ABSTRACT
Aim. The purpose of this study was to determine the response of circulating markers of lipid and protein oxidation following an incremental test to exhaustion before and after 4 weeks of high-intensity interval training performed in the heat. Methods. To address this question, 16 physically active men (age = 23 ± 2 years; body mass = 73 ± 12 kg; height = 173 ± 6 cm; % body fat = 12.5 ± 6 %; body mass index = 24 ± 4 kg/m2) were allocated into 2 groups: control group (n = 8) performing high-intensity interval training at 22°C, 55% relative humidity and heat group (n = 8) training under 35°C, 55% relative humidity. Both groups performed high-intensity interval training 3 times per week for 4 consecutive weeks, accumulating a total of 12 training sessions. Before and after the completion of 4 weeks of high-intensity interval training, participants performed an incremental cycling test until exhaustion under temperate environment (22°C, 55% relative humidity) where blood samples were collected after the test for determination of exercise-induced changes in oxidative damage biomarkers (thiobarbituric acid reactive species and protein carbonyls). Results. When high-intensity interval training was performed under control conditions, there was an increase in protein carbonyls (p < 0.05) following the incremental test to exhaustion with no changes in thiobarbituric acid reactive species. Conversely, high-intensity interval training performed in high environmental temperature enhanced the incremental exercise-induced increases in thiobarbituric acid reactive species (p < 0.05) with no changes in protein carbonyls. Conclusion. In conclusion, 4 weeks of high-intensity interval training performed in the heat enhances exercise-induced lipid peroxidation, but prevents protein oxidation following a maximal incremental exercise in healthy active men.
KEYWORDS: exercise, hyperthermia, oxidative stress, protein carbonyls, TBARS
Introduction
High-intensity interval training (HIIT) is a chronic exercise in which an acute bout consists of brief sessions of intermittent vigorous activities interspersed by periods of rest or low intensity exercise. Evidence suggests that HIIT may be an alternative to the traditional continuous endurance training, provoking similar physiological adaptations despite the low volume and time necessary to perform the training.1-4 The physiological remodeling induced by HIIT is attributed to the high intensity, corresponding to >200% of the power output eliciting maximal oxygen uptake (VO2max), resulting in large changes in ATP:ADP/AMP ratio and activation of 5'adenosine monophosphate-activated protein kinase (AMPK) and peroxisome-proliferator activated receptor- γ coactivator-1α (PGC-1α).5-7 Although the precise mechanism by which HIIT activates these adaptive pathways remains elusive; it may involve the formation of reactive oxygen species (ROS).8 Likewise, the potential interactions between HIIT and environmental factors (e.g. high environmental temperature) that might impact physiological outcomes remain unknown.
Studies demonstrate that HIIT increases the exercise-induced formation of ROS.9-12 The rise in exercise-induced ROS after HIIT possibly arises from higher activities of xanthine and NADPH oxidases due to the large increase in metabolism induced by anaerobic stimulus.13 Although ROS can have positive effects on exercise training adaptations,14,15 when ROS formation overcomes the cellular antioxidant system, it causes structural oxidative damage to lipids (e.g., cell membranes), proteins and DNA.16,17 Similarly, exercise performed under high environmental temperatures has been shown to acutely enhance the production of oxidants in humans.18-23 For instance, McAnulty et al. demonstrated that men exercising on a treadmill at 39.5°C for 50 min displayed increased circulating concentrations of lipid peroxides in comparison with controls exercising for the same time at normal environmental temperature.18 Laitano et al. found increases in markers of oxidative stress in the circulation of heat stressed humans (~1.3°C elevation in core and 6°C increase in skin temperature) after short-term one-legged knee extensor exercise.19 Both Sureda et al and Mestre-Alfaro et al showed that circulatory markers of oxidative stress were significantly increased after 45 minutes at 75–80% VO2max in a hot environment (30–32°C and 75–78% RH).21,22 More recently, it has been hypothesized that increased ROS production observed during exercise in the heat reflects skeletal muscle requirements of adapting to osmotic challenges, hyperthermia challenges, and loss of circulating fluid volume.23 Based on the abovementioned studies, it is reasonable to hypothesize that HIIT performed under high environmental temperatures would enhance oxidation of lipids and proteins, but this has not been systematically tested before.
The main feature of increased cellular ROS formation is the oxidation of lipids and proteins. Typically, measurements of circulating products of oxidative stress, such as thiobarbituric acid reactive substances (TBARS) and protein carbonyls (PC), are used to determine the extent of lipid and protein oxidation, respectively.15,24 Therefore, we measured plasma TBARS and PC in men following a maximal incremental exercise before and after 4 weeks of HIIT performed in the heat, in order to assess its effects on lipid and protein oxidation.
Materials and methods
Participants
Sixteen young healthy male adults (age = 23 ± 2 years; body mass = 73 ± 12 kg; height = 173 ± 6 cm; % body fat = 12.5 ± 6 %; body mass index = 24 ± 4 kg/m2) were recruited for this study. Participants were recreationally active 2 to 3 times per week but none were engaged in a structured exercise-training program. They were randomly allocated into 2 groups. One group performed the HIIT in the heat (35°C, 55% relative humidity) and the other group (control) performed HIIT in a temperate environment (22°C, 55% relative humidity). Participants visited the lab on 16 different occasions to perform familiarization sessions, physical assessments and training trials as described in Figure 1. All participants were considered naturally acclimatized to heat as they were born and lived in a tropical climate country. The study protocol was approved by the University's Research Ethical Committee under the number 0005/190313. All participants gave informed consent after being informed about all procedures involved in the experiment. All procedures were in accordance with the code of Ethics of the Medical Association (Declaration of Helsinki).
Figure 1.
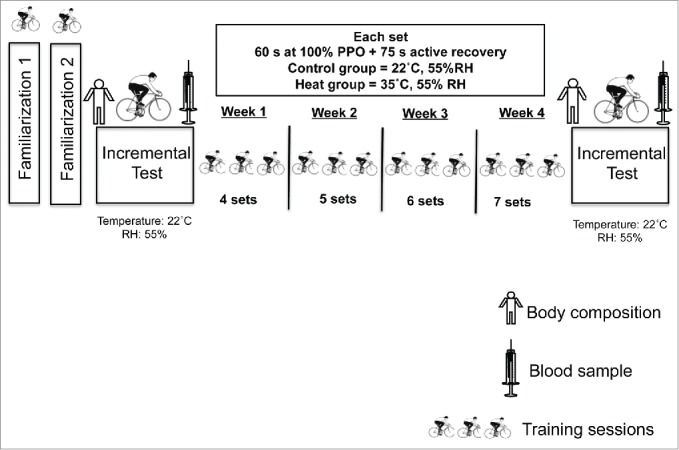
Schematic representation of the experimental design. Participants underwent 2 familiarization sessions on separate days before they performed a body composition assessment, incremental test to exhaustion. A blood sample was taken after the incremental test. HIIT then started for 4 consecutive weeks with 3 sessions per week. Two to 3 d after completion of the training period, body composition, incremental test to exhaustion and blood sampling were performed again. PPO = peak power output, RH = relative humidity
Familiarization sessions and food records
Participants performed 2 familiarization sessions before participating in the study (Fig. 1). Sessions were designed to familiarize the participants to the cycle ergometer and the type of exercise performed in the following sessions. Participants warmed-up by cycling for 3 minutes at a cadence of 60 rpm with a light workload and then performed sprints similar to the training sessions described below. During the familiarization trials, intensity was set at 5% of participants' individual body mass. All participants performed one session under temperate environmental temperature and another in the high environmental temperature, as at this stage they were not assigned to any study group. In order to standardize diet before both incremental tests to exhaustion, participants were instructed by a trained qualified dietitian to report their food consumption 24 h before the first incremental test. They then replicated the same diet 24 h before the second incremental test. None of the participants were taking any antioxidant supplements and were asked to avoid drastic changes in regular diet habits throughout the training period.
Incremental test to exhaustion
After completion of the familiarization sessions, participants underwent a body composition assessment and an incremental cycling test to exhaustion. Body composition was assessed through measurements of height and nude body weight and 7 skinfold sites (triceps, subscapular, chest, abdominal, suprailiac, thigh and calf) by a trained professional with certified experience. Percentage of body fat was then estimated by using the Jackson & Pollock equation.25 Thereafter, participants performed an incremental test to exhaustion. The aim of this test was to determine the intensity of the following training sessions. Participants performed a warm-up for 3 min with a light workload before starting an incremental protocol that consisted of an initial workload of 30 W with increments of 30 W/min to exhaustion. The peak power output (PPO) achieved during this incremental test was used as the workload for the upcoming training sessions. Toe clips were used and the feet were strapped to the pedal. The saddle height and harness setting for each subject were recorded and used for all trials. To ensure euhydration during the tests, participants drank a volume of plain water (in mL) correspondent to 0.5% of their body mass 20 min before starting the incremental test. Participants repeated this same protocol 2 to 3 d after completion of the 4 weeks of HIIT. Blood samples were taken after the incremental test, before and after 4 weeks of training, to determine the effects of training on the selected biomarkers. Regardless of the training condition (e.g control or heat), both groups performed the incremental cycling test in a temperate environment (22°C, 55% RH).
High intensity interval training protocol
To avoid carry-over effect from the previous incremental test, participants started the HIIT at least 2 and at most 7 d after the incremental test. Training sessions were performed 3 times a week for 4 consecutive weeks and were supervised by at least one member of the research team. Individuals of the control group performed the training protocol at a temperate environment (22°C, 55% RH), while the experimental group ones trained in the heat (35°C, 55% RH). The warm temperature was achieved by pre-heating the room using an electric heater device while control condition was achieved by using an air conditioning unit set at the desired temperature. Environmental temperature and relative humidity were monitored with a thermo hygrometer (Impac, IP-780). In each training session, participants performed a standardized 3 min warm-up period followed by 4 sets of 60 s efforts of high-intensity cycling at a workload that corresponded to the PPO achieved at the end of the previous incremental test to exhaustion.26 These intervals were interspersed by 75 s of active low intensity (30 W) recovery. The number of sets performed increased from 4 to 7 since one set was added to the protocol after each week of training, according to the principle of progressive training overload (Fig. 1). Therefore, at completion of the HIIT both groups accomplished the same training volume of 12 sessions in total as shown in Table 1.
Table 1.
Training volumes per session of HIIT throughout 4 weeks. Each set comprised 60 s of cycling at 100% power output followed by 75 s of active recovery. Participants performed 3 sessions per week. The HIIT protocol was adapted from Little et al.26
| Warm-up (s) | Number of Sets | Active Training (s) | Active Recovery (s) | Total Training Duration (s) | |
|---|---|---|---|---|---|
| Week 1 | 180 | 4 | 240 | 225 | 645 |
| Week 2 | 180 | 5 | 300 | 300 | 780 |
| Week 3 | 180 | 6 | 360 | 375 | 915 |
| Week 4 | 180 | 7 | 420 | 450 | 1050 |
Blood sampling and analysis
Blood samples were taken immediately after the incremental test, before and after the 4 weeks period of training. Blood was collected via venopuncture in an antecubital vein with a disposable 20-gauge needle attached to a 5 mL syringe. An aliquot of 2 mL was used to analyze hemoglobin in duplicate using the cyanmethemoglobin method, and packed-cell volume was determined in quadruplicate by microcentrifugation to estimate changes in plasma volume, as described by Dill & Costill.27 The remaining 3 mL of blood were transferred to tubes containing 100 μL of 50 IU heparin (Roche) and centrifuged for 2,000 × g for 10 min (room temperature) for plasma separation. At least 1 mL of plasma was stored at -86°C in microcentrifuge tubes containing 10 μL of an ethanolic solution of butylated hydroxytoluene (BHT, 20 μmol/L final concentration) used to prevent in-analysis auto-oxidation in methanol to a final concentration of 20 µmol/L for further analysis of thiobarbituric acid reactive species (TBARS) and PC. For the estimative assessment of oxidative-stress induced lipid peroxidation, TBARS assay was performed according to Ohkawa et al.28 as adapted from De Angelis et al.29 In brief, the samples were mixed with 8.1% sodium dodecyl sulfate (SDS), 0.1% (w/v) BHT in ethanol, 20% (v/v) acetic acid pH 3.5, 0.73% (w/v) thiobarbituric acid (TBA) and boiled at 100°C for 1 h. Absorbance was spectrophotometrically registered at 532 nm. PC were determined according to the protocol proposed by Levine et al.30 in which the samples were incubated in 10 mmol/L 2,4-dinitrophenylhydrazine (DNPH) in 2 mol/L hydrochloric acid, for 1 h, under dark conditions at room temperature, whereas blanks were incubated for the same time in 2 mol/L HCl in the absence of DNPH. After that, proteins were precipitated in 20% (w/v) trichloroacetic acid and the samples were washed 3 times with ethanol/ethyl acetate (1;1, by volume). The pellet was resuspended in 6 mol/L guanidine in 20 mmol/L potassium phosphate pH 2.3 and incubated at 37°C for 15 min. Absorbance was spectrophotometrically read at 370 nm.
Statistical analysis
Normality of data was first assessed using the Shapiro–Wilk test. Data are presented as mean ± SD unless otherwise stated. To identify differences in normally distributed results, 2-way repeated measures analyses of variance (ANOVA) were employed. Where a significant interaction was apparent, pairwise differences were evaluated using Tukey’s post hoc procedure and paired t tests with Holm–Bonferroni adjustment for multiple comparisons. For the purpose of hypothesis testing, the 95% level of confidence was predetermined as the minimum criterion to denote a statistical difference (p < 0.05). All data analyses were undertaken using SPSS 17.0 for Windows (SPSS Inc.., Chicago, IL, USA) and GraphPad Prism.
Results
Both groups were similar in terms of age, body composition, and level of physical activity. As described in Table 2, the participants’ body weight remained the same after the 4 weeks period of HIIT in both groups. Percentage body fat and body mass index did not change significantly throughout the training regardless of the condition (p > 0.05). Changes in plasma volume following the incremental test to exhaustion were not significant in both groups, as hematocrit and hemoglobin measures were not significantly altered by HIIT in both environments (p > 0.05). Training duration per session ranged from ~10 to 17 min (Table 1).
Table 2.
Body weight, % body fat, body mass index (BMI), hematocrit (Htc), hemoglobin (Hb), peak power output (PPO), and maximal heart rate (HRmax) before and after 4 weeks of high intensity interval training in control and hot conditions. * different from pre HIIT (p < 0.05).
| Control |
Heat |
|||||
|---|---|---|---|---|---|---|
| Pre HIIT | Post HIIT | p | Pre HIIT | Post HIIT | p | |
| Height (cm) | 175 ± 6.4 | 175 ± 6.4 | — | 172 ± 5.2 | 172 ± 5.2 | — |
| Body mass (kg) | 73.8 ± 14.4 | 73.3 ± 14.2 | 0.2036 | 69.2 ± 8.7 | 69.4 ± 8.6 | 0.4387 |
| Body fat (%) | 12.3 ± 8.0 | 11.5 ± 6.5 | 0.3622 | 11.3 ± 4.3 | 11.3 ± 4.5 | 0.8483 |
| BMI (kg/m2) | 24.1 ± 4.0 | 23.9 ± 4.0 | 0.1501 | 23.7 ± 3.2 | 23.8 ± 3.1 | 0.7429 |
| Hb (g/dL) | 17.4 ± 1.9 | 18.0 ± 1.1 | 0.4983 | 18.5 ± 2.3 | 17.3 ± 2.7 | 0.3088 |
| Htc (%) | 41.8 ± 4.0 | 45.8 ± 3.3 | 0.0742 | 43.4 ± 3.8 | 45.9 ± 2.7 | 0.1002 |
| PPO (W) | 255 ± 28 | 292 ± 31 | 0.0954 | 259 ± 27 | 304 ± 25* | 0.0026 |
| HRmax (bpm) | 179 ± 11 | 182 ± 9 | 0.1342 | 177 ± 10 | 188 ± 8* | 0.0325 |
When performed under temperate environment, HIIT did not increase PPO achieved in the incremental test. On the other hand, we observed an increase in PPO when HIIT was performed in the heat group (p = 0.026, Table 2). Likewise, there was a significant increase in maximal heart rate achieved in response to incremental test for the group performing HIIT in high environmental temperature (p = 0.032, Table 2) and we observed no changes maximal heart rate when HIIT was performed in temperate environment (p > 0.05).
As reported in Figure 2, HIIT under temperate environment did not alter TBARS values (p = 0.3401) after incremental test to exhaustion. On the other hand, there was an increase in TBARS following the incremental test to exhaustion after 4 weeks of HIIT performed in the heat, thus suggesting that incremental exercise-induced lipid peroxidation was higher after the intervention (p = 0.0177). Conversely, HIIT under temperate environment increased PC values (p = 0.0316) after the incremental test to exhaustion in the control group, whereas plasma PC contents following the incremental test were not affected when HIIT was performed in the heat (p = 0.0722, Fig. 2) suggesting that incremental exercise-induced protein oxidation was prevented after HIIT performed in the heat.
Figure 2.
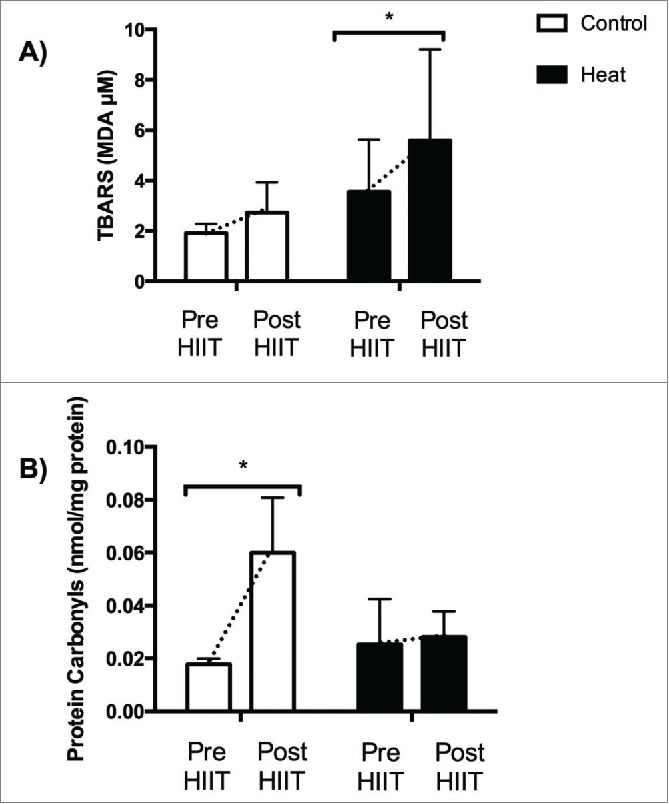
Exercise-induced lipid peroxidation (A) and protein oxidation (panel B) in Control and Heat groups before and after 4 weeks of high intensity interval training. Panel A *p < 0.05; mean of differences = 2.600; 95% confidence interval = 0.63 to 4.56. (B) *p < 0.05; mean of differences = 0.048; 95% confidence interval = 0.0058 to 0.090
Discussion
The primary focus of this study was to investigate the effects of 4 weeks of HIIT performed in high environmental temperature on circulating markers of protein and lipid oxidation in humans. Our main finding was that, unlike HIIT performed in temperate environment, 4 weeks of HIIT performed in the heat enhanced lipid peroxidation but prevented protein oxidation after a maximal incremental exercise. The above circulating parameters are considered classic markers of ROS-induced damage to lipids and proteins. Since ROS can have positive effects on exercise training adaptations,15 it is possible that the environmental temperature in which HIIT is performed may be an additional factor influencing the adaptations promoted by this type of exercise training.
To the best of our knowledge, this is the first study to assess the effects of HIIT performed in the heat on circulatory markers of oxidative damage to lipids and proteins. We used a chronic approach of 4 weeks of HIIT. A study using a single session of high intensity cycling found similar results to our control group, with enhanced protein oxidation, but with no significant effect on lipid peroxidation.10 Although the results are similar, it is difficult to compare and draw firm conclusions since our study used a chronic high intensity exercise and Bloomer et al10 used an acute session of high intensity cycling.
Even though our experimental group trained in a high environmental temperature, the incremental test performed before and after 4 weeks of HIIT occurred in a temperate environment (22°C, 55% RH). One important finding of the present study is that HIIT performed in a high environmental temperature may prevent the increase in PC normally observed.9,10 The mechanisms behind this response were beyond the objective of the current study. Nonetheless, it may involve upregulation of heat shock proteins (HSP), as both heat stress31 and HIIT32 have been found to increase HSP production in the plasma and skeletal muscle. Therefore it is reasonable to consider that the absence of protein oxidation observed after 4 weeks of HIIT performed in the heat could have been a result of protection provided by heat-induced upregulation of HSPs. The physiological impact of each training session on heart rate, core and skin temperatures was not determined in the current study, therefore future studies are warranted to assess these effects.
We observed an increase in PPO when HIIT was performed in the heat, but not after HIIT performed under temperate environment. Given that both groups completed the same training volume and intensity, it is possible to suggest that the temperature in which HIIT is performed can influence the training outcomes (e.g. adaptations). This higher PPO was probably responsible for the higher heart rate achieved after HIIT in the heat. Of note, these physiological adaptations were accomplished after 12 sessions of HIIT performed in the heat. Burgomaster et al. demonstrated that even 6 sessions of HIIT performed under temperate environment can increase skeletal muscle oxidative potential and PPO.33 In the current study we used a modified version of HIIT (60 s at PPO with 75 s active recovery)26 whereas Burgomaster et al. used a higher intensity type of HIIT (Wingate test-based session with 30 s all out cycling).33 These differences in HIIT intensity in both studies may have accounted for the fact that our control group did not increase PPO when training under temperate environment. Importantly, when HIIT was performed in the heat, the increase in PPO mirrored the findings of Burgomaster et al. for PPO despite the differences in intensity and volume between the 2 studies.33
Although HIIT performed in the heat provided protection against protein oxidation, there was an increase in lipid peroxidation after 4 weeks of training (Fig. 2). It is of note that, whereas circulating PC correlate well with skeletal muscle redox imbalances, plasma TBARS are well associated with redox imbalances of heart and liver and may not reflect what occurs in skeletal muscle.34 One of the adaptations observed in the present study was that the maximal heart rate achieved after 4 weeks of HIIT in high environmental temperature was higher than that observed before training. Although not measured in the present study, it is possible that heart rate achieved higher levels during each single HIIT session in the heat than during HIIT in temperate environment. Therefore it is possible that our results for lipid peroxidation might reflect a higher heart oxidant production due to the higher maximal heart rate rather than a skeletal muscle adaptation. However, more studies are required to determine the extent to which circulating TBARS may reflect heart redox imbalances in situations where high intensity exercise and heat stress are chronically superimposed.
Studies indicate that antioxidant defense is modulated by high intensity exercise11,35 and heat stress.19,21,36 A limitation of the present study was that we did not include antioxidant defense in the panel of circulatory biomarkers analyzed. A study11 has recently demonstrated that 3 weeks of HIIT upregulate antioxidant activity after 9 sessions, indicating that the impact of HIIT on antioxidant status might be significant. This corroborates the results reported by an early study35 suggesting that HIIT causes upregulation of antioxidant enzymes in the skeletal muscle. Thus, studies addressing the effects of high intensity training in the heat on antioxidant status are required.
Little et al used a similar type of HIIT employed in our study and observed an increase (~25%) in mitochondria biogenesis master regulator PGC-1α protein abundance.26 PGC-1α signaling pathway in skeletal muscle is redox sensitive and may depend upon ROS formation.8 Although in the present study we assessed only 2 circulatory markers of lipid and protein oxidation likely induced by ROS, they may be related with changes in redox balance required for activating pathways (e.g., PGC-1α) to provoke HIIT-induced adaptations. Nevertheless, studies assessing circulatory and skeletal muscle biomarkers of ROS during HIIT performed in the heat are required to shed light into the precise mechanism involved in differential HIIT adaptations in the heat herein described.
In the present study, blood samples were obtained before and after 4 weeks of HIIT performed in the heat or under temperate environment. While this is the first study to determine this response, blood samples could have been taken half way through the HIIT period to establish a time-course response regarding the circulating markers herein studied. This could be considered a limitation of the present study and the time-course response of resting TBARS and PC to HIIT performed in the heat is still to be determined.
Our study involved subjects who were born and lived in a tropical climate country and thus were considered naturally acclimatized to heat. It is well known that heat acclimatization improves thermal comfort and submaximal as well as maximal exercise performance in the heat as recently reviewed by Racinais et al.37 Whether the responses observed in the present study holds true for non-acclimatized subjects is a matter that requires further investigation.
Conclusion
In conclusion, our results suggest that chronic HIIT performed under high environmental temperature may enhance exercise-induced lipid peroxidation, but prevent protein oxidation following a maximal incremental exercise in healthy active men. The major implication of these findings is that the environmental temperature in which HIIT is performed may be an important factor influencing the adaptations promoted by this type of exercise training.
Abbreviations
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- AMPK
activated protein kinase
- ATP
adenosine triphosphate
- BHT
butylated hydroxytoluene
- DNA
deoxyribonucleic acid
- DNPH
2,4-dinitrophenylhydrazine
- g
gravity
- HCl
hydrochloric acid
- HIIT
high-intensity interval training
- HSP
heat shock proteins
- NADPH oxidases
Nicotinamide adenine dinucleotide phosphate-oxidase
- PC
protein carbonyls
- PGC1-α
peroxisome-proliferator activated receptor-γ coactivator-1α
- Ph
potential hydrogen
- PPO
peak power output
- RH
relative humidity
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid reactive species
- VO2max
maximal oxygen consumption
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Charles de Souza Vieira, Marcos Vinicius Oliveira Carneiro, and Gabriel Lucas Leite da Silva Santos for their excellent technical support.
Funding
This study was funded by the Brazilian National Council for Scientific and Technological Development (CNPq), grant #480227/2012–8 to OL. AASS and EM were supported by scholarships from CNPq-PIBIC. CMS and PIHBJ are also grateful to CNPq for research support (grant #402626/2012–5 and #402634/2012–0).
References
- [1].Gibala MJ, Little JP, Van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint intervalversustraditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 2006; 575:901-11; PMID:16825308; http://dx.doi.org/ 10.1113/jphysiol.2006.112094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burgomaster KA, Heigenhauser GJ, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol (1985) 2006; 100:2041-7; PMID:16469933; http://dx.doi.org/ 10.1152/japplphysiol.01220.2005 [DOI] [PubMed] [Google Scholar]
- [3].Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 2008; 586:151-60; PMID:17991697; http://dx.doi.org/ 10.1113/jphysiol.2007.142109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol 2008; 295:R236-42; PMID:18434437; http://dx.doi.org/ 10.1152/ajpregu.00069.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 2012; 590:1077-84; PMID:22289907; http://dx.doi.org/ 10.1113/jphysiol.2011.224725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol (1985) 2009; 106:929-34; PMID:19112161; http://dx.doi.org/ 10.1152/japplphysiol.90880.2008 [DOI] [PubMed] [Google Scholar]
- [7].Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1alpha and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1303-10; PMID:21451146; http://dx.doi.org/ 10.1152/ajpregu.00538.2010 [DOI] [PubMed] [Google Scholar]
- [8].Kang C, O'Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med 2009; 47:1394-400; PMID:19686839; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.08.007 [DOI] [PubMed] [Google Scholar]
- [9].Bloomer RJ, Goldfarb AH, McKenzie MJ. Oxidative stress response to aerobic exercise: comparison of antioxidant supplements. Med Sci Sports Exerc 2006; 38:1098-105; PMID:16775552; http://dx.doi.org/ 10.1249/01.mss.0000222839.51144.3e [DOI] [PubMed] [Google Scholar]
- [10].Bloomer RJ, Fry AC, Falvo MJ, Moore CA. Protein carbonyls are acutely elevated following single set anaerobic exercise in resistance trained men. J Sci Med Sport 2007; 10:411-7; PMID:16949870; http://dx.doi.org/ 10.1016/j.jsams.2006.07.014 [DOI] [PubMed] [Google Scholar]
- [11].Bogdanis GC, Stavrinou P, Fatouros IG, Philippou A, Chatzinikolaou A, Draganidis D, Ermidis G, Maridaki M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem Toxicol 2013; 61:171-7; PMID:23747717; http://dx.doi.org/ 10.1016/j.fct.2013.05.046 [DOI] [PubMed] [Google Scholar]
- [12].Deminice R, Trindade CS, Degiovanni GC, Garlip MR, Portari GV, Teixeira M, Jordao AA. Oxidative stress biomarkers response to high intensity interval training and relation to performance in competitive swimmers. J Sports Med Phys Fitness 2010; 50:356-62; PMID:20842099 [PubMed] [Google Scholar]
- [13].Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol 2004; 29:245-63; PMID:15199226; http://dx.doi.org/ 10.1139/h04-017 [DOI] [PubMed] [Google Scholar]
- [14].Finaud J, Lac G, Filaire E. Oxidative stress : relationship with exercise and training. Sports Med 2006; 36:327-58; PMID:16573358; http://dx.doi.org/ 10.2165/00007256-200636040-00004 [DOI] [PubMed] [Google Scholar]
- [15].Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008; 88:1243-76; PMID:18923182; http://dx.doi.org/ 10.1152/physrev.00031.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol (1985) 2008; 104:853-60; PMID:18006866; http://dx.doi.org/ 10.1152/japplphysiol.00953.2007 [DOI] [PubMed] [Google Scholar]
- [17].Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008; 295:C849-68; PMID:18684987; http://dx.doi.org/ 10.1152/ajpcell.00283.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McAnulty SR, McAnulty L, Pascoe DD, Gropper SS, Keith RE, Morrow JD, Gladden LB. Hyperthermia increases exercise-induced oxidative stress. Int J Sports Med 2005; 26:188-92; PMID:15776334; http://dx.doi.org/ 10.1055/s-2004-820990 [DOI] [PubMed] [Google Scholar]
- [19].Laitano O, Kalsi KK, Pook M, Oliveira AR, Gonzalez-Alonso J. Separate and combined effects of heat stress and exercise on circulatory markers of oxidative stress in euhydrated humans. Eur J App Physiol 2010; 110:953-60; PMID:20658249; http://dx.doi.org/ 10.1007/s00421-010-1577-5 [DOI] [PubMed] [Google Scholar]
- [20].Laitano O, Kalsi KK, Pearson J, Lotlikar M, Reischak-Oliveira A, Gonzalez-Alonso J. Effects of graded exercise-induced dehydration and rehydration on circulatory markers of oxidative stress across the resting and exercising human leg. Eur J Appl Physiol 2012; 112:1937-44; PMID:21932069; http://dx.doi.org/ 10.1007/s00421-011-2170-2 [DOI] [PubMed] [Google Scholar]
- [21].Mestre-Alfaro A, Ferrer MD, Banquells M, Riera J, Drobnic F, Sureda A, Tur JA, Pons A. Body temperature modulates the antioxidant and acute immune responses to exercise. Free Radic Res 2012; 46:799-808; PMID:22448737; http://dx.doi.org/ 10.3109/10715762.2012.680193 [DOI] [PubMed] [Google Scholar]
- [22].Sureda A, Mestre-Alfaro A, Banquells M, Riera J, Drobnic F, Camps J, Joven J, Tur JA, Pons A. Exercise in a hot environment influences plasma anti-inflammatory and antioxidant status in well-trained athletes. J Therm Biol 2015; 47:91-8; PMID:25526659; http://dx.doi.org/ 10.1016/j.jtherbio.2014.11.011 [DOI] [PubMed] [Google Scholar]
- [23].King MA, Clanton TL, Laitano O. Hyperthermia, dehydration, and osmotic stress: unconventional sources of exercise-induced reactive oxygen species.. Am J Physiol Regul Integr Comp Physiol 2016; 310:R105-R114; PMID:26561649; http://dx.doi.org/23771832 10.1152/ajpregu.0039.2015 [DOI] [PubMed] [Google Scholar]
- [24].Spirlandeli AL, Deminice R, Jordao AA. Plasma malondialdehyde as biomarker of lipid peroxidation: effects of acute exercise. Int J Sports Med 2014; 35:14-8; PMID:23771832 [DOI] [PubMed] [Google Scholar]
- [25].Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 1978; 40:497-504; PMID:718832; http://dx.doi.org/ 10.1079/BJN19780152 [DOI] [PubMed] [Google Scholar]
- [26].Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 2010; 588:1011-22; PMID:20100740; http://dx.doi.org/ 10.1113/jphysiol.2009.181743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974; 37:247-8; PMID:4850854 [DOI] [PubMed] [Google Scholar]
- [28].Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95:351-8; PMID:36810; http://dx.doi.org/ 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- [29].De Angelis KL, Cestari IA, Barp J, Dall'Ago P, Fernandes TG, de Bittencourt PI, Bello-Klein A, Bello AA, Llesuy S, Irigoyen MC. Oxidative stress in the latissimus dorsi muscle of diabetic rats. Braz J Med Biol Res 2000; 33:1363-8; PMID:11050669; http://dx.doi.org/ 10.1590/S0100-879X2000001100016 [DOI] [PubMed] [Google Scholar]
- [30].Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 1994; 233:346-57; PMID:8015469; http://dx.doi.org/ 10.1016/S0076-6879(94)33040-9 [DOI] [PubMed] [Google Scholar]
- [31].Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol (1985) 1997; 83:1413-7; PMID:9375300 [DOI] [PubMed] [Google Scholar]
- [32].Carmeli E, Beiker R, Maor M, Kodesh E. Increased iNOS, MMP-2, and HSP-72 in skeletal muscle following high-intensity exercise training. J Basic Clin Physiol Pharmacol 2010; 21:127-46; PMID:20853596; http://dx.doi.org/ 10.1515/JBCPP.2010.21.2.127 [DOI] [PubMed] [Google Scholar]
- [33].Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol (1985) 2005; 98:1985-90; PMID:15705728; http://dx.doi.org/ 10.1152/japplphysiol.01095.2004 [DOI] [PubMed] [Google Scholar]
- [34].Veskoukis AS, Nikolaidis MG, Kyparos A, Kouretas D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic Biol Med 2009; 47:1371-4; PMID:19616614; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.07.014 [DOI] [PubMed] [Google Scholar]
- [35].Hellsten Y, Apple FS, Sjodin B. Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J Appl Physiol (1985) 1996; 81:1484-7; PMID:8904557 [DOI] [PubMed] [Google Scholar]
- [36].Ohtsuka Y, Yabunaka N, Fujisawa H, Watanabe I, Agishi Y. Effect of thermal stress on glutathione metabolism in human erythrocytes. Eur J Appl Physiol Occup Physiol 1994; 68:87-91; PMID:8162928; http://dx.doi.org/ 10.1007/BF00599247 [DOI] [PubMed] [Google Scholar]
- [37].Racinais S, Alonso JM, Coutts AJ, Flouris AD, Girard O, Gonzalez-Alonso J, Hausswirth C, Jay O, Lee JK, Mitchell N, et al.. Consensus Recommendations on Training and Competing in the Heat. Sports Med 2015; 45:925-38; PMID: 26002286; http://dx.doi.org/ 10.1007/s40279-015-0343-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


