Abstract
Steatosis is a major risk factor for complications after liver surgery. Since neutrophil cytotoxicity is critical for ischemia-reperfusion injury in normal livers, the aim of the present study was to evaluate whether an exaggerated inflammatory response could cause the increased injury in steatotic livers. In C57Bl/6 mice, 60 min of warm hepatic ischemia triggered a gradual increase in hepatic neutrophil accumulation during reperfusion with peak levels of 100-fold over baseline at 12 h of reperfusion. Neutrophil extravasation and a specific neutrophil-induced oxidant stress (immunostaining for hypochlorous acid-modified epitopes) started at 6 h of reperfusion and peaked at 12–24 h. Ob/ob mice, which had a severe macrovesicular steatosis, suffered significantly higher injury (alanine transaminase activity: 18,000 ± 2,100 U/l; 65% necrosis) compared with lean littermates (alanine transaminase activity: 4,900 ± 720 U/l; 24% necrosis) at 6 h of reperfusion. However, 62% fewer neutrophils accumulated in steatotic livers. This correlated with an attenuated increase in mRNA levels of several proinflammatory genes in ob/ob mice during reperfusion. In contrast, sham-operated ob/ob mice had a 50% reduction in liver blood flow and 35% fewer functional sinusoids compared with lean littermates. These deficiencies in liver blood flow and the microcirculation were further aggravated only in ob/ob mice during reperfusion. The attenuated inflammatory response and reduced neutrophil-induced oxidant stress observed in steatotic livers during reperfusion cannot be responsible for the dramatically increased injury in ob/ob mice. In contrast, the aggravated injury appears to be mediated by ischemic necrosis due to massive impairment of blood and oxygen supply in the steatotic livers.
Keywords: liver blood flow, microvascular dysfunction, heme oxygenase-1, neutrophils, hypochlorous acid, steatosis
nonalcoholic hepatic steatosis is a common problem in the developed world, affecting between 10 and 24% of the general population (4). The presence of moderate to severe steatosis (>30%) results in a higher risk of liver failure and mortality after major hepatic resection (7). In liver transplantation, even mild steatosis (<30%) worsens graft and patient survival (35). One of the main reasons for the vulnerability of steatotic liver may be the increased susceptibility to ischemia-reperfusion injury (28, 45).
In lean livers, reperfusion injury is caused by an excessive inflammatory response and by microcirculatory dysfunction (8, 17, 29, 39, 42). The inflammatory response involves early activation of Kupffer cells leading to the first phase of the postischemic oxidant stress and liver injury (19, 21, 31). Cell contents release from necrotic parenchymal cells causes activation of complement factors (22) and, through high-mobility group box-1, which interacts with the Toll-like receptor 4 on Kupffer cells (53), cytokine formation. Activated Kupffer cells and CD4+ lymphocytes are the main sources of proinflammatory cytokines, which induce chemokine formation in parenchymal cells (9, 54). Cytokines, CXC chemokines, and activated complement factors all contribute to activation and recruitment of neutrophils in the postischemic liver (5, 22, 30, 42). Neutrophils cause cell damage during the second phase of reperfusion injury (24). These phagocytes kill predominantly through generation of reactive oxygen species such as superoxide anion radicals, H2O2, and hypochlorous acid (HOCl, formed only from H2O2 by myeloperoxidase in the presence of chloride ions), and the release of proteases (18, 25). The spontaneous generation of superoxide anion radicals by neutrophils isolated from postischemic livers at 6–24 h (20, 23) as well as immunostaining for HOCl-modified proteins during this time period (14) provided the direct evidence for a specific neutrophil-derived oxidant stress during the second phase of reperfusion injury. Thus, under conditions of mild to moderate ischemic stress, the inflammatory response with activation of Kupffer cells and in particular neutrophils is the dominant mechanism of injury in lean livers. Although microvascular dysfunction can affect reperfusion injury (37, 39), its contribution is small after shorter periods of ischemia. However, with increasing ischemic stress microcirculatory blood flow is more and more impaired, thereby causing cell injury through ischemic necrosis. Thus the longer the ischemic stress the more prominent the contribution of microcirculatory dysfunction to the overall reperfusion injury.
It is widely accepted that steatotic livers are more susceptible to hepatic ischemia-reperfusion injury (17, 27, 45). Increased microcirculatory dysfunction (44, 51, 52) and impairment of regeneration (45) were suggested as reasons for the increased liver damage in steatotic livers. Although neutrophils appear to be also present in the steatotic liver during reperfusion (40), a specific neutrophil-induced oxidant stress and its potential contribution has not been evaluated. Therefore, the objectives of the present investigation were 1) to verify that the time course of hepatic neutrophil infiltration and the development of a neutrophil-specific oxidant stress are similar in a mouse model of warm hepatic ischemia-reperfusion compared with the previously studied rat model (14); 2) to evaluate the inflammatory response in lean livers compared with steatotic livers from ob/ob mice, which represent a genetic model of steatosis similar to the frequently used Zucker rats; and 3) to evaluate the importance of potential microcirculatory dysfunction in lean livers and in steatotic livers from ob/ob mice.
MATERIALS AND METHODS
Animals
Male C57Bl/6J wild-type mice (22–28 g body wt), C57Bl/6J-ob/ob mice (43–50 g), and their lean littermates (22–28 g) were purchased from Jackson Laboratory (Bar Harbor, ME) and allowed free access to food and water. The experimental protocols, which were reviewed and approved by the Institutional Animal Care and Use Committee, followed the criteria of the University of Arizona and the National Research Council for the Care and Use of Laboratory Animals in Research. Under anesthesia with pentobarbital sodium solution (60 or 100 mg/kg ip in lean mice or obese mice, respectively), a laparotomy was performed, and the blood supply to the median and left hepatic lobes was occluded with an atraumatic vascular clamp. Reperfusion was initiated by removal of the clamp. The body temperature was maintained with a heating lamp. After reperfusion was initiated, 4 ml/kg of physiological saline was injected intraperitoneally, the abdominal incision was closed with 4-0 silk and wound clips, and the animals were allowed to recover.
Time course experiments
Wild-type C57Bl/6J mice underwent 60 min of ischemia. The blood was drawn and the liver was excised before ischemia, at the end of ischemia, and at 1, 6, 12, and 24 h of reperfusion. The plasma was used for determination of alanine transaminase (ALT) activity. A part of the excised liver was fixed in a phosphate-buffered 10% (vol/vol) formalin solution and embedded in paraffin for histological evaluations, and the remaining tissue was snap frozen in liquid nitrogen for several assays described below.
Steatotic liver experiments
Ob/ob mice and lean littermates were subjected to 40 or 20 min of ischemia. At 2 h of reperfusion, hepatic microcirculatory alterations were evaluated by laser Doppler flow-metry and a high-resolution in vivo microscopic method. Before ischemia and 2.5 and 6 h after reperfusion, the plasma and the liver were collected. In some experiments, 5 mg/kg of cobalt protoporphyrin (Co-P) (Sigma, St. Louis, MO), an inducer of heme oxygenase-1 (HO-1), or 4 ml/kg of its vehicle was subcutaneously injected to ob/ob mice 24 h before ischemia. In another experiment, 15 mg/kg of tin protoporphyrin (Sn-P) (Alexis, San Diego, CA), an inhibitor of HO-1, or 4 ml/kg of its vehicle was subcutaneously injected to lean litter-mates 4 h before ischemia. Co-P and Sn-P solutions were prepared as described previously (1, 3, 11).
Quantitation of liver injury, lipid peroxidation, and hepatic neutrophil accumulation
Plasma ALT activity was measured with a commercially available test kit (Biotron Diagnostics, Hemet, CA). Lipid peroxidation in the tissue was evaluated by measuring malondialdehyde (MDA) with the BIOXYTECH MDA-586 assay kit (Oxis Research, Portland, OR) as described (14). The MDA-586 method and the specific assay conditions applied here allow a selective determination of MDA with minimal interference of other aldehydes, e.g., 4-hydroxyalkenals. Briefly, for evaluation of free MDA, 1 volume of liver tissue was homogenized in 4 volumes of 25 mM HEPES buffer (pH 7.5) containing 5 mM EDTA and 5 mM butylated hydroxytoluene. For evaluation of total MDA content, 20% liver homogenate in 0.1 N HCl solution including 5 mM butylated hydroxytoluene was prepared (pH was ~2). The homogenate was incubated for 80 min at 60°C to hydrolyze Schiff base adducts of MDA. The samples for each assay were centrifuged at 3,000 g for 10 min at 4°C. The supernatant was used to determine concentrations of MDA with the BIOTECH MDA-586 kit. Protein-bound MDA was calculated by subtracting free MDA value from total MDA value. Protein concentration in the supernatant was measured with a bicinchoninic acid reagent kit (Pierce, Rockford, IL).
Liver sections stained with hematoxylin-eosin (H&E) were used for histological determination of liver injury. The percentage of necrosis was estimated by assessing the area of necrosis compared with the entire histological section (14). The pathologist (A. Farhood), who was blinded to the treatment of the animals, performed the histological examinations. For neutrophil quantitation, deparaffinized and rehydrated thin sections were stained with a commercially available kit using the naphthol AS-D chloroacetate esterase technique (Sigma). The number of neutrophils present in sinusoidal spaces or extravasated into hepatic parenchyma was counted in 20 high-power fields (×400) with a light microscope (14). The sum of them was expressed as the total neutrophil sequestration in the liver.
Detection of neutrophil-mediated oxidant stress
Presence of neutrophil-mediated oxidant stress was evaluated with immunohistochemistry against protein epitopes modified by HOCl, the major neutrophil-derived oxidant. HOCl-modified proteins were stained as described previously with some modifications (14). Deparaffinized and rehydrated thin sections were incubated sequentially with ImmunoPure peroxidase suppressor (Pierce) and a mouse IgG blocking reagent (M.O.M. immunodetection kit, Vector Laboratories, Burlingame, CA). The sections were then incubated (2 h at room temperature) with a mouse monoclonal antibody supernatant against HOCl-modified proteins [clone 2D10G9, at a 1:500 dilution, not cross-reacting with other oxidative (lipo)protein modifications, e.g., MDA modification] (33, 34). For a competition experiment, the primary antibody was preincubated with HOCl-modified low-density lipoprotein for 20 min at the molar ratio of 1:20 (34). Thereafter, a biotinylated anti-mouse IgG reagent and an ABC reagent (M.O.M. immunodetection kit, Vector) were used. Color was developed with diaminobenzidine chromogen (Dako, Carpentaria, CA).
Evaluation of fat accumulation in the liver
Blocks of liver tissue were frozen in optimum cutting temperature embedding medium (Tissue-Tek O.C.T. compound; Sakura Finetek, Torrance, CA). Five-micrometer cryostat sections were prepared and stained with Oil red O only, because counterstaining with hematoxylin stained nuclei and interfered with image analysis. Image analysis was performed within 24 h. Analysis of size (area) of fat droplets was performed with Simple PCI software by Compix (Cranberry, PA). Image acquisition was done with an Olympus IMT-2 inverted microscope equipped with a Hamamatsu digital camera that can capture 1,280 × 1,024 10-bit images of bright-field, phase-contrast, or fluorescence images. The images were captured at a magnification of 40 × 1.5, and for the purpose of measurement the fat droplets were pseudocolored and the background was subtracted. Twenty fields from each liver section were measured. The program gives detailed summary of statistics of each field, including the area of the field, mean area occupied by fat droplets, object count, minimum and maximum areas, as well as standard error of the mean and standard deviation.
Expression of mRNA in liver tissue
Expressions of the selected genes were quantified by real-time RT-PCR analysis as previously described (12). Briefly, total RNA was reversely transcribed with Moloney murine leukemia virus reverse transcriptase and oligo(dT) primers. The forward and reverse primers for the genes were designed by use of Primer Express software (Applied Biosystems, Foster City, CA). All primer sequences are published (12). The SYBRgreen DNA PCR kit (Applied Biosystems) was used for real-time RT-PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values. Ct values for the various genes were first normalized with that of β-actin in the same sample, and then relative differences between groups were expressed as relative increases or decreases, with the lean control group set as 1.00. Assuming the Ct value is reflective of the initial starting copy and that there is 100% efficacy, a difference of 1 cycle is equivalent to a twofold difference in starting copy. Standard curve analysis was also performed according to the method of Liu and Saint (32).
Hepatic microcirculation
Hepatic tissue blood flow was examined with a laser Doppler flowmeter (Moor Instruments, Wilmington, DE). Animals were anesthetized with 2 g/kg of urethane (Sigma). The probe (type P5a/P5b) was placed on the surface of the median or the left hepatic lobe, and the widths of the signals were calculated with Moorsoft for moorLAB version 1.1 (Moor Instruments). After the blood flow measurement, the sinusoidal microcirculatory status was evaluated by an established high-resolution in vivo microscopic method (36, 38). Briefly, a compound binocular microscope (Leitz, Wetzlar, Germany) adopted for in vivo microscopy was equipped to provide either transillumination or epi-illumination, as well as video microscopy using a charge-coupled device camera (MTI, Michigan City, IN). After intravenous injection of 100 mg/kg of fluorescein isothiocyanate-labeled dextran (MW 150,000, Sigma), the liver was exteriorized through a left subcostal incision, positioned over a window of optical-grade mica in a specially designed tray mounted on a microscopic stage, and covered with a piece of Saran wrap (Dow Chemical, Midland, MI), which held the liver in position and limited movement. Homeostasis was ensured by a constant suffusion of the organ with Ringer solution maintained at body temperature. Epi-illumination images were observed with a water-immersion objective lens (Leitz ×40/0.65, Wetzlar, Germany) and were recorded for subsequent off-line analysis using a Sony Betacam videotape recorder (Sony Medical Electronics, Park Ridge, NJ). Hepatic sinusoids containing blood flow were determined as “functioning sinusoids.” The number of functioning sinusoids, which run across a 250-µm-long bar, was counted.
Statistical analysis
Data are given as means ± SE. Comparisons between two groups were performed with the unpaired Student’s t-test or Cochran-Cox test. Comparisons among multiple groups were done with one-way ANOVA followed by Newman-Keuls test. Difference in survival rates was evaluated with χ2 test. P < 0.05 was considered significant.
RESULTS
Liver injury and hepatic neutrophil accumulation in C57Bl/6 mice
After 60 min of ischemia, plasma ALT activities progressively increased during reperfusion, reaching a peak at 6 h (Fig. 1A). As a further alternative indicator of hepatocellular injury, necrosis was estimated in histological sections of the liver (Figs. 1B, 2A, 2B). Patchy areas of coagulative necrosis appeared 6 h after reperfusion. Thereafter, necrotic areas were cumulatively enlarged until 24 h of reperfusion. Only few neutrophils were present in sinusoids before ischemia (Fig. 2C). However, neutrophils started to accumulate in sinusoids at 1 h of reperfusion (Fig. 3A). Thereafter, the number of accumulated neutrophils increased until 24 h of reperfusion. Neutrophils extravasated into the hepatic parenchyma appeared at 6 h and then increased until 24 h of reperfusion (Fig. 3A). Extravasated neutrophils were seen exclusively around and within necrotic areas (Fig. 2D).
Fig. 1.
Time course of liver injury during reperfusion after 60 min of ischemia (I) in C57Bl/6 mice. Alanine aminotransferase (ALT) activities were measured in plasma, and the area of necrosis was estimated in hematoxylin-eosin (H&E)-stained tissue sections. Values represent means ± SE of 4–5 animals per time point. *P < 0.05 compared with controls (Cont).
Fig. 2.
Representative pictures of liver sections of C57Bl/6 mice before ischemia (A, C, E) and 12 h after reperfusion following 60 min of ischemia (B, D, F). In sections stained with H&E, no structural change could be seen before ischemia (A, magnification ×100), but extensive coagulative necrosis occurred after 12 h of reperfusion (B, magnification ×100). Neutrophils were stained red with a naphthol AS-D chloroacetate esterase method. Only a few neutrophils (arrow) were observed in hepatic sinusoids before ischemia (C, magnification ×400). However, many neutrophils were present around and within the necrotic area, and some of them extravasated into hepatic parenchyma (D, magnification ×400). Sections were immunohistochemically stained for hypochlorous acid (HOCl)-modified proteins (E and F, magnification ×400). Although no significant staining was observed before ischemia (E), almost all necrotic hepatocytes were stained 12 h after reperfusion (F).
Fig. 3.
Time course of hepatic neutrophil accumulation and neutrophil-mediated oxidant stress in liver during reperfusion after 60 min of ischemia in C57Bl/6 mice. A: neutrophils were stained the naphthol AS-D chloroacetate esterase method and counted in 20 high-power fields (HPFs). B: hepatocytes stained for HOCl-modified epitopes and the number of positive cells compared with the total number of hepatocytes per field. Values represent means ± SE of 4–5 animals per group. *P < 0.05 (compared with respective control groups).
Immunohistochemical detection of neutrophil-mediated oxidant stress
No significant staining for HOCl-modified epitopes was observed before ischemia (Fig. 2E), at the end of ischemia or at 1 h of reperfusion (data not shown). At 6 h of reperfusion, some necrotic hepatocytes were stained (Fig. 3B). Almost all necrotic hepatocytes and some hepatocytes around necrotic areas were stained at 12 h (Figs. 2F and 3B) and 24 h (Fig. 3B) of reperfusion. Omission of the primary antibody (clone 2D10G9) or preabsorption with HOCl-modified low-density lipoprotein (data not shown) prevented antibody binding, indicating that the staining was specific for HOCl-modified epitopes generated in vivo by the MPO-H2O2-chloride system of activated phagocytes. These results with the mouse model of ischemia-reperfusion injury are consisted with those of our earlier paper using rats (14), which showed that the accumulation and extravasation of neutrophils and production of HOCl by neutrophils (containing up to 5% MPO of total cell protein content) played a major role in hepatocyte necrosis in the later phase of reperfusion injury after hepatic ischemia. To test for the presence of a general oxidant stress during reperfusion, free and protein-bound MDA was measured in livers of controls, at the end of ischemia, and at different times during reperfusion. The assay kit used for these measurements allows a selective quantitation of MDA with minimal interferences from other lipid aldehydes. A significant increase in total and protein-bound MDA was found only during reperfusion as early as 1 h after ischemia (Fig. 4).
Fig. 4.
Time course of hepatic malondialdehyde (MDA) levels during reperfusion after 60 min of ischemia in C57Bl/6 mice. Protein-bound, free, and total MDA levels were measured in frozen tissue. Values represent means ± SE of 4–5 animals per group. *P < 0.05 (compared with respective control groups).
Steatosis in ob/ob mice
To first quantify steatosis in ob/ob mice vs. lean littermates, liver sections were stained with Oil red O. In the livers of lean littermates, a few small fat droplets were detected (Fig. 5A). In contrast, the livers of ob/ob mice showed massive steatosis throughout the hepatic lobules (Fig. 5B). The size of the fat droplets ranged from moderate to large (Fig. 5B). Quantitative assessment of fat accumulation in lean animals indicated that <5% of liver sections contained fat (Fig. 5C). In contrast, the Oil red O-positive area was 50% in livers of ob/ob mice (Fig. 5C).
Fig. 5.
Fat accumulation in livers of ob/ob mice and lean littermates. Liver sections of lean littermates (A) and ob/ob mice (B) were stained with Oil red O. C: comparison of positively stained areas between ob/ob mice and lean littermates. Oil red O-positive areas were quantitated with image analysis as described in materials and methods. Values represent means ± SE of 3 animals. *P < 0.05 (compared with lean group).
Liver injury and survival rate
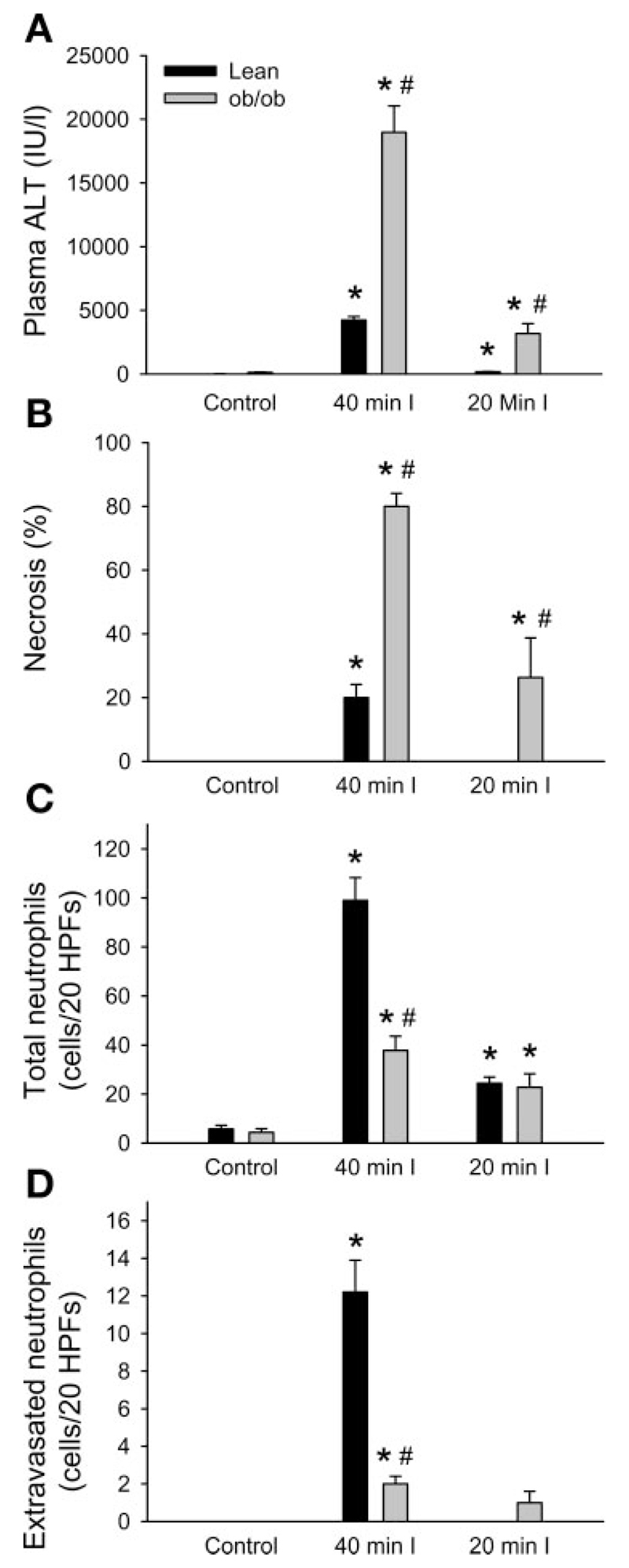
Since 60 min of ischemia caused very severe liver injury and a high mortality in ob/ob mice, the time of ischemia was reduced. At 6 h of reperfusion after 40 or 20 min of ischemia, liver injury estimated with plasma ALT activities and histological evaluation of the necrotic areas was significantly more severe in ob/ob mice than in lean littermates (Fig. 6, A and B). Survival rate of ob/ob mice or lean littermates 6 h after reperfusion following 40 min of ischemia was 56% (n = 5/9) or 100% (n = 5/5), respectively (p < 0.05). In contrast, all animals in both groups survived and most of them were active at 6 h after reperfusion following 20 min of ischemia. Both total neutrophils and extravasated neutrophils were significantly increased 6 h after reperfusion following 40 or 20 min of ischemia in lean littermates (Fig. 6, C and D). Neutrophils were densely accumulated around and within necrotic areas as observed in the time course experiments mentioned above. On the other hand, in ob/ob mice, the number of neutrophils was significantly less or similar compared with lean littermates after 40 or 20 min of ischemia, respectively (Fig. 6, C and D). In contrast to lean littermates, few neutrophils were observed in necrotic areas of ob/ob mice despite the fact that still a significant number of neutrophils was accumulating within sinusoids in nonnecrotic areas. Although there was no relevant neutrophil-specific oxidant stress in these livers (i.e., staining for HOCl-modified epitope-positive hepatocytes), there was a significant increase in total and protein-bound MDA levels in the postischemic livers (Fig. 7). However, despite the drastic difference in injury, MDA levels were not significantly different between lean and ob/ob mice (Fig. 7).
Fig. 6.
Liver injury and hepatic neutrophils accumulation 6 h after reperfusion following 40 or 20 min of ischemia in ob/ob mice and lean littermates. ALT activities were measured in plasma (A) and the area of necrosis was estimated in H&E-stained tissue sections (B). Neutrophils were stained with the naphthol AS-D chloroacetate esterase method and counted in 20 HPFs. All neutrophils were counted irrespective of their location (total neutrophils, C), and neutrophils extravasated into the parenchyma were counted separately (D). Values are means ± SE of 4–5 animals. *P < 0.05 (compared with respective control groups), #P < 0.05 (compared with respective lean groups).
Fig. 7.
Hepatic MDA levels 6 h after reperfusion following 40 or 20 min of ischemia in ob/ob mice and lean littermates. Protein-bound, free, and total MDA levels were measured in frozen tissue. Values represent means ± SE of 4–5 animals per group. *P < 0.05 (compared with respective control groups), #P < 0.05 (compared with respective lean groups).
mRNA expression of proinflammatory genes in ob/ob mice
To evaluate the overall inflammatory and stress responses to ischemia-reperfusion in lean vs. ob/ob mice, the mRNA levels of inflammatory genes were determined in liver tissues. In principle, genes analyzed by real-time RT-PCR were clustered into four major groups, i.e., cytokines, chemokines, adhesion molecules, and others (Table 1). In lean littermates, there was a substantial upregulation of many proinflammatory genes including IL-1β, IL-1 receptor, macrophage inflammatory protein-2, and murine keratinocyte-derived chemokine mRNA at 6 h of reperfusion (Table 1). However, the induction of IL-1β, IL-1 receptor, and murine keratinocyte-derived chemokine was significantly impaired in ob/ob mice compared with lean mice. Expression of tumor necrosis factor-α mRNA had tendencies to be upregulated in lean littermates and downregulated in ob/ob mice after reperfusion, which resulted in a significant difference between both groups after reperfusion. Expression level of intercellular adhesion molecule-1 mRNA after reperfusion in ob/ob mice was also significantly lower than in the respective lean group. Only interferon-γ, among proinflammatory mediators, was more highly expressed in ob/ob mice after reperfusion compared with lean littermates. Among others, there was no significant difference in mRNA expressions of inducible nitric oxide synthase, cyclooxygenase 2, thrombospondin 1, early growth response factor-1, and superoxide dismutase-1 between ob/ob mice and lean littermates. However, upregulation of HO-1 mRNA observed after reperfusion in lean littermates was completely prevented in ob/ob mice (Table 1). Uncoupling protein-2 mRNA levels in ob/ob mice were significantly higher than those in respective lean mice both before ischemia and after reperfusion. These gene expression data demonstrate an overall lower inflammatory response in ob/ob mice despite the much more severe reperfusion injury. Since there was a striking difference in HO-1 mRNA induction between lean and ob/ob mice, we hypothesized that aggravation of microcirculatory deficiencies rather than the inflammatory response may be the reason for the increased injury in ob/ob mice.
Table 1.
Real-time RT-PCR analysis of liver tissue in ob/ob mice and lean littermates before ischemia and after 40 min of ischemia and 6 h of reperfusion
| Lean Control | Lean I-R | ob/ob Control | ob/ob I-R | |
|---|---|---|---|---|
| Cytokines | ||||
| TNF-α | 1.00±0.07 | 1.21±0.17 | 1.00±0.17 | 0.64±0.09‡ |
| TNFR1 | 1.00±0.43 | 0.85±0.17 | 0.65±0.06 | 0.53±0.18 |
| IL-1β | 1.00±0.18 | 8.60±1.88* | 1.89±0.22† | 4.29±0.15*‡ |
| IL-1R | 1.00±0.07 | 2.89±0.37* | 0.29±0.01† | 0.62±0.19‡ |
| IL-6 | 1.00±0.07 | 0.81±0.15 | 0.82±0.04 | 0.86±0.12 |
| IFN-γ | 1.00±0.32 | 1.21±0.30 | 1.61±0.64 | 3.87±0.72‡ |
| Chemokines | ||||
| MIP-2 | 1.00±0.15 | 4.74±0.83* | 1.17±0.21 | 4.03±0.63* |
| mKC | 1.00±0.40 | 8.08±1.24* | 0.25±0.09 | 2.83±0.72*‡ |
| Adhesion molecules | ||||
| ICAM-1 | 1.00±0.33 | 1.82±0.20 | 0.85±0.16 | 1.17±0.18‡ |
| VCAM-1 | 1.00±0.07 | 0.66±0.11 | 0.94±0.05 | 0.82±0.07 |
| Others | ||||
| iNOS | 1.00±0.29 | 1.04±0.19 | 0.95±0.39 | 1.24±0.36 |
| COX-2 | 1.00±0.13 | 0.60±0.09* | 0.94±0.06 | 0.82±0.06 |
| TSP-1 | 1.00±0.07 | 6.35±0.96* | 1.96±0.49 | 7.99±1.69* |
| HO-1 | 1.00±0.20 | 6.22±0.70* | 1.20±0.05 | 1.65±0.33‡ |
| UCP-2 | 1.00±0.20 | 0.64±0.09* | 3.21±0.28† | 1.55±0.06* |
| EGR-1 | 1.00±0.28 | 10.38±1.80* | 1.64±0.52 | 12.42±2.36* |
| SOD-1 | 1.00±0.12 | 0.55±0.16 | 1.12±0.03 | 0.79±0.08* |
The expression level of each gene was first normalized to that of β-actin in each individual sample, and then the relative differences between groups were expressed as relative increases or decreases setting the lean control group as 1.00. Values are presented as means ± SE of 3–5 animals per group. TNF-α, tumor necrosis factor-α; TNFR1, TNF receptor type 1; IL-1β, interleukin 1β; IL-1R, IL-1 receptor; IL-6, interleukin 6; IFN-γ, interferon γ; MIP-2, macrophage inflammatory protein-2; mKC, murine keratinocyte-derived chemokine; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase 2; TSP-1, thrombospondin 1; HO-1, heme oxygenase-1; UCP-2, uncoupling protein-2; EGR-1, early growth response factor-1; SOD-1, superoxide dismutase-1.
P < 0.05 vs. respective control groups
P < 0.05 vs. lean control group
P < 0.05 vs. lean ischemia-reperfusion (I-R) group.
Hepatic microcirculation in lean vs. ob/ob mice
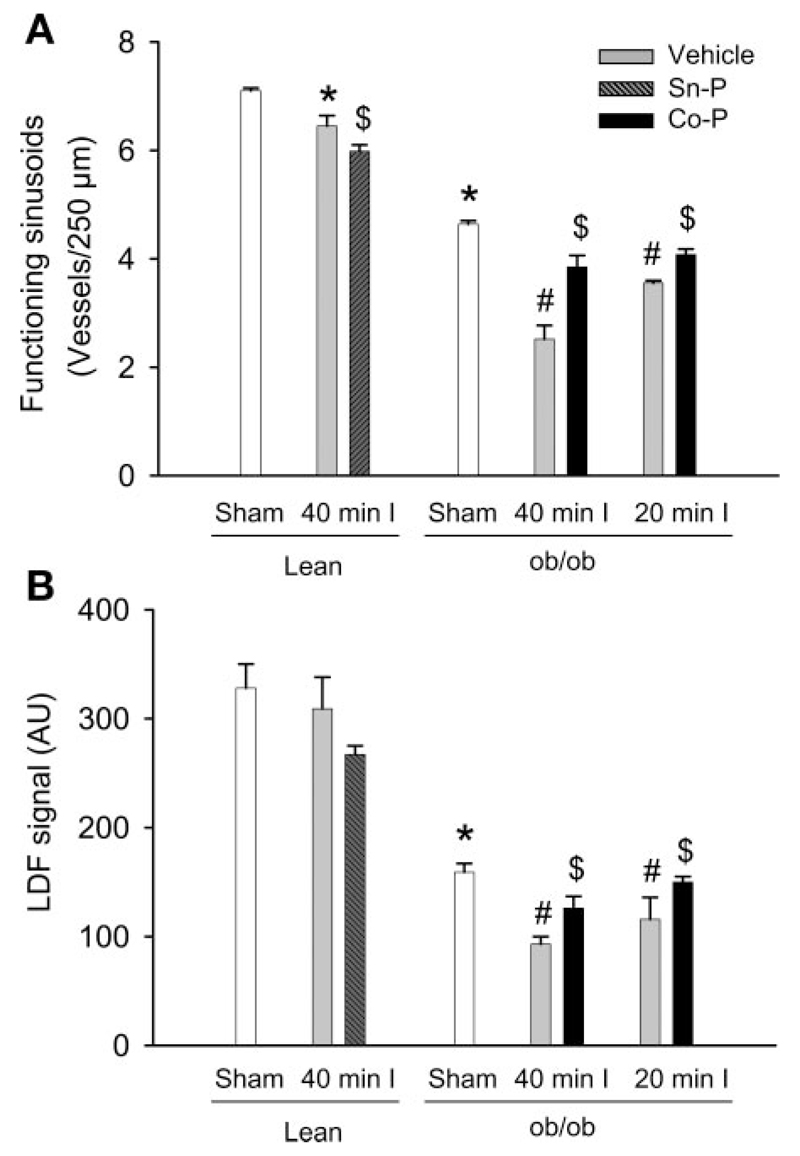
A high-resolution in vivo microscopic method was used to evaluate changes in the microcirculation during ischemia-reperfusion (36, 38). There was no significant difference in the diameter of hepatic sinusoids between ob/ob mice and lean littermates (data not shown). Almost all sinusoids contained blood flow in both sham-operated lean mice and ob/ob mice (97.3 and 97.0%, respectively) and were considered as functioning sinusoids. However, the number of functioning sinusoids in livers of ob/ob mice was significantly less (by 35%) than that of lean littermates because accumulated fat considerably enlarged hepatic cords (Fig. 8A). After 40 min of ischemia and 2 h of reperfusion, the number of functioning sinusoids significantly decreased by 9% in lean littermates and by 45% in ob/ob mice compared with the respective sham-operated controls (Fig. 8A). Total liver blood flow measured by laser Doppler flowmetry supported these findings (Fig. 8B). There was a 50% lower hepatic blood flow in ob/ob mice compared with lean litter-mates. Whereas no significant reduction in liver blood flow was observed in lean mice during reperfusion, the blood flow in ob/ob mice was further reduced by 42% (Fig. 8B). These data suggest that impaired liver blood flow may be a critical factor in the aggravation of liver injury during reperfusion in ob/ob mice. Since HO-1 may affect liver blood flow, we evaluated whether manipulation of HO-1 expression can modulate reperfusion injury in these animals.
Fig. 8.
Number of functioning sinusoids (A) and liver blood flow (B) in ob/ob mice or lean littermates. Blood flow (laser Doppler flowmetry, LDF) and the microcirculation (intravital microscopy) were evaluated in sham-operated animals and at 2 h of reperfusion after 40 or 20 min of ischemia. Some of the lean animals were treated with the heme oxygenase-1 (HO-1) inhibitor tin protoporphyrin (Sn-P), and some of the ob/ob mice were treated with the HO-1 inducer cobalt protoporphyrin (Co-P) before ischemia. Values represent means ± SE of 3–4 animals per group. *P < 0.05 (compared with lean sham-operated group), #P < 0.05 (compared with ob/ob sham-operated group), $P < 0.05 (compared with respective vehicle-treated groups).
Effect of HO-1 modulation on liver injury and neutrophil accumulation
1) Sn-P inhibits HO-1 activity in a competitive fashion (3). Treatment of lean littermates with Sn-P caused a minor reduction in the number of functioning sinusoids and a trend to a lower liver blood flow during reperfusion (Fig. 8, A and B). Sn-P significant aggravated liver injury (Fig. 9, A and B) but did not increase hepatic neutrophil accumulation (Fig. 9, C and D) after 40 min of ischemia and 6 h of reperfusion. On the other hand, in the absence of relevant reperfusion injury after 20 min of ischemia, Sn-P treatment did neither induce liver injury nor modulate neutrophil accumulation (Fig. 9, A–D). 2) Pretreatment with Co-P induces hepatic HO-1 expression in steatotic livers (1). In ob/ob mice, Co-P treatment resulted in a significant increase in the number of functioning sinusoids and improvement of liver blood flow at 2 h of reperfusion after 40 or 20 min of ischemia (Fig. 8, A and B). Treatment of Co-P significantly improved liver injury at 6 h of reperfusion after 20 min of ischemia but not after 40 min of ischemia (Fig. 10, A and B). However, hepatic neutrophil accumulation was not altered by Co-P after either ischemic period (Fig. 10, C and D).
Fig. 9.
Liver injury and hepatic neutrophils accumulation 6 h after reperfusion following 40 or 20 min of ischemia in lean littermates. Some of the animals were treated with the HO-1 inhibitor Sn-P or vehicle before ischemia. ALT activities were measured in plasma (A), and the area of necrosis was estimated in H&E stained tissue sections (B). Neutrophils were stained with the naphthol AS-D chloroacetate esterase method and counted in 20 HPFs. All neutrophils were counted irrespective of their location (total neutrophils, C), and neutrophils extravasated into the parenchyma were counted separately (D). Values are means ± SE of 4–5 animals. *P < 0.05 (compared with vehicle-treated groups).
Fig. 10.
Liver injury and hepatic neutrophil accumulation 6 h after reperfusion following 40 or 20 min of ischemia in ob/ob mice. Some of the animals were treated with the HO-1 inducer Co-P or vehicle before ischemia. ALT activities were measured in plasma (A), and the area of necrosis was estimated in H&E-stained tissue sections (B). Neutrophils were stained with the naphthol AS-D chloroacetate esterase method and counted in 20 HPFs. All neutrophils were counted irrespective of their location (total neutrophils, C), and neutrophils extravasated into the parenchyma were counted separately (D). Values are means ± SE of 4–5 animals. *P < 0.05 compared with vehicle-treated groups.
DISCUSSION
The main objectives of this investigation were 1) to evaluate hepatic neutrophil accumulation and a specific neutrophil-induced oxidant stress in a mouse model of hepatic ischemia-reperfusion injury, 2) to assess the inflammatory response in steatotic livers of ob/ob mice compared with lean littermates, and 3) to determine a potential contribution of microcirculatory disturbances to the postischemic liver injury in ob/ob mice. Our data clearly demonstrate that the time course of hepatic neutrophil infiltration and the occurrence of a neutrophil-derived oxidant stress in the mouse model of warm ischemia-reperfusion injury are very similar to the previously described sequence of events in rats (14). However, this inflammatory response appears significantly reduced in steatotic livers of ob/ob mice despite a drastic increase in reperfusion injury during the first 6 h. The aggravated injury in ob/ob mice correlated with a substantial reduction in overall liver blood flow and sinusoidal perfusion, suggesting ischemic necrosis as the main mechanism of reperfusion injury in these mice.
Neutrophil response in a mouse model of ischemia-reperfusion injury
Despite the increased use of mouse models of hepatic warm ischemia-reperfusion injury during the last 10 years, the question when neutrophils accumulate in the postischemic liver and when these phagocytes actually contribute to the postischemic oxidant stress has been controversially discussed (9, 13, 15, 30, 41). Lentsch and coworkers (9, 30, 41) provided evidence for hepatic neutrophil infiltration at 6–8 h of reperfusion and further found that these neutrophils are activated and generate increased amounts of reactive oxygen species (41), similar to previous findings in the rat model (19, 20). On the other hand, there are reports showing no neutrophil infiltration during the first 6 h of reperfusion and no evidence of a neutrophil-induced oxidant stress (13, 15). Our data, which relied on the identification of neutrophils in tissue sections based on characteristic morphology as well as specific staining, demonstrated that these cells started to accumulate in sinusoids within the first hour of reperfusion. The neutrophil recruitment into the liver continued during the entire 24-h reperfusion period. On the other hand, neutrophil extravasation into the parenchyma began around 6 h and continued during the remaining reperfusion time. In addition, a neutrophil-specific oxidant stress, identified by the occurrence of HOCl-modified epitopes in the liver, correlated with the extravasation of neutrophils between 6 and 24 h of reperfusion. In contrast, protein-bound MDA levels were increased during the entire reperfusion period. This suggests that this marker of a general oxidant stress reflects more the activity of Kupffer cells at least during the early reperfusion period (13, 19, 21). Thus our data demonstrate that, in the mouse model of warm ischemia-reperfusion injury, neutrophil accumulation occurs during the entire reperfusion period but the neutrophil contribution to the postischemic oxidant stress occurs mainly after 6 h of reperfusion. Since these data are very similar to the time course of events described in a rat model of warm ischemia-reperfusion injury (14, 19, 21), one can conclude that there appears to be no significant difference in the postischemic neutrophil-derived inflammatory response between rat and mouse models. However, in contrast to the rat model (23, 24, 31), direct proof for a neutrophil-mediated injury mechanism is lacking in the mouse model. Nevertheless, substantial indirect evidence, mainly based on the neutralization of mediators known to activate neutrophils, supports the hypothesis that neutrophils also contribute to the pathogenesis in the mouse model after 6 h of reperfusion (42).
Inflammatory response in steatotic livers of ob/ob mice
Zucker rats and ob/ob mice are genetic models of obesity and steatosis. Both genetic models are frequently used to study mechanisms of reperfusion injury in steatotic livers (28, 46–49, 51). However, the role of neutrophils in the pathophysiology of ischemia-reperfusion injury is not well defined (17). Our data with the ob/ob mice, which have a very severe steatosis, confirmed previous findings that animals with steatotic livers suffer from a much more severe reperfusion injury compared with lean animals (16, 28, 44, 46, 47, 51, 52). However, despite the massive aggravation of liver injury, the number of neutrophils accumulating in the postischemic liver was substantially lower compared with the lean littermates. This finding correlated with the reduced expression of a number of proinflammatory genes including cytokines and chemokines, which are thought to be involved in hepatic neutrophil recruitment (5, 30). Because these events occurred during the first 6 h of reperfusion, i.e., before neutrophils actually contribute to reperfusion injury even in lean mice, we conclude that this aggravation of reperfusion injury in steatotic livers of ob/ob mice was not caused by an enhanced neutrophil response. In fact, since 44% of ob/ob animals died after less than 6 h of reperfusion (40 min ischemia) and none of the animals survived longer than 10 h, neutrophils may not contribute at all to the severe reperfusion injury in these animals.
Microcirculatory disturbances in steatotic livers of ob/ob mice
Evaluation of liver blood flow and functional sinusoidal density demonstrated that ob/ob mice show impaired liver blood supply even before ischemia. Whereas a moderate ischemia time of 40 min did not significantly affect postischemic liver blood flow in lean mice, it substantially further impaired these parameters in ob/ob mice. These findings are consistent with previous reports in Zucker rats, ob/ob mice, or animals with a diet-induced steatosis showing disturbances of the hepatic microcirculation during reperfusion (44, 47, 51, 52). The combination of a reduced inflammatory response with the severe impairment of liver blood flow in ob/ob mice suggests that microcirculatory problems resulting in ischemic necrosis are the dominant mechanisms of reperfusion injury under these conditions. Since there was no induction of HO-1, which could have produced the vasodilator carbon monoxide (50) in ob/ob mice during reperfusion, we hypothesized that this could have been the reason for the reduced liver blood flow. Consistent with this hypothesis is the well-established fact that there is an imbalance between vasodilator and vasoconstrictor responses in the postischemic liver (10). Using a HO-1 inhibitor in lean mice, we could mimic the increased reperfusion injury in ob/ob mice with no changes of neutrophil accumulation. However, the changes in liver blood flow in response to Sn-P treatment were very minor, suggesting that HO-1 inhibition did not aggravate reperfusion injury by impairing liver blood flow. Since carbon monoxide and biliverdin/bilirubin were shown to protect against a variety of insults by directly interfering with intracellular signaling mechanisms of cell death (2, 6, 11, 26, 43), this seems to be the more likely explanation for the increased injury in Sn-P-treated lean animals. On the other hand, induction of HO-1 with Co-P before ischemia (1, 11, 26, 43) partially restored liver blood flow toward values in sham-operated animals but had an effect neither on reperfusion injury nor on the inflammatory response after 40 min of ischemia and only a minor protective effect after 20 min of ischemia. This confirms the effect on the impaired microcirculation as the main mechanism of injury in ob/ob mice. Since HO-1 induction had only a moderate effect on the microcirculatory dys-function and reperfusion injury, one can conclude that in ob/ob mice the reduced hepatic blood flow seems to be less a vasoconstrictor issue but may be more related to the mechanical obstruction due to excessive fat accumulation in hepatocytes. Alternatively, ob/ob mice could be more susceptible to the activation of the coagulation cascade, which also may contribute to the impairment of blood flow.
In summary, we demonstrated a progressive neutrophil accumulation in sinusoids during the entire reperfusion period and extravasation and a neutrophil-specific oxidant stress after 6 h of reperfusion in lean mice. In contrast, the inflammatory response in steatotic livers of ob/ob mice was attenuated despite the massive aggravation of reperfusion injury in these animals within 6 h after ischemia. The increased injury in ob/ob mice compared with lean littermates is correlated with the severe impairment of liver blood flow. Since these microcirculatory problems in livers of ob/ob mice appear to be caused mainly by mechanical obstruction due to the excessive fat accumulation and only to a lesser degree by increased vasoconstriction, the only way to reduce reperfusion injury in these animals is to limit the time of ischemia. These results in ob/ob mice are different compared with reports in Zucker rats (1) or mice fed a choline-methionine-deficient diet (47) or the Lieber DeCarli diet (unpublished observations). Although more detailed studies on the mechanisms of reperfusion injury in steatotic livers are necessary, our data suggest that mechanisms obtained with specific models may not be applicable to all models of steatosis and the relevance of the model for the human pathophysiology needs to be carefully assessed before therapeutic recommendations can be considered.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
GRANTS
This work has been supported in part by National Institutes of Health (NIH) Grants AA-12916 (H. Jaeschke), DK-070195 (H. Jaeschke), and AA-12436 (R. S. McCuskey); the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (J. Liu); and the Austrian Science Fund (FWF P17013-B05) (E. Malle).
References
- 1.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, Kupiec-Weglinski JW. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KE, Simionatto CS, Drummond GS, Kappas A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J Pharmacol Exp Ther. 1984;228:327–333. [PubMed] [Google Scholar]
- 4.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 5.Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Bauer I. Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 2002;4:749–758. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- 7.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig F, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 8.Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508–515. doi: 10.1016/s0168-8278(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G969–G976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- 10.Clemens MG, Bauer M, Pannen BH, Bauer I, Zhang JX. Remodeling of hepatic microvascular responsiveness after ischemia/reperfusion. Shock. 1997;8:80–85. doi: 10.1097/00024382-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dorman RB, Bajt ML, Farhood A, Mayes J, Jaeschke H. Heme oxygenase-1 induction in hepatocytes and non-parenchymal cells protects against liver injury during endotoxemia. Comp Hepatol. 2004;3(Suppl 1):S42. doi: 10.1186/1476-5926-2-S1-S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57Bl/6J-lpr mice after bile duct ligation. Hepatology. 2004;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- 13.Harada H, Hines IN, Flores S, Gao B, McCord J, Scheerens H, Grisham MB. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch Biochem Biophys. 2004;423:103–108. doi: 10.1016/j.abb.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hines IN, Harada H, Bharwani S, Pavlick KP, Hoffman JM, Grisham MB. Enhanced post-ischemic liver injury in iNOS-deficient mice: a cautionary note. Biochem Biophys Res Commun. 2001;284:972–976. doi: 10.1006/bbrc.2001.5069. [DOI] [PubMed] [Google Scholar]
- 16.Hui AM, Kawasaki S, Makuuchi M, Nakamura J, Ikegami T, Miyagawa S. Liver injury following normothermic ischemia in steatotic rat liver. Hepatology. 1994;20:1287–1293. doi: 10.1002/hep.1840200528. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–382. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol Gastrointest Liver Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 22.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol Gastrointest Liver Physiol. 1993;264:G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17:915–923. [PubMed] [Google Scholar]
- 24.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 25.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–128. [PubMed] [Google Scholar]
- 27.Koneru B, Dikdan G. Hepatic steatosis and liver transplantation current clinical and experimental perspectives. Transplantation. 2002;73:325–330. doi: 10.1097/00007890-200202150-00001. [DOI] [PubMed] [Google Scholar]
- 28.Koneru B, Reddy MC, dela Torre AN, Patel D, Ippolito T, Ferrante RJ. Studies of hepatic warm ischemia in the obese Zucker rat. Transplantation. 1995;59:942–946. doi: 10.1097/00007890-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 30.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 31.Liu P, McGuire GM, Fisher MA, Farhood A, Smith CW, Jaeschke H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62. [PubMed] [Google Scholar]
- 32.Liu W, Saint DA. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal Biochem. 2002;302:52–59. doi: 10.1006/abio.2001.5530. [DOI] [PubMed] [Google Scholar]
- 33.Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- 34.Malle E, Woenckhaus C, Waeg G, Esterbauer H, Grone EF, Grone HJ. Immunological evidence for hypochlorite-modified proteins in human kidney. Am J Pathol. 1997;150:603–615. [PMC free article] [PubMed] [Google Scholar]
- 35.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Poyayko MK, Hay JE, Gores GJ, Krom RAF. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246–1251. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]
- 36.McCuskey RS. Physical Techniques in Biology and Medicine Microvascular Technology. New York: Academic; 1986. Microscopic methods for studying the microvasculature of internal organs; pp. 247–264. [Google Scholar]
- 37.McCuskey RS. Hepatic and splanchnic microvascular responses to inflammation and shock. Hepatogastroenterology. 1999;46(Suppl 2):1464–1467. [PubMed] [Google Scholar]
- 38.McCuskey RS, Ito Y, Robertson GR, McCuskey MK, Perry M, Farrell GC. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386–393. doi: 10.1002/hep.20302. [DOI] [PubMed] [Google Scholar]
- 39.Menger MD, Vollmar B. Role of microcirculation in transplantation. Microcirculation. 2000;7:291–306. [PubMed] [Google Scholar]
- 40.Nakano H, Nagasaki H, Barama A, Boudjema K, Jaeck D, Kumada K, Tatsuno M, Baek Y, Kitamura N, Suzuki T, Yamaguchi M. The effect of N-acetylcysteine and anti-intercellular adhesion molecule-1 monoclonal antibody against ischemia-reperfusion injury of the rat steatotic liver produced by a choline-methionine-deficient diet. Hepatology. 1997;26:670–678. doi: 10.1053/jhep.1997.v26.pm0009303498. [DOI] [PubMed] [Google Scholar]
- 41.Okaya T, Blanchard J, Schuster R, Kuboki S, Husted T, Caldwell CC, Zingarelli B, Wong H, Solomkin JS, Lentsch AB. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock. 2005;24:421–427. doi: 10.1097/01.shk.0000181282.14050.11. [DOI] [PubMed] [Google Scholar]
- 42.Okaya T, Lentsch AB. Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J Invest Surg. 2003;16:141–147. [PubMed] [Google Scholar]
- 43.Sass G, Soares MC, Yamashita K, Seyfried S, Zimmermann WH, Eschenhagen T, Kaczmarek E, Ritter T, Volk HD, Tiegs G. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003;38:909–918. doi: 10.1053/jhep.2003.50386. [DOI] [PubMed] [Google Scholar]
- 44.Seifalian AM, Piasecki C, Agarwal A, Davidson BR. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999;68:780–784. doi: 10.1097/00007890-199909270-00009. [DOI] [PubMed] [Google Scholar]
- 45.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 46.Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 47.Selzner N, Selzner M, Jochum W, Amann-Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol. 2006;44:694–701. doi: 10.1016/j.jhep.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 48.Serafín A, Roselló-Catafau J, Prats N, Xaus C, Gelpí E, Peralta C. Ischemic preconditioning increases the tolerance of fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587–601. doi: 10.1016/S0002-9440(10)64214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serafín A, Roselló-Catafau J, Prats N, Gelpí E, Rodés J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia-reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- 50.Suematsu M, Wakabayashi Y, Ishimura Y. Gaseous monoxide: a new class of microvascular regulator in the liver. Cardiovasc Res. 1996;32:679–686. [PubMed] [Google Scholar]
- 51.Sun CK, Zhang XY, Zimmermann A, Davis G, Wheatley AM. Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of the Zucker rat. Transplantation. 2001;72:1625–1631. doi: 10.1097/00007890-200111270-00008. [DOI] [PubMed] [Google Scholar]
- 52.Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076–1082. doi: 10.1097/00007890-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4+ T-lymphocytes mediate ischemia/reperfusion induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]