Comment on: Sanchez-Alavez M, et al. Monoacylglycerol lipase regulates fever response. PloS One 2015; 10:e0134437; http://dx.doi.org/10.1371/journal.pone.0134437; Kita Y, et al. Fever is mediated by conversion of endocannabinoid 2-arachidonoylglycerol to prostaglandin E2. PloS One 2015; 10:e0133663; http://dx.doi.org/10.1371/journal.pone.0133663
It was recently shown that genetic or pharmacological inhibition of monoacylglycerol lipase (MAGL) blunted the fever response in mice. MAGL hydrolyzes the endocannabinoid 2-arachidonoylglycerol into arachidonic acid, providing a phospholipase-independent source of substrate for the synthesis of prostaglandin E2 (PGE2). These findings bring new light on the mechanisms of fever and identify MAGL as an antipyretic target.
Perhaps it is surprising that a phenomenon so common, so extensively investigated and for the most part well controlled pharmacologically such as fever has something new and important to reveal. Or perhaps, as it is sometimes the case, the knowledge acquired on the subject is considered so solid that new findings appear unlikely or after all, marginally relevant. However, two recent studies show that the mechanisms of fever are not to be taken for granted.1,2
Fever is an old companion of humans who consider it a sign of sickness and learned to treat it before they understood it. Eventually, it was elegantly and exhaustively demonstrated that fever occurs when prostaglandin E2 act via the specific EP3 receptor to affect hypothalamic neurons that regulate thermoregulation.3,4 It was also discovered that the synthesis of PGE2 is triggered by endogenous pyrogens, i.e. interleukin 1 among other cytokines, as well as by exogenous pyrogens, i.e., bacterial lipopolysaccharides, which acted at least in part by stimulating the production of endogenous pyrogenic cytokines. This demonstrated that the pathways leading to fever can converge into a unique mechanism and identified the biochemical pathway for the synthesis of PGE as a suitable pharmacological target for the control of fever response. Indeed, fever can be controlled by inhibiting cyclooxygenases (COXs), the enzymes that convert arachidonic acid (AA) into prostaglandin G2, the precursor of PGE2 and of other prostanoids.
AA can be derived from membrane phospholipids by the action of phospholipases A2 (PLA2) a calcium-dependent enzyme that has generally been considered to be the main source of AA for prostaglandin production. However, less than a decade ago, Rosenberg and colleagues found that PLA2-deficient mice had unaltered brain levels of AA,5 suggesting the existence of an additional source of AA. Consistent with this premise, studies in the 1970s and 80s pointed to the existence of potentially alternative AA-prostaglandin pathways that involve neutral lipases converting diacylglycerols and monoacylglycerols to AA.6,7 The physiological significance of these alternative pathways, especially as pertains to the nervous system, however, remained unknown. More recently, Nomura and colleagues discovered that hydrolysis of the endocannabinoid 2-arachidonoylglycerol (2-AG) by mono-acylglycerol lipase (MAGL) serves as a major source for AA in the brain.8 (Fig. 1). This novel and PLA-independent route for AA production is biologically significant as genetic or pharmacological inhibition of MAGL effectively reduced PGE2 and neuroinflammation in mice.
Figure 1.
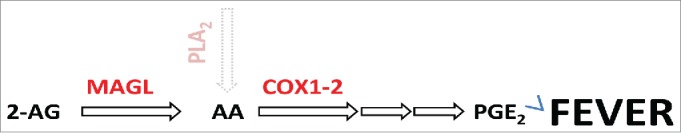
The endocannabinoid 2-AG is hydrolyzed by MAGL providing a PLA-independent source of AA. AA is the substrate for the synthesis of prostaglandin PGE2 (and other prostanoids). Genetic or pharmacological inhibition of MAGL strongly reduced fever response to LPS or IL-1β.
The role of the 2-AG-MAGL pathway in fever was investigated independently by Kita and colleagues at the University of Tokyo and by Sanchez-Alavez and colleagues at The Scripps Research Institute.1,2 Both groups found that mice null for MAGL (Mgll−/−) and their wild-type littermates had similar circadian profile of their core body temperature (CBT), indicating that MAGL is not required for the normal basal maintenance of temperature. On the other hand, the fever response to LPS was significantly attenuated in Mgll−/− mice. The results were similar in both studies that used the same source of LPS (Sigma, St Louis) albeit different serotype, 0127:B8 vs 0111:B4, and slightly different doses, 100 vs ˜66 µg/kg. Sanchez-Alavez also found similar effects when fever was elicited by intracerebroventricular injection of the endogenous pyrogen interleukin 1β, a design used to mimic neuroinflammation and to specifically assess the contribution of central 2-AG/PGE2.
Since Mgll−/− mice also have strongly elevated 2-AG content in the brain, the two groups then used different approaches to investigate the possibility that the reduction of fever response could also be mediated by endocannabinoid action. Sanchez-Alavez ruled this out pharmacologically in animals treated with the cannabinoid receptor CB1 inverse agonist rimonabant. Kita obtained similar results testing Mgll−/− mice that also lacked functional CB1 signaling. In addition, intracerebroventricular injection of PGE2 induced similar fever response in both Mgll−/− and Mgll+/+ mice.
Importantly, Kita compared the fever response of mice lacking cPLA2 and their wild type littermates and found that the activity of this enzyme was not required for a full fever response. Finally, using transgenic mice and biochemical measurements, the authors demonstrated that lack of diacylglycerol lipase α (DAGLα), one of the two enzymes known to synthesize 2-AG in the nervous system, did not impact fever.
Several outstanding questions remain to be addressed. One is the source of 2-AG in fever states: if DAGLα is not necessary, to what extent does DAGLβ contribute to the 2-AG-MAGL pathway relevant for fever? Are there other yet unidentified pathways for the synthesis of 2-AG? Although Akira did not find evidence that the liver could significantly contribute to the synthesis of PGE2 during fever, Nomura demonstrated that the 2-AG-MAGL pathway is active in some peripheral tissues, such as the lung. In addition, when considering the possible contribution of peripheral PGE2, the role of cPLA2, which is the main contributor to AA production in several peripheral tissues, such as the intestine and the spleen, should be evaluated. Second, is there a 2-AG independent source of AA? For instance, could the other known endocannabinoid anandamide contribute to a specific pool of AA via hydrolysis mediated by fatty acid amide hydrolase? Finally, is MAGL a suitable pharmacological target for controlling fever? Sanchez-Alavez provided some important and encouraging evidence that it might. For instance, fever response was significantly attenuated in mice receiving a single intraperitoneal injection of JZL184 in both LPS- and interleukin 1β fever models. Targeting MAGL could thus lead to novel antipyretic/anti-neuroinflammatory therapeutics devoid of the gastrointestinal effects of COX-inhibitors. Further investigations of this concept will need to determine the precise contribution of 2-AG and MAGL to the distinct phases that characterize the fever response.
These findings, taken together, demonstrate that fever still has secrets to reveal and that their discovery may lead to novel and better antipyretic/anti-inflammatory therapies.
References
- [1].Sanchez-Alavez M, Nguyen W, Mori S, Moroncini G, Viader A, Nomura DK, Cravatt BF, Conti B. Monoacylglycerol Lipase Regulates Fever Response. PloS One 2015; 10:e0134437; PMID:26287872; http://dx.doi.org/ 10.1371/journal.pone.0134437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kita Y, Yoshida K, Tokuoka SM, Hamano F, Yamazaki M, Sakimura K, Kano M, Shimizu T. Fever Is Mediated by Conversion of Endocannabinoid 2-Arachidonoylglycerol to Prostaglandin E2. PloS One 2015; 10:e0133663; PMID:26196692; http://dx.doi.org/ 10.1371/journal.pone.0133663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nature Neurosci 2007; 10:1131-3; PMID:17676060; http://dx.doi.org/ 10.1038/nn1949 [DOI] [PubMed] [Google Scholar]
- [4].Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, et al.. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 1998; 395:281-4; PMID:9751056; http://dx.doi.org/ 10.1038/26233 [DOI] [PubMed] [Google Scholar]
- [5].Rosenberger TA, Villacreses NE, Contreras MA, Bonventre JV, Rapoport SI. Brain lipid metabolism in the cPLA2 knockout mouse. J Lipid Res 2003; 44:109-17; PMID:12518029; http://dx.doi.org/ 10.1194/jlr.M200298-JLR200 [DOI] [PubMed] [Google Scholar]
- [6].Bell RL, Kennerly DA, Stanford N, Majerus PW. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A 1979; 76:3238-41; PMID:290999; http://dx.doi.org/ 10.1073/pnas.76.7.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Broekman MJ. Stimulated platelets release equivalent amounts of arachidonate from phosphatidylcholine, phosphatidylethanolamine, and inositides. J Lipid Res 1986; 27:884-91; PMID:3021886 [PubMed] [Google Scholar]
- [8].Nomura DK, Morrison B, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Lichtman AH, Hahn JK, Conti B, et al.. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 2011; 334:809-13; PMID:22021672; http://dx.doi.org/ 10.1126/science.1209200 [DOI] [PMC free article] [PubMed] [Google Scholar]


