ABSTRACT
Severe hypothermia has been shown to influence the levels of catecholamines and thrombomodulin, an endothelial protein essentially involved in the regulation of haemostasis and inflammation. A link between thrombomodulin and catecholamines during cold exposure has also been previously suggested. The aim of this study was to assess the influence of short-term cold exposure without hypothermia on catecholamines and the circulating and urinary thrombomodulin levels. Seven healthy male subjects were immersed in cold water (+10°C) for 10 minutes followed by a 20-minute immersion in +28°C water. Warm water immersion was performed separately for each subject (+30°C for 30 minutes). Thrombomodulin and catecholamine concentrations were measured from pre- and post-immersion (up to 23 hours) samples. In urine, the thrombomodulin level correlated strongly with adrenaline (ρ = 0.806) and noradrenaline (ρ = 0.760) levels. There were no significant differences in thrombomodulin levels between immersion temperatures. Post-immersion urinary thrombomodulin levels were significantly lower than the pre-immersion level at both immersion temperatures. Median concentrations of plasma noradrenaline and urinary adrenaline were higher after exposure to +10°C than to +30°C. Thus, further evidence of the association between thrombomodulin and catecholamines was gained in a physiologically relevant setting in humans. Additionally, it is evident that a short-term cold exposure was not able to elicit changes in the thrombomodulin levels in a follow-up period of up to 23 hours. These findings provide further understanding of the physiological responses to cold during immersion, and of the potential influence of stress on haemostatic and inflammatory responses.
KEYWORDS: catecholamines, cold stress, haemostasis, hypothermia, immersion, inflammation, thrombomodulin
Introduction
Thrombomodulin (TM) is a transmembrane protein expressed in the endothelial cells of most blood vessels.1 TM functions essentially as anticoagulant and anti-inflammatory regulator by acting as cofactor for thrombin.2 The TM-thrombin complex elicits these effects mainly by activating protein C.3 However, the complex also inhibits fibrinolysis through the activation of thrombin-activatable fibrinolysis inhibitor.4 TM is found in the circulation and urine as soluble fragments which retain these functionalities.5
Recently, we have shown that rats exposed to severe hypothermia had low TM levels in circulation and myocardium while the myocardial TM transcript level was increased.6 In autopsy material, lethal hypothermia was associated with low myocardial TM transcript and TM protein levels as well as low urinary TM level compared with other causes of death.7 Furthermore, the circulating TM levels have been shown to increase in patients with induced milder hypothermia compared with normothermic patients after approximately a 2-hour period.8 However, no changes were seen in healthy subjects immediately after a 10-minute dry cold exposure.9
Cold exposure is also known to increase the levels of catecholamines, adrenaline and noradrenaline.10 High urinary adrenaline and adrenaline-to-noradrenaline levels have been suggested as indicators of lethal hypothermia.11-13 Furthermore, high circulating adrenaline level was associated with lowered myocardial TM expression in rats, and positive correlation was found between thrombomodulin and adrenaline concentrations in plasma.6 Significant correlations between TM and catecholamine levels were also seen in autopsy material.7 These findings suggested that there could be a regulatory link between catecholamines and TM during severe cold exposure, possibly via protein kinase A signaling pathway.
Although an association between TM and catecholamines has been described in hypothermia-related settings, it is unclear whether this connection also exists in physiological conditions. Furthermore, the influence of short-term cold stress on the circulating and urinary TM levels in a prolonged follow-up is unresolved. More detailed knowledge of TM secretion during and after cold exposure, as well as its possible regulatory pathways would give valuable information about the effects of cooling on haemostasis and other essential functions. Thus, the aim of this study was to test the hypotheses that TM and catecholamine levels are related in non-hypothermic conditions, and that the correlations might differ in cold and warm water exposures. We also hypothesized that short-term cold exposure could cause changes in TM levels during a follow-up period of up to 23 hours.
Material and methods
Subjects
Following approval of the experimental protocol by the University of Oulu and Northern Ostrobothnia Hospital research ethics committee and obtaining written informed consent, 7 healthy, non-smoking male participants volunteered for the study. Only males were recruited to reduce the dispersion of the results in a small study population. The characteristics (mean ± standard deviation) of the participants were: age, 26 ± 2 years; height, 178 ± 4 cm; weight, 75 ± 7 kg; body mass index, 23.7 ± 1.6 kg/m2. Maximal exercise tests were conducted to assess their physical condition. The mean maximal oxygen consumption was 52.3 ± 5.2 ml/kg/min. The subjects were informed of the nature and risks of the experiment.
Experimental protocol
The experiment was carried out in the laboratory of physiology at the Finnish Institute of Occupational Health, Oulu, Finland (65°N 25°E). Any potential effects of cold acclimatisation were controlled by carrying out the experiments in late spring and early summer (between 6th May and 27th June). The subjects participated in the tests at least 2 hours after a light breakfast and were instructed not to use coffee or alcohol the evening before the measurements (>12 hours). They were instructed to avoid strenuous exercise and to sleep normally (e.g. 8 hours) during the preceding night. Two immersions of different exposure temperatures were conducted in random order for each subject. The immersions started at 9:00 a.m. (UTC +2).
The immersions were accomplished with head-out immersion in upright posture in a pool of water with a temperature of either +10°C or +30°C. The duration of the +10°C exposure was 10 minutes followed by an immersion in a pool with water temperature of +28°C for 20 minutes. This was done so as to limit the severity of the exposure, and also to allow further heat loss in a slightly below thermoneutral temperature. In the +30°C exposure, the same water temperature was used for the corresponding time (10 + 20 minutes). The total immersion time was thus 30 minutes. Blood and urine samples were taken before and after the immersions according to the schedule presented in Table 1.
Table 1.
The schedule of immersions and sampling.
| Samples taken |
|||
|---|---|---|---|
| Cumulative time from the start of immersion (h:min) | Blood | Urine | |
| −1:00 | x | x | |
| 0:00 (start of immersion) | x | ||
| 0:30 (end of immersion) | x | ||
| 1:00 | x | x | |
| 2:00 | x | ||
| 4:00 | x | x | |
| 6:00 | x | x | |
| 23:00 | x | x | |
Temperature measurements
Skin temperatures were measured from 10 sites: forehead, upper back, chest, abdomen, upper arm, lower arm, back of the hand, anterior thigh, dorsal side of the foot and calf (NTC DC 95, Digi Key, USA). The thermistors were attached to the skin with adhesive tape. Rectal temperature (Trect) was measured 10 cm beyond the anal sphincter with an YSI 401 probe (Yellow Springs Instrument Co., Yellow Springs, USA). Skin and rectal temperature values were recorded at 30-s intervals with a datalogger (SmartReader 8+, ACR Systems, Canada) throughout the cold and warm exposure. Mean skin temperature (Tsk) was calculated as an area-weighted average according to the following formula: 0.07 • (Tforehead) + 0.35 • mean(Tchest, Tscapula, Tabdomen) + 0.14 • mean(Tupper arm, Tlower arm) + 0.05 • (Tdorsal hand) + 0.19 • (Tthigh), + 0.13 • (Tcalf) + 0.07 • (Tdorsal side of foot).14 Mean body temperature (Tb) was calculated by the equation: Tb = 0.65 • Trect + 0.35 • Tsk.15
TM and catecholamine analyses
TM and catecholamine (adrenaline and noradrenaline) concentrations in blood and urine samples were measured with enzyme-linked immunosorbent assay (TM: Quantikine Human Thrombomodulin Immunoassay kit, R&D Systems, Inc.., Minneapolis, MN, USA; catecholamines: Cat Combi kit, DRG, Marburg, Germany) according to the instructions given by the manufacturers. According to the manufacturer, the mean minimum detectable dose of TM with the assay was 7.82 pg/ml. Coefficients of variance for intra/inter-assay precisions were <4% and <8%, respectively. The analytical sensitivities for adrenaline and noradrenaline were 0.011 ng/ml and 0.044 ng/ml, respectively. Coefficients of variance for intra-assay precisions were 15.0% for adrenaline and 16.1% for noradrenaline at 2.5 ng/ml and 24.4 ng/ml levels, respectively. The analyses of TM and catecholamines were done in the laboratory of the Department of Forensic Medicine, University of Oulu. Osmolality of the urine samples were measured in the laboratory of Oulu University Hospital (NordLab, Oulu). Haematocrit was analyzed with a Coulter Hematology Analyzer (Beckman Coulter Inc.., Brea, CA, USA).
Statistical analyses
Statistical analyses were done with IBM SPSS Statistics 21 (IBM, Armonk, NY, USA). Values from different time points were analyzed as pre- and post-immersion sample groups. Differences between median values of TM, adrenaline and noradrenaline were analyzed with Mann-Whitney non-parametric test, and the Benjamini-Hochberg corrections procedure was used to control false discovery rate in multiple comparisons. Correlations between TM and catecholamines were analyzed with Spearman's correlation coefficient. Data is given as medians and range unless stated otherwise.
Results
Changes in temperature
The +10°C immersion decreased the mean Tsk (Fig. 1A) and Tb (Fig. 1C) maximally by 11.1°C and 3.8°C, respectively. There were no obvious effects on Tsk and Tb at +30°C. Trect remained fairly constant in both exposure temperatures (Fig. 1B).
Figure 1.
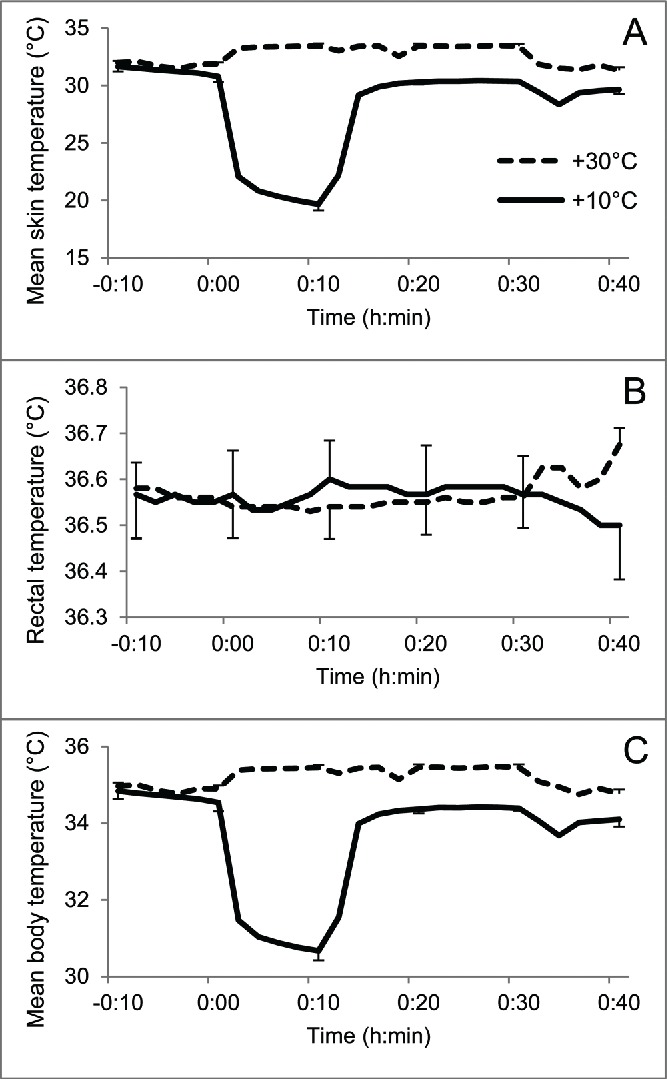
The temperature data during immersions. Mean skin (A) and body temperatures (C) decreased during immersion in +10°C water, but the rectal temperature (B) was unaffected. No obvious changes were seen in immersion to +30°C water. Error bars represent standard error.
Haematocrit and urine osmolality
Median urinary osmolality was higher before immersion (658.0, 250.0–924.0 mosm kg[H2O]−1) than after exposure to +10°C (421.0, 140.0–822.0 mosm kg[H2O]−1) or +30°C (338.5, 143.0–955.0 mosm kg[H2O]−1). Mean (±SE) haematocrit was higher after exposure to +10°C (46.6 ± 0.4%) than to +30°C (44.7 ±0.4%). The pre-immersion haematocrit was 46.1 ± 0.5%.
TM concentrations
The pre-immersion median TM concentration was 4.54 (3.34–6.41) ng/ml in plasma and 15.97 (1.05–32.04) ng/ml in urine for both immersions. The post-immersion osmolality-corrected urinary TM levels were significantly lower in both exposure temperatures compared with the pre-immersion level (Fig. 2A). There were no significant differences between exposure to +10°C or +30°C, or in the plasma TM levels. Proportioning the plasma TM levels with haematocrit did not cause significant changes to the results (data not shown).
Figure 2.
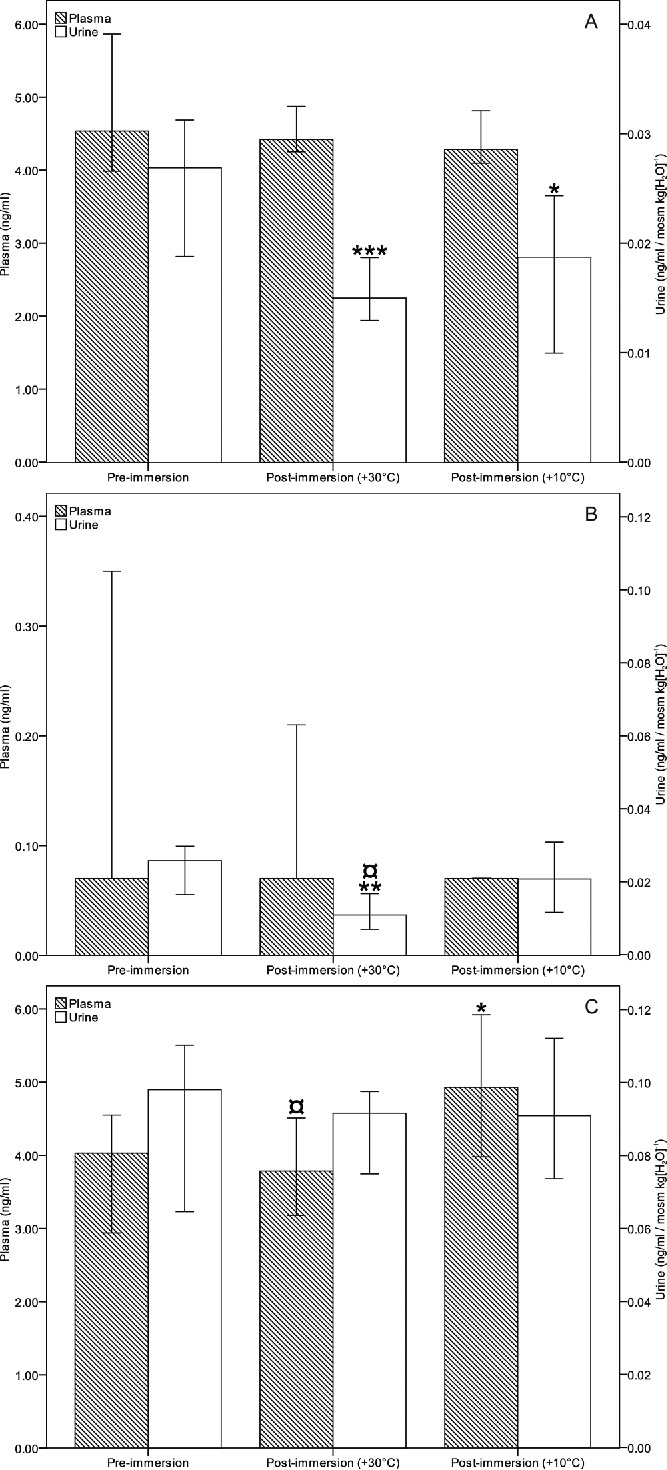
Median levels of thrombomodulin (A), adrenaline (B) and noradrenaline (C) in plasma and urine (corrected with osmolality). *P < 0.05, **P < 0.01, ***P < 0.001 compared with pre-immersion level; ¤P < 0.05 compared with +10°C immersion. Error bars represent 95% confidence interval.
Catecholamine concentrations
The median plasma noradrenaline concentration (Fig. 2C) was increased after immersion to +10°C (4.93, 1.55–12.29 ng/ml) compared with the pre-immersion level (4.03, 1.64–6.98 ng/ml; p = 0.017) or the exposure to +30°C (3.79, 1.97–7.24 ng/ml; p = 0.021). There were no significant differences between plasma adrenaline levels. Proportioning the plasma catecholamine levels with haematocrit did not cause significant changes to the results (data not shown). Urinary adrenaline level (Fig. 2B) was lower in +30°C exposure (4.04, 0.72–40.81 ng/ml) than the pre-immersion level (13.27, 1.66–39.20 ng/ml) or in the +10°C exposure (7.91, 0.94–40.31 ng/ml). The differences were significant after osmolality correction (p = 0.003 and p = 0.011 compared with pre-immersion level and +10°C, respectively).
Correlations between TM and catecholamines
Urinary adrenaline and noradrenaline levels correlated strongly with the urinary TM concentration (Table 2). The correlations were observed in both pre- and post-immersion data, and the correlations were similar at both exposure temperatures. Plasma TM level exhibited a weak to moderate inverse relation to urinary adrenaline and plasma noradrenaline level.
Table 2.
The correlations between thrombomodulin (TM) and catecholamine parameters.
| Urine TM | Plasma TM | |||
|---|---|---|---|---|
| ρ | p | ρ | p | |
| Urine adrenaline | 0.806 | < 0.001 | −0.471 | 0.003 |
| Urine noradrenaline | 0.760 | < 0.001 | −0.323 | 0.048 |
| Plasma adrenaline | 0.094 | ns | 0.121 | ns |
| Plasma noradrenaline | −0.107 | ns | −0.385 | 0.002 |
ns, not significant.
Discussion
The urinary TM and catecholamine levels correlated significantly in pre- and post-immersion samples. Similar correlations have been demonstrated in plasma and urine in both cold-related6,7 and non-cold-related16 settings. In rats, a high circulating adrenaline level correlated with low cellular TM transcript levels.6 Based on these findings, it is plausible that adrenaline — and possibly also noradrenaline — increase the release of TM from endothelium to circulation and inhibit the transcriptional activation of TM.
The short-term cold exposure with significant superficial cooling but without decrease in core temperature was not able to produce significant changes in the TM levels in plasma or urine in this study. In our previously conducted study with rats, mild hypothermia was associated with lower urinary and higher circulating TM levels compared with severe hypothermia.6 However, the urine of lethal hypothermia victims contained lower TM concentrations7 than the baseline values measured in the present study. These findings would suggest that the elimination of TM is diminished in the first phases of cold exposure, possibly reflecting an increased need in the circulation. In prolonged, severe exposure, the production mechanisms are probably slowed down or exhausted.
The TM levels in urine were significantly lower during the follow-up period of both exposure temperatures than before immersion. It is, thus, possible that the immersion itself might have influence on the results. There is no published data on the effects of water immersion on TM expression or secretion, but it is known to affect hemodynamic parameters. Immersion increases central plasma volume and cardiac output, and causes a relative hyperperfusion of peripheral tissues.17-19 Some changes in haemostatic potential may also occur during head-out immersion in thermoneutral water, demonstrated by a decrease in prothrombin time and the activity of plasminogen activator inhibitor.20 Thus, the influence of water immersion and the subsequent rise in external hydrostatic pressure seems to be the main contributing factor affecting the concentrations of circulating and urinary TM.
An increase in the plasma noradrenaline level was produced by exposure to +10°C, but not with the exposure to +30°C. Also, the post-immersion levels of urinary adrenaline were higher in +10°C than +30°C temperature. It is well-known that the circulating noradrenaline level reacts more sensitively to cold stress,10 and adrenomedullary activation generally requires a more severe stimulation.21 However, high levels of urinary adrenaline have been regarded more specific to severe hypothermia than noradrenaline.12,13
The cold-induced increase in diuresis is a well-known phenomenon,22 associated with a concomitant decrease in urine osmolality.23 To eliminate the influence of dilution on the results, the TM and catecholamine concentrations were proportioned to the respective osmolality of the samples. Thus, the pre- and post-immersion analyses can be regarded comparable. Urine creatinine has been proposed for the same purpose,24 but osmolality could be less sensitive to changes in renal function. Proportioning the plasma levels of TM and catecholamines with haematocrit, in order to control for haemodilution or haemoconcentration, did not cause significant changes to the results.
In conclusion, further evidence of the association between TM and catecholamines was gained in healthy subjects, suggesting that these potential regulatory mechanisms are valid in a physiological setting. A short-term cold exposure causing superficial cooling and increase in catecholamine levels was not able to elicit changes in TM levels during a follow-up of up to 23 hours. These observations provide further understanding of the responses of the body to stress factors such as cold and immersion. The findings could be useful, for instance, in developing diagnostic methods for assessing cold stress, or therapeutic tools concerning haemostatic and inflammatory processes. However, future studies are needed to reveal the exact regulatory pathways of TM expression and secretion on cellular level.
Abbreviations
- Tb
mean body temperature
- Trect
rectal temperature
- Tsk
mean skin temperature
- TM
thrombomodulin
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Ms Helmi Konola for her assistance in analyzing the samples.
References
- [1].Maruyama I, Bell CE, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol 1985; 101:363-71; PMID:2991298; http://dx.doi.org/ 10.1083/jcb.101.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol 2004; 24:1374-83; PMID:15178554; http://dx.doi.org/ 10.1161/01.ATV.0000134298.25489.92 [DOI] [PubMed] [Google Scholar]
- [3].Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A 1981; 78:2249-52; PMID:7017729; http://dx.doi.org/ 10.1073/pnas.78.4.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem 1996; 271:16603-8; PMID:8663147; http://dx.doi.org/ 10.1074/jbc.271.28.16603 [DOI] [PubMed] [Google Scholar]
- [5].Ishii H, Majerus PW. Thrombomodulin is present in human plasma and urine. J Clin Invest 1985; 76:2178-81; PMID:3001144; http://dx.doi.org/ 10.1172/JCI112225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kaija H, Pakanen L, Uusitalo J, Nikkilä S, Kortelainen M-L, Porvari KS. Changes in cardiac thrombomodulin and heat shock transcription factor 1 expression and peripheral thrombomodulin and catecholamines during hypothermia in rats. Stress 2014; 17:504-11; PMID:25109347; http://dx.doi.org/ 10.3109/10253890.2014.953477 [DOI] [PubMed] [Google Scholar]
- [7].Pakanen L, Kaija H, Kortelainen M-L, Särkioja T, Porvari K. Victims of lethal hypothermia have decreased levels of thrombomodulin in myocardium and urine. Int J Legal Med 2015; 129:289-96; PMID:25543320; http://dx.doi.org/ 10.1007/s00414-014-1138-2 [DOI] [PubMed] [Google Scholar]
- [8].Boldt J, Knothe C, Welters I, Dapper FL, Hempelmann G. Normothermic versus hypothermic cardiopulmonary bypass: Do changes in coagulation differ? Ann Thorac Surg 1996; 62:130-5; PMID:8678631; http://dx.doi.org/ 10.1016/0003-4975(96)00239-1 [DOI] [PubMed] [Google Scholar]
- [9].Matsuda J, Tsukamoto M, Gohchi K, Saitoh N, Miyajima Y, Kazama M. Effect of total-body cold exposure on plasma concentrations of von Willebrand factor, endothelin-1 and thrombomodulin in systemic lupus erythematosus patients with or without Raynaud's phenomenon. Acta Haematol 1992; 88:189-93; PMID:1337960; http://dx.doi.org/ 10.1159/000204684 [DOI] [PubMed] [Google Scholar]
- [10].Pääkkönen T, Leppäluoto J. Cold exposure and hormonal secretion: a review. Int J Circumpolar Health 2002; 61:265-76. [DOI] [PubMed] [Google Scholar]
- [11].Hirvonen J, Huttunen P. Increased urinary concentration of catecholamines in hypothermia deaths. J Forensic Sci 1982; 27:264-71; PMID:7097201 [PubMed] [Google Scholar]
- [12].Pakanen L, Kortelainen M-L, Särkioja T, Porvari K. Increased adrenaline to noradrenaline ratio is a superior indicator of antemortem hypothermia compared with separate catecholamine concentrations. J Forensic Sci 2011; 56:1213-8; PMID:21595691; http://dx.doi.org/ 10.1111/j.1556-4029.2011.01805.x [DOI] [PubMed] [Google Scholar]
- [13].Palmiere C, Bardy D, Letovanec I, Mangin P, Augsburger M, Ventura F, Iglesias K, Werner D. Biochemical markers of fatal hypothermia. Forensic Sci Int 2013; 226:54-61; PMID:23313602; http://dx.doi.org/ 10.1016/j.forsciint.2012.12.007 [DOI] [PubMed] [Google Scholar]
- [14].Hardy JD, Du Bois EF. The technic of measuring radiation and convection. J Nutr 1938; 15:461-75. [Google Scholar]
- [15].Burton AC. Human calorimetry II. The average temperature of the tissues of the body. J Nutr 1935; 9:261-80. [Google Scholar]
- [16].Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. High circulating adrenaline levels at admission predict increased mortality after trauma. J Trauma Acute Care Surg 2012; 72:428-36; PMID:22439205 [DOI] [PubMed] [Google Scholar]
- [17].Arborelius M Jr, Ballidin UI, Lilja B, Lundgren CEG. Hemodynamic changes in man during immersion with the head above water. Aerosp Med 1972; 43:592-8; PMID:5035546 [PubMed] [Google Scholar]
- [18].Park KS, Choi JK, Park YS. Cardiovascular regulation during water immersion. Appl Human Sci 1999; 18:233-41; PMID:10675972; http://dx.doi.org/ 10.2114/jpa.18.233 [DOI] [PubMed] [Google Scholar]
- [19].Choukroun ML, Guenard H, Varene P. Pulmonary capillary blood volume during immersion in water at different temperatures. Undersea Biomed Res 1983; 10:331-42; PMID:6675229 [PubMed] [Google Scholar]
- [20].Boldt L-H, Fraszl W, Röcker L, Schefold JC, Steinach M, Noack T, Gunga H-C. Changes in the haemostatic system after thermoneutral and hyperthermic water immersion. Eur J Appl Physiol 2008; 102:547-54; PMID:18043935; http://dx.doi.org/ 10.1007/s00421-007-0620-7 [DOI] [PubMed] [Google Scholar]
- [21].Frank SM, Cattaneo CG, Wieneke-Brady MB, El-Rahmany H, Gupta N, Lima JA, Goldstein DS. Threshold for adrenomedullary activation and increased cardiac work during mild core hypothermia. Clin Sci 2002; 102:119-25; PMID:11749669; http://dx.doi.org/ 10.1042/CS20010073 [DOI] [PubMed] [Google Scholar]
- [22].Granberg P-O. Human physiology under cold exposure. Arctic Med Res 1991; 50 Suppl 6:23-7; PMID:1811574 [PubMed] [Google Scholar]
- [23].Sun Z, Zhang Z, Cade R. Renal responses to chronic cold exposure. Can J Physiol Pharmacol 2003; 81:22-7; PMID:12665254; http://dx.doi.org/ 10.1139/y03-002 [DOI] [PubMed] [Google Scholar]
- [24].Sadler DW, Pounder DJ. Urinary catecholamines as markers of hypothermia. Forensic Sci Int 1995; 76:227-30; PMID:8566925; http://dx.doi.org/ 10.1016/0379-0738(95)01814-X [DOI] [PubMed] [Google Scholar]


