Abstract
The absorption of dietary fat is of increasing concern given the rise of obesity not only in the United States but throughout the developed world. This review explores what happens to dietary fat within the enterocyte. Absorbed fatty acids and monoacylglycerols are required to be bound to intracellular proteins and/or to be rapidly converted to triacylglycerols to prevent cellular membrane disruption. The triacylglycerol produced at the level of the endoplasmic reticulum (ER) is either incorporated into prechylomicrons within the ER lumen or shunted to triacylglycerol storage pools. The prechylomicrons exit the ER in a specialized transport vesicle in the rate-limiting step in the intracellular transit of triacylglycerol across the enterocyte. The prechylomicrons are further processed in the Golgi and are transported to the basolateral membrane via a separate vesicular system for exocytosis into the intestinal lamina propria. Fatty acids and monoacylglycerols entering the enterocyte via the basolateral membrane are also incorporated into triacylglycerol, but the basolaterally entering lipid is much more likely to enter the triacylglycerol storage pool than the lipid entering via the apical membrane.
Keywords: lipid absorption, transport vesicles, chylomicrons, complex lipid synthesis
INTRODUCTION
The adequate absorption of lipids is a requirement for all mammalian species because of their inability to synthesize essential fatty acids (FA), such as ω-3 and ω-6, and fat-soluble vitamins. This review covers the complex process by which this absorption occurs, with an emphasis on the production of the end product: the chylomicron. Why should such an important function as lipid absorption be so complex? The answer lies in the innate incompatibility between the lipid-soluble products of the diet and the aqueous environment of the organism. The lipids follow their physicochemical path while the organism attempts to control their movement through a series of protein-controlled interactions that make the lipids either more or less aqueous interactive, depending on the compartment in which the reactions occur. In addition to their control function, proteins play a crucial role in directing the lipids to the next sequential compartment on their metabolic path.
The clinical relevance of understanding and potentially controlling this process is evidenced by the epidemic of obesity that is sweeping the United States and other developed countries. According to the Centers for Disease Control in 2006 (http://www.cdc.gov/obesity/data/trends.html), in 26 states, mostly in the middle of the country and in the southeast, 25–29% of the adult population consisted of individuals with a body mass index (BMI) greater than 30. In six additional states, 30% or greater of the adult population had a similar BMI. Only one state had an adult population of which less than 19% were obese.
MOVEMENT OF DIETARY FATTY ACIDS TO THE ENDOPLASMIC RETICULUM
There are few robust data that show how absorbed FA move to the endoplasmic reticulum (ER). What is known is that they must be bound nearly completely so that their free cytosolic concentration remains low. If not, the FA would perturb cellular membranes, potentially leading to cell death (1). This finding has recently been highlighted in cell culture experiments, in which the inability to adequately dispose of absorbed FA resulted in cell death (2). One mechanism to blunt the toxic effects of FA absorption would be to protein bind incoming FA, a function performed by the FA-binding proteins (FABPs) present in enterocytes. The large amount of both liver and intestinal FA-binding proteins (L- and I-type FABPs, respectively), which constitute 5–6% of cytosolic proteins, supports this contention. In rat intestine, I- and L-FABP are present in approximately equal amounts (3), whereas human autopsy studies show that L-FABP clearly predominates (4). Additionally, both FABPs are found mainly in the proximal intestine (5), the site of dietary lipid absorption in both humans (6) and rats (7), and they are both increased 50% in rats fed a high-fat diet (8, 9). In mice, only L-FABP is increased by fat feeding (10). In sum, these data support an active role for I-and L-FABP in dietary lipid absorption.
These two FABPs deliver their ligands differently, despite their similarities. I-FABP donates its ligand to a target membrane by a collision model, whereas L-FABP donates its ligand by diffusion (11), a more typical method of ligand delivery that is used by other FABPs (12). In the diffusion model, the FA is delivered randomly by diffusion through the cytosol as it releases from L-FABP, and in the collision model, targeted delivery of the FA is provided by attachment of the I-FABP to the ER membrane. Thus, as it pertains to the enterocyte, I-FABP is proposed to function in removing FA from the apical membrane and delivering them to their target membrane, the ER, whereas L-FABP is proposed to function as a reservoir with respect to FA transport (11). These speculations are supported by the threefold-greater binding of FA to L- versus I-FABP under conditions of fat feeding and by an even greater disparity during fasting (13).
Another potential mechanism for delivering absorbed dietary FA to the enterocyte ER is the acylation of FA by FA-transport protein 4 (FATP4). This protein, which may be responsible for up to 60% of the FA uptake from the intestinal lumen, may also serve to activate the FA to FA-CoA and thus may function as a FA-CoA synthetase (14). Recent evidence has challenged the apical location of FATP4, instead placing it at the ER membrane (15). Thus, at the level of the ER, FATP4 in effect captures an absorbed FA (delivered to it by acylating the FA to their CoA derivative) because FA-CoA cannot cross the apical membrane. This process is termed vectorial acylation (15). Because FA-CoA also forms micelles and thus may perturb cellular membranes above its critical micellar concentration (CMC), FA-CoA—like FA—needs to be protein bound to lessen its potential for disrupting cellular membranes, a function that can be performed in the cytosol by L-FABP as well as by acyl-CoA-binding protein (16). On the ER membrane itself, FA-CoA can of course be handed off to the complex lipid–synthesizing enzymes, as described below.
The membrane by which FA enter the cell also influences its ultimate disposition, although the incorporation of the FA into complex lipids occurs at the level of the ER regardless of the portal of entry. FA entering from the apical membrane are more likely to enter the triacylglycerol (TAG) synthetic pathway than if they enter via the basolateral membrane, presumably from the circulation (17, 18). By contrast, FA entering from the basolateral membrane are more likely to enter the phospholipid synthetic pathway or to be oxidized (17, 18). In contrast to these studies, in which the radiolabeled FA tracer was given as an acute (up to 2 min) injection to conventionally fed rats, if the tracer (19) was constantly given intravenously to rats receiving a large intraduodenal TAG infusion until a steady-state-specific activity of mucosal TAG and FA was obtained (6 h), the tracer was predominantly found in the TAG fraction. Under these conditions, the FA presumably entered the enterocyte from the basolateral membrane and was converted to TAG.
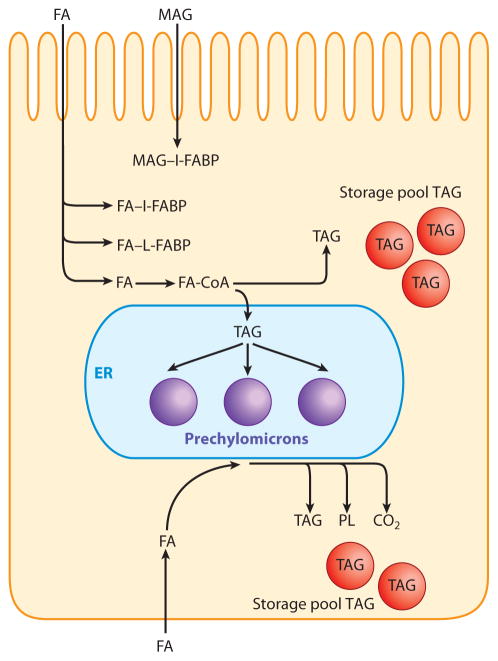
In sum, ligand binding is one method available to the enterocyte to mitigate the adverse effects of the surface-active molecules FA and FA-CoA. A second available method is the conversion of these compounds to the much more physicochemically inert TAG. The conversion of FA to TAG occurs at the level of the ER, a process detailed in the following section. A summary of these steps is shown in Figure 1.
Figure 1.
Absorption of dietary lipid and its conversion to triacylglycerol (TAG). Fatty acids (FA) and sn-2-monoacylglycerol (MAG) resulting from pancreatic TAG lipase hydrolysis of dietary TAG are absorbed by the enterocytes. The FA are then bound to either intestinal-type FA-binding protein (I-FABP) or liver-type FABP (L-FABP), and the MAG is bound to L-FABP. The FA may instead acylate CoA via the action of FA-transport protein 4 (FATP4) at the level of the endoplasmic reticulum (ER). Both FA and MAG are converted to TAG by the complex lipid–synthesizing enzymes of the ER and are incorporated into chylomicron TAG in the ER lumen. Alternatively, especially if large lipid loads are presented to the ER for TAG synthesis, the TAG formed on the cytosolic side of the ER may phase separate as TAG droplets and enter the TAG storage pool. FA entering the enterocyte via the basolateral membrane are transported to the ER, perhaps via FABPs, and are also converted to TAG. However, this TAG is more likely to enter the TAG storage pool than to be incorporated into chylomicron TAG. The FA may also be either incorporated into phospholipids (PL) or metabolized to CO2 in the mitochondria (not shown).
Overview
Because the complex lipids of the diet need to undergo lipolysis in order to be efficiently absorbed, the resulting FA and sn-2-monoacylglycerol (MAG) require resynthesis to TAG for two purposes, the first to produce a physicochemically more inert product (TAG) and the second to enable the eventual direction of the dietary lipid to its intended target tissues (adipose and muscle). Lipid absorbed as FA alone goes to the liver via the portal vein, whereas as TAG the lipid can be included in chylomicrons and directed to its targeted destination by the lipoproteins on the chylomicron surface. In addition, FA delivered to the liver are in part exported as very low-density lipoprotein (VLDL), the major liver TAG-rich lipoprotein, which on its metabolism by lipoprotein lipase ultimately produces low-density lipoprotein (LDL), the major cholesterol-carrying lipoprotein in the plasma. By contrast, TAG that is absorbed as chylomicron TAG, when metabolized, produces not only chylomicron remnants but also phospholipid discs from the excess surface phospholipids as the TAG is removed (20). These discs take up cholesterol and acquire apolipoprotein AI (apoAI), resulting in high-density lipoprotein (HDL), the so-called reverse cholesterol transporter. In humans (21) and mice (22), up to 70% of circulating HDL is produced by this mechanism.
Acyl-CoA Synthetases
The acyl-CoA synthetases (Acsl) are a family of CoA synthetases that activate long-chain FA by forming the acyl-AMP derivative, which acylates CoA. This process results in the end products acyl-CoA, AMP, and PPi. There are five members of this family, which have 30 to 60% homology. Acsl are tissue specific; only Acsl3 and Acsl5 are significantly expressed in the intestine (23). Acsl function as the initiator of acylation in complex lipid synthesis, each step of which requires activated FA. This process is similar to what has been proposed for FATPs. Of the six members of this family, only FATP4 is expressed in the intestine (24).
In addition to functioning as FA activators, Acsl may also influence the pathway by which TAG is synthesized (25). As discussed further below, TAG can be synthesized either by the progressive acylation of MAG or via the acylation of glycerol-3-phosphate (G-3-P), with subsequent acylation and dephosphorylation. The common product of the two pathways, diacylglycerol (DAG), is not metabolically equivalent in that DAG synthesized from MAG can only be acylated, whereas DAG synthesized from G-3-P either can be acylated or can enter the phospholipid synthetic pathway (26, 27). Because the substrates between the two pathways are not interchangeable, the pathways may be geographically separated, a suggestion that has received recent support from study of the Tung tree (28). In addition to this separation, FA enter different TAG synthetic pathways both in vivo and in vitro, depending on their route of entry. For example, FA entering via the apical membrane are preferentially shunted to the TAG pathway that provides TAG for incorporation into chylomicrons (7, 17, 29, 30). By contrast, the TAG coming from the basolateral membrane enters the pathway that provides TAG for the intracellular storage pool (19). Eventually, the storage pool is depleted of TAG, which exits the cell not as TAG in chylomicrons but presumably as FA in the portal vein (31). This function could be performed by pancreatic TAG lipase, which is expressed in the intestine (32). In vivo evidence for this supposition comes from rats that received 3H-oleate either by jugular vein or intraduodenally as [3H-oleate]-glyceryltrioleate (TO). The radiolabeled oleate was infused by vein, whereas the nonradiolabeled TO was infused intraduodenally. In the former method, the enterocyte TAG became radiolabeled, but the specific activity of the resulting TAG was less than half that of the TAG in mesenteric lymph chylomicrons (19). One interpretation of these data is that the TAG pool that was labeled by the FA entering from the basolateral side of the cell was not selected for transport from the cell in lymph chylomicrons. On the basis of this finding, we proposed that this TAG formed part of the TAG storage pool in enterocytes (19). By contrast, when the 3H-TO was given intraduodenally, the specific activity of the mesenteric lymph chylomicron TAG was close to what was infused and was nearly twice that of the mucosal TAG (7). An interpretation of these data is that lymph chylomicron TAG’s acyl groups are predominantly composed of the subset of mucosal TAG derived from infusate TAG. This interpretation is supported by experiments that directly tested this hypothesis by intraduodenally infusing linoleate after infusing TO, whose exit as chylomicron TAG was blocked by Pluronic L-81. On recovery of chylomicron output into the lymph by removal of the inhibitor, the lymph chylomicron TAG acyl groups remained mostly oleate with little linoleate (33). Additional studies in Caco-2 cells (34) also support the concept that dietary FA are primarily substrates for chylomicron TAG.
Monoacylglycerol Acyltransferase
In the intestine, TAG is hydrolyzed to two FA and one MAG by pancreatic TAG hydrolase (pancreatic lipase). This enzyme strongly prefers primary alcohols for hydrolysis, leaving the secondary alcohol in the sn-2 position of glycerol still esterified to the FA available for absorption. Although there is some isomerization of sn-2- to sn-1-MAG, the reaction is slow. Whether or not the MAG remains as sn-2 or sn-1, it can be hydrolyzed within the intestinal lumen to FA and glycerol by cholesterol esterase. If the sn-2 acyl group has migrated to the sn-1 position, it may be hydrolyzed by pancreatic TAG lipase. Despite these hydrolytic potentials, most sn-2-MAG remains unhydrolyzed and is absorbed as sn-2-MAG, resulting in 75% of it appearing in the lymph as chylomicron sn-2-TAG (35). The small amount of sn-1-MAG that may be absorbed could potentially be hydrolyzed by MAG hydrolase, present in intestinal ER (36), to FA and glycerol.
The MAG that is presented to the ER for acylation is acylated by monoacylglcyerol acyltransferase (MGAT). MGAT has three isoforms: MGAT1, -2, and -3. MGAT1 is not expressed in the intestine (37). MGAT2 is expressed exclusively in the small intestine of mice and more ubiquitously in humans (38). Interestingly, although transcripts for MGAT2 are found in human liver in contrast to rodent liver, no MGAT activity is expressed (39). In intestine, MGAT2 is very responsive to a high-fat diet and increases in activity as much as threefold, although MGAT2 mRNA does not change, suggesting posttranslational regulation (36, 40). The gradient of MGAT2 mRNA along the small intestine also parallels the proximal-to-distal gradient of lipid absorption in both rats and humans (6, 7, 40). By contrast, MGAT3 is expressed five times more in the ileum than in the proximal intestine in higher species, but it is not expressed in rodents (41). Consistent with its greater sequence homology to DGAT2 than to either MGAT1 or -2, MGAT3 has a much greater ability to acylate DAG than does either MGAT1 or -2 (42). MGAT2, however, does have considerable DGAT activity (43). In sum, these data suggest that MGAT2 is the only MGAT that functions in rodent intestinal mucosa, which helps elucidate data interpretation of studies dependent on this enzyme. In addition, gene disruption of MGAT2 supports the central role of this isoform in the distribution of dietary fat to peripheral tissues as, presumably, chylomicron TAG (44). MGAT2−/− mice do not gain weight, and unlike wild-type mice they do not develop aspects of the metabolic syndrome on a high-fat diet.
As with FA entry into enterocytes, the metabolic fate of MAG also depends on its site of entry. MAG entering from the apical membrane is predominantly utilized for TAG synthesis, whereas if it enters from the basolateral membrane phospholipid synthesis predominates, especially that of phosphatidylethanolamine (17).
Because of isomerization or other mechanisms, when sn-1- or sn-3-MAG results from TAG hydrolysis in the intestinal lumen and is absorbed, it is acylated by MGAT, resulting in sn-1,3-MAG (37, 45). This DAG isomer is a metabolic dead end (46) in rodents but not in higher species, where TAG can be produced from the sn-1,3 isomer (42).
The sum of the data on MAG absorption suggest that the FA esterified in the sn-2 position of dietary TAG are preserved to be incorporated into chylomicron TAG. This preservation of the FA at the sn-2 position has led to the proposal that one way to deliver essential FA to the liver is by this mechanism. In this scenario, essential FA esterified at the sn-2 position in chylomicrons could, after lipoprotein lipase attack on the chylomicron resulting in a chylomicron remnant, be retained as TAG in the remnant particle and taken up by the liver’s chylomicron remnant uptake mechanism.
Diacylglycerol Acyltransferase
Acetylation of DAG to TAG in the intestine is performed by acyl-CoA:diacylglycerol acyltransferase (DGAT). DGAT comprises three enzymes: DGAT1, DGAT2, and MGAT3. MGAT3, although it acylates MAG to DAG, as suggested by its name, also has considerable DGAT ability (42). DAG, from whatever source, is a branch point in complex lipid synthesis. Either the DAG can be acylated to TAG by a member of the DGAT family, or it can enter the phospholipid synthetic pathway through, for example, CDP-ethanolamine:diacylglycerol ethanolaminephosphotransferase or CDP-choline:diacylglycerol cholinephosphotransferase. In the intestine, synthesis of TAG from DAG clearly predominates over the synthesis of phospholipids (47); nevertheless, both processes are very active. This synthesis of TAG and phospholipids results in the quick disposal of DAG, which might otherwise engage in its function as a second messenger. The fact that it does not, despite its presence in reasonable concentrations during fat absorption, is likely due to compartmentalization of the DAG to the ER in the enzyme-bound state.
Although it is not clear which enzyme with DGAT activity is regulated, chronic (7 days) (48) but not acute (24 h) (49) fat feeding increases total DGAT activity, suggesting transcriptional regulation rather than a more acute regulatory mechanism such as phosphorylation.
DGAT1
In the overall synthesis of TAG, the function of DGAT1—first cloned in 1998 (50)—was not understood until a DGAT1−/− mouse model was constructed (51). Preliminary data from these studies showed that DGAT mRNA was expressed predominantly in the villi of the proximal intestine, as would be expected for an enzyme engaged in active lipid absorption. On gene disruption, although the knockout mice absorbed fat normally, the fat-bolused knockout mice were unable to mount the chylomicronemia observed in wild-type mice (51). Despite the absence of DGAT1, lipid droplets were found in the cytosol in chronically high-fat-fed knockout mice, suggesting the presence of TAG in the mucosa, which was confirmed by Oil Red O studies. The data further suggested that a TAG synthetic route other than DGAT1 was present in the intestine. This route was shown to be DGAT2. Unexplained in the DGAT1−/−gene–disrupted mice was the excessive use of energy for heat production associated with an increase in uncoupling protein 1, a protein that results in the generation of heat rather than ATP (52). As expected, the DGAT1−/− mice tolerated fasting poorly. In addition to TAG synthesis, DGAT1 expresses MGAT activity, synthesizes wax mono- and diesters, and can function as a retinol acyl transferase, resulting in retinol ester synthesis (53).
DGAT2
The Farese group (54) cloned and expressed DGAT2, a 388-amino-acid enzyme, which was present in mice in high concentrations in actively TAG-synthesizing tissues such as liver, white adipose, small intestine, and mammary gland. This DGAT was functionally separate from DGAT1 in that it was sensitive to high concentrations of Mg2+ and was from a different gene family. Whereas DGAT1 is related to the acyl CoA cholesterol acyl transferase 1 and 2 family, DGAT2 is related to the MGAT2 (39) and the multifunctional O-acyltransferase (MFAT) families (55). MFAT has been shown to synthesize DAG, wax monoesters, and retinol esters, depending on the acyl substrate presented to the enzyme (55). These activities are similar to those performed by DGAT1 (53).
The membrane orientation of DGAT2 is organized such that both the N and C termini are cytosolically disposed with two transmembrane domains (amino acids 66–115, 86–93) joined by a short bridge (56). The transmembrane domains and bridge are located near the N terminus. Unfortunately, the experiments designed to more precisely localize the bridge could not differentiate between an ER luminal and an intramembrane location (56).
DGAT2 overexpression in McA-RH7777 cells resulted in TAG accumulation in the cytosol to a greater extent than when DGAT1 was overexpressed, emphasizing the role of the former in TAG synthesis (57). However, when DGAT2−/− mice were constructed, the mice died shortly after birth. In the gene-disrupted mice, circulating TAG, FA, and glucose were all severely reduced at birth (57); the mice were underweight; and there was a severe reduction in carcass TAG. Their lack of fat stores was not the only cause of early death, however. There were gross, microscopic, and chemical changes in their skin that resulted in transdermal water loss, which, when reduced by keeping the mice in a more humid atmosphere, resulted in a few more hours of life (57). These data indicate that DGAT1 does not compensate for the loss of DGAT2 activity, a hypothesis supported by the severe reduction in VLDL TAG output by the liver in DGAT2−/− mice despite adequate functioning of DGAT1 (58).
Sn-Glycerolphosphate-3-Acyltransferase
The synthetic pathway utilizing MGAT and DGAT is the predominant pathway used by dietary FA and MAG to produce TAG. Separate from this pathway, however, is a second pathway that produces TAG de novo. This pathway begins with G-3-P, which is acylated at the sn-1 position to sn-1–lysophosphatidic acid (LPA) by G-3-P acyltransferase. The next enzyme acylates LPA to phosphatidic acid, which is mediated by sn-1-acylglycerol-3-phosphate-O-acyltransferase (AGPAT). Phosphatidic acid phosphohydrolase or lipin removes the phosphate group to form DAG, which is then acylated to TAG by DGAT.
There are no data in the intestine that elucidate the rate-limiting step in TAG synthesis by the de novo pathway. However, in liver, both PAP (59) and DAG (60) have been proposed to be limiting. Current data favor DAG.
Formation of the Prechylomicron in Intestinal Endoplasmic Reticulum
The apoB48 found in chylomicrons is the N-terminal 48% translation of the whole apoB gene (apoB100) and is the only form of apoB synthesized in the adult human and rat intestines. This truncation occurs because of the action of apoB-editing catalytic component 1 (APOBEC1), which deaminates cytidine 6666 of apoB100 DNA to produce uridine, resulting in a stop codon (61). Thus far, two major functional differences between apoB48 and apoB100 have been elucidated. Mice whose APOBEC1 gene was disrupted and thus make only apoB100 in their intestine have a reduced chylomicron output into the lymph and TAG accumulation in the mucosa on feeding lipid loads as compared with wild-type mice (62). Secondly, apoB48 lacks the low-density lipoprotein receptor recognition site (63).
The biogenesis of the prechylomicron takes place in a two-step process (64). Each step is described separately here. The first portion of the lipoprotein that forms is the primordial chylomicron, which consists of apoB48 (65), phospholipids, cholesterol, and minor amounts of TAG and cholesterol ester. This particle is dense with a buoyant density similar to HDL (64). The most important part of the particle is apoB48. Its synthesis is constitutive (66), but interestingly the amount surviving is regulated by the availability of lipid on the luminal side of the ER membrane to rescue it from being degraded by the ubiquitin-proteasome degradation pathway (67). Both the fully and partially translated forms of apoB are susceptible to degradation (68). Because of its extreme insolubility in water, as the apoB48 comes slowly through the ER membrane translocon as it is being translated, both the lipid, two chaperones [MTP (69) and BiP], and perhaps other chaperones such as HSP110 (70) are required to prevent its degradation. At least one function of MTP is to prolong the time that apoB48 has to interact with lipids before being degraded (71). When the apoB48 is finished with its translation and movement through the translocon, the apoB48-containing primordial particle detaches from the membrane and is present in the ER lumen, ready to fuse with the lipid-rich, protein-poor particle in the second step of prechylomicron formation.
The lipid-rich particle utilized in the second step of prechylomicron formation is composed mostly of TAG and cholesterol ester. Its protein composition is unknown, but it does not contain apoB48 or apoAI. The TAG in the particle is synthesized at least in part on the cytosolic side of the ER membrane and must traverse the ER membrane to its cytosolic face. This transfer is verapamil sensitive, indicating that more than diffusion of the TAG through the membrane occurs (72). The rapidity by which the TAG can traverse the membrane is called into question by studies showing that, at high TAG intraduodenal input loads, TAG piles up on the cytosolic side of the ER membrane and is susceptible to lipolytic attack (73). This problem would be obviated if at least some of the TAG were synthesized on the luminal side of the membrane, a possibility raised using the carnitine palmitoyl transferase 1 inhibitor etomoxir (74). Once the TAG is on the ER luminal surface, it is presumed that MTP ferries it to the enlarging neutral lipid-rich particle (75). What controls the size of the particle other than substrate availability (76) is not clear, but increasing the expression of apolipoprotein-AIV (apoAIV) is known to greatly increase the size of the secreted chylomicron (77).
There is no information on how the dense and lipid-rich particles merge. Presumably there is target recognition and some mechanism to reduce the surface pressure of the lipid-rich particle, enabling the surface of the dense particle to combine with it. Once formed, there is only one apoB48 per chylomicron (78), so studies that show an alteration in lymph apoB48 output imply that the number of chylomicrons secreted into the lymph is altered as well.
EXIT OF PRECHYLOMICRONS FROM THE ENDOPLASMIC RETICULUM
Following their biosynthesis in the ER lumen, prechylomicrons are transported to their next destination, the cis-Golgi, along the secretory pathway. The movement of prechylomicrons from the ER to the Golgi consists of a cascade of complex events; however, their exit from the ER and delivery to the Golgi are the two main steps (81–84). The ER exit of prechylomicrons is defined as the rate-limiting step in their overall secretion from the enterocytes (30, 85). Because of their very large size (average diameter ~250 nm) (83), how prechylomicrons traverse the ER and Golgi membranes is unclear. Spatial constraints impede their transit across the ER membrane. Furthermore, the prechylomicrons require information on their surface that would target them to the Golgi, and their TAG is susceptible to lipolysis from pancreatic lipase, expressed in the intestine and present in the cytosol (32). These concerns could be easily addressed if a membrane-enclosed space large enough to contain specific cargo and able to contain targeting proteins on its surface were available to perform these functions. A well-described paradigm for this transport mechanism, in which newly synthesized proteins are carried from the ER to the Golgi in membrane-bound vesicles (referred to herein as protein transport vesicles), already exists. Because coat protein complex II (COPII) regulates the biogenesis of protein transport vesicles, these vesicles are often termed COPII vesicles (86–89).
The COPII coat is composed of five different cytosolic proteins. The orderly recruitment of these proteins to the ER membrane leads to vesicle-cargo selection and generation (90–92). The biogenesis of these vesicles is highly organized and occurs at distinct sites on the ER membrane known as ER exit sites (93–95). The assembly of COPII begins with the binding of Sar1-GDP to Sec12, an integral ER-membrane protein that functions as a guanine nucleotide exchange factor for Sar1 (96–98). Sec12 catalyzes the conversion of Sar1-GDP to Sar1-GTP, which causes the exposure of an alpha-helical component of Sar1 that inserts into the ER membrane (99, 100). The membrane-bound Sar1 recruits Sec23-Sec24 as a heterodimer, which forms the inner layer of the COPII coat. This membrane-bound Sar1-Sec23-Sec24 complex is often termed the prebudding complex (100, 101). Sec24 helps select proteins to be transported in the vesicle by interacting with the cytosolic domains of specific, potential cargo proteins (101, 102). Although Sec24 plays a major role in cargo recruitment, Sar1 and Sec23 also participate in the cargo-selection process (103). Once bound to the ER membrane, the Sar1-Sec23-Sec24 complex recruits Sec13-Sec31 as a heterotetramer (104), which forms the outer layer of the coat and mediates membrane deformation, thereby leading to vesicle generation (105). The size of these protein transport vesicles, however, ranges between 55 and 70 nm in diameter (106). Recent studies from Balch’s group (107) employing cryoelectron microscopy have elucidated the architecture of the COPII coat. These studies revealed that the geometry of the COPII coat is flexible enough to accommodate cargos that are large in size, but not greater than 100 μm. Therefore, the size of the coat is not sufficient for much larger cargos such as prechylomicrons (107).
Biogenesis of the Prechylomicron Transport Vesicle
The egress of prechylomicrons from the ER does not follow the canonical COPII-mediated ER-budding process (83). In a study of how nascent prechylomicrons do exit the ER membrane, in vitro assays revealed the existence of a novel vesicle, the prechylomicron transport vesicle (PCTV) (83). PCTVs were able to be isolated from other vesicles by their light buoyant density, which is due to their TAG-rich cargo. Together, electron microscopy and size-exclusion chromatography data suggested that the average PCTV is ~250 nm in diameter—large enough to accommodate prechylomicron-sized particles (83). Studies aiming to characterize PCTV as a bona fide transport vesicle suggest that PCTVs (a) can contain prechylomicrons, as indicated by the presence of apoB48 and apoAIV, marker proteins of prechylomicrons, (b) do not carry ER-resident proteins calnexin or calreticulin, (c) are not fragmented ER membranes (prechylomicron apoB48 within PCTVs is protected from proteolysis by strong proteases), and (d) can fuse with and deliver their cargo, prechylomicrons, to the Golgi lumen (108). These studies also reveal that PCTVs differ from protein transport vesicles with regard to size, density, cargo, and, most strikingly, biogenesis from the ER membranes (83). Protein vesicles require Sar1 for their formation. By contrast, through use of Sar1-depleted cytosol and ER membranes, our laboratory has shown that PCTV generation not only continued but was enhanced several-fold in the absence of Sar1 (83). In the absence of Sar1, the formation of protein transport vesicles was completely abrogated. However, addition of recombinant Sar1 to the Sar1-depleted system not only restored protein transport vesicle formation, it also brought PCTV generation back to the level found in native cytosol.
Electron microscopy studies that used immunogold labeling, coupled with immunoblotting data, show that PCTVs generated in the presence of wild-type intestinal cytosol contain all five components of the COPII coat (83). These data raise the valid question of why COPII proteins are present on PCTVs if they are not involved in their biogenesis. At least a partial answer can be found in additional studies showing that when PCTVs generated via the Sar1-depleted system were used in an in vitro PCTV-Golgi fusion assay they failed to fuse with Golgi and did not deliver prechylomicrons to the Golgi lumen (83). Through use of blocking antibodies to individual COPII components, it has also been shown that the PCTV-Golgi fusion event could be stopped. Together, these results indicate that the COPII proteins, although not necessary for PCTV formation, are essential for PCTV fusion with the Golgi, suggesting a role for the COPII proteins in targeting PCTVs to the Golgi.
Studies focusing on discovering how PCTVs bud off the ER membrane suggest that prechylomicrons utilize a new paradigm to exit the ER in which megasized vesicles are required to be produced from the ER to transport prechylomicrons. To determine which proteins drive PCTV formation, intestinal cytosol was fractionated on size-exclusion chromatography, and each fraction was tested in a PCTV-budding assay (109). In contrast to the COPII coat, whose relative molecular mass (Mr) is ~576 kDa, the active fraction that could generate PCTVs with an efficiency equal to native cytosol eluted off the column in the range of very low Mr proteins. Additional chromatographic refinement of the active fraction and subsequent identification of its proteins revealed that L-FABP is the only fully active cytosolic protein that can generate PCTVs on its own (109). That vesicles generated using recombinant L-FABP alone were bona fide PCTVs was supported by their size (average ~250 nm), buoyant density, and the presence of apoB48, apoAIV, and vesicle-associated membrane protein 7 (VAMP7), marker proteins for PCTVs (109). Interestingly, L-FABP-generated PCTVs were not fusion competent and, therefore, could not deliver chylomicrons to the Golgi. This could be because these PCTVs do not contain COPII proteins, which are required for PCTV-Golgi fusion. Although L-FABP is clearly a key player in PCTV biogenesis, other proteins are likely to play important roles in targeting, docking, and eventual fusion of PCTVs with the Golgi.
Regulation of Prechylomicron Transport Vesicle Biogenesis
PCTV budding does not require GTP, whereas protein transport vesicle biogenesis is GTP dependent (88). However, PCTV formation is ATP dependent, as was determined by complete cessation of PCTV biogenesis when (a) ATP was replaced with apyrase or (b) an in vitro budding assay was carried out in the absence of ATP (83). A clear requirement of ATP in PCTV biogenesis suggests a role for a kinase and/or a phosphorylation event. A variety of inhibitors of protein kinase A and protein kinase B did not have any effect on PCTV budding. However, ER membranes and cytosol that had been pretreated with calphostin C, a common inhibitor of all protein kinase C (PKC) isoforms, displayed a significant reduction in PCTV formation, suggesting the involvement of a PKC (110). Further studies from our laboratory have revealed that PKCζ plays an essential role in the regulation of PCTV formation (110). More specifically, PKCζ-mediated phosphorylation of a small Mr protein is shown to be critical to PCTV generation (110). Identification of this protein would provide a better understanding of the underlying regulatory mechanism.
Fusion of the Prechylomicron Transport Vesicle with cis-Golgi
Once the PCTVs are released into the cytosol, the next steps on their itinerary are targeting to and fusing with cis-Golgi. Because PCTVs shuttle unique cargo and appear to be morphologically and biochemically different from protein transport vesicles, it is likely that they utilize different fusion machinery. In general, specific soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins facilitate the targeting and fusion of a specific vesicle with its specific target membrane (111–115). SNAREs are membrane proteins that are localized to vesicles (v-SNAREs) and their cognate target membranes (t-SNAREs). Each SNARE protein contains a characteristic ~70-amino-acid sequence referred as the SNARE motif (116). Based on the presence of arginine (R) or glutamine (Q) residues in their centers, SNAREs are classified either as R-SNARE (localized to vesicles) or as Q-SNARE (localized to target membranes) (117). Q-SNAREs are further divided into Qa, Qb, and Qc subtypes. The R-SNARE docks with its cognate target membrane by an intricate mechanism in which the R-SNARE binds to its cognate Q-SNAREs to form a four-member alpha-helix coiled-coil structure necessary for fusion. The SNARE complex formed between two membranes is known as a SNARE-pin (118). All SNARE complexes are composed of one R-SNARE and one each of Qa-, Qb-, and Qc-SNAREs (116). Not only does the SNARE complex bring two membranes into close proximity, but the complex alone is able to initiate fusion between the two membranes (113, 114). Studies to determine the precise molecular mechanism underlying PCTV-Golgi fusion event revealed that PCTVs utilize VAMP7 as a v-SNARE (108). Although VAMP7 normally localizes to endosomes and the Golgi and is involved in post-Golgi secretory events (119–121), in the intestine it is uniquely localized to the ER (108). Our studies have demonstrated that PCTVs utilize VAMP7 as an R-SNARE for their fusion with Golgi (108). To investigate the SNARE complex formation between PCTV and Golgi membranes, an in vitro docking assay that enables SNARE complex assembly but restricts the actual fusion process was developed. This was accomplished by eliminating Mg-ATP and incubating PCTVs with Golgi at 4° C. Under these conditions, the SNARE complex forms, but fusion of the PCTV and the Golgi membranes does not occur because the act of fusion is ATP driven (114, 122). Our studies have shown that PCTVs utilize a unique set of SNARE proteins for their docking and fusion with the Golgi. The components of the SNARE complex formed are VAMP7 as the R-SNARE and syntaxin 5, rbet1, and vti1a as the Q-SNAREs (108). Under PCTV-Golgi docking conditions, these proteins constitute a 112-kDa SNARE complex that can be dissociated, on boiling, to their monomeric forms. The functionality of this complex was demonstrated by blocking each of its components, which resulted in significant inhibition of PCTV-Golgi fusion.
Chylomicron Transformation in the Golgi and Post-Golgi Transport
Once fused with the Golgi membrane, a PCTV delivers its prechylomicron cargo to the Golgi lumen. In the Golgi lumen, two transformational events occur. In the first, apoAI—which is not on the prechylomicrons in PCTVs—is acquired by the prechylomicrons in the Golgi (123). In the second, the glycosylation of apoB48 is altered such that it becomes endoH resistant (124), indicating replacement of mannose residues with other carbohydrates. More controversial is the question of the expansion of the developing chylomicron by additional TAG within the Golgi. Some of the requirements for this potential are present. Microsomal triacylglycerol transfer protein (MTP) has been shown to be present in the Golgi (125), although it is considered primarily an ER-localized protein (126) and although most of the lipid transfer activity is located in the microsomes (127). However, the possibility of Golgi localization is enhanced by its lack of the C-terminal ER-retention signal, KDEL, which is present on its heterodimer partner, protein disulfide isomerase (PDI) (79), but not on MTP (126). A newly described splice variant of MTP, MTP-B, is poorly localized to the intestine and likely has little physiological relevance, although it is found in the Golgi (128). Prechylomicrons in PCTVs have the same average diameter as mature chylomicrons (83), suggesting that if additional TAG is added to the chylomicrons in the Golgi, the amount is small.
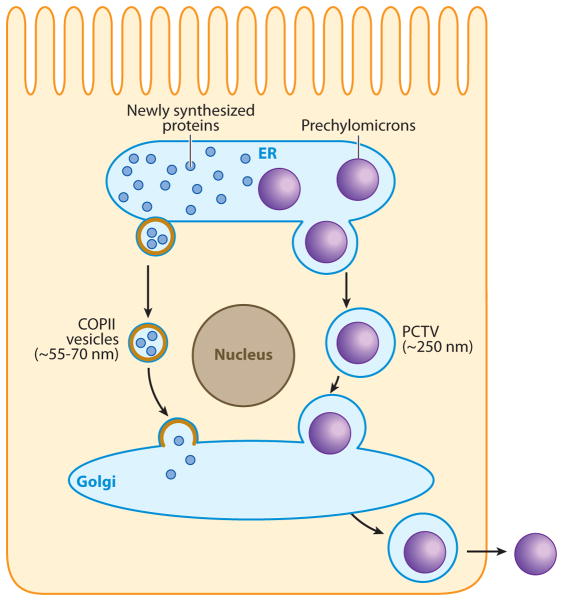
Little is known about the exit of chylomicrons from the Golgi, but morphological data suggest that a vesicle transports them, several at a time, to the basolateral membrane (129). There, the vesicle fuses with the basolateral membrane, and the chylomicrons are released into the lamina propria by reverse exocytosis (129). The transit of chylomicrons from their formation in the ER to their exit from the enterocyte at the basolateral membrane is summarized in Figure 2.
Figure 2.
Intracellular prechylomicron transport. The assembly of prechylomicrons occurs in the lumen of the endoplasmic reticulum (ER). After their biogenesis in the ER lumen, prechylomicrons are packaged into specialized vesicles known as prechylomicron transport vesicles (PCTVs). The average diameter of the PCTVs is ~250 nm, which is sufficient to enclose chylomicron-sized particles. PCTVs bud off the ER membrane and move to and fuse with the cis-Golgi, delivering their prechylomicron cargo to the Golgi lumen. Nascent proteins are transported from the ER to the Golgi in coat protein complex II (COPII) vesicles. Their size ranges between ~55 and 70 nm. PCTVs bud from the ER membrane in the absence of COPII proteins, whereas the protein vesicles require the COPII machinery for their budding. After processing in the Golgi, mature chylomicrons are transported to the basolateral membrane via a separate vesicular system.
SUMMARY
In sum, dietary lipid absorption is the complex process whereby water-insoluble lipids such as TAG are made more water interactive, enabling absorption into enterocytes. Enterocytes convert these hydrolytic products rapidly back into water-insoluble TAG to avoid membrane disruption and package the TAG into a transport vehicle, the chylomicron, so that ultimately the dietary lipid can be directed to specific organs in the body for metabolism or storage. How FA and MAG are desorbed from the apical membrane of enterocytes is controversial, but the process has been shown to involve several proteins that transport the FA and MAG to the ER. There, the FA and MAG are rapidly resynthe-sized to TAG, in part to avoid their membrane-disruptive effects and in part to enable their inclusion in the unique intestinal TAG-rich lipoprotein, the chylomicron. Within the ER lumen, the mature chylomicron is formed in a two-step process and, in the rate-limiting step, exits from the ER in a specialized transport vesicle, PCTV, and is transported to the Golgi. There the chylomicron matures by the addition of apoA1, constituent proteins undergo glycosylation changes, and the mature chylomicron is exported into the lamina propria from the basolateral membrane to join the circulation via the thoracic duct lymph.
FUTURE DIRECTIONS
Research in the area of lipid absorption is of critical interest with the belief that better understanding of this process will lead to new ways to combat the obesity epidemic. At each step, important questions remain to be answered. A few are highlighted here. During the entry of FA into the enterocyte, what mechanism drives the FA to the ER? Although diffusion is certainly one potential mechanism, the random nature of diffusion and the rapidity of the process by which FA become incorporated into TAG at the level of the ER suggest the possibility of a vesicle or some other structure that offers a targeting mechanism to move the FA more quickly to the ER. Another important area of research is which DGAT is the main provider of TAG for chylomicrons. There is an apparent limit on the mass of TAG that can cross the ER membrane from the cytosolic to the luminal side for eventual incorporation into chylomicrons. Can TAG be formed on the luminal side of the ER, thus bypassing the ER membrane transit requirement of the TAG being synthesized on the cytosolic side? The mechanisms by which chylomicrons are formed are incompletely understood. The approach taken by McKnight and Hussain’s groups (71) offers new informative ways of understanding the initial steps in forming the primordial chylomicron. There is no information on how the primordial chylomicron, once formed, combines with the TAG-rich, apoB48-absent, lipid droplet to form the prechylomicron. The conjoining of these two particles requires target recognition and fusion steps, both of which have not been addressed. Another area open for exploration is the control of the prechylomicron exit from the ER in PCTV. Protein phosphorylation is one possibility, as suggested by the crucial role played by PKCζ in PCTV budding (80). These are just some of the many areas that are open for exploration in this exciting field of research.
Acknowledgments
The project described was supported by award numbers RO1 DK81413 (S.A.S.) and RO1 DK38760 and RO1 DK074565 (C.M.M.) from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Charles M. Mansbach, Email: cmansbach@utmem.edu.
Shadab A. Siddiqi, Email: ssiddiqi@mail.ucf.edu.
LITERATURE CITED
- 1.Stralfors P. Autolysis of isolated adipocytes by endogenously produced fatty acids. FEBS Lett. 1990;263:153–54. doi: 10.1016/0014-5793(90)80726-y. [DOI] [PubMed] [Google Scholar]
- 2.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, et al. Triglyceride accumulation protects against fatty acid–induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–82. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass NA, Manning JA, Ockner RK, Gordon JI, Seetharam S, Alpers DH. Regulation of the biosynthesis of two distinct fatty acid–binding proteins in rat liver and intestine. J Biol Chem. 1985;260:1432–36. [PubMed] [Google Scholar]
- 4.Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, et al. Intestinal-type and liver-type fatty acid–binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–35. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 5.Shields HM, Bates ML, Bass NM, Best CJ, Alpers DH, Ockner RK. Light microscopic immunocytochemical localization of hepatic and intestinal types of fatty acid–binding proteins in rat small intestine. J Lipid Res. 1986;27:549–57. [PubMed] [Google Scholar]
- 6.Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Investig. 1957;36:1521–36. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansbach CM, II, Arnold A. Steady-state kinetic analysis of triacylglycerol delivery into mesenteric lymph. Am J Physiol. 1986;251:G263–69. doi: 10.1152/ajpgi.1986.251.2.G263. [DOI] [PubMed] [Google Scholar]
- 8.Ockner RK, Manning JA. Fatty acid–binding protein in small intestine: identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Investig. 1974;54:326–38. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass NM. The cellular fatty acid binding proteins: aspects of structure, regulation, and function. Int Rev Cytol. 1988;111:143–84. doi: 10.1016/s0074-7696(08)61733-7. [DOI] [PubMed] [Google Scholar]
- 10.Poirier H, Niot I, Degrace P, Monnot MC, Bernard A, Besnard P. Fatty acid regulation of fatty acid–binding protein expression in the small intestine. Am J Physiol. 1997;273:G289–95. doi: 10.1152/ajpgi.1997.273.2.G289. [DOI] [PubMed] [Google Scholar]
- 11.Hsu K-T, Storch J. Fatty acid transfer from liver and intestinal fatty acid–binding proteins to membranes occurs by different mechanisms. J Biol Chem. 1996;271:13317–23. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 12.Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid–binding proteins. Annu Rev Nutr. 2008;28:73–95. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- 13.Alpers DH, Bass NM, Engle MJ, DeSchryver-Kecskemeti K. Intestinal fatty acid binding protein may favor differential apical fatty acid binding in the intestine. Biochim Biophys Acta. 2000;1483:352–62. doi: 10.1016/s1388-1981(99)00200-0. [DOI] [PubMed] [Google Scholar]
- 14.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 15.Milger K, Herrmann JM, Becker C, Gotthardt D, Zickwolf J, et al. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci. 2006;119:4678–88. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- 16.Frolov A, Schroeder F. Time-resolved fluorescence of intestinal and liver fatty acid binding proteins: role of fatty acyl CoA and fatty acid. Biochemistry. 1997;36:505–17. doi: 10.1021/bi961392i. [DOI] [PubMed] [Google Scholar]
- 17.Storch J, Zhou YX, Lagakos WS. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J Lipid Res. 2008;49:1762–69. doi: 10.1194/jlr.M800116-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangl A, Ockner RK. Intestinal metabolism of plasma free fatty acids: intracellular compartmentation and mechanisms of control. J Clin Investig. 1975;55:803–13. doi: 10.1172/JCI107991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansbach CM, II, Dowell RF. Uptake and metabolism of circulating fatty acids by rat intestine. Am J Physiol. 1992;263:G927–33. doi: 10.1152/ajpgi.1992.263.6.G927. [DOI] [PubMed] [Google Scholar]
- 20.Redgrave TG, Small DM. Quantitation of the transfer of surface phospholipid of chylomicrons in the rat. J Clin Investig. 1979;64:162–71. doi: 10.1172/JCI109435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouma ME, Beucler I, Aggerbeck LP, Infante R, Schmitz J. Hypobetalipoproteinemia with accumulation of an apoprotein B-like protein in intestinal cells. Immunoenzymatic and biochemical characterization of seven cases of Anderson’s disease. J Clin Investig. 1986;78:398–410. doi: 10.1172/JCI112590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young SG, Cham CM, Pitas RE, Burri BJ, Connolly A, et al. A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not the intestine. J Clin Investig. 1995;96:2932–46. doi: 10.1172/JCI118365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mashek DG, Li L, Coleman RA. Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J Lipid Res. 2006;47:2004–10. doi: 10.1194/jlr.M600150-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006;26:3455–67. doi: 10.1128/MCB.26.9.3455-3467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewin TM, Kim JH, Granger DA, Vance JE, Coleman RA. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem. 2001;276:24674–79. doi: 10.1074/jbc.M102036200. [DOI] [PubMed] [Google Scholar]
- 26.Johnston JM, Rao GA, Lowe PA. The separation of the x-α glycerophosphate and monoglyceride pathways in the intestinal biosynthesis of triglycerides. Biochim Biophys Acta. 1967;137:578–80. doi: 10.1016/0005-2760(67)90140-3. [DOI] [PubMed] [Google Scholar]
- 27.Johnston JM, Paultauf F, Schiller CM, Schultz LD. The utilization of the α glycerophosphate and monoglyceride pathways for phosphatidylcholine biosynthesis in the intestine. Biochim Biophys Acta. 1970;218:124–33. doi: 10.1016/0005-2760(70)90099-8. [DOI] [PubMed] [Google Scholar]
- 28.Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, et al. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 2006;18:2294–313. doi: 10.1105/tpc.106.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trotter PJ, Storch J. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J Lipid Res. 1991;32:293–304. [PubMed] [Google Scholar]
- 30.Mansbach CM, II, Nevin P. Intracellular movement of triacylglycerols in the intestine. J Lipid Res. 1998;39:963–68. [PubMed] [Google Scholar]
- 31.Mansbach CM, II, Dowell RF, Pritchett D. Portal transport of absorbed lipids in rats. Am J Physiol. 1991;261:G530–38. doi: 10.1152/ajpgi.1991.261.3.G530. [DOI] [PubMed] [Google Scholar]
- 32.Mahan JT, Heda GD, Rao RH, Mansbach CM., II The intestine expresses pancreatic triacylglycerol lipase: regulation by dietary lipid. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1187–96. doi: 10.1152/ajpgi.2001.280.6.G1187. [DOI] [PubMed] [Google Scholar]
- 33.Halpern J, Tso P, Mansbach CM., II Mechanism of lipid mobilization by the small intestine after transport blockade. J Clin Investig. 1988;82:74–81. doi: 10.1172/JCI113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luchoomun J, Hussain MM. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. J Biol Chem. 1999;274:19565–72. doi: 10.1074/jbc.274.28.19565. [DOI] [PubMed] [Google Scholar]
- 35.Akesson B, Gronowitz S, Herslof B, Ohlson R. Absorption of synthetic, stereochemically defined acylglycerols in the rat. Lipids. 1978;13:338–43. doi: 10.1007/BF02533725. [DOI] [PubMed] [Google Scholar]
- 36.Chon SH, Zhou YX, Dixon JL, Storch J. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J Biol Chem. 2007;282:33346–57. doi: 10.1074/jbc.M706994200. [DOI] [PubMed] [Google Scholar]
- 37.Yen CL, Stone SJ, Cases S, Zhou P, Farese RV., Jr Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc Natl Acad Sci USA. 2002;99:8512–17. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen CL, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem. 2003;278:18532–37. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 39.Lockwood JF, Cao J, Burn P, Shi Y. Human intestinal monoacylglycerol acyltransferase: differential features in tissue expression and activity. Am J Physiol Endocrinol Metab. 2003;285:E927–37. doi: 10.1152/ajpendo.00179.2003. [DOI] [PubMed] [Google Scholar]
- 40.Cao J, Hawkins E, Brozinick J, Liu X, Zhang H, et al. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J Biol Chem. 2004;279:18878–86. doi: 10.1074/jbc.M313272200. [DOI] [PubMed] [Google Scholar]
- 41.Cheng D, Nelson TC, Chen J, Walker SG, Wardwell-Swanson J, et al. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J Biol Chem. 2003;278:13611–4. doi: 10.1074/jbc.C300042200. [DOI] [PubMed] [Google Scholar]
- 42.Cao J, Cheng L, Shi Y. Catalytic properties of MGAT3, a putative triacylgycerol synthase. J Lipid Res. 2007;48:583–91. doi: 10.1194/jlr.M600331-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Cao J, Burn P, Shi Y. Properties of the mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J Biol Chem. 2003;278:25657–63. doi: 10.1074/jbc.M302835200. [DOI] [PubMed] [Google Scholar]
- 44.Yen CL, Cheong ML, Grueter C, Zhou P, Moriwaki J, et al. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med. 2009;15:442–46. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coleman RA, Walsh JP, Millington DS, Maltby DA. Stereospecificity of monoacylglycerol acyl-transferase activity from rat intestine and suckling rat liver. J Lipid Res. 1986;27:158–65. [PubMed] [Google Scholar]
- 46.Weiss SB, Kennedy EP, Kiyasu JY. The enzymatic synthesis of triglycerides. J Biol Chem. 1960;235:40–44. [PubMed] [Google Scholar]
- 47.Mansbach CM., II Complex lipid synthesis in hamster intestine. Biochim Biophys Acta. 1973;296:386–402. doi: 10.1016/0005-2760(73)90097-0. [DOI] [PubMed] [Google Scholar]
- 48.Mansbach CM. Effect of fat feeding on complex lipid synthesis in hamster intestine. Gastroenterology. 1975;68:708–14. [PubMed] [Google Scholar]
- 49.Mansbach CM., II Effect of acute dietary alteration upon intestinal lipid synthesis. Lipids. 1975;10:318–21. doi: 10.1007/BF02532452. [DOI] [PubMed] [Google Scholar]
- 50.Cases S, Smith SJ, Zheng Y-W, Myers HM, Lear SR, et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA. 1998;95:13018–23. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, et al. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem. 2002;277:25474–79. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 52.Chen HC, Ladha Z, Smith SJ, Farese RV., Jr Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol Endocrinol Metab. 2003;284:E213–18. doi: 10.1152/ajpendo.00248.2002. [DOI] [PubMed] [Google Scholar]
- 53.Yen CL, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res. 2005;46:1502–11. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Cases S, Stone SJ, Zhou R, Yen E, Tow B, et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase and related family members. J Biol Chem. 2001;276:38870–76. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 55.Yen CL, Brown CH, Monetti M, Farese RV., Jr A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J Lipid Res. 2005;46:2388–97. doi: 10.1194/jlr.M500168-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone SJ, Levin MC, Farese RV. Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. J Biol Chem. 2006;281:40273–82. doi: 10.1074/jbc.M607986200. [DOI] [PubMed] [Google Scholar]
- 57.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–76. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Millar JS, Cromley DA, Graham M, Crooke R, et al. Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim Biophys Acta. 2008;1781:97–104. doi: 10.1016/j.bbalip.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Brindley DN. Intracellular translocation of phosphatidate phosphohydrolase and its possible role in the control of glycerolipid synthesis. Prog Lipid Res. 1984;23:115–33. doi: 10.1016/0163-7827(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 60.Mayorek N, Grinstein I, Bar-Tana J. Triacylglycerol synthesis in cultured rat hepatocytes. The rate-limiting role of diacylglycerol acyltransferase. Eur J Biochem. 1989;182:395–400. doi: 10.1111/j.1432-1033.1989.tb14844.x. [DOI] [PubMed] [Google Scholar]
- 61.Chen SH, Li XX, Liao WS, Wu JH, Chan L. RNA editing of apolipoprotein B mRNA. Sequence specificity determined by in vitro coupled transcription editing. J Biol Chem. 1990;265:6811–16. [PubMed] [Google Scholar]
- 62.Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, et al. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol. 2008;294:G344–52. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 63.Kita T, Goldstein JL, Brown MS, Watanabe Y, Hornick CA, Havel RJ. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci USA. 1982;79:3623–27. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cartwright IJ, Higgins JA. Direct evidence for a two-step assemble of apoB48-containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes. J Biol Chem. 2001;276:48048–57. doi: 10.1074/jbc.M104229200. [DOI] [PubMed] [Google Scholar]
- 65.Kane JP, Hardman DA, Paulus HE. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci USA. 1980;77:2465–69. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davidson NO, Kollmer ME, Glickman RM. Apolipoprotein B synthesis in rat small intestine. Regulation by dietary triglyceride and biliary lipid. J Lipid Res. 1986;27:30–39. [PubMed] [Google Scholar]
- 67.Sakata N, Wu X, Dixon JL, Ginsberg HN. Proteolysis and lipid-facilitated translocation are distinct but competitive processes that regulate secretion of apolipoprotein B in Hep G2 cells. J Biol Chem. 1993;268:22967–70. [PubMed] [Google Scholar]
- 68.Liao W, Yeung SC, Chan L. Proteasome-mediated degradation of apolipoprotein B targets both nascent peptides cotranslationally before translocation and full-length apolipoprotein B after translocation into the endoplasmic reticulum. J Biol Chem. 1998;273:27225–30. doi: 10.1074/jbc.273.42.27225. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Zhou M, Huang L-S, Wettereau J, Ginsberg HN. Demonstration of a physical interaction between microsomal triacylglyceride transfer protein and apolipoprotein B during the assembly of apo B-containing lipoproteins. J Biol Chem. 1996;271:10277–81. doi: 10.1074/jbc.271.17.10277. [DOI] [PubMed] [Google Scholar]
- 70.Hrizo SL, Gusarova V, Habiel DM, Goeckeler JL, Fisher EA, Brodsky JL. The Hsp110 molecular chaperone stabilizes apolipoprotein B from endoplasmic reticulum-associated degradation (ERAD) J Biol Chem. 2007;282:32665–75. doi: 10.1074/jbc.M705216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang ZG, Liu Y, Hussain MM, Atkinson D, McKnight CJ. Reconstituting initial events during the assembly of apolipoprotein B-containing lipoproteins in a cell-free system. J Mol Biol. 2008;383:1181–94. doi: 10.1016/j.jmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higashi Y, Itabe H, Fukase H, Mori M, Fujimoto Y, Takano T. Transmembrane lipid transfer is crucial for providing neutral lipids during very low density lipoprotein assembly in endoplasmic reticulum. J Biol Chem. 2003;278:21450–58. doi: 10.1074/jbc.M301376200. [DOI] [PubMed] [Google Scholar]
- 73.Mansbach CM, Dowell R. Effect of increasing lipid loads on the ability of the endoplasmic reticulum to transport lipid to the Golgi. J Lipid Res. 2000;41:605–12. [PubMed] [Google Scholar]
- 74.Washington L, Cook GA, Mansbach CM., 2nd Inhibition of carnitine palmitoyltransferase in the rat small intestine reduces export of triacylglycerol into the lymph. J Lipid Res. 2003;44:1395–403. doi: 10.1194/jlr.M300123-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Kulinski A, Rustaeus S, Vance JE. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J Biol Chem. 2002;277:31516–25. doi: 10.1074/jbc.M202015200. [DOI] [PubMed] [Google Scholar]
- 76.Hayashi H, Fujimoto K, Cardelli JA, Nutting DF, Bergstedt S, Tso P. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am J Physiol. 1990;259:G709–19. doi: 10.1152/ajpgi.1990.259.5.G709. [DOI] [PubMed] [Google Scholar]
- 77.Lu S, Yao Y, Cheng X, Mitchell S, Leng S, et al. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J Biol Chem. 2006;281:3473–83. doi: 10.1074/jbc.M502501200. [DOI] [PubMed] [Google Scholar]
- 78.Phillips ML, Pullinger C, Kroes I, Kroes J, Hardman DA, et al. A single copy of apolipoprotein B-48 is present on the human chylomicron remnant. J Lipid Res. 1997;38:1170–17. [PubMed] [Google Scholar]
- 79.Wetterau JR, Combs KA, McLean LR, Spinner SN, Aggerbeck LP. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991;30:9728–35. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- 80.Siddiqi SA, Mansbach CM., 2nd PKCzeta-mediated phosphorylation controls budding of the pre-chylomicron transport vesicle. J Cell Sci. 2008;121:2327–38. doi: 10.1242/jcs.022780. [DOI] [PubMed] [Google Scholar]
- 81.Kumar NS, Mansbach CM., II Determinants of triacylglycerol transport from the endoplasmic reticulum to the Golgi in intestine. Am J Physiol. 1997;273:G18–30. doi: 10.1152/ajpgi.1997.273.1.G18. [DOI] [PubMed] [Google Scholar]
- 82.Kumar NS, Mansbach CM., II Prechylomicron transport vesicle: isolation and partial characterization. Am J Physiol. 1999;276:G378–86. doi: 10.1152/ajpgi.1999.276.2.G378. [DOI] [PubMed] [Google Scholar]
- 83.Siddiqi SA, Gorelick FS, Mahan JT, Mansbach CM., II COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the prechylomicron transport vesicle. J Cell Sci. 2003;116:415–27. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- 84.Siddiqi SA, Kumar NS, St Hilaire RJ, Nutting DF, Mansbach CM., II Nutrient absorption. Curr Opin Gastroenterol. 2000;16:147–53. doi: 10.1097/00001574-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Mansbach CM, II, Dowell R. Effect of increasing lipid loads on the ability of the endoplasmic reticulum to transport lipid to the Golgi. J Lipid Res. 2000;41:605–12. [PubMed] [Google Scholar]
- 86.Rowe T, Aridor M, McCaffery JM, Plutner H, Nuoffer C, Balch WE. COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fromme JC, Schekman R. COPII-coated vesicles: flexible enough for large cargo? Curr Opin Cell Biol. 2005;17:345–52. doi: 10.1016/j.ceb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 89.Barlowe C. COPII: a membrane coat that forms endoplasmic reticulum–derived vesicles. FEBS Lett. 1995;369:93–96. doi: 10.1016/0014-5793(95)00618-j. [DOI] [PubMed] [Google Scholar]
- 90.Barlowe C. COPII and selective export from the endoplasmic reticulum. Biochim Biophys Acta. 1998;1404:67–76. doi: 10.1016/s0167-4889(98)00047-0. [DOI] [PubMed] [Google Scholar]
- 91.Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, et al. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- 92.Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 2002;21:6105–13. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hughes H, Stephens DJ. Assembly, organization, and function of the COPII coat. Histochem Cell Biol. 2008;129:129–51. doi: 10.1007/s00418-007-0363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang BL, Wang Y, Ong YS, Hong W. COPII and exit from the endoplasmic reticulum. Biochim Biophys Acta. 2005;1744:293–303. doi: 10.1016/j.bbamcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–49. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 97.Barlowe C, d’Enfert C, Schekman R. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;268:873–79. [PubMed] [Google Scholar]
- 98.Aridor M, Balch WE. Kinase signaling initiates coat complex II (COP II) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem. 2000;275:35673–76. doi: 10.1074/jbc.C000449200. [DOI] [PubMed] [Google Scholar]
- 99.Huang M, Weissman JT, Beraud-Dufour S, Luan P, Wang C, et al. Crystal structure of Sar1-GDP at 1.7 Å resolution and the role of the NH2 terminus in ER export. J Cell Biol. 2001;155:937–48. doi: 10.1083/jcb.200106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–77. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 101.Kuehn MJ, Herrmann JM, Schekman R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature. 1998;391(6663):187–90. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- 102.Allan BB, Weissman J, Aridor M, Moyer B, Chen CD, et al. Stage-specific assays to study biosynthetic cargo selection and role of SNAREs in export from the endoplasmic reticulum and delivery to the Golgi. Methods. 2000;20:411–16. doi: 10.1006/meth.2000.0954. [DOI] [PubMed] [Google Scholar]
- 103.Aridor M, Fish KN, Bannykh S, Weisman J, Roberts TH, et al. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152:213–29. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lederkremer GZ, Cheng Y, Petre BM, Vogan E, Springer S, et al. Structure of the Sec23p/24p and Sec13p/31p complexes of COPII. Proc Natl Acad Sci USA. 2001;98(19):10704–9. doi: 10.1073/pnas.191359398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stagg SM, Gurkan C, Fowler DM, LaPointe P, Foss TR, et al. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–38. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 106.Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, et al. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–75. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 107.Stagg SM, LaPointe P, Razvi A, Gurkan C, Potter CS, et al. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–84. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siddiqi SA, Mahan J, Siddiqi S, Gorelick FS, Mansbach CM., II Vesicle-associated membrane protein 7 is expressed in intestinal ER. J Cell Sci. 2006;119:943–50. doi: 10.1242/jcs.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, et al. Liver fatty acid–binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem. 2007;282:17974–84. doi: 10.1074/jbc.M610765200. [DOI] [PubMed] [Google Scholar]
- 110.Siddiqi SA, Mansbach CM., II PKC ζ–mediated phosphorylation controls budding of the pre-chylomicron transport vesicle. J Cell Sci. 2008;121:2327–38. doi: 10.1242/jcs.022780. [DOI] [PubMed] [Google Scholar]
- 111.Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–12. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- 112.Sollner TH, Rothman JE. Molecular machinery mediating vesicle budding, docking and fusion. Cell Struct Funct. 1996;21:407–12. doi: 10.1247/csf.21.407. [DOI] [PubMed] [Google Scholar]
- 113.Sollner T, Whitehart SW, Brunner M, Erdjumont-Bromage GS, Tempst P, Rothman JB. SNAP receptors implicated in vesicle targeting and infusion. Nature. 1993;362:318–24. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 114.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–77. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 116.Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell. 2007;18:3463–71. doi: 10.1091/mbc.E07-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–86. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weber T, Zemelman BV, McNew JA, Wetermann B, Gmachi M, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–72. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 119.Pryor PR, Jackson L, Gray SR, Edeling MA, Thompson A, et al. Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell. 2008;134:817–27. doi: 10.1016/j.cell.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galli T. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–38. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Advani R, Prekeris R, Lee K, Klumperman J, Scheller R. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J Cell Biol. 1999;146:765–75. doi: 10.1083/jcb.146.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Söllner T, Bennett MK, Whiteheart S, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–18. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 123.Siddiqi SA, Siddiqi S, Mahan J, Peggs K, Gorelick FS, Mansbach CM., II The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J Biol Chem. 2006;281:20974–82. doi: 10.1074/jbc.M601401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berriot-Varoqueaux N, Dannoura A, Moreau A, Verthier N, Sassolas A, et al. Apolipoprotein B48 glycosylation in abetalipoproteinemia and Anderson’s disease. Gastroenterology. 2001;121:1101–8. doi: 10.1053/gast.2001.29331. [DOI] [PubMed] [Google Scholar]
- 125.Levy E, Stan S, Delvin E, Menardi D, Shoulders C, et al. Localization of microsomal triglyceride transfer protein in the Golgi. J Biol Chem. 2002;277:16470–77. doi: 10.1074/jbc.M102385200. [DOI] [PubMed] [Google Scholar]
- 126.Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136–50. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 127.Wetterau JR, Zilversmit DB. Localization of intracellular triacylglycerol and cholesterol ester transfer activity in rat tissues. Biochim Biophys Acta. 1986;875:610–17. doi: 10.1016/0005-2760(86)90084-6. [DOI] [PubMed] [Google Scholar]
- 128.Mohler PJ, Zhu MY, Blade AM, Ham AJ, Shelness GS, Swift LL. Identification of a novel isoform of microsomal triglyceride transfer protein. J Biol Chem. 2007;282:26981–88. doi: 10.1074/jbc.M700500200. [DOI] [PubMed] [Google Scholar]
- 129.Sabesin SM, Clark SB, Holt PR. Ultrastructural features of regional differences in chylomicron secretion by rat intestine. Exp Mol Pathol. 1977;26:277–89. doi: 10.1016/0014-4800(77)90055-7. [DOI] [PubMed] [Google Scholar]