Abstract
FGF-2 stimulates preosteoblast replication, and knockout of the Fgf2 gene in mice resulted in osteopenia with age, associated with decreased Wnt–β-Catenin signaling. In addition, targeted expression of FGF-2 in osteoblast progenitors increased bone mass in mice via Wnt-β-Catenin signaling. We posited that diminution of the intrinsic proliferative capacity of human mesenchyme-derived progenitor cells (HMDPCs) with age is due in part to reduction in FGF-2. To test this hypothesis HMDPCs from young (27~38), middle aged (47~56) and old (65~76) female human subjects were isolated from bone discarded after orthopedic procedures. HMDPCs cultures were mostly homogeneous with greater than 90% mesenchymal progenitor cells, determined by fluorescence-activated cell sorting. There was a progressive decrease in Fgf2 and FGFR1 mRNA and protein in HMDPCs with age. Since FGF-2 activates β-catenin, which can enhance bone formation, we also assessed its age-related expression in HMDPCs. An age-related decrease in total-β-Catenin mRNA and protein expression was observed. However there were increased levels of p-β-Catenin and decreased levels of activated-β-Catenin in old HMDSCs. FGF-2 treatment increased FGFR1 and β-Catenin protein, reduced the level of p-β-Catenin and increased activated-β-Catenin in aged HMDPCs. In conclusion, reduction in Fgf2 expression could contribute to age-related impaired function of HMDPCs via modulation of Wnt-β-catenin signaling.
Keywords: Aging, Endogenous Fibroblast growth factor-2, Fibroblast growth factor Receptor, β-catenin, Human mesenchyme-derived progenitor cells
INTRODUCTION
The fibroblast growth factor family of ligands is the product of 22 genes that signal via low affinity heparin sulfate proteoglycans and high affinity tyrosine kinase FGF receptors [Coutu and Galipeau, 2011; Hurley MM, 2002]. Fibroblast growth factor 2 (FGF-2) is synthesized by many cell types including osteoblasts and chondrocytes [Hurley MM, 2002]. It can have multiple effects on bone by stimulating osteoblast replication and proliferation as well as osteoclast differentiation [Hurley MM, 2002]. We recently reported that FGF-2 stimulates the proliferation of mesenchymal-derived progenitor cells from aging mouse and human bone [Kuhn et al., 2013; Ou et al., 2010]. We also found that disruption of the Fgf2 gene in mice resulted in decreased bone mineral density (BMD) and bone mineral content (BMC) [Montero et al., 2000] that became more pronounced with age [Xiao et al., 2010]. FGF-2 expression is increased by other factors including parathyroid hormone (PTH) and bone morphogenetic protein 2 (BMP-2) that play important roles in bone homeostasis [Hurley MM, 2002]. Furthermore endogenous FGF-2 is necessary for the maximal bone anabolic response to both PTH [Sabbieti et al., 2009] and BMP-2 [Fei et al., 2013; Naganawa et al., 2008].
Few studies on age-induced changes in FGF-2 and resulting effects on bone can be found. Primary osteoblasts derived from adult (60-day-old) rat calvaria demonstrated decreased cell proliferation and differentiation compared to primary osteoblasts from juvenile (2-day-old) rat calvaria with a differential expression of the FGF2 isoforms with age [Cowan et al., 2003]. In response to FGF-2 the osteoblasts from juvenile bone increased expression of more matrix proteins than FGF-2 stimulated adult progenitors, however, adult osteoblasts were more differentiated at the start. [Cowan et al., 2003]. Another study, comparing FGF gene expression in skin of young and old mice revealed a decline in both the number of wound-responsive FGF and FGFR genes in skin of aged mice [Komi-Kuramochi et al., 2005].
We previously reported that human osteoblasts synthesize FGF-2 [Sobue et al., 2001], however there are no reported studies on changes in FGF-2 expression in human mesenchyme-derived progenitor cells (HMDPCs) with age. In this study we used HMDPCs isolated from adult human bone discarded after orthopedic surgery. Initially, these isolated cells are multipotential and have been shown to have the ability to become either osteoblasts, adipocytes or chondrocytes and, therefore, were coined “multipotential mesenchymal progenitor cells from adult bone” [Song et al., 2005; Tuli et al., 2003]. Interestingly when differentiated in culture, these cells have been shown to be identical in phenotype to mesenchymal stem cells obtained from bone marrow aspirates [Robey and Termine, 1985; Sakaguchi et al., 2004]. Our studies from 102 subjects have also shown that HMDPCs from elderly female subjects grown in differentiation medium have a reduced capacity to make bone relative to age-matched cells from male subjects and young female subjects [Zhang et al., 2004]. Our hypothesis is that an age-induced change in FGF-2 and FGF receptors and/or signaling contributes to a decrease in bone-forming capacity.
FGF2 signals via FGF receptor tyrosine kinases (FGFR) to affect multiple downstream signaling pathways including Wntless and Int-1 (Wnt) [Fei et al., 2013]. The Wnt signaling pathway plays an important role in the maintenance of bone mass in rodents and humans [Krishnan et al., 2006] and in regulating FGF-2 mediated bone formation in mice [Fei et al., 2011; Xiao et al., 2009] However, it is not clear if the decreased bone mass with aging in humans is related in part to FGF-2 reduction in HMDPCs. Furthermore, there are also no reported studies that examined whether expression of Wnt-related genes such as β-catenin correlated with changes in FGF-2 expression in HMDPCs with age. β-catenin is found in the cytoplasm of many types of cells. When β-catenin levels rise high enough to saturate all binding sites in the cytoplasm, it translocates to the nucleus where it engages numerous transcription factors to regulate gene transcription, in particular genes involved in bone formation.
MATERIALS AND METHODS
Isolation and Culture of Human Mesenchyme-Derived Progenitor Cells
HMDPCs were isolated from adult human bone as previously described [Ou et al., 2010]. Briefly, bone discarded from orthopedic procedures (Institutional Review Board exempt) was obtained from young (32, 27, 38, 24, 35, 29 -year-old), middle aged (47, 47, 56, 42, 45-year old) and older (66, 66, 72, 76, 69, 65-year-old) female subjects. After the bones were aseptically harvested, the soft tissues were scraped off, and the bone was chopped into 1-2 mm fragments. Bone fragments were transferred to 100-mm dishes and were cultured in Dulbecco’s minimum essential medium/F-12 (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Gibco/Invitrogen, Rockville, MD), 100 U/mL penicillin-G (Sigma-Aldrich, St. Louis, MO), and 100 m g/mL streptomycin (Sigma). Culture medium was replaced two times per week. HMDPCs started to migrate from the bone chips after 1–2 weeks. On days 12~14, bone fragments were removed, and at near confluence, the cells were passaged using 0.25% trypsin and 0.1% EDTA (GIBCO) in PBS. Cells were re-plated to expand the cultures and then plated for gene expression, protein and immunofluorescence experiments. Cells at passage 2-3 were used for the experiments and were switched to heat inactivated FBS for the studies. For the experiments without FGF-2 treatment, cells were plated at 30000 cells/cm2 in 6-well culture dishes with 10% heat-inactivated fetal bovine serum (HIFBS; Gibco/Invitrogen, Rockville, MD). Whenever cells reached the stage of contact inhibition they were extracted for assay. Cells from young subjects reached contact inhibition at about 14 days, cells from old subjects reached contact inhibition at about 21 days. For the experiments with FGF-2 treatment, cells were plated at 50000 cells/cm2 on a coverslip in 6-well culture dishes. When they reached about 50-80% confluence at about day 10 after plating, cells were serum-deprived overnight, and then treated with vehicle for FGF-2.
RNA isolation, RT-PCR and PrimePCR Array
Total RNA was extracted from the dishes by using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) as previously described [Fei et al., 2011]. For real time quantitative reverse transcription-PCR analysis, RNA was reverse-transcribed by the Super-ScriptTM first-strand synthesis system for reverse transcription-PCR. Quantitative PCR (qPCR) was performed using the QuantiTectTM SYBR Green PCR kit (BIO-RAD Laboratories Inc. Hercules, CA) on a MyiQTM instrument. β-actin was used as an internal reference. Primers used for qPCR are shown in Supplemental Table 1.
For PrimePCR Array analysis, PrimePCR 96-well Custom Plates were purchased from Bio-Rad (BIO-RAD Laboratories Inc. Hercules, CA). RT samples were loaded on plates and run on MyiQ-96 instrument. Data was analyzed using PrimePCR™ Analysis Software #GeneStudy_1.0.030.1023.
Protein isolation and Western Blot
Total protein was extracted from dishes using a RIPA buffer (Cell Signaling Technology, Inc. Danvers, MA). After separation on 4-15% gradient gels, proteins were transferred to ImmobilonTM (BIO-RAD Laboratories Inc. Hercules, CA) and blocked in 5% nonfat milk. Membranes were incubated with the anti-FGF-2 (1:1000) (Santa Cruz Biotechnology, Inc. Dallas, Texas), anti-total-b -Catenin antibody (5 µg/ml) (BD Transduction Laboratory), anti-p-b-Catenin (Ser33/37/Thr41) antibody (Cell Signaling), or anti-activated-b-Catenin (Ser33/37/Thr41) antibody (Cell Signaling) for 1 h, washed, and then incubated with anti-rabbit IgG, HRP-linked secondary antibody (Cell Signaling Technology, Inc. Danvers, MA) or anti-mouse IgG, HRP-linked secondary antibody (GE Healthcare Life Sciences, Pittsburgh, PA) and washed. SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL) was used for detection.
Immunocytochemistry
Cells cultured on coverslip were serum deprived overnight and then the cells were treated with 1nM of FGF-2 for 30 min or 4 h. Then cells were briefly rinsed with PBS and fixed in 4% paraformaldehyde for 20 min. Cells were washed three times in PBS, permeabilized with 0.3% Triton X-100 for 30 min, and incubated with 0.5% bovine serum albumin diluted in PBS for 20 min. Finally, cells were incubated with anti FGFR1 (Flg, Santa Cruz) 1:50, or anti-total-b-Catenin antibody (BD Transduction Lab) at 5ug/ml, anti-p-b-Catenin (Ser33/37/Thr41) antibody (Cell Signaling), or anti-activated-b-Catenin (Ser33/37/Thr41) antibody (Cell Signaling) in PBS for 2 h at room temperature. After rinsing, cells were incubated with Texas Red-conjugated goat anti-rabbit IgG (Invitrogen, Grand Island, NY) or Texas Red-conjugated goat anti-mouse IgG (Invitrogen, Grand Island, NY). After washing, coverslips were mounted on slides and nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI). Cells were photographed with a Nikon fluorescence microscope. Photomicrographs were evaluated and analyzed with ImageJ software to count the number of positive/total cells.
Fluorescence-activated cell sorting (FACS)
Cells were trypsinized, washed in PBS containing 0.2% bovine serum albumin and 0.1% NaN3. Aliquots containing 106 cells were incubated with 100 µl of appropriately diluted antibodies and isotype control antibody for 30 min at 4 °C in the dark. The following monoclonal antibodies were used for human cellular surface staining: CD11b (APC), CD31 (Pacific Blue), CD34 (Fitc), CD44 (PerCP/Cy5.5), CD45 (Alexa Fluor 700), CD73 (PE), CD90 (Fitc), CD105 (PE-Cy7), CD146 (Alexa Fluor 647), CD166 (PE) and were purchased from eBioscience and Biolegend (San Diego, CA). Cells were fixed with 2% paraformaldehyde in PBS. Relative fluorescence intensities were determined on a 4-decade log scale by flow cytometric analysis, using an LSRII (Becton Dickinson, San Jose, CA). At least one to three hundred thousand cell events were collected per sample. Analysis was carried out with FlowJo software (Version7.6.5).
Statistical Analysis
The statistical analysis of the results was performed by ANOVA followed by Least Significant Difference (LSD) for Post Hoc Multiple Comparisons with the conventional 0.05 level considered to reflect statistical significance. At least three independent experiments from different isolations of HMDPCs from bones from at least three young, three middle aged and three old subjects were performed. Data are expressed as mean ± SEM unless otherwise specified.
RESULTS
Characterization of HMDPCs
Prior to the start of the aging studies, flow cytometric analysis was undertaken to assess the homogeneity of the HMDPCs population, and any changes in mesenchymal progenitor cell (MSC) markers with subsequent passage of the cells (Table 1). P0 describes the cells that grow out of the bone chips obtained from the subjects, and P1-P5 describes subsequent cell passages. The macrophage marker, CD11b, was 0.04% at P0 and then further decreased. At P0 the endothelial cell lineage marker, CD146, varied with each patient ranging from 0.8% to 32.5%, dropping to an average of 10.3% at P3 and 0.2% at P5. Similar to CD146, the MSC marker CD105, which is also associated with angiogenesis, initially differed in expression depending on the patient, but then rapidly increased with subsequent passages. CD105 is endoglin and is part of the TGF beta receptor complex, which has an important role in endochondral and intramembranous ossification and is expressed by osteoblasts [Chen et al., 2012]. MSC markers; CD90, CD44, CD166, and CD73 were greater than 83% at P1 and were more than 90% by P3. However, MSC markers varied among each other in expression from greater than 95% for CD166 and CD73 from P0-P5. Expression of hematopoietic markers, CD34, CD45 and CD31, were less than 0.1% for cells obtained from all subjects even at P0.
Table 1.
FACS analysis was performed on human mesenchyme-derived progenitor cells from human bone from passage 0 (P0), 1, 3 and 5. The following markers were assessed CD11b, CD146, CD105, CD90, CD44, CD166, CD34, CD45, CD31 and CD73 and their % in the culture are shown. Cells were obtained from three human samples of bone from 56 year old, 58 year old and 62 year old females, and were separately cultured and analyzed for their expression of each marker. Values are means ± SEM.
| P0 | P1 | P3 | P5 | |
|---|---|---|---|---|
| CD11b | 0.04±0.02 | 0.01±0.03 | 0.01±0.00 | 0.01±0.00 |
| CD146 | 12.0±5.95 | 10.3±0.75 | 10.3±0.75 | 0.20±0.06 |
| CD105 | 36.8±16.1 | 86.6±6.87 | 77.4±8.03 | 90.7±3.00 |
| CD90 | 82.7±3.23 | 94.7±1.04 | 88.7±1.56 | 92.1±2.19 |
| CD44 | 77.0±4.97 | 83.6±4.45 | 91.4±2.94 | 94.8±1.10 |
| CD166 | 95.1±1.56 | 98.7±0.52 | 99.4±0.06 | 99.4±0.12 |
| CD34 | 0.01±0.01 | 0.02±0.01 | 0.07±0.01 | 0.15±0.05 |
| CD45 | 0.50±0.19 | 0.03±0.01 | 0.02±0.01 | 0.02±0.00 |
| CD31 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 |
| CD73 | 98.9±0.06 | 99.17±0.00 | 99.8±0.17 | 99.7±0.00 |
Age-related changes in FGF2 mRNA and protein expression in HMDPCs
We assessed whether Fgf2 mRNA and protein expression in HMDPCs declined with age. Pooled data from three independent experiments with mean age of Young=32yo Middle aged=50yo, Old=68yo showed that Fgf2 mRNA expression was 53% lower in cells from the middle aged group (p<0.05), and 63% lower in cells from the old group (p<005) compared with the young group (Figure 1A). A representative Western blot showed progressive lower FGF2 protein in old versus young subjects (Figure 1B). Pooled data of Western blot analysis from five independent experiments showed FGF-2 protein expression was 59% lower in cells from the old group compared with the young group (p<005) (Figure 1C). Individual data points of Fgf2 and Fgfr1 mRNA for each group from different experiments showed the reproducibility for similar age ranges (Supplemental Figure 1).
Figure 1. Age-related changes in Fgf2 mRNA, protein and Fgfr1 mRNA expression in human mesenchyme-derived progenitor cells.
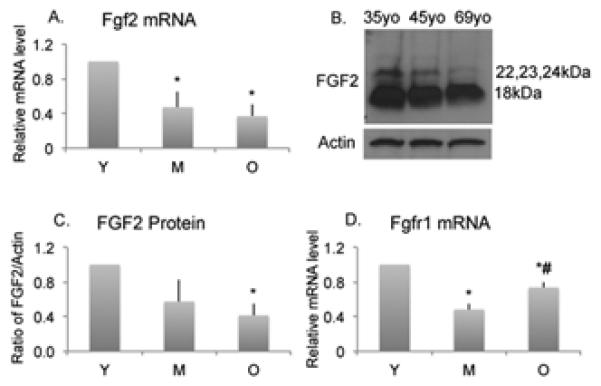
(A). Fgf2 mRNA expression was lower in cells from old (O) compared to young (Y) subjects. Data are from 3 independent experiments. Mean age of Young=32yo (32, 27, 38yo), Middle-aged=50yo (47, 47, 56yo), Old=68yo (66, 66, 72yo). (B). Representative Western blot showed FGF-2 protein expression was lower in cells from old (69yo) compared with middle aged (45yo) or young (35yo). (C). Quantitative analysis showed FGF-2 protein was lower in cells from old (O) compared with young subjects. Data are from independent experiments. Mean age of Young=31yo (32, 27, 38, 24, 35yo), Middle-aged=47yo (47, 47, 56, 42, 45yo), Old=70yo (66, 66, 72, 76, 79yo). (D). Fgfr1 mRNA expression was significantly lower in cells from middle aged (M) and old (O) compared to young (Y) subjects. Data are from 3 independent experiments. Mean age of Young=32yo (32, 27, 38yo), Middle aged=50yo (47, 47, 56yo), Old=68yo (66, 66, 72yo). Values are mean± SEM. * Significantly different in cells from Y subjects (p<0.05). # Significantly different from M subjects (p<0.05).
Age-related changes in FGF Receptor mRNA expression
Since FGF-2 signals via FGF receptors (FGFRs), we determined whether FGFR expression was also modulated with age. As shown in Figure 1D, pooled data from three independent experiments showed that Fgfr1 mRNA expression was 52% lower in cells from the middle aged group (p<0.05), and 27% lower in cells from the old group (p<0.05) compared with the young group. Interestingly, Fgfr1 mRNA was 53% higher in the old group compared with the middle-aged group (p<0.05). We also examined Fgfr2 and Fgfr3 but there were no significant differences between the groups (data not shown).
Effect of exogenous FGF-2 treatment on FGFR1 protein expression
Immunofluorescence staining was performed for FGFR1 protein in HMDPCs of all ages after treatment with vehicle or 1nM FGF-2 for 30 minutes or 4h. As shown in Figure 2A, basal expression of FGFR1 was lower in HMDPCs from old compared to young subjects. Quantitative analysis from three independent experiments. (Figure 2B) showed that basal level of FGFR1 protein was 49% lower in the old group compared with the young group (p<0.05). Treatment of HMDPCs from old subjects with FGF-2 for 30 minutes resulted in a 124% increase in FGFR1 protein immunostaining in the old-FGF2 group compared with the old-vehicle group (p<0.05), which was a greater percent increase than the response to FGF-2 in the other groups.
Figure 2. Effect of FGF-2 treatment on FGFR1 protein expression in human mesenchyme-derived progenitor cells.
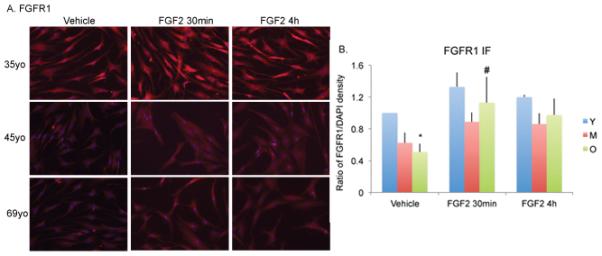
(A). Representative immunofluorescence staining for FGFR1 protein in HOBs after treatment with vehicle or 1nM FGF-2. Young=35yo, Middle aged=45yo, Old=69yo. Photomicrographs were taken at 200x. (B). Quantitative analysis shows FGFR1 expression was lower in vehicle-treated cells from old (O) subjects compared with young (Y) subjects. There was a significant increase in FGFR1 protein in FGF-2 treated cells in old (O) subjects at 30 min. Data are from 3 independent experiments. Mean age of Young=32yo (38, 24, 35yo), Middle-aged=48yo (56, 42, 45yo), Old=72yo (72, 76, 69yo). Values are mean±SEM. * Significantly different from corresponding Y (p<0.05). # Significantly different from corresponding vehicle group (p<0.05).
Age-related changes in total-β-Catenin mRNA and protein expression
Since we previously reported that knockout or overexpression of FGF-2 in murine osteoblast progenitors modulated β-catenin expression and bone formation, we examined whether age-related changes in FGF-2 expression in HMDPCs was associated with altered total-β-catenin expression. As shown in Figure 3A, data from three independent experiments showed β-Catenin mRNA expression was 63% lower in the cells from the old group compared with the young group (p<0.05). A representative Western blot analysis showed total-β-Catenin protein expression was lower in cells from the old group compared with young (Figure 3B). Quantitative analysis of Western blots from five independent experiments with mean age of Young=31yo (32, 27, 38, 24, 35yo), Middle aged=47yo (47, 47, 56, 42, 45yo), Old=70yo (66, 66, 72, 76, 79yo)(Figure 3C) showed that total-β-Catenin protein was 32% lower in the cells from the old group compared with the young group (p<0.05).
Figure 3. Age-related changes in β-catenin mRNA and protein expression in human mesenchyme-derived progenitor cells.

(A). β-catenin mRNA expression was lower in cells from old (O) compared to young (Y) subjects. Data are from 3 independent experiments. Mean age of Young=32 yo (32, 27, 38yo), Middle-aged=50yo (47, 47, 56yo), Old=68yo (66, 66, 72yo). (B). Total- β-catenin protein expression by Western blot showed the expression level was lower in old (O) subjects. Young=35yo, middle aged=45yo, old=69yo. (C). Quantitative analysis shows total-β-catenin protein expression was lower in cells from old (O) subjects compared with young. Data are from 5 independent experiments. Mean age of Young=31yo (32, 27, 38, 24, 35yo), Middle-aged=47yo (47, 47, 56, 42, 45yo), Old=70yo (66, 66, 72, 76, 79yo). * Significantly different from Y (p<0.05).
Effect of exogenous FGF-2 treatment on total-β-Catenin protein expression
β-Catenin labeling in HMDPCs after treatment with vehicle or 1nM FGF-2 for 30 min or 4 h was determined by immunofluorescence (Figure 4A). It appears that total-β-catenin labeling was lower in vehicle-treated HMDPCs from old subjects compared with young. FGF-2 resulted in an increase in total β-catenin staining in HMDPCs from all subjects after 30 min. Quantitative analysis (Figure 4B) from three independent experiments with mean age of Young=32yo (38, 24, 35yo), Middle aged=48yo (56, 42, 45yo), Old=72yo (72, 76, 69yo) shows that basal levels of total-β-catenin was 41% lower in the old-vehicle group compared with the young-vehicle group (p<0.05). Treatment with FGF-2 for 30 minutes resulted in a significant 76% increase in total-β-catenin in old-FGF2 group compared with the old-vehicle group (p<0.05) and nuclear accumulation of β-catenin in HMDPCs from old subjects (Figure 4C). By 4 h totalβ-catenin levels returned to baseline.
Figure 4. Effect of FGF-2 treatment on total-β-catenin protein expression in human mesenchyme-derived progenitor cells.
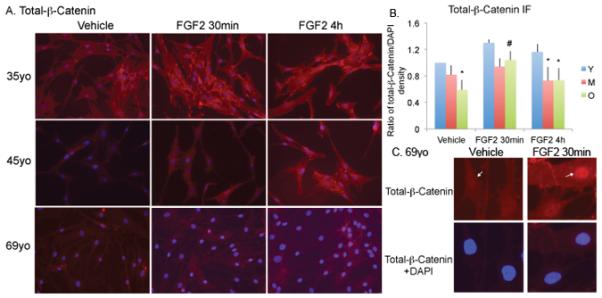
(A). Immunofluorescence staining for total-β-catenin in cells after treatment with vehicle or 1nM FGF-2. Young=35yo, middle aged=45yo, old=69yo. Photomicrographs are taken at 200x. (B). Quantitative analysis shows total-β-catenin expression was lower in old-vehicle compared with young-vehicle group. There was a significant increase in total-β-catenin protein in FGF-2-treated cells in old (O) subjects at 30 min and 4 h. Data are from 3 independent experiments. Mean age of Young=32yo (38, 24, 35yo), Middle-aged=48yo (56, 42, 45yo), Old=72yo (72, 76, 69yo). * Significantly different from corresponding Y (p<0.05). # Significantly different from corresponding vehicle (p<0.05). (C). High magnification image of immunofluorescence staining shows the nuclear accumulation of total-β-catenin (arrow) in old (69yo) cells after 30min treatment with FGF-2.
Age related changes and effect of exogenous FGF-2 treatment on p-β-catenin and activated-β-catenin protein expression
To determine whether changes in total-β-catenin observed were due to alteration in degradation of this protein, the status of p-β-catenin (Ser33/37/Thr41) protein, a destabilized form, and stabilized/activated form -β-catenin (Ser33/37/Thr41) protein, were investigated in cells from an old (65yo) subject and a young (29yo) subject. Figure 5 A, B and C shows the effect of FGF-2 treatment on p-β-catenin and activated-β-catenin protein expression. Western blot analysis (Figure 5A) and immunofluorescence staining (Figure 5B and C) showed that p-β-catenin protein expression was higher in cells from the old (65yo) subject compared with the young (29yo). FGF-2 treatment resulted in a decrease in p-β-catenin in cells from the old subject. In contrast, activated-β-catenin protein expression was lower in cells from the old (65yo) subject compared with the young (29yo). FGF-2 treatment resulted in an increase in activated-β-catenin in cells from both young and old subjects. These data suggest that there is less active-β-catenin in cells from the old subject due to greater breakdown of this protein. FGF-2 treatment reducedβ-catenin breakdown and therefore increased the amount of the active form of this protein.
Figure 5. Effect of FGF-2 treatment on p-β-catenin and activated-β-catenin protein expression in human mesenchyme-derived progenitor cells.
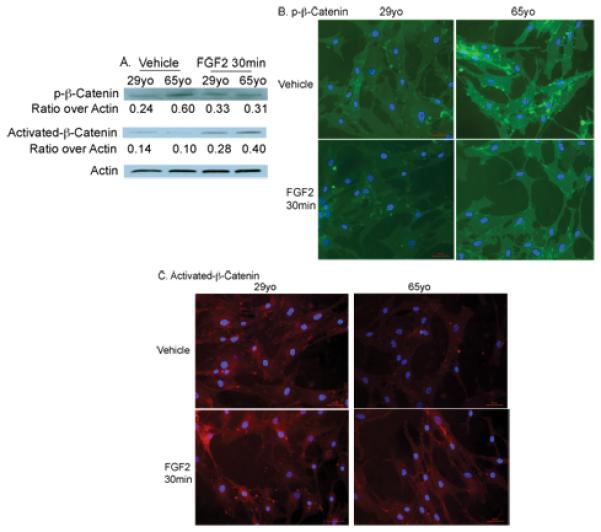
Western blot analysis (A) and immunofluorescence staining (IF) (B, C) showed that p-β-catenin protein expression was higher in cells from an old (65yo) subject compared with a young (29yo). FGF-2 treatment resulted in a decrease in p-β-catenin in cells from both young and old subjects. In contrast, activated-β-catenin protein expression was lower in cells from the old subject compared with the young. FGF2 treatment resulted in an increase in activated-p-β-catenin in cells from both young and old subjects.
Age related changes of Wnt cascade genes expression
We next examined whether there were age-related changes in Wnt cascade genes expression in HMDPCs by PrimePCR Array and qPCR. As shown in Supplemental Table 2, β-catenin (gene Ctnnb1), Lrp6, Wnt7b, Wnt16 expression was lower in cells from the old subject (65yo) compared with Young (29yo). The expression of the Wnt inhibitor Frzb (SFRP3) was 6-fold higher in old. QPCR analysis showed that Wnt 10b mRNA expression was similar between young and old (data not shown).
DISCUSSION
Maintenance of bone mass is a complex highly coordinated process involving proliferation and differentiation of osteoblast progenitors mediated in part by production of polypeptide growth factors and their receptors by the osteoblasts themselves [Ornitz and Marie, 2002; Robubi et al., 2014]. Among these polypeptide growth factors is FGF-2, which, we have shown to be a potent stimulator of proliferation and maintenance of ALCAM+ mesenchyme-derived osteoprogenitors from both aging human and mouse bones [Ou et al., 2010]. We hypothesized that the decrease in bone mass with age is due in part to reduction in FGF-2 expression. We report here that Fgf2 and FGFR1 mRNA and protein decreases with age in HMDPCs. Earlier studies examined Fgf2 and FGFR gene expression in skin of young and aged mice and reported that both were decreased with age [Komi-Kuramochi et al., 2005;] and FGF-2 expression was found to be decreased in hippocampus of aged rats [Salik et al., 2005], surprisingly a review of the literature revealed no reports of comparative age-dependent changes in expression of FGF-2 and its receptors in human osteoblasts or their precursors. Thus this report is the first comparison of FGF-2 expression in HMDPCs from young, middle-aged and old subjects.
Characterization of HMDPCs by FACS analysis demonstrated that the HMDPCs used for these studies were very homogenous for MSC markers. They expressed greater that 90% MSC markers and less than 0.1% hematopoietic and osteoclast markers. FACS is an inexact tool but our data demonstrates relative homogeneity for MSC markers by P3. Therefore, differences in FGF-2 and receptor expression can be attributed to age-related differences in mesenchyme-derived progenitor cell populations, and not differences due to contamination from other cell populations. Thus these age-related differences are found mainly in osteoblast progenitors from human bone, and may explain in part the reduced capacity for bone formation.
FGF-2 signals via high affinity tyrosine kinase FGFRs that play important roles in bone homeostasis [Hurley MM, 2002]. In particular FGFR1 and FGFR2 are important in FGF-2 downstream signaling in osteoblasts [Hurley MM, 2002]. Studies have shown that FGFR1 increases mesenchymal progenitor proliferation and survival and that it is also necessary for progenitor commitment to the osteoblast lineage [Coutu and Galipeau, 2011]. We examined gene expression for FGFR1, FGFR2 and FGFR3 and observed a decrease in FGFR1 mRNA and protein in HMDPCs from old compared with young subjects. Furthermore FGF-2 treatment caused a significant increase in FGFR1 in HMDPCs from old subjects within 30 minutes. This finding is interesting since we previously reported that mesenchymal derived progenitor cells from human bone were more sensitive to FGF-2 than mouse osteoblasts and demonstrated increased proliferation with lower doses of FGF-2 treatment [Ou et al., 2010] which could be due in part to increased FGFR1 signaling.
A single Fgf2 gene encodes for multiple protein isoforms [Abraham et al., 1986; Florkiewicz and Sommer, 1989]. In humans, there are high molecular weight (HMW) intracellular /nuclear isoforms of 22, 23 and 24 kDa that have nuclear localization sequences and a low molecular weight (18kDa, LMW) FGF2 protein that is exported from cells. The HMW isoforms of FGF2 are usually localized to the nucleus and function in an intracrine manner. In contrast although the LMW FGF2 isoform can accumulate in the nucleolus, it signals in an autocrine and paracrine manner. In the current studies we found the expression level of both HMW and LMW FGF2 isoforms along with signaling receptor FGFR1was lower in cells from old subjects. Interestingly, exogenously added FGF-2 resulted in an increase in Fgfr1 and activated-β-Catenin in old HMDPCs. This finding suggests that an FGF2/FGFR1 autocrine activation pathway may exist in cells from older humans to modulate Wnt/β-catenin autocrine signaling.
Although many downstream signaling pathways have been linked to age-related bone loss in humans [Roforth et al., 2014], the molecular mechanism of impaired osteoblast function is not fully defined [Roforth et al., 2014]. β-catenin is a cytoplasmic and nuclear protein encoded by the Ctnnb1 gene [Monroe et al., 2012]. It is a key link in numerous signaling cascades, including the “canonical Wnt pathway” that regulates bone mass [Krishnan et al., 2006; Monroe et al., 2012]. We previously reported that there is cross talk between Wnt-β-catenin and FGF-2 signaling pathways in murine osteoblasts [Fei et al., 2011]. Specifically in Fgf2 ko mice [Montero et al., 2000] that develop progressive decrease in bone mass and increased bone marrow adiposity with age, along with reduced osteoblast number and activity [Xiao et al., 2010], we observed a significant decrease in several Wnt related genes including Lrp-6, Wnt10b and β-catenin. There was also a significant decrease in the inactive form of Gsk3β and reduced accumulation β-catenin in nuclei of osteoblasts [Fei et al., 2011]. Interestingly, in the old HMDPCs in addition to reduced β-Catenin, we observed a decrease in other components of the Wnt signaling pathway including Lrp6, Gsk3β, Wnt3a, Wnt7b, and Wnt16 that are important in bone formation and maintenance of bone mass [Chen et al., 2014; Eijken et al., 2008; Monroe et al., 2012; Moverare-Skrtic et al., 2014].
We previously reported that treatment with exogenous FGF-2 increased β-catenin nuclear accumulation in Fgf2ko bone marrow stromal cell cultures [Fei et al., 2011]. In this report we examined whether the age-related decrease in FGF-2 expression in HMDPCs was associated with decreased β-catenin. We observed a progressive decrease in β-catenin mRNA and protein in HMDPCs with age, similar to our observations in mouse osteoblasts [Fei et al., 2011]. Treatment with FGF-2 increased β-catenin in HMDPCs in middle aged and old subjects.
In conclusion, reduction in endogenous Fgf2 expression and receptors could contribute to the age-related impairment of the bone forming capacity of mesenchyme-derived osteoblast progenitor cells via modulation of Wnt-β-catenin signaling.
Supplementary Material
Acknowledgments
FUNDING
This work was supported in part by the National Institute Of Dental & Craniofacial Research of the National Institutes of Health (NIDCR NIH) under award number R01DE021103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- Abraham JA, Whang JL, Tumolo A, Mergia A, Fiddes JC. Human basic fibroblast growth factor: nucleotide sequence, genomic organization, and expression in mammalian cells. Cold Spring Harbor symposia on quantitative biology. 1986;51:657–668. doi: 10.1101/sqb.1986.051.01.078. Pt 1. [DOI] [PubMed] [Google Scholar]
- Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem cells and development. 2009;18:955–968. doi: 10.1089/scd.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. International journal of biological sciences. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, Ruegg MA, Hall MN, Ma L, Long F. WNT7B promotes bone formation in part through mTORC1. PLoS genetics. 2014;10:e1004145. doi: 10.1371/journal.pgen.1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu DL, Galipeau J. Roles of FGF signaling in stem cell self-renewal, senescence and aging. Aging. 2011;3:920–933. doi: 10.18632/aging.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CM, Quarto N, Warren SM, Salim A, Longaker MT. Age-related changes in the biomolecular mechanisms of calvarial osteoblast biology affect fibroblast growth factor-2 signaling and osteogenesis. The Journal of biological chemistry. 2003;278:32005–2013. doi: 10.1074/jbc.M304698200. [DOI] [PubMed] [Google Scholar]
- Eijken M, Meijer IM, Westbroek I, Koedam M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Wnt signaling acts and is regulated in a human osteoblast differentiation dependent manner. Journal of cellular biochemistry. 2008;104:568–579. doi: 10.1002/jcb.21651. [DOI] [PubMed] [Google Scholar]
- Fei Y, Gronowicz G, Hurley MM. Fibroblast growth factor-2, bone homeostasis and fracture repair. Current pharmaceutical design. 2013;19:3354–3363. doi: 10.2174/1381612811319190002. [DOI] [PubMed] [Google Scholar]
- Fei Y, Xiao L, Doetschman T, Coffin DJ, Hurley MM. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. The Journal of biological chemistry. 2011;286:40575–40583. doi: 10.1074/jbc.M111.274910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz RZ, Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MM, Marie PJ, Florkiewicz RZ. Fibroblast growth factor (FGF) and FGF receptor families in bone. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Second Academic Press; San Diego, CA, USA: 2002. pp. 825–851. [Google Scholar]
- Komi-Kuramochi A, Kawano M, Oda Y, Asada M, Suzuki M, Oki J, Imamura T. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. The Journal of endocrinology. 2005;186:273–289. doi: 10.1677/joe.1.06055. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. The Journal of clinical investigation. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn LT, Ou G, Charles L, Hurley MM, Rodner CM, Gronowicz G. Fibroblast growth factor-2 and bone morphogenetic protein-2 have a synergistic stimulatory effect on bone formation in cell cultures from elderly mouse and human bone. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013;68:1170–1180. doi: 10.1093/gerona/glt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero A, Okada Y, Tomita M, Ito M, Tsurukami H, Nakamura T, Doetschman T, Coffin JD, Hurley MM. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. The Journal of clinical investigation. 2000;105:1085–1093. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Henning P, Liu X, Nagano K, Saito H, Borjesson AE, Sjogren K, Windahl SH, Farman H, Kindlund B, Engdahl C, Koskela A, Zhang FP, Eriksson EE, Zaman F, Hammarstedt A, Isaksson H, Bally M, Kassem A, Lindholm C, Sandberg O, Aspenberg P, Savendahl L, Feng JQ, Tuckermann J, Tuukkanen J, Poutanen M, Baron R, Lerner UH, Gori F, Ohlsson C. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nature medicine. 2014;20:1279–1288. doi: 10.1038/nm.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa T, Xiao L, Coffin JD, Doetschman T, Sabbieti MG, Agas D, Hurley MM. Reduced expression and function of bone morphogenetic protein-2 in bones of Fgf2 null mice. Journal of cellular biochemistry. 2008;103:1975–1988. doi: 10.1002/jcb.21589. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes & development. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- Ou G, Charles L, Matton S, Rodner C, Hurley M, Kuhn L, Gronowicz G. Fibroblast growth factor-2 stimulates the proliferation of mesenchyme-derived progenitor cells from aging mouse and human bone. The journals of gerontology. Series A, Biological sciences and medical sciences. 2010;65:1051–1059. doi: 10.1093/gerona/glq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey PG, Termine JD. Human bone cells in vitro. Calcified tissue international. 1985;37:453–460. [PubMed] [Google Scholar]
- Robubi A, Berger C, Schmid M, Huber KR, Engel A, Krugluger W. Gene expression profiles induced by growth factors in in vitro cultured osteoblasts. Bone & joint research. 2014;3:236–240. doi: 10.1302/2046-3758.37.2000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roforth MM, Fujita K, McGregor UI, Kirmani S, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S. Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone. 2014;59:1–6. doi: 10.1016/j.bone.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbieti MG, Agas D, Xiao L, Marchetti L, Coffin JD, Doetschman T, Hurley MM. Endogenous FGF-2 is critically important in PTH anabolic effects on bone. Journal of cellular physiology. 2009;219:143–151. doi: 10.1002/jcp.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004;104:2728–2735. doi: 10.1182/blood-2003-12-4452. [DOI] [PubMed] [Google Scholar]
- Salik E, Ercan F, Sirvanci S, Cetinel S, Onat F, San T. Effect of aging on the distribution of basic fibroblast growth factor immunoreactive cells in the rat hippocampus. Brain Res Bull. 2005;64:409–415. doi: 10.1016/j.brainresbull.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Sobue T, Zhang X, Florkiewicz RZ, Hurley MM. Interleukin-1 regulates FGF-2 mRNA and localization of FGF-2 protein in human osteoblasts. Biochemical and biophysical research communications. 2001;286:33–40. doi: 10.1006/bbrc.2001.5343. [DOI] [PubMed] [Google Scholar]
- Song L, Young NJ, Webb NE, Tuan RS. Origin and characterization of multipotential mesenchymal stem cells derived from adult human trabecular bone. Stem cells and development. 2005;14:712–21. doi: 10.1089/scd.2005.14.712. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG, Tuan RS. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem cells. 2003;21:681–693. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- Xiao L, Liu P, Li X, Doetschman T, Coffin JD, Drissi H, Hurley MM. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. The Journal of biological chemistry. 2009;284:3170–3182. doi: 10.1074/jbc.M804900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, Hurley MM. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone. 2010;47:360–370. doi: 10.1016/j.bone.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lewis CG, Aronow MS, Gronowicz GA. The effects of patient age on human osteoblasts' response to Ti-6Al-4V implants in vitro. Journal of orthopaedic research. 2004;22:30–38. doi: 10.1016/S0736-0266(03)00155-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


