Abstract
Drug delivery to the brain is severely restricted by formation of tight junctions between adjacent brain capillary endothelial cells (BCEC). In the present study we have evaluated the effects of protamine–oligonucleotide nanoparticles (proticles) on the functional properties of primary porcine BCEC and characterized uptake and transcytosis of proticles by these cells. Proticles had no adverse effects on BCEC properties relevant to blood–brain barrier (BBB) function. Transcytosis of 125I-labeled proticles across polarized BCEC cultures occurred in a time- and concentration-dependent manner. As apolipoproteins were suggested to enhance cellular proticle uptake, proticle coating was performed with apoA-I, the major apolipoprotein component of high density lipoproteins. Adsorption of apoA-I on the surface of proticles resulted in significantly improved uptake and transcytosis properties as compared to uncoated proticles. ApoA-I coating enhanced proticle delivery to astrocytes in an in vitro model of the BBB almost twofold. Blocking of scavenger receptor class B, type I (the prime receptor for high density lipoprotein/apoA-I that is expressed on BCEC) reduced transcytosis of apoA-I-coated proticles to levels observed for uncoated proticles. Our data indicate that apoA-I-coating of proticles could be a feasible targeting technology to improve delivery across the BBB.
Keywords: HDL, SR-BI, Proticles
1. Introduction
Drug delivery to the brain is severely hampered due to the exceptionally low permeability of the blood–brain barrier (BBB). Brain capillary endothelial cells (BCEC) form the physical basis of the BBB and are connected by tight junctions that are responsible for the highly selective permeability properties of the cerebrovasculature [1,2]. Although this is necessary to maintain the finely tuned microenvironment of the central nervous system (CNS) it poses a major obstacle on the efficient delivery of potential neuropharmaceuticals to the brain. Consequently only a limited number of drugs, i.e. small, lipophilic compounds with a molecular mass <400–500 Da, cross the endothelial cell layer of the BBB and, unfortunately, only a small number of CNS diseases respond to this compound class [3]. In the human brain the total length of brain capillaries is estimated to be in the range of 400 miles and the surface area of the brain endothelium is approximately 20 m2 [4]. Thus the cerebrovasculature would provide a huge delivery platform for transendothelial administration of drugs to the CNS; however, this would require that the restrictive permeability properties of the BBB could be overcome. One promising approach to compensate permeability restrictions is the application of targeted release systems that are recognized by a BBB-located receptor that mediates transcytosis (in the luminal-to-abluminal direction). This is achieved by certain peptidomimetic antibodies that recognize epitopes on transcytotic BBB receptors that are different from the ligand-binding domain (e.g. against the insulin or the transferrin receptor). Coupling of these antibodies to large-molecule drugs facilitates transcytosis via the corresponding, antibody-targeted receptor. This technology was used in several successful approaches in different sandwich constructions, including nanoparticles and immunoliposomes [5].
Next to other colloidal drug delivery vehicles, nanoparticles appear to be a promising class of carriers that enable transport of drugs (that are not transported across the cerebrovasculature after intravenous injection) across the BBB. A number of technologies to improve the transcytotic properties of biodegradable nanoparticles with different chemical composition across the BBB have been published (for review see [6–8]). Among the nanoparticle formulations that have been successfully used to deliver therapeutically useful compounds are protamine–oligonucleotide nanoparticles, so called proticles. These nanoparticles consist of protamine, which is a relatively small polycationic peptide with a molecular weight of approximately 4000 Da. In the sperm of salmon, protamine condenses DNA and delivers it to the nucleus of the egg after fertilization. This property of protamine has been exploited to condense plasmid DNA into compact particles. In combination with antisense oligonucleotides, proticles increased particle stability against nuclease activity, and improved cellular uptake of antisense molecules [9]. Proticles have several properties that make them potential candidates for drug delivery: i) The production is simple and rapid and is based on self-assembly of charged macromolecules [9]. ii) Proticles display extremely low (cyto)toxicity in vitro as compared to other, non-viral transfection vehicles such as cationic liposomes [10]. Nanoparticle coating with apolipoproteins (representing the protein moiety of circulating lipoproteins) is an experimental approach to improve uptake efficacy across the BBB. This was demonstrated for dalargin- or loperamide-loaded nanoparticles after coating with apolipoproteins: Only apolipoprotein (apo)B- or apoE-containing particles (but not their apoA-II-, apoC-II-, or apoJ-containing counterparts) were able to achieve an anti-nociceptive effect in mice as measured by the tail-flick test [11]. These results were interpreted in that way that apoB- and apoE-coated nanoparticles mimic lipoprotein particles that could be taken up by BCEC via low-density lipoprotein (LDL or apoB/E)-receptor-mediated endocytosis [11]. Indeed the LDL-receptor is expressed on the apical membrane and mediates transcytosis of LDL across BCEC monolayers [12].
We have previously demonstrated that scavenger receptor class B, type I (SR-BI) the prime receptor for high density lipoprotein (HDL) is also expressed at the apical membrane of primary porcine BCEC [13]. SR-BI facilitates uptake of HDL-associated cholesteryl ester and Vitamin E across BCEC in vitro [13] and in vivo [14]. Although SR-BI is a multiligand receptor, apoA-I (the major apolipoprotein of HDL) is a high affinity ligand. Based on these findings the aim of this study was to investigate the uptake and transcytosis properties of apoA-I-coated protamine–oligonucleotide nanoparticles in an in vitro model of the BBB.
2. Materials and methods
2.1. Materials
Earl’s medium M199 was obtained from Gibco (Vienna, Austria). Penicillin/streptomycin (P/S), gentamycin, and trypsin were from Biochrom (Berlin, Germany). Ox serum was from PAA Laboratories (Linz, Austria). Plastic ware for cell culture was from Costar (Vienna). PD-10 size-exclusion columns, and ECL+ Plus Western Blotting Detection Reagents were from Amersham Pharmacia Biotech Ltd. (Vienna). Polyvinylidene Fluoride transfer membrane (BioTrace™ PVDF) was from Pall Corporation (Vienna). Microcon centrifugal filter units were from Millipore (Vienna). Oligonucleotides were obtained from Biospring GmbH (Frankfurt, Germany). The sequence 5′ ACG TTG GTC CTG CGG GAA 3′ was found to efficiently assemble proticles by complexation with protamine free base (Sigma, Vienna). 125INa was from New England Nuclear (Vienna; specific activity 629 GBq/mmol). The MTT test kit was from Sigma (Vienna). The monoclonal anti-vascular endothelial (VE)-cadherin antibody (raised against a C-terminal peptide of human VE-cadherin) used for immunofluorescence studies of BCEC was from Santa Cruz (Santa Cruz, CA; sc9989). Polyclonal anti-human zonula occludens (ZO)-1 was from Zymed (Vienna), polyclonal anti-SR-BI was from Abcam (Cambridge, UK), and cyanine (Cy)-3-labeled secondary antibodies were from Jackson Dianova (Hamburg, Germany). All other chemicals were from Sigma.
2.2. Methods
2.2.1. Preparation and characterization of proticles
Nanoparticles were prepared in aqueous solution by mixing the indicated mass ratios of oligonucleotides (ON) and protamine to achieve final concentrations of 100 μg/ml ON and 200, 300 or 500 μg/ml protamine in MilliQ water (Millipore, Vienna). The proticles were formed by self-assembly of the compounds within the first few minutes of incubation. Reproducibility of proticle formation was ascertained by incubation of proticle solutions for 1 h at room temperature on an orbital shaker (300 rpm) [9]. To obtain apoA-I-coated proticles, preassembled proticles were incubated in the presence of the indicated amounts of apoA-I (isolated as described below) for 1 h at room temperature on an orbital shaker.
The particle size (hydrodynamic diameter) and size distribution (polydispersity index) of the primary complex between ON and protamine as well as the size of apoA-I-coated proticles were determined by dynamic light scattering. The surface charge (zeta-potential) was determined by measuring their electrophoretic mobility. Both measurements were performed on a Zetasizer HSA 3000 (Malvern, Herrenberg, Germany). Data evaluation was performed as described [15].
To determine the binding capacity of Cy-3-labeled apoA-I and its effect on particle size, proticles were incubated with various amounts of Cy-3-labeled apoA-I (0–50 μg apoA-I/10 μg ON). Proticles were centrifuged at 20,000×g at 4 °C for 2 h (Eppendorf centrifuge 5804 R). The amount of Cy-3-labeled apoA-I in the supernatant was determined using a fluorescence plate reader (544/590 nm; Fluostar Galaxy, BMG Labtechnologies) and the amount of bound apoA-I was calculated by the difference between fluorescence intensities before and after addition of proticles.
2.2.2. Radio- and fluorescence-labeling of proticle preparations
For radioactive labeling, NaHCO3 was added to 300 μg ON in 500 μl aqueous solution to get a final concentration of 170 mM. This solution was incubated with 10 μl N-Br-succinimide (1 mg/ml) and labeled with 500 μCi of 125INa [16], mixed and incubated at ambient temperature for 5 min. Radiolabeled ON were transferred to a Slide-A-Lyzer®Dialysis Cassette from Pierce with 3500 Da cutoff and dialyzed against desalted H2O (3 days). To determine the free, not ON-associated radioactivity, 100 μl ON were centrifuged in microcon-filtration tube at 13,000 rpm for 20 min (Millipore, USA; cutoff 3000 Da). The flow through was diluted (1:1000) and counted on a γ-counter. When free radioactivity reached a value less than 5% of total radioactivity, labeled ON were used for proticle formation as described above. Cy-3-labeling of apoA-I was performed with the FluoroLink MAb™ Cy3™ Labeling Kit (Amersham Life Science). The 18-mer oligonucleotide 5′-ACG TTG GTC CTG CGG GGA-3′ was labeled with Cy-5 at the 5′-end (custom synthesis by Biospring, Frankfurt, Germany). Protamine free base was labeled with tetramethylrhodamine–isothiocyanate and purified by dialysis using Slide-A-Lyzer cassettes (cutoff 3.5 kDa) [15].
2.2.3. Isolation of human HDL and apoA-I
Human, apoE-poor HDL (d=1.125–1.21 g/ml) was prepared by discontinuous density ultracentrifugation of plasma from normolipidemic donors [17]. HDL was recovered from the tubes and desalted on PD-10 columns. The protein content was determined according to Lowry [18]. Delipidation of HDL was performed as described [19]. Delipidated apolipoproteins were redissolved in 50 mM glycine, 4 mM NaOH, 0.5 M NaCl, and 6 M urea (pH 8.8). HDL apolipoproteins were then separated by size exclusion chromatography on a Sephacryl S-200 column (3 × 150 cm) [19]. The fractions containing the major peak were pooled and dialyzed against 50 mM NH4HCO3. The product was homogenous as assessed by SDS–PAGE, reversed phase HPLC and MS analysis.
2.2.4. ApoA-I characterization
2.2.4.1. Western blotting
ApoA-I samples were run on SDS–PAGE gels (12%) for 90 min at 150 V in a Bio-Rad Miniprotean chamber (Bio-Rad, Vienna) and proteins were electrophoretically transferred to membranes (150 mA, 4 °C, 90 min). ApoA-I was detected with a polyclonal rabbit anti-human apoA-I antiserum, followed by a horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma). Detection was performed using the ECL method.
2.2.4.2. HPLC separation and ESI–MS of apoA-I
About 5 μg of apoA-I were separated by RP–HPLC (Waters Alliance 2690 separation module) on a Vydac C 18 column [20]. MS analysis was performed on a LC–MS combination consisting of a Flux Rheos 4000 binary gradient pump (Munich, Germany), an ERC vacuum degasser (Spectronex, Vienna), a Vydac C18 column (4.6 × 150 mm), and a Finnigan LCQ ion trap mass spectrometer equipped with an electrospray source (San Jose, CA, USA). The settings of the ion source were: spray voltage 3.5 kV, capillary temperature 220 °C, capillary voltage 13.6 V and a tube lens offset of 30 V [21]. N2 was used as sheath gas at a flow rate of 100 units. Samples were injected in the moving solvent (10 μl/min; H2O/acetonitrile, 1:1 (v/v), 0.05% TFA).
2.2.5. Isolation and culture of BCEC
Porcine brains were obtained from freshly slaughtered pigs. After removal of the meninges and secretory areas the gray and white matter of the brain cortex was minced. BCEC were isolated by enzymatic digestion and subsequent centrifugation steps as described [22]. Cells from one brain were seeded in six to eight collagen-coated 75 cm2 flasks (M199 containing 10% ox serum, 1% gentamycin, and 1% P/S). On the following day cells were washed with phosphate-buffered saline (PBS), and gentamycin-free medium was added. After 3–7 days in vitro, cells were washed, trypsinized, and seeded on collagen-coated 12-well cell culture plates at a density of 40,000 cells/cm2 in M199 (containing 10% ox serum, 1% P/S) for monolayer experiments.
2.2.6. Transwell cultures
Cells were seeded on calf-skin collagen (12 μg in 100 μl PBS; aspirated after 5 min) coated 12-well Transwell culture trays at a density of 40,000 cells/cm2 [23]. The medium (0.5 ml in the apical and 1.5 ml in the basolateral compartment) used to induce the formation of tight monolayers was added after 2 days, and consisted of DMEM/Ham's F12 supplemented with 150 nM hydrocortisone, 1% P/S, and 0.25% glutamine. After 3 days in culture, before starting the experiment, the tightness of the monolayer was assessed by measuring the transendothelial electrical resistance (TEER) using an Endohm tissue resistance measurement chamber. TEERs of coated, cell-free filters were subtracted from Transwell TEER to obtain the resistance of the cell monolayers. Results are expressed in Ω cm2 or in % of controls. Only wells with TEER higher than 300 Ω cm2 were used. [14C]Sucrose permeability was used as a second parameter to characterize the tightness of the monolayers. 200 μl of the medium in the apical compartment was removed and substituted with assay medium containing 2.5 μCi [14C] sucrose. 50 μl samples were taken in duplicate from the basolateral compartment every 20 min (over 2 h) and replaced by 100 μl fresh medium. Permeability coefficients (Pe) were calculated as described [24].
2.2.7. BCEC–astrocyte cocultures
To determine whether proticles that underwent transcytosis are subjected to concomitant uptake by astrocytes, cocultures were established. BCEC were isolated as described above, plated on Transwells and assembled with a human astrocytic cell line CCF–STTG (ATCC, Rockville, MD), that were cultured separately in monolayers in RPMI 1640 medium (containing 1% P/S, 10% FCS). When BCEC reached TEER values of 200 Ω cm2 and astrocytes were almost confluent, cocultures were assembled (Transwell system). Cocultures were incubated in M199 (containing 10% ox serum, 1% P/S (v/v)) for 1 day, then M199 medium was replaced by DMEM/Ham’s F12 medium supplemented with 150 nM hydrocortisone, 1% P/S, and 0.25% glutamine.
2.2.8. Uptake and transcytosis experiments
For uptake experiments BCEC were cultured on monolayer 6-well trays in medium M199 (containing 10% ox serum, 1% P/S (v/v)). At confluency, cells were loaded with radiolabeled proticles (200 μl/2 ml medium) that were either apoA-I-coated (5 μg apoA-I/10 μg ON) or uncoated. A 1:3 mass ratio between ON and protamine was chosen for these preparations. After the indicated time periods cells were washed twice with washing buffer and cell lysis was performed with 0.3 M NaOH at 4 °C for 1 h under shaking. Cellular lysates were collected in counter vials and cell-associated radioactivity was counted. Cell protein content was measured using the Lowry method [18]. For transcytosis experiments, proticles were prepared in the same way as described above. Cells were plated on collagen-coated (12 μg/100 μl) filter inserts of Transwells and cultured in M199 (containing 10% ox serum, 1% P/S (v/v)) for three days as described under isolation and culture of BCEC. Radiolabeled proticles (see below) containing various mass ratios (ON: protamine 1:2, 1:3 or 1:5) with or without apoA-I coating were added to the apical medium (corresponding to the luminal, ‘blood side’ of the brain in this system). Time-dependent transcytosis (1, 2 and 4 h) was investigated by collecting 200 μl of the medium from the basolateral side (corresponding to the ‘brain side’) and counting the radioactivity. NaOH lysis (0.3 M) was performed at 4 °C for 4 h and cell-associated radioactivity was measured.
2.2.9. Immunofluorescence studies
BCECs were cultured on collagen-coated (12 μg/100 μl) Permanox chamber slides. The cells were incubated with proticle preparations (mass ratio of 1:3; Cy-5-ON/protamine; absence or presence of 5 μg apoA-I/10 μg Cy-5-ON) for 4 h. Coverslips were washed twice in PBS and dried for 2 h, fixed in acetone for 5 min at 25 °C, rehydrated in PBS for 5 min, and treated with protein blocking solution for 20 min. Coverslips were incubated with primary antibodies (anti-ZO-1, 1:50; mouse anti-VE-cadherin, 1:200) for 30 min. Coverslips were rinsed with PBS and incubated with Cy-3-conjugated secondary antibodies (1:300) for 30 min, rinsed again and mounted with Moviol. Confocal laser scanning microscopy was performed on a Leitz/Leica TCSSP2 microscope (Leica Lasertechnik GmbH, Heidelberg, Germany).
2.2.10. MTT test
To test effects of proticles on BCEC viability the MTT system was used. Briefly, 50 μl of the MTT solution were added to cells (in 500 μl medium), incubated for 1 h at 37 °C under standard conditions. Monolayers were washed with PBS, and cell lysis was performed with 300 μl of the lysis solution (propan-2-ol/HCl (0.4 M), 25:1, v/v). The lysates were transferred to Eppendorf tubes and centrifuged to remove cellular debris (13,000 rpm, 2 min). 100 μl of the supernatant were transferred to 96-well microtiter plates and absorbance was measured at 570 nm and corrected for background absorption (650 nm).
2.2.11. Statistical analysis
All data are expressed as mean±S.D. Data sets were compared using Student’s t-test and differences were considered as significant at p<0.05.
3. Results
3.1. Characterization of proticles
Photon correlation spectroscopy measurements (hydrodynamic diameter) of uncoated proticles (ON/protamine ratio 1:2, 1:3 and 1:5, respectively) showed a mean particle size between 120 and 150 nm. Zeta-potential (surface charge) determination lead to values of 10, 13 and 20 mV for mass ratios of 1:2, 1:3 and 1:5, which is due to the increasing protamine concentration within the formulations.
3.2. Effects of proticles on junctional morphology, barrier function and viability of BCEC
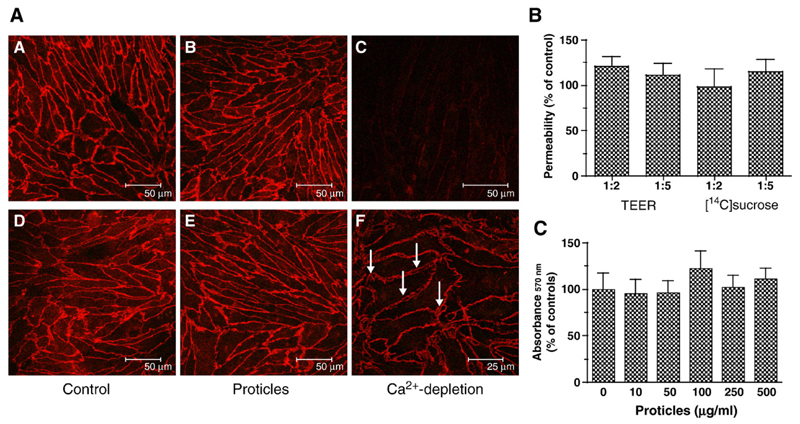
One of the cardinal features of BCEC is the formation of tight and adherens junctions that restrict the permeability of metabolites in the apical-to-basolateral direction. In a first set of experiments we have assessed the effects of proticles on the intactness of cellular junctions (ZO-1 was used as a tight junction, and VE-cadherin as an adherens junction marker), TEER, [14C]sucrose permeability, and cell viability (Fig. 1). Immunofluorescence studies revealed that proticles (100 μg ON/ml medium) had no adverse effects on the cellular localization of ZO-1 and VE-cadherin, indicating that proticles do not display adverse effects on the junctional architecture of BCEC (Fig. 1A). Depletion of extracellular Ca2+ (known to disrupt endothelial barrier function) resulted in pronounced gap formation between adjacent BCEC but did not alter cellular localization of ZO-1. In contrast, Ca2+-depletion resulted in complete loss of VE-cadherin staining at the plasma membrane (Fig. 1A), findings in line with others [25].
Fig. 1.
Effects of proticles on tight and adherens junctions, transendothelial electrical resistance (TEER) and sucrose permeability, and viability of BCEC. (A) BCEC were cultured on collagen-coated chamber slides as described in Materials and methods, incubated (4 h) in the presence of proticles (2.5 μg ON, ON/protamine mass ratio of 1:5) and processed for immunocytochemistry. Labeling of ZO-1 (A–C) was performed with anti-human ZO-1 antiserum (1:50) followed by Cy-3-labeled goat anti-rabbit IgG (1:300). Immunostaining of VE-cadherin (D–F) was performed with a mouse monoclonal antibody (1:200) and a Cy-3-labeled goat anti-mouse IgG (1:300). Tight junctions were disrupted by Ca2+-depletion in serum-free medium containing EDTA (2.5 mM; arrow in F indicates gap formation between adjacent cells). (B) BCEC were cultured in 12-well Transwell clusters in the presence of proticles (2.5 μg ON/ml; ON/protamine mass ratio of 1:2 and 1:5; 4 h). TEER was measured with an Endohm electrode, [14C]sucrose permeability was assessed as described in Materials and methods. Results are mean±S.D. (n = 4). TEER of controls was 590 ± 125 Ω cm2, [14C]sucrose permeability of control cultures was 1.2 ×10−6 cm/s. (C) BCEC were incubated in the presence of the indicated proticle concentration (ON/protamine mass ratio of 1:5, 4 h), washed, and 50 μl of the MTT solution was added to cells in 500 μl medium. After 1 h, the cells were washed, lysed, and processed for measurement as described in Materials and methods. Results are mean±S.D. (n = 3).
Quantitation of BCEC monolayer permeability in the absence or presence of proticles was performed by measuring the TEER and [14C]sucrose permeability. To test the influence of proticles on barrier function, BCEC were incubated in Transwell cultures exposed to proticles consisting of ON and protamine. TEER values observed in Transwell cultures exposed to proticles (ON/protamine ratio 1:2 and 1:5, respectively) showed slightly (but statistically not significant) enhanced resistance values (approximately 1.2-fold) over control cultures. [14C]Sucrose permeability of Transwell cultures was not affected by the presence of nanoparticles (Fig. 1B).
Next, effects of proticles on cell viability were evaluated using the MTT test. During these experiments it became apparent that ON/protamine proticles over a wide concentration range (10–500 μg ON/ml medium; mass ratio of 1:5) had no adverse effects on BCEC cell viability (Fig. 1C). Thus the experiments described above demonstrate that proticles (under the experimental conditions applied) have no detrimental effects on BCEC properties.
3.3. Uptake and transcytosis of proticles
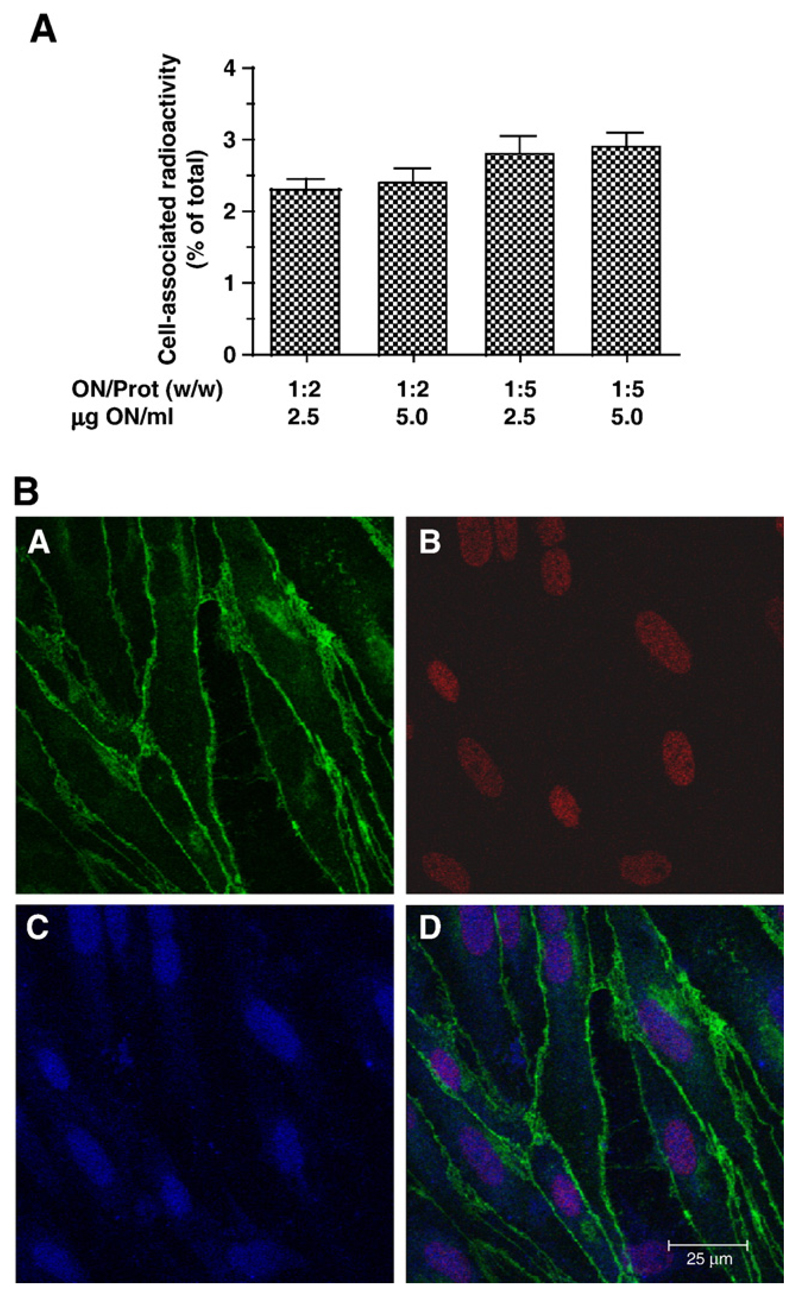
The next set of experiments aimed to clarify uptake of 125I-labeled proticles in BCEC monolayers and transcytosis rates in Transwell cultures and to analyze the intracellular distribution of fluorescently labeled proticles. Uptake experiments in conventional BCEC monolayer cultures indicated that the ON/protamine ratio was a determining factor for uptake efficacy: The uptake of proticles with a higher ON/protamine mass ratio (1:5) was on average 1.2-fold higher than proticles with lower protamine content (1:2) (Fig. 2A). Moreover cellular uptake of proticles was confirmed by confocal microscopy (Fig. 2B). These studies revealed that proticles are subjected to internalization by BCEC and, that protamine and oligonucleotides colocalize in the nucleus where protamine and oligonucleotides colocalize (Fig. 2B). After 4 h, the majority of proticles was detected in the nucleus of BCEC.
Fig. 2.
Uptake of 125I- and fluorescence-labeled proticles by BCEC. (A) BCEC were cultured on 6-well trays and incubated in the presence of 125I-labeled proticle preparations at the indicated ON/protamine mass ratios and concentrations. After 4 h, incubation cells were washed, lysed in NaOH (0.3 M) and the radioactivity counted in a γ-counter. An aliquot was used to determine the cellular protein content. Results are mean±S.D. (n = 3). (B) BCEC were cultured on collagen-coated chamber slides in the presence of proticles that were labeled in the protamine-moiety (Rhodamine; red) or in the oligonucleotide moiety (Cy-5; blue). After 4 h, the cells were washed, fixed in acetone and immunostaining of VE-cadherin (green) was performed as described in Fig. 1.
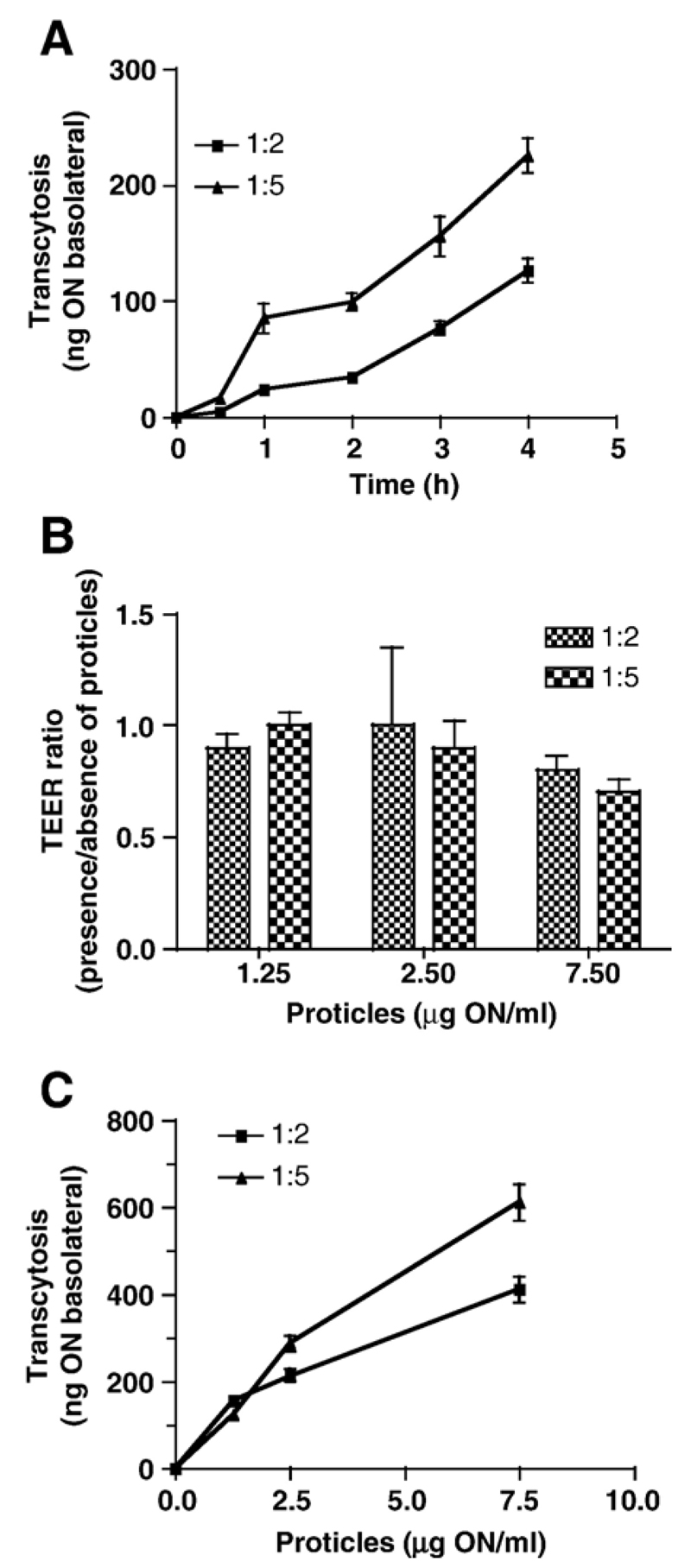
In time-dependent studies, transcytosis rates of proticles with varying protamine content have been compared in Transwell cultures. During these experiments a comparable tendency as seen in monolayer uptake experiments was observed (Fig. 3A): Proticles with an ON/protamine ratio of 1:5 showed a higher transcytosis rate (225 ng basolateral; Pe= 6.9 × 10−6 cm/s) than those with a lower protamine content (127 ng ON basolateral; Pe= 3.1 × 10−6 cm/s). These findings suggest that the most efficient uptake and transcytosis properties are observed for particles with a high ON/protamine ratio.
Fig. 3.
125I-Proticles are subjected to time- and concentration-dependent transcytosis across BCEC Transwell cultures. (A) Transwell cultures were incubated in the presence of 125I-proticles (2.5 μg/ml; ON/protamine mass ratio of 1:2 and 1:5). At the indicated time points 100 μl of the basolateral medium was removed for counting and replaced by fresh medium. Results are mean±S.D. (n = 3). (B) Cells were incubated exactly as described in A. TEER was measured at the start and end (4 h) of the experiment. Results are shown as TEER ratios (TEERend/TEERstart) and represent mean±S.D. (n = 4). (C) BCEC were cultured on polycarbonate filter inserts. After induction of tight junctions, 125I-labeled proticles (ON/protamine mass ratio of 1:2 and 1:5) were added at indicated concentrations. After 4 h the basolateral medium was removed and counted on a γ-counter. Results are mean±S.D. (n = 3).
Prior to concentration-dependent transcytosis experiments, TEER values have been measured before and after incubation in the presence of the indicated proticle concentrations (Fig. 3B). While low proticle concentrations (1.25 and 2.5 μg ON/ml) were without effect on TEER values, the electrical resistance dropped slightly after 4 h incubation in the presence of 7.5 μg ON/ml (on average by 20%). Concentration-dependent transcytosis is shown in Fig. 3C. Again, transcytosis rates were clearly dependent on the ON/protamine mass ratio (611 vs. 411 ng ON basolateral; 1:5 and 1:2, respectively).
3.4. Isolation of apoA-I and conjugation of proticles with apoA-I
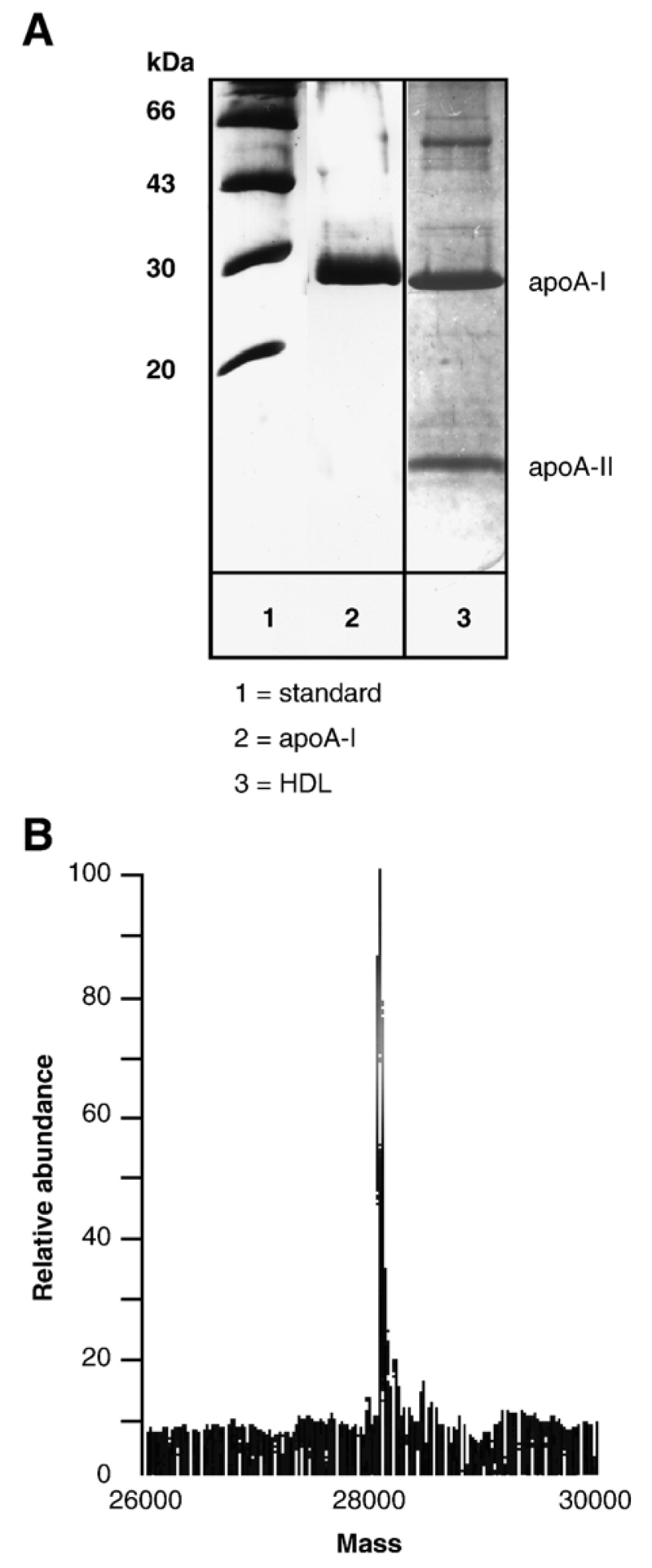
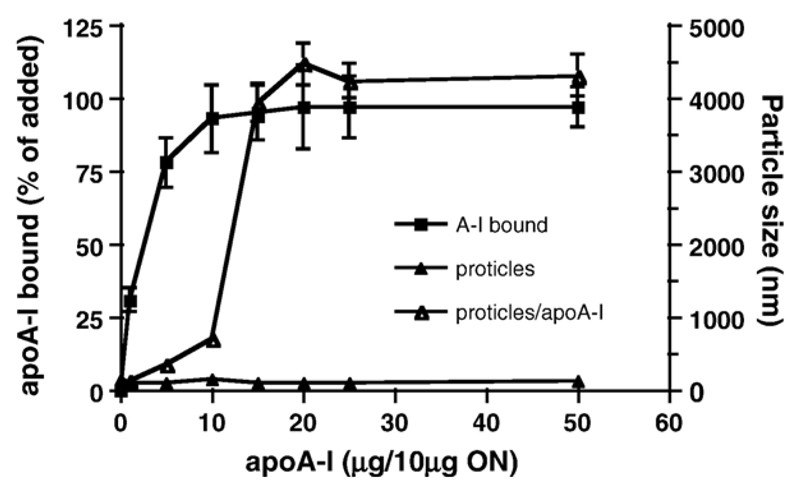
Having established that proticles do cross an in vitro BBB model, we hypothesized that apoA-I could provide a suitable targeting sequence to enhance proticle transcytosis across BCEC, probably via an SR-BI-mediated pathway [24]. Therefore, apoA-I was purified from human HDL (Fig. 4A; apparent molecular mass on 15% SDS–PAGE gels = 29 kDa); ESI–MS analysis of purified apoA-I revealed the expected mass of 28072 Da (Fig. 4B) deduced from the primary structure. Proticles were then incubated with purified apoA-I and the apoA-I binding capacity and effects on proticle size distribution were investigated (Fig. 5). An incubation of proticles (ON/protamine ratio of 1:3) with fluorescently labeled apoA-I revealed an exceptionally high binding capacity of proticles towards apoA-I: at concentrations >5 μg/10 μg ON more than 90% of apoA-I was recovered in association with proticles. However, it became evident that the particle size of uncoated proticles (125 nm) increased dramatically above a threshold concentration of 10 μg apoA-I/10 μg ON and resulted in particle aggregation within a size range of 300 to 5000 nm, a fact most probably due to hydrophobic interaction between adjacent apoA-I molecules. Based on this finding, proticles (10 μg ON) were coated with 5 μg apoA-I (1.8 × 10−10 mol) to avoid particle aggregation. Proticles coated with 5 μg apoA-I/10 μg ON showed a particle size of 280 nm, while for those coated with 10 μg apoA-I the size increased above 1000 nm due to aggregation. Under these experimental conditions on average 80% of added apoA-I (corresponding to 1.4 × 10−10 mol) was bound to proticles (10 μg ON, corresponding to 1 × 10−14 mol proticles) resulting in a proticle population containing approximately 14,000–15,000 apoA-I molecules per nanoparticle (this calculation is based on a molar proticle mass of 4 × 109 Da).
Fig. 4.
Characterization of human apoA-I HDL was isolated by density gradient ultracentrifugation, dialyzed, and delipidated apolipoproteins were separated by size exclusion chromatography as described in Materials and methods. (A) SDS–PAGE (12%) of purified human apoA-I (10 μg) and HDL (containing apoA-I and apoA-II; 10 μg protein) was performed under the conditions described in Materials and methods. The molecular mass of the marker proteins is indicated. (B) ApoA-I (5 μg) was separated by RP–HPLC (Vydac C 18 column) and analyzed by ESI–MS.
Fig. 5.
Characterization of apoA-I-coated proticles The binding capacity of proticles was determined using Cy-3-labeled apoA-I. Proticles were incubated in the presence of indicated amounts of apoA-I (1 h, ambient temperature, 300 rpm) and centrifuged (20,000×g, 2 h, 4 °C). 100 μl aliquots were removed from the supernatant and fluorescence determined using a fluorescence plate reader (544/590 nm, Ex/Em). The percentage of apoA-I bound to proticles (filled squares) was calculated from the difference in fluorescence intensities measured prior to and after the coincubation of proticles and Cy-3-labeled apoA-I. Effects of apoA-I conjugation on particle size were determined by dynamic light scattering prior and after the incubation of proticles in the absence (filled triangles) or presence (open triangles) of the indicated mass ratios of apoA-I/ON. Results are mean±S.D. from three different experiments performed in triplicates.
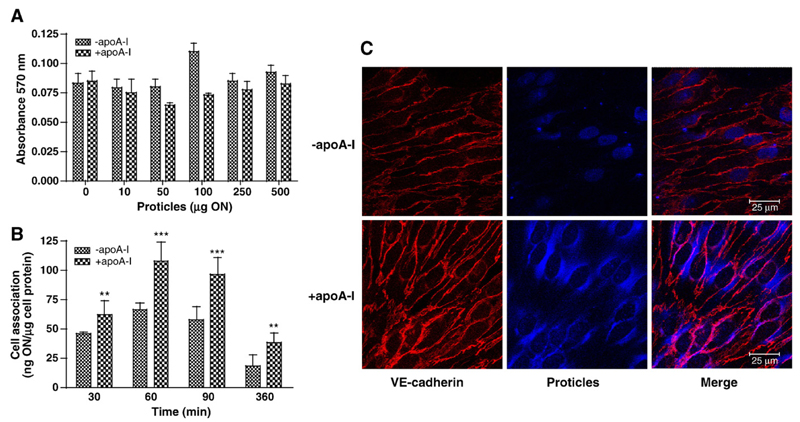
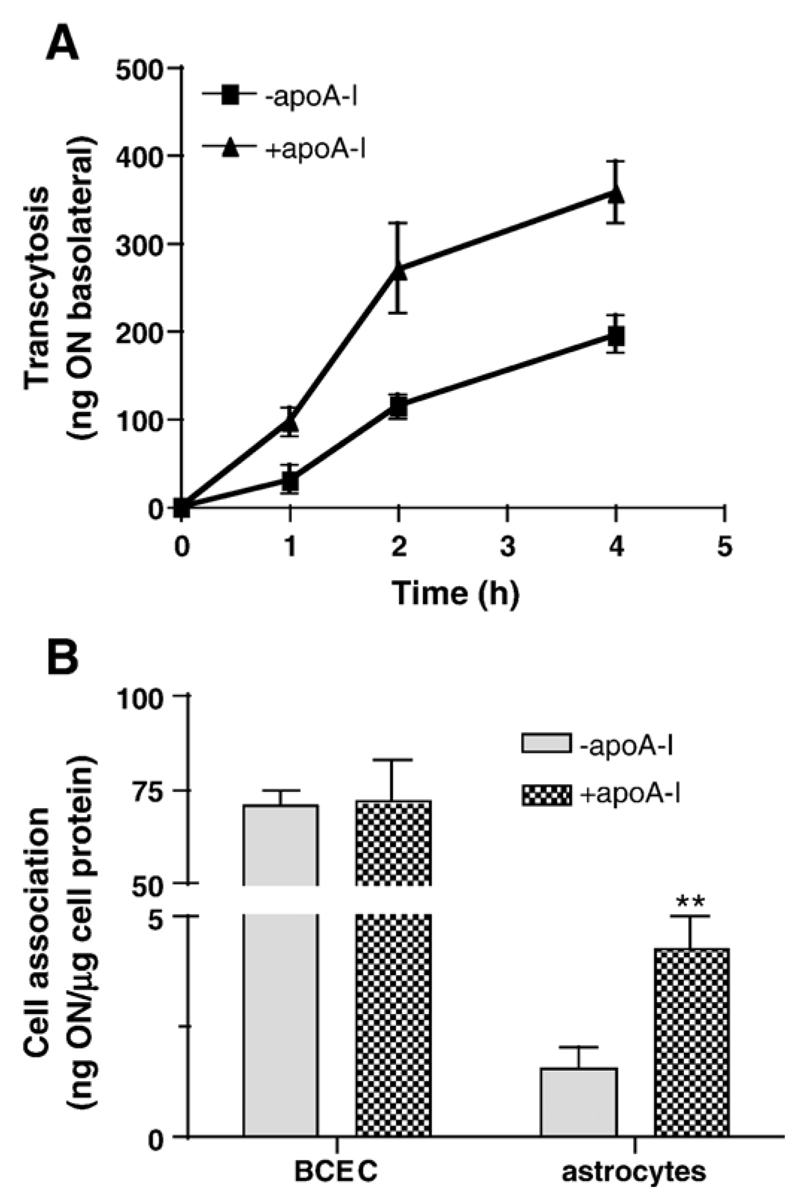
We next assessed the effects of apoA-I-coated proticles on BCEC viability, uptake by BCEC and intracellular distribution (Fig. 6). An incubation of BCEC in the presence of 10 to 500 μg ON (uncoated or apoA-I-coated) had no inadvertent effect on cell viability (Fig. 6A), findings in line with data shown in Fig. 1. In comparison to uncoated proticles, uptake of apoA-I-coated proticles by BCEC monolayer cultures was between 1.4- and 2.1-fold higher at the time points analyzed (Fig. 6B). To get an indication about altered subcellular distribution in response to apoA-I coating, uptake of fluorescently labeled proticles was followed by confocal microscopy (Fig. 6C). These experiments revealed that uncoated particles accumulated mainly in the nuclei of BCEC (Fig. 6C, upper panel), while apoA-I-coated proticles were primarily located in perinuclear regions of BCEC (Fig. 6C, lower panel). Next the effects of apoA-I coating on proticle transcytosis were investigated in an in vitro BBB model (BCEC grown on polycarbonate filter inserts and a confluent layer of human astrocytes grown on the bottom of the basolateral compartment). In line with results obtained in monolayer experiments (Fig. 6B), apoA-I-coating led to significantly increased proticle transcytosis across BCEC: The differences were most pronounced at the shorter incubation times (3.2- and 2.4-fold higher transcytosis at 1 and 2 h, respectively) and transcytosis of apoA-I-coated proticles was still 1.8-fold higher after 4 h in comparison to controls (Fig. 7A). Measurement of astrocyte-associated radioactivity revealed that cellular uptake of apoA-I-coated proticles exceeded uptake of uncoated proticles by 2-fold (Fig. 7B).
Fig. 6.
ApoA-I coating of proticles does not affect BCEC viability, results in increased uptake rates and alters subcellular distribution of proticles in BCEC monolayers. (A) BCEC were incubated in the presence of the indicated concentration of proticles or apoA-I-coated proticles and cell viability was determined as described in the legend to Fig. 1C. Results shown are mean±S.D. (n = 4). (B) BCEC were incubated in the presence of 125I-labeled proticles and 125I-labeled apoA-I-coated proticles (2.5 μg/ml) for the indicated times. Cells were washed, lysed in NaOH (0.3 M) at 4 °C for 8 h and the radioactivity was counted. An aliquot of the lysate was used to determine the cellular protein content. Results shown are mean±S.D. (n = 3). **p<0.05;***p<0.005. (C) BCEC were cultured on chamber slides and incubated with Cy-5-labeled uncoated or apoA-I-coated proticles (shown in blue) for 4 h. Following the incubations (4 h) cells were fixed and VE-cadherin staining (red) was performed as described in Materials and methods. Detection was performed with a Cy-3-labeled goat anti-mouse antibody.
Fig. 7.
ApoA-I coating of proticles results in enhanced transcytosis rates and increased uptake by astrocytes in a coculture model of the BBB. (A) BCEC were cultured on polycarbonate filter inserts and CCF–STT astrocytes were cultured on the bottom of the basolateral compartment. After induction of tight junctions, uncoated or apoA-I-coated 125I-labeled proticles were added to the apical medium was removed for counting of the radioactivity and replaced with fresh medium. Results are mean±S.D. (n = 3). (B) After 4 h the polycarbonate filter inserts containing BCEC were washed, cut out, transferred to a new insert and lysed in NaOH (0.3 M) to determine the cell-associated radioactivity. An aliquot was used to determine the cellular protein content. Astrocytes were washed, lysed in NaOH and processed as described for BCEC. Results are mean±S.D. (n = 3). **p<0.05.
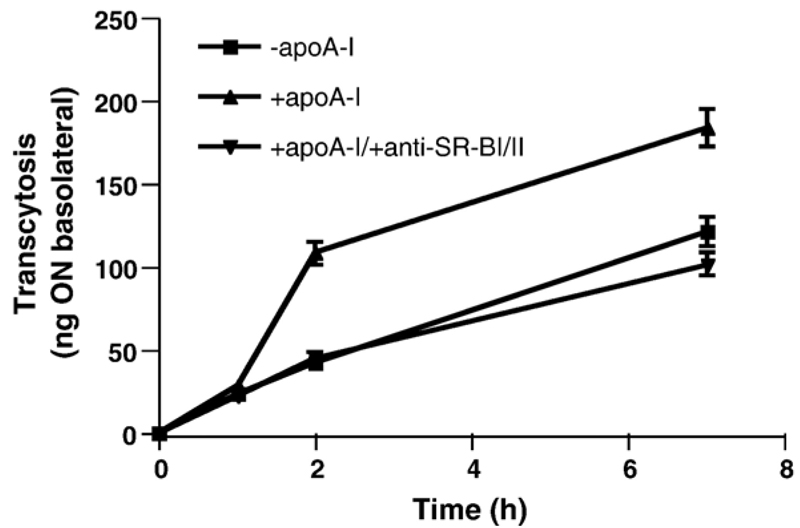
To clarify whether uptake of apoA-I-coated proticles is mediated by SR-BI a specific antibody raised against the extracellular receptor loop was added. Fig. 8 shows that the increase of proticle transcytosis in response to apoA-I-coating was completely abolished by anti SR-BI IgG and reduced to levels observed with uncoated proticles.
Fig. 8.
Transcytosis of apoA-I-coated 125I-proticles is reduced in the presence of an SR-BI neutralizing antibody BCEC were cultured on collagen-coated 12-well Transwell clusters. Where indicated a polyclonal blocking antiserum (1 μl) against SR-BI was added to the apical compartment 60 min prior to the addition of 2.5 μg of apoA-I-coated 125I-proticles/ml to the apical compartment. Uncoated 125I-proticles were used as controls (2.5 μg/ml). Cells were incubated at 37 °C and at indicated time points, 200 μl aliquots were removed from the basolateral compartment, replaced with fresh medium, and the radioactivity was determined by γ-counting. Data shown represent mean±S.D. from one experiment performed in triplicates.
To get an indication whether proticles are secreted as intact particles into the basolateral compartment BCEC Transwell cultures were incubated in the presence of 125I-labeled uncoated or apoA-I-coated proticles. The basolateral medium was applied to Microcon® centrifugal filter units with a cutoff of 3 (retaining intact ON and intact proticles in the upper chamber) and 100 kDa (containing only intact proticles in the retentate). These experiments revealed that the majority (93–94%) of the radioactive material recovered from the basolateral compartment was <3 kDa, indicating that most of the proticles are degraded after passage across polarized BCEC cultures (Table 1). Only 6–7% were recovered in the retentate, corresponding most likely to a mixed population of undegraded ON and intact proticles. Using Microcon units with a cutoff of 100 kDa the radioactivity recovered in the retentate dropped to 2%. These results indicate, that after passage over Transwell cultures, approximately 93% of proticles are degraded, with the remaining material consisting of 5% intact ON and 2% intact proticles. Comparable observations were made after size exclusion chromatography on Sephadex G75 columns (data not shown).
Table 1.
Size-fractionation of basolateral media on Microcon® centrifugal filter units
| Cutoff (kDa) |
Uncoated proticles |
ApoA-I-coated proticles |
||
|---|---|---|---|---|
| Eluate | Retentate | Eluate | Retentate | |
| 3 | 93 | 7 | 94 | 6 |
| 100 | 98 | 2 | 98 | 2 |
BCEC Transwell cultures were incubated in the presence of 125I-labeled uncoated or apoA-I-coated proticles (2.5 μg ODN/ml; 4 h). To determine the integrity of proticles after transcytosis across BCEC Transwell cultures, the basolateral media were applied to Microcon® centrifugal filter units with a cut off of 3 and 100 kDa. After centrifugation (25 min, 10,000 rpm) the radioactivity from the lower (‘eluate’) and the upper compartment (including the ultrafiltration membrane; ‘retentate’) was determined on a γ-counter. Results are expressed as percentage of total radioactivity (total radioactivity detected in the eluate, membrane, and retentate) and represent mean values from duplicate determinations.
4. Discussion
The rationale for the present study comes from findings that besides passive diffusion, tight junction opening, inhibition of efflux pumps by nanoparticles, and transcytosis of suitably engineered nanoparticles via the insulin or transferrin–receptor system, the conjugation of nanoparticles with specific apolipo-proteins proved a successful strategy to obtain improved delivery properties of drug-loaded nanocarriers across the BBB [26]. In this context proticles are potent drug delivery vehicles with a clear drug targeting option. These nanoparticles have a large surface area which is able to adsorb other proteins due to strong electrostatic interactions [27]. Therefore it is possible to modify the surface structure of nanoparticles without chemical crosslink which will be beneficial in terms of immunological side reactions and enables drug release by dissociation [28]. Our findings demonstrate that proticles do not affect physiological properties of BCEC that are relevant to functions of the BBB. Proticles are subjected to efficient uptake by BCEC, accumulate intracellularly and are subjected to transcytosis in Transwell cultures. Coating of proticles with apoA-I does not affect BCEC viability, results in significantly increased uptake and transcytosis of these proticles, and enhanced intracellular accumulation in astrocytes that are grown in the basolateral compartment below a BCEC monolayer. This points towards the ability of these particles to reach deeper regions of the brain more efficiently than uncoated nanoparticles.
Kreuter and colleagues [11] have demonstrated that drugs that are normally unable to cross the BBB (following intravenous injection) can be transported across this barrier by binding to poly(butylcyanoacrylate) nanoparticles that were coated with polysorbate 80. During that study it was demonstrated that dalargin-loaded and polysorbate 80-coated nanoparticles displayed pronounced antinociceptive properties in vivo. The efficacy was significantly enhanced after apoB- or apoE-overcoating. These authors [11] suggested that polysorbate 80-coated nanoparticles adsorb these apolipoproteins in the circulation thus mimicking lipoprotein particles that are taken up by BCEC via apoB/E-mediated endocytosis. Efficacy of brain uptake of apoE-conjugated nanoparticles was dependent on the apoE isoform specifically recognized by the apoB/E-receptor [29] expressed in a regulated manner on BCEC [30]. Dehouck and colleagues [14] have demonstrated that LDL-transcytosis by bovine BCEC is regulated in a similar manner as apoB/E-receptor expression. In this system, LDL-particles entered a non-lysosomal pathway different from the classical LDL-pathway indicating that this transcytotic route is unique to BCEC [12].
The expression of SR-BI at the luminal membrane of BCEC [13,24] prompted us to study the effects of apoA-I-coating of proticles on transcytosis in an in vitro BBB model. ApoA-I is responsible for solubilization of lipids in HDL particles, activation of lecithin/cholesterol acyltransferase, mobilization of cellular cholesterol, and binding of HDL to cells. Secondary structure prediction of apoA-I revealed repeated amphipathic helices forming the lipid-binding domain of this apolipoprotein [31]. It has been suggested that the N-terminal portion of apoA-I is responsible for the maintenance of a stable, lipid-free structure of apoA-I [32]. In plasma approximately 4% of total apoA-I is not lipoprotein-associated and transported in lipidfree form [33]. The pattern of hydrophobic residues in the helical repeats is similar to the canonical heptad repeat of α-helical coiled coil proteins [34] and probably mediates proticle binding.
As observed during the present study the association of apoA-I with proticles was extremely efficient. However, at a certain threshold molar ratio (approximately 15,000 apoA-I molecules per nanoparticle) a massive increase of the hydrodynamic diameter of the originally nanostructured system is indicative for apoA-I-mediated aggregation. ApoA-I exhibits different structural conformations in the lipid-bound and in the lipid-free state and transition between the two forms governs lipid-binding ability, receptor interaction, or protein misfolding leading to amyloidosis [35]. ApoA-I undergoes concentration-dependent self-association with dimer formation at low protein concentrations and tetra- or pentamer formation at high protein concentrations [36,37]. This ability for self-aggregation in conjunction with the sterical proximity of (exposed) apoA-I residues on proticles is a likely explanation for the high tendency of proticle aggregation at high apoA-I concentrations.
Coating of proticles with apoA-I resulted in significantly enhanced transcytosis across BCEC. This is most probably due to the fact that SR-BI (besides mediating endocytosis-independent selective lipid uptake [38]) is able to enter the endocytic recycling compartment as shown e.g. in polarized hepatocytes couplets [39] or BCEC in vitro [24] and in vivo [14]. We previously reported that SR-BI mediates HDL-transport across BCEC Transwell cultures and this transport could be blocked by an antibody directed against the extracellular loop of this receptor [24]. Comparable observations were made in the present study where an SR-BI blocking antiserum reduced transcytosis rates of apoA-I-coated proticles.
Another observation of the present study is altered subcellular trafficking of apoA-I-coated proticles compared with uncoated proticles. While a significant proportion of uncoated proticles ended up in the nucleus of BCEC (most probably due to the nuclear targeting sequence present in protamine [40]) apoA-I-coated proticles bypassed this compartment. The majority of apoA-I-coated proticles was localized in both, perinuclear and cytosolic compartments, thus enabling transcytosis of these nanocarriers. This observation is comparable to what has been shown for cellular SR-BI-trafficking in hepatocytes where SR-BI plus cargo was detected in the endosomal recycling compartment (see above [41]). This bypass due to apoA-I coating in conjunction with SR-BI-mediated internalization is also the most plausible explanation for increased uptake of apoA-I-coated proticles by astrocytoma cells that were cocultured with BCEC.
A question remaining was the nature of the material that is released basolaterally after passage through the in vitro BBB model used during the present study. Our results suggest that most of the proticles that were added to the apical compartment are degraded and released as radiolabeled compound with a molecular mass <3 kDa (Table 1). This indicates that, after uptake and transcytosis across polarized BCEC Transwell cultures proticles are degraded intracellularly which would result in immediate release of proticle-associated therapeutic compounds in the CNS.
In summary, our in vitro data indicate that apoA-I coating of proticles might provide a useful strategy to facilitate nanoparticle transport across the BBB and further transport to deeper regions of the brain.
Acknowledgements
Financial support by the Austrian BioNanoNet and the Austrian Nanoinitiative within the NanoHealth Project N203-NAN, N251-NAN, NanoProt and NanoBrain, the Austrian Research Promotion Agency (FFG; Bridge P810994), and the Austrian Science Fund (FWF, P17013-B05, P19074-B05, and P17474-B09) is acknowledged.
References
- 1.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci. 2001;24:719. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 2.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 3.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1:131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. Targeting neurotherapeutic agents through the blood–brain barrier. Arch Neurol. 2002;59:35–40. doi: 10.1001/archneur.59.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 6.Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2:108–119. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A, Mallikarjuana NN, Manohar S, Liang HF, Kulkarni AR, Sung HW, et al. Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s disease. J Control Release. 2005;108:193–214. doi: 10.1016/j.jconrel.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 9.Junghans M, Kreuter J, Zimmer A. Antisense delivery using protamine–oligonucleotide particles. Nucleic Acids Res. 2000;28:E45. doi: 10.1093/nar/28.10.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyermann J, Lochmann D, Zimmer A. Comparison of antisense oligonucleotide drug delivery systems. J Control Release. 2004;100:411–423. doi: 10.1016/j.jconrel.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 12.Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood–brain barrier. J Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goti D, Hrzenjak A, Levak-Frank S, Frank S, van der Westhuyzen DR, Malle E, Sattler W. Porcine scavenger reeptor class B, type I is expressed at the blood–brain barrier and contributes to selective uptake of HDL-associated vitamin E. J Neurochem. 2001;76:498–508. doi: 10.1046/j.1471-4159.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 14.Mardones P, Strobel P, Miranda S, Leighton F, Quinones V, Amigo L, Rozowski J, Krieger M, Rigotti A. Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J Nutr. 2002;132:443–449. doi: 10.1093/jn/132.3.443. [DOI] [PubMed] [Google Scholar]
- 15.Lochmann D, Vogel V, Weyermann J, Dinauer N, von Briesen H, Kreuter J, Schubert D, Zimmer A. Physicochemical characterization of protamine–phosphorothioate nanoparticles. J Microencapsul. 2004;21:625–641. doi: 10.1080/02652040400000504. [DOI] [PubMed] [Google Scholar]
- 16.Sinn HJ, Schrenk HH, Friedrich EA, Via DP, Dresel HA. Radioiodination of proteins and lipoproteins using N-bromosuccinimide as oxidizing agent. Anal Biochem. 1988;170:186–192. doi: 10.1016/0003-2697(88)90107-8. [DOI] [PubMed] [Google Scholar]
- 17.Sattler W, Mohr D, Stocker R. Rapid isolation of lipoproteins and assessment of their peroxidation by high-performance liquid chromatography postcolumn chemiluminescence. Methods Enzymol. 1994;233:469–489. doi: 10.1016/s0076-6879(94)33053-0. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Brewer BH, Ronan R, Meng M, Bishop C. Isolation and characterization of apolipoproteins A-I, A-II and A-IV. Methods Enzymol. 1986;128:223–246. doi: 10.1016/0076-6879(86)28070-2. [DOI] [PubMed] [Google Scholar]
- 20.Anantharamaiah GM, Hughes TA, Iqbal M, Gawish A, Neame PJ, Medley MF, Segrest JP. Effect of oxidation on the properties of apolipoproteins A-I and AII. J Lipid Res. 1988;29:309–318. [PubMed] [Google Scholar]
- 21.Bergt C, Oettl K, Keller W, Andreae F, Leis HJ, Malle E, Sattler W. Reagent or myeloperoxidase-generated hypochlorite affects discreteregions in lipid-free and lipid-associated human apolipoprotein A-I. Biochem J. 2000;346:345–354. [PMC free article] [PubMed] [Google Scholar]
- 22.Sovic A, Panzenboeck U, Wintersperger A, Kratzer I, Hammer A, Levak-Frank S, Frank S, Rader DJ, Malle E, Sattler W. Regulated expression of endothelial lipase by porcine brain capillary endothelial cells constituting the blood–brain barrier. J Neurochem. 2005;94:109–119. doi: 10.1111/j.1471-4159.2005.03175.x. [DOI] [PubMed] [Google Scholar]
- 23.Panzenboeck U, Kratzer I, Sovic A, Wintersperger A, Bernhart E, Hammer A, Malle E, Sattler W. Regulatory effects of synthetic liver X receptor- and peroxisome-proliferator activated receptor agonists on sterol transport pathways in polarized cerebrovascular endothelial cells. Int J Biochem Cell Biol. 2006;38:1314–1329. doi: 10.1016/j.biocel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, Malle E, Sattler W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood–brain barrier model. J Neurochem. 2004;89:939–950. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- 25.Schnittler HJ, Puschel B, Drenckhahn D. Role of cadherins and plakoglobin in interendothelial adhesion under resting conditions and shear stress. Am J Physiol. 1997;273:H2396–H2405. doi: 10.1152/ajpheart.1997.273.5.H2396. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari SB, Amiji MM. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 2006;3:219–232. doi: 10.2174/156720106776359230. [DOI] [PubMed] [Google Scholar]
- 27.Lochmann D, Weyermann J, Georgens C, Prassl R, Zimmer A. Albumin–protamine–oligonucleotide nanoparticles as a new antisense delivery system: Part 1. Physicochemical characterization. Eur J Pharm Biopharm. 2005;59:419–429. doi: 10.1016/j.ejpb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Weyermann J, Lochmann D, Georgens C, Zimmer A. Albumin–protamine–oligonucleotide-nanoparticles as a new antisense delivery system: Part 2. Cellular uptake and effect. Eur J Pharm Biopharm. 2005;59:431–438. doi: 10.1016/j.ejpb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, Kreuter J, Langer K. Covalent linkage of apolipoprotein E to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–1253. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- 30.Dehouck B, Dehouck MP, Fruchart JC, Cecchelli R. Upregulation of the low-density lipoprotein receptor at the blood–brain-barrier: inter-communications between capillary endothelial cells and astrocytes. J Cell Biol. 1994;126:465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borhani DW, Rogers DP, Engler JA, Brouillette CG. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc Natl Acad Sci U S A. 1997;94:12291–12296. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers DP, Roberts LM, Lebowitz J, Engler JA, Brouillette CG. Structural analysis of apolipoprotein A-I: effects of amino- and carboxy-terminal deletions on the lipid-free structure. Biochemistry. 1998;37:945–955. doi: 10.1021/bi9713512. [DOI] [PubMed] [Google Scholar]
- 33.Ishida BY, Frolich J, Fielding CJ. Prebeta-migrating high density lipoprotein: quantitation in normal and hyperlipidemic plasma by solid phase radioimmunoassay following electrophoretic transfer. J Lipid Res. 1987;28:778–786. [PubMed] [Google Scholar]
- 34.Andreola A, Bellotti V, Giorgetti S, Mangione P, Obici L, Stoppini M, Torres J, Monzani E, Merlini G, Sunde M. Conformational switching and fibrillogenesis in the amyloidogenic fragment of apolipoprotein A-I. J Biol Chem. 2003;278:2444–2451. doi: 10.1074/jbc.M204801200. [DOI] [PubMed] [Google Scholar]
- 35.Bellotti V, Mangione P, Stoppini M. Biological activity and pathological implications of misfolded proteins. Cell Mol Life Sci. 1999;55:977–991. doi: 10.1007/s000180050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swaney JB, O’Brien K. Cross-linking studies of the self-association properties of apo-A-I and apo-A-II from human high density lipoprotein. J Biol Chem. 1978;253:7069–7077. [PubMed] [Google Scholar]
- 37.Calabresi L, Vecchio G, Longhi R, Gianazza E, Palm G, Wadensten H, Hammarstrom A, Olsson A, Karlstrom A, Sejlitz T, et al. Molecular characterization of native and recombinant apolipoprotein A-I Milano dimer. The introduction of an interchain disulfide bridge remarkably alters the physicochemical properties of apolipoprotein A-I. J Biol Chem. 1994;269:32168–32174. [PubMed] [Google Scholar]
- 38.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 39.Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem. 2006;281:4739–4745. doi: 10.1074/jbc.M509042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noguchi A, Hirashima N, Nakanishi M. Cationic cholesterol promotes gene transfection using the nuclear localization signal in protamine. Pharm Res. 2002;19:933–938. doi: 10.1023/a:1016449902541. [DOI] [PubMed] [Google Scholar]
- 41.Silver DL, Wang N, Xiao X, Tall AR. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type I results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J Biol Chem. 2001;276:25287–25293. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]