Abstract
Engagement of the receptor for advanced glycation end products (RAGE) by its signal transduction ligands evokes inflammatory cell infiltration and activation of the vessel wall. However, soluble RAGE (sRAGE), the truncated form spanning the extracellular binding domain of RAGE, has potent anti-inflammatory properties by acting as a decoy for RAGE ligands. We now show that sRAGE binds with high affinity to atherogenic low-density lipoprotein (LDL) modified by hypochlorous acid (HOCl), the major oxidant generated by the myeloperoxidase-H2O2-chloride system of phagocytes activated during inflammation. We further demonstrate that sRAGE can be coprecipitated with HOCl-LDL from spiked serum. To determine the functional significance of sRAGE binding to HOCl-LDL, cell association studies with macrophages were performed. sRAGE effectively inhibited cellular uptake of HOCl-LDL and subsequent lipid accumulation. Using Chinese hamster ovary cells overexpressing class B scavenger receptor CD36 or SR-BI, two preferential scavenger receptors for HOCl-LDL, we demonstrate that sRAGE only interferes with CD36-mediated uptake of HOCl-LDL. The present findings indicate that sRAGE acts as a sink for HOCl-LDL, which is abundantly present in human atherosclerotic lesions. We propose that sRAGE represents a physiological antagonist that interferes with scavenger receptor-mediated cholesterol accumulation and foam cell formation of macrophages.
Keywords: myeloperoxidase, inflammation, oxidized LDL, foam cells
The receptor for advanced glycation end products (RAGE) was initially described as a central surface interaction site for advanced glycation end products (AGEs), the products of nonenzymatic glycooxidation of proteins/lipids that accumulate in the tissue and plasma of patients with diabetes (for reviews, see refs. 1–3). In principle, RAGE does not act as a clearance receptor. However, RAGE engagement amplifies immune and inflammatory responses, cell mobility, arterial injury, and atherogenesis in diabetes and diabetic complications via sustained postreceptor signaling (4). Overexpression of human RAGE in transgenic mice results in advanced nephropathy under diabetic conditions (5). RAGE is expressed in nondiabetic and diabetic human atherosclerosis (4, 6), and to an enhanced degree in diabetes (7). A variety of therapeutic approaches aim to impair the pathophysiological effects of AGE (3). For example, AGE cross-link formation may be blocked or, when already formed, AGE cross-link breakers may be used. A promising approach for inhibiting AGE effects is to target RAGE by infusing a soluble extramembrane portion of the receptor (sRAGE) that acts as a decoy (8). Administration of sRAGE has been shown to improve wound healing and nephropathy in diabetic db+/db+ mice (9, 10) to suppress inflammation and acceleration of early atherosclerosis (11, 12) and to stabilize established atherosclerosis in diabetic and euglycemic apolipoprotein (apo) E-null mice (6).
In addition to AGEs, RAGE binds several other proinflammatory non-AGE molecules, such as S100/calgranulins, high-mobility group box chromosomal protein-1, and amyloid β peptide, which mediate diverse pathological functions in disease stages (e.g., atherosclerosis, diabetes, kidney disease, Alzheimer’s disease, and others (1, 3). We recently demonstrated that albumin, when oxidized by hypochlorous acid [HOCl, formed only from H2O2 by myeloperoxidase (MPO) in the presence of physiological chloride concentrations], is a high-affinity ligand for RAGE and promotes RAGE-mediated downstream signaling events (13). Immunofluorescence studies revealed colocalization of HOCl-modified epitopes with albumin and RAGE in human atherosclerotic lesions (13). RAGE and MPO are highly expressed in human diabetic and nondiabetic lesions (7), and colocalize with monocytes/macrophages (14). MPO is an inflammatory mediator of atherosclerosis, and increased plasma MPO levels are a determinant factor for cardiovascular disease (for a review, see ref. 15). MPO is abundantly present in neutrophils and monocytes, and leukocyte-derived MPO associates with circulating albumin (16). However, MPO also binds to low-density lipoprotein (LDL) (17), and MPO-catalyzed oxidation products are present on LDL particles extracted from human atherosclerotic lesion material (18, 19). Density gradient centrifugation of human plaque homogenate and subsequent immunoblot analysis of the LDL buoyant fraction further revealed that apoB-100, the major apolipoprotein of LDL, is modified by the MPO-H2O2-chloride system (18). In vitro experiments demonstrated that modification of LDL by HOCl, when added as reagent or generated by the MPO-H2O2-chloride system, promotes scavenger receptor-mediated uptake of the modified LDL particle by monocytes/macrophages, leading to uncontrolled accumulation of cholesterol and cholesteryl esters (20, 21).
As HOCl-albumin binds with high affinity to RAGE (13), the present study was aimed to investigate whether sRAGE could act as a decoy for HOCl-LDL. HOCl-LDL exerts an array of adverse biological effects (for a review, see ref. 22), including foam cell formation; therefore, we were interested in whether sRAGE interferes with scavenger receptor-mediated uptake of HOCl-LDL.
MATERIALS AND METHODS
Human lipoprotein
LDL (d=1.035–1.065 g/ml) was isolated by ultracentrifugation as described (23). The protein of the final LDL preparation consisted of 96–98% apoB-100 as measured immunochemically. LDL concentrations are expressed in micrograms of protein/ml and were determined according to the Lowry method using bovine serum albumin as a standard. Prior to modification, native LDL was desalted and the preservatives were removed by dialysis or size exclusion chromatography on Econopac 10-DG columns (Bio-Rad, Hercules, CA, USA) (23).
Oxidative modifications
HOCl-LDL was prepared as described (21). Briefly, 500 µg of LDL protein/ml of phosphate-buffered saline (PBS, pH 7.4) was incubated with HOCl (oxidant:protein molar ratio of 200:1, 400:1, and 800:1) in the absence of free amino acids/carbohydrate at 4°C for up to 1 h at pH 7.4.
HOCl-albumin was prepared as described (13). Briefly, 500 mg of fatty acid-free bovine serum albumin (fatty acid content≤0.005%, low endotoxin; Sigma, St. Louis, MO, USA) was incubated with HOCl (oxidant:protein molar ratio of 100:1) in PBS (pH 7.4, 1 h, 4°C) in the absence of amino acids/carbohydrate/lipids to exclude formation of AGE-like structures.
AGE-albumin was prepared as described previously (13, 24). Briefly, 500 µg of albumin was dissolved with 3.0 g of d-glucose in 10 ml of 500 mM PBS (pH 7.4) containing 0.05% NaN3. The solution was deoxygenated with N2, sterilized by ultrafiltration, and incubated for 90 days at 37°C in the dark.
All modified (lipo)protein preparations were passed over a PD10 column (Amersham, Arlington Heights, IL, USA) immediately before use to remove unreacted HOCl or glucose. The electrophoretic mobility of HOCl-modified (lipo)protein preparations was assessed by agarose gel electrophoresis using the Lipidophor system (23).
Oxidation of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) (Avanti Polar Lipids, Alabaster, AL, USA) was performed as described (25). Briefly, 1 mg PAPC dissolved in 100 µl of chloroform was transferred to a 125 mm glass test tube and evaporated under a stream of nitrogen. The lipid residue was allowed to autoxidize while being exposed to air for 24–48 h.
Expression and isolation of recombinant human sRAGE
sRAGE was isolated from the cell supernatant of human embryonic kidney (HEK) -293 cells (ATCC), stably transfected with vector pSecTag2B (Invitrogen, Carlsbad, CA, USA) containing sRAGE cDNA. The recombinant sRAGE (a C-terminal truncated protein and identical to the extracellular domain of full-length RAGE) containing a C-terminal His6-tag was purified as described (26).
Radioactive labeling
Labeling of sRAGE, native, and modified LDL preparations was performed with Na125I (PerkinElmer Life Sciences, Norwalk, CT, USA) using N-bromosuccinimide as the coupling agent. Routinely, 200 µCi of Na125I was used to label 1 mg of protein. This procedure resulted in specific activities between 200 and 400 cpm/ng of protein (27).
sRAGE binding assay
Microtiter wells (Nunc Maxisorp, VWR International, Mississauga, ON, Canada) were coated with albumin [native albumin, HOCl-albumin (oxidant:protein molar ratio of 100:1), and AGE-albumin] or LDL [native and HOCl-LDL (oxidant: protein molar ratio of 800:1), each 100 µg/ml (15 mM sodium carbonate, 35 mM sodium bicarbonate, pH 9.6] overnight at 4°C. Subsequently, the wells were washed with washing buffer (ice-cold PBS containing 0.05% Tween-20). In some experiments, LDL was coated to the wells and directly oxidized by adding 100 µl of HOCl solutions (1–100 µM in PBS, pH 7.4) for 1 h at 4°C. After washing and blocking [2 h at 37°C with PBS containing albumin (10 mg/ml)], the wells were incubated with 125I-sRAGE (100 µl at the concentrations indicated either alone or in the presence of an excess of unlabeled competitors) in Dulbecco’s minimum essential medium (DMEM, containing 1 mg albumin/ml) for 2 h at 37°C. Then the supernatant was removed and the wells were washed four times with washing buffer. Bound 125I-sRAGE was quantified by γ-counting (13, 27).
Precipitation techniques
Lipoproteins (LDL or HOCl-LDL) and 125I-sRAGE were added to 1 ml of human serum. Subsequently, MgCl2 (25 µl of a 2 M stock solution) and phosphotungstate (100 µl of a 4% stock solution in 0.19 M NaOH) were added to precipitate LDL and HOCl-LDL. The samples were centrifuged and radioactivity was measured in the supernatants and the pellets, respectively.
Alternatively, human serum was treated with polyethylen-glycol to precipitate lipoproteins (LDL or HOCl-LDL) according to the manufacturer’s instructions (Quantolip, Immuno AG, Vienna, Austria).
Cell lines and cell culture experiments
RAW264.7 macrophages (ATCC) were cultured in DMEM containing 10% (v/v) fetal calf serum (FCS), 2 mM glutamine, 50 U/ml penicillin, and 50 µg/ml streptomycin. Stable transfectant Chinese hamster ovary (CHO) cells expressing murine scavenger receptor class B, type I (SR-BI, termed CHO-SR-BI), or human CD36 (termed CHO-CD36) (ATCC) were cultured in Ham’s-F-12K medium containing 5% (v/v) fetal calf serum (FCS), 2 mM glutamine, 50 U/ml penicillin, and 50 µg/ml streptomycin and maintained in medium containing 0.5 mg/ml G-418.
Cell association studies of 125I-labeled lipoprotein particles (LDL or HOCl-LDL) were performed at 37°C in the absence (total cell association) or presence of 1 mg of protein/ml (nonspecific cell association) of unlabeled autologous LDL or HOCl-LDL particles or the indicated concentrations of sRAGE and/or ox-PAPC in DMEM (without FCS). After this incubation, the medium was aspirated and the cells were rinsed twice with TBS [Tris-buffered saline containing 5% (w/v) bovine serum albumin], followed by two washes with TBS only. Cells were lysed with 0.3 N NaOH. The radioactivity and the protein content of the cell lysate were measured in the same aliquot. Specific cell association was calculated as the difference between total and nonspecific cell association (27).
Degradation of 125I-labeled lipoprotein particles by RAW cells was estimated by measuring nontrichloroacetic acid-precipitable radioactivity in the medium as described (27). Specific degradation was calculated as the difference between total and nonspecific degradation (measured in the presence of 1 mg/ml unlabeled autologous lipoproteins).
To follow lipid accumulation and lipid uptake in RAW macrophages, cells were plated onto 24-well plates, grown for 2 days in DMEM [10% (v/v) FCS], then incubated in the absence (controls) or presence of 50 µg/ml HOCl-LDL (oxidant:protein molar ratio of 200:1, 400:1, and 800:1) and/or 20 µg/ml sRAGE up to 72 h. To measure accumulated neutral lipids, 2.5 µg Nile red in acetone was added to macrophage cultures for 5 min and processed for fluorescence microscopy. Each condition was measured in triplicate.
RESULTS
HOCl-LDL/sRAGE interaction
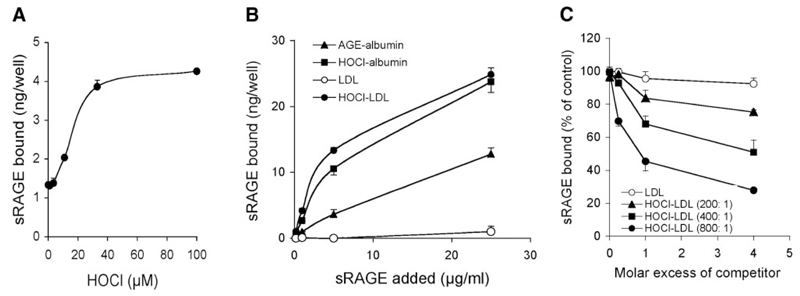
RAGE is composed of a short cytosolic tail (that is essential for RAGE signaling), a transmembrane domain, and a positively charged extracellular domain (1). As modification by HOCl alters the net charge of the protein, we investigated whether an increasing oxidant:lipoprotein molar ratio (paralleled by a decrease in positively-charged lysine residues) (28) affects the binding capacity of HOCl-LDL to sRAGE. A microplate-based cell-free assay was used for these studies (13); LDL was bound to the plates and directly modified by increasing HOCl concentrations. Figure 1A shows that binding of 125I-sRAGE to LDL increased in an oxidant:lipoprotein-dependent manner. Only weak interaction of 125I-sRAGE with native LDL could be observed.
Figure 1. Interaction of lipoproteins with sRAGE.
A) LDL was immobilized onto wells of plastic dishes and modified by indicated HOCl concentrations. After washing and blocking of excess binding sites with native albumin, 125I-sRAGE (3 µg/ml) was added to coated wells. Subsequently wells were washed and radioactivity was counted. B) LDL and freshly prepared HOCl-LDL (oxidant:protein molar ratio of 800:1), AGE-albumin (AGE-Alb), and HOCl-Alb (oxidant:protein molar ratio of 100:1) were immobilized onto wells of plastic dishes. After washing and blocking of excess binding sites with native albumin, 125I-sRAGE was added to coated wells. Subsequently wells were washed and radioactivity was counted. C) Dose response curves for 125I-sRAGE (3 µg/ml) binding inhibition to AGE-albumin-coated microtiter wells by LDL and HOCl-LDL (at the indicated oxidant:protein molar ratio). Results represent mean ± sd (n=3) of 1 experiment of 3.
To further determine 125I-sRAGE binding properties toward HOCl-LDL, microtiter wells were coated with native or HOCl-LDL. As controls, AGE-albumin and HOCl-albumin, two specific ligands for sRAGE/RAGE (13, 29), were used. Subsequently, increasing concentrations of 125I-sRAGE were added. As shown in Fig. 1B, the binding affinity of 125I-sRAGE to HOCl-LDL or HOCl-albumin was significantly higher than observed with AGE-albumin. The corresponding KD values are listed in Table 1.
TABLE 1.
Binding constants for HOCl-LDL:sRAGE interaction
| Kd (µg/ml) | |
|---|---|
| AGE-Alb | 40.1 ± 4.3 |
| HOCl-Alb (100:1) | 11.5 ± 1.0 |
| LDL | n.c. |
| HOCl-LDL (800:1) | 6.7 ± 0.8 |
| IC50 (µg/ml) | |
| LDL | n.c. |
| HOCl-LDL (200:1) | 700 ± 40 |
| HOCl-LDL (400:1) | 125 ± 14 |
| HOCl-LDL (800:1) | 25.6 ± 4.3 |
Kd values for 125I-sRAGE binding to microtiter wells coated with the indicated LDL and albumin preparations are listed. IC50 values for 125I-sRAGE binding inhibition to AGE-albumin-coated microtiter wells by increasing HOCl-LDL concentrations (at indicated oxidant: protein molar ratio) are given. Calculations were performed by nonlinear regression analysis (Graph Pad Prism). n.c. = noncalculable. Results represent means ± sd (n=3) of 1 experiment of 4.
Next we tested whether HOCl-LDL is able to compete for 125I-sRAGE binding to AGE-albumin. This was determined on the basis of the ability of LDL preparations to inhibit 125I-sRAGE binding to AGE-albumin-coated microtiter wells. Dose-response curves show that HOCl-LDL preparations effectively blocked 125I-sRAGE binding to AGE-albumin; native LDL showed no effect (Fig. 1C). The ability of the HOCl-LDL preparations to inhibit 125I-sRAGE binding to AGE-albumin was strongly dependent on the oxidant:lipoprotein molar ratio. The calculated IC50 values are listed in Table 1. From these experiments, we conclude that HOCl-LDL is a preferential ligand for sRAGE.
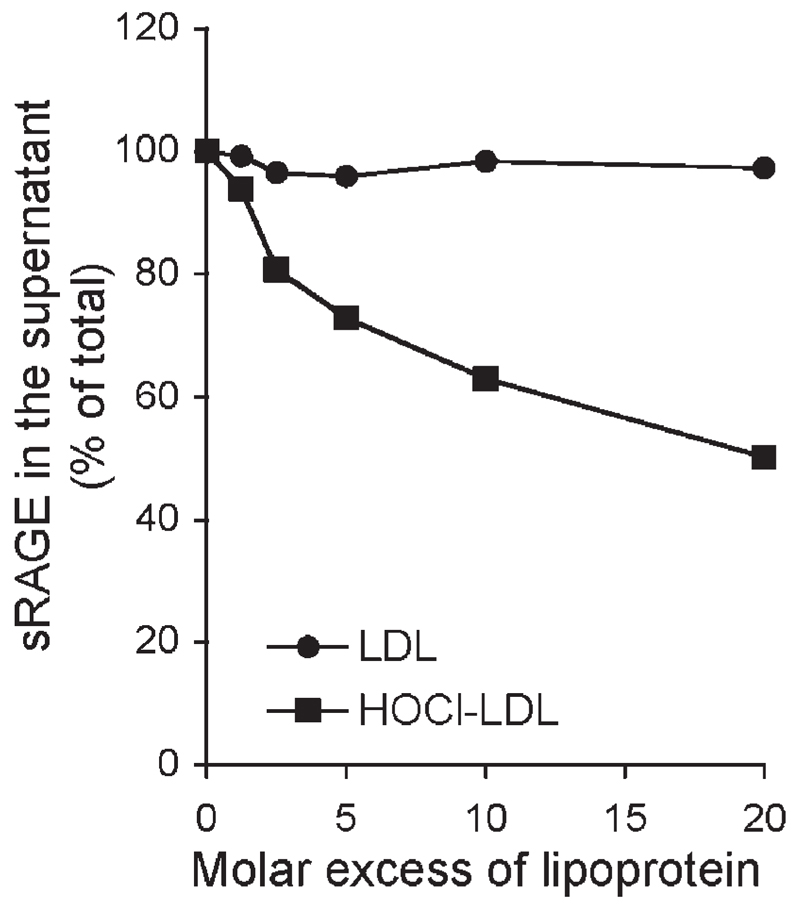
To examine whether sRAGE also associates with HOCl-LDL in biological specimens, serum from normolipidemic healthy subjects was spiked with 125I-sRAGE and lipoprotein preparations (of up to 600 µg/ml); then, the lipoprotein fraction was precipitated and the radioactivity was counted in the pellet and in the supernatant. Figure 2 shows that HOCl-LDL efficiently pulled down 125I-sRAGE in serum while native LDL failed to precipitate sRAGE from the biological specimen. Similar results were obtained when polyethylenglycol was used to precipitate the corresponding lipoprotein species (data not shown).
Figure 2. Coprecipitation of lipoproteins with sRAGE.
125I-labeled sRAGE (3 µg/ml) was incubated with LDL or HOCl-LDL in serum at the indicated molar excess and treated with phosphotungstate to precipitate LDL or HOCl-LDL (oxidant: protein molar ratio of 800:1). Radioactivity was then measured in the supernatant and the pellet. Results are presented as percent of sRAGE remaining in the supernatant. Results represent mean ± sd (n=3) of 1 experiment of 4.
Uptake studies of HOCl-LDL by macrophages in the presence of sRAGE
Modification of LDL by HOCl leads to uncontrolled accumulation of the modified LDL particles within monocytes/macrophages, and hence loading with excess of lipids, including cholesterol and cholesteryl esters (20, 21). Given the high-affinity binding of sRAGE to HOCl-LDL (Fig. 1), we were interested in whether sRAGE could interfere with the uptake of HOCl-LDL by macrophages. Therefore the protein moiety of LDL and HOCl-LDL was labeled with Na125I, and cell association studies were performed in the absence or presence of sRAGE. As shown in Fig. 3A, the presence of 20 µg/ml sRAGE markedly decreased 125I-HOCl-LDL cell association to macrophages but had no effect on cell association of 125I-LDL. In Fig. 3B, we further show that sRAGE dose-dependently inhibited the specific 125I-HOCl-LDL cell association. Of note, a 3-fold molar excess of sRAGE over HOCl-LDL had already blocked ~50% of the specific cell association of 125I-HOCl-LDL (Fig. 3B).
Figure 3. Inhibition of HOCl-LDL cell association with macrophages by sRAGE.
A) RAW macrophages were incubated with 1 µg/ml of 125I-labeled LDL and HOCl-LDL (at the indicated oxidant:protein molar ratio) at 37°C for 4 h in the absence or presence of 20 µg/ml sRAGE, and cell association was measured. B) RAW macrophages were incubated with 20 µg/ml 125I-labeled HOCl-LDL (oxidant:protein molar ratio of 400:1) at 37°C for 2 h in the presence of the indicated concentrations (molar excess) of sRAGE and specific cell association was measured. C) RAW macrophages were incubated with 1 µg/ml 125I-labeled HOCl-LDL (oxidant:protein molar ratio of 400:1) at 37°C for 4 h in the absence or presence of 20 µg/ml sRAGE, and cell degradation was measured. Nonspecific cell association (B) and degradation (C, measured in the presence of 1 mg/ml unlabeled HOCl-LDL; oxidant:protein molar ratio of 400:1) were subtracted to obtain specific cell association or degradation. Values are averages of triplicate determinations from 1 representative experiment of 3; error bars represent ± sd. D) RAW macrophages were incubated in the absence (a, controls) or presence of HOCl-LDL (oxidant:protein molar ratio of 400:1) and/or sRAGE for 72 h; lipid staining was performed with Nile red. b) 20 µg/ml sRAGE; c) 50 µg/ml HOCl-LDL; d) 50 µg/ml HOCl-LDL and 20 µg/ml sRAGE.
In a parallel series of experiments, the degradation of 125I-HOCl-LDL by macrophages was estimated by the nontrichloroacetic acid-precipitable radioactivity in the medium. From Fig. 3C it is evident that degradation of 125I-HOCl-LDL was inhibited by sRAGE up to 70%. Staining of lipid droplets in macrophages with Nile red confirmed that foam cell formation by HOCl-LDL in vitro is remarkably impaired when cells were coincubated with sRAGE (Fig. 3D).
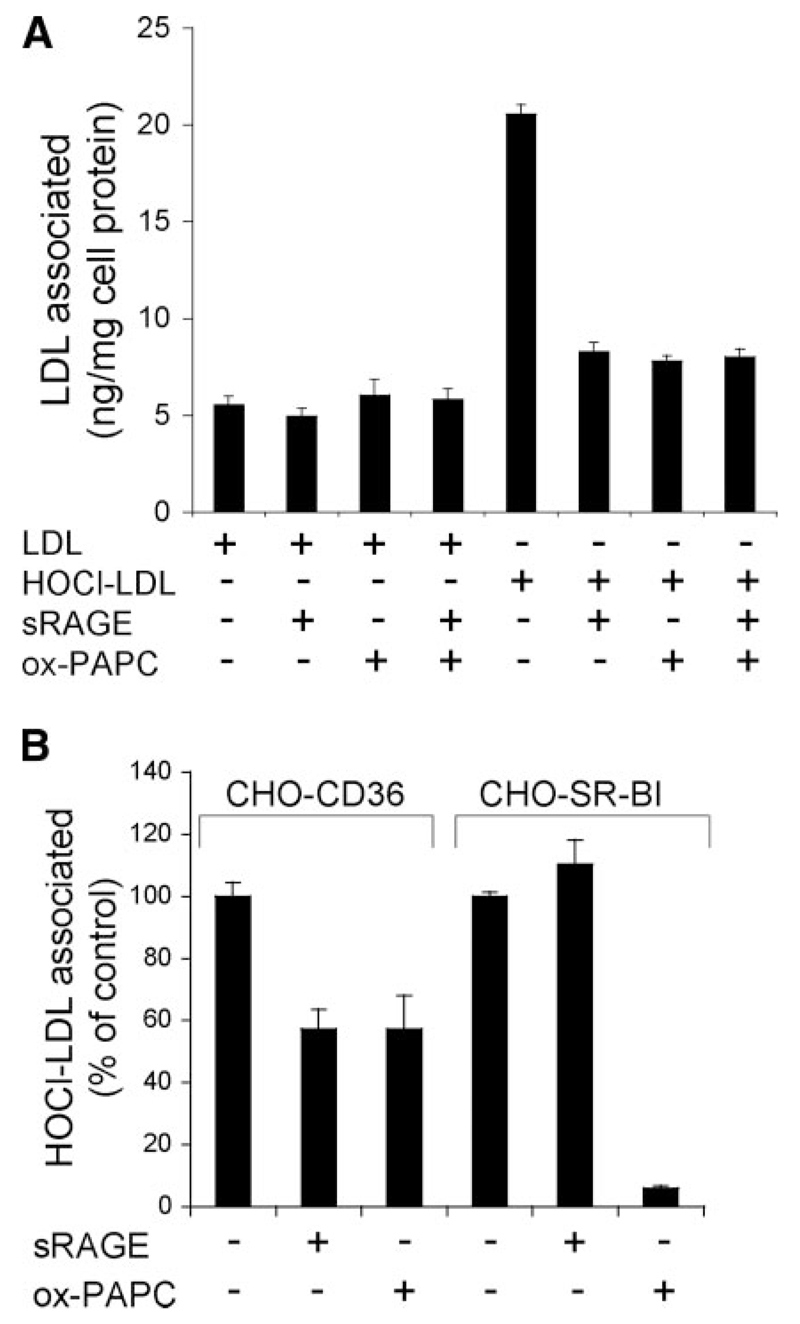
sRAGE interferes with scavenger receptor-mediated uptake of HOCl-LDL
Class A and class B scavenger receptors are candidate receptors on macrophages mediating uptake of modified LDL (30). Our previous experiments (i.e., competition studies of bound HOCl-LDL with acetylated-LDL, a ligand to class A scavenger receptor, or copper-oxidized LDL, a ligand for class B scavenger receptor) revealed that class B scavenger receptors are likely to mediate binding and uptake of LDL modified with physiologically relevant HOCl concentrations (21). In line with our previous findings (21), we now show that cell association of HOCl-LDL to macrophages (RAW cells) is effectively blocked by ox-PAPC (Fig. 4A), a potent inhibitor of scavenger receptor class B (31). The specificity of ox-PAPC for class B scavenger receptors (CD36 and SR-BI) is shown in Supplemental Fig. 1. sRAGE showed the same inhibitory activity as ox-PAPC and coincubation of sRAGE, and ox-PAPC did not further decrease HOCl-LDL cell association. Thus, sRAGE interferes with SR-B-mediated uptake of HOCl-LDL.
Figure 4. sRAGE interferes with scavenger receptor-mediated uptake of lipoproteins.
A) RAW macrophages were incubated with 1 µg/ml of 125I-labeled LDL and HOCl-LDL (oxidant: protein molar ratio of 400:1) at 37°C for 2 h in the absence or presence of sRAGE (20 µg/ml) and/or ox-PAPC (50 µg/ml), and cell association was measured. B) CHO cells overexpressing CD36 (CHO-CD36) or SR-BI (CHO-SR-BI) were incubated with 125I-labeled HOCl-LDL (1 µg/ml; oxidant:protein molar ratio of 400:1) at 37°C for 2 h in the absence or presence of sRAGE (20 µg/ml) or ox-PAPC (50 µg/ml) and cell association was measured. Values are averages of triplicate determinations from 1 representative experiment of 2; error bars represent ± sd.
To confirm the results obtained with macrophages (Fig. 4A), experiments were performed with CHO cells overexpressing CD36, the major class B scavenger receptor on macrophages mediating uptake of modified/oxidized LDL particles. In line with findings obtained with macrophages, sRAGE inhibited 125I-HOCl-LDL cell association to CHO-CD36 cells to the same extent as ox-PAPC (Fig. 4B).
We were able to show earlier that HOCl-LDL also acts as a high-affinity ligand for SR-BI (21), another class B scavenger receptor (32) that is also expressed by monocytes/macrophages (33). To investigate whether sRAGE also interferes with SR-BI-HOCl-LDL binding, experiments were performed with SR-BI-overexpressing CHO cells. The presence of sRAGE showed no effect on the association of 125I-HOCl-LDL to SR-BI, whereas ox-PAPC effectively blocked 125I-HOCl-LDL association (Fig. 4B). Thus, sRAGE only interferes with CD36-mediated uptake of HOCl-LDL.
DISCUSSION
The idea that sRAGE may act as a decoy has been supported by numerous studies demonstrating that administration of sRAGE prevents the development of micro- and macrovascular complications in animal models of vascular disorders (6, 8, 10–12). These data are consistent with the concept that sRAGE neutralizes inflammatory ligands and thus represents a suitable therapeutic approach for a variety of “RAGE-mediated” disorders. The hypothesis that sRAGE may act as a decoy for AGEs and other RAGE ligands has been strengthened by low endogenous plasma sRAGE concentrations in diabetes and renal disease (34). We now show that physiologically relevant HOCl concentrations convert LDL into a ligand for sRAGE with an affinity even higher than that observed for AGE-albumin (a preferential (s)RAGE ligand; see ref. 29). This indicates that sRAGE could act as a decoy for MPO-modified/oxidized (lipo)proteins generated under inflammatory conditions such as atherosclerosis (7, 18, 35), glomer-ulosclerosis (36), and ischemia-reperfusion injury (37).
Engagement of RAGE activates key signal transduction pathways that modulate fundamental cellular properties, thereby leading to vascular and inflammatory cell perturbation. We previously reported that HOCl-albumin, an advanced protein oxidation product lacking AGE structures (38, 39), colocalizes with RAGE in the artery wall and promotes MCP-1 expression via the RAGE-ERK1/2 MAP kinase pathway (13). As the antibody used during this study (13) recognizes both full-length RAGE and sRAGE, part of the observed colocalization might be due to sRAGE bound to HOCl-modified epitopes/(lipo)proteins. HOCl-modified (lipo)-proteins are mostly found to be associated with monocytes and macrophages (35), and HOCl-modified LDL is also present in human atherosclerotic lesions (18). Analysis of LDL extracted from human atherosclerotic lesions revealed an oxidant:LDL molar ratio of at least 100:1 (calculated on the basis of the extent of 3-chlo-rotyrosine formation in apoB-100; ref. 40). However, LDL extracted from lesion material represents a mixture of unmodified and modified lipoprotein species; therefore, an oxidant:LDL molar ratio of >100:1 is likely to occur in vivo. This assumption is supported by previous findings (18) that a monoclonal antibody raised against HOCl-LDL (oxidant:LDL molar ratio of 800:1) strongly cross-reacted with apoB-100 extracted from human lesions. In vitro studies have shown that HOCl-LDL displays a number of pathophysiological effects on phagocytes and vascular cells, contributing to initiation and maintenance of the inflammatory process during atherosclerotic lesion development (22). LDL modified with physiologically relevant HOCl concentrations is taken up by macrophages via class B but not class A scavenger receptors (21); most importantly, the protein but not the lipid moiety of HOCl-LDL is recognized by the corresponding class B scavenger receptor (21). CD36, which is involved in lipid metabolism, inflammation, and atherosclerosis (41), has been regarded as a major clearance receptor for (lipo)proteins, modified by the MPO-H2O2-nitrite system (42, 43), and thus promotes lipid accumulation and macrophage foam cell formation in vitro and in vivo (44–46). Our previous findings (21) and present data (Fig. 4B) show that LDL, when modified by the MPO product HOCl, is also a specific CD36 ligand. A main and novel finding of the present study is that sRAGE interferes with receptor-mediated uptake of the modified LDL particle probably by masking the binding motif of HOCl-LDL. Therefore, it is conceivable that sRAGE interferes directly with foam cell formation in the artery wall. This hypothesis is supported by our own observations (Fig. 3D) and by studies performed in animal models demonstrating that sRAGE administration stabilizes established atherosclerosis and suppresses accelerated diabetic lesion formation (12). The concentrations of sRAGE administration during these in vivo studies (100 µg sRAGE/mouse/day) are comparable to the highest concentrations used in our in vitro experiments. We further show that a 3-fold molar excess of sRAGE over HOCl-LDL impairs CD36-mediated cell association by ~50% (Fig. 3B). This indicates that sRAGE has potent atheroprotective properties besides acting as a decoy for AGE ligands. To date, no data are available on the concentration of endogenous sRAGE in the subendothelial space; sRAGE may be expressed either as a splice variant of full-length RAGE (47) or generated by extracellular cleavage of full-length RAGE by metalloproteinases (34). Full-length RAGE is highly expressed in diabetic and nondiabetic lesions, and a similar expression pattern of sRAGE seems plausible. Therefore, the endogenous sRAGE concentration in lesions could be sufficient to interfere locally with the uptake of HOCl-LDL by macrophages.
sRAGE does not interfere with SR-BI-mediated binding and uptake of HOCl-LDL. This may be explained by the fact that the binding affinity of HOCl-LDL to SR-BI is ~8-to 10-fold higher than CD36 (21); therefore, it is conceivable that sRAGE is not able to compete with SR-BI for HOCl-LDL binding, at least at the concentrations of sRAGE tested. Another possibility is that SR-BI and sRAGE recognize different protein epitopes on HOCl-LDL. However, the low levels of SR-BI expression on macrophages compared with CD36 expression suggest a quantitatively less important role for SR-BI in mediating the clearance of modified particles.
Plasma levels of sRAGE decrease in coronary artery disease and diabetic patients (34), and are negatively correlated with the development of complications. This suggests that sRAGE apparently is trapped in the circulation through interaction with or “lost” through removal of (s)RAGE ligands. Summarizing, we show that HOCl-LDL, which is present in human atherosclerotic lesions (18), is a high-affinity ligand to sRAGE. Most important, sRAGE blocks CD36 scavenger receptor-mediated uptake of HOCl-LDL and reduces foam cell formation, suggesting that this axis could be a possible therapeutic approach.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. M. Krieger (MIT, Cambridge, MA, USA) for providing scavenger receptor-overexpressing CHO cells (ldlA[mSR-BI]) and to Dr. M. Vadon (MUG, Graz, Austria) for providing human plasma. The expert technical assistance of M. Sundl and S. Zirkl is appreciated. This work was supported by grants from the Austrian Science Fund (FWF, P17013-B05, P19074-B05, F3007-B05) to E.M and W.S.
References
- 1.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 3.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 4.Ramasamy R, Yan SF, Schmidt AM. The RAGE axis and endothelial dysfunction: maladaptive roles in the diabetic vasculature and beyond. Trends Cardiovasc Med. 2005;15:237–243. doi: 10.1016/j.tcm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–268. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong L, Goova MT, Moser B, Kislinger T, Lee DC, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 7.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 8.Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM. RAGE axis: animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1342–1349. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- 9.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kislinger T, Tanji N, Wendt T, Qu W, Lu Y, Ferran LJ, Jr, Taguchi A, Olson K, Bucciarelli L, Goova M, et al. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001;21:905–910. doi: 10.1161/01.atv.21.6.905. [DOI] [PubMed] [Google Scholar]
- 12.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 13.Marsche G, Semlitsch M, Hammer A, Frank S, Weigle B, Demling N, Schmidt K, Windischhofer W, Waeg G, Sattler W, Malle E. Hypochlorite-modified albumin colocalizes with RAGE in the artery wall and promotes MCP-1 expression via the RAGE-Erk1/2 MAP-kinase pathway. FASEB J. 2007;21:1145–1152. doi: 10.1096/fj.06-7439com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuccurullo C, Iezzi A, Fazia ML, De Cesare D, Di Francesco A, Muraro R, Bei R, Ucchino S, Spigonardo F, Chiarelli F, et al. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26:2716–2723. doi: 10.1161/01.ATV.0000249630.02085.12. [DOI] [PubMed] [Google Scholar]
- 15.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 16.Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci U S A. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr AC, Myzak MC, Stocker R, McCall MR, Frei B. Myeloperoxidase binds to low-density lipoprotein: potential implications for atherosclerosis. FEBS Lett. 2000;487:176–180. doi: 10.1016/s0014-5793(00)02227-4. [DOI] [PubMed] [Google Scholar]
- 18.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazell LJ, Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J. 1993;290:165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsche G, Zimmermann R, Horiuchi S, Tandon NN, Sattler W, Malle E. Class B scavenger receptors CD36 and SR-BI are receptors for hypochlorite-modified low density lipoprotein. J Biol Chem. 2003;278:47562–47570. doi: 10.1074/jbc.M308428200. [DOI] [PubMed] [Google Scholar]
- 22.Malle E, Marsche G, Arnhold J, Davies MR. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim Biophys Acta. 2006;1761:392–415. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- 24.Takata K, Horiuchi S, Araki N, Shiga M, Saitoh M, Morino Y. Endocytic uptake of nonenzymatically glycosylated proteins is mediated by a scavenger receptor for aldehyde-modified proteins. J Biol Chem. 1988;263:14819–14825. [PubMed] [Google Scholar]
- 25.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh HL, Schafer BW, Weigle B, Heizmann CW. S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun. 2004;316:949–959. doi: 10.1016/j.bbrc.2004.02.135. [DOI] [PubMed] [Google Scholar]
- 27.Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol. 2000;20:763–772. doi: 10.1161/01.atv.20.3.763. [DOI] [PubMed] [Google Scholar]
- 28.Opper C, Schuüssler G, Sattler W, Malle E. Effects of hypochlorite-modified low density lipoproteins and high density lipoproteins on platelet function. Platelets. 1998;9:339–341. doi: 10.1080/09537109876582. [DOI] [PubMed] [Google Scholar]
- 29.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 30.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 31.Hartwich J, Dembinska-Kiec A, Gruca A, Motyka M, Partyka L, Skrzeczynska J, Bzowska M, Pryjma J, Huber J, Leitinger N, Schmitz G. Regulation of platelet adhesion by oxidized lipoproteins and oxidized phospholipids. Platelets. 2002;13:141–151. doi: 10.1080/09533710022149368. [DOI] [PubMed] [Google Scholar]
- 32.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, Okamoto Y, Matsuyama A, Matsumoto K, Miyagawa J, Matsuzawa Y. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ Res. 1999;85:108–116. doi: 10.1161/01.res.85.1.108. [DOI] [PubMed] [Google Scholar]
- 34.Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr Med Chem. 2006;13:1971–1978. doi: 10.2174/092986706777585013. [DOI] [PubMed] [Google Scholar]
- 35.Malle E, Waeg G, Schreiber R, Groöne E, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267:4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 36.Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int. 2003;64:1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol. 2005;289:G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- 38.Capeillere-Blandin C, Gausson V, Descamps-Latscha B, Witko-Sarsat V. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim Biophys Acta. 2004;1689:91–102. doi: 10.1016/j.bbadis.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR, Liu ZQ, Zhou ZM, Zhou M, Xie D, Wang GB, Zhang X. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:1156–1162. doi: 10.1161/01.ATV.0000214960.85469.68. [DOI] [PubMed] [Google Scholar]
- 40.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 44.Han J, Hajjar DP, Tauras JM, Nicholson AC. Cellular cholesterol regulates expression of the macrophage type B scavenger receptor, CD36. J Lipid Res. 1999;40:830–838. [PubMed] [Google Scholar]
- 45.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- 46.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.