ABSTRACT
Arterio-venous anastomoses (AVAs) are direct connections between small arteries and small veins. In humans they are numerous in the glabrous skin of the hands and feet. The AVAs are short vessel segments with a large inner diameter and a very thick muscular wall. They are densely innervated by adrenergic axons. When they are open, they provide a low-resistance connection between arteries and veins, shunting blood directly into the venous plexuses of the limbs. The AVAs play an important role in temperature regulation in humans in their thermoneutral zone, which for a naked resting human is about 26°C to 36°C, but lower when active and clothed. From the temperature control center in the hypothalamus, bursts of nerve impulses are sent simultaneously to all AVAs. The AVAs are all closed near the lower end and all open near the upper end of the thermoneutral zone. The small veins in the skin of the arms and legs are also contracted near the lower end of the thermoneutral zone and relax to a wider cross section as the ambient temperature rises. At the cold end of the thermoneutral range, the blood returns to the heart through the deep veins and cools the arterial blood through a countercurrent mechanism. As the ambient temperature rises, more blood is returned through the superficial venous plexuses and veins and heats the skin surface of the full length of the 4 limbs. This skin surface is responsible for a large part of the loss of heat from the body toward the upper end of the thermoneutral zone.
KEYWORDS: arteriovenous anastomoses, human skin, laser Doppler, temperature control, thermoneutral zone, ultrasound Doppler
Introduction
Arterio-venous anastomoses (AVAs) are direct connections between small arteries and small veins, with no capillary section between them. Since they contain no capillary segment, they cannot transport dissolved substances to or from the tissues. The only transport function they could possibly have is the transport of heat from the body core to surface areas containing AVAs.
Functional anatomy
AVAs are found in many organs and tissues in vertebrate bodies, but usually in low numbers.1 The exceptions are certain areas of the skin and mucous membranes in mammals and birds, where they may be numerous. Results of thorough light microscopic investigations were first published by Hoyer in 1877 2 and later by Grosser in 1902.3 Later anatomical investigations have mainly confirmed their findings.1,4,5 The AVAs are typically short vessel segments with a very thick muscular wall. The walls are usually much thicker than those of the small arteries which feed into them. The AVAs are densely innervated by adrenergic axons.6 The inner diameter is described as being from 10 to 150 µm and the thickness of the wall from 40 to 60 µm. The length is typically from 200 to 500 µm. The wall is composed of circular smooth muscle cells as is the case for arterioles, but in addition there is usually an inner layer of longitudinal smooth muscle cells. This suggested to the early anatomists that the AVAs could act as sphincters, in other words that they could close down the vessels completely (Fig. 1).
Figure 1.
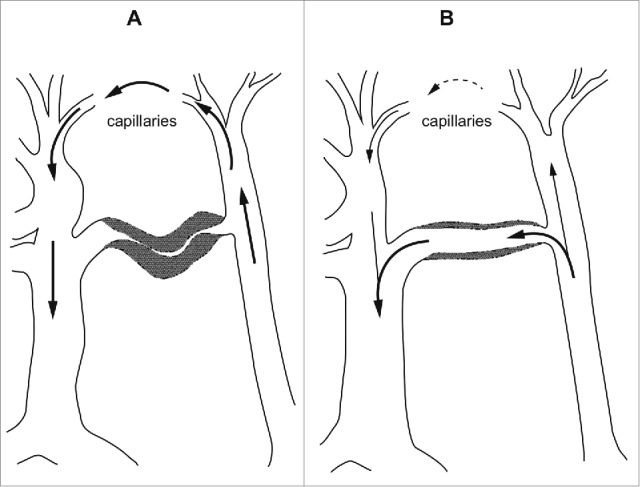
Diagram showing the effect of a closed (A) and an open (B) arterio-venous anastomosis. (After Boyd5).
In humans, the AVAs are abundant in the nail-beds of the fingers and toes and in the glabrous skin of the hands and feet. The density is described as being between 600 AVAs/cm2 in nail-beds and 100 AVAs/cm2 in the skin of the thenar and hypothenar regions of the hand.4 AVAs have not been demonstrated by histological techniques in the skin of human fetuses,4 but are physiologically present 2 days after birth and further developed at 3 months of age.7
Large numbers of AVAs have not been described anywhere else than in certain areas of the skin and mucous membranes in warm-blooded (homeothermic) animals, that is mammals and birds.3,8 Prior to the 1930s, all discussion of the possible functions of AVAs was based on this comparative fact, on their distribution in different skin areas in different animal species, and on their microscopic structure. Hoyer 2 suggested that AVAs are important in temperature regulation in mammals and birds. Others have suggested that AVAs are important in blood pressure regulation.3,8
Early physiological observations
The first physiological observations of AVAs were obtained in the early 1930s from the rabbit ear by Grant and Clark independently of each other.9-12 Grant observed AVAs through the intact skin after the removal of the hairs and noted ‘oscillations in caliber’ with a frequency of one or 2 oscillations per minute. When the body temperature of the animal was raised, the AVAs dilated, and with cooling they constricted. Clark made similar observations through a transparent chamber operated into the rabbit ear.
Physiological measurements
The first physiological blood flow measurements which could be related to the opening and closing of AVAs in humans were obtained by Burton using a finger plethysmograph.13 He showed that successive determinations of finger blood flow only a few seconds apart could give very different values of flow. He also showed that a series of such measurements obtained over several minutes exhibits characteristic rhythmic oscillations with a frequency of between one and 3 fluctuations per minute (Fig. 2). Such fluctuations obtained from the big toe were closely correlated with fluctuations in the same subject's finger. The fluctuations were not present in the fingers of a sympathectomized arm. The plethysmographic method has some serious limitations in these studies. It is not possible to measure flow values more often than every 6 seconds, and longer intervals may be needed to obtain precise measurements. This means that more rapid changes cannot be detected. Another difficulty, which is specific to the AVAs, is that they are very sensitive to sensory and psychic stimuli. Only a slight disturbance, for instance the sound of the inflation of the plethysmographic cuff, may result in the instantaneous closure of all AVAs. In practice, no other methods of measuring blood flow through tissues with a high density of AVAs in humans were available until the late 1970s.
Figure 2.
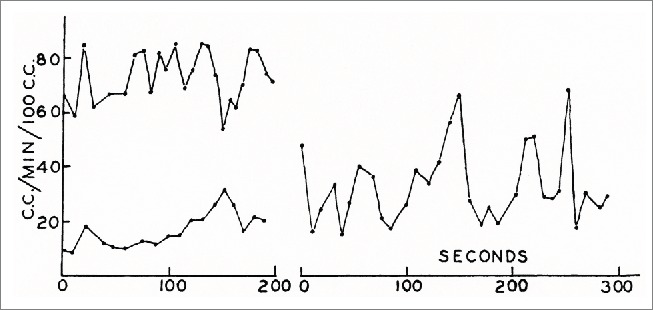
Fluctuations in blood flow in a finger at 3 different levels of ambient temperature measured by a venous occlusion plethysmograph. Each point represents a new measurement. (From Burton13). © The American Physiological Society. Reproduced by permission of The American Physiological Society. Permission to reuse must be obtained from the rightsholder.
Measurement methods
In the 1970s, 2 methods which could be used to give qualitative and semi-quantitative measurements of blood flow to regions with a high density of AVAs became available, the ultrasound Doppler method and the laser Doppler method.14,15 Both methods have clear limitations. The ultrasound Doppler method only gives quantitative measurements if the entire cross section of the vessel is ensonicated at constant intensity and if the cross-sectional area of the vessel is known and constant during the measurement period. For large vessels down to the size of the radial and ulnar arteries, these assumptions can be controlled, but this is not possible for smaller arteries like the finger arteries. Nevertheless, it is usually assumed that the ultrasound Doppler signal is proportional to flow even in small arterial vessels. One advantage of this method is that no zero-flow calibration is necessary. The laser Doppler method suffers from the problem that the flux signal increases linearly, but not proportionally, with flow. The number of capillaries and AVAs in the investigated tissue volume will vary between measurement sites. In addition, some of the high velocities in the AVAs may be filtered away.16 Both these methods have the advantage over the plethysmographic method that they do not give any sensory input to the experimental subjects.
Thoresen and Walløe confirmed many of Burton's findings using ultrasound Doppler measurements.14 However, they found more rapid rhythmic fluctuations with a much greater amplitude than Burton was able to detect given the limited time resolution of the plethysmographic method (Fig. 3). With a subject in the middle of the thermoneutral zone, the velocity increased sevenfold from the bottom of a velocity cycle to the top. Such large fluctuations cannot possibly be the result of vasomotion in arterioles, but must be the result of the opening and closure of the AVAs, as Burton also stated. Since blood from the ulnar artery also perfuses skin capillaries and some connective tissue and bone, presumably at constant flow, it can safely be assumed that nearly all AVAs in the region supplied by the ulnar artery close down simultaneously and that many of them at least also open simultaneously. The investigation also showed that the AVAs in one hand close as a result of sensory stimulus to another part of the body (immersion of the other hand in cold water for one minute), and that mental disturbance also results in closure of the AVAs (Fig. 4).
Figure 3.
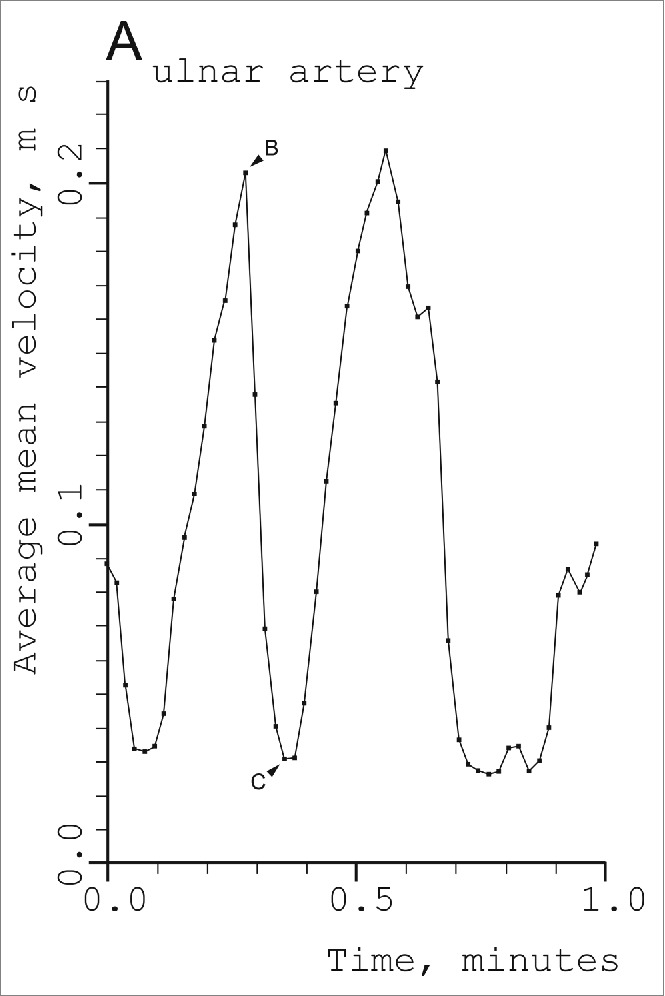
Fluctuations in blood velocity in the right ulnar artery in a 27-year-old male subject in a comfortably warm environment (24–28° C). The points show the time average for each cardiac cycle of the cross-sectional mean of the blood of the blood velocities in the vessel as a function of time. (From Thoresen and Walløe14). © Blackwell Publishing. Reproduced by permission of Blackwell Publishing. Permission to reuse must be obtained from the rightsholder.
Figure 4.
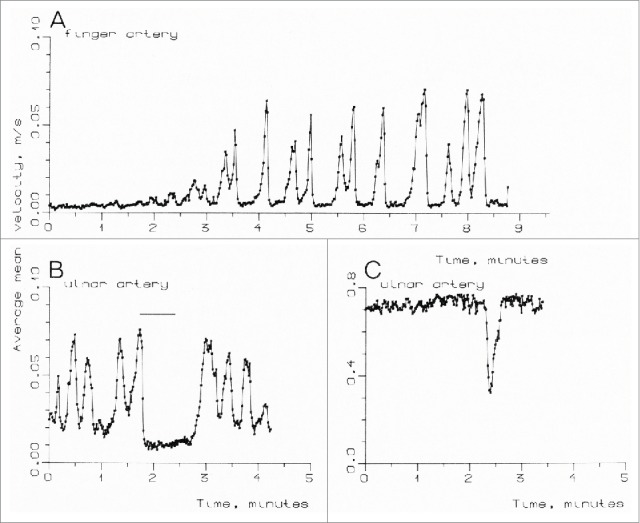
(A) Blood velocity in a finger artery in a 29-year-old male subject, measured over a period when ambient temperature was being increased. (B) Blood velocity in the right ulnar artery in a 26-year-old male subject. The subject's opposite hand was submerged in cold water for 40 s. (C) Blood velocity in the right ulnar artery in a 27-year-old male subject. The vasoconstriction was caused by the sight of a girl in a neighboring tropical greenhouse. (From Thoresen and Walløe14). © Blackwell Publishing. Reproduced by permission of Blackwell Publishing. Permission to reuse must be obtained from the rightsholder.
Thermoneutral zone
The concept of a thermoneutral zone goes back to investigations by Scholander and his collaborators in the 1940s and 50s.17 In a resting mammal, the basal metabolism was shown to be approximately constant over an ambient temperature interval from a little below the central body temperature (37 °C) down to a lower temperature dependent on the insulation of the animal body (fur, subcutaneous fat). Above the upper end of the thermoneutral zone, the metabolic rate increases due to the production of sweat (in man) or panting (in many mammals). Below the lower end of the thermoneutral zone, the metabolic rate increases proportionally to the difference between the ambient temperature and the central body temperature due to increased production of heat from shivering or voluntary muscle work (Fig. 5). In a resting naked human the thermoneutral zone is usually found to be from around 26° C (lower in subjects with a thicker layer of subcutaneous fat) to about 36°C. Clothing shifts the thermoneutral zone, especially the lower end, downwards. Thoresen and Walløe found that the amplitude of the fluctuations in the ulnar artery was larger when the subjects were in the middle of their thermoneutral zone (∼28-30 °C) than when they were near one end of it. Burton made similar observations.
Figure 5.
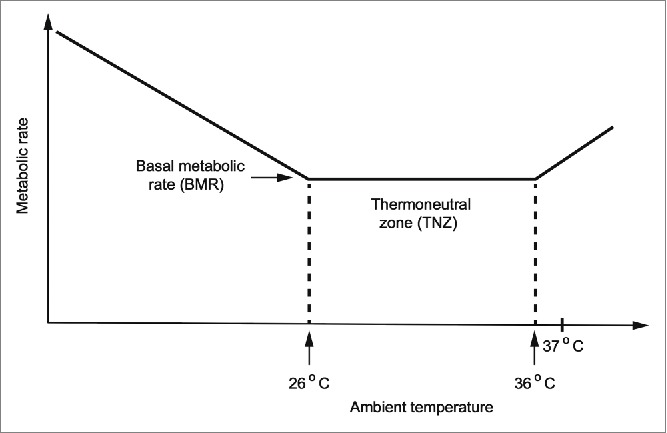
Schematic drawing of the metabolic rate of a resting naked human as a function of ambient temperature.
All early authors using anatomical methods agreed that there are AVAs in the glabrous skin of the hands and feet of humans. However, there has been disagreement about AVAs in other skin areas. Some authors claim to have found AVAs in the human forehead, nose and ears.5 Bergersen18 therefore searched for AVAs in these regions using Doppler ultrasound with simultaneous ultrasound Doppler recordings from a radial artery as a control. She found temporal correlation between fluctuations in the angular artery which perfuses the nose and fluctuations in the radial artery, showing that there probably are AVAs in the skin of the nose, but no similar correlation with blood velocities in the facial artery or the temporal artery.
Correlation between AVA flows in different skin areas and effect of nerve block
Burton13 observed that the fluctuations in blood flow were “closely correlated in parts of the body as widely separated as a finger of the right hand and the left big toe.” This finding has been confirmed in many later studies by both the ultrasound Doppler and the laser Doppler method.19-21 The vasoconstrictions have a characteristic shape, as seen in recordings from the radial, ulnar and dorsalis pedis arteries and also from the finger arteries (Fig. 6). In the middle of the subject's thermoneutral zone, blood velocity fluctuates between a high level and a very low level, with about 2 to 3 vasoconstrictions per minute. The high level corresponds to a state when all AVAs are open. This can clearly be seen from recordings made from women during labor at term (Fig. 7). It is usually quite warm in a birthing room, and the women show the characteristic AVA fluctuations in the radial and dorsalis pedis arteries. After epidural block, the velocity in the dorsalis pedis artery remained steady at the same high level as at the top of the fluctuations before the block was given, or a little higher.22 Thus, it was not only the pain fibers that were blocked, but also the sympathetic nerves to the AVAs and to the arterioles in the feet. The vasoconstrictions in the radial artery remained unchanged in these women.
Figure 6.
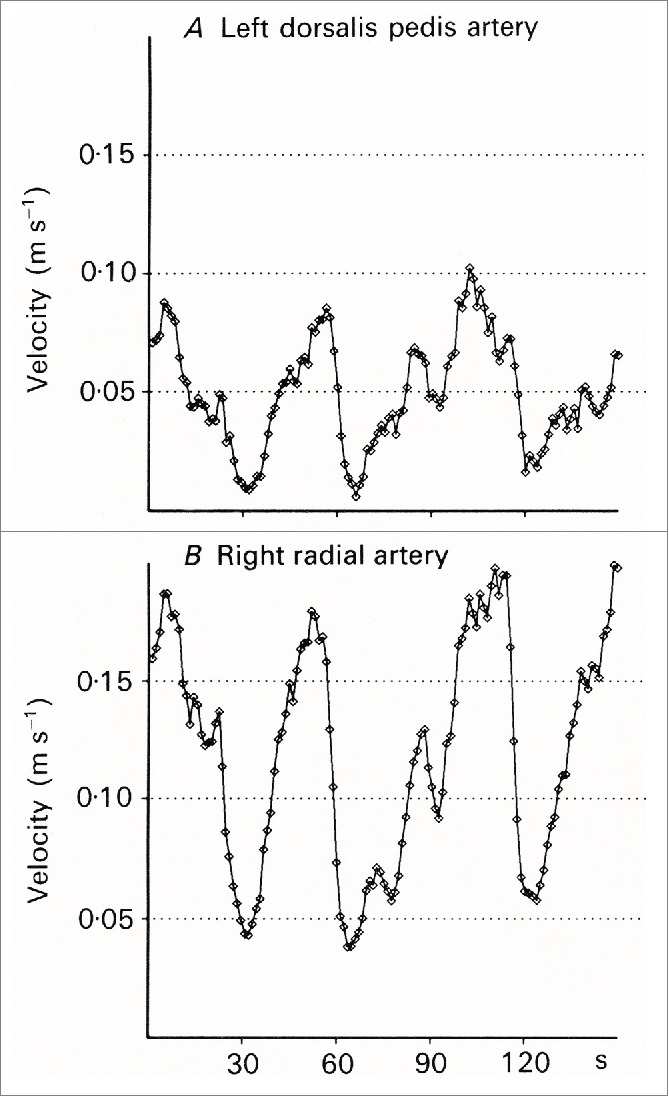
Simultaneous measurements from the left dorsalis pedis artery (A) and in the right radial artery (B) (From Lossius, Eriksen and Walløe20) © The Physiological Society. Reproduced by permission of The Physiological Society. Permission to reuse must be obtained from the rightsholder.
Figure 7.
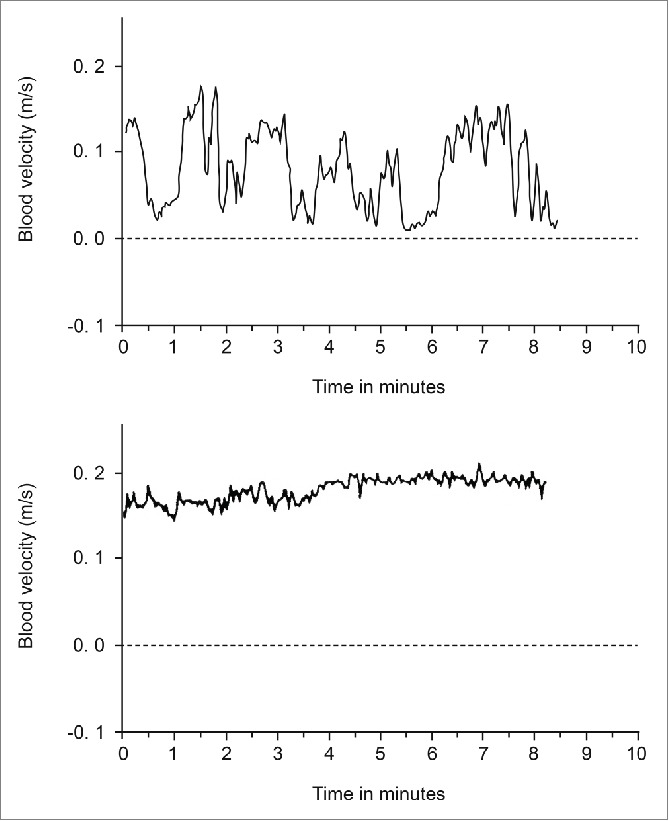
Blood velocity in the right dorsalis pedis artery of a woman during labor, but between contractions. Upper panel: Fluctuations typical of temperature regulation in a fairly warm delivery room. Lower panel: Same woman in the same environment after epidural block. (Graphs from unpublished recordings obtained during the investigations described in Janbu22).
The characteristic vasoconstrictions occur rapidly, within 2 to 4 heartbeats, while dilatation is a slower process. The interpretation is that the vasoconstrictions are caused by a burst of impulses in the adrenergic axons innervating the AVAs and that the increase in flow afterwards is caused by a passive release of the contractions in the AVA smooth muscles. These bursts of activity in adrenergic neurons to AVAs must occur simultaneously in both hands and feet and also in the angular artery in the face. The blood flow fluctuations were correlated in all skin areas with AVAs, with positive correlation coefficients of the order of 0.7 to 0.95 with subjects in the middle of their thermoneutral zone.
Correlation with arterial blood pressure
The AVA flow fluctuations are also strongly negatively correlated with mean arterial blood pressure (MAP) fluctuations, with correlation coefficients of the order of −0.80 to −0.3. The blood pressure was recorded by finger plethysmography and was time averaged and recorded beat-by-beat in the same way as the blood velocity signals. The MAP fluctuations often had a peak-to-trough magnitude of 15 mmHg, again largest in the middle of the thermoneutral zone (Fig. 8). These findings suggest either a causal relationship between skin vascular conductance and MAP fluctuations, or a common cause for fluctuations in both variables.
Figure 8.
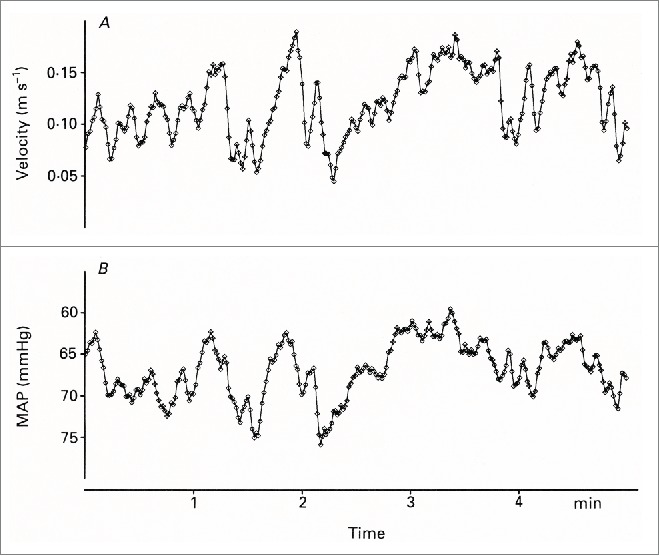
Simultaneous measurements of velocity in the right radial artery (A) and mean arterial pressure (MAP) (B) in the left third finger. The MAP axis is inverted. (From Lossius, Eriksen and Walløe20). © The Physiological Society. Reproduced by permission of The Physiological Society. Permission to reuse must be obtained from the rightsholder.
Time-domain transfer function analysis revealed the time sequence and magnitude of the heart rate (HR) and MAP changes linked with the AVA blood flow fluctuations. An increase in HR and MAP started 1-4 seconds before a step decrease in skin vascular conductance, but was followed by a transient HR decrease a few seconds later, probably caused by baroreceptor stimulation from the increased blood pressure. These findings indicate that that the HR and MAP responses are not passive effects of changes in peripheral resistance, but are probably explained by the presence of an autonomic rhythm originating in the central nervous system. The time lag between the HR and AVA responses could then be explained by the different latencies in the sympathetic and the parasympathetic nervous system; long latencies in the sympathetic innervation of the AVAs and very short latencies in the parasympathetic innervation of the heart. This interpretation was later supported by further studies of these fluctuations with subjects in a cool environment and during parasympathetic blockade by atropine.23 The large fluctuations in radial artery velocity were retained after atropine administration, as was the amplitude of the associated MAP fluctuations, despite a large reduction in HR variability. In a cool environment, presumably near the lower end of the thermoneutral zone, the fluctuations in radial artery velocity were nearly abolished. In some subjects the fluctuations also disappeared near the upper end of the thermoneutral zone, and the flow, presumably mainly through the AVAs, was nearly constant at a level corresponding to the peaks of the fluctuations seen at lower ambient temperatures.14 In other subjects with a lower threshold for sweating, some fluctuations continued after sweating began.
Quantitative estimates of flow fluctuations
Large fluctuations in blood velocity such as have been measured in arteries supplying the hands and feet have never been seen in arteries supplying skin without AVAs or in other tissues.14 Nor have they been detected by laser Doppler measurements from skin areas, including the dorsal side of the hands, where AVAs are few or absent.21,24 From measurements and assumptions about the internal diameters of the 8 arteries supplying the AVAs in the 2 hands and the 2 feet, Lossius et al 20 estimated the sum of the flow fluctuations in a resting adult subject in the middle of the thermoneutral zone to be of the order of 250-500 ml min−1, corresponding to 5-10 % of cardiac output. Assuming that the peak of the velocity fluctuations when the subject is in the middle of the thermoneutral zone corresponds to opening of all the AVAs (Fig. 3), the flow through the AVAs can be estimated to be about twice this value at the upper end of the thermoneutral zone. This corresponds to up to about 20 % of the resting cardiac output, or of the order of magnitude of 1 l/min in an adult subject.
Lossius and Eriksen also made recordings from different skin areas using laser Doppler, using ultrasound Doppler recordings from the radial artery as a control.21 In addition they recorded blood pressure by finger plethysmography. In skin areas with AVAs, they found large fluctuations in laser flux that were strongly, but negatively, correlated with blood pressure fluctuations. This was true even though multiple regression analyses sometimes revealed that a component of the flux variability was positively correlated with variations in blood pressure. Such a positive component has never been detected in ultrasound Doppler velocity recordings from finger arteries. Presumably the laser Doppler flux is more strongly influenced by the skin capillary blood flow in the measurement volume than by the flow through the AVAs, which are situated deeper in the skin. The fluctuations in capillary flow could perhaps be a result of a ‘steal’ effect.
Effect of local heating
Interactions between local heating of the human hand and the centrally induced rhythmic fluctuations in blood velocity in the AVAs has been investigated by Bergersen and collaborators.19 During the experiments, the subjects were dressed only in shorts and rested on a bench in a climatic chamber in a semisupine position. The room temperature was 35 °C and the relative humidity 40 %. The subject was acclimatized and stable close to the upper end of the thermoneutral zone, with large fluctuations in both radial arteries as measured by Doppler ultrasound. Each hand was immersed in a separate thermostat-controlled water bath. Initially, both water baths were kept at 35 °C. After a steady-state period, the temperature in one of the baths was linearly raised to 43 °C and then maintained at this temperature for a second steady-state period. All instrument and temperature adjustments were made from outside the climatic chamber, leaving the subject completely undisturbed. The blood velocity fluctuations in the control hand remained unchanged, while the mean velocity in the heated hand showed an increase with increasing water temperature (Fig. 9). However, the blood velocity fluctuations remained unchanged and closely correlated with those in the control radial artery (Fig. 10). The most likely explanation of this finding is that the pattern of central nervous control of the AVA vasomotion is unaffected by local warming, and that some other part of the vascular bed, such as the arterioles in the skin, dilates when heated. The authors were unable to reproduce earlier findings obtained by the plethysmographic method showing that heat-induced vasoconstriction (HIVC) occurs in the human finger.25-27 The vasoconstrictions described by Nagasaka and co-workers occur only under very restricted circumstances. Briefly, they observed vasoconstrictions in the finger at water temperatures of 39 and 41 °C only, not at 37 or 43 °C (Fig. 11). The vasoconstrictions were brief, appearing at the time of and just after the stepwise increase in water bath temperature, not later, and were accompanied by a brief increase in local thermal sensation. The forearm skin showed no HIVC. The effect was not present in the finger after digital nerve blockade,28 showing that it must be initiated from the central nervous system, in contrast to the HIVC observed in certain other mammals such as the sheep.29 When Nagasaka and coworkers used laser Doppler with a gap between the measurement probe and the skin, so that they could assume that only blood movement in the skin capillaries was measured, and not flow in the deeper AVAs, they saw no HIVC.27 Because the ultrasound Doppler method and the plethysmographic method gave different results, the ultrasound Doppler measurements were repeated with subjects in a hyperthermic state.30 Again the results showed no indication of HIVC. The main difference between the 2 experimental protocols was that Nagasaka and co-workers increased the temperature in steps, while Bergersen and coworkers increased the temperature gradually. Reflexes from the increase in local thermal sensation connected to each step may perhaps explain the conflicting results. In any case, the effects reported by Nagasaka and co-workers are so limited in effect that they are unlikely to have any important function in human temperature control. This is in contrast to the importance of HIVC in some mammal species, for example the sheep 29 and the jack rabbit.31
Figure 9.
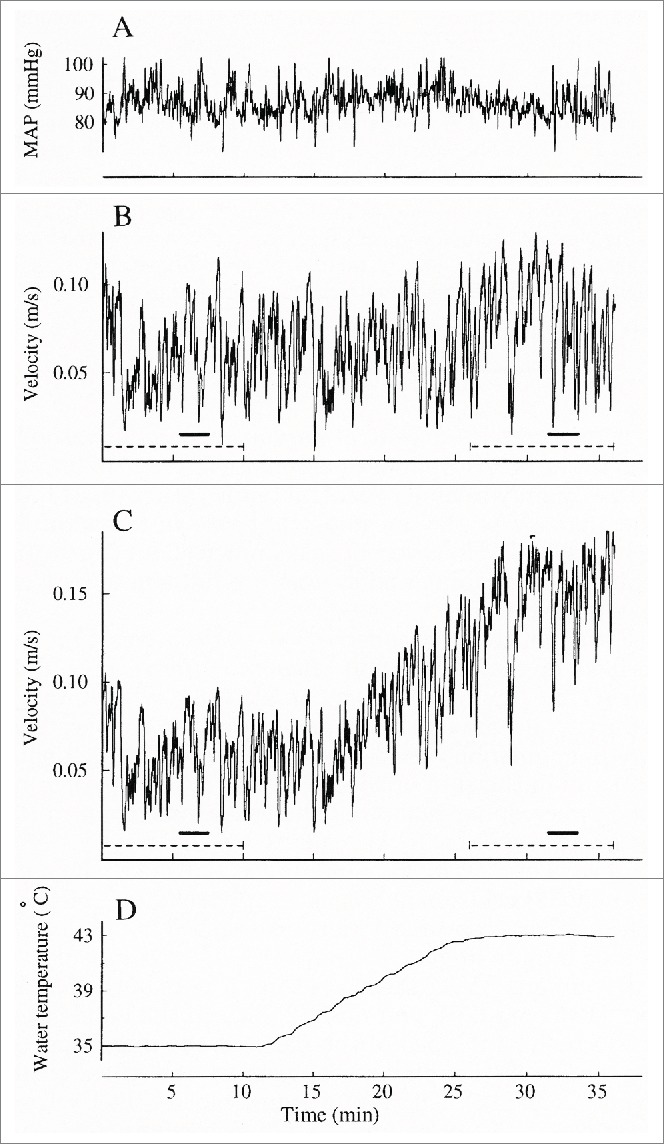
Simultaneous beat-by-beat averaged arterial blood velocity and mean arterial pressure (MAP) from one subject during local warming from 35 to 42°C (room temperature 24°C). (A) mean arterial pressure (MAP), (B) blood velocity in control radial artery, (C) blood velocity in radial artery of heated hand, (D) water temperature. (From Bergersen, Eriksen and Walløe19). © The American Physiological Society. Reproduced by permission of The American Physiological Society. Permission to reuse must be obtained from the rightsholder.
Figure 10.
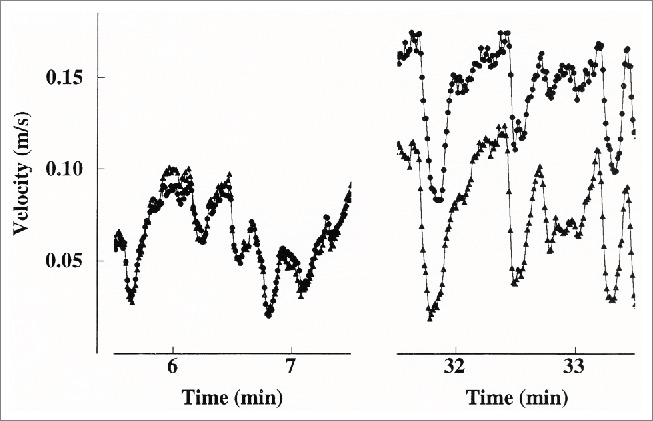
Two shorter blood velocity recordings from bilateral arteries sampled beat by beat, also displayed in Figures 9, B and C, marked by solid bars. Left panel: before heating. Right panel: after heating. Circles: heated finger artery. Triangles: control finger artery. (From Bergersen, Eriksen and Walløe19) © The American Physiological Society. Reproduced by permission of The American Physiological Society. Permission to reuse must be obtained from the rightsholder.
Figure 11.
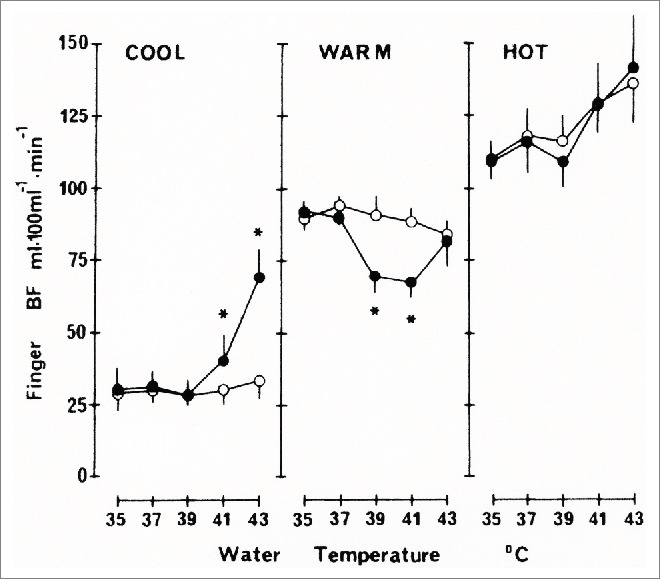
Changes in finger blood flow (BF) in locally heated hand (filled circles) and control hand (open circles) measured by finger occlusion plethysmography plotted against water temperature for the subject in 3 different thermal states. Values are means ± SE of 6 individual average values for each set of water temperatures. * Significantly different from corresponding value in control hand (p <0.05) (From Nagasaka26). © The American Physiological Society. Reproduced by permission of The American Physiological Society. Permission to reuse must be obtained from the rightsholder.
Effect of local cooling
Interactions between local cooling of the human finger and the centrally induced rhythmic fluctuations in blood velocities in the AVAs have also been investigated by Bergersen and collaborators.32 The experimental setup was similar to that used to investigate the effect of local heating. To keep the body core temperature as constant as possible, only a small area of skin was cooled, and only one finger was submerged in the water bath. On two consecutive days, the temperature of the water bath was lowered from 35 °C to 27 °C and from 27 °C to 19 °C respectively. The characteristic blood velocity fluctuations were unaltered both in the finger artery of the cooled finger and in the finger artery of the control finger on the opposite hand during cooling from 35 °C to 27 °C. When the temperature of the water bath was lowered from 27 °C on the second day, the fluctuations in the cooled finger stopped quite abruptly in all experimental subjects when the temperature dropped below ∼21.5 °C (Figs. 12 and 13). The blood velocity in the finger artery of the cooled finger remained low while the velocity fluctuations in the finger artery of the control hand were unchanged. Presumably the AVAs remained closed at a local temperature below 21.5 °C.
Figure 12.
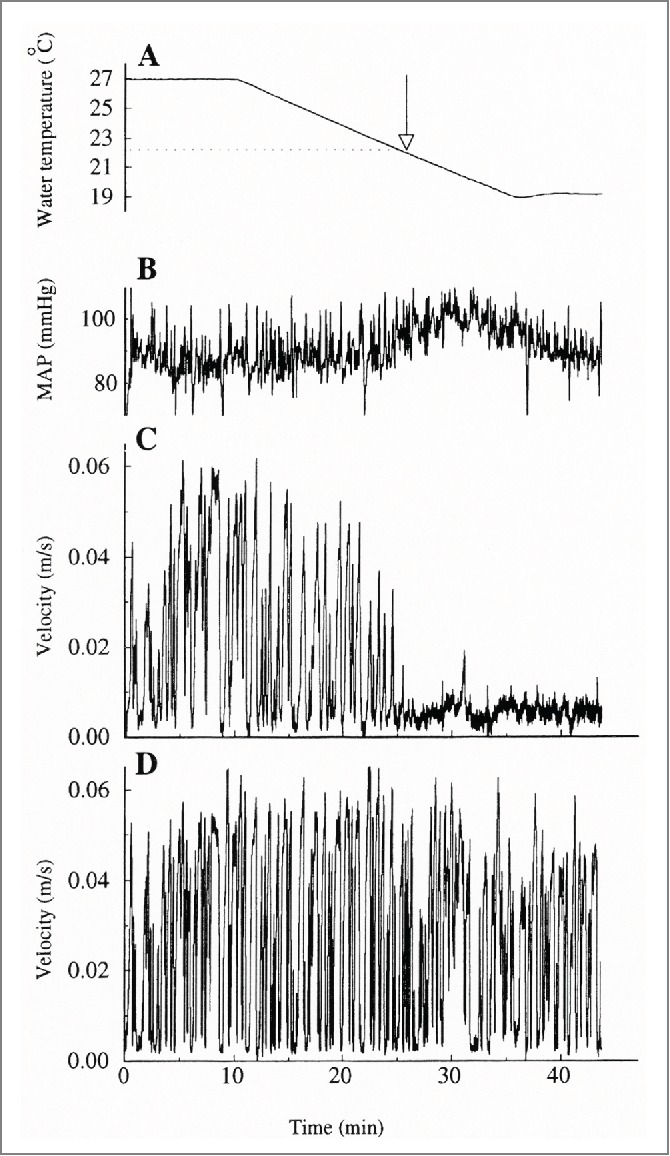
Simultaneous beat-by-beat averaged arterial blood velocity and mean arterial pressure (MAP) from one subject during local cooling from 27 to 19°C (room temperature 25°C). (A) water temperature, (B) MAP, (C) blood velocity in 3rd finger artery of cooled hand, (D) blood velocity in 3rd finger artery of control hand (From Bergersen, Eriksen and Walløe32). © The American Physiological Society. Reproduced by permission of The American Physiological Society. Permission to reuse must be obtained from the rightsholder.
Figure 13.
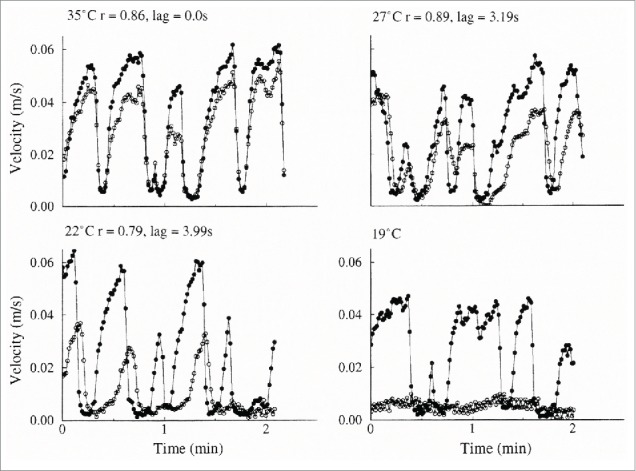
Two-minute periods of simultaneous bilateral blood velocity fluctuations at 4 different local temperatures. Same experiment as in Figure 12. Open circle: cooled hand. Filled circle: control hand. (From Bergersen, Eriksen and Walløe32). © The American Physiological Society. Reproduced by permission of The American Physiological Society. Permission to reuse must be obtained from the rightsholder.
Bergersen and her coworkers have also investigated finger blood flow during local cooling in a water bath kept at a steady temperature of 3 °C.33 Lewis first reported that when after immersion in cold water, finger skin temperature falls and remains low for 5-10 minutes, but then increases abruptly.34 Lewis interpreted this as being due to local vasodilatation. If the exposure to cold continues for some 10 to 20 minutes, another period of vasoconstriction occurs, and then a second period of cold-induced vasodilatation (CIVD). Ultrasound Doppler recordings showed that velocity fluctuations in the finger artery during CIVD were similar to those in the control hand, but with a smaller amplitude. Thus, vasodilatation occurs in the AVAs when the low temperature has acted on the contracted smooth muscle cells in the AVAs for some minutes. For some reason, the contraction cannot be sustained and the AVAs open up. A study of the responses of the hands and feet to cold exposure has recently been published.35
Arterio-venous anastomoses and human temperature control
On the basis of the information discussed above about the physiological responses of the AVAs and current knowledge of the physiological responses of the ordinary arterioles in the glabrous and non-glabrous skin of the human body, it is possible to give an overview of the role of AVAs in temperature regulation in humans. When the ambient temperature is below the lower end of the thermoneutral zone, all AVAs are closed. Above the upper end of the thermoneutral zone they are probably open most of the time, but this is of little importance, since the vasodilatation caused by cholinergic mechanisms related to sweat production results in a large increase in blood flow to all skin areas. Johnson and co-workers have investigated the vasodilator mechanisms in the human skin in detail, and have shown the importance of these mechanisms in situations when the body has a pressing need to rid itself of excess heat.36 As a result of general full vasodilatation, all venous plexuses in the skin are filled with hot blood. This heat evaporates the sweat and is also transported to the surroundings by conduction, convection, and radiation. In this situation, total blood flow to the skin in adult humans has been estimated to be about 8 l/min,37 and blood flow through the AVAs is only about 10 % of this.
However, the most important mechanism in human temperature control is conscious behavioral changes. We put on or take off clothing, move between sunlit areas and shadow, and adjust our physical activity level if possible. By means of these behavioral changes we try to avoid the 2 extremes of sweating and shivering, which we perceive as unpleasant, and try to keep the body within the thermoneutral zone. From an evolutionary perspective it also makes sense for the body to use other physiological mechanisms than the metabolically more demanding solutions of shivering or sweating as far as possible. Within the thermoneutral zone, the AVAs play a major role in human temperature control. In this temperature range, body temperature is controlled solely by adjusting blood flow to the skin, without any accompanying changes in metabolic rate. In the 4 limbs, hot blood is transported through the AVAs to the veins in the fingers and toes and in the palms and soles of the hands and feet. This is brought about by rhythmic active closing and passive opening of the AVAs triggered by bursts of impulses in the adrenergic vasoconstrictor axons to the AVAs, probably originating in the temperature control center in the hypothalamus. The frequency and the amplitude of the flow peaks increase from the lower end to the middle of the thermoneutral zone, but decrease again as the ambient temperature rises further toward the upper end of the thermoneutral zone, where all AVAs are open. Thus, blood flow through the AVAs increases from near zero toward the lower end of the thermoneutral zone to perhaps 1 l/min toward the upper end. Using thermal biophysical arguments, Taylor et al 38 conclude that the hands and feet in humans are important as insulators, radiators and evaporators. I agree with this, particularly as regards insulation and radiation. However, the small veins in the skin of the arms and legs are also contracted near the lower end of the thermoneutral zone and relax to a wider cross section as the ambient temperature increases.37 At the cold end of the thermoneutral range, the blood returns to the heart through the deep veins and cools the arterial blood through a countercurrent mechanism. As the ambient temperature rises, more blood is returned through the superficial venous plexuses and veins and heats the skin surface of the full length of the 4 limbs. This skin surface makes up about 50 % of the body surface, and is responsible for a large part of the loss of heat from the body toward the upper end of the thermoneutral zone.39 Thus, the AVAs of the hands and feet and the superficial veins of the limbs may be regarded as a single entity responsible for control of body temperature in most ordinary situations.
Abbreviations
- AVA
atreriovenous anastomoses
- CIVC
cold induced vasoconstriction
- HIVC
heat induced vasoconstriction
- HR
heart rate
- MAP
mean arterial blood pressure
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I would like to thank Maja Elstad for valuable comments on the manuscript and Alison Coulthard for correcting the English.
Reference
- [1].Clara M. Die arterio-venösen Anastomosen. Wien: Springer, 1956. [Google Scholar]
- [2].Hoyer H. Ueber unmittelbare Einmündung kleinster Arterien in Gefässäste venösen Charakters Archiv für mikroskopische Anatomie 1877; 13:603-46; http://dx.doi.org/ 10.1007/BF02933950 [DOI] [Google Scholar]
- [3].Grosser O. Ueber arterio-venöse Anastomosen an den Extremitätsenden beim Menschen und den krallentragenden Säugethieren. Arch Mikro Anat Entw 1902; 60:191-216; http://dx.doi.org/ 10.1007/BF02978384 [DOI] [Google Scholar]
- [4].Popoff NW. The digital vascular system - With reference to the state Glomus in inflammation Arteriosclerotic gangrene, diabetic gangrene thrombo-angiitis obliterans and supernumerary digits in man. Arch Pathol 1934; 18:295-330 [Google Scholar]
- [5].Boyd JD. Arterio-venous anastomoses. London Hospital Gazette 1939; 42:2-8 [Google Scholar]
- [6].Donadio V, Nolano M, Provitera V, Stancanelli A, Lullo F, Liguori R, Santoro L. Skin sympathetic adrenergic innervation: An immunofluorescence confocal study. Ann Neurol 2006; 59:376-81; PMID:16437571; http://dx.doi.org/ 10.1002/ana.20769 [DOI] [PubMed] [Google Scholar]
- [7].Lossius K, Eriksen M. Connection between skin arteriovenous shunt flow fluctuations and heart rate variability in infants. Early human development 1994; 39:69-82; PMID:7843046; http://dx.doi.org/ 10.1016/0378-3782(94)90071-X [DOI] [PubMed] [Google Scholar]
- [8].Clara M. Die arterio-venösen Anastomosen der Vögel und Säugetiere. Ergebnisse der Anatomie 1927:246-301 [Google Scholar]
- [9].Grant RT. Observations on direct communications between arteries and veins in the rabbit's ear. Heart-J Stud Circ 1930; 15:281-303 [Google Scholar]
- [10].Clark ER, Clark EL. Observations on living arterio-venous anastomoses as seen in transparent chambers introduced into the rabbit's ear. Am J Anat 1934; 54:229-86; http://dx.doi.org/ 10.1002/aja.1000540204 [DOI] [Google Scholar]
- [11].Grant RT, Pearson RSB. The blood circulation in the human limb; Observations on the differences between the proximal and distal parts and remarks on the regulation of body temperature. Clin Sci 1938; 3:119-39 [Google Scholar]
- [12].Clark ER. Arterio-venous anastomoses. Physiol Rev 1938; 18:229-47 [Google Scholar]
- [13].Burton AC. The range and variability of the blood flow in the human fingers and the vasomotor regulation of body temperature. Am J Physiol 1939; 127:437-53 [Google Scholar]
- [14].Thoresen M, Walloe L. Skin blood flow in humans as a function of environmental temperature measured by ultrasound. Acta physiologica Scandinavica 1980; 109:333-41; PMID:7446176; http://dx.doi.org/ 10.1111/j.1748-1716.1980.tb06604.x [DOI] [PubMed] [Google Scholar]
- [15].Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol Respir Environ Exerc Physiol 1984; 56:798-803; PMID:6706783; http://dx.doi.org/ 10.1063/1.334009 [DOI] [PubMed] [Google Scholar]
- [16].Eriksen ML, K. Inadequacy of laser Doppler flowmetry in skin areas of the human hand. Med Biol Eng Comput 1993; 31:311-8; PMID:8412386; http://dx.doi.org/ 10.1007/BF02458052 [DOI] [PubMed] [Google Scholar]
- [17].Scholander PF, Hock R, Walters V, Johnson F, Irving L. Heat regulation in some arctic and tropical mammals and birds. Biol Bull 1950; 99:237-58; PMID:14791422; http://dx.doi.org/ 10.2307/1538741 [DOI] [PubMed] [Google Scholar]
- [18].Bergersen TK. A search for arteriovenous anastomoses in human skin using ultrasound Doppler. Acta physiologica Scandinavica 1993; 147:195-201; PMID:8475746; http://dx.doi.org/ 10.1111/j.1748-1716.1993.tb09489.x [DOI] [PubMed] [Google Scholar]
- [19].Bergersen TK, Eriksen M, Walloe L. Effect of local warming on hand and finger artery blood velocities. Am J Physiol 1995; 269:R325-30; PMID:7653653 [DOI] [PubMed] [Google Scholar]
- [20].Lossius K, Eriksen M, Walloe L. Fluctuations in blood flow to acral skin in humans: connection with heart rate and blood pressure variability. J Physiol 1993; 460:641-55; PMID:8487211; http://dx.doi.org/ 10.1113/jphysiol.1993.sp019491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lossius K, Eriksen M. Spontaneous flow waves detected by laser Doppler in human skin. Microvascular Res 1995; 50:94-104; PMID:7476583; http://dx.doi.org/ 10.1006/mvre.1995.1041 [DOI] [PubMed] [Google Scholar]
- [22].Janbu T. Blood velocities in the dorsal pedis and radial arteries during labour. Br J Obstet Gynaecol 1989; 96:70-9; PMID:2647132; http://dx.doi.org/ 10.1111/j.1471-0528.1989.tb01579.x [DOI] [PubMed] [Google Scholar]
- [23].Lossius K, Eriksen M, Walloe L. Thermoregulatory fluctuations in heart rate and blood pressure in humans: effect of cooling and parasympathetic blockade. J Auton Nerv System 1994; 47:245-54; PMID:8014382; http://dx.doi.org/ 10.1016/0165-1838(94)90185-6 [DOI] [PubMed] [Google Scholar]
- [24].Eriksen M, Lossius K. Inadequacy of laser Doppler flowmetry in skin areas of the human hand. Med Biol Eng Comput 1993; 31:311-8; PMID:8412386; http://dx.doi.org/ 10.1007/BF02458052 [DOI] [PubMed] [Google Scholar]
- [25].Nagasaka T, Cabanac M, Hirata K, Nunomura T. Heat-induced vasoconstriction in the fingers: a mechanism for reducing heat gain through the hand heated locally. Pflugers Arch 1986; 407:71-5; PMID:3737384; http://dx.doi.org/ 10.1007/BF00580723 [DOI] [PubMed] [Google Scholar]
- [26].Nagasaka T, Cabanac M, Hirata K, Nunomura T. Control of local heat gain by vasomotor response of the hand. J Appl Physiol 1987; 63:1335-8; PMID:3693165 [DOI] [PubMed] [Google Scholar]
- [27].Nagasaka T, Hirata K, Nunomura T. Contribution of arteriovenous anastomoses to vasoconstriction induced by local heating of the human finger. Jpn J Physiol 1987; 37:425-33; PMID:2960840; http://dx.doi.org/ 10.2170/jjphysiol.37.425 [DOI] [PubMed] [Google Scholar]
- [28].Sakurada S, Shido O, Yamamoto K, Sugimoto N, Kobayashi T, Nagasaka T. Effect of digital nerve blockade on heat-induced vasoconstriction in the human finger. J Appl Physiol 1995; 78:746-9; PMID:7759449; http://dx.doi.org/ 10.1063/1.360333 [DOI] [PubMed] [Google Scholar]
- [29].Hales JRS, Jessen C, Fawcett AA, King RB. Skin Ava and Capillary Dilatation and Constriction Induced by Local Skin Heating. Pflug Arch Eur J Phy 1985; 404:203-7; http://dx.doi.org/ 10.1007/BF00581240 [DOI] [PubMed] [Google Scholar]
- [30].Rein EBW, L. No evidence of heat-induced vasoconstriction in human skin using ultrasound Doppler after passive whole-body heat stess -in manuscript 2015. [Google Scholar]
- [31].Schmidt-Nielsen KD, TJ, Hammel HT, Hinds D, Jackson DC. The Jack rabbit - a study in desert survival. Hvalrådets skrifter 1965; 48:125-42 [Google Scholar]
- [32].Bergersen TK, Eriksen M, Walloe L. Local constriction of arteriovenous anastomoses in the cooled finger. Am J Physiol 1997; 273:R880-6; PMID:9321863 [DOI] [PubMed] [Google Scholar]
- [33].Bergersen TK, Hisdal J, Walloe L. Perfusion of the human finger during cold-induced vasodilatation. Am J Physiol 1999; 276:R731-7; PMID:10070133 [DOI] [PubMed] [Google Scholar]
- [34].Lewis T. Observations upon the reactions of the vessels of the human skin to cold. Heart-J Stud Circ 1930; 15:177-208 [Google Scholar]
- [35].Cheung SS. Responses of the hands and feet to cold exposure. Temperature 2015; 2:105-20; http://dx.doi.org/ 10.1080/23328940.2015.1008890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Johnson JM, Minson CT, Kellogg DL. Cutaneous Vasodilator and Vasoconstrictor Mechanisms in Temperature Regulation. Compr Physiol 2014; 4:33-89; PMID:24692134; http://dx.doi.org/ 10.1002/cphy.c130015 [DOI] [PubMed] [Google Scholar]
- [37].Rowell LB. Reflex control of the cutaneous vasculature. J Investig Dermatol 1977; 69:154-66; PMID:326990; http://dx.doi.org/ 10.1111/1523-1747.ep12497938 [DOI] [PubMed] [Google Scholar]
- [38].Taylor NA, Machado-Moreira CA, van den Heuvel AM, Caldwell JN. Hands and feet: physiological insulators, radiators and evaporators. Eur J Appl Physiol 2014; 114:2037-60; PMID:25011493; http://dx.doi.org/ 10.1007/s00421-014-2940-8 [DOI] [PubMed] [Google Scholar]
- [39].Elstad M, Vanggaard L, Lossius AH, Walloe L, Bergersen TK. Responses in acral and non-acral skin vasomotion and temperature during lowering of ambient temperature. J Ther Biol 2014; 45:168-74; PMID:25436967; http://dx.doi.org/ 10.1016/j.jtherbio.2014.09.003 [DOI] [PubMed] [Google Scholar]


