RdPM CV correlated with lower (worse) conflicting and contralateral MRT scores (r = −0.55, p = 0.04 and r = −0.56, p = 0.04 respectively; Figure 1), suggesting that increased DMN-RdPM connectivity was associated with worse motor inhibition.
DMN-RSM1
The DMN-RSM1 CV positively correlated with PANESS timed total (r = 0.58, p = 0.03) indicating increased connectivity between these regions was associated with worse motor speed and control.
DAN-LSM1
As depicted in Figure 2, CV between the DAN and LSM1 negatively correlated with PANESS total overflow (r = −0.59, p = 0.028) and dysrhythmia (r = −0.54, p = 0.048), revealing that increased DAN–LSM1 connectivity was associated with better motor coordination and control in children with TBI.
There were no significant correlations between CV in regions of between-group differences and scores on D-KEFS Trails, LGP, or PANESS total gaits and stations.
Relationship between DMN-RdPM and DAN-LSM1 connectivity
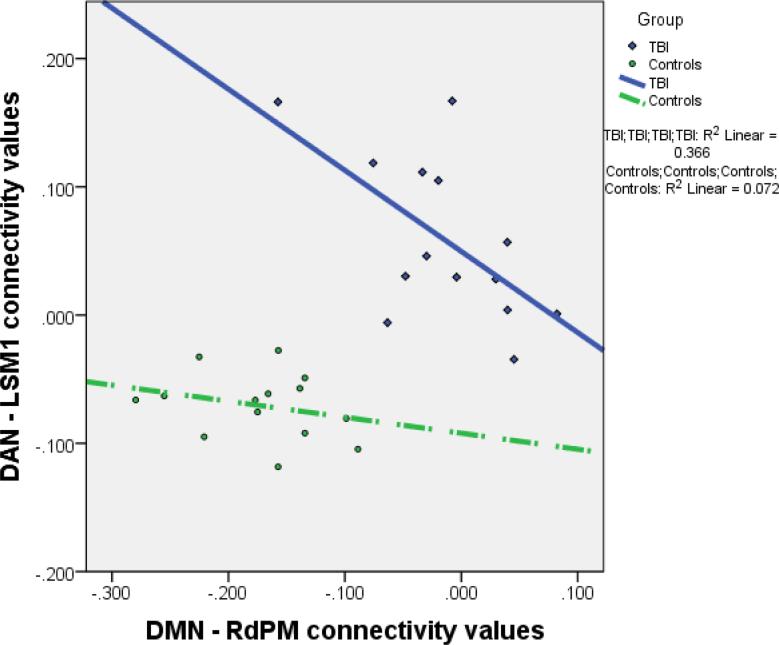
As connectivity of the DMN-RdPM and DAN-LSM1 showed correlations in opposing directions with PANESS measures of overflow and dysrhythmia, the relationship of the CV between these two regions was further explored. The CV between the DMN-RdPM and DAN-LSM1 were negatively correlated in children with TBI (r = −0.61, p = 0.02); however, this relationship was not present in controls (Figure 3).
Figure 3. Relationship between DMN-RdPM connectivity and DAN-LSM1 connectivity in children with TBI and CONTROLS.
Plot of the correlation between the connectivity values derived from DMN-RdPM and DAN-LSM1 for both the TBI and CONTROLS cohorts. Children within the TBI cohort have distinctly different connectivity values in both regions with very minimal overlap of connectivity values between TBI and CONTROLS cohorts. There is a significant negative correlation in IC values for children with TBI but not CONTROLS.
Correlations of connectivity with clinical measures
Within the TBI cohort, DAN-RSM1 connectivity negatively correlated with the time from injury to imaging, indicating reduced IC between these regions with increased days from injury (r = −0.59, p = 0.027). The presence of symptoms at study image acquisition and abnormal study structural MRI were both associated with increased DAN-LC connectivity (respectively p = 0.007 and p = 0.056, trend level of significance). There were no significant associations between connectivity and the other clinical variables.
Discussion
To better understand the pathophysiological changes after TBI, we aimed to evaluate IC of two fundamental networks, the DMN and DAN, in children within the subacute recovery phase after mild-complicated to moderate TBI compared to an age and sex matched cohort. While this study was not designed specifically to examine motor network connectivity, our rs-fMRI analyses of two independent networks revealed robust altered connectivity in children with TBI within only four brain regions, all of which are associated with motor control and coordination (right dorsal premotor cortex, bilateral primary sensorimotor cortex, left caudate). The significant changes in connectivity were particularly notable in that the TBI and control groups largely showed an opposite pattern of connectivity between these regions (e.g., regions that were correlated with the DMN in controls were anti-correlated with the DMN in the TBI group). Brain-behavior analyses suggested that the strength of these connectivity changes is associated with motor function in children with TBI, which further underscored the functional relevance of these alterations in connectivity. That these functionally-related connectivity changes were observed in our TBI cohort more than 2 months, on average, from the time of injury is an important clinical consideration when evaluating children likely to be returning to higher-risk activities.
As a group, children with TBI showed increased connectivity between the DMN and RdPM. Furthermore, the children with TBI with the highest (most abnormal) DMNRdPM connectivity values had worse subconscious motor control, evidenced by increased overflow and dysrhythmia on the PANESS, as well as worse conscious motor control, reflected in the increased errors during conflicting and contralateral MRT. The RdPM, which includes the supplementary motor complex (SMC), is known to be important for control and selection of motor actions and has been found to be involved in inhibition of mirror overflow movements (33-35). Additionally, impulsivity in adolescents has been related to increased connectivity specifically between the DMN and bilateral dorsolateral premotor cortex (36). Similarly, the current findings suggest an adverse effect of more DMN connectivity with the RdPM for inhibitory motor control, whether at a conscious or subconscious level, and may represent a disadvantageous change in connectivity following TBI in children.
While between-group connectivity revealed overall reduced DMN-RSM1 connectivity in children with TBI compared to controls, greater correlation of DMN-RSM1 IC in children within the TBI cohort (i.e., more similar to controls) was associated with poorer performance on PANESS timed motor tasks, again suggesting a disadvantage of DMN-motor network connectivity after TBI. It is possible that reduced (more anti-correlated) DMN-RSM1 connectivity may reflect better suppression of the DMN during motor task performance, thereby supporting improved motor control; conversely, increased IC between the DMN and motor regions may impair task-related recruitment of motor regions needed for optimal performance.
In contrast to the DMN-behavioral analyses, increased DAN-LSM1 connectivity (less similar to controls) in children with TBI was associated with better PANESS performance as measured by reduced subconscious extraneous (overflow) movements. The increased connectivity between these regions may function as a compensatory mechanism after TBI, recruiting attentional networks to enhance top-down control of motor actions. This hypothesis is supported by prior work demonstrating that more attention to task is associated with fewer overflow movements (37). The current data suggest that greater DAN-LSM1 connectivity is associated with suppression of subtle motor abnormalities even in the absence of external attention being called to these movements during testing.
These brain-behavior findings are concordant with studies of adults with TBI in the acute and chronic phase of recovery. Shumskaya et al (2012) described reduced IC within the motor networks in adults with acute mTBI as well as deficits on measures of psychomotor and processing speed (38). While we did not set out to specifically examine motor network connectivity, our whole-brain analyses ended up revealing altered connectivity after TBI that was particular to motor regions. It is possible that increased DMN-motor network connectivity may suppress IC within motor networks, and increased DAN-LSM1 IC may compensate for reduced connectivity within the motor network occurring after TBI. Additionally, task-based fMRI studies in adults and children with TBI have reported altered motor network organization with compensatory recruitment of additional brain regions during task performance (39-41). The altered connectivity patterns seen within our TBI cohort at rest may reflect these functionally based changes reported in task-based studies.
The negative correlation between DMN-RdPM CV and DAN-LSM1 CV, seen only within the TBI cohort, suggests a divergent pattern of altered connectivity after brain injury, with some children establishing more DMN-RdPM connectivity (which is related to worse motor function) while others develop apparent compensatory DAN-LSM1 connectivity after injury. As the DMN and DAN are anti-correlated networks, these regions may compete for motor network connectivity after injury, with other unknown factors perhaps further mediating the pattern of altered connectivity.
While both the left and right SM1 demonstrate increased IC with the DAN in children with TBI, only DAN-LSM1 connectivity was related to motor function. This finding may be secondary to the predominance of right-handedness in our sample and the known dominance of left hemisphere systems in motor control (particularly in right-handed individuals). Furthermore, only DAN-RSM1 connectivity significantly decreased with increased time from injury, becoming more similar to controls over time. This may suggest that DAN-RSM1 connectivity is not functionally reinforced at this stage after injury.
While altered DAN-LC connectivity was not related to motor performance on the tasks examined, that DAN-LC CV were stable over time may reflect that this altered connectivity is reinforced throughout recovery. A recent task-based fMRI study suggests that children with concussion have reduced activation of the left caudate during non-verbal working memory tasks (42); thus, connectivity between the DAN and LC after TBI may support cognitive function not measured in our study. Furthermore, children who had experienced resolution of injury-related symptoms by study participation and those with normal MRI demonstrated more abnormal DAN-LC connectivity. This altered network connectivity in clinically-recovered children is consistent with that observed in asymptomatic adults following sports-related concussions (6). The association between DAN-LC IC and clinical recovery may indicate other functional relevance; alternatively, it may be that greater injury severity, suggested by the ongoing presence of symptoms or abnormalities on brain MRI, may interfere with the ability to establish potentially beneficial patterns of functional connectivity. Future work will be important to better understand the persistence of these findings and their relevance to longer-term outcomes.
Altered IC within our TBI cohort was not related to performance D-KEFS Trails tests and LGP, each of which require visuomotor coordination, suggesting changes in connectivity at this stage after injury may support more basic motor control. Likewise, LOC, PTA, and abnormal acute head CT were not related to altered connectivity in children with TBI in our sample; though classically considered indicators of injury severity, recent literature notes that these variables have not been consistently associated with outcome after milder injuries in children (43). This suggests that there are likely other factors influencing connectivity changes after TBI; future work to elucidate variables that impact neural reorganization after injury may provide an opportunity for interventions promoting more adaptive recovery patterns and improving outcome.
Limitations
While the TBI and control cohorts were age- and sex- matched, our study is limited by the small sample size common to studies of advanced neuroimaging techniques. Because of the small study size, post-hoc tests were not corrected for multiple comparisons. Additionally, the small sample size affects the interpretation of negative findings and lacking associations, such as between connectivity values and clinical variables. It is possible that the task of “resting” in the scanner is more challenging for children after TBI, potentially influencing rs-fMRI brain activation patterns; however, the relationship between altered IC and performance on tasks outside of the scanner reinforces the broader relevance of these findings. While fMRI is based on cerebral blood flow (CBF) which may be altered acutely after TBI (44), the focality and bi-directionality of altered connectivity suggest that our findings are not solely related to potential changes in CBF.
Conclusions and Future Direction
These data suggest that intrinsic neural connectivity is altered in children with mild-complicated to moderate TBI months after injury occurs. Furthermore, these data suggest that the strength and pattern of altered connectivity in children with TBI are associated with behavioral findings. Continuing to explore patterns of neural connectivity is essential to understanding the pathophysiological changes, both maladaptive and compensatory, that occur throughout recovery from TBI.
Acknowledgements
N/A
Dr. Risen received funding from NIH grant T32HD007414-20.
Dr. Mostofsky received funding from RC2DA029475 and remains funded by NIH R01MH078160 and R01MH085328.
Dr. Suskauer was funded by NIH K23HD061611 and received research support from NIH/NCRR CTSA Program UL1TR001079-01.
Footnotes
Statement of Conflicts of Interest and Statement of Funding
Dr. Barber reports no disclosures.
References
- 1.Faul MXL, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. 2010:1–21. [Google Scholar]
- 2.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30(1):179–88, xi. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front Aging Neurosci. 2013;5:41. doi: 10.3389/fnagi.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J, et al. Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol. 2011;82(1):115–23. doi: 10.1016/j.ijpsycho.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, et al. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. NeuroImage. 2012;59(1):511–8. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkatesan UM, Dennis NA, Hillary FG. Chronology and chronicity of altered resting-state functional connectivity after traumatic brain injury. Journal of neurotrauma. 2014 doi: 10.1089/neu.2013.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borich M, Babul AN, Huang PH, Boyd L, Virji-Babul N. Alterations in resting state brain networks in concussed adolescent athletes. Journal of neurotrauma. 2014 doi: 10.1089/neu.2013.3269. [DOI] [PubMed] [Google Scholar]
- 9.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53(1):303–17. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31(38):13442–51. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32(11):1825–35. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, et al. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 2013;70(7):845–51. doi: 10.1001/jamaneurol.2013.38. [DOI] [PubMed] [Google Scholar]
- 17.Pandit AS, Expert P, Lambiotte R, Bonnelle V, Leech R, Turkheimer FE, et al. Traumatic brain injury impairs small-world topology. Neurology. 2013;80(20):1826–33. doi: 10.1212/WNL.0b013e3182929f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134(Pt 8):2233–47. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- 19.Sours C, Zhuo J, Janowich J, Aarabi B, Shanmuganathan K, Gullapalli RP. Default mode network interference in mild traumatic brain injury - a pilot resting state study. Brain Res. 2013;1537:201–15. doi: 10.1016/j.brainres.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, et al. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265(3):882–92. doi: 10.1148/radiol.12120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Bie HMA, Boersma M, Adriaanse S, Veltman DJ, Wink AM, Roosendaal SD, et al. Resting-state networks in awake five- to eight-year old children. Hum Brain Mapp. 2012;33(5):1189–201. doi: 10.1002/hbm.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51(1):156–67. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiebel SJ, Poline JB, Friston KJ, Holmes AP, Worsley KJ. Robust smoothness estimation in statistical parametric maps using standardized residuals from the general linear model. NeuroImage. 1999;10(6):756–66. doi: 10.1006/nimg.1999.0508. [DOI] [PubMed] [Google Scholar]
- 26.Denckla MB. Revised Neurological Examination for Subtle Signs (1985). Psychopharmacol Bull. 1985;21(4):773–800. [PubMed] [Google Scholar]
- 27.Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB, Mahone EM. Effects of gender and age on motor exam in typically developing children. Developmental neuropsychology. 2007;32(1):543–62. doi: 10.1080/87565640701361013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahone EM, Powell SK, Loftis CW, Goldberg MC, Denckla MB, Mostofsky SH. Motor persistence and inhibition in autism and ADHD. J Int Neuropsychol Soc. 2006;12(5):622–31. doi: 10.1017/S1355617706060814. [DOI] [PubMed] [Google Scholar]
- 29.Christensen AL. Luria's neuropsychological investigation. Spectrum; New York: 1975. [Google Scholar]
- 30.Watson RT, Miller BD, Heilman KM. Nonsensory neglect. Annals of neurology. 1978;3(6):505–8. doi: 10.1002/ana.410030609. [DOI] [PubMed] [Google Scholar]
- 31.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System: Technical Manual. Harcourt Assessment Company; San Antonio, TX: 2001. [Google Scholar]
- 32.Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. Multi-Health Systems; North Tonawanda: 1997. [Google Scholar]
- 33.Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. Motor “dexterity”?: Evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 2012;22(1):51–9. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaule V, Tremblay S, Theoret H. Interhemispheric control of unilateral movement. Neural Plast. 2012;2012:627816. doi: 10.1155/2012/627816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. NeuroImage. 2013;67:283–97. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, et al. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci U S A. 2011;108(27):11241–5. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Addamo PK, Farrow M, Hoy KE, Bradshaw JL, Georgiou-Karistianis N. The effects of age and attention on motor overflow production--A review. Brain Res Rev. 2007;54(1):189–204. doi: 10.1016/j.brainresrev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Shumskaya E, Andriessen TM, Norris DG, Vos PE. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology. 2012;79(2):175–82. doi: 10.1212/WNL.0b013e31825f04fb. [DOI] [PubMed] [Google Scholar]
- 39.Caeyenberghs K, Wenderoth N, Smits-Engelsman BC, Sunaert S, Swinnen SP. Neural correlates of motor dysfunction in children with traumatic brain injury: exploration of compensatory recruitment patterns. Brain. 2009;132(Pt 3):684–94. doi: 10.1093/brain/awn344. [DOI] [PubMed] [Google Scholar]
- 40.Jantzen KJ, Steinberg FL, Kelso JA. Brain networks underlying human timing behavior are influenced by prior context. Proc Natl Acad Sci U S A. 2004;101(17):6815–20. doi: 10.1073/pnas.0401300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasahara M, Menon DK, Salmond CH, Outtrim JG, Taylor Tavares JV, Carpenter TA, et al. Altered functional connectivity in the motor network after traumatic brain injury. Neurology. 2010;75(2):168–76. doi: 10.1212/WNL.0b013e3181e7ca58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keightley ML, Saluja RS, Chen JK, Gagnon I, Leonard G, Petrides M, et al. A functional magnetic resonance imaging study of working memory in youth after sports-related concussion: is it still working? Journal of neurotrauma. 2014;31(5):437–51. doi: 10.1089/neu.2013.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babcock L, Byczkowski T, Wade SL, Ho M, Mookerjee S, Bazarian JJ. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013;167(2):156–61. doi: 10.1001/jamapediatrics.2013.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]