Abstract
In the past, the development of more effective, safe, convenient, broadly applicable, and easy to manufacture vaccines for allergen-specific immunotherapy (AIT) has been limited by the poor quality of natural allergen extracts. Progress made in the field of molecular allergen characterization has now made it possible to produce defined vaccines for AIT and eventually for preventive allergy vaccination based on recombinant DNA technology and synthetic peptide chemistry. Here we review the characteristics of recombinant and synthetic allergy vaccines that have reached clinical evaluation and discuss how molecular vaccine approaches can make AIT more safe and effective and thus more convenient. Furthermore, we discuss how new technologies can facilitate the reproducible manufacturing of vaccines of pharmaceutical grade for inhalant, food, and venom allergens. Allergy vaccines in clinical trials based on recombinant allergens, recombinant allergen derivatives, and synthetic peptides allow us to target selectively different immune mechanisms, and certain of those show features that might make them applicable not only for therapeutic but also for prophylactic vaccination.
Keywords: Allergy, allergen, allergen-specific immunotherapy, allergy vaccine, preventive allergy vaccine
Allergen-specific immunotherapy (AIT) was reported first by Leonard Noon in 1911.1 Noon injected grass pollen extract into allergic patients and, despite the occurrence of side effects, observed clinical improvement for almost 1 year in the treated patients. In his article Noon quotes earlier work by William Dunbar,2 who had shown that anti-sera raised against pollen allergen extract could neutralize allergen-induced conjunctival inflammation. The early work of Dunbar had already indicated that a major effect of AIT was caused by induction of allergen-specific blocking antibodies. In 1935, more than 40 years before the identification of IgE antibodies, Cooke et al3 reported a series of elegant experiments showing that allergen-specific IgG antibodies induced by AIT can suppress allergen-induced skin inflammation. Since then, the importance of allergen-specific IgG antibodies that compete with IgE for binding to the allergens has been demonstrated by numerous studies as a major mechanism of the mode of action of AIT,4 and therefore one might consider AIT and in particular the traditional form of subcutaneous AIT as a therapeutic vaccine.5
There are several important features that suggest that AIT has many advantages over symptomatic treatment with anti-inflammatory drugs and biologics when applied as recommended according to guidelines.6 First of all, AIT functions in an allergen-specific and thus causative manner as a therapeutic vaccine. It uses the immune system of the patient to establish a counterimmune response, antagonizing the allergic immune response by vaccination with the disease-causing allergens or derivatives thereof. Therefore, as with other vaccines, allergy vaccines can be relatively easily produced, and the costs of AIT are low, in particular when compared with those of treatment with biologic agents, such as anti-cytokine antibodies.7 Unlike anti-inflammatory treatment, AIT can stop the progression of mild forms (ie, rhinitis) of allergy toward severe forms (ie, asthma) and thus modifies the natural course of disease.8,9 Furthermore, AIT has long-lasting effects, even after discontinuation of treatment, which cannot be achieved with symptomatic treatment.10 The achievement of long-term “clinical tolerance” can be achieved through induction of long-lived B cells or plasma cells secreting high-affinity antibodies11 and/or through a reduction in boosts of allergen-specific IgE, which occurs after natural allergen contact.12-14
Diagnosis of the disease-causing allergens and monitoring of treatment have been greatly facilitated through the availability of molecular allergy diagnosis, also termed component-resolved allergy diagnosis.15-18 For example, microarrayed allergen molecules allow detection of IgE reactivities and treatment-induced IgG antibody responses toward a comprehensive set of allergens.16 Thus tools are available for more precise prescription of allergy vaccines and for controlling the effects of the vaccine.16,19
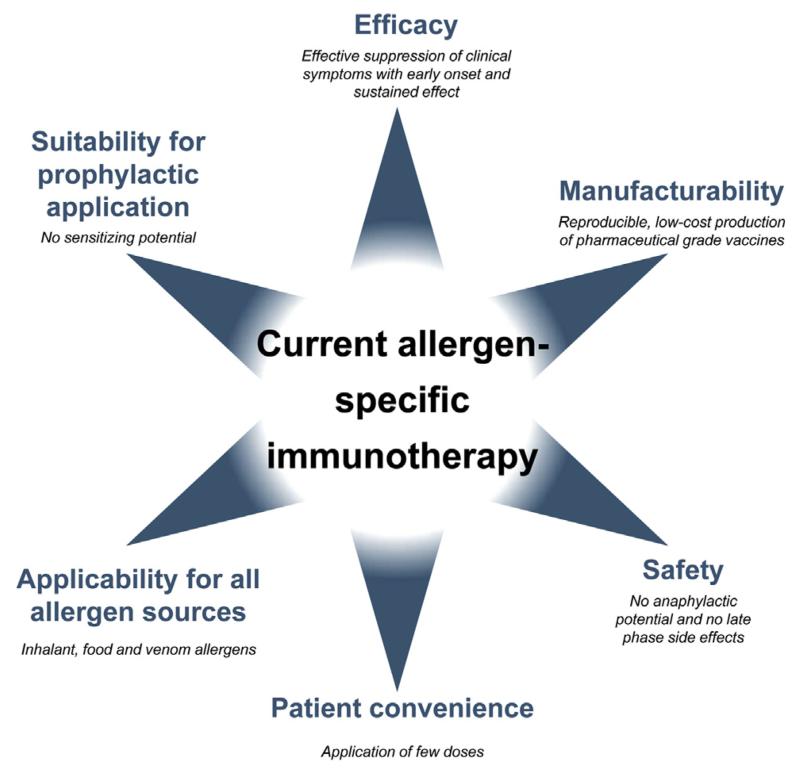
However, there are several important bottlenecks (Fig 1) that limit the broad applicability of AIT for allergy treatment. In this review we will discuss how these bottlenecks have been addressed in the past with traditional technologies and how modern technologies of molecular treatment might lead to a breakthrough of AIT not only for global allergy treatment but also ultimately for allergy prevention.
FIG 1.
Requirements for improved allergy vaccines.
AREAS OF AIT THAT NEED IMPROVEMENT
Fig 1 provides an overview of areas in which improvement of AIT is needed. Some of these areas (safety, efficacy, and convenience) are closely connected to each other. A major problem in AIT is that administration of allergens can induce side effects in patients, which, in the worst-case scenario, can lead to anaphylactic shock and death.20 Side effects can be classified as immediate side effects, which are caused by allergen-induced cross-linking of mast cell– and basophil-bound IgE antibodies. These side effects occur within 30 minutes after administration of the vaccine and, when induced systemically, can give rise to life-threatening anaphylactic shock. Systemic activation of mast cells and basophils occurs mainly when relevant doses of IgE-reactive allergens are distributed systemically in the body.
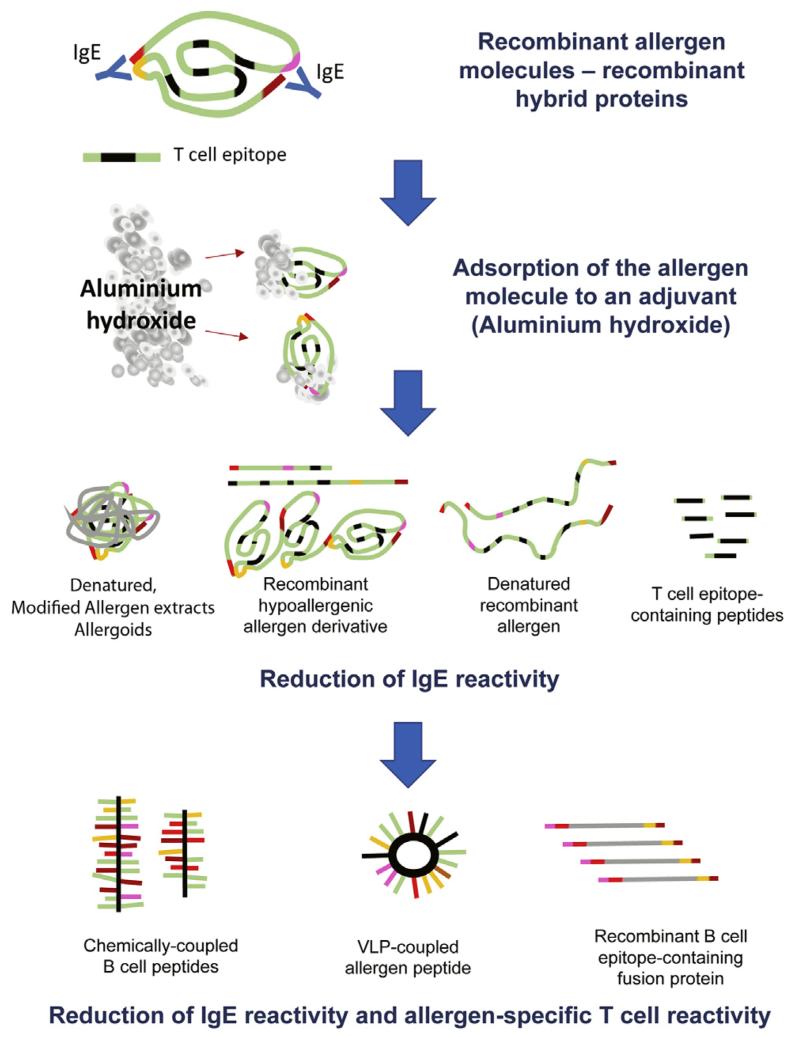
A reduction in the risk of immediate systemic side effects can be achieved by keeping allergens locally bound at the application site, such as through the use of certain adjuvants, such as aluminum hydroxide, which has been introduced already in 1935 and led to a profound reduction of severe systemic side effects (Fig 2).21 Another way to reduce side effects has been reduction of the IgE reactivity of allergen extracts, which in the past has been achieved by chemical modification, such as denaturation with aldehydes.22 Such modified allergen extracts that exhibit reduced IgE reactivity are termed “allergoids” (Fig 2). Interestingly, even strong reduction of IgE reactivity cannot eliminate side effects because late-phase side effects can occur even in the absence of IgE reactivity caused by the presence of un-destroyed T-cell epitopes. In a classical study it has been shown that even non–IgE-reactive T-cell epitope–containing allergen peptides can induce systemic late-phase side effects that occur after hours and are caused by IgE-independent activation of allergen-specific T cells.23 Late-phase side effects have been also reported for AIT with allergoids made from natural allergen extracts.24,25 Because the IgE reactivity of allergoids is usually strongly reduced, these late-phase side effects can be also mediated by activation of allergen-specific T cells because allergoids retain their ability to stimulate T cells.26
FIG 2.
Steps toward improvement of allergy vaccines.
Another possibility to reduce the risk of side effects is to begin treatment with very low doses and to continuously increase the dose until a therapeutically effective maintenance dose has been reached. As a result of the need for updosing, AIT requires multiple administrations, which make the treatment inconvenient. Therefore alternative routes of administration, such as sublingual, oral, and epicutaneous application, were developed, but these treatments also require frequent administration and are inconvenient.27-30 For example, sublingual treatment requires daily administration, and therefore it is not surprising that the compliance of patients receiving AIT is low and particularly low for sublingual AIT.31
Another area of AIT in which improvement is needed is clinical efficacy. Very often it is not possible to reach and maintain the therapeutically active dose in patients because of side effects. Natural allergen extracts contain a variety of allergens with widely varying potency in different quantities. Several studies have shown that natural allergen extracts from different manufacturers contain widely varying contents of allergens and that often important allergens are lacking.32-34 It is also known that individual allergens possess varying immunogenicity (ie, ability to induce IgG responses). For example, certain grass pollen allergens induce high IgG responses (eg, Phl p 5), whereas others induce only low or no IgG responses (eg, Phl p 2 and Phl p 1).12,35,36 Accordingly, allergen extract–based forms of AIT can induce only partial protection. This brings us to the major bottleneck for the further development of AIT, which is related to the use of natural allergen extracts as a source for manufacturing the vaccines. The huge variations regarding amounts, potencies, and immunogenicity of individual allergen molecules in natural allergen extracts cannot be controlled or even manipulated by pharmaceutical production processes. Also, modern technologies, such as proteomics tools or mass spectrometry, do not allow the adequate quantification of immunogenic allergens or allergen derivatives in extracts.34 Therefore one cannot expect any further substantial improvement of AIT based on traditional allergen extract–based forms of treatment.37 Requirements for innovative forms of AIT are summarized in Fig 1 and include high safety. The vaccine should have no anaphylactic potential and induce as few systemic late-phase side effects as possible. AIT vaccines should be effective and induce suppression of clinical symptoms early after the start of the treatment and should have sustained effects, even after discontinuation of the treatment. It should be possible to achieve the therapeutic effect with few doses of application to increase patient compliance. Manufacturing of the vaccine must be reproducible and follow the pharmaceutical requirements set for vaccines and should be possible at low costs. The technology used for the production of the vaccine and the resulting vaccines should be applicable for all allergen sources, including respiratory, food, and venom allergens.
Finally and importantly, it has become clear that one of the big advantages of AIT is that it can prevent the progression of mild to severe forms of allergy. It has been shown that AIT can prevent the progression of rhinitis to asthma when given in children.8,9 Through analysis of the evolution of IgE responses in early childhood with microarrayed allergen molecules in birth cohorts, it seems to become possible to predict the transition of silent and asymptomatic IgE sensitization toward the development of allergic symptoms.38-41 Accordingly, one might consider applying AIT already to children who are sensitized but do not yet have symptoms to prevent the development of allergic symptoms or even as early intervention to prevent the development of allergic sensitization.42 In fact, we are beginning to see the first studies that make an attempt toward prophylactic AIT.43-45 However, it will be necessary to have innovative AIT technologies that selectively induce protective IgG responses and/or tolerance without inducing and/or boosting IgE responses for preventive AIT.
HOW MOLECULAR APPROACHES CAN CONTRIBUTE TO IMPROVEMENT OF AIT
In this article we focus on defined molecular approaches for improvement of specific immunotherapy, which are based on purified recombinant allergens, recombinant allergen derivatives, and allergen-derived peptides. Problems associated with the bad quality of natural allergen extracts can be overcome by the use of purified recombinant allergen molecules, which can be produced in reproducible quality according to pharmaceutical requirements. Fusion of several allergens or of hypoallergenic allergen derivatives in the form of hybrid molecules allows for combining several allergens within one molecule and increasing the immunogenicity of the individual components.35,46
In a classical study Pauli et al47 have demonstrated that rBet v 1 was equally effective as birch pollen extract for subcutaneous AIT of patients with birch pollen allergy. However, rBet v 1 retains the allergenic properties of nBet v 1, and therefore patients had to be treated with a rather inconvenient updosing schedule and monthly maintenance injections in this trial. To increase the safety of AIT based on rBet v 1, tablet formulations of rBet v 1 for sublingual treatment are currently being developed that use pharmaceutical grade recombinant allergen,48 but sublingual treatment will require multiple administrations and therefore remains an inconvenient form of treatment. It is also possible that sublingual application of rBet v 1 will induce oral allergy syndrome as a possible side effect. Finally, SLIT with rBet v 1 can strongly boost allergen-specific IgE production, as was observed for grass pollen allergen extract–containing tablets.30
Currently available data for recombinant birch pollen and recombinant grass pollen allergens thus suggest that recombinant allergens can replace natural allergen extracts.47,49,50 They offer the advantage of being pharmaceutically well-defined allergens that can be easily produced, but side effects will remain similar as for natural allergen extracts. In addition, it will not be possible to reduce the numbers of treatments, and treatment will remain as inconvenient as for allergen extracts. Furthermore, recombinant wild-type allergens boost allergen-specific IgE production and therefore might not be suitable for preventive vaccination.
One possibility to reduce side effects is the genetic engineering of recombinant hypoallergenic allergen derivatives or the chemical denaturation of recombinant allergens. For genetic engineering, many different approaches, such as production of recombinant allergen fragments, mutated allergens, and mosaic approaches, have been used.51 These modifications aim to reduce IgE reactivity and retain T-cell epitopes. The first immunotherapy trial with recombinant allergen derivatives has been carried out with recombinant fragments of rBet v 1 and a rBet v 1 trimer.13 The outcome was that this treatment showed efficacy, induced allergen-specific blocking IgG antibodies, reduced boosts of IgE production caused by seasonal allergen exposure, and eliminated IgE-mediated immediate side effects. Furthermore, because of the hypoallergenic nature of the vaccine, higher doses were tolerated by the patients. However, it was noted that hypoallergenic allergen derivatives induced systemic late-phase side effects in a considerable number of patients.52 A detailed analysis of the potential mechanism underlying the late-phase side effects of the rBet v 1 fragments was then performed by using atopy patch testing, and it seems that the late-phase side effects are IgE independent and mediated by T cells.53 A recent atopy patch test study that used hypoallergenic rBet v 1 fragments in patients with birch pollen–induced rhinoconjunctivitis revealed that late-phase, T cell–mediated side effects are quite common in birch pollen–sensitized patients.54 In agreement with this result is the finding that subcutaneous AIT with Bet v 1–derived long T-cell epitope–containing peptides (AllerT; Anergis, Epalinges, Switzerland), a treatment that is very similar to the first AIT with rBet v 1 fragments, frequently caused late-phase systemic side effects.55 In total, 31 events of late-phase dyspnea and 36 cutaneous late-phase reactions were observed when 75 injections were given.55 It was also noticed that treatment with the hypoallergenic Bet v 1 peptides induced considerable (ie, 3-fold) increases of Bet v 1–specific IgE antibodies.55 AIT with chemically denatured rBet v 1 was effective,56 but it seems that the chemical denaturation of the recombinant allergen represented a difficult production step for the development of a pharmaceutically acceptable vaccine. In fact, chemical denaturation of allergen extracts and allergens yields high-molecular-weight aggregates, which cannot be easily manufactured in a reproducible manner, and the extent of the reduction of IgE reactivity can vary.22
Recombinant hypoallergenic allergen derivatives of the major cat allergen Fel d 1 have been successfully used for intralymphatic AIT,57,58 and there are currently 2 clinical studies ongoing in which a recombinant hypoallergenic mutant of carp parvalbumin, mCyp c 1,59,60 is used for subcutaneous AIT of fish allergy.61
The currently available data from clinical studies with recombinant or synthetic hypoallergenic allergen derivatives containing allergen-specific T-cell epitopes thus indicate that these derivatives induce blocking IgG antibodies and allow injection of higher doses compared with wild-type allergens. Treatment with recombinant hypoallergens is clinically effective, and the risk of immediate allergic side effects can be reduced or eliminated.13 It also seems that hypoallergens can be produced for respiratory and food allergens and that this approach might be generally applicable. However, disadvantages of T-cell epitope–containing hypoallergens are that they can induce T cell–mediated late-phase side effects, and therefore updosing schedules are still needed. Furthermore, hypoallergens induce increases in allergen-specific IgE production, and therefore they might not be useful for preventive vaccination because they can induce allergic sensitizations or boost subclinical IgE sensitizations to become symptomatic. It is also not clear whether it is possible to produce sufficiently immunogenic and nonallergenic derivatives by using recombinant technologies or synthetic peptide chemistry for all allergen sources. For example, it was very difficult to obtain recombinant hypoallergenic allergen derivatives comprising the 4 major timothy grass pollen allergens,62 and we are aware of only one report describing an IgG-inducing hypoallergenic derivative of the major house dust mite allergen Der p 1.63
Another approach for reducing the allergenic activity of AIT is the use of synthetic T-cell epitope–containing peptides derived from major allergens with the aim to induce T-cell tolerance. Results of clinical trials performed with T-cell epitope peptides of the major cat allergen Fel d 1 indicate that the treatment might be clinically effective and that treatment effects can be long-lasting.64-66 Furthermore, this treatment requires only a few injections, and it seems that it has been possible to overcome the originally observed late-phase T cell–mediated side effects.23 However, it is presently unclear what the immunologic mechanism of this treatment is because no induction of allergen-specific blocking IgG has been observed because the peptides are too short to induce IgG responses. Another challenge is that AIT with T-cell epitopes requires a large number of peptides to address MHC diversity in patients, particularly when several important allergens need to be covered by this approach. Furthermore, not all of the peptides are easy to manufacture and might present problems regarding solubility and aggregation. Because T-cell epitope–containing peptides do not induce IgE sensitizations, they might well be suited for preventive treatment.42
B-CELL EPITOPE–BASED ALLERGY VACCINES: A NEW KID ON THE BLOCK
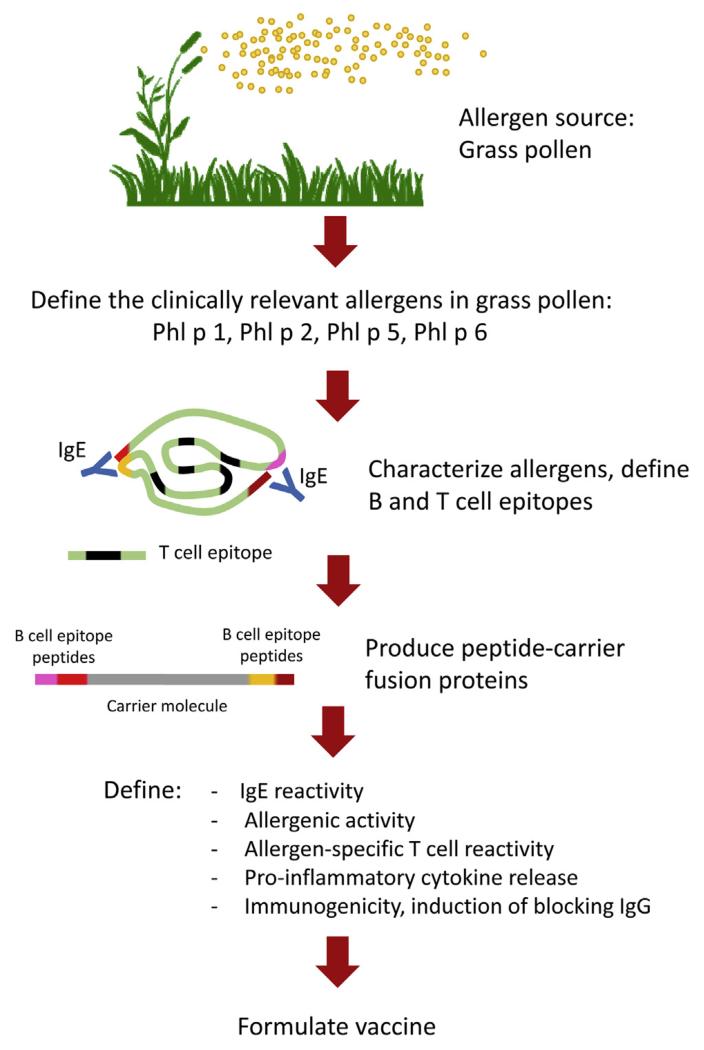
B-cell epitope–based allergy vaccines contain intrinsically non–IgE-reactive peptides of a length of approximately 20 to 40 amino acids, which are derived from the IgE-binding sites of the major allergens of a given allergen source.37,67-69 To render these peptides immunogenic so that they induce a robust IgG antibody response, which blocks IgE binding to the corresponding allergen, the peptides need to be covalently linked to a protein carrier that is not related to the allergens and provides carrier-specific T-cell help (Figs 2 and 3). The first proof-of-principle studies showing that it is possible to use carrier-bound allergen peptides to induce allergen-specific IgG antibodies were made with allergen peptides from the major timothy grass pollen allergen Phl p 1 and from the major birch pollen allergen Bet v 1, which were chemically coupled to keyhole limpet hemocyanin.68,69 It was found that these conjugates showed an even greater reduction of allergenic activity compared with recombinant hypoallergens. Because of the fact that non–IgE-reactive peptides were selected from the IgE-binding sites, it was possible to eliminate allergen-specific T-cell epitopes to a large extent. Thus B-cell epitope–based allergy vaccines were found to exhibit a strongly reduced potential to activate allergen-specific T cells and to induce proinflammatory cytokine production in PBMCs from allergic patients.70 In a skin test study performed in patients with grass pollen allergy at the height of the grass pollen season, it was found that the B-cell epitope–based grass pollen allergy vaccine BM32 did not induce relevant IgE-mediated immediate-type skin reactions, and the reduction of allergen-specific T-cell epitopes in the vaccine rendered the vaccine negative in atopy patch testing.71 These results indicated that B-cell epitope–based allergy vaccines might have a reduced capacity to induce late-phase, T cell–mediated side effects, which was confirmed subsequently in clinical AIT studies (ClinicalTrials.gov nos. NCT01445002 and NCT01538979). Thus B-cell epitope–based vaccines appear to be more safe than vaccines based on recombinant or synthetic allergy vaccines, which contain allergen-specific T-cell epitopes. Chemical coupling to keyhole limpet hemocyanin or viral particles was left68,72,73 and vaccines were produced as recombinant fusion proteins in Escherichia coli to yield defined products that are easy to manufacture in a reproducible and pharmaceutical grade. As carrier proteins, the viral proteins VP1 from human rhinovirus74 and PreS from hepatitis B75 were used, and it was found that the fusion proteins not only induced allergen-specific IgG responses on immunization but also virus-specific antibodies, which even had virus-neutralizing activities.76 Thus it is quite possible that B-cell epitope–based allergy vaccines based on viral carrier proteins might offer an antiviral effect in addition to the AIT effect.
FIG 3.
Schematic representation of the development of BM32, a recombinant B-cell epitope–based grass pollen allergy vaccine.
An important feature of the B-cell epitope–based vaccines is that the use of a strongly immunogenic carrier allows induction of allergen-specific IgG antibodies also against allergens that intrinsically are poorly immunogenic and thus would induce a poor blocking IgG response when used as wild-type allergen. Furthermore, the selective inclusion of peptides from the IgE-binding sites allows us to better focus allergen-specific IgG responses toward the IgE-binding sites of allergens, which might result in better protection.77 Because B-cell epitope–based allergy vaccines show a strongly reduced allergenic activity, it is possible to inject high doses into patients. Together with the good immunogenicity of these vaccines, this indicates that only few (ie, approximately 3) injections per year will be needed for treatment and that the vaccines would thus be extremely convenient for the patient.
The concept of using the hepatitis B–derived capsid protein PreS as a carrier for B-cell epitope–based vaccines has been found to be applicable to different allergens,70,75,77,78 and thus it appears that it will be possible to use the PreS-based technology for construction of vaccines for allergens from different sources, such as inhalant, food, and venom allergens. The analysis of serum samples from patients who have been treated with BM32, a grass pollen vaccine based on the B-cell epitope concept, has shown that the vaccine induces a highly selective allergen-specific IgG response (ie, IgG1 = IgG4 > IgG2) but does not boost allergen-specific IgE responses. Thus it is quite possible that B-cell epitope–based allergy vaccines can be used for preventive vaccination because they seem to have no IgE-sensitizing potential. Although the clinical relevance of AIT-induced IgE sensitizations has not been demonstrated, they might become a potential bottleneck for the application of AIT in a preventive setting. In fact, we found that AIT with BM32, even when adsorbed to the TH2-driving adjuvant aluminum hydroxide, did not boost allergen-specific IgE responses. Indeed, experiments performed in mice indicate that immunization with B-cell epitope–based allergy vaccines might be useful to prevent allergic sensitization.79
According to currently available in vitro, animal, and in vivo data from clinical studies, B-cell epitope–based vaccines have several modes of action. The vaccines induce allergen-specific IgG antibodies that inhibit allergen-induced cross-linking of IgE on mast cells and basophils and thus immediate allergic inflammation. Furthermore, the IgG antibodies inhibit IgE-facilitated allergen presentation and thus will likely suppress also T cell–mediated allergic inflammation. Finally, it seems that vaccine-induced IgG antibodies also reduce the boosts of IgE production induced by allergen exposure, and thus treatment might have a long-lasting effect because of a reduction in allergen-specific IgE levels.
In summary, recombinant B-cell epitope allergy vaccines are the new kid on the block and hold great promise to profoundly improve AIT and become applicable also for preventive allergy vaccination.
Acknowledgments
This study was supported by research grants F4605 and F4613 from the Austrian Science Fund (FWF) and by a research grant from BIOMAY AG, Vienna, Austria.
Abbreviation used
- AIT
Allergen-specific immunotherapy
Footnotes
Disclosure of potential conflict of interest: R. Valenta has received grants and consulting fees or honoraria from Biomay AG, ThermoFisher, and Fresenius Medical Care. V. Niederberger has received a grant from the Austrian Science Fund (FWF; SFB4613). The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–3. [Google Scholar]
- 2.Dunbar WP. Zur Frage betreffend die Ätiologie und spezifische Therapie des Heufiebers. Berliner klin. Wochenschr. 1903;24:6. [Google Scholar]
- 3.Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity coexisting sensitization in a type of human allergy (hay fever) J Exp Med. 1935;62:733–50. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 6.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Cox L, Calderón M, Pfaar O. Subcutaneous allergen immunotherapy for allergic disease: examining efficacy, safety and cost-effectiveness of current and novel formulations. Immunotherapy. 2012;4:601–16. doi: 10.2217/imt.12.36. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 9.Valovirta E. Effect of AIT in children including potential to prevent the development of asthma. Allergy. 2011;66(suppl 95):53–4. doi: 10.1111/j.1398-9995.2011.02640.x. [DOI] [PubMed] [Google Scholar]
- 10.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 11.James LK, Shamji MH, Walker SM, Wilson D, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–16. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 12.Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 13.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vacci-nation with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101(suppl 2):14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 15.Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–6. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 16.Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canonica GW, Ansotequi IL, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sastre J, Landivar ME, Ruiz-García M, Andregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67:709–11. doi: 10.1111/j.1398-9995.2012.02808.x. [DOI] [PubMed] [Google Scholar]
- 19.Wollmann E, Lupinek C, Kundi M, Selb R, Niederberger V, Valenta R. Reduction in allergen-specific IgE binding as measured by microarray: a possible surrogate marker for effects of specific-immunotherapy. J Allergy Clin Immunol. 2015;136:806–9. doi: 10.1016/j.jaci.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winther L, Arnved J, Malling HJ, Nolte H, Mosbech H. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254–60. doi: 10.1111/j.1365-2222.2006.02340.x. [DOI] [PubMed] [Google Scholar]
- 21.Sledge RF. Treatment of hay-fever with alum-precipitated pollen extract. U S Naval Bull. 1938;36:18–29. [Google Scholar]
- 22.Marsh DG, Lichtenstein LM, Campbell DH. Studies on “allergoids” prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970;18:705–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Haselden BM, Kay AB, Larché M. Immunoglobulin E-independent major histo-compatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman PS, Lichtenstein LM, Marsh DG. Studies on allergoids from naturally occurring allergens. IV. Efficacy and safety of long-term allergoid treatment of ragweed hay fever. J Allergy Clin Immunol. 1981;68:460–70. doi: 10.1016/0091-6749(81)90200-1. [DOI] [PubMed] [Google Scholar]
- 25.Bousquet J, Hejjaouim A, Soussana M, Michel FB. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. IV. Comparison of safety and efficacy of two dosages of a high-molecular-weight allergoid. J Allergy Clin Immunol. 1990;85:490–7. doi: 10.1016/0091-6749(90)90160-6. [DOI] [PubMed] [Google Scholar]
- 26.Kahlert H, Grage-Griebenow E, Stüwe HT, Cromwell O, Fiebig H. T cell reactivity with allergoids: influence of the type of APC. J Immunol. 2000;165:1807–15. doi: 10.4049/jimmunol.165.4.1807. [DOI] [PubMed] [Google Scholar]
- 27.von Moos S, Johansen P, Tay F, Graf N, Kündig TM, Senti G. Comparing safety of abrasion and tape-stripping as skin preparation in allergen-specific epicutaneous immunotherapy. J Allergy Clin Immunol. 2014;134:965–7. doi: 10.1016/j.jaci.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Spina L, Weisskopf M, von Moos S, Graf N, Kündig TM, Senti G. Comparison of microneedles and adhesive-tape stripping in skin preparation for epicutaneous allergen delivery. Int Arch Allergy Immunol. 2015;167:103–9. doi: 10.1159/000434681. [DOI] [PubMed] [Google Scholar]
- 29.Sato S, Yanagida N, Ogura K, Imai T, Utsunomiya T, Iikura K, et al. Clinical studies in oral allergen-specific immunotherapy: differences among allergens. Int Arch Allergy Immunol. 2014;164:1–9. doi: 10.1159/000361025. [DOI] [PubMed] [Google Scholar]
- 30.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immuno-therapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–9. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 31.Keil MA, Röder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Mölken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353–60. doi: 10.1016/j.jaci.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–8. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 33.Casset A, Mari A, Purohit A, Resch I, Weghofer M, Ferrara R, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–62. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casset A, Valenta R, Vrtala S. Allergen content and allergenic activity of house dust mite extracts. Int Arch Allergy Immunol. 2013;161:287–8. doi: 10.1159/000347047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linhart B, Jahn-Schmid B, Verdino P, Keller W, Ebner C, Kraft D, et al. Combination vaccines for the treatment of grass pollen allergy consisting of genetically engineered hybrid molecules with increased immunogenicity. FASEB J. 2002;16:1301–3. doi: 10.1096/fj.01-1012fje. [DOI] [PubMed] [Google Scholar]
- 36.Gadermaier E, Staikuniene J, Scheiblhofer S, Thalhamer J, Kundi M, Westritschnig K, et al. Recombinant allergen-based monitoring of antibody responses during injection grass pollen immunotherapy and after 5 years of discontinuation. Allergy. 2011;66:1174–82. doi: 10.1111/j.1398-9995.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- 37.Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–97. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 38.Bousquet J, Anto J, Sunyer J, Nieuwenhuijsen M, Vrijheid M, Keil T, et al. Pooling birth cohorts in allergy and asthma: European Union-funded initiatives—a MeDALL, CHICOS, ENRIECO, and GA2LEN joint paper. Int Arch Allergy Immunol. 2013;161:1–10. doi: 10.1159/000343018. [DOI] [PubMed] [Google Scholar]
- 39.Westman M, Lupinek C, Bousquet J, Andersson N, Pahr S, Baar A, et al. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135:1199–206. doi: 10.1016/j.jaci.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asarnoj A, Hamsten C, Wadén K, Lupinek C, Andersson N, Kull I, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a Barn/Children Allergy/Asthma Milieu Stockholm Epidemiologic (BAMSE) mechanisms for the development of allergy study. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.09.052. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Custovic A, Sonntag HJ, Buchan IE, Belgrave D, Simpson A, Prosperi MC, et al. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol. 2015;136:1645–52.e8. doi: 10.1016/j.jaci.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 42.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272:144–57. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szépfalusi Z, Bannert C, Ronceray L, Mayer E, Hassler M, Wissmann E, et al. Preventive sublingual immunotherapy in preschool children: first evidence for safety and pro-tolerogenic effects. J Allergy Clin Immunol. 2014;25:788–95. doi: 10.1111/pai.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zolkipli Z, Roberts G, Cornelius V, Clayton B, Pearson S, Michaelis L, et al. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J Allergy Clin Immunol. 2015;136:1541–7.e11. doi: 10.1016/j.jaci.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 45.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linhart B, Hartl A, Jahn-Schmid B, Verdino P, Keller W, Krauth MT, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115:1010–6. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 47.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Nony E, Bouley J, Le Mignon M, Lemoine P, Jain K, Horiot S, et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen-allergic patients. Allergy. 2015;70:795–804. doi: 10.1111/all.12622. [DOI] [PubMed] [Google Scholar]
- 49.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Klimek L, Schendzielorz P, Pinol R, Pfaar O. Specific subcutaneous immuno-therapy with recombinant grass pollen allergens: first randomized dose-ranging safety study. Clin Exp Allergy. 2012;42:936–45. doi: 10.1111/j.1365-2222.2012.03971.x. [DOI] [PubMed] [Google Scholar]
- 51.Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2012;30:4328–35. doi: 10.1016/j.vaccine.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–25. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 53.Campana R, Vrtala S, Maderegger B, Jertschin P, Stegfellner G, Swoboda I, et al. Hypoallergenic derivatives of the major birch pollen allergen Bet v 1 obtained by rational sequence reassembly. J Allergy Clin Immunol. 2010;126:1024–31. doi: 10.1016/j.jaci.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 54.Campana R, Moritz K, Marth K, Neubauer A, Huber H, Henning R, et al. Frequent occurrence of T cell-mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J Allergy Clin Immunol. 2016;137:601–9. doi: 10.1016/j.jaci.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spertini F, Perrin Y, Audran R, Pellaton C, Boudousquié C, Barbier N, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–40. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Klimek L, Bachert C, Lukat KF, Pfaar O, Meyer H, Narkus A. Allergy immuno-therapy with a hypoallergenic recombinant birch pollen allergen rBet v. 1-FV in a randomized controlled trial. Clin Transl Allergy. 2015;5:28. doi: 10.1186/s13601-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–6. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 58.Zaleska A, Eiwegger T, Soyer O, van de Veen W, Rhyner C, Soyka MB, et al. Immune regulation by intralymphatic immunotherapy with modular allergen translocation MAT vaccine. Allergy. 2014;69:1162–70. doi: 10.1111/all.12461. [DOI] [PubMed] [Google Scholar]
- 59.Swoboda I, Bugajska-Schretter A, Linhart B, Verdino P, Keller W, Schulmeister U, et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J Immunol. 2007;178:6290–6. doi: 10.4049/jimmunol.178.10.6290. [DOI] [PubMed] [Google Scholar]
- 60.Douladiris N, Linhart B, Swoboda I, Gstöttner A, Vassilopoulou E, Stolz F, et al. In vivo allergenic activity of a hypoallergenic mutant of the major fish allergen Cyp c 1 evaluated by means of skin testing. J Allergy Clin Immunol. 2015;136:493–5. doi: 10.1016/j.jaci.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuidmeer-Jongejan L, Huber H, Swoboda I, Rigby N, Versteeg SA, Jensen BM, et al. Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int Arch Allergy Immunol. 2015;166:41–51. doi: 10.1159/000371657. [DOI] [PubMed] [Google Scholar]
- 62.Linhart B, Focke-Tejkl M, Weber M, Narayanan M, Neubauer A, Mayrhofer H, et al. Molecular evolution of hypoallergenic hybrid proteins for vaccination against grass pollen allergy. J Immunol. 2015;194:4008–18. doi: 10.4049/jimmunol.1400402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen KW, Blatt K, Thomas WR, Swoboda I, Valent P, Valenta R, et al. Hypoallergenic Der p 1/Der p 2 combination vaccines for immunotherapy of house dust mite allergy. J Allergy Clin Immunol. 2012;130:435–43. doi: 10.1016/j.jaci.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Worm M, Lee HH, Kleine-Tebbe J, Hafner RP, Laidler P, Healey D, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 65.Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–9. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 66.Couroux P, Patel D, Armstrong K, Larché M, Hafner RP. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45:974–81. doi: 10.1111/cea.12488. [DOI] [PubMed] [Google Scholar]
- 67.Valenta R, Vrtala S, Focke-Tejkl M, Bugajska-Schretter, Ball T, Twardosz A, et al. Genetically engineered and synthetic allergen derivatives: candidates for vaccination against type I allergy. Biol Chem. 1999;380:815–24. doi: 10.1515/BC.1999.101. [DOI] [PubMed] [Google Scholar]
- 68.Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 69.Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, et al. Nonanaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–33. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 70.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–17. e1–11. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136:1101–3. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kündig TM, Senti G, Schnetzler G, Wolf C, Prinz Vavricka BM, Fulurija A, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in health adults. J Allergy Clin Immunol. 2006;117:1470–6. doi: 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 73.Schmitz N, Dietmeier K, Bauer M, Maudrich M, Utzinger S, Muntwiler S, et al. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206:1941–5. doi: 10.1084/jem.20090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edlmayr J, Niespodziana K, Linhart B, Focke-Teijkl M, Westritschnig K, Schiblhofer S, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 75.Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–70. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edlmayr J, Niespodziana K, Focke-Tejkl M, Linhart B, Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol. 2011;352:121–40. doi: 10.1007/82_2011_130. [DOI] [PubMed] [Google Scholar]
- 77.Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to an tolerogenic and Th1 phenotype. J Immunol. 2013;190:3068–78. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banerjee S, Weber M, Blatt K, Swoboda I, Focke-Tejkl M, Valent P, et al. Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol. 2014;192:4867–75. doi: 10.4049/jimmunol.1400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linhart B, Narayanan M, Focke-Tejkl M, Wrba F, Vrtala S, Valenta R. Prophylactic and therapeutic vaccination with carrier-bound Bet v 1 peptides lacking allergen-specific T cell epitopes reduces Bet v 1-specific T cell responses via blocking antibodies in a murine model for birch pollen allergy. Clin Exp Allergy. 2014;44:278–87. doi: 10.1111/cea.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]