Summary
Hypermethylation of CpG islands in the RASSF1 promoter is one of the most frequent events identified in human cancer [1–3]. The epigenetic-driven loss of RASSF1A protein expression is observed more often in tumors of higher grade and correlates with a decreased responsiveness to DNA-damaging therapy [4–6]. Ras association domain-containing family 1A (RASSF1A) promotes apoptosis by signaling through the MST2 and LATS1 kinases, leading to stabilization of the YAP1/p73 transcriptional complex [7]. Here we provide evidence for a new pathway linking DNA damage signaling to RASSF1A via the main sensor of double-strand breaks in cells, ataxia telangiectasia mutated (ATM). We show that, upon DNA damage, RASSF1A is phosphorylated by ATM on Ser131 and is involved in the activation of both MST2 and LATS1, leading to the stabilization of p73. Furthermore, lung and ovarian tumor cell lines that retain RASSF1A expression commonly harbor polymorphisms in the region of Ser131 [8], and our analysis shows that the S131F polymorphism conveys resistance to DNA-damaging agents. Thus, we present a novel DNA damage pathway emanating from ATM that is frequently disabled in tumors via epigenetic silencing of RASSF1 or mutation of an ATM phosphorylation site.
Results and Discussion
RASSF1A and MST2 Pathway Signaling Is Activated following DNA Damage
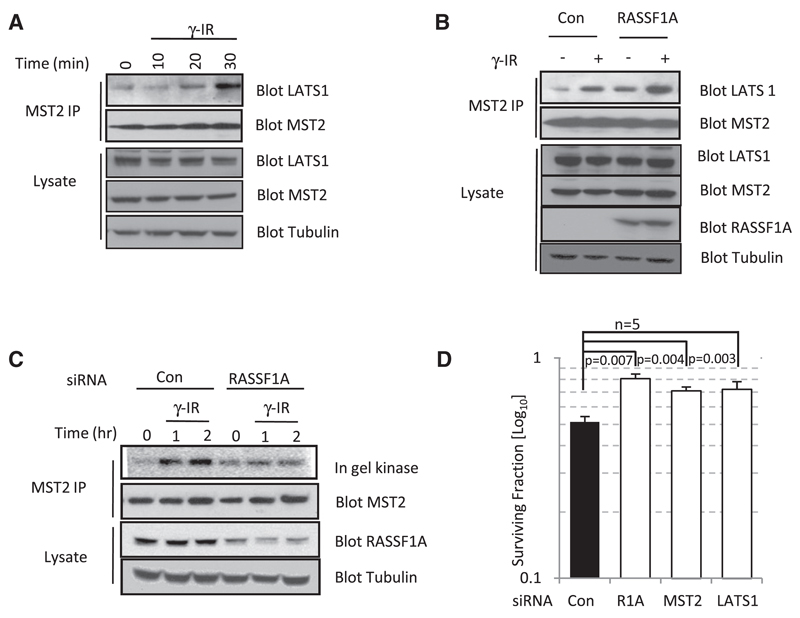
To determine whether the Ras association domain-containing family 1A (RASSF1A)/MST2 pathway is activated upon DNA damage, we first addressed whether activation of pathway signaling could be stimulated in response to double-strand breaks (DSBs) generated by exposure to ionizing radiation. In U2OS and HeLa cells (data not shown), both of which retain RASSF1A expression, MST2 associates with LATS1 within 20–30 min of exposure to ionizing radiation (Figure 1A). In MCF7 cells, where RASSF1A is undetectable because of promoter methylation, exogenous expression of FLAG-RASSF1A enhances the induced binding of MST2 and LATS1 observed following exposure to ionizing radiation (Figure 1B). RASSF1A and MST2 have previously been described to respond to receptor signaling events [7, 9, 10]; therefore, we tested whether the MST2 kinase activity was similarly induced in response to DNA damage. MST2 immunoprecipitates from HeLa cells demonstrated an increase in kinase activity in response to ionizing radiation, whereas knockdown of RASSF1A by small interfering RNA (siRNA) abrogated any response of MST2 to DNA damage (Figure 1C). Concomitant activation of LATS1 kinase was also observed and was dependent on RASSF1A and DNA damage (see Figure S1A available online). We next examined whether the clonogenic potential of irradiated tumor cells would be enhanced by removal of the individual components of this pathway via siRNA. The surviving fraction is directly proportional to the number of cells present posttransfection, therefore removing any variation due to inherent tumor-suppressor capacity unrelated to DNA damage. HeLa cells ablated of RASSF1A by siRNA and exposed to a clinically relevant dose of 2 Gy ionizing radiation that sensitizes xenografts to methylation inhibitors in vivo [6] had increased colony survival compared to controls (Figure 1D; Figure S1B). Similarly, removal of MST2 and LATS1 also demonstrated enhanced clonogenic survival in response to treatment with ionizing radiation compared to the nontargeting control (Figure 1D; Figure S1B). Therefore, loss of RASSF1A (as in RASSF1 methylated tumors) or the downstream signaling pathway reduces the ability to respond to DNA damage signals.
Figure 1. DNA Damage Activates the RASSF1A/ MST2 Signaling Pathway.
(A) Endogenous MST2 was immunoprecipitated from U2OS cells irradiated (10 Gy) and harvested at the indicated time points. Immunoprecipitates and lysates were probed with the indicated antibodies.
(B) MCF7 cells were transfected with pcDNA3 or FLAG-RASSF1A. Endogenous MST2 was immunoprecipitated 30 min after irradiation (10 Gy). Immunoprecipitates and lysates were probed with the indicated antibodies.
(C) HeLa cells transfected with small interfering RNA (siRNA) against RASSF1A or a nontargeting control were irradiated (4 Gy) and harvested at the indicated time points. Endogenous MST2 was immunoprecipitated, and MST2 activity in immunoprecipitates was determined via in gel kinase assay.
(D) HeLa cells transfected with two different siRNAs against RASSF1A, MST2, LATS1, or a nontargeting control were seeded at 400 cells per 6 cm dish. Results are representative of two independent siRNAs for each target; siRNAs ablated protein levels in HeLa cells as described previously [7]. Cells were exposed to γ-irradiation (2 Gy), and surviving colonies were counted 14 days postirradiation. Error bars represent standard error.
RASSF1A Is Phosphorylated on Ser131 in a DNA Damage-Dependent Manner
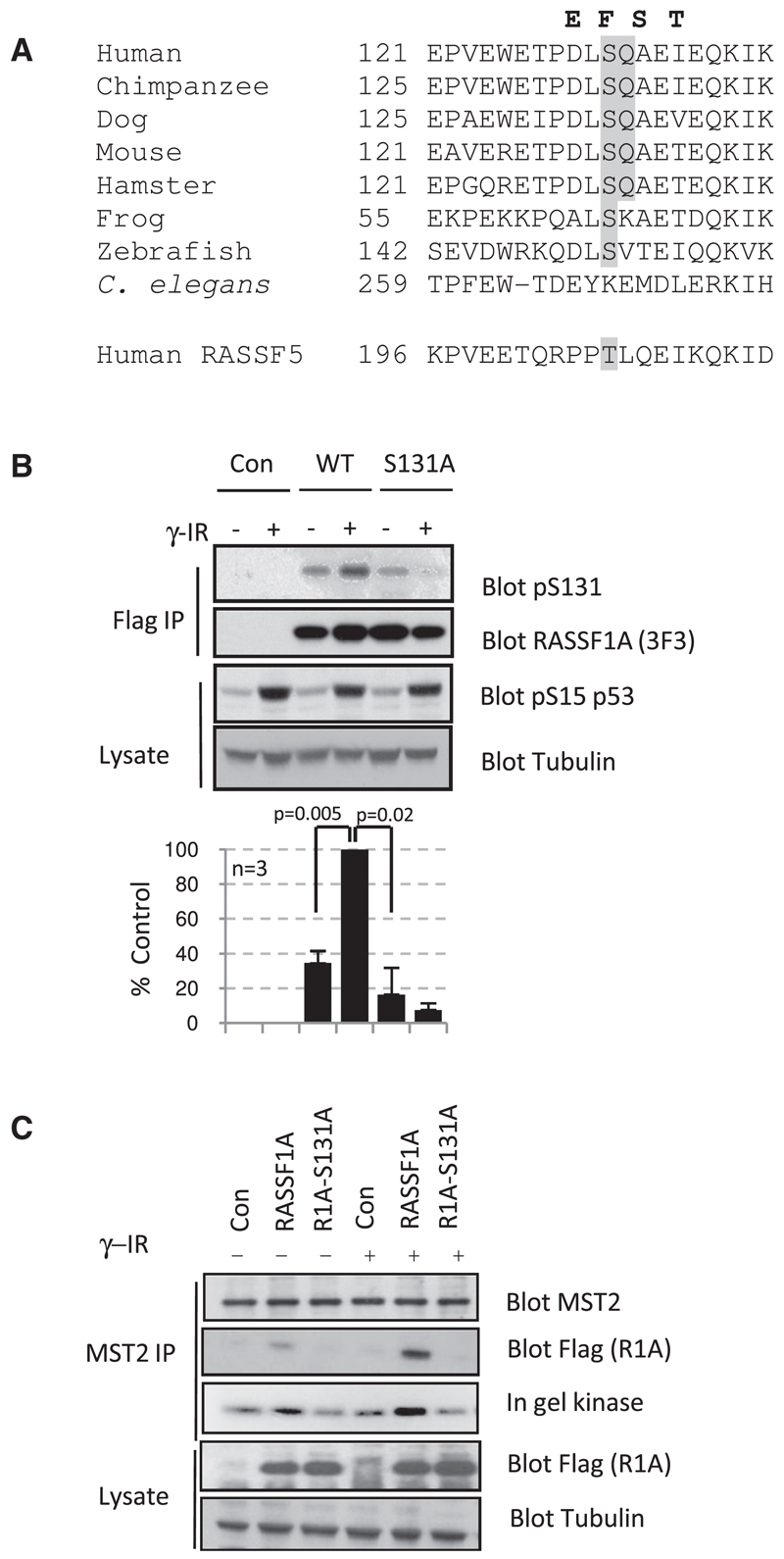
We next sought to establish the molecular mechanism as to how RASSF1A was integrating signals from DSBs to the activation of MST2. The major kinases (ataxia telangiectasia mutated [ATM], ATM- and RAD51-related [ATR], and DNA protein-kinase catalytic subunit [DNA-PKcs]) involved in transmitting signals from DSBs to cell-cycle checkpoints, activation of DNA repair, and/or apoptosis belong to the phosphoinositide 3-kinase-related kinase (PIKK) family [11–13]. ATM, ATR, and DNA-PKcs are Ser/Thr kinases that share a common SQ/TQ substrate recognition motif [14, 15]. RASSF1A possesses a single SQ motif at Ser131 that is evolutionarily conserved among RASSF1A homologs but absent from related family members whose expression is less frequently altered in tumors [1] (Figure 2A). After exposure to ionizing radiation, endogenous RASSF1A reacted to a generic phospho-SQ/TQ antibody (Figure S2A) and a specific antibody raised against a phospho-Ser131 peptide containing residues 126–137 of RASSF1A (Figure 2B). Mutation of the SQ site abolishes reactivity toward the phospho-specific antibody, and phospho-RASSF1A is no longer detected (Figure 2B). Similarly, phosphorylation was removed by pretreatment with the PIKK inhibitor caffeine or lambda protein phosphatase (Figures S2A and S2B). This is in agreement with previous observations that suggested that the SQ site on a RASSF1A peptide may be a substrate for the PIKK family [14, 15].
Figure 2. RASSF1A Is Phosphorylated at Ser131 following Ionizing Radiation.
(A) Alignment of a conserved SQ motif in RASSF1A sequences from vertebrates; the matching sequence in RASSF5 is included for comparison. Mutations that have been identified in human lung and ovarian cancer cells [8, 19] in proximity to Ser131 are highlighted in bold.
(B) MCF7 cells were transfected with pcDNA3 or plasmid expressing FLAG-RASSF1A or S131A-mutated FLAG-RASSF1A. Cells were harvested 30 min post γ-irradiation (10 Gy). FLAG-tagged proteins were immunoprecipitated and probed by western blot with the phosphospecific antibody raised against a phospho-Ser131 peptide spanning residues 125–136 of RASSF1A. Lysates and immunoprecipitates were blotted with the indicated antibodies. The data shown are representative of three independent experiments. Phospho-Ser131 bands were normalized to the loading controls, and bands were quantitated with ImageJ software. Error bars represent standard error.
(C) MCF7 cells were transfected with either pcDNA3 or plasmid expressing FLAG-RASSF1A or S131A-mutated FLAG-RASSF1A. Cells were irradiated (10 Gy) and harvested 30 min following irradiation. Endogenous MST2 was immunoprecipitated and probed by western blot for anti-MST2 and anti-FLAG. The kinase activity of MST2 in immunoprecipitates was determined by in gel kinase assay. Lysates were probed with indicated antibodies.
To verify that the phosphorylation of Ser131 is required for DNA damage-dependent activation of MST2, we tested the ability of RASSF1A-S131A to enhance MST2 kinase activity upon ionizing radiation. Figure 2C shows that an inability to phosphorylate S131 correlates with decreased MST2 association and lower kinase activity. Taken together, these data suggest that DNA damage-induced phosphorylation of RASSF1A-Ser131 promotes MST2 binding to RASSF1A and is required to subsequently promote MST2 kinase activity.
ATM Phosphorylates RASSF1A-Ser131
To determine the kinase responsible for Ser131 phosphorylation in response to DNA damage, we pretreated MCF7 cells transfected with RASSF1A with either LY94002 (a pan PIKK inhibitor), KU55933 (a specific ATM inhibitor that, at 10 µM, does not inhibit ATR; Calbiochem), or Nu7026 (a DNA-PKcs inhibitor). Only those cells treated with LY94002 and the ATM inhibitor KU55933 showed reduced phosphorylation of Ser131 in response to DNA damage (Figure S3A). Furthermore, exogenous FLAG-RASSF1A was detected in immunoprecipitates of ATM but not DNA-PKcs (Figure S3B). The lack of an effect seen with Nu7026 on the phosphorylation of RASSF1A-Ser131 combined with the failure of RASSF1A and DNA-PKcs to associate by immunoprecipitation implies that ATM, and not DNA-PKcs, phosphorylates RASSF1A in response to ionizing radiation.
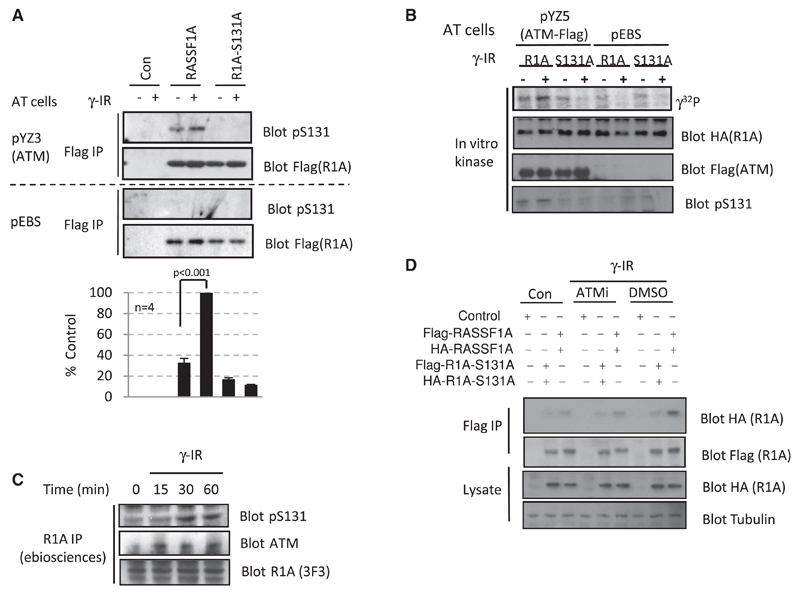
To strengthen the hypothesis that ATM is the kinase responsible for phosphorylation of Ser131, we examined the phospho-RASSF1A status in ATM-deficient (pEBS) and ATM-complemented (YZ3) cells [16], which express ATM to a comparable level of both HeLa and MCF7 cells (Figure S3C). DNA damage failed to induce phosphorylation of RASSF1A in pEBS cells, whereas RASSF1A was efficiently phosphorylated in YZ3 cells (ATM+) in a DNA damage-dependent manner (Figure 3A). RASSF1A-S131A was not phosphorylated in either pEBS or YZ3 (Figure 3A), suggesting that RASSF1A-Ser131 is phosphorylated in an ATM and DNA damage-dependent manner.
Figure 3. ATM Phosphorylates RASSF1A-Ser131 in Response to DNA Damage.
(A) ATM null (pEBS) or ATM reconstituted (YZ3) cells were transfected with pcDNA3 or plasmid expressing FLAG-RASSF1A or S131A-mutated FLAG-RASSF1A. Cells were harvested 30 min post γ-irradiation (10 Gy). FLAG-tagged proteins were immunoprecipitated and probed by western blot with the phosphospecific antibody raised against the phospho-Ser131 peptide. Data shown are representative of four independent experiments. Phospho-Ser131 bands were normalized to the loading controls, and bands were quantitated with ImageJ software. Error bars represent standard error.
(B) In vitro kinase assay of immunoprecipitated FLAG-ATM from YZ5 cells [16] incubated with HA-RASSF1A immunopurified from MCF7 cells in the presence of [γ-32P]ATP. Reactions were resolved by SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was visualized by phosphoimage detection (PMI imager, Bio-Rad) and probed with the indicated antibodies.
(C) HeLa cells were irradiated (10 Gy) and harvested at the indicated time points. Endogenous RASSF1A was immunoprecipitated, and immunoprecipitates were probed by western blot with indicated antibodies.
(D) MCF7 cells were cotransfected with FLAG-RASSF1A and HA-RASSF1A (2.5 µg DNA each), FLAG-RASSF1A-S131A and HA-RASSF1A-S131A (2.5 µg DNA each), or pcDNA3 (5 µg DNA). Cells were preincubated with 10 µM KU55399 (ATMi) or an equivalent volume of dimethyl sulfoxide (DMSO) before irradiation (10 Gy). Cells were harvested 30 min following irradiation. FLAG-tagged proteins were immunoprecipitated and probed by western blot with both anti-FLAG and anti-HA. Lysates were probed with indicated antibodies.
Next we sought to confirm that ATM directly phosphorylates RASSF1A in an in vitro kinase assay. HA-RASSF1A or HA-R-ASSF1A-S131A immunopurified from MCF7 cells was incubated with FLAG-ATM or controls from irradiated and nonirradiated cells. A specific band corresponding to phosphorylated RASSF1A was only detected when HA-RASSF1A was incubated with ATM and was more pronounced upon activation by ionizing radiation (Figure 3B); similar results were observed when cisplatin was used to activate ATM (data not shown).Taken together, these data strongly argue that the RASSF1A protein is a specific and direct substrate for ATM. To verify that endogenous RASSF1A is phosphorylated, we immunoprecipitated RASSF1A from HeLa cells and probed with both the phospho-SQ/TQ generic substrate and the specific phospho-Ser131 antibodies. Figure 3C and Figure S2A show a time-dependent increase in phosphorylation of RASSF1A-Ser131, with initial phosphorylation detected 15–30 min post ionizing radiation, correlating with the association of ATM (Figure 3C). The interaction of endogenous RASSF1A and ATM induced by DNA damage in HeLa cells was also visualized as increased levels of FLAG-RASSF1A in ATM immunoprecipitates from irradiated U2OS cells (Figure S3B). To investigate the mechanism of how ATM phosphorylation may activate MST2, we first determined whether activation of RASSF1A itself could be observed as previously described [17]. Cotransfection of HA-RASSF1A and FLAG-RASSF1A indicated a degree of dimerization that was enhanced by DNA damage (Figure 3D; Figure S3D). Moreover, mutation of the Ser131 site or inhibition of ATM destroyed the DNA damage-mediated dimerization of RASSF1A (Figure 3D), suggesting that failure to dimerize results in a failure to activate MST2 kinase activity (Figure 2C). N-terminal RASSF1A residues have been previously reported to promote self-association independent of the Salvador, RASSF1A, and Hippo (SARAH) domain [17]. Therefore, the enhanced association of MST2 with dimerized and Ser131-phosphorylated RASSF1A (Figure 2C; Figure 3D) may be due to an orientation of the RASSF1A SARAH domains, which have a higher affinity for MST2 than the monomer.
Because ATM is relaying signals detected from the sensing of DNA damage to RASSF1A, we predicted that inhibition of ATM should prevent activation of the kinases downstream of RASSF1A. Indeed, the association of MST2 and LATS1 was impeded either by mutation of the ATM site or by inhibition of ATM (Figure S3E). In contrast, MST2 association with LATS1 was not affected by pretreatment with the DNA-PKcs inhibitor Nu7026 (data not shown). This implies that inhibition of ATM restricts not only RASSF1A phosphorylation but also the ability of MST2 and LATS1 to associate upon DNA damage.
Mutation of RASSF1A-Ser131 Blocks the Response to DNA Damage
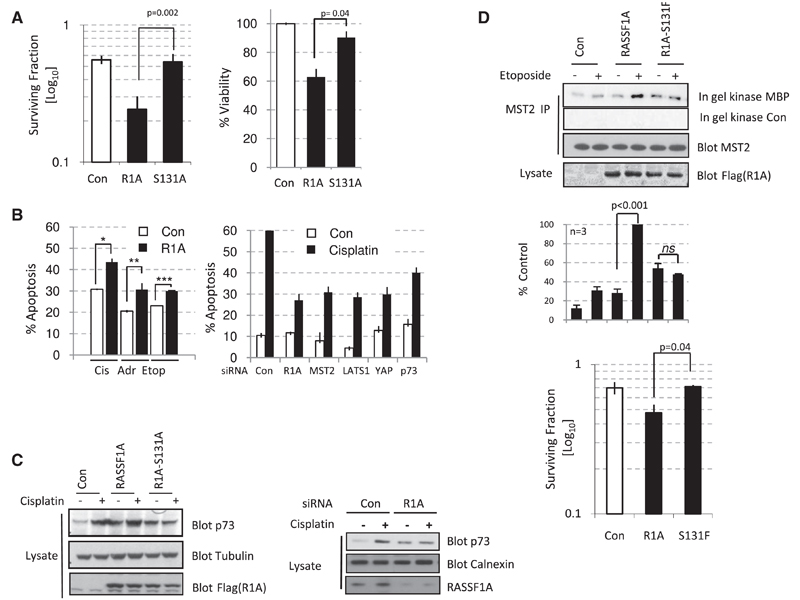
In order to address whether the phosphorylation of RASSF1A-Ser131 has physiological significance, we addressed the ability of MCF7 cells to form colonies after exposure to ionizing radiation. In agreement with Figure 1D, Figure 4A (left) shows a reduction in the ability of RASSF1A-expressing cells to form colonies following ionizing radiation, whereas RASSF1A-S131A-expressing cells are equivalent to controls. In addition, similar results were observed when RASSF1A- and RASSF1A-S131A-expressing cells were tested for viability in a resazurin assay post DNA damage (Figure 4A, right), indicating that RASSF1A has an important tumor-suppressor role in the DNA damage response. Because methylation of RASSF1 appears to affect sensitivity of tumors to both chemo- and radiotherapy, we examined whether RASSF1A could exacerbate DNA damage-induced apoptosis from additional DSB-inducing agents. Exogenous expression of RASSF1A in U2OS cells enhanced the apoptotic response to cisplatin, etoposide, and doxorubicin (Figure 4B, left), indicating a role for RASSF1A in a general response to DNA-damaging agents. The strongest enhancement of apoptosis was seen with cisplatin, which has been previously shown to engage p73 [18]. If this pathway is implicit in a DNA-damage response, then ablation of pathway components should restrict DNA damage-induced apoptosis. Figure 4B (right) shows that RASSF1A depletion reduces cisplatin-induced apoptosis in HeLa cells to a similar extent as observed when p73 itself is depleted. As with RASSF1A, siRNA depletion of MST2, LATS1, and YAP1 also reduced the apoptotic response to cisplatin (Figure 4B, right). Therefore, we were prompted to address whether the phosphorylation of RASSF1A in response to DNA damage mediated the stabilization of p73. As shown previously, expression of RASSF1A enhances the stability of p73 [7], but this stabilization was further enhanced when combined with DNA damage (Figure 4C, left). In agreement with a role for the ATM phosphorylation site in pathway signaling, RASSF1A-S131A blocked the stabilization of p73 in response to cisplatin-mediated DNA damage (Figure 4C, right). Similar results were observed for YAP1 stabilization (Figure S4A) and a transcriptional target of YAP1/p73, p21 (Figure S4B). Interestingly, loss of apoptotic responsiveness (Figure 4B, right) following knockdown of RASSF1A in HeLa cells correlated with the reduced stabilization of p73 in response to cisplatin (Figure 4C, left), an effect that may explain lower cisplatin sensitivity of tumors methylated for RASSF1A [5].
Figure 4. RASSF1A Requires Phosphorylation of S131 to Signal to p73, Induce Apoptosis, and Suppress Colony Formation.
(A) Left: MCF7 cells were transfected with either pcDNA3, FLAG-RASSF1A, or mutated FLAG-RASSF1A-S131A. Cells were seeded at 400 cells per 6 cm dish and exposed to ionizing radiation (2 Gy). Surviving colonies were counted after 14 days. Right: H1299 cells were cultured with 15 µM cisplatin for 48 hr, and cell viability was determined with the resazurin assay. Error bars represent standard error.
(B) Left: U2OS cells transfected with pcDNA3 or FLAG-RASSF1A were treated with DNA damage-inducing drugs cisplatin (25 µM), etoposide (10 µM), or doxorubicin (10 µM) for 40 hr, and apoptotic cells were detected by examining caspase activity in fluorescence-activated cell sorting (FACS) assay.*p < 0.05, **p < 0.05, ***p < 0.05. Right: HeLa cells transfected with siRNA against RASSF1A, MST2, LATS1, YAP1, p73, or a nontargeting control (NT) were treated with 25 µM cisplatin for 24 hr, and apoptotic cells were detected by FACS. Results are representative of two independent siRNAs for each target; siRNAs ablated protein levels in HeLa cells (here and in Figure 1D) as described previously [7]. Error bars represent standard error.
(C) Left: HeLa cells transfected with pcDNA3, FLAG-RASSF1A, or mutated FLAG-RASSF1A-S131A together with HA-P73 were treated with 25 µM cisplatin for 16 hr. Lysates were blotted with the indicated antibodies. Right: HeLa cells transfected with siRNA against RASSF1A or a nontargeting control were treated with 25 µM cisplatin for 24 hr. Lysates were probed with the indicated antibodies.
(D) Top: MCF7 cells were transfected with pcDNA3, FLAG-RASSF1A, or mutated FLAG-RASSF1A-S131F and treated as indicated with 10 µM etoposide. Endogenous MST2 was immunoprecipitated, and activity was determined by in gel kinase assay. Lysates and immunoprecipitates were blotted with the indicated antibodies. Bottom: H1299 cells transfected with pcDNA3, FLAG-RASSF1A, or mutated FLAG-RASSF1A-S131F were seeded at 400 cells per 6 cm dish. Cells were exposed to 25 µM cisplatin for 4 hr, and surviving colonies were counted after 14 days. Error bars represent standard error.
To address whether phosphorylation of RASSF1A-Ser131 has physiological tumor relevance, we took advantage of the fact that RASSF1A variants harboring mutations in and near the ATM site have been isolated from breast, ovarian, and lung cancer patients [ 8, 19] (Figure 2A). Because one mutation directly altered the Ser131 site to Phe (F), we addressed whether this mutation affects MST2 activation in response to DNA damage. As observed in Figure 1B for MST2-LATS1 association in MCF7 cells, low-level MST2 activity can be triggered in response to DNA damage in the absence of exogenous RASSF1A (Figure 4D, top). We interpret these effects to be due to residual expression of RASSF1A because this activity is ablated by siRNA against RASSF1A (Figure S4C).Expression of exogenous RASSF1A at a level that does not elicit maximal MST2 kinase activity allowed the observation of a responsiveness to DSB signaling, and, importantly, mutation of Ser131 to Phe ablated the response (Figure 4D, top). To address whether mutations at the RASSF1A-Ser131 site in tumors might represent an “escape mechanism” for tumor cells in response to DNA damage, we addressed the relevance of this event to colony survival. We again asked whether we could sensitize RASSF1 methylated tumor cells to DNA damage through reexpression of RASSF1A or tumor-derived RASSF1A-S131F. Because RASSF1A has been reported to stabilize p53 [20], we selected H1299 cells, which are p53 null tumor cells that are also methylated at RASSF1, thus ruling out any contribution to colony survival from p53-associated effects. As observed for ionizing radiation, Figure 4D (bottom) shows that expression of RASSF1A reduced the colony-forming ability of cells exposed to cisplatin treatment. Expression of RASSF1A-S131F, however, failed to suppress colony growth, indicating that the tumor cells from which this mutant originally derived are likely to have a defective DNA damage response as a result of RASSF1A mutation. Ultimately, this demonstrates that epigenetic loss or mutation of RASSF1A-Ser131 leads to a reduced response to DNA damage and an increased tumorigenic capacity.
Conclusions
The results described here present a novel role for RASSF1A in responding to DNA damage. This role is dependent on the phosphorylation of Ser131 by ATM and leads to p73 stabilization and activation through the downstream kinases MST2 and LATS1. The RASSF1A pathway has been described to affect DNA damage-dependent p53 stabilization [20–23]; however, p53 is commonly mutated or inactivated in the majority of human tumors. Interestingly, p73 is rarely mutated in human cancers [24], and the activation of this protein is important for the removal of aberrantly proliferating cells when p53 is absent [25]. The activation of p73 has been shown to be key in the cellular response to DNA-damaging drugs such as cisplatin and doxorubicin, irrespective of the p53 status of the cell [25]. The stabilization of p73 and the transcriptional coactivator YAP1 enhance the transcriptional activity of p73 targets in response to DNA damage [26, 27], and we show here that these events appear to require phosphorylation of RASSF1A-Ser131 (Figure 4C; Figures S4A and S4B)
Our previous work demonstrated that RASSF1A can stabilize p73 through regulation of YAP1 and promote apoptosis. ATM phosphorylation of RASSF1A is likely to act concurrently with c-Abl-mediated signaling [28] to stabilize a YAP1/p73 complex to coordinate p73 tumor suppression in response to DNA damage. Song et al. [20] recently showed that, in response to DNA damage, RASSF1A promotes the disruption of the MDM2-DAXX-HAUSP complex, which results in MDM2 self-ubiquitination, p53 stabilization, and subsequent G1/S cell-cycle arrest. Taken together, the regulation of both p53 and p73 implies that RASSF1A may have a dual role in the coordination of p53 and p73 responses. p53 is regulated by a number of additional pathways in response to DNA damage that would make a p53 response less sensitive to RASSF1A loss. However, in tumors where p53 is mutated or deleted, it is p73 that is important for a response to DNA damage. This may provide an explanation as to why tumors that have lost RASSF1A expression (via promoter methylation), and hence the capacity to stabilize p73, appear to have increased resistance to chemo- and radiotherapy [6]. Therefore, understanding the mutational status of the ATM site in RASSF1A in nonmethylated RASSF1 tumors is likely to be a significant factor in predicting either a response to therapy or correlation with DNA repair-deficient phenotypes [29].
Supplemental Data
Supplemental Data include Supplemental Experimental Procedures and four figures and can be found online at http://www.cell.com/current-biology/supplemental/S0960-9822(09)01897-1.
Acknowledgments
The authors would like to thank Y. Shiloh for pEBS, YZ3, and YZ5 cells and K. O’Neill and E. Hammond for critical review and helpful discussions. This work was supported by Cancer Research UK and the Medical Research Council (UK).
References
- 1.Donninger H, Vos MD, Clark GJ. The RASSF1A tumor suppressor. J Cell Sci. 2007;120:3163–3172. doi: 10.1242/jcs.010389. [DOI] [PubMed] [Google Scholar]
- 2.Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005;65:3497–3508. doi: 10.1158/0008-5472.CAN-04-4088. [DOI] [PubMed] [Google Scholar]
- 3.van der Weyden L, Adams DJ. The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta. 2007;1776:58–85. doi: 10.1016/j.bbcan.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catto JW, Azzouzi AR, Rehman I, Feeley KM, Cross SS, Amira N, Fromont G, Sibony M, Cussenot O, Meuth M, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol. 2005;23:2903–2910. doi: 10.1200/JCO.2005.03.163. [DOI] [PubMed] [Google Scholar]
- 5.Honda S, Haruta M, Sugawara W, Sasaki F, Ohira M, Matsunaga T, Yamaoka H, Horie H, Ohnuma N, Nakagawara A, et al. The methylation status of RASSF1A promoter predicts responsiveness to chemotherapy and eventual cure in hepatoblastoma patients. Int J Cancer. 2008;123:1117–1125. doi: 10.1002/ijc.23613. [DOI] [PubMed] [Google Scholar]
- 6.Dote H, Cerna D, Burgan WE, Carter DJ, Cerra MA, Hollingshead MG, Camphausen K, Tofilon PJ. Enhancement of in vitro and in vivo tumor cell radiosensitivity by the DNA methylation inhibitor zebularine. Clin Cancer Res. 2005;11:4571–4579. doi: 10.1158/1078-0432.CCR-05-0050. [DOI] [PubMed] [Google Scholar]
- 7.Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O’Neill E. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962–975. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shivakumar L, Minna J, Sakamaki T, Pestell R, White MA. The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol Cell Biol. 2002;22:4309–4318. doi: 10.1128/MCB.22.12.4309-4318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill E, Rushworth L, Baccarini M, Kolch W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science. 2004;306:2267–2270. doi: 10.1126/science.1103233. [DOI] [PubMed] [Google Scholar]
- 10.Vichalkovski A, Gresko E, Cornils H, Hergovich A, Schmitz D, Hemmings BA. NDR kinase is activated by RASSF1A/MST1 in response to Fas receptor stimulation and promotes apoptosis. Curr Biol. 2008;18:1889–1895. doi: 10.1016/j.cub.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Cimprich KA, Cortez D. ATR: An essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavin MF. Ataxia-telangiectasia: From a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 13.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 14.Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill T, Dwyer AJ, Ziv Y, Chan DW, Lees-Miller SP, Abraham RH, Lai JH, Hill D, Shiloh Y, Cantley LC, et al. Utilization of oriented peptide libraries to identify substrate motifs selected by ATM. J Biol Chem. 2000;275:22719–22727. doi: 10.1074/jbc.M001002200. [DOI] [PubMed] [Google Scholar]
- 16.Ziv Y, Bar-Shira A, Pecker I, Russell P, Jorgensen TJ, Tsarfati I, Shiloh Y. Recombinant ATM protein complements the cellular A-T phenotype. Oncogene. 1997;15:159–167. doi: 10.1038/sj.onc.1201319. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz-Vega S, Khokhlatchev A, Nedwidek M, Zhang XF, Dammann R, Pfeifer GP, Avruch J. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene. 2002;21:1381–1390. doi: 10.1038/sj.onc.1205192. [DOI] [PubMed] [Google Scholar]
- 18.Bertini E, Oka T, Sudol M, Strano S, Blandino G. YAP: At the crossroad between transformation and tumor suppression. Cell Cycle. 2009;8:49–57. doi: 10.4161/cc.8.1.7259. [DOI] [PubMed] [Google Scholar]
- 19.Burbee DG, Forgacs E, Zochbauer-Muller S, Shivakumar L, Fong K, Gao B, Randle D, Kondo M, Virmani A, Bader S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst. 2001;93:691–699. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song MS, Song SJ, Kim SY, Oh HJ, Lim DS. The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2-DAXX-HAUSP complex. EMBO J. 2008;27:1863–1874. doi: 10.1038/emboj.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombani J, Polesello C, Josue F, Tapon N. Dmp53 activates the Hippo pathway to promote cell death in response to DNA damage. Curr Biol. 2006;16:1453–1458. doi: 10.1016/j.cub.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 22.Kawahara M, Hori T, Chonabayashi K, Oka T, Sudol M, Uchiyama T. Kpm/Lats2 is linked to chemosensitivity of leukemic cells through the stabilization of p73. Blood. 2008;112:3856–3866. doi: 10.1182/blood-2007-09-111773. [DOI] [PubMed] [Google Scholar]
- 23.Aylon Y, Michael D, Shmueli A, Yabuta N, Nojima H, Oren M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 2006;20:2687–2700. doi: 10.1101/gad.1447006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichimiya S, Nimura Y, Kageyama H, Takada N, Sunahara M, Shishikura T, Nakamura Y, Sakiyama S, Seki N, Ohira M, et al. p73 at chromosome 1p36.3 is lost in advanced stage neuroblas-toma but its mutation is infrequent. Oncogene. 1999;18:1061–1066. doi: 10.1038/sj.onc.1202390. [DOI] [PubMed] [Google Scholar]
- 25.Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WG., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 26.Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni G, Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 27.Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A, et al. The transcriptional coactivator Yes-associated protein drives p73 genetarget specificity in response to DNA Damage. Mol Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Gao B, Xie XJ, Huang C, Shames DS, Chen TT, Lewis CM, Bian A, Zhang B, Olopade OI, Garber JE, et al. RASSF1A polymorphism A133S is associated with early onset breast cancer in BRCA1/2 mutation carriers. Cancer Res. 2008;68:22–25. doi: 10.1158/0008-5472.CAN-07-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.