Comment on: Cooper EA, et al. You turn me cold: Evidence for temperature contagion. PLoS One 2014; 9:e116126; http://dx.doi.org/10.1371/journal.pone.0116126
Maintaining a stable internal thermal environment is critical to many life-preserving biological processes. Consequently, core body temperature is rigidly regulated by the brains of all homeothermic animals through a variety of involuntary thermoregulatory responses including cutaneous vasomotor responses, shivering, sweating and piloerection as well as behavioral responses such as moving into the sun or shade. Though bottom-up pathways play a central thermoregulatory role, studies using visual imagery, temperature biofeedback and hypnotic suggestion illustrate top-down influences. We have recently extended these findings by showing that thermoregulatory responses can also be modulated by simple observation of another's temperature change.1 A finding similar to effects studied under the rubric of facial feedback.
To investigate whether observing another's temperature change alters observers own peripheral body temperature we showed thirty-six healthy participants 8 purpose-made 3-minute videos depicting actors with their right or left hand in visibly warm (warm video) or cold (cold video) water. During observation of the videos participants' own right and left hand temperature was measured using custom thermometers with a theoretical temperature sensitivity of 0.0001°C. After video playback participants rated how warm or cold the actors hand appeared. Four control videos with the actors' hand placed in front of the water container were also shown.
Participants rated videos showing hands immersed in cold water as being significantly cooler than hands immersed in warm water confirming that they could perceive the differences in observed hand temperature (Fig. 1A). However, more interestingly we also observed a significant change in participants' own hand temperature during videos depicting another's hand immersed in cold/warm water but not control videos. Specifically, participants' own hands became significantly colder when observing cold compared to warm videos F(1,34) = 13.83, p = 0.001. Further exploration showed that this was predominantly driven by reductions in participants' own left (t(35) = 23.54, p = 0.001) and right (t(35) = 22.33, p = 0.026) hand temperature during cold videos (Fig. 1C). Though warm hand videos were associated with an early increase in observers own hand temperature this was not sustained and did not significantly change over total video playback (Fig. 1D).
Figure 1.
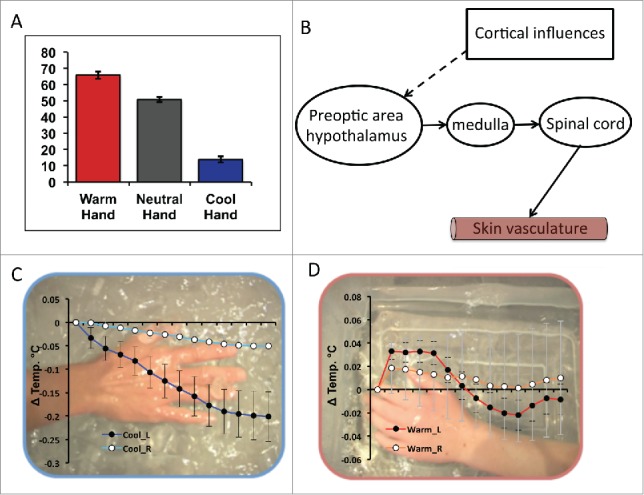
(A) Participants mean subjective ratings for the observed temperature of the actor's hand (“How Hot or Cold is the Actor's Hand?”) reported using a keyboard controlled visual analog scale ranging from ‘Very Cold’ (far left) through ‘Neutral’ (center) to ‘Very Hot’ (far right). (B) Schematic of how cortical (top down) influences may influence thermoregulation. (C) Mean time course response to viewing all cold videos for the left (dark blue) and right (light blue) hand displayed in 10 s epochs. Data are overlaid on a representative frame from one of the cold videos. (D) Mean time course response to viewing all warm videos for the left (red) and right (orange) hand displayed in 10 s epochs. Data are overlaid on a representative frame from one of the warm videos. Data shown are from ref. 1.
The neural circuitry underlying this ‘contagion’ of others peripheral body temperature is currently unknown. Prior brain imaging studies have shown that direct hand cooling increases blood flow (an indirect measure of neuronal activity) within human posterior then subsequently middle and anterior insula.2 This finding is noteworthy, as insula is believed to provide cortical representations of bodily physiological state across physiological domains.3 Whether similar increases in insula activity are also associated with temperature changes observed in others is unknown, though studies showing shared neuronal circuitry (including insula) in the direct and vicarious experience of a related physiological response, pain, suggest they would.4 In this regard, it is instructive to note that in monkeys many thermosensitive neurons in the hypothalamic preoptic area are also sensitive to non-thermal emotional stimuli such as rewards or aversive stimuli.5 This suggests that effector pathways from the preoptic area to rostral medullary raphe may be recruited by top-down cognitive processes potentially including both anterior insula and anterior cingulate cortices (Fig. 1B).
Interestingly, different thermoregulatory effectors mechanisms are associated with partially separable central control systems, expressed physiologically as a greater sensitivity of vasoconstrictive responses to temperature change.6 This difference in central control mechanism may also underpin why, in our study, we saw isolated changes in hand temperature, which we predict are likely mediated by a direct preoptic to rostral medullary raphe pathway, but not heart rate which is mediated by an intermediate projection to the dorsomedial hypothalamus.6 WARM sensitive neurons projecting via the dorsal parabrachial nucleus play a similar role in orchestrating cutaneous vasodilation and tachycardia in response to environmental warming.
Insight into the mechanisms underlying temperature contagion may also be usefully informed by studies of disrupted body ownership induced experimentally using the rubber hand illusion (where simultaneous stroking of a visible rubber hand and hidden own hand create the illusion of rubber hand ownership), and the clinical disorder cold-type complex regional pain syndrome, a neurological disorder associated with pain, abnormal temperature regulation and often dystonia in a single limb. In both conditions unilateral disruption of body ownership is associated with a localized reduction in body temperature suggesting that the conscious sense of our physical self and its physiological regulation are linked.7
During experimental induction of the rubber hand illusion activity changes are observed within insula as well as premotor and intra-parietal cortex suggesting a potential role for the insula in reported temperature changes. In cold-type complex regional pain syndrome, changes in limb temperature are reported to be dependent on its physical location in space. For example, when the affected (cool) limb is moved across the midline its temperature spontaneously increases with a converse effect described for the healthy limb.7 Based on this finding, these authors argued for a space-based rather than self-related (somatotopic) frame of reference, with descending projections from parietal cortex onto brainstem autonomic centers hypothesized as the mechanism mediating spatially dependent temperature change. It is possible that inter-personal comparator processes within the intra-parietal junction play a similar role in the social contagion of temperature responses.
A final finding from our study was a nuanced relationship between sensitivity to temperature contagion and self-reported empathy. Individuals high in empathic concern yet low in a broader measure of empathy (balanced emotional empathy scale) showed the greatest sensitivity to temperature contagion, broadly supporting action-perception models of empathy that propose embodiment of another's physiological state as a substrate for empathy. Why we observed this complex relationship rather than a simpler positive correlation with both types of empathy is currently unclear, though may relate to differences in the concepts captured by each scale.
In conclusion, we show that healthy individuals are sensitive to observable signals of another's peripheral body temperature and further, show contagion of their temperature, particularly in the context of cold. Inter-individual differences in temperature contagion are marked and correlate with inter-individual difference in empathy. The neurobiological substrates underpinning these effects are currently unknown though prior studies suggest potential mediation via top-down influences on thermosensitive neurons within the preoptic area of the hypothalamus.
References
- [1].Cooper EA, Garlick J, Featherstone E, Voon V, Singer T, Critchley HD, Harrison NA. You turn me cold: Evidence for temperature contagion. PLoS ONE 2014; 9(12):e116126; PMID:25551826; http://dx.doi.org/ 10.1371/journal.pone.0116126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci 2000; 3:184-90; PMID:10649575; http://dx.doi.org/ 10.1038/72131 [DOI] [PubMed] [Google Scholar]
- [3].Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655-66; PMID:12154366; http://dx.doi.org/ 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- [4].How do you feel? Interoception: the sense of the physiological condition of the body. Neuroimage 2011; 54:2492-502; PMID:20946964; http://dx.doi.org/ 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- [5].Hori T, Kiyohara T, Shibata M, Oomura Y, Nishino H, et al. Responsiveness of monkey preoptic thermosensitive neurons to nonthermal emotional stimuli. Brain Research Bulletin 1986; 17, 75-82; PMID:3756547; http://dx.doi.org/ 10.1016/0361-9230(86)90163-2 [DOI] [PubMed] [Google Scholar]
- [6].Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: A thermosensor it is not. Pharmacol Rev 2009; 61:228-61; PMID:19749171; http://dx.doi.org/ 10.1124/pr.109.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moseley GL, Gallace A, Iannetti GD. Spatially defined modulation of skin temperature and hand ownership of both hands in patients with unilateral complex regional pain syndrome. Brain 2012; 135:3676-86; PMID:23250885; http://dx.doi.org/ 10.1093/brain/aws297 [DOI] [PubMed] [Google Scholar]


