Abstract
Women with endometriosis can suffer from decreased fecundity or complete infertility via abnormal oocyte function or impaired placental-uterine interactions required for normal pregnancy establishment and maintenance. Although AT-rich interactive domain 1A (SWI-like) (ARID1A) is a putative tumor suppressor in human endometrial cancers and endometriosis-associated ovarian cancers, little is known about its role in normal uterine function. To study the potential function of ARID1A in the female reproductive tract, we generated mice with a conditional knockout of Arid1a using anti-Müllerian hormone receptor 2-Cre. Female Arid1a conditional knockout mice exhibited a progressive decrease in number of pups per litter, with a precipitous decline after the second litter. We observed no tumors in virgin mice, although one knockout mouse developed a uterine tumor after pregnancy. Unstimulated virgin female knockout mice showed normal oviductal, ovarian, and uterine histology. Uteri of Arid1a knockout mice showed a normal decidualization response and appropriate responses to estradiol and progesterone stimulation. In vitro studies using primary cultures of human endometrial stromal fibroblasts revealed that small interfering RNA knockdown of ARID1A did not affect decidualization in vitro. Timed pregnancy studies revealed the significant resorption of embryos at Embryonic Day 16.5 in knockout mice in the third pregnancy. In addition to evidence of implantation site hemorrhage, pregnant Arid1a knockout mice showed abnormal placental morphology. These results suggest that Arid1a supports successful pregnancy through its role in placental function.
Keywords: ARID1A, female reproductive tract, fertility, genetically engineered mouse models, placenta, tumor suppressor, uterus
INTRODUCTION
The AT-rich interactive domain 1A (SWI-like) (Arid1a) gene encodes a large nuclear protein that is a key subunit of the multiprotein SWI/SNF chromatin-remodeling complex that is present in all eukaryotes. The SWI/SNF complex regulates gene expression for a variety of cellular processes, including differentiation, proliferation, DNA repair, and tumor suppression. The complex uses ATP as energy to mobilize nucleosomes, thereby modulating the accessibility of promoters to transcriptional activation or repression [1, 2].
Loss of ARID1A has been discovered in many human cancers, including renal cell carcinoma, gastric carcinoma, bladder tumors, pancreatic cancer, colon cancer, breast cancer, endometriosis-associated ovarian carcinomas, and uterine endometrioid carcinomas [3–20]. Considering gynecological cancer types, ARID1A mutation is a driver mutation for endometrial cancer, giving an advantage to epithelial cell growth in vitro [19]. Evidence from ovarian cancer cell lines supports the idea that ARID1A functions as a tumor suppressor by binding to tumor protein p53 (TP53) and regulating cyclin-dependent kinase inhibitor 1A (CDKN1A) [20].
Although ARID1A-inactivating mutations occur in a wide variety of tumors of the female reproductive tract in humans, inactivation of ARID1A alone is insufficient for tumor initiation in the female reproductive tract of mice [21, 22]. Studies using adenovirus-driven Cre to delete Arid1a in the ovarian surface epithelium showed that deletion of Arid1a alone is not sufficient for ovarian cancer formation. Rather, female reproductive tract tumor formation required additional mutation in phosphatase and tensin homolog (Pten) or phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (Pik3ca) [22, 23]. Studies using progesterone receptor (Pgr)-driven Cre to delete Arid1a in the uterus showed that deletion of Arid1a alone is not sufficient for endometrial cancer formation [21]. Although these studies suggest the potential cooperation between mutation of Arid1a and other tumor suppressors in tumorigenesis, little is known about the role of ARID1A in the normal female reproductive tract.
ARID1A is essential for mammalian development because deletion of Arid1a in traditional knockout mice causes embryonic lethality resulting from defects in mesoderm development and embryonic stem cell self-renewal, differentiation, and cell lineage decisions [24]. To overcome this early defect and study the global roles of ARID1A in mammals, others have developed Arid1a floxed alleles [24] for the conditional ablation of Arid1a, which has shed light on the role of Arid1a in regulating key genes in cardiac development [25]. Recently, we showed that deletion of Arid1a from the Pgr-positive cells of the uterus resulted in complete sterility with defective embryo implantation and uterine decidualization. The underlying mechanism of this sterility phenotype was from the effects of ARID1A and PGR on expression of Kruppel-like factor 15 (Klf15) and on uterine epithelial cell proliferation [21]. To study the broader role of Arid1a in the female reproductive tract, we used the anti-Müllerian hormone receptor 2 (Amhr2)-Cre mouse model [26], which has Cre recombinase inserted into the Amhr2 gene. Therefore, Cre is expressed in cells that express Amhr2, which include mesenchymal cells of the uterus such as uterine stromal and myometrial cells, oviductal cells, and somatic cells of the ovary such as surface epithelial and granulosa cells [26–30]. Expression of Amhr2 is detectable as early as the 8-cell stage [31], and its expression in the gonad is present beginning at Embryonic Day 12.5 [27]. Amhr2-Cre mice have been successfully used to study the roles of many genes in female reproduction and cancer, including vascular endothelial growth factor a (Vegfa) [32], miR-34c [33], signal transducer and activator of transcription 3 (Stat3) [34], transforming growth factor beta receptor 1 (Tgfbr1) [35], transformation protein related protein 53 (Trp53) [36–38], Pten [28, 36, 37, 39–42], Kirsten rat sarcoma viral oncogene homolog (Kras) [28, 36, 37, 43], beta catenin 1 (Ctnnb1) [29, 39, 44–47], tuberous sclerosis 1 (Tsc1) [48, 49], smoothened, frizzled class receptor (Smo) [50–53], adenomatous polyposis coli (Apc) [54], wingless-type MMTV integration site family, member 4 (Wnt4) [55], breast cancer 1, early onset (Brac1) [38], Dicer1 [42, 56–58], nuclear receptor subfamily 2, group F, member 2 (Nr2f2) [59], splicing factor 1 (Sf1) [60], activins [61], follistatin (Fst) [62], and Smads [63, 64]. Here, using Amhr2-Cre mice, we show that deletion of Arid1a leads to a progressive loss of fertility via placental disruption.
MATERIALS AND METHODS
Generation and Genotyping of Arid1a Conditional Knockout Mice
Arid1a conditional allele (Arid1aflox/flox) mice have been described previously [24] and were maintained in a C57BL/6J; 129S5/Brd mixed hybrid background. Arid1aflox/flox mice were bred to Amhr2cre/+ mice [26] to generate Arid1aflox/+;Amhr2cre/+ mice. Arid1aflox/+;Amhr2cre/+ male mice were crossed to Arid1aflox/flox female mice to generate final breeder pairs of Arid1aflox/flox;Amhr2cre/+ male mice and Arid1aflox/flox female mice. These final breeders were used to generate the experimental female mice: Arid1aflox/flox;Amhr2cre/+ mice (termed Arid1a conditional knockout [cKO] mice) and Arid1aflox/flox mice (termed control mice) (Supplemental Fig. S1A; Supplemental Data are available online at www.biolreprod.org). Arid1aflox/-;Amhr2cre/+ mice were not generated because previous studies show that Arid1a heterozygosity (Arid1a+/−) leads to embryonic lethality in mice, similar to homozygous deletion [24]. All mice were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under an approved protocol. The Baylor College of Medicine Genetically Engineered Mouse Core performed rederivation and initial breeding. Mice were genotyped at 12–14 days of postnatal life from tail biopsies by PCR analyses using specific primers [22, 65] (Supplemental Table S1 and Supplemental Fig. S1B). For the Arid1a floxed allele, the following conditions were used with Arid1a forward and reverse primers: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C (denaturation), 45 sec at 59°C (annealing), and 45 sec at 72°C (extension). For the Cre allele, the following conditions were used with Amhr2-Cre forward and reverse primers: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C (denaturation), 45 sec at 60°C (annealing), and 45 sec at 72°C (extension).
Fertility Analysis and Studies Involving Parous Uteri
To evaluate reproductive performance, 6-wk-old female control and Arid1a cKO mice were bred to wild-type C57BL/6J;129S5Brd hybrid male mice of proven fertility. The numbers of litters and pups were recorded over a 6-mo period. After the 6-mo fertility studies were completed, female mice were rested for 2 mo. Mice were then euthanized, and the parous uteri were fixed for histology.
Tissue Collection, Histological Analysis, and Timed Pregnancy
At times listed in experimental design below, mice were euthanized; reproductive organs were excised and fixed for histology or snap frozen for RNA or protein isolation. Uteri, implantation sites, and placentas were fixed in 4% paraformaldehyde (Sigma), and ovaries were fixed in 10% neutral buffered formalin (EMD Millipore). The Baylor College of Medicine Human Tissue Acquisition and Pathology Core performed tissue processing and paraffin embedding. Sections were cut at 5 μm and stained with hematoxylin (VWR) and eosin (VWR) or periodic acid-Schiff (Sigma) using standard techniques. Tissue and serum samples from timed pregnancies were obtained by mating Arid1a cKO and control female mice with intact wild-type C57BL/6;129S5/Brd hybrid male mice. The morning that the vaginal plug was observed was designated as 0.5 days postcoitus (dpc). Mice were euthanized on Day 4.5, 8.5, 9.5, or 16.5 of pregnancy, and the number of implantation sites was counted.
Steroid Hormone Treatment and Artificial Induction of Decidualization
Beginning at 6 wk of age, Arid1a cKO and control female mice underwent bilateral ovariectomy followed by rest for 2 wk. For steroid hormone responsive studies, mice were treated with either 1 mg progesterone (P4) for 6 h [66, 67] or 100 ng 17β-estradiol (E2) for three consecutive days [68]. Sesame oil was used as a vehicle control (all reagents from Sigma).
The artificial induction of uterine decidualization has been previously described [69]. Briefly, 2 wk after ovariectomy, Arid1a cKO and control female mice were primed with 100 ng E2 per day for 3 days, followed by 2 days of rest and then 3 daily injections of 1 mg P4 and 6.7 ng E2. Six hours after the last injection, the left uterine horn was stimulated by a scratch with a burred needle. Daily injections of 1 mg P4 and 6.7 ng E2 were continued for 5 days. Mice were euthanized on Day 5. The uteri were dissected, weighed, and fixed in 4% paraformaldehyde.
Superovulation
Superovulation experiments were carried out as previously described [70]. At 21 days of age, Arid1a cKO and control female mice were injected intraperitoneally with 5 IU equine chorionic gonadotropin (Calbiochem) for 46 h, followed by intraperitoneal injection with 5 IU human chorionic gonadotropin (Novarel; Ferring Pharmaceuticals). Mice were then bred to wild-type male mice with proven fertility. Mice were dissected at 4.5 dpc.
Serum Analysis
Adult Arid1a cKO and control female mice were anesthetized by isoflurane inhalation (Abbott Laboratories), and blood was collected in microtainer tubes (Becton Dickinson) by closed cardiac puncture. Serum was separated by centrifugation and stored at −20°C until use. The University of Virginia Ligand Assay and Analysis Core performed measurements of follicle stimulating hormone (FSH), luteinizing hormone (LH), anti-Müllerian hormone (AMH), E2, and P4 levels.
Immunohistochemical Staining
Three sections from each of three independent control and Arid1a cKO uteri or ovary were analyzed in parallel. Paraffin-embedded tissues were cut at 5 μm, mounted on silane-coated slides, deparaffinized, rehydrated in graded alcohol series, and boiled in citrate buffer, pH 6.0 (Abcam) prior to blocking with 10% normal goat serum and incubating in primary antibody diluted in 10% normal goat serum (Vector Laboratories) in phosphate-buffered saline (PBS), pH 7.5 (Life Technologies, Inc.) overnight at 4°C. Primary antibodies and conditions are listed in Supplemental Table S2. Sections were washed with PBS and incubated with the appropriate species-specific horseradish peroxidase-conjugated secondary antibody (2 μg/ml; Vector Laboratories) for 1 h at room temperature. Immunoreactivity was detected using the Vectastain Elite DAB kit (Vector Laboratories). Sections were then briefly counterstained with hematoxylin, dehydrated, and mounted. Slides were analyzed by light microscopy.
RNA Isolation and Real-Time Quantitative PCR Analysis
Total RNA was extracted using the mirVana microRNA isolation kit (Life Technologies, Inc.). After nucleic acid quantification on a NanoDrop ND-1000 (Thermo Scientific), RNA was treated with Turbo DNAse (Life Technologies, Inc.) according to the manufacturer's protocol. DNAse-treated RNA (1000 ng) was reverse transcribed in a 50 μl reaction using 250 U Superscript III reverse transcriptase (Life Technologies, Inc.) with random primers (Life Technologies, Inc.). Samples were diluted to 100 μl, and 2 μl was used for each quantitative PCR (qPCR) reaction. Real-time qPCR was performed on the ABI StepOnePlus using either predesigned TaqMan Gene Expression Assays (Life Technologies, Inc.) or custom primers designed using Primer Express software (Life Technologies, Inc.) for SYBR green (Supplemental Table S1). Levels of mouse ribosomal protein L13a (Rpl13a) or 18s were used as endogenous controls for mouse uterus samples as expression of Rpl13a and 18s were shown not to change in response to steroid hormone stimulation [66, 71]. Levels of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) were used for placenta as previous studies have shown this is a reasonable endogenous control for mouse placenta [72, 73]. Levels of human ribosomal protein L19 (RPL19) were used as endogenous controls for human samples [66, 74–76]. TaqMan PCR was performed using TaqMan universal PCR master mix (Life Technologies, Inc.), and PCR with custom primers was performed using SYBR Green PCR master mix (Life Technologies, Inc.) in a 10-μl reaction. The reaction conditions were as follows: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C (denaturation) and 1 min at 60°C (annealing/extension). Each sample was analyzed in duplicate or triplicate, and a nontemplate control (nuclease-free water) was included on each plate for each primer-probe set. All custom primers had an efficiency of 85%–110%. All SYBR green runs had dissociation curves to predict potential primer-dimers. The relative quantity of transcript was calculated using the 2−ΔΔCt method [77].
Institutional Review Board Approval, Collection of Human Tissues, Creation of Primary Cultures, and Transfection of Cells
All human tissues were collected under Baylor College of Medicine Institutional Review Board approval with written informed consent, and cultures were created as described previously [76]. Cells were transfected and underwent in vitro decidualization as described previously [78]. Briefly, 2 days before induction of in vitro decidualization, cells in 6-well plates were treated with Dulbecco-modified Eagle medium/F12 with 2% charcoal-stripped fetal bovine serum (Life Technologies, Inc.) containing 5 μl RNAiMax (Life Technologies, Inc.) with 100 nM of either nontargeting small interfering RNA (siNT) (ON-TARGETplus Non-targeting Control Pool, Thermo Scientific) or small interfering RNA targeting ARID1A (siARID1A) (ON-TARGETplus Human ARID1A small interfering RNA, Thermo Scientific) dissolved in Opti-MEM (Invitrogen). On the day of decidualization induction, which was designated as Day 0, an aliquot of cells for each treatment (siNT or siARID1A) was collected, and the remaining cells were treated with decidual medium containing 10−8 M E2, 10−6 M medroxyprogesterone acetate, and 50 μM 8-bromocyclic AMP in Opti-MEM with 2% charcoal-stripped fetal bovine serum and 1% antibiotic-antimycotic (Invitrogen) as described previously [76]. The medium was changed using freshly prepared decidual medium on Days 2 and 4. On Day 6, cells were washed with PBS, scraped from plates, and harvested in lysis buffer for RNA isolation as described above.
Statistical Analysis
Student t-test or two-way ANOVA with post hoc tests were performed using GraphPad Prism software version 6.0c. For the timed pregnancy study, chi-square analysis was performed. Data are presented as mean ± standard error of the mean, and P < 0.05 was considered statistically significant.
RESULTS
Arid1a cKO Mice Did Not Develop Ovarian Cancer
To generate female mice lacking expression of Arid1a in the reproductive tract, Arid1aflox/flox mice [24] and Amhr2cre/+ mice [26] that express Cre recombinase under the control of the Amhr2 promoter were obtained. Supplemental Figure S1A shows the detailed breeding strategy to generate Arid1aflox/flox; Amhr2cre/+ mice (termed Arid1a cKO mice) and Arid1aflox/flox mice (termed control mice). Previous studies show that similar strategies are successful in disrupting ARID1A function [21, 24, 25].
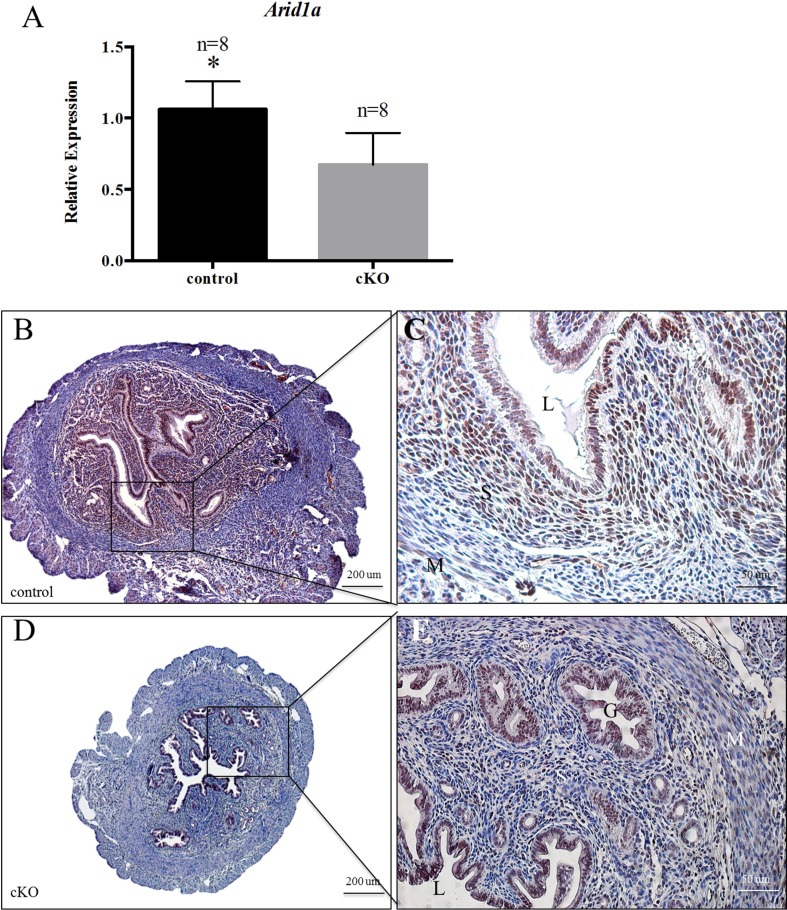
We found that female Arid1a cKO and control mice were born healthy. To confirm tissue-specific recombination and ablation of Arid1a, we performed qPCR and immunohistochemical staining on tissues from virgin 12-wk-old female mice. Quantitative PCR revealed a 1.58-fold decrease (n = 8, Student t-test, P <0.05) in Arid1a in whole uteri of adult Arid1a cKO mice compared with control mice (Fig. 1A). This sizeable but incomplete ablation of Arid1a in the uterus may be due to the lack of Arid1a deletion in the uterine epithelium. Next, we used immunohistochemistry to examine the cell type-specific expression of ARID1A. In uteri from virgin control female mice, ARID1A was expressed in the nuclei of uterine luminal and glandular epithelial cells, uterine stromal cells, and myometrial cells (Fig. 1, B and C). In uteri from virgin Arid1a cKO female mice, there was a lack of ARID1A staining in the uterine stroma but observable staining in uterine luminal and glandular epithelial cells (Fig. 1, D and E). This cell type-specific depletion of ARID1A in the uterus is consistent with previous work showing AMHR2 expression in uterine stromal cells [30]. Thus, as expected, ablation of Arid1a in the uterus of cKO mice was cell type specific.
FIG. 1.
Deletion of Arid1a in the uterine stroma of Arid1a cKO mice. Quantitative PCR showed that uteri from virgin 12-wk-old cKO mice showed decreased expression of Arid1a compared with control mice (A). Comparison of immunohistochemical staining for ARID1A in virgin 12-wk-old control (B, C) and cKO (D, E) mice. Statistical significance (*P < 0.05) was calculated by Student t-test. Relative expression of Arid1a to 18s; G, glandular epithelium; L, lumen; M, myometrium; S, uterine stroma. Bars = 200 μm (B, D) and 50 μm.
Amhr2-Cre should lead to recombination in somatic cells of the ovary, including granulosa cells and ovarian surface epithelial cells [26, 28]. In virgin 12-wk-old female control mice, ARID1A was detected in ovarian theca cells, in somatic cells of ovarian follicles at all stages of follicular development, and in ovarian surface epithelial cells (Supplemental Fig. S2, A and B). In unstimulated virgin female Arid1a cKO mice, there was decreased ARID1A staining in granulosa cells and ovarian surface epithelial cells (Supplemental Fig. S2, C and D). Previous work had shown that deletion of Pten and Dicer using Amhr2-Cre lead to high-grade serous ovarian cancers that arose in the oviduct [42]. ARID1A was detected in nuclei of the columnar epithelium of the oviduct in both virgin control (Supplemental Fig. S2, E and F) and Arid1a cKO mice (Supplemental Fig. S2, G and H).
Because loss of ARID1A in epithelial cells is a common feature of human endometriosis-associated ovarian cancers [9, 11], we performed long-term survival studies with virgin female control and Arid1a cKO mice (n = 20 per genotype). We observed no differences (P = 0.575) between genotypes in body weight over time (Supplemental Fig. S3). No control or Arid1a cKO mice were euthanized due to disease. Furthermore, at 1 yr of age, both virgin control and virgin Arid1a cKO mice had normal reproductive tract histology (data not shown). These results indicate that virgin female Arid1a cKO mice did not develop ovarian cancer.
Arid1a cKO Mice Exhibited a Progressive Decline in Fertility
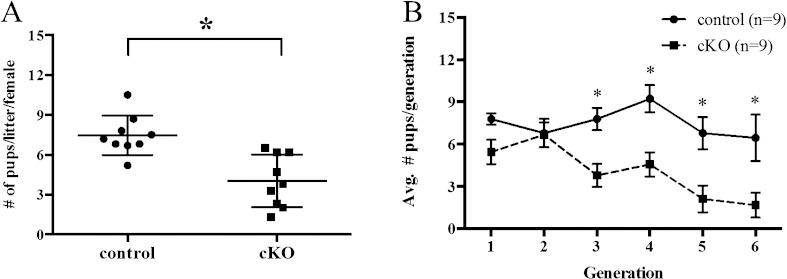
To evaluate fertility, female control and Arid1a cKO mice (n = 9 per genotype) were housed with wild-type males for 6 mo. Arid1a cKO mice were less fertile than control mice, with significantly fewer pups per litter (Student t-test, P < 0.05; Fig. 2A) and significantly fewer litters per month (cKO: 0.7 ± 0.3; control: 1.1 ± 0.1; Student t-test, P < 0.05). Litter size was significantly smaller in Arid1a cKO mice (4.0 ± 1.9 pups per litter) compared with control female mice (7.5 ± 1.5 pups per litter; Student t-test, P < 0.001). Furthermore, this subfertility worsened over time. Although the total number of pups per generation was lower for Arid1a cKO mice than for control mice across all generations (two-way ANOVA, P = 0.0064), there was a progressive decline after the second pregnancy (Fig. 2B). In addition, three out of nine Arid1a cKO mice were infertile by 5 mo of age, whereas all control mice were still fertile at this age, thus demonstrating the importance of Arid1a in pregnancy.
FIG. 2.
Arid1a cKO mice exhibited progressive subfertility. The reproductive ability of mice was tracked over a 6-mo breeding period. Arid1a cKO mice had fewer pups per litter than control mice (A). Conditional knockout mice showed a progressive decline in fertility as evidenced by a lower total number of pups per generation (B). Statistical significance (*P < 0.05) was calculated by Student t-test (A) or two-way ANOVA (B).
Arid1a cKO Mice Had Low Penetrance of Uterine Tumors after Pregnancy
After the fertility experiments, parous female mice were dissected at 8 mo of age to evaluate the reproductive tract. Control mice had grossly normal reproductive tracts, with no uterine or ovarian tumors (Supplemental Fig. S4A and data not shown). Parous control mice also had normal uterine histology, with similar ARID1A expression as virgin female mice (Supplemental Fig. S4B). Parous Arid1a cKO mice had normal uterine histology, with similar ARID1A expression as virgin female mice (Supplemental Fig. S4D). However, of the nine parous Arid1a cKO mice, one mouse had a uterine tumor, although both ovaries, both oviducts, and the opposite uterine horn in this mouse were grossly and histologically normal (Supplemental Fig. S4, A and D, and data not shown). Histological analysis showed that the tumor was a benign smooth muscle tumor with complete ablation of ARID1A in both the uterine stroma and epithelium (Supplemental Fig. S4C). In the normal horn, ARID1A expression was detected in the nuclei of glandular and luminal epithelial cells (Supplemental Fig. S4D), similar to its expression in virgin Arid1a cKO uteri. Overall, other than one benign uterine tumor, parous Arid1a cKO and virgin Arid1a cKO female mice had normal histology of female reproductive tract with no difference in ARID1A expression or localization.
Virgin Arid1a cKO Mice Had Normal Reproductive Tracts, Gonadotropin Levels, and Steroid Hormone Responses
Amhr2-Cre deletes the target allele in mesenchymal cells of the uterus, oviduct, and ovary, all of which may contribute to the subfertility phenotype observed in Arid1a cKO mice. Previous in vivo models of subfertility with Amhr2-Cre have shown defects in virgin or primigravid reproductive tract such as Stat3 [34], Kras [37], Pten [41], Wnt4 [55], and Nr2f2 [59]. Therefore, we undertook a systematic approach to examine the contribution of Arid1a deletion in each organ system in virgin female mice.
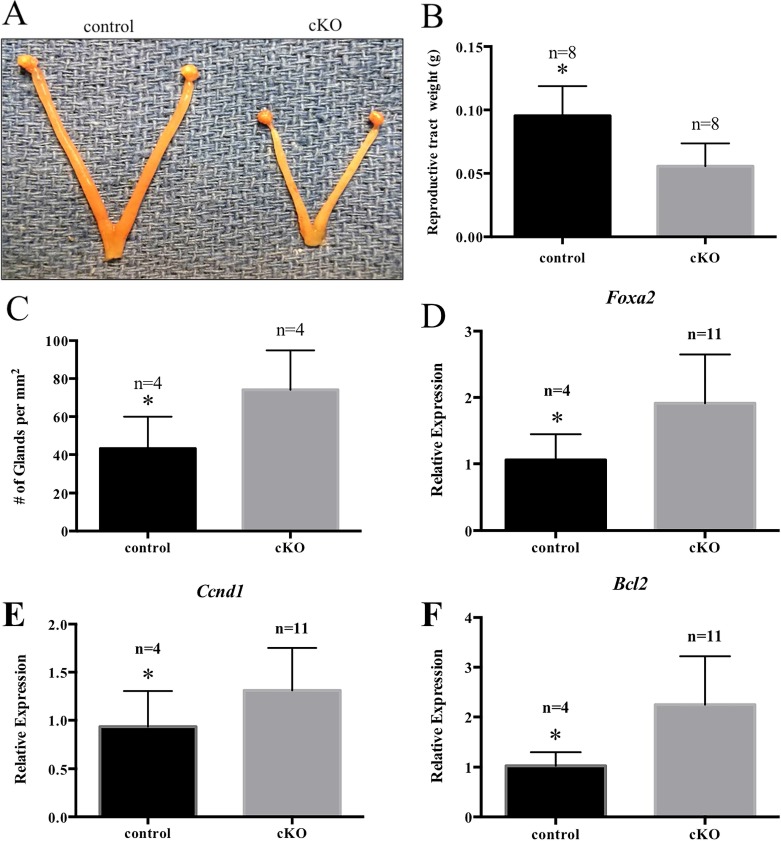
To characterize the uterus of Arid1a cKO mice, virgin mice were euthanized at 12 wk of age. Gross observation showed that the uterus of Arid1a cKO mice was smaller than that of control mice (Fig. 3A), and measurement of uterine weight revealed significantly lighter uteri in Arid1a cKO mice than in control mice (n = 8, Student t-test, P < 0.05; Fig. 3B). Furthermore, Arid1a cKO mice had significantly more uterine glands per mm2 of surface area encapsulated by the myometrium than control mice (n = 4, P < 0.05; Fig. 3C), as well as a 2-fold increase in levels of Foxa2 (n > 4, P < 0.05; Fig. 3D), a marker of glandular epithelium [79]. Previous work shows a critical role of Tgfbr1 in uterine smooth muscle development [80, 81], but we observed no differences between genotypes in levels of Tgfbr1 (data not shown). Both cyclin D1 (CCND1) and B-cell CLL/lymphoma 2 (BCL2) are regulated by ARID1A in human non-small cell lung cancer [82]. We further investigated molecular changes by measuring levels of the cell cycle pathway- and apoptosis-related genes (Ccnd1) and (Bcl2) in virgin uteri. We found a 1.4-fold (n > 4, P < 0.05) and 2.2-fold (n > 4, P < 0.05) increase in Ccnd1 and Bcl2 levels in unstimulated virgin adult Arid1a cKO uteri, respectively (Fig. 3, E and F). However, there was no difference in caspase-3 or Ki-67 staining in unstimulated virgin Arid1a cKO and control uteri (data not shown).
FIG. 3.
Uteri from virgin Arid1a cKO mice were smaller. Uterine horns from 12-wk-old Arid1a cKO mice were shorter than those in control mice (A). Reproductive tracts from 12-wk-old cKO mice weighed less than those in control mice (B). Uteri from cKO mice displayed an increased number of uterine glands (C), consistent with increased levels of Foxa2 expression as revealed by qPCR (D). Uteri from 12-wk-old cKO mice showed up-regulation of Ccnd1 (E) and Bcl2 (F). Statistical significance (*P < 0.05) was calculated by Student t-test. Relative Gene expression was relative to Rpl13a.
Histological analysis demonstrated that at 4, 12, and 52 wk of age, the morphology and histology of virgin ovaries was similar between control and Arid1a cKO mice (Supplemental Fig. S5, A–F). When superovulation was induced in immature mice (n = 23 per genotype), virgin control and Arid1a cKO mice showed similar histology and numbers of corpora lutea (Supplemental Fig. S5, G–L). Furthermore, when we quantified serum AMH levels as a measure of ovarian reserve, we found no significant difference between virgin control (15.64 ± 2.321 ng/ml, n = 9) and virgin Arid1a cKO (12.93 ± 2.268 ng/ml, n = 8; Student t-test, P = 0.179) mice.
To examine gonadotropin hormone levels, we measured FSH and LH serum hormone levels of unstimulated, randomly cycling adult Arid1a cKO and control mice. We observed no significant differences between virgin control and Arid1a cKO mice in FSH levels (control: 5.758 ± 0.625 ng/ml, n = 12; cKO: 7.270 ± 1.789 ng/ml, n = 10; Student t-test, P = 0.402) or LH levels (control: 0.406 ± 0.133 ng/ml, n = 12; cKO: 0.222 ± 0.0633 ng/ml, n = 10; Student t-test, P = 0.253). To evaluate steroid hormone production, we measured serum E2 levels in virgin adult unstimulated, randomly cycling mice. There were no significant differences in E2 levels between control (11.62 ± 7.437 pg/ml, n = 11) and Arid1a cKO (5.200 ± 1.078 pg/ml, n = 10; P = 0.419) mice.
In primigravid mice, at 4.5 dpc, P4 levels in Arid1a cKO pregnant mice (7.641 ± 2.296 ng/ml, n = 12) did not differ from those in control pregnant mice (14.57 ± 4.571 ng/ml, n = 11; P = 0.426), indicating normal luteal function. When Arid1a was deleted from the uterine epithelium using Pgr-Cre, there was a significant loss of Pgr expression as well as loss of expression of P4 responsive genes [21]. Therefore, we more closely examined steroid hormone receptor and steroid hormone response at the molecular level. Levels of Pgr (n > 4, P = 0.194) and estrogen receptor 1 (Esr1) (n > 4; Student t-test, P = 0.817) were similar between unstimulated virgin adult control and Arid1a cKO mice (Supplemental Fig. S6, A and B). To determine whether the uteri of Arid1a cKO mice show an altered response to steroid hormones, virgin unstimulated mice were ovariectomized and treated with E2 for 3 days or P4 for 6 h [67, 83]. The uteri from virgin Arid1a cKO females underwent a 5.2-fold increase in gross weight in response to E2, similar to the 5.8-fold increase observed for virgin control uteri (n = 6, Student t-test, P = 0.3468; Supplemental Fig. S6, C and D). Both control and Arid1a cKO uteri showed a significant increase in the estrogen responsive genes mucin 1 (Muc1), lactotransferrin (Ltf), and leukemia inhibitory factor (Lif) levels after E2 treatment (Supplemental Fig. S6, E–G). Furthermore, both virgin control and Arid1a cKO uteri showed a significant increase in expression of epithelial and stromal P4 responsive genes, Indian hedgehog (Ihh), cytochrome P450, family 26, subfamily A, polypeptide 1 (Cyp26a1), interleukin 13 receptor, alpha 2 (Il13ra2), heart and neural crest derivatives expressed transcript 2 (Hand2), and homeobox A10 (Hoxa10) levels in response to P4 (Supplemental Fig. S6, H–L). These results indicate that virgin Arid1a cKO uteri respond appropriately to E2 and P4.
Arid1a cKO Uteri Showed a Normal Decidualization Response
To further investigate the subfertility phenotype, ovariectomized virgin control and Arid1a cKO mice were treated with hormones, and their uterus was mechanically stimulated to induce decidualization. Gross and histological morphology of the decidual and control horn were similar between virgin control and Arid1a cKO uteri (Supplemental Fig. S7, A–D). In addition, the ratio of decidualized to control horn weight was equivalent in control and Arid1a cKO uteri (n = 7; Student t-test, P = 0.1748; Supplemental Fig. S7E).
Primary cultures of human endometrial stromal fibroblasts (hESCs) have the ability to undergo in vitro decidualization, and this culture system has been used to demonstrate the importance of several factors in uterine decidualization, such as LIF [84], transient receptor potential cation channel, subfamily C, member 1 (TRPC1) [85], transcription factor 23 (TCF23) [86], uterine activing like kinase 2 (ALK2) [78], bone morphogenetic protein 2 (BMP2) [87], and WNT4 [88]. Previous work has shown that ARID1A is deficient in hESCs from women with endometriosis [21] and that in vitro decidualization of hESCs from women with endometriosis have a blunted in vitro decidualization response [89]. To investigate the role of ARID1A in decidualization, we performed small interfering RNA knockdown of ARID1A in hESCs from women without endometriosis and then exposed cells to conditions for in vitro decidualization. HESCs transfected with siARID1A showed a 4.7-fold decrease in ARID1A compared with hESCs transfected with siNT (n = 7, Student t-test, P < 0.001; Supplemental Fig. S8A); hESCs transfected with siARID1A and then exposed to in vitro decidualization conditions showed changes in morphology similar to hESCs treated with siNT (data not shown). In addition, hESCs treated with siNT or siARID1A (n = 7) showed similar increases in levels of decidualization markers prolactin (PRL) (Student t-test, P = 0.1441), a ligand of the Wnt family (WNT4) (Student t-test, P = 0.2146), and insulin-like growth factor binding protein 1 (IGFBP1) (Student t-test, P = 0.2591; Supplemental Fig. S8, B–D). Therefore, knockdown of ARID1A in primary cultures of hESCs did not affect in vitro decidualization, similar to how deletion of Arid1a in stromal cells of the mouse uterus in vivo did not affect artificial decidualization (Supplemental Fig. S7).
Parous Arid1a cKO Mice Exhibited Reduced Fetal Viability
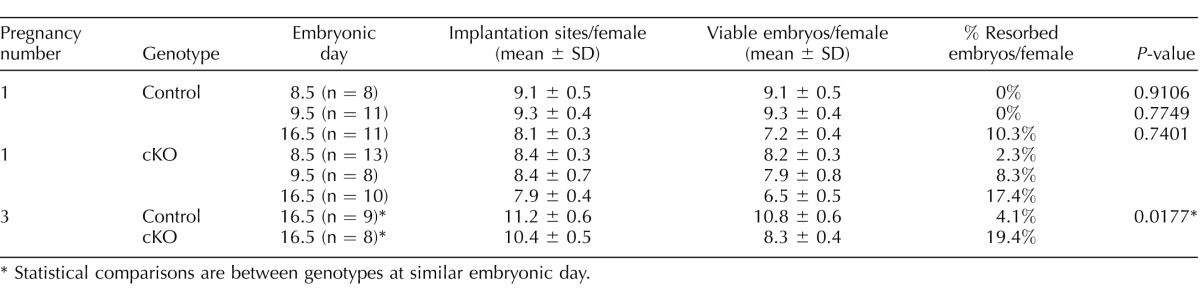
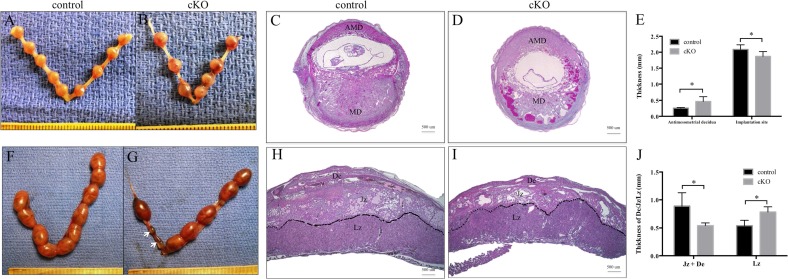
Our fertility studies showed a progressive decline in the number of pups per litter, although female mice maintained normal decidualization. To investigate the time during pregnancy at which this phenotype becomes apparent, we performed timed pregnancy studies by breeding female control and Arid1a cKO mice at 6 wk with wild-type male mice. During the first pregnancy, for control mice, the number of implantation sites/female was similar to the number of viable embryos/female on Embryonic Days 8.5 and 9.5, with no resorbed embryos (Table 1 and Fig. 4A). By Embryonic Day 16.5, control mice had 10.3% resorbed embryos (Table 1). During the first pregnancy for Arid1a cKO mice, fewer implantation sites were observed on Embryonic Days 8.5 and 9.5 (Table 1 and Fig. 4B) compared to control. Additionally, Arid1a cKO females had 17.4% resorbed embryos on Embryonic Day 16.5 (Table 1). However, these differences between genotypes were not statistically significant (Table 1). Given the increase in resorbed embryos at Day 8.5, we examined the histology of implantation sites at this time point in the first pregnancy. We found hemorrhage and disruption in the implantation sites of primigravid Arid1a cKO uteri on Embryonic Day 8.5 (Fig. 4, C and D). Examination of the antimesometrial myometrium showed a significant thickening in primigravid Arid1a cKO mice compared with control mice (Fig. 4E). Although the thickness of the implantation sites was less in primigravid Arid1a cKO mice than in control mice (Fig. 4E), there was no difference in the weight of viable implantation sites (data not shown).
TABLE 1.
Number of implantation sites and resorbed embryos.
Statistical comparisons are between genotypes at similar embryonic day.
FIG. 4.
Arid1a cKO mice showed increased embryo resorption during the third pregnancy. Gross morphology of primigravid control (A) and cKO (B) uteri on Embryonic Day 8.5. Conditional knockout implantation sites (D) on Embryonic Day 8.5 showed evidence of hemorrhage, which was not observed at control implantation sites (C). Conditional knockout antimesometrial decidua was thicker than those in control mice (E). Conditional knockout mice (G) showed more resorbed embryos (arrows) than control mice (F) on Embryonic Day 16.5 of the third pregnancy. Midsagittal sections of placental tissue obtained on Embryonic Day 16.5 from control (H) and cKO (I) mice of third pregnancy. Conditional knockout mice had a thinner junctional zone and decidua compared with control mice (J). AMD, antimesometrial decidua; MD, mesometrial decidua; De, decidua; Jz, junctional zone; Lz, labyrinthine zone; Statistical significance (*P < 0.05) was calculated by two-way ANOVA. Bars = 500 μm (C, D, H, I).
Given the subfertility that began after the second pregnancy, we examined implantation sites in the third pregnancy. We discovered a statistically significant increase in the number of resorbed embryos in multigravida Arid1a cKO mice (19.4%) compared to multigravida control mice (4.1%, chi-square; P = 0.0177, Table 1 and Fig. 4, F and G). We performed histological examination of placentas from Arid1a cKO and control mice. On Embryonic Day 16.5 of the third pregnancy, placentas from Arid1a cKO mice exhibited thinner junctional and decidual zones than control mice (Fig. 4, H–J).
Molecular Differences Existed Between Parous and Virgin Arid1a cKO Mice
To understand what factors contribute to the significant subfertility phenotype beginning with the third litter in Arid1a cKO mice, we studied multiparous females and their placentas. During the third pregnancy, P4 levels in multiparous Arid1a cKO pregnant mice at Embryonic Day 16.5 (23.34 ± 5.030 ng/ml, n = 7) did not differ from those in control pregnant mice (24.14 ± 5.886 ng/ml, n = 6; Student t-test, P = 0.9195), indicating normal luteal function in parous Arid1a cKO mice during pregnancy. Gross morphology and histology of parous ovaries was similar to virgin ovaries for both control and Arid1a cKO mice showing normal corpora lutea and follicular development (data not shown) further supporting normal luteal function in the first pregnancy and subsequent pregnancies. Finally, AMH levels were similar between virgin and multiparous Arid1a cKO mice (data not shown).
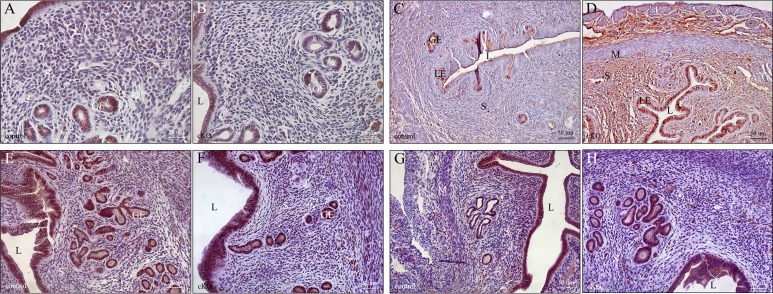
Recently, two different transforming growth factor beta-receptors, uterine activin like kinase 4 (ALK4) and uterine activin like kinase 5 (ALK5), were shown to significantly affect placental function when deleted in the uterus, leading to subfertility and sterility [90, 91]. Given the changes in placental morphology in parous Arid1a cKO females, we examined uterine differences at the molecular level for the underpinnings of this progressive subfertility phenotype. In uteri from 6-mo fertility studies, examination of ARID1A expression in the multiparous uterus showed a slight decrease in ARID1A expression. Expression of ARID1A was present in 50%–80% of epithelial cells in the uteri of Arid1a cKO female mice while expression of ARID1A was present in 80%–95% of epithelial cells in the uteri of control female mice (Fig. 5, A and B). Higher power magnification examination confirmed nuclear ARID1A staining in both Arid1a cKO uteri and control uteri with decreased expression in Arid1a cKO uteri (Supplemental Fig. S9). Previous work had shown that deletion of Arid1a with Pgr-Cre led to proliferation of uterine epithelial cells during early pregnancy, but not uterine stromal cells [21]. In parous uterus, we found that Arid1a cKO uterine epithelial and stromal cells showed increased proliferation compared to multiparous control (Fig. 5, C and D). However, there was no difference in PGR or ESR1 expression (Fig. 5, E–H). These data suggest that ARID1A plays a role in both epithelial and stromal cell proliferation in the uterus. Given the subtle changes in thickness of antimesometrium at Embryonic Day 8.5 in the first pregnancy and the more significant changes in placental morphology at Embryonic Day 16.5 in the third pregnancy, we focused our molecular studies on the placenta.
FIG. 5.
After 6-mo fertility studies, parous uteri from Arid1a cKO mice showed increased proliferation. ARID1A expression was present in >95% of epithelial cells from control (A) but only 50%–80% of epithelial cells from cKO (B) uteri. However, increased proliferation in both epithelium and stromal compartments was apparent in cKO (D) compared to control (C) parous uteri by Ki67 staining. Expression of ESR1was similar between control (E) and cKO (F) parous uteri. Similarly, expression of PGR was similar between control (G) and cKO parous uteri (H). GE, glandular epithelium; L, lumen; LE, luminal epithelium; M, myometrium; S, uterine stroma. Bars = 25 μm (A, B) and 50 μm (C–H).
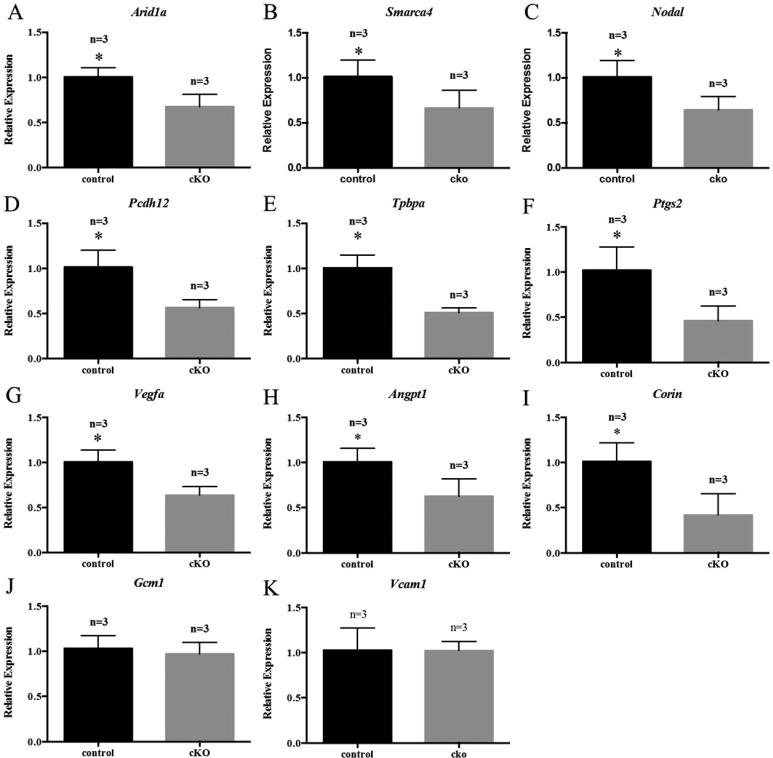
We discovered a 33% decrease in Arid1a expression in the placentas from Arid1a cKO mothers during the third pregnancy (Fig. 6A), likely an indirect effect and not an effect of knockout on the placenta. Similarly, there was a significant decrease in SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 4 (Smarca4), an ARID1A binding partner, expression (Fig. 6B). To gain insight into the molecular underpinnings of the placental phenotype, we examined the levels of molecules involved in placental structure and function. We discovered decreased expression of nodal growth differentiation factor (Nodal), a transforming growth factor beta-receptor ligand (Fig. 6C) in placentas from Arid1a cKO female mice. Additionally, placentas from Arid1a cKO mice exhibited decreased protocadherin 12 (Pcdh12), a marker of glycogen trophoblast cells, and trophoblast specific protein alpha (Tphpa), a marker of spongiotrophoblast cells compared to placentas from control female mice (Fig. 6, D and E). Compared with placentas from control female mice, placentas from Arid1a cKO female mice showed significantly lower levels of Ptgs2 (Fig. 6F), which is important for ectoplacental cone formation [92]. Levels of molecules involved in vasculogenesis (Vegfa) and angiogenesis (Angpt1) were also significantly decreased in placentas from cKO females (Fig. 6, G and H). Furthermore, levels of Corin, which is critical for trophoblast invasion [93], were significantly reduced in placentas from Arid1a cKO females (Fig. 6I). There were no differences between genotypes in levels of Gcm1, which is important for chorioallontoic branching [94], or Vcam1, which is critical for vascular endothelium formation [95] (Fig. 6, J and K).
FIG. 6.
Placentas from Arid1a cKO mice showed molecular dysregulation. Placental mRNA levels of Arid1a (A), Smarca4 (B), Nodal (C), Pcdh12 (D), Tpbpa (E), Ptgs2 (F), Vegfa (G), Angpt1 (H), and Corin (I) were decreased in cKO mice on Embryonic Day 16.5 of the third pregnancy. There was no difference in levels of Gcm1 (J) or Vcam1 (K). Statistical significance (*P < 0.05) was calculated by Student t-test. Gene expression was measured relative to Gapdh.
DISCUSSION
Many previous studies have focused on the role of ARID1A in cancer, particularly as a tumor suppressor or driver mutation in tumors of the female reproductive tract [9, 11, 13, 15, 20, 23]. Thus, our original hypothesis was that deletion of Arid1a with Amhr2-Cre would give rise to ovarian tumors in mice. Here, by conditionally deleting Arid1a in mesenchymal-derived cells of the female reproductive tract, we provide evidence that loss of Arid1a is not sufficient for tumor formation, suggesting that additional mutations are required for tumor formation in the mouse female reproductive tract. However, we show that ARID1A is important for normal placental morphology and fertility in parous female mice. Female mice with conditional deletion of Arid1a show a progressive decline in fertility with higher parity, with a loss of pups in the postimplantation phase beginning with the third pregnancy.
Studies suggest that ARID1A plays a key role in the proliferation of several types of cancers and is associated with genetic aberrations in cyclin-dependent kinases [96]. For colon cancer, non-small cell lung cancer, and gastric cancer, knockdown of ARID1A enhances cellular proliferation in vitro, and restoration of ARID1A expression suppresses cellular proliferation [82, 97, 98]. Likewise, we found that loss of Arid1a up-regulated Ccnd1 in the virgin uterus in vivo. Additionally, we also observed up-regulation of the pro-apoptotic gene Bcl2 in the Arid1a cKO uterus. Therefore, we believe that the balance of proliferation and apoptosis may preclude the development of cancer in virgin female Arid1a cKO mice. Other studies using adenovirus-driven Cre to delete Arid1a from the ovarian surface epithelium showed that Arid1a depletion is not sufficient to induce ovarian tumors in mice [22]. Additionally, our work deleting Arid1a in the uterus using Pgr-Cre did not develop endometrial tumors [21]. Consistently, we found that Arid1a deletion from the ovarian surface epithelium using Amhr2-Cre did not induce ovarian tumors in virgin female mice. Thus, we speculate that female reproductive tract cancers in mice require additional genetic hits along with loss of Arid1a to give rise to malignant tumors.
Interestingly, Arid1a cKO female mice had significant subfertility that became apparent after the second pregnancy. Translationally, this may be comparable to secondary infertility although most of our Arid1a cKO mice were not completely infertile. Male factors did not contribute because we were using fertility-proven intact wild-type male mice for our experiments. Given the deletion of Arid1a in the somatic cells of the ovary, ovarian dysfunction was critically evaluated. In primigravid and multigravida female Arid1a cKO mice, we found similar P4 levels as control as well as similar numbers of corpora lutea. AMH levels, a measure of ovarian reserve, were also similar between Arid1a cKO and control for both virgin and parous female mice. Gonadotropin levels were also similar between virgin Arid1a cKO and control female mice. Thus, we do not see an obvious luteal phase defect or other ovulatory dysfunction in either virgin or parous Arid1a cKO female mice. However, it remains possible that a more subtle luteal defect may contribute to the reduced pups per litter. Anatomically, Arid1a cKO had normal oviductal, uterine, and cervical histology for both virgin and parous female mice, and we found no gross or histological evidence of endometriosis. Thus, we do not believe that anatomical factors lead to secondary subfertility. In women undergoing an evaluation for secondary infertility, this would fall under the category of unexplained infertility and is typically thought to be caused by endometrial dysfunction. Only recently have genomewide profiling techniques been used to identify these subtle molecular changes [99–101].
Our virgin Arid1a cKO female mice had normal implantation, normal decidualization, and normal pregnancy outcomes in the first pregnancy. However, examination of parous uteri showed an increase in both epithelial and stromal proliferation (Fig. 5) with deletion of ARID1A that was not present in virgin uteri even at 1 yr of age (data not shown). Little has been published about the molecular differences between virgin and parous uteri. We can only speculate that the deletion of Arid1a led to indirect changes in gene expression that affected uterine remodeling after pregnancy. We speculate that subtle changes in the uterine stroma after remodeling postpartum lead to changes in placentation with subsequent pregnancies. However, significant work needs to be done to understand the subtle molecular changes between the parous and the virgin uterus before we can tease out the specific changes associated with loss of Arid1a.
Only after pregnancy, one female Arid1a cKO mouse was found to have a benign uterine tumor that exhibited a lack of ARID1A in epithelial cells. Given the low penetrance (one in nine) of tumors in parous uteri, a significantly larger number of female mice would need to be examined to determine the statistical significance of this result. Previous studies suggest that glandular and luminal epithelial cells of the uterus are regenerated from uterine stromal cells after pregnancy in Amhr2-Cre mice [102]. After one pregnancy, at most 50% of epithelial cells were regenerated from stroma in a process of mesenchymal epithelial transition (MET) [103]. Thus, depletion of ARID1A in the epithelium could result from Arid1a deletion in uterine stromal cells. This could account for the loss of ARID1A in the one female mouse with a tumor after pregnancy. Interestingly, recurrent copy number variants of Arid1a have recently been discovered in uterine leiomyomata tumors [104], which are the most translationally equivalent human disease to the one tumor. However, other parous Arid1a cKO female mice examined had expression of ARID1A in the epithelium and no tumors. Deletion of Arid1a with Pgr-Cre deleted Arid1a in the epithelium and did not result is similar tumors. However, these female mice were completely sterile and thus could not be studied in the multiparous state [21].
Indeed, we did see a decrease in ARID1A staining in the epithelium after repetitive pregnancy (Fig. 5 and Supplemental Fig. 9) in Arid1a cKO uteri, suggesting that MET is occurring in this model. Similar to published work, we saw at most 50% loss of ARID1A staining in epithelial cells after repetitive pregnancy. While previous work had only examined MET after 1 pregnancy, we have shown that MET remains after repetitive pregnancies and may be long lasting. The MET effect does not seem to be exponential with each pregnancy (i.e., loss of 50% with first pregnancy, loss of another 50% with second pregnancy). However, we cannot speculate as to whether repetitive pregnancy allows for regeneration of ARID1A-positive epithelial cells from ARID1A-positive epithelial cells based on our experimental time point. While a partial deletion of ARID1A does occur in the epithelial cells of the uterus in the Arid1a cKO mice, the phenotype is different than the complete deletion of ARID1A in the epithelial cells of the uterus of the Pgr-Cre female mice. However, it remains possible that the epithelial cell phenotype in the Amhr2-Cre female mice contributes to the reduced pups per litter.
Finally, multiparous Arid1a cKO female mice developed significantly abnormal placentas. The placenta is critical for proper fetal development. Translationally, placental abnormalities can lead to significant maternal morbidities such as pre-eclampsia and fetal complications such as fetal growth restriction or fetal demise. While we did not find gross differences in size of live born pups (data not shown), we did discover significant resorption of embryos at Embryonic Day 16.5 in the third litter of Arid1a cKO female mice (Table 1and Fig. 4). The placental morphology was abnormal (Fig. 4), and molecular markers of abnormal placental morphology confirmed these differences (Fig. 6). ARID1A is a critical player in chromatin remodeling, cellular proliferation, and gene regulation [2, 104–108]. Although multiple studies have focused on the role of epigenetic markers in chromatin remodeling in the placenta, little is known about the actual gene products involved in placental chromatin remodeling. One study shows that in vitro depletion of SMARCA4 (also known as BRG1), which is a critical member of the SWI/SNF chromatin-remodeling complex and a known protein-binding partner for ARID1A [109] that plays a key role in trophoblast stem cell self-renewal [110], has a similar effect on cellular proliferation as the depletion of ARID1A [111]. We found a decrease in both Arid1a and Smarca4 expression in placentas from multiparous Arid1a cKO females (Fig. 6). In the present study, the molecular markers examined in placentas from Arid1a cKO mice are at best suggestive of abnormal placental morphology. Although they do not provide a complete understanding of the molecular mechanism of the effect of Arid1a deletion, they suggest that loss of Arid1a in the uterus impairs placental function, evidenced by a subfertility phenotype, increased resorbed embryos, and a thinner junctional zone at implantation sites.
In conclusion, we found that Arid1a deletion alone is insufficient to induce tumorigenesis in the female reproductive tract of mice. ARID1A regulates embryo implantation and development of a functional placenta. Future studies of our Arid1a cKO mouse model should provide molecular insight into the delicate function of SWI/SNF-mediated chromatin remodeling in placental function.
Supplementary Material
ACKNOWLEDGMENT
We thank Monica Logan, A. Theresa Wittman, and Mahitha Ravi for their technical support.
Footnotes
This work was supported by The Liz Tilberis Scholarship Ovarian Cancer Research Fund through the Estate of Agatha Fort and an MD Anderson Cancer Center SPORE in Ovarian Cancer Developmental Research Project Award (grant number P50-CA83639) to S.M.H., and funding from the National Institutes of Health (grant number HL109054) to W.Z. The Pathology and Histology Core and the Genetically Engineered Mouse Core at Baylor College of Medicine also supported this project with funding from the National Institutes of Health (NCI P30-CA125123). This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreements U54HD028934 (to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, Charlottesville, VA) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. Presented in part at the 45th Annual Meeting of the Society for the Study of Reproduction, 12–15 August 2012, State College, Pennsylvania.
REFERENCES
- Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl NG, Jr, Patsialou A, Haines DS, Dallas PB, Beck GR, Jr, Moran E. The p270 (ARID1A/SMARCF1) subunit of mammalian SWI/SNF-related complexes is essential for normal cell cycle arrest. Cancer Res. 2005;65:9236–9244. doi: 10.1158/0008-5472.CAN-05-1225. [DOI] [PubMed] [Google Scholar]
- Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, Schmidt MK, Markowitz S, Yan H, Bigner D, Hruban RH, Eshleman JR, Iacobuzio-Donahue CA, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33:100–103. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee C, Suh JH, Chae JY, Kim HW, Moon KC. Decreased ARID1A expression correlates with poor prognosis of clear cell renal cell carcinoma. Hum Pathol. 2015;46:454–460. doi: 10.1016/j.humpath.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY, Lee SP, Ho SL, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–1223. doi: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- Balbas-Martinez C, Rodriguez-Pinilla M, Casanova A, Dominguez O, Pisano DG, Gomez G, Lloreta J, Lorente JA, Malats N, Real FX. ARID1A alterations are associated with FGFR3-wild type, poor-prognosis, urothelial bladder tumors. PLoS One. 2013;8:e62483. doi: 10.1371/journal.pone.0062483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo A, Cavallone L, Tuzmen S, Chabot C, Ferrario C, Hassan S, Edgren H, Kallioniemi O, Aleynikova O, Przybytkowski E, Malcolm K, Mousses S, et al. An integrated genomic approach identifies ARID1A as a candidate tumor-suppressor gene in breast cancer. Oncogene. 2012;31:2090–2100. doi: 10.1038/onc.2011.386. [DOI] [PubMed] [Google Scholar]
- Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, Steidl C, Wiseman SM, Gascoyne RD, Gilks B, Huntsman DG. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224:328–333. doi: 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- Wiegand KC, Hennessy BT, Leung S, Wang Y, Ju Z, McGahren M, Kalloger SE, Finlayson S, Stemke-Hale K, Lu Y, Zhang F, Anglesio MS, et al. A functional proteogenomic analysis of endometrioid and clear cell carcinomas using reverse phase protein array and mutation analysis: protein expression is histotype-specific and loss of ARID1A/BAF250a is associated with AKT phosphorylation. BMC Cancer. 2014;14:120. doi: 10.1186/1471-2407-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Wang TL, Ie-Ming S, Mao T-L, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum DJ, Adelaide J, Mamessier E, Finetti P, Lagarde A, Monges G, Viret F, Goncalves A, Turrini O, Delpero JR, Iovanna J, Giovannini M, et al. Genome profiling of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 2011;50:456–465. doi: 10.1002/gcc.20870. [DOI] [PubMed] [Google Scholar]
- Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, Chen E, Jeng YM, Wang TL, Ie-Ming S. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhao YL, Li Y, Fletcher JA, Xiao S. Genomic and functional evidence for an ARID1A tumor suppressor role. Genes Chromosomes Cancer. 2007;46:745–750. doi: 10.1002/gcc.20459. [DOI] [PubMed] [Google Scholar]
- Liang H, Cheung LW, Li J, Ju Z, Yu S, Stemke-Hale K, Dogruluk T, Lu Y, Liu X, Gu C, Guo W, Scherer SE, et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 2012;22:2120–2129. doi: 10.1101/gr.137596.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Cheung HW, Subramanian A, Sharifnia T, Okamoto M, Yang X, Hinkle G, Boehm JS, Beroukhim R, Weir BA, Mermel C, Barbie DA, et al. Highly parallel identification of essential genes in cancer cells. Proc Natl Acad Sci U S A. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier N, Yang W, Assem M, Panetta JC, Pei D, Paugh SW, Cheng C, Den Boer ML, Relling MV, Pieters R, Evans WE, Cheok MH. The SWI/SNF chromatin-remodeling complex and glucocorticoid resistance in acute lymphoblastic leukemia. J Natl Cancer Inst. 2008;100:1792–1803. doi: 10.1093/jnci/djn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Nagl NG, Jr, Flowers S, Zweitzig D, Dallas PB, Moran E. Expression of p270 (ARID1A), a component of human SWI/SNF complexes, in human tumors. Int J Cancer. 2004;112:636. doi: 10.1002/ijc.20450. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network , Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, et al. Integrated genomic characterization of endometrial carcinoma Nature 2013. 497 67 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan B, Wang TL, Ie-Ming S. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718–6727. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Yoo JY, Wang Z, Lydon JP, Khatri S, Hawkins SM, Leach RE, Fazleabas AT, Young SL, Lessey BA, Ku BJ, Jeong JW. ARID1A is essential for endometrial function during early pregnancy. PLoS Genet. 2015;11:e1005537. doi: 10.1371/journal.pgen.1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan B, Rahmanto YS, Wu RC, Wang Y, Wang Z, Wang TL, Ie-Ming S. Roles of deletion of Arid1a, a tumor suppressor, in mouse ovarian tumorigenesis J Natl Cancer Inst 2014. 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, Starmer J, Serber D, Yee D, Xiong J, Darr DB, Pardo-Manuel de Villena F, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6:6118. doi: 10.1038/ncomms7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A. 2008;105:6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei I, Gao X, Sham MH, Wang Z. SWI/SNF protein component BAF250a regulates cardiac progenitor cell differentiation by modulating chromatin accessibility during second heart field development. J Biol Chem. 2012;287:24255–24262. doi: 10.1074/jbc.M112.365080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol Cell Endocrinol. 2003;211:15–19. doi: 10.1016/j.mce.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Mullany LK, Fan HY, Liu Z, White LD, Marshall A, Gunaratne P, Anderson ML, Creighton CJ, Xin L, Deavers M, Wong KK, Richards JS. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene. 2011;30:3522–3536. doi: 10.1038/onc.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher E. Hung-Chang Yao H. Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev Biol. 2007;307:227–236. doi: 10.1016/j.ydbio.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Sargent KM, Lu N, Clopton DT, Pohlmeier WE, Brauer VM, Ferrara N, Silversides DW, Cupp AS. Loss of vascular endothelial growth factor A (VEGFA) isoforms in granulosa cells using pDmrt-1-Cre or Amhr2-Cre reduces fertility by arresting follicular development and by reducing litter size in female mice. PLoS One. 2015;10:e0116332. doi: 10.1371/journal.pone.0116332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Kim J, He L, Creighton CJ, Gunaratne PH, Hawkins SM, Matzuk MM. Functional analysis of miR-34c as a putative tumor suppressor in high-grade serous ovarian cancer. Biol Reprod. 2014;91:113. doi: 10.1095/biolreprod.114.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker RL, Watson LN, Robertson SA, Dunning KR, McLaughlin EA, Russell DL. Identification of sites of STAT3 action in the female reproductive tract through conditional gene deletion. PLoS One. 2014;9:e101182. doi: 10.1371/journal.pone.0101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Li S, Li Q. Uterine epithelial cell proliferation and endometrial hyperplasia: evidence from a mouse model. Mol Hum Reprod. 2014;20:776–786. doi: 10.1093/molehr/gau033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany LK, Liu Z, King ER, Wong KK, Richards JS. Wild-type tumor repressor protein 53 (Trp53) promotes ovarian cancer cell survival. Endocrinology. 2012;153:1638–1648. doi: 10.1210/en.2011-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Paquet M, Wang J, Lydon JP, DeMayo FJ, Richards JS. Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009;69:6463–6472. doi: 10.1158/0008-5472.CAN-08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Scangas G, Nitta M, He L, Xu X, Ioffe YJ, Aspuria PJ, Hedvat CY, Anderson ML, Oliva E, Karlan BY, Mohapatra G, et al. A role for BRCA1 in uterine leiomyosarcoma. Cancer Res. 2009;69:8231–8235. doi: 10.1158/0008-5472.CAN-09-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lague MN, Paquet M, Fan HY, Kaartinen MJ, Chu S, Jamin SP, Behringer RR, Fuller PJ, Mitchell A, Dore M, Huneault LM, Richards JS, et al. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29:2062–2072. doi: 10.1093/carcin/bgn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Jackson L, Besnard V, Whitsett J, Ellenson LH, Dey SK. Cell-specific conditional deletion of Pten in the uterus results in differential phenotypes. Gynecol Oncol. 2011;122:424–429. doi: 10.1016/j.ygyno.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lague MN, Detmar J, Paquet M, Boyer A, Richards JS, Adamson SL, Boerboom D. Decidual PTEN expression is required for trophoblast invasion in the mouse. Am J Physiol Endocrinol Metab. 2010;299:E936–E946. doi: 10.1152/ajpendo.00255.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A. 2012;109:3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany LK, Liu Z, Wong KK, Deneke V, Ren YA, Herron A, Richards JS. Tumor repressor protein 53 and steroid hormones provide a new paradigm for ovarian cancer metastases. Mol Endocrinol. 2014;28:127–137. doi: 10.1210/me.2013-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Wang Y, Bonilla-Claudio M, Martin JF, Gonzalez G, Taketo MM, Behringer RR. CTNNB1 in mesenchyme regulates epithelial cell differentiation during Mullerian duct and postnatal uterine development. Mol Endocrinol. 2013;27:1442–1454. doi: 10.1210/me.2012-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Patterson AL, Zhang L, Teixeira JM, Pru JK. Endometrial stromal beta-catenin is required for steroid-dependent mesenchymal-epithelial cross talk and decidualization. Reprod Biol Endocrinol. 2012;10:75. doi: 10.1186/1477-7827-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS. Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 2005;65:9206–9215. doi: 10.1158/0008-5472.CAN-05-1024. [DOI] [PubMed] [Google Scholar]
- Boerboom D, White LD, Dalle S, Courty J, Richards JS. Dominant-stable beta-catenin expression causes cell fate alterations and Wnt signaling antagonist expression in a murine granulosa cell tumor model. Cancer Res. 2006;66:1964–1973. doi: 10.1158/0008-5472.CAN-05-3493. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Yoshie M, Xie H, Sun X, Cha J, Ellenson LH, Dey SK. Conditional deletion of Tsc1 in the female reproductive tract impedes normal oviductal and uterine function by enhancing mTORC1 signaling in mice. Mol Hum Reprod. 2013;19:463–472. doi: 10.1093/molehr/gat016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Park JH, Tanwar PS, Kaneko-Tarui T, Mittal S, Lee HJ, Teixeira JM. Deletion of tuberous sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility. Endocrinology. 2012;153:404–416. doi: 10.1210/en.2011-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone FF, Ren Y, Cowan RG, Harman RM, Nikitin AY, Quirk SM. Dominant activation of the hedgehog signaling pathway alters development of the female reproductive tract. Genesis. 2012;50:28–40. doi: 10.1002/dvg.20786. [DOI] [PubMed] [Google Scholar]
- Ren Y, Cowan RG, Migone FF, Quirk SM. Overactivation of hedgehog signaling alters development of the ovarian vasculature in mice. Biol Reprod. 2012;86:174. doi: 10.1095/biolreprod.112.099176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman RM, Cowan RG, Ren Y, Quirk SM. Reduced signaling through the hedgehog pathway in the uterine stroma causes deferred implantation and embryonic loss. Reproduction. 2011;141:665–674. doi: 10.1530/REP-10-0468. [DOI] [PubMed] [Google Scholar]
- Ren Y, Cowan RG, Harman RM, Quirk SM. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol Endocrinol. 2009;23:711–723. doi: 10.1210/me.2008-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jia Y, Franken P, Smits R, Ewing PC, Lydon JP, DeMayo FJ, Burger CW. Anton Grootegoed J, Fodde R, Blok LJ. Loss of APC function in mesenchymal cells surrounding the Mullerian duct leads to myometrial defects in adult mice. Mol Cell Endocrinol. 2011;341:48–54. doi: 10.1016/j.mce.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Boyer A, Goff AK, Boerboom D. WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol Metab. 2010;21:25–32. doi: 10.1016/j.tem.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK. The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol. 2010;315:63–73. doi: 10.1016/j.mce.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Behringer RR. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev. 2009;76:678–688. doi: 10.1002/mrd.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ, Matzuk MM. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai MJ, Tsai SY. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci U S A. 2007;104:6293–6298. doi: 10.1073/pnas.0702039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–1619. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Tran M, Agno J, Li X, Brown CW, Kumar TR, Matzuk MM. Intraovarian activins are required for female fertility. Mol Endocrinol. 2007;21:2458–2471. doi: 10.1210/me.2007-0146. [DOI] [PubMed] [Google Scholar]
- Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol. 2004;18:953–967. doi: 10.1210/me.2003-0301. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol. 2006;20:1406–1422. doi: 10.1210/me.2005-0462. [DOI] [PubMed] [Google Scholar]
- Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev. 2005;71:480–488. doi: 10.1002/mrd.20338. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, Andreu-Vieyra CV, Kim TH, Jeong JW, Hodgson MC, Chen R, Creighton CJ, Lydon JP, Gunaratne PH, DeMayo FJ, Matzuk MM. Dysregulation of uterine signaling pathways in progesterone receptor-Cre knockout of dicer. Mol Endocrinol. 2012;26:1552–1566. doi: 10.1210/me.2012-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–3505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadgill DW, Lydon JP, Jeong JW, DeMayo FJ. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014;10:e1004451. doi: 10.1371/journal.pgen.1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7:82–86. doi: 10.1093/biolreprod/7.1.82. [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- Schroder AL, Pelch KE, Nagel SC. Estrogen modulates expression of putative housekeeping genes in the mouse uterus. Endocrine. 2009;35:211–219. doi: 10.1007/s12020-009-9154-6. [DOI] [PubMed] [Google Scholar]
- Sato TA, Gupta DK, Keelan JA, Marvin KW, Mitchell MD. Cytosolic phospholipase A(2)and 15-hydroxyprostaglandin dehydrogenase mRNA expression in murine uterine and gestational tissues during late pregnancy. Prostaglandins Leukot Essent Fatty Acids. 2001;64:247–251. doi: 10.1054/plef.2001.0267. [DOI] [PubMed] [Google Scholar]
- Rampon C, Bouillot S, Climescu-Haulica A, Prandini MH, Cand F, Vandenbrouck Y, Huber P. Protocadherin 12 deficiency alters morphogenesis and transcriptional profile of the placenta. Physiol Genomics. 2008;34:193–204. doi: 10.1152/physiolgenomics.00220.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayakannu T, Taylor AH, Willets JM, Brown L, Lambert DG, McDonald J, Davies Q, Moss EL, Konje JC. Validation of endogenous control reference genes for normalizing gene expression studies in endometrial carcinoma. Mol Hum Reprod. 2015;21:723–735. doi: 10.1093/molehr/gav033. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, Conti M, Giudice LC. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod. 2011;84:801–815. doi: 10.1095/biolreprod.110.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Clementi C, Tripurani SK, Large MJ, Edson MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP, Pangas SA, DeMayo FJ, Matzuk MM. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet. 2013;9:e1003863. doi: 10.1371/journal.pgen.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5:193–208. doi: 10.1016/j.modgep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Gao Y, Bayless KJ, Li Q. TGFBR1 is required for mouse myometrial development. Mol Endocrinol. 2014;28:380–394. doi: 10.1210/me.2013-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Duran S, Lydon JP, DeMayo FJ, Burghardt RC, Bayless KJ, Bartholin L, Li Q. Constitutive activation of transforming growth factor Beta receptor 1 in the mouse uterus impairs uterine morphology and function. Biol Reprod. 2015;92:34. doi: 10.1095/biolreprod.114.125146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu X, Zhang M, Bai X, Li H, Kan L, Niu H, He P. ARID1A is downregulated in non-small cell lung cancer and regulates cell proliferation and apoptosis. Tumour Biol. 2014;35:5701–5707. doi: 10.1007/s13277-014-1755-x. [DOI] [PubMed] [Google Scholar]
- Wang XN, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, Dimitriadis E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One. 2011;6:e25288. doi: 10.1371/journal.pone.0025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi Y, Hai L, Honda A, Horiuchi S, Tsujioka H, Ichikawa J, Inoue R. Critical role of TRPC1-mediated Ca(2)(+) entry in decidualization of human endometrial stromal cells. Mol Endocrinol. 2012;26:846–858. doi: 10.1210/me.2011-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Kovanci E, Creighton CJ, O'Malley BW, Demayo FJ, Lydon JP. A murine uterine transcriptome, responsive to steroid receptor coactivator-2, reveals transcription factor 23 as essential for decidualization of human endometrial stromal cells. Biol Reprod. 2014;90:75. doi: 10.1095/biolreprod.114.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282:31725–31732. doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009;80:105–114. doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Fullerton PT, Jr, Monsivais D, Clementi C, Su GH, Matzuk MM. Uterine activin-like kinase 4 regulates trophoblast development during mouse placentation. Mol Endocrinol. 2015;29:1684–1693. doi: 10.1210/me.2015-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Monsivais D, You R, Zhong H, Pangas SA, Matzuk MM. Uterine activin receptor-like kinase 5 is crucial for blastocyst implantation and placental development. Proc Natl Acad Sci U S A. 2015;112:E5098–E5107. doi: 10.1073/pnas.1514498112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Xie H, Yang J, Wang H, Bradshaw HB, Dey SK. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc Natl Acad Sci U S A. 2010;107:16887–16892. doi: 10.1073/pnas.1010892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang S, Nakano H, Simmons DG, Wang S, Kong S, Wang Q, Shen L, Tu Z, Wang W, Wang B, Wang H, et al. A positive feedback loop involving Gcm1 and Fzd5 directs chorionic branching morphogenesis in the placenta. PLoS Biol. 2013;11:e1001536. doi: 10.1371/journal.pbio.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse A, Martens N, Fernekorn U, Hallmann R, Butcher EC. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol Reprod. 2002;66:333–345. doi: 10.1095/biolreprod66.2.333. [DOI] [PubMed] [Google Scholar]
- Kato S, Schwaederle M, Daniels GA, Piccioni D, Kesari S, Bazhenova L, Shimabukuro K, Parker BA, Fanta P, Kurzrock R. Cyclin-dependent kinase pathway aberrations in diverse malignancies: clinical and molecular characteristics. Cell Cycle. 2015;14:1252–1259. doi: 10.1080/15384101.2015.1014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Fu L, Han Y, Li Q, Wang E. Decreased ARID1A expression facilitates cell proliferation and inhibits 5-fluorouracil-induced apoptosis in colorectal carcinoma. Tumour Biol. 2014;35:7921–7927. doi: 10.1007/s13277-014-2074-y. [DOI] [PubMed] [Google Scholar]
- Wang DD, Chen YB, Pan K, Wang W, Chen SP, Chen JG, Zhao JJ, Lv L, Pan QZ, Li YQ, Wang QJ, Huang LX, et al. Decreased expression of the ARID1A gene is associated with poor prognosis in primary gastric cancer. PLoS One. 2012;7:e40364. doi: 10.1371/journal.pone.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martinez-Conejero JA, Alama P, Garrido N, Pellicer A, Simon C. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99:508–517. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- Garrido-Gomez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simon C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril. 2013;99:1078–1085. doi: 10.1016/j.fertnstert.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simon C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–824. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7:e44285. doi: 10.1371/journal.pone.0044285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XS, Trojer P, Matsumura T, Treisman JE, Tanese N. Mammalian SWI/SNF–a subunit BAF250/ARID1 is an E3 ubiquitin ligase that targets histone H2B. Mol Cell Biol. 2010;30:1673–1688. doi: 10.1128/MCB.00540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buza N, Xu F, Wu W, Carr RJ, Li P, Hui P. Recurrent chromosomal aberrations in intravenous leiomyomatosis of the uterus: high-resolution array comparative genomic hybridization study. Hum Pathol. 2014;45:1885–1892. doi: 10.1016/j.humpath.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Dallas PB, Pacchione S, Wilsker D, Bowrin V, Kobayashi R, Moran E. The human SWI-SNF complex protein p270 is an ARID family member with non-sequence-specific DNA binding activity. Mol Cell Biol. 2000;20:3137–3146. doi: 10.1128/mcb.20.9.3137-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsker D, Patsialou A, Zumbrun SD, Kim S, Chen Y, Dallas PB, Moran E. The DNA-binding properties of the ARID-containing subunits of yeast and mammalian SWI/SNF complexes. Nucleic Acids Res. 2004;32:1345–1353. doi: 10.1093/nar/gkh277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl NG, Jr, Zweitzig DR, Thimmapaya B, Beck GR, Jr, Moran E. The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer Res. 2006;66:1289–1293. doi: 10.1158/0008-5472.CAN-05-3427. [DOI] [PubMed] [Google Scholar]
- Nagl NG, Jr, Wang X, Patsialou A, Van Scoy M, Moran E. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J. 2007;26:752–763. doi: 10.1038/sj.emboj.7601541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Arakawa N, Hirano H. Mass spectrometric analysis of the phosphorylation levels of the SWI/SNF chromatin remodeling/tumor suppressor proteins ARID1A and Brg1 in ovarian clear cell adenocarcinoma cell lines. J Proteome Res. 2014;13:4959–4969. doi: 10.1021/pr500470h. [DOI] [PubMed] [Google Scholar]
- Kidder BL, Palmer S. Examination of transcriptional networks reveals an important role for TCFAP2C, SMARCA4, and EOMES in trophoblast stem cell maintenance. Genome Res. 2010;20:458–472. doi: 10.1101/gr.101469.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Mei P, Zhang C, Chen F, Li C, Pan Z, Liu H, Zheng J. BRG1 is a prognostic marker and potential therapeutic target in human breast cancer. PLoS One. 2013;8:e59772. doi: 10.1371/journal.pone.0059772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.