Abstract
Anatomical, molecular, and physiological interactions between astrocytes and neuronal synapses regulate information processing in the brain. The fruit fly Drosophila melanogaster has become a valuable experimental system for genetic manipulation of the nervous system and has enormous potential for elucidating mechanisms that mediate neuron glia interactions. Here, we show the first electrophysiological recordings from Drosophila astrocytes and characterize their spatial and physiological relationship with particular synapses. Astrocyte intrinsic properties were found to be strongly analogous to those of vertebrate astrocytes, including a passive current-voltage relationship, low membrane resistance, high capacitance, and dye-coupling to local astrocytes. Responses to optogenetic stimulation of glutamatergic pre-motor neurons were correlated directly with anatomy using serial electron microscopy reconstructions of homologous identified neurons and surrounding astrocytic processes. Robust bidirectional communication was present: neuronal activation triggered astrocytic glutamate transport via Eaat1, and blocking Eaat1 extended glutamatergic interneuron-evoked inhibitory post-synaptic currents in motor neurons. The neuronal synapses were always located within a micron of an astrocytic process, but none were ensheathed by those processes. Thus, fly astrocytes can modulate fast synaptic transmission via neurotransmitter transport within these anatomical parameters.
Keywords: electrophysiology, optogenetics, electron microscopy, coupling, SCR_007353, AB_528235, AB_221477, AB_2313643, AB_528122, AB_2490070
Graphical Abstract
The authors show the first recordings from Drosophila astrocytes and find that their electrophysiological properties are strongly analogous to those of vertebrate astrocytes. Using correlative electron microscopy and physiology, they found no glial ensheathment of glutamatergic synapses but demonstrate that perturbing astrocyte responses to synaptic activity impacts fast glutamatergic signaling.
Introduction
The fruit fly Drosophila melanogaster offers sophisticated tools for genetic manipulation of the nervous system and has proven to be a robust and relevant system for elucidating neuronal function at the levels of genes, cellular physiology, and neuronal circuits (Venken et al., 2011). More recently, flies have become a valuable experimental system for identifying glial genes and signaling pathways that mediate neuron-glia interactions (Egger et al., 2002; Freeman et al., 2003; Altenhein et al., 2006; Coutinho-Budd and Freeman, 2013). One glial subtype has been of particular interest as a model for astrocyte development and function. These fly astrocyte-like glial cells have come to be called simply astrocytes; there are six astrocytes per hemisegment in the larval ventral nerve cord (VNC) (Stork et al., 2014).
Morphologically, the highly branched processes of fly astrocytes strongly resemble those of vertebrate protoplasmic astrocytes, and their presence throughout the synaptic neuropil suggests they may functionally regulate mature neuronal synapses (Awasaki et al., 2008). Molecular evidence indicates that fly astrocytes express neurotransmitter transporters (glutamate transporter, Eaat1, and GABA transporter, Gat) (Rival et al., 2004; Thimgan et al., 2006) and receptors (metabotropic GABA receptors) (Mezler et al., 2001; Muthukumar et al., 2014), as well as gap-junction forming innexins (Stebbings et al., 2002), much like their mammalian counterparts (Kimelberg and Nedergaard, 2010). Several complex behaviors have been shown to be influenced by genes expressed in Drosophila astrocytes. Larval locomotion rate is regulated by Eaat1 (Stacey et al., 2010), courtship behavior is controlled by the amino acid transporter genderblind (Grosjean et al., 2008), and age-related memory impairment is sensitive to the pyruvate carboxylase dPC (Yamazaki et al., 2014). In addition, misexpression of the endocytosis mutant allele shits1 or the heat-activated cation channel TrpA1 in astrocytes yields aberrant circadian locomotor rhythms (Ng et al., 2011) and olfactory processing (Liu et al., 2014), respectively. Each of these results provides indirect evidence that astrocytes modulate synapses in the fly, although they do not address which endogenously expressed astrocyte genes and proteins regulate synapses.
While anatomical and molecular features have been identified and a role in larval behavior has been demonstrated, the cellular physiology of Drosophila astrocytes has not been explored in any previous study, leaving basic questions about the anatomy and physiology of neuron-astrocyte interactions in the fly unanswered. Although there is a growing appreciation of heterogeneity within mammalian astrocyte populations (Matyash and Kettenmann, 2010; Oberheim et al., 2012), core electrophysiological features are shared across brain regions and have served as reliable identifiers of astrocytic cells. These features include a resting membrane potential that is more hyperpolarized than that of neuronal cells, a passive current-voltage relationship, and, for mature astrocytes examined in vivo, a lack voltage-gated currents (Kafitz et al., 2008; Verkhratsky and Butt, 2013). Astrocyte electrical membrane responses to neuronal activity have been observed in rodent preparations and include potassium buffering currents (Meeks and Mennerick, 2007) as well as glutamate transport (Bergles and Jahr, 1997; Clark and Barbour, 1997; Lalo et al., 2006; Meeks and Mennerick, 2007; Zhang et al., 2009; Huda et al., 2013).
Viewed at high magnification, virtually all synaptic neuropils across species feature a feltwork of veil-like astrocytic processes that interdigitate between neuronal elements, such that no neuronal synapse operates at a great distance from an astrocytic process. Although astrocytic coverage of synapses has been examined in detail only in a few brain regions, at least in certain neuropils, astrocytic processes completely or partially enwrap synapses (Grosche et al., 1999; Xu-Friedman et al., 2001). However, the degree and nature of association between the two cell types vary, and the implications of these variations are poorly understood (Chao et al., 2002; Bernardinelli et al., 2014). Electron microscopic examination has revealed that fly synapses exhibit little astrocyte contact and no ensheathment (Stork et al., 2014; this paper). The Drosophila neuropil is thus well-suited to address questions about the spatial relationships needed to permit fast, physiological synapse-astrocyte interactions. This is critical, because although the notion that astrocytes are an essential third synaptic element (forming a “tripartite synapse”) is well documented (Verkhratsky and Nedergaard, 2014), no prior study has directly linked the anatomical proximity of the astrocytic element to a given pre-synaptic element with functional, in situ recording. We asked whether Drosophila astrocytes and synapses form functional tripartite synapses, i.e., whether perturbing astrocyte function can impact fast signal transmission, even in the absence of classical tripartite anatomy.
Materials and Methods
Transgenic flies
The following stocks were obtained from the Bloomington Drosophila Stock Center (Indiana University): UAS-CD4-tdGFP (Han et al., 2011), UAS-ChR2-H134R (Pulver et al., 2009), UAS GFP.nls, LexAop2-CsChrimson, and GMR50G08-LexA (Pfeiffer et al., 2010), which was used to drive expression in looper interneurons. UAS-dEaat1RNAi flies (transformant ID 109401) were obtained from the Vienna Drosophila Resource Center (Dietzl et al., 2007). The D42-GAL4 (Yeh et al., 1995) stocks used to drive expression in subsets of motor neurons, bipolar sensory neurons, and interneurons (Iyengar et al., 2011) was a gift from R.B. Levine (University of Arizona). The alrm-GAL4 (Doherty et al., 2009) flies used to drive expression of Green Fluorescent Protein (GFP) in Drosophila astrocytes were a gift from M Freeman (University of Massachusetts). For D42>ChR2-H134R experiments, homozygous animals were used to achieve sufficient expression and subsequent depolarization to trigger neuronal action potentials. The remaining experimental animals used in this study were trans-heterozygous for GAL4/UAS insertions. Animals were reared on standard cornmeal-agar yeast fly food at 25° C on a 12:12 light:dark cycle; except those expressing channelrhodopsins, which were raised on food containing 1 mM all-trans-retinal at 25°C in constant darkness.
Solutions
Extracellular recording saline was composed of: (in mM) 1.8 CaCl2 (dihyd), 118 NaCl, 2 NaOH, 2 KCl, 4 MgCl2 (hexahyd), 25 sucrose, 5 trehalose, and 5 HEPES, and pH was adjusted to 7.2 and osmolarity to 290 mOsm using sucrose. To reduce muscle contractions during dissection, CaCl2 was replaced with MgCl2 in the dissecting solution. To block calcium channels during physiological recordings, CaCl2 was replaced with MgCl2, and 1 mM CdCl2 also was added. Intracellular recording saline included 5 KCl, 2 MgCl2 (hexahyd), 2 EGTA, 20 HEPES, 133 K-gluconate, and 2 NaATP, and pH was adjusted to 7.4 using KOH, osmolarity to 290 mOsm using glucose. For some experiments, KCl was replaced with CsCl and K-gluconate was replaced with CsOH and D-gluconic acid to block potassium channels. In some cases, Lucifer Yellow (2.7 mM), Alexa Fluor 568 (1 mM, Thermo Fisher Scientific), or Alexa Fluor 647 (1mM) hydrazide was added to the pipette solution. Drugs were used at the following concentrations: tetrodotoxin (TTX), 1 μM; tetraethylammonium (TEA), 50 mM; 4-Aminopyridine (4-AP), 1.5 mM; DL-threo-β-benzyloxyaspartic acid (TBOA) (Tocris, UK), 100 μM; picrotoxin (PTX), 100 μM.
Whole-cell recordings
Wandering third-instar larvae were dissected in calcium-free A-solution (Jan and Jan, 1976). Central nervous systems were isolated, pinned in a Sylgard dish using pins made from 0.002-inch diameter stainless steel wire, and continuously perfused with extracellular recording saline. Electrodes were pulled from thin-walled borosilicate glass capillaries (Fredrick and Dimmock, Millville, NJ, USA; ID 0.9 mm OD 1.2 mm) using a Narishige PP-83 electrode puller, and were fire-polished to a resistance of 10-12 MΩ (astrocyte recordings) or 8-10 MΩ (motor neuron recordings). Preparations were visualized in the recording chamber using infrared and water-immersion optics. Recordings were made from astrocytes in the 2nd and 3rd thoracic segments (T2,3) or from the 1st abdominal segment (A1), where the space between the lateral and medial motor neuron clusters is larger, making the astrocyte cell bodies easier to identify than in the more posterior abdominal segments. Additionally, motor neuron physiology has been studied in these thoracic (Worrell and Levine, 2008) and upper abdominal (Baines and Bate, 1998) segments. All recordings were collected from one of the four astrocytes located on the dorsal surface of the neuropil of each hemisegment because the lateral and ventral astrocytes are too deep to access in whole-mount tissue. Focal protease application (Protease XIV, Sigma) was used to expose the cells for recording. Enzyme (1-2 mg/mL) was delivered via gently broken recording electrodes, which also were used subsequently to remove overlying perineurial and subperineurial sheath-cell debris, following a widely-used method to obtain motor neurons recordings in this preparation (Rhorbough and Broadie, 2002; Choi et al., 2004; Worrell and Levine, 2008; Pulver et al., 2009; Marley and Baines, 2011). Signals were acquired with an Axopatch 200B amplifier (Molecular Devices, CA, USA), filtered using a 1 kHz low-pass Bessel filter, digitized using a Digidata 1440A A-D converter (Molecular Devices), and collected at 10 kHz using pClamp 10 software (Molecular Devices). Leak subtraction was performed online using the pClamp P/4 opposite-polarity algorithm. In effort to approximate the degree of error inherent to leak subtraction, manual post hoc leak subtraction was carried out by scaling the current responses to +10-mV and −10-mV steps proportionally to the voltage-step in question and performing subtraction (e.g., 2× the +10mV step would be subtracted from the +20mV step, 3× for a +30mV step, etc.). Notably, different leak subtraction protocols affected the shape and amplitude of the peak, plateau, and tail currents, indicating that significant leak subtraction error is inherent in these astrocyte recordings (not shown). In some cases, a notch filter centered at 585 kHz was used. Access resistances ranged from 20 to 50 MΩ; any cells with RA beyond this range were excluded from analysis. 470-nm light pulses were delivered using a CoolLED Pe-2 (Andover, UK) and 470-nm LP excitation filters. Measurements of voltage-gated and neuronal-activity-induced astrocyte currents were averaged across 2 and 4 trials, respectively, within each cell. These responses were then averaged across cells and plotted using Prism software (GraphPad Software, La Jolla, CA, USA). Looper-evoked motor neuron IPSC kinetics were analyzed in pClamp’s Clampfit application, rise and decay times were measured from 1/3-2/3 peak amplitude and 2/3-1/3 peak amplitude, respectively, and fit using a standard exponential with 1-term. Motor neurons were identified by referencing the morphological profiles of motor neurons with medially-located cell bodies described in Choi et al. (2004).
Tissue preparation, Immunolabeling, and Imaging
Brains were fixed in 4% paraformaldehyde in 0.1M PO4 buffer, washed in PBS, and mounted in glycerol, except for tissues with anti-dEaat1 immunolabeling, which were fixed in Bouin’s fixative solution (Ramachandran and Budnik, 2010). Whole-mount preparations were mounted between two coverslips, using #1 coverslips as spacers to preserve tissue shape. For cross-sections, brains were embedded in 8% low-melting-point agarose and cut at 80-μm thickness using a vibrating microtome. The following primary antibodies were used: anti-Fasciclin II, anti-GFP, anti-dvGAT, anti-ChAT, anti-dvGLUT, and anti-dEAAT1 (Table 2). For anti-FasII, anti-GFP, and anti-dEaat1 labeling, 2% BSA was used in a pre-incubation blocking step as well as during primary-antibody incubation. For anti-dvGAT and anti-ChAT labeling, 5% BSA was included in blocking and primary incubation steps. No BSA was used for anti-dvGLUT .Secondary antibodies included goat-anti-mouse Cy-3 secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) used at 1:200, and goat-anti-rabbit Alexa Fluor 647 (Thermo Fischer Scientific) used at 1:400. Triton-100X was included in all pre-incubation, primary, and secondary antibody steps to permeabilize tissue, at 0.2% for cross-sectioned and 0.5% for whole-mounted tissue. Syto 59 (1:20K, 30 min. Thermo Fischer Scientific) was used to label cell nuclei. Confocal images were collected on a Zeiss 510 Laser Scanning Confocal Microscope and adjusted in Adobe Photoshop using linear and/or gamma corrections.
Table 2.
Primary Antibodies Used in this Study
| Antibody name |
Immunogen | Source, catalog No., species, type |
Dilution | Reference, RRID |
|---|---|---|---|---|
| anti-FasII | Drosophila fasciclin II | DSHB, Cat# 1D4 concentrate, mouse, monoclonal, MIgG1 |
1:400 |
Grenningloh et al., 1991 AB_528235 |
| anti-GFP | Green Fluorescent Protein |
Molecular Probes, Cat# A21311, rabbit, polyclonal, Alexa Fluor 488 conjugated |
1:450 | AB_221477 |
| anti-dvGAT |
Drosophila vesicular GABA transporter C- terminal peptide (− CDSGNALINAFEIGLPF) |
David Krantz, David Geffen School of Medicine at UCLA |
1:400 |
Fei et al., 2010 AB_2313643 |
| anti-ChAT |
Drosophila choline acetyl transferase |
DSHB, Cat# chat4b1 supernatant, mouse, monoclonal |
1:10 |
Takagawa, K, and P Salvaterra. 1996. AB_528122 |
| anti- dvGLUT |
Drosophila vesicular glutamate transporter N- terminal fragment (amino acids 2–87) |
Hermann Aberle, Heinrich-Heine- Universität Düsseldorf, rabbit, polyclonal |
1:200 |
Mahr and Aberle, 2006 AB_2490070 |
| anti- dEAAT1 |
Drosophila excitatory amino acid transporter 1 (amino acids 117-195) |
Donald van Meyel, McGill University Centre for Research in Neuroscience, rabbit, polyclonal |
1:5K | Peco et al., 2016. |
Antibody Characterization
Anti-FasII
The anti-FasII antibody labeled a 97-kDa band in Western blot, which was absent in FasII null mutants (Grenningloh et al., 1991; Mathew et al., 2003). The labeling pattern observed in this study is identical to previous reports (Grenningloh et al., 1991; Landgraf et al., 2003; Mathew et al., 2003).
Anti-GFP
No labeling was observed in larval brains from flies lacking genetically encoded GFP expression (w1118) (our observation, not shown).
Anti-dvGAT
Western blots of head homogenates probed with an antibody to dvGAT showed a single major band at 60 kDa. Cultured S2 cells expressing dvGAT cDNA show punctate intracellular labeling with this antibody (Fei et al., 2010).
Anti-ChAT
The anti-ChAT antibody was shown to label a single band at a position of about 80 kDa in crude fly head samples (Takagawa and Salvaterra, 1996).
Anti-dvGLUT
No labeling was observed in embryos homozygous for OK371ΔD, a chromosomal deficiency covering dvGLUT (Mahr and Aberle, 2006).
Anti-Eaat1
Sera were tested by immunolabeling dissected CNS tissues. Specificity of Eaat1 antiserum was confirmed by loss of Eaat1 immunoreactivity in Eaat1 null mutants. (Peco et al., 2016).
Three-dimensional Reconstruction from Electron Microscopy Data Set
The third-instar larval VNC EM dataset was generated according to methods in Ohyama et al. (2015). Images were aligned using TrakEM software and annotated in the CATMAID user environment (Saalfeld et al., 2009), in which the looper population member A02m_a3l Pseudolooper-3 (located in segment A3, left) was skeletonized completely according to criteria in Ohyama et al. (2015), including full annotation of pre- and post- synaptic connections. Synapse-rich regions were identified, and image subsets that included all 67 pre-synaptic sites on the neuron were exported into Reconstruct (Fiala, 2005) for volumetric reconstruction. The profile of the looper neurite, all looper presynaptic sites, and the astrocytic processes surrounding the neurite were traced in each Z section. An x-y area of at least 5 μm × 5 μm was reconstructed. These objects were exported to Amira v. 5.6 (http://www.amiravis.com, RRID:SCR_007353), where measurements from each point on the surface of the presynaptic site to the nearest astrocytic process were calculated. These distances were displayed visually using a colorimetric heat-map, where red represents a distance of 0 μm; yellow, 0.5 μm; blue, 1 μm. The smallest distance value for each presynaptic site is represented in the summary histogram.
Locomotion Assays
Animals were reared and tested in conditions as described above, but in an environmental chamber with 50-70% humidity. Locomotion assays were performed on grape-juice agar (3%) plates by an individual who had been blinded to genotype. Female late third-instar larvae were collected from vials, transferred to plates, and then allowed to acclimate for at least 30 seconds before testing. Larvae were placed under a dissecting microscope to enable observation and counting of peristaltic contractions. A digital handheld counter and timer were used to record the number of contractions per minute. For each animal, three sequential one-minute trials were conducted and the results of each were averaged to yield a mean contraction rate.
Statistical Analysis
Statistical analysis and graphing was carried out in GraphPad Prism unless otherwise noted. Astrocyte dye-coupling frequency in stimulated and unstimulated preparations was compared using Fischer’s Exact Test. Recording duration distribution for coupled and uncoupled results were compared using an independent sample Kolmogorov-Smirnov test. The relationship between astrocyte holding potential and D42 neuron-evoked responses was examined using a bivariate correlation. Looper-evoked motor neuron IPSC parameters recorded in standard saline vs. TBOA were compared using a paired two-tailed t-test. Looper-evoked motor neuron IPSC recorded in control (alrm-GAL4) and Eaat1 knockdown conditions were compared using an unpaired two-tailed t-test. Locomotion assay data was analyzed using a one-way ANOVA test.
Results
Identification and intrinsic properties of third-instar larval astrocytes in vivo
The larval central nervous system consists of two brain lobes and the VNC. Each VNC segment corresponds with a segment of the larval body wall and is divided into two symmetrical hemisegments. The architecture of the VNC follows the general plan of the insect CNS (Figure 1A). It is organized into two distinct compartments: the cortex, which contains all neuronal and glial cell bodies (nuclei in blue), and the neuropil, which contains neuronal processes, synapses, and astrocytic processes (green). Fasciclin II-positive axon tracts (magenta) are shown to provide landmarks within the larval VNC neuropil (Landgraf et al., 2003). Drosophila astrocytes were labeled by using the astrocyte-specific driver, alrm-GAL4, to express membrane-targeted GFP (Doherty et al., 2009). In the cross-sectional view of the third thoracic segment in Figure 1A, the cell bodies of 3 of the 12 astrocytes residing in that segment are visible; dorsal (d), lateral (l), and ventral (v) astrocyte cell bodies appear in the left hemisegment. Typically, an entire thoracic hemisegment includes three additional dorsal astrocytes but no additional lateral or ventral cells (our observation). These numbers are in close agreement with Stork et al. 2014, where a dorsomedial group (3 cells), a dorsolateral group (2 cells), and a single ventrally positioned cell were described.
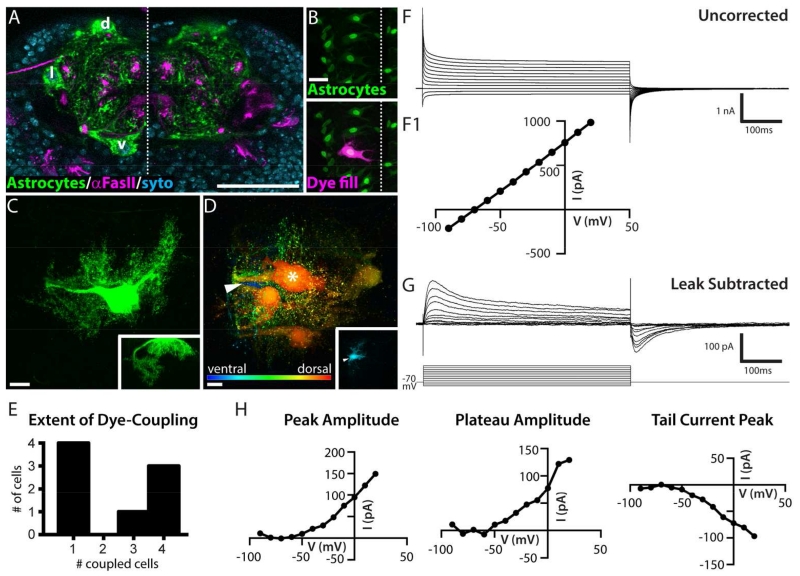
Figure 1. Drosophila astrocytes exhibit dye-coupling and passive membrane properties.
A. Cross-sectional view of larval ventral nerve cord (VNC), 3rd thoracic segment, showing astrocyte membrane (green, alrm-GAL4>CD4-GFP), Fasciclin II-positive axon tracts (magenta) and cell nuclei (blue, Syto dye). Total substack thickness = 2.5 μm, astrocyte cell bodies located d, dorsally; l, laterally; v, ventrally; dotted line, midline. Scale bar = 50 μm. B. Dorsal view of whole-mount preparation showing astrocyte nuclei (green, alrm-GAL4>GFP.nls) and a dye-filled astrocyte (magenta). Dotted line, midline. Scale bar = 20 μm. C. Maximal projection of a dye-fill resulting in a single labeled astrocyte, dorsal view. Inset, cross-sectional view. Scale bar = 10 μm. D. Depth-coded image (red, dorsal; blue ventral) of a maximal projection of a dye-fill showing 4 astrocytes in addition to the primary astrocyte from which the recording was made (asterisk). Arrowhead, blue cell body located ventrally. Inset, substack showing the same ventral cell body; overlying dorsal optical sections are omitted. Scale bar = 10 μm. 8 of the 26 cells examined displayed coupling. E. Histogram showing the extent of dye-coupling observed in 8 cases where coupling was present. F. Uncorrected whole-cell currents (mean, n = 8) recorded from a holding potential of −70 mV. F1. The corresponding current-voltage (I-V) plot indicates a linear I-V relationship (current measured 490 ms into the 500 ms pulse). G. Whole-cell currents (mean, n = 20) recorded as in E with leak current subtracted (online, P/4, opposite polarity). H. I-V plots for mean leak subtracted trace shown in F, current amplitude was measured at the peak of the outward current, the plateau (10 ms before termination of the pulse), and the peak tail current.
Drosophila astrocytes were targeted for recording by labeling cells with nuclear GFP expressed under the control of alrm-GAL4. Inclusion of dye in the recording electrode allowed us to confirm the identity of the recorded cell after each experiment (Figure 1B). In the majority of cases (70% of astrocytes, n = 26), only the primary, recorded cell was filled with dye (Figure 1C). In the remaining 30% of cases, Drosophila astrocytes displayed dye-coupling to 1-4 additional astrocytes within the hemisegment (Figure 1D,E). The cell bodies of the dye-coupled astrocytes could be dorsal, lateral, or ventral in the hemisegment (Figure 1D, inset). Dye-coupling was never observed to extend to astrocytes with cell bodies located in anterior, posterior, or contralateral hemisegments, nor was any dye-coupling observed between astrocytes and other types of glial cells, such as ensheathing and cortex glia. This suggests that the functional organization of the astrocyte network corresponds with the segmentally repeated neuronal circuitry, or “anatomo-functional compartments”, of the larval VNC.
We hypothesized that the subset of astrocytes that were filled with dye during experiments in which neuronal activity was artificially increased (via channelrhodopsin expression under the control of the D42-GAL4 driver, see “Astrocyte response to broad neuronal network activity” below) would display a higher frequency of astrocyte dye-coupling (Roux et al., 2011). However, there was no difference between our sample of unstimulated preparations, where 4 of 14 astrocytes were dye-coupled, and stimulated preparations, where 4 of 12 astrocytes were dye-coupled. This result does not eliminate the possibility that activity from a certain neuronal type, or patterned in a certain manner, may dynamically regulate astrocyte coupling. In any case, our results are the first to demonstrate dye-coupling between Drosophila astrocytes, a hallmark feature of vertebrate astrocytes.
The mean membrane resistance (Rm) of a Drosophila astrocyte was 48.1 MΩ +/− 27.8 SD, a value significantly lower than that of neighboring motor neurons, whose resistance was in the 1 – 1.5 GΩ range, as also reported by Choi et al. (2004). Measurements collected immediately after breaking into the cell show that Drosophila astrocyte membranes rest at a potential of ~−70mV. This potential is more hyperpolarized than that recorded in larval motor neurons (−55 to −60 mV, our observations, and Choi et al., 2004). The mean membrane capacitance (Cm) of individual (not dye-coupled) Drosophila astrocytes was 138.5 pF +/− 44.9, and at −70 mV, the time constant (tau) for a 10-mV depolarizing pulse was 2.57 ms +/− 1.16. This membrane capacitance value is approximately 3× greater than what we have observed for motor neurons in this system. These parameters indicate that astrocytes possess a large, but leaky, surface area. Their morphology reinforces this notion; each astrocyte has three to four thick primary processes, some of which travel along the cortex-neuropil boundary for some distance before entering the neuropil. Once in the neuropil, astrocytic processes exhibit a tufted morphology with finely branched, often veil-like, distal processes (Figure 1C). Given these morphological and electrical properties, we assume that our cell-body recordings are unlikely to detect activity in the distalmost branches.
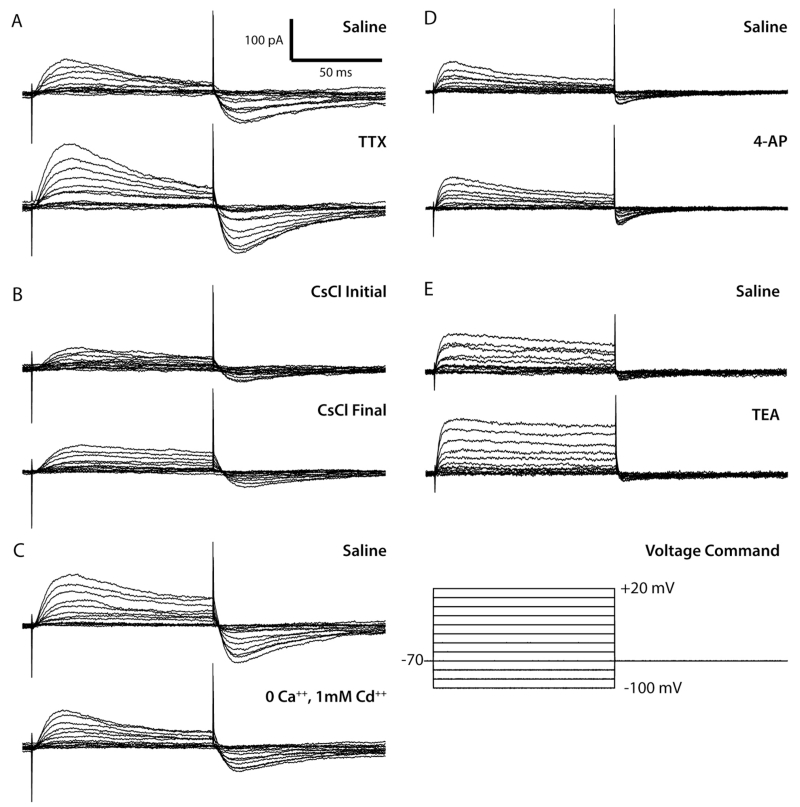
Drosophila astrocytes lack detectable voltage-gated currents
Astrocytes were voltage-clamped at −70 mV and stepped from −90 mV to +20 mV for 500 ms in 10 mV increments. The resulting current-voltage (I-V) relationship is roughly linear and indicative of a large leak current (Figure 1F). Subtraction of the leak current eliminated the linear current response between −90 and −60 mV and revealed a small net-outward current with two apparent temporal components in response to depolarizing pulses (Figure 1G): a fast, transient current that reaches its peak amplitude 16-20 ms after depolarization, and a sustained component. Upon returning to −70 mV, an inward tail current is activated. These leak-subtracted currents are ten-fold smaller than the uncorrected amplitudes, and retain an approximately linear I-V relationship between −50 mV and +20 mV (Fig 1H).
We investigated the ionic bases of the fast peak and the plateau using 100-ms voltage steps and a battery of ion-substitution protocols and well-characterized pharmacological agents that block voltage-gated sodium and potassium channels. The recordings showed modest variation in the absolute and relative amplitude of each component across individual cells “before” drug, and subtle, but inconsistent changes in the amplitude and kinetics of the fast transient, sustained outward, and fast inward tail current in response to the particular saline solution or agent applied (Figure 2A-E). No systematic effect of any drug was observed across cells. These observations led us to propose that the signal remaining after leak subtraction does not contain any significant voltage-gated current; rather, the membrane properties of astrocytes, which allow 10- to 20-fold more current leakage than do neurons in this system, perhaps generate an axial-current flow between non-isopotential cell compartments (ΔVm at the soma and proximal processes > ΔVm at distal processes, creating a potential difference that drives negative current toward the electrode) in the voltage-clamp configuration that is not accurately subtracted by our methods. The transient, larger amplitude current observed in leak-subtracted traces during the first 20 ms of a depolarizing step may thus represent the larger initial potential difference between the soma and distal processes, which eventually reaches equilibrium at around 40 ms. In summary, we did not find evidence of any significant voltage-gated current in third-instar larval VNC astrocytes.
Figure 2. Voltage-gated currents were not detected in larval VNC astrocytes.
All data shown in this figure are representative traces from one of a group of 3-5 astrocytes in each set. Traces were generated using online P/4 opposite polarity leak subtraction. A. Top: whole-cell voltage-clamp current recorded from a single cell in standard solution. Bottom: current recorded during subsequent perfusion with TTX. B. Top: current recorded immediately after breaking into the cell with a CsCl pipette solution. Bottom: current recorded from the same cell ten minutes after cell break in. C. Top: current recorded in standard solution. Bottom: current recorded during subsequent perfusion with 0 Ca++, 1 mM Cd++. D. Top: current recorded in standard solution. Bottom: whole-cell current recorded during subsequent perfusion with 4-AP. E. Top: current recorded in standard solution. Bottom: current recorded during subsequent perfusion with TEA.
Astrocyte response to broad neuronal network activity
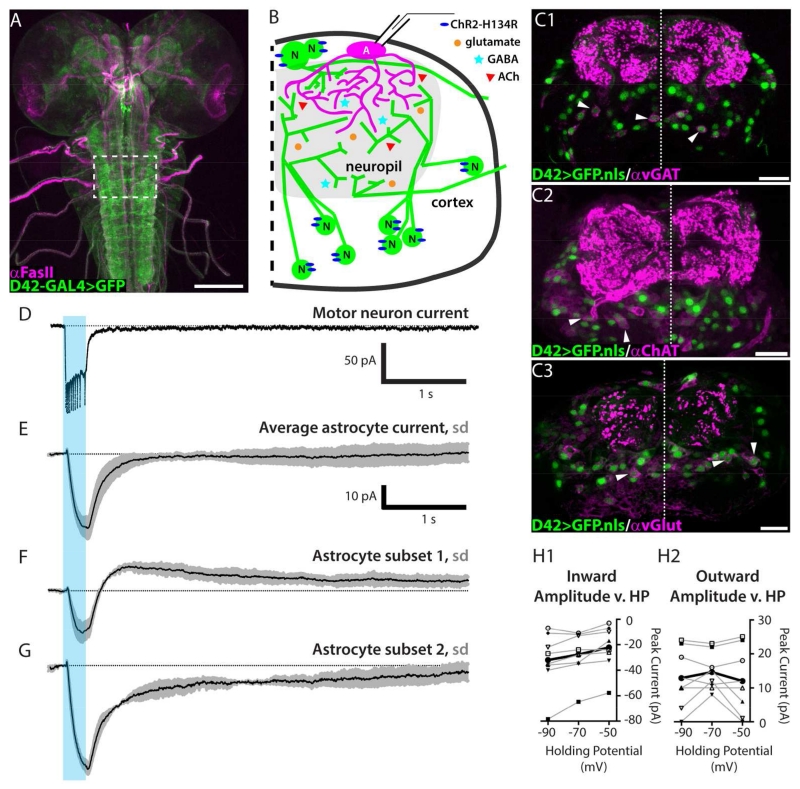
We next asked whether Drosophila astrocytes respond to neuronal activity. Neurons were activated optogenetically using the D42-GAL4 neuronal driver, which was selected because it has high GAL4 expression levels and defines a restricted yet heterogeneous population of larval ventral nerve cord neurons (Figure 3A-C). D42-GAL4 expressing neurons (“D42 neurons”) are heterogeneous in transmitter type; immunolabeling for the vesicular GABA and glutamate transporters, as well as choline acetyl transferase, indicate that the D42 population comprises at least cholinergic, glutamatergic, and GABAergic neurons (Figure 3C). D42 neurons are also heterogeneous with respect to neuronal cell class, including many interneurons, a subset of sensory neurons, and some motor neurons. The motor neurons, which are glutamatergic, are not known to make any presynaptic connections within the CNS, and thus may not be relevant to local astrocyte responses to neuronal activity (Schneider-Mizell et al., in press). Channelrhodopsin (ChR2-H134R, Pulver et al., 2009) was used to stimulate D42 neurons. To determine an optimal stimulus duration we presented optical stimuli of different durations while recording from select D42-GAL4 expressing motor neurons with medially located cell bodies (RP2 and aCC). Attenuation of neuronal firing rate was used to determine that 250 ms was an appropriate stimulus duration to elicit maximal firing with minimal adaptation of firing frequency (Figure 3D). Note that in the D42-GAL4>ChR2-H134R experiments, two copies of the GAL4 driver and UAS construct were needed to obtain sufficient expression levels / depolarization to trigger motor neuron spikes.
Figure 3. Neuronal activity induces an inward current as well as a current with slow kinetics and variable polarity.
A. D42 neuronal driver expression pattern (green) and Fas II landmarks (magenta) in a whole-mount 3rd-instar larval CNS. Scale bar = 100 μm. B. Schematic diagram for experiments: channelrhodopsin is expressed in a heterogeneous neuronal population using the D42 driver; whole-cell recordings are collected from dorsal astrocytes. C1-C3. Cross sectional view of D42-GAL4>nuclear GFP (green) and anti-vGAT (C1), anti-ChAT (C2), or anti-vGlut (C3) immunolabeling pattern (magenta). Arrowheads highlight selected D42 nuclei surrounded by transmitter identifying immunolabeling. Dotted line, midline. Total substack thickness = 2.5 μm. Scale bars = 20 μm. D. Voltage-clamp recording from a channelrhodopsin-expressing motor neuron (representative trace, n = 10 preparations). Unclamped spikes are triggered by a 250-ms blue-light stimulus (470 nm, blue bar). Dotted line, baseline. E. Mean current from 31 astrocytes. Blue light activation of neurons yields an inward glial current that follows by 25 ms. Grey, S.D. F-G. Mean current from two subsets of cells sorted by response profile to optogenetic stimulation. F. In one subset, the current transiently overshoots baseline before returning over 4 s, n = 18. G. In the second subset, the current rises to baseline over 4 s, n = 13. H1. Shows a weak positive relationship between holding potential and peak amplitude of the fast inward current (r = 0.219). H2. No relationship between holding potential and the amplitude of the slow, variable polarity current (r =−0.046). Black, mean of 8 cells. Grey, individual cells.
In subsequent experiments, D42 neurons were again stimulated for 250 ms, but the patch electrode was targeted instead to astrocytes (Figure 3B,E). Astrocytes were reliably identified by using the intrinsic properties (Rm, Cm, Tau, non-spiking, linear voltage-activated currents) defined earlier and subsequently confirmed using Alexa Fluor dye fills. D42 neuronal activity elicited an inward current present for the duration of the light stimulus (Figure 3E). The mean amplitude of this inward current was −37.7 pA +/− 17.3. The onset of astrocyte depolarization was delayed significantly relative to neuronal activity, occurring 35 ms after ChR2-mediated neuronal depolarization began and 25 ms after the first spike was recorded from the medial motor neurons.
The astrocyte current shown in Figure 3E represents the mean of all 31 cells in this experimental series. For the group, the mean current returns to baseline 1.5 seconds after termination of the stimulus. However, the current of an individual cell rarely returned to baseline within 1.5 seconds. We classified the responses into categories that illustrate the two extremes; these profiles are shown separately in Figures 3F (n = 18) and 3G (n = 13). In one subset (Figure 3F), the peak of the fast inward current slightly precedes the offset of light-induced neuronal activity and is followed by a net outward current that slowly returns to baseline, reaching it about 4 seconds after stimulus offset. In the second subset (Figure 3G), the peak inward current coincides with the termination of the stimulus, and there is a net inward current that slowly returns to baseline over an identical time course of about 4 seconds. We note that a given cell’s current profile could be dynamic during the session. Namely, the polarity and amplitude of the slow current changed over time in 12 of 31 cells examined.
Fast inward neuronal-activity-evoked astrocyte currents are mediated by glutamate transporters
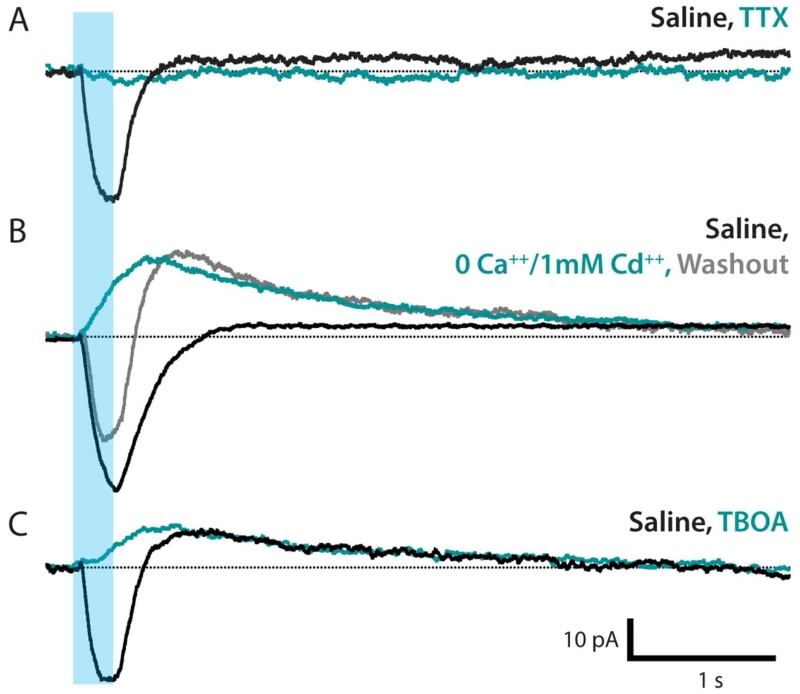
Next, we asked whether the astrocyte response to neuronal activity is dependent on neurotransmitter release. Bath application of TTX during optical-stimulation experiments blocked all astrocyte current in all trials (Figure 4A). This effect is mediated by blockade of Nav channels in neurons, as we did not find evidence for Nav in astrocytes (Figure 2A). This blockade did not reverse upon washout with normal saline, consistent with observations in other insect nervous systems that TTX binds the neuronal voltage-gated sodium channel irreversibly (Hayashi and Levine, 1992). Blocking neurotransmitter release with a zero Ca++ and 1 mM Cd++solution blocked the fast, inward current in all trials; the slow current either appeared with outward polarity or was preserved as an outward current. Upon washout with normal saline, the fast inward current returned; the slow outward current remained at the conclusion of a ten-minute washout (Figure 4B).
Figure 4. Neurotransmitter release evokes an inward glutamate transporter current.
Representative traces, n = 5-6 cells in each group. A. TTX completely blocks the inward current associated with neuronal stimulation (teal). B. Neurotransmitter release blocked using a 0 Ca++, 1mM Cd++ bath (teal). Inward current is abolished and an outward current is induced/potentiated. Saline washout restores the inward current; outward current remains (gray). C. Inward current is completely blocked by glutamate transporter antagonist TBOA (teal); slow outward current is not affected.
Because there are glutamatergic synapses in the Drosophila VNC neuropil (Figure 3C,C3 and Mahr and Aberle, 2006), we hypothesized that this fast inward current would be mediated by a glutamate transporter. Glutamate transporter currents derive from the co-transport of three Na+, one H+, and one glutamate (−) into the cell and one K+ out of the cell and are thus net-inward (Shimamoto et al., 1998). We applied D,L-TBOA, a high-affinity competitive antagonist of excitatory amino acid transporters that does not affect ionotropic or metabotropic receptors (Shimamoto et al., 1998). We found that TBOA completely blocked the fast inward current in Drosophila astrocytes, supporting the conclusion that this current is mediated by glutamate transporters (Figure 4C). Consistent with its dependence on ionic gradients, we find a weak positive relationship between the amplitude of the inward glutamate transporter current and holding potential (r = 0.219, Figure 3H,H1).
Slow variable polarity neuronal-activity-evoked astrocyte currents reflect potassium buffering
TBOA did not block the slow neuronal-activity-induced current (Figure 4C), and we found no correlation between the amplitude of the outward slow current and holding potential (r = −0.046, Figure 3H,H2); inward slow currents were excluded from this analysis due to the confounding effect of the fast inward current’s offset kinetics. This insensitivity to holding potential suggests that this signal originates beyond the spatial extent of the voltage-clamped membrane. Taking together the variable polarity of the slow current and its sensitivity to TTX, but not Cd++ or TBOA, these data indicate that the slow current depends on the Na+/K+ flux generated by neuronal activity. Our results are consistent with the hypothesis that the slow astrocyte current reflects a dynamic response to neuronal-activity-mediated changes in the concentration and distribution of extracellular potassium. Thus, we suggest this current is indicative of potassium-buffering.
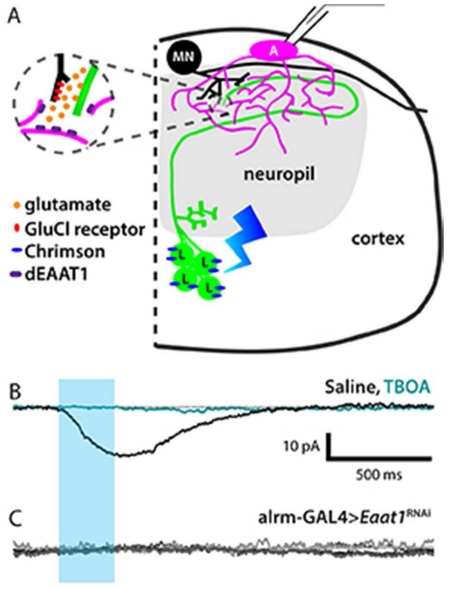
Looper-to-motor neuron connections are mediated by glutamate-gated chloride channels
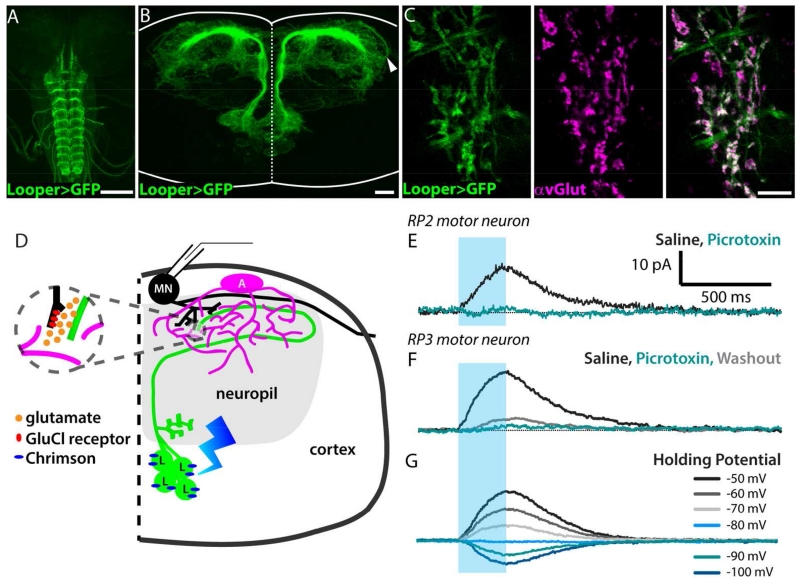
To further examine the astrocytes’ response to synaptically-released glutamate, we performed a series of experiments where we expressed a channelrhodopsin with improved properties, Chrimson (Klapoetke et al., 2014), in a population of glutamatergic interneurons. The neurons in this population are called “loopers” here, in reference to the looped morphology of their primary neurite (Figure 5A,B). Loopers have ventro-medially located somas (14 cells per hemisegment), receive inputs in the sensory neuropil, and make outputs, as well as receive some additional inputs, in the dorsal motor neuropil (Figure 5D). The wiring diagram derived from a first-instar 3-D electron-microscopy data set confirms that loopers make direct synaptic connections with some medial motor neurons, including RP2 (also named as MNISN-Is) (Schneider-Mizell et al., in press). Terminal looper neurites located in the motor neuropil were labeled by an antibody to the vesicular glutamate transporter protein dvGLUT (Figure 5C).
Figure 5. Looper-motor neuron synaptic connection is mediated by a glutamate-gated chloride channel receptor.
A. Whole-mount 3rd-instar larval CNS showing the VNC expression pattern of the Looper driver (green, Looper-LexA>membrane GFP). Scale bar = 100 μm. B. Cross-sectional view of the looper neurons (green); dotted line, midline; solid line, tissue boundary. Substack encompasses cells in thoracic segment 2, total thickness = 73 μm. Arrowhead, looped tract shown in schematic diagram in E. Scale bar = 10 μm. C. Dorsal view of a single optical section (z = 0.5 μm) showing colocalization of looper membrane (green) and dvGlut antibody labeling (magenta). Green-only regions in merged image are looper tracts, where no synapses are expected. Scale bar = 10 μm. D. Schematic diagram illustrating the presynaptic glutamatergic neuron (Looper, “L”, green) expressing Chrimson (blue), an adjacent astrocyte (“A”, magenta), and a postsynaptic motor neuron (“MN”, black) from which recordings shown in E-G were collected. E. Optical stimulation of looper neurons (blue bar, stimulus window) during a whole-cell voltage-clamp recording (holding potential = −60mV) from motor neuron RP2 reveals an IPSC in standard saline (black) that is blocked by bath-applied picrotoxin (teal). No washout was observed after 10 minutes. F. As in E, but recorded from motor neuron RP3. Some current returns upon washout (gray). G. Looper-evoked RP3 motor neuron IPSCs reverse at a holding potential of −80mV.
Our physiological data reaffirms that the looper-to-RP2 connection persists in the third-instar (Figure 5E). In addition to RP2, we also detected looper-evoked IPSCs in whole-cell recordings from RP3 (MN6/7-Is) (Figure 5F,G). Note that glutamate signaling in the fly CNS differs from vertebrates due to the presence of inhibitory glutamate receptors. Previous studies have shown that motor neurons express glutamate-gated chloride channels (Rohrbough and Broadie 2002), which have been characterized in other invertebrates such as C. elegans (Vassilatis et al., 1997) but are absent from vertebrate genomes (Wolstenholme, 2012). The Drosophila homolog of this receptor, GluCl-alpha, produces a glutamate-gated chloride current when cloned and expressed in Xenopus oocytes (Cully et al., 1996).
In agreement with each of these findings, and other studies concluding that glutamate’s action in the Drosophila CNS is inhibitory (Liu and Wilson, 2013; Kohsaka et al., 2014), looper-evoked motor neuron IPSCs are blocked by picrotoxin and have a reversal potential of −80mV (Figure 5E-G). Taken together with this literature, our immunolabeling, electrophysiology, and pharmacology results show that synaptic communication between looper neurons and motor neurons is mediated by glutamate-gated chloride channels.
Astrocyte glutamate transport is mediated by Eaat1
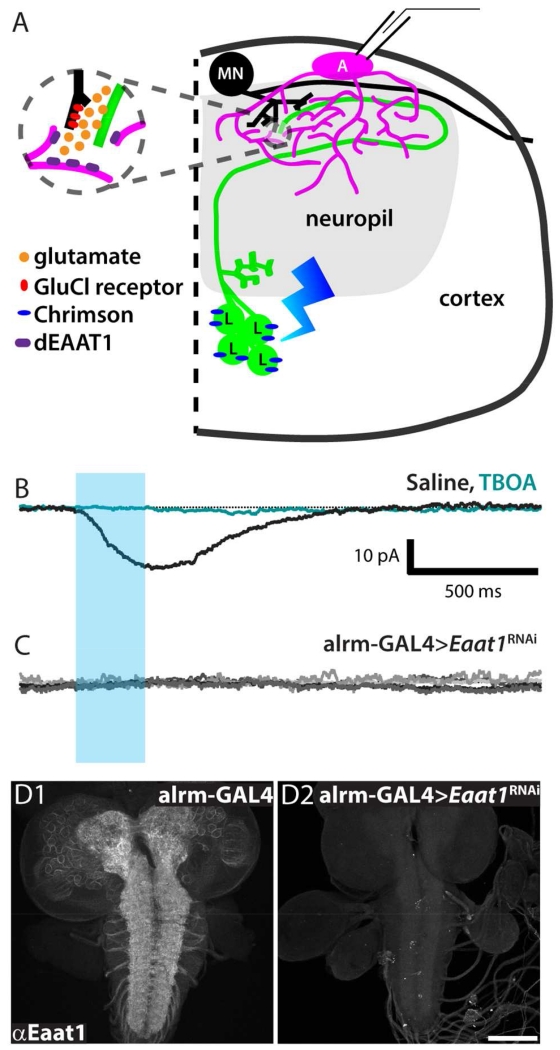
One would expect looper activation, which releases glutamate in the dorsal neuropil, to activate a glutamate transporter current in nearby astrocytes. To test this, whole-cell patch clamp recordings were collected from astrocytes while activating looper neurons (Figure 6A). This elicited an inward current with a mean amplitude of −15.57 pA, +/− 3.75 that was completely blocked by bath application of TBOA (Figure 6B). The mean amplitude is smaller and shows less variation than the mean inward amplitude for D42 neuronal activation, as would be expected with activation of a smaller group of glutamatergic neurons. The slow current with variable polarity (potassium-buffering current) observed in response to activation of D42 neurons was not observed in response to looper activation. This is consistent with the idea that looper activation recruits far fewer neurons, which would elevate [Ko] to a lesser degree.
Figure 6. Astrocytes respond to glutamatergic neuronal activity with an Eaat1-mediated transporter current.
A. Schematic diagram illustrating the presynaptic glutamatergic neuron (Looper, “L”, green) expressing Chrimson (blue), the postsynaptic motor neuron (“MN”, black), and an adjacent astrocyte (“A”, magenta), from which recordings shown in F-H were collected. B. Representative whole-cell astrocyte recording in control genotype (Looper-LexA>Chrimson, alrm-GAL4). Black, standard saline perfusion. Teal, during perfusion with TBOA. Blue bar, optical stimulus window. n = 5. C. Whole-cell astrocyte recordings collected from experimental genotype (Looper-LexA>Chrimson, alrm-GAL4>Eaat1RNAi). Five overlaid traces show no response to looper activation. D. Whole-mount 3rd-instar larval CNS labeled with rabbit-anti-Eaat1. D1, alrm-GAL4 control shows labeling throughout neuropil regions. D2, alrm-GAL4>Eaat1RNAi shows little to no Eaat1 labeling. Scale bar = 100 μm.
In these experiments, we expressed Chrimson in looper neurons using the LexA system, while the GAL4/UAS binary system was used to manipulate the glutamate transporter Eaat1 (excitatory amino acid transporter 1) specifically in astrocytes with alrm-GAL4. In the presence of looper-LexA>Chrimson, the knockdown of astrocyte Eaat1 completely eliminated the inward current observed in response to looper activation in controls (Figure 6C). To confirm the specificity and efficacy of the RNAi hairpin, we performed Eaat1 immunolabeling in both control and alrm-GAL4>Eaat1RNAi larval brains. Eaat1 labeling was robust in controls and almost completely eliminated in alrm-GAL4>Eaat1RNAi samples (Figure 6D). Taken together, these experiments identify glutamate transporters as a highly conserved mediator of neuron-astrocyte interaction.
The known lethality of null mutations in Eaat1 (Stacey et al., 2010) and pronounced phenotypes in flies with pan-glial-cell RNAi knockdown of Eaat1 (repo-GAL4) (Rival et al., 2004; Rival et al., 2006) led us to test alrm-GAL4> Eaat1 RNAi larvae for gross locomotor defects. We did not detect any changes in contraction rate (mean +/− SD contractions per minute = 40.2 +/− 20.0 for UAS Eaat1RNAi controls, 46.6 +/− 10.5 for alrm-GAL4 controls, and 41.2 +/− 13.1 for alrm-GAL4> Eaat1RNAi; n = 7 in each group, n.s.). Locomotor behavior, measured as contractions per minute, is thus grossly normal in alrm-GAL4> Eaat1RNAi larvae.
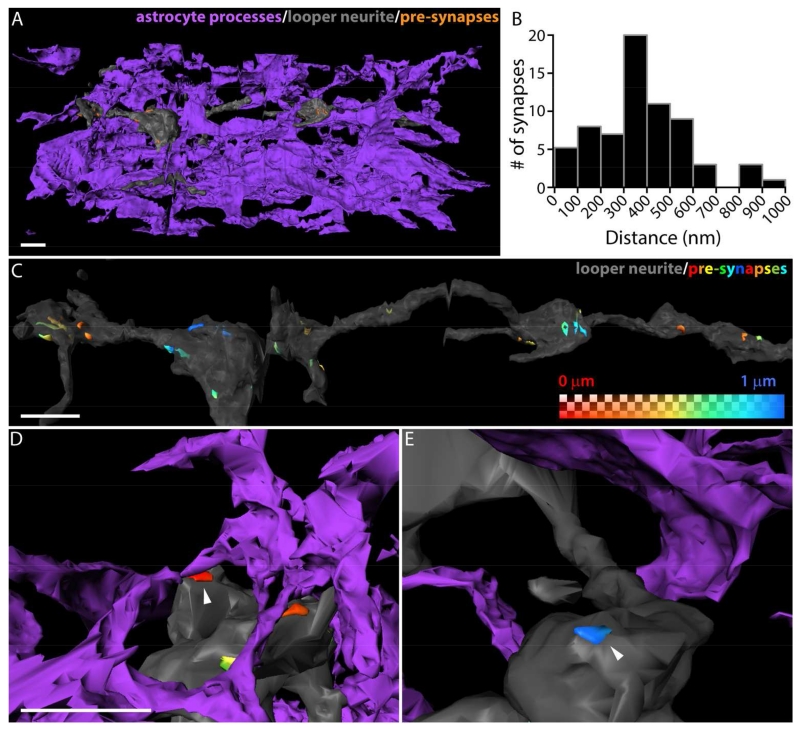
Drosophila astrocytic processes are found within 1 micron of looper synapses but do not ensheathe them
After observing astrocyte responses to looper neurons’ synaptically-released glutamate, we wanted to investigate the anatomical relationship between Drosophila astrocytic processes and looper synapses in this neuropil. We identified one member of the same population of neurons (loopers) used in the electrophysiology experiments described above in a 3D electron microscopic dataset that spans third-instar larval VNC segments A2-A4. In order to link the specific anatomy of looper synapses and astrocytic processes to our experimental physiology results, we first identified all 67 presynaptic sites on this particular looper neuron. Then we volumetrically reconstructed the looper neurite, all of its presynaptic sites, and the surrounding astrocytic processes. The resultant three-dimensional model (Figure 7A) illustrates the dense feltwork of astrocytic processes present within the third-instar larval VNC neuropil. This model was used to measure the distance from each point on the extracellular surface of the presynaptic site to the nearest astrocytic process. The shortest-distance value for each presynaptic site ranged from 0.0006 - 0.93 μm, with a mean of 0.375 μm +/− 0.2 (Figure 7B). These distances were used to generate a heat map that illustrates the range across presynaptic sites as well as within a given presynaptic site (Figure 7C-E). Complete ensheathment of a looper presynaptic site was never observed; in 2 cases (out of 67) the astrocytic process was close enough to the presynaptic site to be considered in direct contact. Our physiology results demonstrating astrocyte glutamate transporter currents in response to looper activation thus suggest that distances of up to at least a micron allow for communication between neurons and astrocytes.
Figure 7. Astrocytic processes are found within 1 micron of looper presynaptic sites but do not ensheathe them.
A. Three-dimensional reconstruction of a portion of the looper neurite (gray), and the surrounding astrocytic processes (purple). Some of the looper presynaptic sites are visible (orange). Scale bar = 1 μm. B. Distribution of shortest distance from a given active zone to nearest astrocytic process. All presynaptic sites on a fully reconstructed cell are included (mean = 375 nm +/− 200 nm, n = 67). C.-E. Reconstructed looper neurite, presynaptic sites are labeled with a heat map indicating the distance to the nearest astrocytic process. C. Astrocyte-process-to-presynaptic-site distance does not vary uniformly with location of the synapse on the neurite. Scale bar = 1 μm. D. Synapse at the minimum distance in the distribution (0.0006 μm, arrowhead) makes glancing contact with the presynaptic site. The astrocytic process does not ensheathe the synapse. Scale bar = 1 μm in D and E. E. Synapse from another neurite at the maximum distance in the distribution (0.93 μm, arrowhead).
Loss of astrocyte glutamate transport extends looper-evoked motor neuron IPSCs
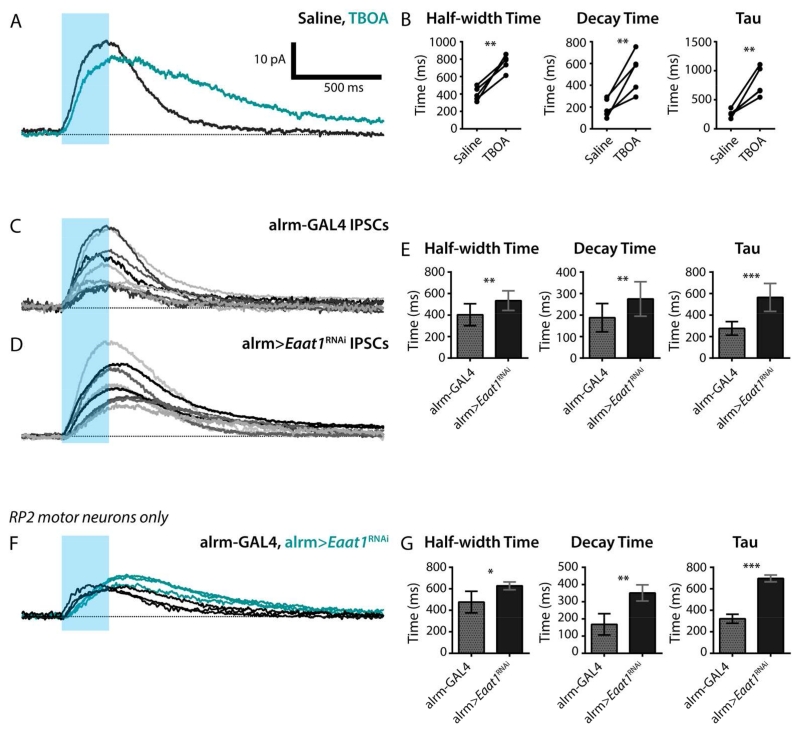
Given these anatomical parameters, we next asked what effect blocking astrocyte glutamate transporters, either pharmacologically or genetically, has on looper-to-motor neuron communication. To address this question, looper-evoked motor neuron inhibitory post-synaptic currents (IPSCs) were recorded in standard saline, and then exposed to TBOA, the same drug that blocked looper-evoked glutamate transporter currents in the astrocytes. This acute glutamate transporter block slowed the decay of IPSCs (Figure 8A), significantly increasing IPSC half-width time, decay time, and the time constant resulting from a single exponential fit (Figure 8B); no changes were found in IPSC rise time or amplitude (not shown).
Figure 8. Astrocyte-specific Eaat1 knockdown significantly prolongs IPSCs at the looper-MN synapse.
A. Optical stimulation of looper neurons (blue bar, stimulus window) during a whole-cell voltage-clamp recording results in motor neuron IPSCs. Standard saline (black), bath-application of the glutamate-transporter blocker TBOA (teal). Representative trace from 1 of 5 preparations. B. IPSC half-width time, decay time, and time constant (fit with a single exponential function) are shown for 5 data pairs in control and TBOA conditions. **, p < 0.01; ***, p < 0.001. C. Looper-evoked motor neuron IPSCs from 10 control larval VNCs. D. Looper-evoked motor neuron IPSCs from 8 larval VNCs with reduced astrocyte Eaat1. note: both control and experimental genotypes also include Looper-LexA>Chrimson. E. Mean half-width time, decay time, and time constant for control and alrm-GAL4>Eaat1RNAi. Error bars, +/− SD. **, p < 0.01; ***, p < 0.001. F-G. A subset of the data shown in C and D demonstrates that the kinetic parameters in E remain significant when only recordings from motor neuron RP2 are considered. F. Recordings from control (black, n = 3) and alrm GAL4>Eaat1RNAi (teal, n = 3) are overlaid to facilitate direct comparison of IPSC kinetics. Blue bar, light stimulus. G. Analysis of kinetics of looper-evoked motor neuron IPSCs. Mean +/− SD. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
A similar, but less dramatic, extension of the decay kinetics was observed when looper-evoked motor neuron IPSCs from control brains (normal Eaat1 levels) were compared with those from experimental brains with reduced Eaat1 levels (alrm-GAL4>Eaat1RNAi) (Figure 8C,D). Although the degree of change was attenuated in this chronic Eaat1 knockdown condition as compared to the acute, pharmacological block, changes in the IPSC half-width time, decay time, and the time constant of a single exponential fit remained significant (Figure 8E), and again, rise time and amplitude were unchanged (not shown). Among the ten motor neuron recordings shown for the control and eight motor neuron recordings shown for the experimental genotype in Figure 5C and D, respectively, the resultant dye-fills confirmed that three of the recordings in each group were from the motor neuron RP2 (MNISN-Is). Four of the remaining motor neurons in each group were identified as RP3/MN6/7-Ib, one was MNSNb/d-Is, and the remaining 3 were unidentifiable post-experiment due to incomplete dye-fills. This subset of RP2-only recordings was analyzed independently and replicated the results of the larger dataset containing motor neurons of heterogeneous identity (Figure 8F,G), except for the addition of a significant increase in IPSC amplitude in alrm-GAL4>Eaat1RNAi preparations (p<0.05, not shown).
Discussion
The subset of Drosophila glial cells examined in this study has been named ‘interface glia’ (Ito et al., 1995), ‘longitudinal glia’ (Beckervordersandforth et al., 2008), ‘astrocyte-like glia’ (Awasaki et al., 2008), and, most recently, just ‘astrocytes’ (Stork et al., 2014; Muthukumar et al., 2014; Peco et al., 2016), based on their morphology and molecular identity. The present study is the first to characterize the electrical membrane properties of Drosophila astrocytes. These whole-cell recordings indicate strong conservation of basic astrocyte properties across fly and vertebrate nervous systems, thus strengthening the argument that Drosophila astrocytes can be used to address very targeted and complex questions about general neuron-glia interactions. With these similarities in mind, our pairing of neuronal optogenetics and astrocyte electrophysiology with three-dimensional electron microscopy provides insight into critical anatomical determinants of neuron-glia interactions.
Drosophila astrocyte intrinsic properties are similar to those of vertebrate astrocytes
The linear I-V relationship, indicating passive membrane conductance as first noted by Kuffler and Nicholls (1966), has emerged as a common property of astrocytes across species (humans: Schroder et al., 2000; Han et al., 2013; rat: Steinhauser et al., 1994; Kressin et al., 1995; Bergles and Jahr, 1997; Clark and Barbour, 1997; Zhou and Kimelberg, 2000, 2001; Zhang et al., 2009; Uwechue et al., 2012; Huda et al., 2013; mouse: Matthias et al., 2003; Lalo et al., 2006; caiman: Zayas-Santiago et al., 2014). Much like the astrocyte membranes in each of these species, the fly astrocyte has a resting membrane potential that is similar to or more negative than the potassium equilibrium potential, low membrane resistance, and high membrane capacitance. While there has been some debate as to whether the passive-membrane property of astrocytes is truly cell-intrinsic, or rather, derives from an extensively coupled astrocyte network, we note in our recordings from Drosophila astrocytes that the large leak currents found in uncoupled cells were comparable to those recorded from cells later found to be dye-coupled to other astrocytes. This result is in agreement with a careful study of rat astrocytes conducted by Schools et al. (2006) and we similarly conclude that these passive properties are cell intrinsic and do not derive from the coupled network.
While astrocytes studied in vitro or in immature brain tissues display voltage-gated currents, those in mature, in vivo tissues overwhelmingly do not (Kafitz et al., 2008; reviewed in Ransom and Giaume, 2013). Here, we find little evidence for a significant voltage-gated current in third-instar Drosophila VNC astrocytes. The voltage-activated current remaining after leak subtraction is insensitive to Ca++ and K+ ion substitution and pharmacological agents that block voltage-gated sodium, calcium, and potassium currents. However, the membrane properties of the cell (low Rm) severely limit the spatial extent of the voltage-clamp, meaning that there is a potential difference between the soma and distal processes which in turn generates a significant axial-current flow, makes accurate leak subtraction difficult, and yields the current profile with transient and sustained epochs presented here. Because these same properties limit the spatial extent of the voltage clamp, we cannot rule out the presence of voltage-gated channels on fine, distal processes, where somatic voltage commands would have little influence on the local membrane potential.
Local dye-coupling was observed in some Drosophila astrocytes
The restriction of astrocyte coupling to cells within a hemisegment is reminiscent of findings in the olfactory bulb (Roux et al., 2011) and barrel cortex (Houades et al., 2008), where astrocytes were preferentially coupled within, and not across, anatomo-functional compartments (also reviewed in Giaume and Liu, 2012). We found no difference in the frequency of coupling in channelrhodopsin-stimulated and unstimulated preparations. Isolated larval CNS preparations do vary in their degree of spontaneous, rhythmic activity (Choi et al., 2004; Worrell and Levine, 2008; Berni et al., 2012), and it is plausible that these differences in spontaneous activity across preparations are causally related to the mixed dye-coupling results we obtained. We do not yet know the characteristics of patterns of neuronal activity that might influence changes in glial network connectivity; it is possible that the time course of the ChR2-mediated stimulation protocol we used was insufficient to affect coupling. Nevertheless, the presence of coupling in some preparations strongly suggests that dynamic coupling is present in Drosophila astrocytes and establishes that another defining feature of the vertebrate astrocyte is conserved in flies (coupling in mammals: reviewed in Ransom and Giaume, 2013; birds: Kafitz et al., 1999).
Glutamate transporter currents are mediated by Eaat1
Tight regulation of glutamate levels in the synaptic space is paramount to brain health across a variety of species; EAATs (excitatory amino acid transporters) regulate glutamate-mediated neuroplasticity, protect neurons from excitotoxicity caused by excessive glutamate, and have been implicated in a variety of neurodegenerative and neurological diseases (Nakagawa and Kaneko, 2013). Much like astrocytes in the rodent hippocampus (Bergles and Jahr, 1997; Meeks and Mennerick, 2007), cerebellum (Clark and Barbour, 1997), cortex (Lalo et al., 2006), spinal cord (Zhang et al., 2009), and brainstem (Huda et al., 2013), Drosophila astrocytes show an inward glutamate transporter current in response to neuronal activity. The current is completely blocked by astrocyte-specific expression (alrm-GAL4) of an RNAi hairpin targeting Eaat1. In addition to confirming the RNAi target using Eaat1 immunolabeling, we attempted to rescue the looper-evoked astrocyte current by adding a tagged construct (UAS-Eaat1-GFP) in addition to the RNAi, but this yielded very little GFP and no significant astrocyte current. Given this cellular phenotype, it is somewhat surprising that the larvae exhibit grossly normal locomotion and development. In contrast, flies carrying null mutations in Eaat1 do not survive beyond the first-instar larval stage (Stacey et al., 2010), and those with pan-glial-cell RNAi knockdown of Eaat1 (repo-GAL4) are viable but exhibit a shortened lifespan, neuropil degeneration, and locomotor phenotypes (namely, they can walk but cannot fly; Rival et al., 2004; Rival et al., 2006). This is unexpected given that Eaat1 immunolabeling is localized almost entirely to astrocytes; and alrm-driven RNAi knockdown eliminates any detectable labeling. Presumably, alrm-GAL4 mediated knockdown permits just enough protein production, at the necessary time(s) in embryonic and/or larval development, to allow grossly normal development and locomotor behavior, despite the demonstrated defect in third-instar astrocyte membrane currents.
Spatial relationships that support astrocyte regulation of synaptic signals
Astrocytic processes are found in close proximity to synapses in all brain regions investigated across species, but the degree of contact between astrocytes and synapses varies and is distributed nonuniformly within a region (Bernardinelli et al., 2014). At one extreme, cerebellar synapses formed by climbing fibers in rodents show an average degree of enwrapping around 87% (Xu-Friedman et al., 2001), whereas estimates of degree of enwrapping in the neocortex are lower and suggest that ensheathment is rare (<10% of synapses), and contact with the dendritic spine (but not the axon-spine interfaces) dominates (70%) (Genoud et al., 2006). The significance of these differences is poorly understood, but may involve 1) the stability and maturity of the synapse (Nishida and Okabe, 2007; Medvedev et al., 2014), 2) patterns of neuronal connectivity, such that some areas are by design more disposed to transmitter spillover (Bernardinelli et al., 2014), and 3) dynamic astrocytic process extension and retraction in response to neuronal activity and hormonal changes (Theodosis et al., 2008). These distances, however, should be viewed as relative, since these methods do not account for the actual diffusion pathway, which is tortuous, and all measurements taken from chemically-fixed EM preparations are subject to non-uniform shrinkage, making astrocytes appear to be located closer to synapses than they are in vivo (Korogod et al., 2015).
As in other species, the spatial relationships between synapses and astrocytic processes in the Drosophila larval VNC neuropil are non-uniform, even when considering the population of pre-synaptic sites along a single neuron. Our analysis of all 67 presynaptic-sites on the selected looper neuron does not offer any examples of complete ensheathment and indicates that direct astrocytic contact with the pre-synaptic site is also rare; yet no synapses were found at a distance greater than one micron from an astrocytic process. This upper limit of astrocyte-presynaptic distance (approximately one micron) may represent an important parameter for synapse function.
Although they did not examine the underlying anatomy, results of Liu et al. (2014) in the olfactory pathway of Drosophila lend support to the notion that astrocytes regulate synaptic signaling in this species: they overexpressed the TrpA1 channel in astrocytes and found that thermogenetic astrocyte activation modulates excitatory post-synaptic potentials in antennal-lobe projection neurons. The nature of the anatomical relationship between astrocytes and those synapses, however, was not investigated.
The current study clearly demonstrates that neurotransmitter homeostasis and fast regulation of the synaptic signal by transmitter transport are present in the neuropil of the Drosophila larval VNC. Our analysis of the same synapse-astrocyte interaction from both an anatomical and physiological perspective demonstrates that astrocytes modulate synaptic communication between looper neurons and motor neurons, despite minimal direct apposition to synaptic elements and a mean separation distance of 0.375 μm between looper presynaptic sites and astrocytic processes. We conclude that even without extensive contact, robust communication between these two cell types is present.
Summary
Taken with their previously noted gross anatomical and molecular similarities with astrocytes across the vertebrate phylum, the presence of shared core electrophysiological features in Drosophila astrocytes lends strong support to the argument that these astrocytes are functionally analogous to vertebrate astrocytes. This finding enhances the significance and broad predictive value of mechanistic, molecular genetic studies of astrocytes in the fly system. Our detailed exploration of the anatomical and physiological interactions between synapses and astrocytic processes demonstrates that astrocytes in Drosophila respond to activity at synapses and that glutamate transport by astrocytes affects the post-synaptic response in an anatomical configuration that features no ensheathment and infrequent direct contact. Thus, Drosophila gives us an example of a functionally tripartite interaction without a close, classically tripartite, anatomical relationship between synapses and astrocytic processes.
Table 1.
Summary of Experimental Genotypes
| Figures | Genotype |
|---|---|
| 1A | yw; P{w[+mC]=UAS-CD4-tdGFP}8M2; alrm-GAL4 |
| 1B-2E | w; P{w[+mC]=UAS-GFP.nls}14; alrm-GAL4 |
| 3A | yw; P{w[+mC]=UAS-CD4-tdGFP}8M2; D42-GAL4 |
| 3C,C1-3 | w; P{w[+mC]=UAS-GFP.nls}14/+; D42-GAL4/+ |
| 3D-4C | w; UAS-ChR2-H134R; D42-GAL4 w[1118]; P{y[+t7.7] w[+mC]=GMR50G08-lexA}attP40/+ ; |
| 5A-5C | P{y[+t7.7] w[+mC]=13XLexAop2-IVS-myr::GFP}attP2 / + w[1118]; P{y[+t7.7] w[+mC]=GMR50G08-lexA}attP40, alrm- GAL4 / + ; P{y[+t7.7] w[+mC]=13XLexAop2-IVS- |
| 5E,F; 6B | Chrimson.mVenus}attP2 / + w[1118]; P{y[+t7.7] w[+mC]=GMR50G08-lexA}attP40, alrm- GAL4 / UAS-RNAiCG3747{attP,y[+],w[3`]} ; P{y[+t7.7] |
| 6C | w[+mC]=13XLexAop2-IVS-Chrimson.mVenus}attP2 / + |
| 6D, D1 | w[1118]; +; alrm-GAL4/+ |
| 6D, D2 | w[1118]; UAS-RNAiCG3747{attP,y[+],w[3`]} ; alrm-GAL4/+ w[1118]; P{y[+t7.7] w[+mC]=GMR50G08-lexA}attP40, alrm- GAL4 / + ; P{y[+t7.7] w[+mC]=13XLexAop2-IVS- |
| 8A; 8C | Chrimson.mVenus}attP2 / + w[1118]; P{y[+t7.7] w[+mC]=GMR50G08-lexA}attP40, alrm- GAL4 / UAS-RNAiCG3747{attP,y[+],w[3`]} ; P{y[+t7.7] |
| 8D | w[+mC]=13XLexAop2-IVS-Chrimson.mVenus}attP2 / + |
| 8F | black traces: same as in 8C. Teal traces: same as in 8D. |
Other Acknowledgements
We thank Patricia Jansma, for her assistance with all things microscopy; Marvin Landis, for extensive guidance on 3D reconstruction; Casey Schneider-Mizell, for review of the looper skeleton; Kimberly Lance, for skillful lab management; and Emilie Peco and Don van Meyel, for kindly sharing reagents prior to publication. We also thank undergraduate lab members Si Woo Lee, Ernesto Hernandez, Leah Kaplan, and Neha Godbole for their skillful assistance throughout this project. SEM gratefully acknowledges the 2012 CSHL Drosophila Neurobiology Course (supported by NSF IOS-1238832 and NIDA 1R13DA034437-03) for invaluable training and networking.
Funding was provided by NSF grant IOS-1353739 to LAO, funds from the University of Arizona to LPT, HHMI Janelia funding to AC, and Swiss NSF grant 31003A_132969 to AC. SEM was supported by an NSF Graduate Research Fellowship, ARCS Foundation Phoenix Scholar Award, a University of Arizona Gruener Travel Award. KEL and CTT were supported by University of Arizona Neuroscience and Cognitive Science Summer Research Program funding, and KEL was additionally supported by a Microscopy Society of America Undergraduate Research Scholarship.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
Data Accessibility
Data generated in the lab and the subsequent analyses will be made available upon request to the Oland/Tolbert laboratory by qualified individuals as long as doing so would not compromise intellectual property interests or interfere with publication. Any shared data would include notations, standards, and the like that are necessary for interpretation of the data.
Literature Cited
- Altenhein B, Becker A, Busold C, Beckmann B, Hoheisel JD, Technau GM. Expression profiling of glial genes during Drosophila embryogenesis. Dev Biol. 2006;296:545–560. doi: 10.1016/j.ydbio.2006.04.460. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Lai S-L, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Bate M. Electrophysiological Development of Central Neurons in the Drosophila Embryo. J Neurosci. 1998;18:4673–4683. doi: 10.1523/JNEUROSCI.18-12-04673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth RM, Rickert C, Altenhein B, Technau GM. Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech Dev. 2008;125:542–557. doi: 10.1016/j.mod.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic Activation of Glutamate Transporters in Hippocampal Astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Muller D, Nikonenko I. Astrocyte-synapse structural plasticity. Neural Plast. 2014;2014:232105. doi: 10.1155/2014/232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni J, Pulver SR, Griffith LC, Bate M. Autonomous circuitry for substrate exploration in freely moving drosophila larvae. Curr Biol. 2012;22:1861–1870. doi: 10.1016/j.cub.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T, Rickmann M, Wolff J. The synapse-astrocyte boundary: an anatomical basis for an integrative role of glia in synaptic transmission. In: Volterra A, Magistretti P, Haydon P, editors. The tripartite synapse: glia in synaptic transmission. Oxford University Press; New York, NY: 2002. pp. 3–23. [Google Scholar]
- Choi JC, Park D, Griffith LC. Electrophysiological and morphological characterization of identified motor neurons in the Drosophila third instar larva central nervous system. J Neurophysiol. 2004;91:2353–2365. doi: 10.1152/jn.01115.2003. [DOI] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol. 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Budd J, Freeman MR. Probing the enigma: unraveling glial cell biology in invertebrates. Curr Opin Neurobiol. 2013;23:1073–9. doi: 10.1016/j.conb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully DF, Paress PS, Liu KK, Schaeffer JM, Arena JP. Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J Biol Chem. 1996;271:20187–91. doi: 10.1074/jbc.271.33.20187. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–6. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Doherty J, Logan MA, TaYdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Leemans R, Loop T, Kammermeier L, Fan Y, Radimerski T, Strahm MC, Certa U, Reichert H. Gliogenesis in Drosophila: genome wide analysis of downstream genes of glial cells missing in the embryonic nervous system. Development. 2002;129:3295–3309. doi: 10.1242/dev.129.14.3295. [DOI] [PubMed] [Google Scholar]
- Fei H, Chow DM, Chen A, Romero-Calderón R, Ong WS, Ackerson LC, Maidment NT, Simpson JH, Frye MA, Krantz DE. Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. J Exp Biol. 2010;213:1717–30. doi: 10.1242/jeb.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4:e343. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Liu X. From a glial syncytium to a more restricted and specific glial networking. J Physiol Paris. 2012;106:34–9. doi: 10.1016/j.jphysparis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rehm EJ, Goodman CS. Genetic Analysis of Growth Cone Guidance in Drosophila: Fasciclin II Functions as a Neuronal Recognition Molecule. Cell. 1991;67(1):45–57. doi: 10.1016/0092-8674(91)90571-f. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Möller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–43. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Grosjean Y, Grillet M, Augustin H, Ferveur J-F, Featherstone DE. A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat Neurosci. 2008;11:54–61. doi: 10.1038/nn2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Jan LY, Jan Y-N. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc Natl Acad Sci U S A. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–53. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J, Levine RB. Calcium and potassium current in leg motoneurons during postembryonic development in the hawkmoth Manduca Sexta. J exp Biol. 1992;171:15–42. doi: 10.1242/jeb.171.1.15. [DOI] [PubMed] [Google Scholar]
- Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. Gap junction mediated astrocytic networks in the mouse barrel cortex. J Neurosci. 2008;28:5207–17. doi: 10.1523/JNEUROSCI.5100-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda R, McCrimmon DR, Martina M. pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol. 2013;110:368–77. doi: 10.1152/jn.01074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux’s Arch Dev Biol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Iyengar BG, Chou CJ, Vandamme KM, Klose MK, Zhao X, Akhtar-Danesh N, Campos a. R, Atwood HL. Silencing synaptic communication between random interneurons during Drosophila larval locomotion. Genes, Brain Behav. 2011;10:883–900. doi: 10.1111/j.1601-183X.2011.00729.x. [DOI] [PubMed] [Google Scholar]
- Jan L, Jan Y. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Güttinger HR, Müller CM. Seasonal changes in astrocytes parallel neuronal plasticity in the song control area HVc of the canary. Glia. 1999;27:88–100. [PubMed] [Google Scholar]
- Kafitz KW, Meier SD, Stephan J, Rose CR. Developmental profile and properties of sulforhodamine 101--Labeled glial cells in acute brain slices of rat hippocampus. J Neurosci Methods. 2008;169:84–92. doi: 10.1016/j.jneumeth.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–53. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GKS, Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–46. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka H, Takasu E, Morimoto T, Nose A. A group of segmental premotor interneurons regulates the speed of axial locomotion in Drosophila larvae. Curr Biol. 2014;24:2632–42. doi: 10.1016/j.cub.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Korogod N, Petersen CCH, Knott GW. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife. 2015:4. doi: 10.7554/eLife.05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressin K, Kuprijanova E, Jabs R, Seifert G, Steinhäuser C. Developmental regulation of Na+ and K+ conductances in glial cells of mouse hippocampal brain slices. Glia. 1995;15:173–87. doi: 10.1002/glia.440150210. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG. The physiology of neuroglial cells. Ergebnisse der Physiol Biol Chemie und Exp Pharmakologie. 1966;57:1–90. [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–83. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Sánchez-Soriano N, Technau GM, Urban J, Prokop A. Charting the Drosophila neuropile: A strategy for the standardised characterisation of genetically amenable neurites. Dev Biol. 2003;260:207–225. doi: 10.1016/s0012-1606(03)00215-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhou B, Yan W, Lei Z, Zhao X, Zhang K, Guo A. Astrocyte-like glial cells physiologically regulate olfactory processing through the modification of ORN PN synaptic strength in Drosophila. Eur J Neurosci. 2014;40:2744–54. doi: 10.1111/ejn.12646. [DOI] [PubMed] [Google Scholar]
- Liu WW, Wilson RI. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc Natl Acad Sci U S A. 2013;110:10294–9. doi: 10.1073/pnas.1220560110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr A, Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: A marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr Patterns. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Marley R, Baines RA. Whole-cell patch recording from Drosophila larval neurons. Cold Spring Harb Protoc. 2011 doi: 10.1101/pdb.prot065664. 2011. [DOI] [PubMed] [Google Scholar]
- Mathew D, Popescu A, Budnik V. Drosophila amphiphysin functions during synaptic Fasciclin II membrane cycling. J Neurosci. 2003;23:10710–6. doi: 10.1523/JNEUROSCI.23-33-10710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated Expression of AMPA-Type Glutamate Receptors and Glutamate Transporters Defines Distinct Astrocyte Populations in the Mouse Hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Medvedev N, Popov V, Henneberger C, Kraev I, Rusakov D a, Stewart MG. Glia selectively approach synapses on thin dendritic spines. Philos Trans R Soc Lond B Biol Sci. 2014;369:20140047. doi: 10.1098/rstb.2014.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Astrocyte membrane responses and potassium accumulation during neuronal activity. Hippocampus. 2007;17:1100–1108. doi: 10.1002/hipo.20344. [DOI] [PubMed] [Google Scholar]
- Mezler M, Muller T, Raming K. Cloning and functional expression of GABAB receptors from Drosophila. Eur J Neurosci. 2001;13:477–486. doi: 10.1046/j.1460-9568.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- Muthukumar AK, Stork T, Freeman MR. Activity-dependent regulation of astrocyte GAT levels during synaptogenesis. Nat Neurosci. 2014;17:1340–1350. doi: 10.1038/nn.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kaneko S. SLC1 glutamate transporters and diseases: psychiatric diseases and pathological pain. Curr Mol Pharmacol. 2013;6:66–73. doi: 10.2174/18744672113069990033. [DOI] [PubMed] [Google Scholar]
- Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol. 2011;21:625–34. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27:331–40. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of Astrocytic Form and Function. In: Milner R, editor. Astrocytes. Vol. 814. Humana Press; 2012. pp. 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, Mensh BD, Branson KM, Simpson JH, Truman JW, Cardona A, Zlatic M. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]
- Peco E, Davla S, Camp D, Stacey S, Landgraf M, van Meyel D. Drosophila astrocytes cover specific territories of CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development. 2016 doi: 10.1242/dev.133165. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity P a, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–88. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Budnik V. Embryonic and Larval Neuromuscular Junction. In: Zhang B, Freeman MR, Waddell S, editors. Drosophila Neurobiology: A Laboratory Manual. 1st ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2010. pp. 93–123. [Google Scholar]
- Ransom BR, Giaume C. Gap Junction and Hemichannels. In: Kettenmann H, Ransom BR, editors. Neuroglia. 3rd ed. Oxford University Press; New York, NY: 2013. pp. 292–305. [Google Scholar]
- Rival T, Soustelle L, Cattaert D, Strambi C, Iché M, Birman S. Physiological requirement for the glutamate transporter dEAAT1 at the adult Drosophila neuromuscular junction. J Neurobiol. 2006;66:1061–1074. doi: 10.1002/neu.20270. [DOI] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson M-T, Iché M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol. 2004;14:599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. J Neurophysiol. 2002;88:847–60. doi: 10.1152/jn.2002.88.2.847. [DOI] [PubMed] [Google Scholar]
- Roux L, Benchenane K, Rothstein JD, Bonvento G, Giaume C. Plasticity of astroglial networks in olfactory glomeruli. Proc Natl Acad Sci U S A. 2011;108:18442–6. doi: 10.1073/pnas.1107386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfeld S, Cardona A, Hartenstein V, Tomancak P. CATMAID: collaborative annotation toolkit for massive amounts of image data. Bioinformatics. 2009;25:1984–6. doi: 10.1093/bioinformatics/btp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Mizell CM, Gerhard S, Longair M, Kazimiers T, Li F, Zwart MF, Champion A, Midgley F, Fetter R, Saalfeld S, Cardona A. Quantitative neuroanatomy for connectomics in Drosophila. eLIFE. doi: 10.7554/eLife.12059. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J Neurophysiol. 2006;96:1383–92. doi: 10.1152/jn.00449.2006. [DOI] [PubMed] [Google Scholar]
- Schroder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP, Steinhauser C. Functional and Molecular Properties of Human Astrocytes in Acute Hippocampal Slices Obtained from Patients with Temporal Lobe Epilepsy. Epilepsia. 2000;41:S181–S184. doi: 10.1111/j.1528-1157.2000.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, LeBrun B, Yasuda-kamatani Y, Sakaitani M, Shigeri Y. A Potent Blocker of Excitatory Amino Acid Transporters. Mol. Pharmacol. 1998;201:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Stacey SM, Muraro NI, Peco E, Labbé A, Thomas GB, Baines R a, van Meyel DJ. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J Neurosci. 2010;30:14446–14457. doi: 10.1523/JNEUROSCI.1021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings LA, Todman MG, Phillips R, Greer CE, Tam J, Phelan P, Jacobs K, Bacon JP, Davies JA. Gap junctions in Drosophila: Developmental expression of the entire innexin gene family. Mech Dev. 2002;113:197–205. doi: 10.1016/s0925-4773(02)00025-4. [DOI] [PubMed] [Google Scholar]
- Steinhäuser C, Kressin K, Kuprijanova E, Weber M, Seifert G. Properties of voltage activated Na+ and K+ currents in mouse hippocampal glial cells in situ and after acute isolation from tissue slices. Pflugers Arch. 1994;428:610–20. doi: 10.1007/BF00374585. [DOI] [PubMed] [Google Scholar]
- Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR. Neuron Glia Interactions through the Heartless FGF Receptor Signaling Pathway Mediate Morphogenesis of Drosophila Astrocytes. Neuron. 2014;83:388–403. doi: 10.1016/j.neuron.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagawa K, Salvaterra P. Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci Res. 1996;24:237–43. doi: 10.1016/0168-0102(95)00999-x. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SHR. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Thimgan MS, Berg JS, Stuart AE. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J Exp Biol. 2006;209:3383–404. doi: 10.1242/jeb.02328. [DOI] [PubMed] [Google Scholar]
- Uwechue NM, Marx M-C, Chevy Q, Billups B. Activation of glutamate transport evokes rapid glutamine release from perisynaptic astrocytes. J Physiol. 2012;590:2317–31. doi: 10.1113/jphysiol.2011.226605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilatis DK, Elliston KO, Paress PS, Hamelin M, Arena JP, Schaeffer JM, Van der Ploeg LH, Cully DF. Evolutionary relationship of the ligand-gated ion channels and the avermectin-sensitive, glutamate-gated chloride channels. J Mol Evol. 1997;44:501–8. doi: 10.1007/pl00006174. [DOI] [PubMed] [Google Scholar]
- Venken KJT, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Butt AM. Glial Physiology and Pathophysiology. Wilet-Blackwell; 2013. [Google Scholar]
- Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc B Biol Sci. 2014;369:20130595–20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme AJ. Glutamate-gated chloride channels. J Biol Chem. 2012;287:40232–8. doi: 10.1074/jbc.R112.406280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell JW, Levine RB. Characterization of voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J Neurophysiol. 2008;100:868–878. doi: 10.1152/jn.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman M a, Harris KM, Regehr WG. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci. 2001;21:6666–6672. doi: 10.1523/JNEUROSCI.21-17-06666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Horiuchi J, Ueno K, Ueno T, Saeki S, Matsuno M, Naganos S, Miyashita T, Hirano Y, Nishikawa H, Taoka M, Yamauchi Y, Isobe T, Honda Y, Kodama T, Masuda T, Saitoe M. Glial Dysfunction Causes Age-Related Memory Impairment in Drosophila. Neuron. 2014;84:753–763. doi: 10.1016/j.neuron.2014.09.039. [DOI] [PubMed] [Google Scholar]
- Yeh E, Gustafson K, Boulianne GL. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci U S A. 1995;92:7036–40. doi: 10.1073/pnas.92.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas-Santiago A, Agte S, Rivera Y, Benedikt J, Ulbricht E, Karl A, Dávila J, Savvinov A, Kucheryavykh Y, Inyushin M, Cubano LA, Pannicke T, Veh RW, Francke M, Verkhratsky A, Eaton MJ, Reichenbach A, Skatchkov SN. Unidirectional photoreceptor-to-Müller glia coupling and unique K+ channel expression in Caiman retina. PLoS One. 2014;9:e97155. doi: 10.1371/journal.pone.0097155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xin W, Dougherty PM. Synaptically evoked glutamate transporter currents in Spinal Dorsal Horn Astrocytes. Mol Pain. 2009;5:36. doi: 10.1186/1744-8069-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly Isolated Astrocytes From Rat Hippocampus Show Two Distinct Current Patterns and Different [K+]o Uptake Capabilities. J Neurophysiol. 2000;84:2746–2757. doi: 10.1152/jn.2000.84.6.2746. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly Isolated Hippocampal CA1 Astrocytes Comprise Two Populations Differing in Glutamate Transporter and AMPA Receptor Expression. J Neurosci. 2001;21:7901–7908. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]