Summary
Anorexia nervosa (AN) is characterized by severe dietary restriction or other weight loss behaviors and exhibits the highest mortality rate of any psychiatric disorder. Therapeutic renourishment in AN is founded primarily on clinical opinion and guidelines, with a weak evidence base. Genetic factors do not fully account for the etiology of AN, and non-genetic factors that contribute to the onset and persistence of this disease warrant investigation. Compelling evidence that the intestinal microbiota regulates adiposity and metabolism, and more recently, anxiety behavior, provides a strong rationale for exploring the role of this complex microbial community in the onset, maintenance of, and recovery from AN. This review explores the relationship between the intestinal microbiota and AN and a potential role for this enteric microbial community as a therapy for this severe illness.
Keywords: Anorexia nervosa, intestinal microbiota, behavior, refeeding, adiposity, metabolism
Introduction
Anorexia nervosa (AN) is a highly morbid disease that affects approximately 0.9% of women and 0.3% of men in the United States.[1] The disorder is characterized by severe dietary restriction or other weight-loss behaviors, such as purging or excessive physical activity, an intense fear of weight gain, and a disturbed body image.[2] In addition to the obvious sequelae of poor energy intake, the disorder has several psychological consequences, including social isolation, feelings of worthlessness, and resistance to treatment.[3] For this reason and others, outcomes are generally poor, and AN exhibits the highest mortality rate of any psychiatric disorder.[4,5] Despite significant morbidity and mortality,[1,4-6] the evidence base for the treatment of AN is meager.[7,8]
Approaches to weight restoration are rarely evidence-based but rather based on clinical opinion and experience, and are reported to be uncomfortable and distressing to patients.[8] Additionally, these approaches often fail to restore a healthy body fat distribution. Redistribution after refeeding is not uniform, with disproportionate central adipose tissue deposition. This unequal distribution is marked by an elevated waist-to-hip ratio, which indicates central obesity, as well as increased total trunk fat and visceral adipose tissue.[9] Poor treatment outcome is, in part, due to the fact that the biology of AN and the physical adaptations that occur during weight restoration are inadequately understood, rendering it difficult to design effective treatment interventions that target the core features of the illness.
Genetic epidemiological investigations indicate that AN has a strong genetic etiology.[10,11] Indeed, a significant familial association has been reported for AN,[12,13] and twin studies estimate heritability at over 60%.[10,11,14,15] Genes do not act alone: environmental factors—either directly or via gene-environment co-action—also influence risk. The intestinal microbiota, which describes the community of microorganisms living within the intestinal tract, has been shown to have a crucial role in metabolic function, accumulation and storage of fat, and behavior (e.g., anxiety and stress).[16-21] This highlights the intestinal microbiota as a compelling topic of exploration, as our understanding of the manner in which the intestinal microbiota affects the development and maintenance of AN may allow us to effectively improve our treatment of AN. The following sections review the current evidence for the role of the intestinal microbiota in regulating several elements of AN—the development of adiposity and behavior control—and explore the direct role of the intestinal microbiota in AN.
The Intestinal Microbiota and Adiposity
The intestinal microbiota plays an important role in weight regulation in both humans and animals, and consistent evidence implicates this enteric microbial community in obesity—though the extent of that contribution is controversial. Early work by Ley et al. reported that differences in weight were associated with differential composition of the intestinal microbiota, with the enteric microbiota in genetically obese mice (ob/ob) demonstrating a 50% reduction in abundance of the Bacteroidetes phylum and a parallel increase in Firmicutes phylum compared with wild-type siblings fed the same fat-heavy diet.[22] Similarly, studies in humans revealed that obese individuals have relatively fewer Bacteroidetes and more Firmicutes than lean controls,[23] but a one-year low-calorie diet (either low-fat or low-carbohydrate) can raise the relative level of Bacteroidetes and lower the relative level of Firmicutes in obese individuals. Subsequent research both confirmed[24-26] and refuted[18,27-29] the association between obesity and increased Firmicutes/decreased Bacteroidetes. Taken together, these data suggest that weight affects the composition of the intestinal microbiota, and that diet alone may alter this composition as well.
Recent studies changed focus from observational to experimental, introducing mouse:mouse, human:mouse, and human:human fecal transplants. A key study showed that the intestinal microbiotas of obese mice are more effective at extracting energy from food than those of lean mice, and that this trait can be passed to germ-free (GF) mice via microbial transplantation, causing increased adiposity.[30] Transferring fecal samples from obese adult females into GF mice also reveals that increased body fat, fat mass, and obesity-associated metabolic phenotypes can be transferred via the intestinal microbiota.[31] Furthermore, studies suggest that the presence of a “normal” microbiota can reverse or negate the direct effects of possessing an “obese” microbiota. Wild-type mice harboring an obese mouse's microbiota (Ob) cohoused with wild-type mice harboring microbiota from the Ob's lean co-twin (Ln) prevented the development of increased body mass and obesity-associated metabolic phenotypes in Ob cage-mates.[31] Fecal transplants from healthy human male donors (BMI <23) to obese males with metabolic syndrome led to improved insulin sensitivity after six weeks compared with individuals who received transplants created from their own stool samples.[32]
The Intestinal Microbiota and Behavior
Mood and behavior significantly impact maintenance of a healthy weight.[33] For example, dysregulated affective states (e.g., increased stress and anxiety) are prevalent in patients prior to bariatric surgery, and negative affective states (e.g., anxiety) can adversely influence the maintenance of weight loss.[34] While the relationship between mood and weight is still unclear, a growing body of evidence indicates a strong connection between the brain and the intestinal microbiota.[35,36] Early findings in animal models suggest a direct association between the intestinal microbiota and stress-like and/or depressive behaviors,[36] and the collective body of evidence suggests bidirectional communication between the intestinal microbiota and brain function in times of both homeostasis and disease.[37] This communication appears to occur primarily through complex interactions along the hypothalamic-pituitary-adrenal (HPA) axis and structures in the central nervous system (CNS) that can affect cognition, mood, and emotion. For example, Sudo and colleagues[20] investigated the potential role of the intestinal microbiota in altering HPA axis function, which is purported to be involved in depression. These investigators found that inducing stress in GF mice caused an exaggerated hormonal response of the HPA axis compared with conventionally raised mice. Notably, stress is also implicated in the pathogenesis of both depression and compositional changes in the intestinal microbiota, which may confound these findings. Nonetheless, the exaggerated hormonal response was reversed by introduction of Bifidobacterium infantis, a member of the normal intestinal microbiota, to GF mice. Similarly, a series of studies by Lyte et al.[38] demonstrated that oral administration of Campylobacter jejuni, a bacterium commonly associated with gastroenteritis, caused behaviors suggestive of anxiety in mice, as measured by exploratory behavior on an elevated plus-maze. A more recent study found that diversifying the intestinal microbiota through alteration of the diet resulted in improvements in working memory and reduced anxiety-like behavior in mice.[21] As AN patients exhibit elevated levels of anxiety,[39] complex enteric microbial communities may potentially influence this behavior in patients with AN (Figure 1). Although further studies are needed to elucidate the complex interactions between the intestinal microbiota and mood/behavior, the extant literature suggests this field is particularly rich for exploration into the causes and potential treatment of mood disorders.
Figure 1.
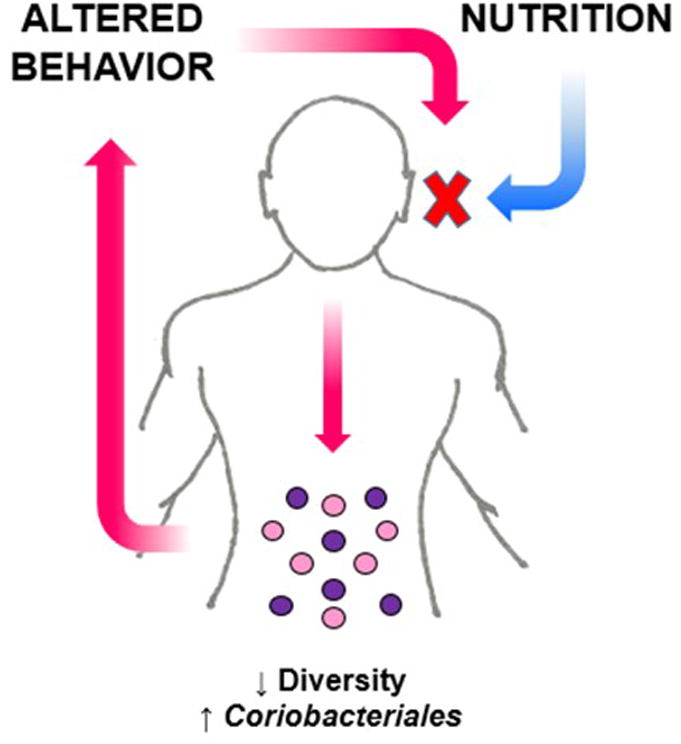
Potential relationship between the intestinal microbiota and behavior in patients with Anorexia Nervosa. Restricting calorie intake can have a profound impact on enteric microbes resulting in decreased microbial richness and a bloom of bacteria that can flourish in a nutrient depleted environment. This altered composition in the intestinal microbiota could possibly impact the behavior of a patient with Anorexia Nervosa, perpetuating restriction of food intake.
The Intestinal Microbiota and Anorexia Nervosa
Although the composition of the intestinal microbiota differs between obese and lean individuals, and obese (versus lean) individuals may extract more energy from a given diet, very little is known about the intestinal microbiota in individuals with eating disorders—and the limited research in this area has focused on AN. In a small cohort, one study characterized bacterial composition in AN inpatients (n=16) versus healthy controls (n=12), and found that AN patients had significantly lower alpha (within-sample) diversity than healthy controls, as well as differences between groups in taxa abundance.[19] To more specifically characterize the bacterial species in AN, another study used traditional microbial culturing techniques of a single stool sample from a patient with acute AN and identified 11 completely new species in the phyla Firmicutes (n=7), Bacteroidetes (n=2), and Actinobacteria (n=2).[40] This suggests distinct characteristics of the intestinal microbiota in AN, an illness that truly represents a unique nutrient-impoverished state. Further research is needed to investigate whether these new species are specifically associated with AN.
In addition, a cross-sectional study analyzing the intestinal microbiota of nine patients with AN found increased levels of the archaeon Methanobrevibacter smithii, which plays an important role in efficiency of microbial fermentation and associated energy yield from one's diet. This difference could reflect an adaptive response in those individuals with AN to a prolonged very low-calorie diet.[24] Because this study analyzed a limited number of microbial groups, further studies are needed to more comprehensively characterize the composition of the intestinal microbiota in individuals with AN, and any changes in this composition as they undergo therapeutic re-nourishment. A summary of these findings is provided in Table 1.
Table 1. Studies that have characterized the enteric microbial communities in patients with AN.
| Author | Journal | Publication Year | Population | Results |
|---|---|---|---|---|
| Kleiman et al. [19] | Psychosomatic Medicine | 2015 | AN inpatients (n=16) vs. healthy controls (n=12) | Patients with AN demonstrate significant changes between hospital admission and discharge in taxa abundance and beta (between-sample) diversity; patients with AN demonstrate lower alpha (within-sample) diversity than healthy controls. AN and healthy controls demonstrate differences in taxa abundance |
| Pfleiderer et al. [40] | European Journal of Clinical Microbiology & Infectious Diseases | 2013 | Single AN patient | Identified 133 bacterial species, including 19 species never before isolated from the human gut, and sequenced 11 of these new species in the phyla Firmicutes, Bacteroides & Actinobacteria |
| Armougom et al. [24] | PLoS One | 2009 | Obese patients (n=20), AN patients (n=9) & healthy controls (n=20) | AN patients demonstrated reduced levels of Lactobacillus species compared with obese individuals or healthy controls, and increased levels of the archaeon Methanobrevibacter smithii |
Early mechanistic research in animal models also suggests that the intestinal microbiota plays a role in satiety via interaction with peptide signaling,[41] which may be sex-specific. These early, promising findings suggest that research into the role of the intestinal microbiota in the development, maintenance, and recovery from eating disorders will reveal exciting advances in our understanding of the role of the intestinal microbiota in the development and treatment of AN. Likewise, next generation research on this topic will expand beyond studies of patients with AN to include other disorders, such as bulimia nervosa, binge-eating disorder, and avoidant/restrictive food intake disorder.
Five Year View
Studies involving transplantation of intact, uncultured microbiotas from healthy humans to individuals with Clostridium difficile-induced colitis or patients with metabolic syndrome have yielded proof-of-principle that the intestinal microbiota represents a valid therapeutic target for treating or preventing disease.[32,42,43] These findings provide a basis for the use of fecal microbiota transplantation beyond the treatment of C. difficile and metabolic syndrome. Supporting this concept, manipulation of the intestinal microbiota via administration of probiotics has also been shown to be effective in several disease states, including acute childhood diarrhea.[44] Likewise, a probiotic originally isolated from the intestinal microbiota of a healthy individual (Lactobacillus rhamnosus JB-1) reduced anxiety- and stress-related behavior in mice via modulation of the expression of gamma-aminobutyric acid (GABA) in the brain.[45] These biological and behavioral effects were not seen in vagotomized mice, illustrating the critical role of microbe-gut-brain communication.[45] Given the impact that enteric microbial communities appear to have on traits that are commonly seen in individuals with AN and that a dysbiotic intestinal microbiota has been reported in this patient population, a potential to develop safe and effective therapies using or targeting gut microbes exists. However, it remains to be determined which specific bacterial strains or the metabolites they produce are responsible for dysregulated weight, adiposity, and behavior in AN patients. Additionally, one of the most important questions regarding the relationship between alterations in the intestinal microbiota and disease is whether a dysbiotic enteric microbial community is a cause or consequence of health status. Additional studies are required to delineate whether changes in the composition of the intestinal microbiota are a cause or consequence of AN. Future studies will likely identify dysregulated host-microbe interactions that may ultimately be targeted leading to novel therapies for this serious illness.
Expert Commentary
The composition of the intestinal microbiota has been a subject of study for decades, and many factors are known to affect the survival of microbes in the human gut. However, the body of research examining the relationship between intestinal microbiota and eating disorders is in its infancy. Enteric microbial communities can directly affect adiposity and weight regulation, although the degree and mechanism of this influence is still under investigation. Adiposity and weight are not the only intestinal microbiota-influenced traits that are relevant to eating disorders. Mood and behavior are integral features of eating disorders and evidence is rapidly accumulating documenting the impact of this complex microbial community on anxiety, depression, and other psychological symptoms.[16,36,45-49]
Extending research on the intestinal microbiota to AN and other eating disorders is logical and inevitable. AN is a debilitating and potentially lethal disorder characterized by severe and often prolonged dietary restriction that drastically alters the intestinal environment. Findings in this area could have immediate relevance to the prognosis, treatment, and process of recovery from AN. Specifically, based on the profile of the intestinal microbiota (presence or absence of specific enteric microbes or composition and/or diversity) in AN patients, it may become possible to identify which individuals will experience difficulties with weight restoration, and who will succeed in maintaining therapeutically restored weight versus relapse. Ultimately, we may identify specific bacterial taxa whose promotion or elimination would improve the efficiency of therapeutic weight restoration, as well as the psychological and physical treatment experience of patients.
Key Issues.
Anorexia nervosa is a serious illness with the highest mortality rate of any psychiatric disorder; the evidence base for treatment, especially in adults, is scant.
Renourishment is a critical first step in treatment but can cause discomfort and result in central distribution of body fat.
As the intestinal microbiota affects metabolism, fat accumulation and storage, and behavior, it is worthy of investigation in anorexia nervosa.
The intestinal tract of patients with AN is a nutrient-poor environment and may select for microorganisms that can survive under those conditions.
Two hypotheses exist regarding the differences between the intestinal microbiotas of lean (or anorexic) and obese humans: (i) differing microorganism compositions may harvest energy from food with different efficiencies; or (ii) certain microorganisms may produce metabolites that differentially impact feelings of satiation.
Research into the relationship between eating disorders such as AN and the intestinal microbiota continues. More knowledge about the roles that specific microorganisms play in the “microbe-gut-brain axis” and the mechanisms by which these interactions occur could lead to innovative therapies for AN.
Acknowledgments
Financial Disclosure: Dr. Carroll is a consultant for Salix Pharmaceuticals, Inc. Dr. Bulik is a grant recipient from Shire and a consultant for Ironshore Pharmaceutical & Development, Inc.
Footnotes
No other authors have any conflicts to declare.
References
- 1.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2(12):1099–1111. doi: 10.1016/S2215-0366(15)00356-9. [DOI] [PubMed] [Google Scholar]
- 3.Keel P, McCormick L. Diagnosis, assessment, and treatment planning for anorexia nervosa. In: Grilo C, Mitchell JE, editors. The Treatment of Eating Disorders: A Clinical Handbook. The Guilford Press; New York: 2010. p. 3. [Google Scholar]
- 4.Birmingham CL, Su J, Hlynsky JA, Goldner EM, Gao M. The mortality rate from anorexia nervosa. Int J Eat Disord. 2005;38(2):143–146. doi: 10.1002/eat.20164. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos FC, Ekbom A, Brandt L, Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br J Psychiatry. 2009;194(1):10–17. doi: 10.1192/bjp.bp.108.054742. [DOI] [PubMed] [Google Scholar]
- 7.Hart LM, Granillo MT, Jorm AF, Paxton SJ. Unmet need for treatment in the eating disorders: a systematic review of eating disorder specific treatment seeking among community cases. Clin Psychol Rev. 2011;31(5):727–735. doi: 10.1016/j.cpr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 8.NICE. Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. National Institute for Clinical Excellence. 2004 [PubMed] [Google Scholar]
- 9.Mayer L, Walsh BT, Pierson RN, Jr, et al. Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr. 2005;81(6):1286–1291. doi: 10.1093/ajcn/81.6.1286. [DOI] [PubMed] [Google Scholar]
- 10.Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychol Med. 2001;31(4):737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- 11.Wade TD, Bulik CM, Neale M, Kendler KS. Anorexia nervosa and major depression: shared genetic and environmental risk factors. Am J Psychiatry. 2000;157(3):469–471. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- 12.Lilenfeld LR, Kaye WH, Greeno CG, et al. A controlled family study of anorexia nervosa and bulimia nervosa: psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch Gen Psychiatry. 1998;55(7):603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- 13.Strober M, Freeman R, Lampert C, Diamond J, Kaye W. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am J Psychiatry. 2000;157(3):393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- 14.Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63(3):305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 15.Kortegaard LS, Hoerder K, Joergensen J, Gillberg C, Kyvik KO. A preliminary population-based twin study of self-reported eating disorder. Psychological medicine. 2001;31(2):361–365. doi: 10.1017/s0033291701003087. [DOI] [PubMed] [Google Scholar]
- 16.Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 17.Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes (Lond) 2013;37(2):216–223. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutrit DIab. 2014;4:e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom Med. 2015;77(9):969–981. doi: 10.1097/PSY.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96(4-5):557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. PNAS USA. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 24.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PloS one. 2009;4(9):e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo HJ, Xie ZM, Zhang WW, et al. Gut bacteria alteration in obese people and its relationship with gene polymorphism. World J Gastroenterol. 2011;17(8):1076–1081. doi: 10.3748/wjg.v17.i8.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88(4):894–899. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 28.Mai V, McCrary QM, Sinha R, Glei M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutrit J. 2009;8:49. doi: 10.1186/1475-2891-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 30.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 31.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterol. 2012;143(4):913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Cardi V, Leppanen J, Treasure J. The effects of negative and positive mood induction on eating behaviour: A meta-analysis of laboratory studies in the healthy population and eating and weight disorders. Neurosci Biobehav Rev. 2015;57:299–309. doi: 10.1016/j.neubiorev.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Sheets CS, Peat CM, Berg KC, et al. Post-operative psychosocial predictors of outcome in bariatric surgery. Obes Surg. 2015;25(2):330–345. doi: 10.1007/s11695-014-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exper Med Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 36.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav. 1998;65(1):63–68. doi: 10.1016/s0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- 39.Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161(12):2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 40.Pfleiderer A, Lagier JC, Armougom F, Robert C, Vialettes B, Raoult D. Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. Eur J Clin Microbiol Infect Dis. 2013;32(11):1471–1481. doi: 10.1007/s10096-013-1900-2. [DOI] [PubMed] [Google Scholar]
- 41.Tennoune N, Chan P, Breton J, et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide alpha-MSH, at the origin of eating disorders. Translat Psychiatry. 2014;4:e458. doi: 10.1038/tp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterol. 2013;145(5):946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 43.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 44.Caffarelli C, Cardinale F, Povesi-Dascola C, Dodi I, Mastrorilli V, Ricci G. Use of probiotics in pediatric infectious diseases. Expert Rev Anti Infect Ther. 2015;13(12):1517–1535. doi: 10.1586/14787210.2015.1096775. [DOI] [PubMed] [Google Scholar]
- 45.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 47.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 49.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]


