Abstract
VLDLs (very-low-density lipoproteins) are synthesized in the liver and play an important role in the pathogenesis of atherosclerosis. Following their biogenesis in hepatic ER (endoplasmic reticulum), nascent VLDLs are exported to the Golgi which is a physiologically regulatable event. We have previously shown that a unique ER-derived vesicle, the VTV (VLDL-transport vesicle), mediates the targeted delivery of VLDL to the Golgi lumen. Because VTVs are different from other ER-derived transport vesicles in their morphology and biochemical composition, we speculated that a distinct set of SNARE (soluble N-ethylmaleimide-sensitive factor-attachment protein receptor) proteins would form a SNARE complex which would eventually facilitate the docking/fusion of VTVs with Golgi. Our results show that Sec22b is concentrated in VTVs as compared with the ER. Electron microscopic results show that Sec22b co-localizes with p58 and Sar1 on the VTV surface. Pre-treatment of VTV with antibodies against Sec22b inhibited VTV–Golgi fusion, indicating its role as a v-SNARE (vesicle SNARE). To isolate the SNARE complex, we developed an in vitro docking assay in which VTVs were allowed to dock with the Golgi, but fusion was prevented to stabilize the SNARE complex. After the docking reaction, VTV–Golgi complexes were collected, solubilized in 2% Triton X-100 and the SNARE complex was co-immunoprecipitated using anti-Sec22b or GOS28 antibodies. A ~ 110 kDa complex was identified in non-boiled samples that was dissociated upon boiling. The components of the complex were identified as Sec22b, syntaxin 5, rBet1 and GOS28. Antibodies against each SNARE component significantly inhibited VTV–Golgi fusion. We conclude that the SNARE complex required for VTV–Golgi fusion is composed of Sec22b, syntaxin 5, rBet1 and GOS28.
Keywords: apolipoprotein B (apoB), endoplasmic reticulum (ER), Sec22b, triacylglycerol (TAG), very-low-density lipoprotein-transport vesicle (VTV)
INTRODUCTION
The liver plays a central role in lipid metabolism. It intermittently experiences heavy influxes of NEFAs [non-esterified (‘free’) fatty acids] from a variety of sources. The end product of NEFA influxes into the liver is TAG (triacylglycerol)-rich VLDL (very-low-density lipoprotein), which is eventually secreted into the blood. On its metabolism in the blood, VLDL produces IDL (intermediate-density lipoprotein) and LDL (low-density lipoprotein) which contributes to the pathogenesis of atherosclerosis. The formation of VLDL occurs in the ER (endoplasmic reticulum) and is mediated by microsomal triacylglycerol-transfer protein [1–7]. Once synthesized in the ER lumen, VLDL particles are transported to the cis-Golgi, where their essential structural protein apoB100 (apolipoprotein B100) becomes further glycosylated and phosphorylated [8–11]. It has been well documented that VLDLs are first exported to the Golgi lumen before their secretion from hepatocytes [8–13]. Although the movement of VLDLs from the ER to the Golgi is required for their eventual secretion into the blood, this important step has been scarcely studied.
Transport of nascent proteins along the secretory pathway is highly regulated and mediated by a well-defined COPII (coat complex II) vesicular system [14–20]. Because of their large size and on and off nature of their production depending on TAG supply to the liver, it was initially thought that a different vesicular system might be involved for the ER–Golgi transport of VLDL. Findings from both our laboratory [21] and Fisher’s group [22] have shown that the movement of VLDL from the ER to the Golgi is mediated by distinct vesicles, the VTVs (VLDL-transport vesicles). The biogenesis of VTVs from the ER membranes requires COPII machinery; however, VTVs are different from vesicles that transport newly synthesized proteins from the ER to the Golgi (referred here as protein-transport vesicles) in their size, buoyant density, cargo and protein composition [21].
Specific soluble SNARE (N-ethylmaleimide-sensitive factor-attachment protein receptor) proteins mediate the targeting of transport vesicles to their destinations and play a key role in their docking and fusion with their target membranes [23–26]. SNAREs are membrane-bound proteins and each of them contains a characteristic ‘SNARE motif’ consisting of ~ 70 amino acids [27]. SNAREs are generally referred to as v-SNARE and t-SNARE on the basis of their localization to either vesicles or their target membranes respectively. Transport vesicles dock with their target membranes by an intricate mechanism in which one v-SNARE interacts with its cognate three t-SNAREs to form a four-member α-helix coiled-coil structure necessary for fusion. Each SNARE complex contains one arginine and three glutamine residues in the centre of the complex. On the basis of the presence of an arginine (R) or glutamine (Q) residue, Fasshauer et al. [28] described the SNAREs as R-SNARE (localized to vesicles) or Q-SNARE (localized to target membranes). The Q-SNAREs are subdivided further into Qa-, Qb- and Qc-SNAREs. One R-SNARE and one each of Qa-, Qb- and Qc-SNAREs form a SNARE complex, also called the SNAREpin [28,29], which brings two membranes into close proximity and initiate the fusion process [23,26].
Because VTVs are different morphologically and biochemically from the ER-derived protein-transport vesicles [21,22], it is likely that they utilize a different fusion machinery. We have shown previously that a unique composition of SNARE complex is required for the fusion of PCTVs (pre-chylomicron transport vesicles) with intestinal cis-Golgi [30,31]. The PCTV is the largest ER-derived vesicle [32,33] that uniquely utilizes VAMP7 (vesicle-associated membrane protein 7) as a v-SNARE [31,34]. Although both VTVs and PCTVs are the large ER-derived vesicles engaged in ER–Golgi transport of TAG-rich lipoproteins, VLDL and chylomicrons respectively, both vesicles appeared to be fundamentally different in their budding and fusion mechanisms. The generation of VTVs from the ER membranes requires GTP and COPII proteins, whereas the formation of PCTVs from the intestinal ER requires protein kinase Cζ and L-FABP (liver fatty-acid-binding protein) [35,36]. Even though PCTV-budding is GTP- or COPII-independent [21,30], COPII proteins are required for the fusion of PCTVs with intestinal cis-Golgi [33,37]. In contrast with PCTVs, VTVs do not contain VAMP7. Because of these differences, we hypothesize that VTVs utilize a unique targeting mechanism and fusion machinery.
The present paper describes our endeavours to identify the components of a functional SNARE complex that is involved in targeting and fusion of VTVs with cis-Golgi in primary hepatocytes. We have developed an in vitro VTV–Golgi docking assay that allows the VTV to dock with cis-Golgi, but prevents the fusion event. We report that Sec22b serves as a functional v-SNARE on VTVs. We show further that Sec22b interacts with Syn5 (syntaxin 5), rBet1 and GOS28 to form a fusion complex.
EXPERIMENTAL
Materials
[3H]Oleic acid (9.2 Ci/mM), [14C]oleic acid (56 mCi/mM) and [3H]leucine (180 Ci/mM) were procured from PerkinElmer Life Sciences. Gel electrophoresis and Western blot reagents were obtained from Bio-Rad Laboratories. ECL (enhanced chemiluminescence) reagents were purchased from GE Healthcare. Protease inhibitor cocktail tablets were procured from Boehringer Mannheim. Other biochemicals used were of analytical grade and were purchased from local companies. Sprague–Dawley rats, 150–200 g, were obtained from Harlan. All procedures involving animals were conducted according to the guidelines of the University of Central Florida’s Institutional Animal Care and Use Committee (IACUC) and strictly following the IACUC-approved protocol.
Antibodies
Rabbit polyclonal antibodies against rat VAMP7 (amino acids 105–123) have been described previously [31]. Polyclonal antibodies against mammalian Sar1 have been characterized previously [33]. Mouse monoclonal anti-Sec22b antibodies were purchased from Santa Cruz Biotechnology. Rabbit polyclonal anti-Syn5 antibodies were a gift from Dr William Balch (Scripps Research Institute, La Jolla, CA, U.S.A.), rabbit polyclonal anti-Sec22b antibodies were a gift from Dr Jesse Hay (Division of Biological Sciences, University of Montana, Missoula, MT, U.S.A.) and rabbit polyclonal anti-apoB antibodies were a gift from Dr Larry Swift (Department of Pathology, Vanderbilt University, Nashville, TN, U.S.A.). Mouse monoclonal anti-GOS28, anti-rBet1 and anti-membrin antibodies were procured from StressGen Biotechnologies. Rabbit anti-albumin antibodies were purchased from Bethyl Laboratories. Goat anti-calnexin, anti-Rab11, goat anti-(rabbit IgG), goat anti-(mouse IgG) and goat anti-(rabbit IgG) antibodies conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology. Goat anti-(rabbit IgG) antibodies conjugated to agarose beads were purchased from Sigma Chemical Co.
Preparation of radiolabelled ER
Double-labelled ER containing [14C]TAG/[3H]proteins or single-labelled ER having [14C]TAG only was prepared using essentially the same procedure as described previously [21]. Briefly, freshly isolated small pieces of rat liver were washed thoroughly with ice-cold 0.25 M sucrose in 10 mM Hepes (pH 7.2) and incubated with albumin-bound [14C]oleate (100 μCi) and [3H]leucine (50 μCi) for 30 min at 37°C and washed twice with PBS containing 2% (w/v) BSA to remove the excess [14C]oleate and [3H]leucine, homogenized in buffer A (10 mM Hepes, pH 7.2, 0.25 M sucrose, 0.5 mM EGTA and protease inhibitors) using a Parr bomb, and the ER was isolated using a sucrose step gradient [21,33] that was repeated to purify the ER. The purified ER contained calnexin, a marker protein for the ER, but was devoid of GOS28 and Rab11, markers for cis-Golgi and endosomes/lysosomes respectively, as determined by Western blotting (Figure 1A).
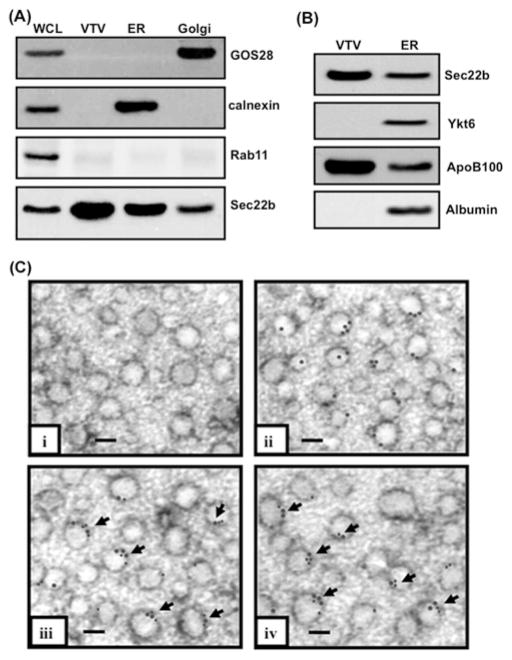
Figure 1. Sec22b concentrates on VTVs and co-localizes with p58 and Sar1 on their surface.
(A) Samples containing hepatic whole-cell lysate (WCL), VTVs, ER and Golgi (each containing30 μg of protein) were separated by SDS/PAGE (12 % gel), transblotted on to a nitrocellulose membrane and probed with specific antibodies against GOS28, calnexin, and Rab11. For details, see the Experimental section. Protein detection was by ECL. (B) Samples of ER and VTVs (30 μg of protein each) were separated by SDS/PAGE (5–15 % gel), transblotted on to a nitrocellulose membrane and probed with specific antibodies against Sec22b, Ykt6, ApoB100 and albumin. Protein detection was by ECL. (C) Immunoelectron microscopy of VTVs utilizing the negative-staining technique. VTVs were adsorbed on formvar-carbon-coated nickel grids and were treated with: (i) anti-rabbit pre-immune IgG; (ii) rabbit polyclonal anti-Sec22b antibodies detected with anti-(rabbit IgG) labelled with 15 nm gold particles; (iii) mouse anti-Sec22b antibodies detected with anti-(mouse IgG) labelled with 15 nm gold particles and anti-Sar1 antibodies detected with anti-(rabbit IgG) labelled with 10 nm gold particles (arrows show co-localization of Sec22b with Sar1); and (iv) anti-p58 antibodies detected with anti-(rabbit IgG) labelled with 10 nm gold particles and mouse anti-Sec22b antibodies detected with anti-(mouse IgG) labelled with 15 nm gold particles (arrows show co-localization of Sec22b with p58). Scale bars, 100 nm.
Preparation of hepatic cytosol
Rat liver cytosol was prepared using the same methodology as detailed previously [21]. In order to remove endogenous ATP and GTP, cytosol was dialysed twice against ice-cold buffer [25 mM Hepes, pH 7.2, 125 mM KCl, 2.5 mM MgCl2, 0.5 mM DTT (dithiothreitol) and protease inhibitors] for 6 h at 4°C [21].
Isolation of cis- and trans-Golgi
The preparation of non-radiolabelled cis- and trans-Golgi was carried out as described in [21]. The purity of cis-Golgi was assessed using marker protein [21] (Figure 1A).
In vitro VTV formation
VTVs containing either [14C]TAG or [14C]TAG/[3H]proteins were generated from single- or double-labelled ER respectively [21]. In brief, radiolabelled ER membranes (500 μg of protein) were incubated at 37°C for 30 min with rat liver cytosol (1 mg of protein), 1 mM GTP and an ATP-regenerating system (1 mM ATP, 5 mM phosphocreatine and 5 units of creatine kinase) in the absence of Golgi acceptor (total reaction volume of 0.5 ml). After incubation, the reaction mixture was resolved on a continuous sucrose density gradient (0.1–1.15 M sucrose) and VTVs isolated from the light portions of the gradient. VTVs thus formed were concentrated using YM-10 centricons (Millipore).
In vitro VTV–Golgi docking assay
In vitro docking of VTV with cis-Golgi was accomplished utilizing a similar methodology with modifications as described by Siddiqi et al. [30]. In brief, VTVs (150 μg of protein) containing [14C]TAG/[3H]protein were incubated with cis-Golgi (300 μg of protein), 1 mM ATP[S] (adenosine 5′-[γ-thio]triphosphate), 1 mM EDTA and cytosol (500 μg of protein) in a buffer containing (30 mM Hepes, pH 7.2, 2.5 mM Ca2+, 2 mM DTT and 30 mM KCl; total reaction volume of 500 μl) for 45 min at 4°C. After incubation, 500 μl of cold buffer (10 mM Hepes, pH 7.2) was added to the reaction mixture and the density was adjusted to 1.22 M sucrose (total volume of 3 ml). This was overlaid with 2.6 ml each of 1.15, 0.86 and 0.25 M sucrose, and the gradient was centrifuged at 25900 rev./min for 3 h at 4°C using an SW41 rotor (Beckman) [21,30]. Under these conditions, VTVs docked with cis-Golgi become isodense, whereas VTVs not associated with the cis-Golgi float to the top of the gradient [21,30]. VTVs associated with the cis-Golgi fraction were collected by aspiration [21]. To assess the docking, two parameters ([14C]TAG and [3H]apoB100) were taken into consideration; [14C]TAG was extracted and [3H]apoB100 was immunoprecipitated from the VTV–Golgi complex and the relative radioactivity was measured.
Isolation of the SNARE complex
To isolate the SNARE complex, VTVs were first docked with hepatic cis-Golgi as described above, and the putative VTV–Golgi complex was isolated on a sucrose step gradient [30]. Under these conditions, undocked VTVs float to the top of the gradient and were removed by aspiration. Putative VTV–Golgi complexes were solubilized using 2% (v/v) Triton X-100 in PBS and were incubated overnight at 4°C with either rabbit anti-Sec22b or anti-GOS28 antibodies complexed to agarose beads. After incubation, beads were washed thoroughly to remove unbound proteins. Complete removal of unbound protein was assessed by the absence of protein content in final washes as determined by protein assay or by the absence of Sec22b as judged by Western blotting.
The beads were either boiled or not boiled in 2× Laemmli buffer, and the immunoprecipitated proteins were separated by SDS/PAGE (5–15% gel) followed by transblotting on to nitrocellulose membranes (Bio-Rad Laboratories) [21]. The same blot was probed with antibodies against the indicated proteins. For re-probing membranes with different antibodies, membranes were washed three times with PBS-T (PBS containing 0.05% Tween 20), incubated in stripping buffer (62.5 mM Tris/HCl, pH 6.7, 100 mM 2-mercaptoethanol and 2% SDS) for 45 min at 56°C, and washed three times with PBS-T, which was followed by blocking in 5% (v/v) Blotto (Bio-Rad Laboratories) in PBS-T overnight.
In vitro VTV–Golgi fusion assay
To examine the fusion of VTVs with hepatic cis-Golgi, VTVs (150 μg of protein) containing [14C]TAG/[3H]protein were incubated with cis-Golgi (300 μg of protein), an ATP-generating system, rat liver cytosol (500 μg of protein), in a buffer containing 1 mM GTP, 2.5 mM Ca2+, 5 mM Mg2+, 2 mM DTT, 30 mM KCl and 30 mM Hepes (pH 7.2) for 30 min at 37°C. After incubation, the reaction mixture was resolved on a sucrose step gradient and the cis-Golgi fraction obtained by aspiration [21]. [14C]TAG was extracted [21] from the cis-Golgi and the radioactivity was measured. [3H]ApoB100 was immunoprecipitated as described below and the radioactivity was determined.
Measurement of TAG activity and protein radioactivity
TAG was extracted and radioactivity was quantified as described previously [21,33]. Proteins were precipitated with TCA (trichloroacetic acid) and radioactivity was measured [21,33]. When samples were double-labelled, the dual-isotope mode was used on the scintillation analyser (TriCarb, Model 2900, PerkinElmer).
Immunoprecipitation and endo H (endoglycosidase H) treatment of apoB100
ApoB100 was immunoprecipitated from either the Golgi or VTV–Golgi complexes applying the same methodology as described previously [21]. Immunoprecipitated [3H]apoB100 was treated with endo H (500 units) for 20 h at 37°C, separated by SDS/PAGE (5–15% gel) and autoradiographed [21].
SDS/PAGE, Western blotting and autoradiography
Proteins were solubilized in Laemmli buffer, and separated by SDS/PAGE (12% gel) [21]. Molecular masses of proteins were calculated as described previously [30].
For Western blotting, proteins were separated by SDS/PAGE (5–15% gel) and transblotted on to nitrocellulose membranes [21]. After blocking with 5% (w/v) non-fat dried skimmed milk in PBS, membranes were incubated with specific primary and then secondary antibodies. Proteins were detected using ECL reagents and exposing the developed blots to Biomax film (Eastman Kodak) [21].
Autoradiography was performed as described previously [21]. In brief, proteins were separated by SDS/PAGE (5–15% gels), gels were rinsed with distilled water and stained with Simply Blue™ SafeStain (Invitrogen). After destaining, gels were dried and autoradiographed using Kodak Biomax film at −70°C.
MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight)
The VTV and ER membrane proteins were separated by two-dimensional SDS/PAGE (8–18% gel), stained with Simply Blue™ SafeStain, and the VTV protein band (molecular mass 24 kDa; pI 8.85) was excised from the gel and destained with 50% acetonitrile and 100 mM ammonium bicarbonate (pH 8.0). The gel pieces were dried in a vacuum centrifuge and digested overnight with trypsin (0.5 mg) at 37°C. The digested peptides were extracted with 60% acetonitrile and 5% TFA (trifluoroacetic acid) with sonication and dried in a vacuum centrifuge. The peptides were suspended in 0.1% TFA, desalted with a ZipTip and applied to a CHCA (α-cyano-4-hydroxycinnamic acid) matrix. MALDI–TOF was performed on a Voyager-DERP mass spectrometer (Applied Biosystems).
Immunoelectron microscopy
To examine VTVs by electron microscopy, we employed the negative-staining technique [21,33]. Briefly, VTVs were generated and concentrated, and a formvar-carbon-coated nickel grid was placed on a drop of concentrated VTV for 2–3 min. The grids were rinsed with PBS and water, stained with 0.5% aqueous uranyl acetate, air-dried and examined using a JEOL 1200 EX electron microscope at 12000× magnification.
Immunolabelling of the VTVs was performed as described in [33,38]. Briefly, samples were incubated with 10% (w/v) BSA containing (i) anti-rabbit pre-immune IgG (1:100), and (ii) rabbit polyclonal anti-Sec22b antibodies (1:100) for 3–4 h and subsequently with anti-rabbit IgG (1:50) conjugated with 15 nm colloidal gold. The samples were fixed in 1% (w/v) glutaraldehyde in PBS for 10 min and stained with 0.5% aqueous uranyl acetate for 1 min, and examined under the JEOL 1200 EX electron microscope at 12000× magnification.
Co-localization of Sec22b with Sar1 and p58 on VTVs was determined by immunogold labelling as described in [33,38]. Briefly, samples were incubated with 10% (w/v) BSA containing (iii) mouse anti-Sec22b (1:100) and rabbit anti-Sar1 (1:100) antibodies or (iv) mouse anti-Sec22b (1:100) and rabbit anti-p58 (1:100) antibodies for 3–4 h and subsequently with anti-(mouse IgG) (1:50) conjugated with 15 nm colloidal gold and anti-(rabbit IgG) (1:50) conjugated with 10 nm colloidal gold. The samples were fixed, stained and examined at 12000× magnification as described above.
Statistical analysis
Comparisons between means were carried out using the Instat statistical package supplied by GraphPad Software using a two-tailed Student’s t test.
RESULTS
Sec22b is present on the VTV
VTVs were prepared from hepatic ER that was free from Golgi and endosomal/lysosomal contamination as determined by the absence of GOS28 and Rab11 respectively (Figure 1A). However, these ER membranes were enriched with calnexin, an ER marker protein, as shown in Figure 1(A). To identify the v-SNARE present on VTVs, we carried out a comparative analysis of VTV and ER membrane proteins; we reasoned that a potential v-SNARE would be more concentrated on VTVs than the ER because VTVs are ER-derived. Since SNAREs are membrane proteins, we decided to collect VTV and ER membrane proteins by incubating them with 100 mM sodium carbonate solution (pH 11) which releases peripheral proteins and vesicle luminal proteins. To detect the protein of interest, we resolved VTV and ER membrane proteins by two-dimensional SDS/PAGE (results not shown) and compared two-dimensional gels of the VTV and the ER, which revealed that a few proteins were concentrated in VTVs. Of the concentrated proteins, the most prominent protein band (molecular mass 24 kDa; pI 8.85), when submitted to the SWISS-PROT database, gave several possible proteins, including Ykt6, Sec22b and VAMP7. These three proteins were of great interest because these are known v-SNAREs that are involved in ER–Golgi transport of secretory proteins and intestinal lipoprotein, the chylomicron [30,31,39–41]. As a first approach to determine which one of these three v-SNAREs is present on VTVs, we carried out MALDI–TOF analysis. MS results identified the protein band of interest as Sec22b with a Z score of 2.19.
To confirm that Sec22b is enriched in VTVs, we performed Western blotting using specific antibodies against Sec22b, Ykt6 and VAMP7 proteins. As shown in Figure 1(B), Sec22b was concentrated in VTVs as compared with the ER membranes. Neither Ykt6 nor VAMP7 was detected in the VTV membranes (Figures 1B and 5B). Ykt6 was present in the ER (Figure 1B), but VAMP7 was not found in the ER (Figure 5B), supporting our previous findings that hepatic ER does not contain VAMP7 [31]. However, VAMP7 was present in hepatic whole-cell lysate and in hepatic Golgi (Figure 5B). To ascertain that our vesicular fraction contain bona fide VTVs, we probed for apoB100, a marker protein for both VLDL and VTVs. As shown in Figure 1(B), apoB100 is concentrated in VTVs [21]. To assess the purity of our VTV fractions and to make sure that VTVs are not contaminated with protein-transport vesicles, we immunoblotted for albumin. Our results indicate that albumin was not present in VTVs, whereas it was present in the ER (Figure 1B). These results indicate that the VTV concentrates Sec22b on its surface as a putative v-SNARE.
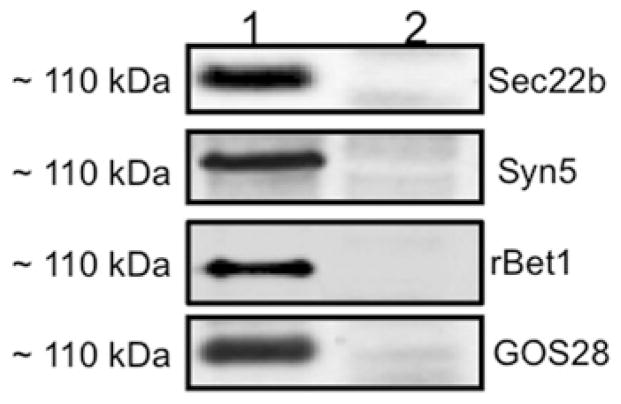
Figure 5. Proteins co-immunoprecipitated with Sec22b in a ~110 kDa complex after VTV–Golgi docking.
(A) VTVs (150 μg) were incubated at 4 °C with hepatic cis-Golgi (300 μg), cytosol (500 μg of protein), and without Mg2+-ATP. VTV–Golgi complexes were isolated on a sucrose step gradient. VTV–Golgi complexes were solubilized in 2 % (v/v) Triton X-100 and incubated with anti-Sec22b antibodies bound to agarose beads at 4 °C overnight. The beads were washed and either not boiled (lane 1) or boiled (lane 2) in Laemmli buffer. The proteins were separated by SDS/PAGE (5–15 %) and probed with antibodies against the indicated proteins. A single membrane was used, which was sequentially probed with the indicated antibodies after washing. Only protein bands that migrated at ~ 110 kDa are shown. Detection was carried out by ECL. The results are representative of four experiments. (B) Samples of hepatic whole-cell lysate (WCL), VTVs, ER and Golgi (each containing 30 μg of protein) were separated by SDS/PAGE (12 % gel), transblotted on to a nitrocellulose membrane and probed with specific antibodies against the indicated proteins. Protein detection was by ECL.
To provide additional morphological evidence for the localization of Sec22b to the VTV, we utilized immunogold labelling of Sec22b on the VTV and examined the results by electron microscopy using the negative-staining technique. The results presented in Figure 1(C) show that Sec22b is localized on the surface of VTV as determined by immunogold labelling (Figure 1C, panel ii), whereas control studies using pre-immune IgG showed no immunogold labelling (Figure 1C, panel i). Since VTVs are ER-derived vesicles, we sought to demonstrate the co-localization of Sec22b with Sar1 and p58, because these two are the marker proteins for ER-derived vesicles [15,42,43]. Both Sar1 and p58 are co-localized with Sec22b on VTVs as depicted in Figure 1(C), panels iii and iv. Taken together, these biochemical and morphological results provide compelling evidence that the VTV contains as well as concentrates the v-SNARE protein Sec22b on its surface.
Sec22b functions as a v-SNARE for ER-derived VTVs
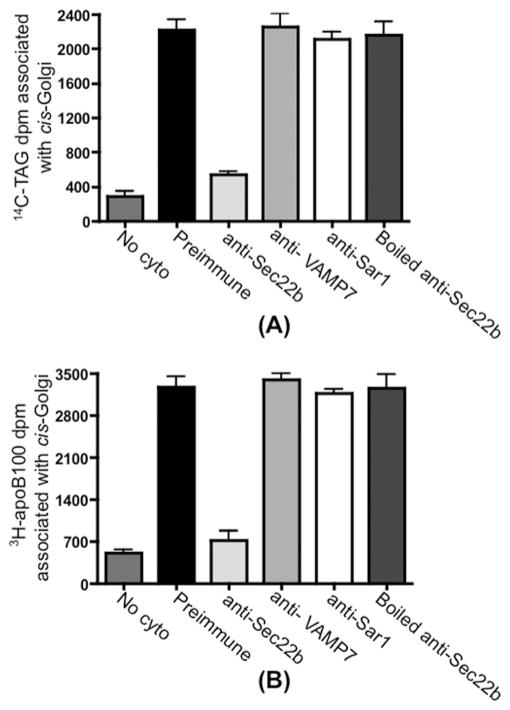
The concentration of Sec22b and its co-localization with Sar1 and p58 on the VTV surface indicate that Sec22b may serve as a v-SNARE for VTVs during ER–Golgi transport of VLDL in primary hepatocytes. To establish the functional role of Sec22b as the potential v-SNARE for the VTVs, the effect of blocking Sec22b on the delivery of VLDL to the cis-Golgi was determined. To achieve this, we utilized an in vitro VTV–Golgi fusion assay which has been established previously [21]. In this assay, [14C]TAG/[3H]protein-containing VTVs were pre-treated with either pre-immune IgG or anti-Sec22b antibodies and excess antibodies were then removed by washing. The treated VTVs were incubated with non-radiolabelled hepatic cis-Golgi in the absence or the presence of hepatic cytosol and an ATP-regenerating system at 37°C for 30 min. After incubation, the cis-Golgi was isolated on a sucrose step density gradient. Under these conditions, unreacted VTVs were expected to be floating on the top of the gradient and were removed by aspiration, whereas the cis-Golgi was at the 0.86/1.15 M sucrose interface. [14C]TAG was extracted and [3H]apoB100 was immunoprecipitated from the Golgi membranes (see the Experimental section). As shown in Figure 2, the treatment of VTVs with anti-Sec22b antibodies greatly reduced the delivery of both [14C]TAG and [3H]apoB100 to the cis-Golgi, whereas pre-immune IgG treatment of VTVs did not have any effect on either [14C]TAG (Figure 2A) or [3H]apoB100 (Figure 2B) delivery to the cis-Golgi.
Figure 2. VTVs utilize Sec22b as a functional v-SNARE to fuse with hepatic cis-Golgi.
VTVs (150 μg of protein) containing [14C]TAG and [3H]apoB100 were incubated at 4 °C with 10 μl of pre-immune IgG, 10 μl of anti-Sec22b antibodies, 10 μl of anti-VAMP7 antibodies, 10 μl of anti-Sar1 antibodies or 10 μl of boiled anti-Sec22b antibodies. Antibodies in each case were removed by washing, and the treated VTVs were incubated with non-radiolabelled cis-Golgi (300 μg of protein) at 37 °C in the presence or absence (no cyto) of cytosol (0.5 mg of protein). (A) After incubation, Golgi membranes were separated and the Golgi-associated [14C]TAG was extracted (A) or the Golgi-associated [3H]apoB100 was immunoprecipitated (B). Total [14C]TAG (A) or [3H]apoB100 (B) levels (in d.p.m.) were determined. For details, see the Experimental section. Results are means + S.E.M. (n = 4).
To determine whether the reduction in the delivery of [14C]TAG and [3H]apoB100 to the cis-Golgi by Sec22b antibodies is because of steric hindrance or not, we examined the effect of pre-treatment of VTVs with anti-VAMP7 antibodies or anti-Sar1 antibodies on the VTV–Golgi fusion event. We selected anti-VAMP7 and anti-Sar1 antibodies because Sar1 is present on VTVs [21] (Figure 1C, panel iii), whereas VAMP7 serves as a functional v-SNARE in the ER–Golgi transport of nascent lipoproteins (pre-chylomicrons) in enterocytes [30]. Both anti-VAMP7 and anti-Sar1 antibodies did not inhibit VTV–Golgi fusion, indicating no effect on the delivery of [14C]TAG and [3H]apoB100 to the cis-Golgi (Figure 2). These results suggest that inhibition of VTV–Golgi fusion is not due to steric hindrance. To rule out the possibility of anti-Sec22b antibody-mediated non-specific inhibition, we boiled anti-Sec22b antibodies for 5 min and then incubated with VTVs. VTVs were washed to remove antibodies and used in a fusion assay. Figure 2 shows that boiled anti-Sec22b antibodies had no effect on the delivery of [14C]TAG and [3H]apoB100 to the cis-Golgi. These results strongly suggest that Sec22b serves as a functional v-SNARE for VTVs to fuse with hepatic cis-Golgi.
VTVs dock with hepatic cis-Golgi
To identify the components of minimal fusion machinery or the SNARE complex, it was necessary to establish an in vitro VTV–Golgi docking assay. Docking of VTVs with cis-Golgi can be defined as the event where VTVs bind to the cis-Golgi, but do not fuse [23,44]. Under docking conditions, we expected that a shift of VTVs from light-density fractions to high-density fractions containing Golgi would occur and VTVs would become isodense with cis-Golgi in a sucrose gradient, whereas unreacted VTVs would be floating at the top of the gradient.
To achieve the VTV–Golgi docking, VTVs containing [14C]TAG and [3H]apoB were incubated with non-radiolabelled acceptor hepatic cis-Golgi and hepatic cytosol for 45 min at 4°C in the absence of Mg2+-ATP. To determine VTV–Golgi docking activity, two variables were considered: (i) measurement of VTV–[14C]TAG (in d.p.m.) present in the VTV–Golgi complex; and (ii) endo H treatment of [3H]apoB100 immunoprecipitated from the VTV–Golgi complex. We took advantage of the fact that VTV–apoB100 is endo H-sensitive, whereas apoB100 becomes endo H-resistant in the Golgi lumen [8,21]. Therefore when VTVs are being docked with the Golgi, VTV–[3H]apoB100 will remain endo H-sensitive; however, VTV–[14C]TAG will be associated with the VTV–Golgi complex.
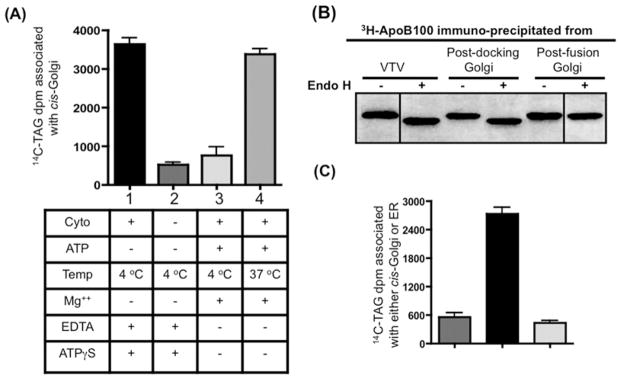
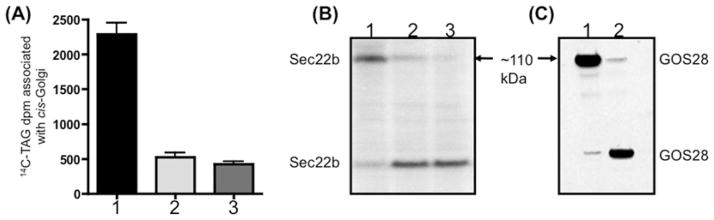
Figure 3(A), shows that VTVs interact with the cis-Golgi upon incubation at 4°C in the presence of cytosol and in the absence of Mg2+-ATP as judged by the presence of [14C]TAG in the VTV–Golgi complex (Figure 3A, bar 1). However, the association of VTVs with Golgi was greatly reduced when incubation occurred without Mg2+-ATP at 4°C, but cytosol was not included in the assay (Figure 3A, bar 2). These results indicate that VTV docking with the cis-Golgi requires cytosolic factors, which is consistent with other reports [30,45].
Figure 3. VTVs dock at hepatic cis-Golgi and require cytosol for the docking and fusion process.
(A) VTVs (150 μg of protein) containing [14C]TAG and [3H]apoB100 were incubated with non-radiolabelled cis-Golgi (300 μg of protein) with or without cytosol at either 4 or 37 °C in the presence or absence of Mg2+ and ATP as indicated. After incubation for 30 min, the cis-Golgi fraction was isolated on a sucrose step gradient and collected by aspiration of the 0.86/1.15 M interface, and the amount of [14C]TAG was measured. The Golgi was separated from VTVs and the Golgi-associated [14C]TAG was extracted and levels were measured. Results are means + S.E.M. (n = 4). ATPγS, adenosine 5′-[γ-thio]triphosphate. (B) Exactly the same experiment was carried out as in (A) and the [3H]apoB100 was immunoprecipitated either from the VTVs, post-docking Golgi (A, bar 1) or the post-fusion Golgi (A, bar 4). Immunoprecipitated [3H]apoB100 was incubated with endo H (500 units) for 20 h at 37 °C, separated by SDS/PAGE (5–15 % gel), and autoradiographed. (C) VTVs (150 μg of protein) containing [14C]TAG were incubated with either non-radiolabelled cis-Golgi (300 μg of protein) (left-hand and middle bars) or non-radiolabelled hepatic ER (right-hand bar) at 4 °C in the presence (middle and right-hand bars) or absence (left-hand bar) of cytosol. Mg2+-ATP were excluded in each case. After incubation, the cis-Golgi fraction was isolated and the [14C]TAG levels were determined. Results are means + S.E.M. (n = 4).
Next, we decided to observe the effect of Mg2+-ATP on VTV–Golgi docking. As shown in Figure 3(A), bar 3, addition of Mg2+-ATP greatly reduced docking activity. These results are consistent with our previously published work in small intestine [30] and also with results from the Rothman group, as these authors suggested that the addition of Mg2+-ATP even at 4°C causes dissociation of SNARE complex [46]. Also, when VTVs were incubated with Golgi in the presence of cytosol and Mg2+-ATP at 37°C, conditions favourable for the VTV–Golgi fusion event (Figure 3A, bar 4), increased [14C]TAG levels were associated with post-fusion Golgi, indicating VTV–Golgi fusion. However, these results (Figure 3A, bars 1 and 4) do not discriminate between VTVs being docked with Golgi and VTVs actually fused with Golgi.
To show more precisely that VTVs are able to dock with Golgi, [3H]apoB100 was immunoprecipitated from the VTV–Golgi complex and treated with endo H. As shown in Figure 3(B), [3H]apoB100 was sensitive to endo H treatment (lane 3 compared with lane 4), when docking conditions (as in Figure 3A, bar 1) were used. Under conditions that support fusion (Figure 3A, bar 4), [3H]apoB100 was resistant to endo H treatment, as shown in Figure 3(B), lane 6, suggesting that [3H]apoB100 has entered the Golgi lumen. Results shown in Figure 3(B) indicate that VTV–apoB100 is endo H-sensitive (lane 1 compared with lane 2), confirming our previous findings [21]. These results provide strong evidence that VTVs dock at, but do not fuse with, hepatic cis-Golgi in the absence of Mg2+-ATP at 4°C. To find out whether VTV–Golgi docking is specific or not, we incubated VTVs with hepatic ER membranes under conditions that favour docking. The results presented in Figure 3(C) suggest that VTVs did not dock with the ER membrane (right-hand bar); however, incubation of VTVs with Golgi under similar conditions gave a robust signal (middle bar). These results are consistent with our previous observations showing that VTV-mediated VLDL export is unidirectional in nature [21].
Isolation of the SNARE complex and identification of its components
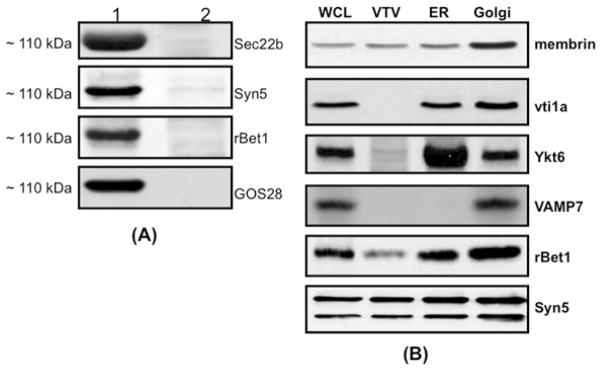
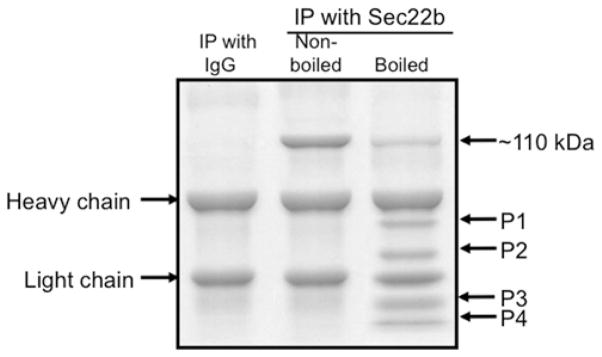
We rationalized that the proteins facilitating the VTV–Golgi docking would be the components of the SNARE complex. In an attempt to isolate the SNARE complex which facilitates the fusion of VTVs with hepatic cis-Golgi, we performed an in vitro docking assay as described above. The VTV–Golgi complexes were separated on a sucrose step gradient, solubilized in 2% (v/v) Triton X-100 and incubated with anti-Sec22b antibodies bound to agarose beads. We selected anti-Sec22b antibodies to pull-down the putative SNARE complex because Sec22b serves as a functional v-SNARE for VTVs; we used pre-immune IgG as control. After an overnight incubation, beads were washed, and the Sec22b-binding proteins were separated by SDS/PAGE followed by Coomassie Blue staining (Figure 4). Under non-boiling conditions, a single strong protein band corresponding to ~ 110 kDa was detected when anti-Sec22b antibodies were used for immunoprecipitation (Figure 4, middle lane), suggesting that Sec22b has formed a complex with other proteins. To find out whether Sec22b exists in a ~ 110 kDa protein complex, we boiled the sample in Laemmli buffer before loading on to the gel. Upon boiling, the ~ 110 kDa band was greatly reduced and dissociated to four low-molecular-mass proteins (Figure 4, right-hand lane); one of the four proteins migrated at 24 kDa which corresponds to the molecular mass of Sec22b. These results suggest that the dissociated proteins (protein bands P1–P4) would be the components of SNARE complex. Pre-immune IgG did not immunoprecipitate either the ~ 110 kDa band or low-molecular-mass proteins under the same conditions (Figure 4, left-hand lane), suggesting that co-precipitation with anti-Sec22b antibodies was specific. These results demonstrate that Sec22b forms a stable high-molecular-mass multi-protein complex during VTV–Golgi docking. Furthermore, these results indicate that the complex is SDS-resistant, a criterion of SNARE complex formation [47,48].
Figure 4. Sec22b pulls down a ~110 kDa protein complex from post-docking VTV–Golgi complexes.
VTVs (150 μg) were incubated at 4 °C with hepatic cis-Golgi (300 μg), cytosol (500 μg of protein) and without Mg2+-ATP. The VTV–Golgi complexes were isolated on a sucrose step gradient. The VTV–Golgi complexes were solubilized in 2 % (v/v) Triton X-100 and incubated with either pre-immune IgG or anti-Sec22b antibodies bound to agarose beads at 4 °C overnight. The beads were washed and either boiled or not, as indicated, in Laemmli buffer. The proteins were separated by SDS/PAGE (5–15 % gel) and the gel was stained. For further details, see the Experimental section. IP, immunoprecipitation.
To determine what proteins are present in the ~ 110 kDa complex, we first identified protein bands P1–P4 (Figure 4, right-hand lane) by MS and Western blotting. Protein bands P1–P4 were identified as Syn5, GOS28, Sec22b and rBet1 respectively. The presence of Syn5, GOS28, Sec22b and rBet1 in a large complex (corresponding to ~ 110 kDa) was confirmed by Western blots using specific antibodies when the samples were not boiled (Figure 5A, lane 1). These results suggest that these four SNARE proteins formed a heteromeric SDS-resistant complex. Essentially, a single membrane was sequentially probed with the indicated antibodies after stripping. Upon boiling, the complex disassembled and each of the SNARE proteins (disassociated from the ~ 110 kDa complex) migrated at its expected monomeric molecular mass (results not shown). The collective molecular mass of these four SNARE proteins is 111 kDa, which is very close to 110 kDa, the observed molecular mass of the SNARE complex.
Next, we considered the possibility that the ~ 110 kDa complex may be the result of detergent-mediated formation of multi-protein complex because SDS and Triton X-100 are known to induce intramolecular disulfide bonding [47]. To address this issue, we probed the same membrane for Ykt6, VAMP7, membrin and vti1a, which are known to be involved in ER–Golgi transport of proteins and lipoproteins [30,39–41]. We found that Ykt6, VAMP7, membrin and vti1a do not exist in the ~ 110 kDa complex (results not shown); however, these proteins were present in hepatic whole-cell lysates and Golgi (Figure 5B). Interestingly, membrin, rBet1 and Syn5 were present in VTV fractions, but were not concentrated as compared with the ER (Figure 5B), whereas Sec22b gave the most prominent signal in VTVs (Figure 5B). These observations lead us to conclude that the ~ 110 kDa complex is not generated by non-specific detergent-mediated intermolecular protein–protein interactions.
To ascertain that Sec22b, Syn5, GOS28 and rBet1 are the components of SNARE complex required for the VTV–Golgi docking/fusion, we cross-checked our results using anti-GOS28 antibodies bound to agarose beads for co-immunoprecipitation. Figure 6 shows GOS28-associated proteins, which were co-immunoprecipitated from solubilized VTV–Golgi complexes. As shown in Figure 6, the same SNARE proteins were co-precipitated with anti-GOS28 antibodies as with anti-Sec22b antibodies. These proteins migrated at ~ 110 kDa, suggesting that the ~ 110 kDa SNARE complex is specific. Taken together, our findings support the conclusion that Sec22b, Syn5, GOS28 and rBet1 form a stable SNARE complex as a result of the interaction of VTVs with cis-Golgi.
Figure 6. Proteins co-immunoprecipitated with GOS28 in a ~110 kDa complex after VTV–Golgi docking.
A similar VTV–Golgi docking assay was performed as in Figure 5(A), and the VTV–Golgi complexes were isolated. The VTV–Golgi complexes were solubilized in 2 % (v/v) Triton X-100 and incubated with either pre-immune IgG or anti-GOS28 antibodies bound to agarose beads at 4 °C overnight. The beads were washed and either not boiled (lane 1) or boiled (lane 2) in Laemmli buffer. The proteins were separated by SDS/PAGE (5–15 %) and probed with antibodies against the indicated proteins. A single membrane was used, which was sequentially probed with the indicated antibodies after washing. Only protein bands migrated at ~ 110 kDa are shown. Detection was carried out by ECL. The results are representative of four independent trials.
Effect of antibodies against SNARE proteins on complex formation
Next, we sought to determine the effect of blocking the SNARE proteins by specific antibodies on complex formation. We incubated either VTVs with anti-Sec22b antibodies or the Golgi membranes with antibodies against GOS28 and then removed the unbound antibodies by washing. Either the treated VTVs and non-treated Golgi or the treated Golgi membranes and non-treated VTVs were used in docking assay. The results presented in Figure 7(A) reveal that the antibodies against Sec22b (bar 2) and GOS28 (bar 3) inhibited VTV–Golgi docking. Treatment of VTVs and Golgi membranes with pre-immune IgG had no effect on VTV–Golgi docking (Figure 7A, bar 1). To demonstrate the effect of blocking the Sec22b or GOS28 on SNARE complex formation, we performed co-immunoprecipitation using anti-Sec22b and -GOS28 antibodies. As shown in Figure 7(B), Sec22b migrated at its monomeric molecular mass when either VTVs with anti-Sec22b antibodies (Figure 7B, lane 2) or the Golgi membranes with antibodies against GOS28 (Figure 7B, lane 3) were treated before the docking assay. However, we found a strong protein band corresponding to ~ 110 kDa when VTVs were treated with pre-immune IgG (Figure 7B, lane 1). Similarly, GOS28 was not detected in ~ 110 kDa complex and migrated at its monomeric molecular mass when cis-Golgi was treated with anti-GOS28 antibodies before the docking assay (Figure 7C, lane 2), whereas pre-immune IgG had no effect on complex formation as was evident by the presence of GOS28 in the ~ 110 kDa complex (Figure 7C, lane 1). Treatment of Golgi membranes with anti-Syn5 or anti-rBet1 antibodies before their use in the docking assay had similar effects as observed with anti-GOS28 antibody treatment (results not shown). To assess the specificity of these findings, we treated VTVs or the Golgi with pre-immune IgG or antibodies against VAMP7, membrin and vti1a, which had no effect on complex formation (results not shown). These results provide strong evidence that all four identified proteins are required for the VTV–Golgi interaction that leads to the SNARE complex formation.
Figure 7. Incubation of VTVs with anti-Sec22b antibodies or hepatic Golgi with anti-GOS28 antibodies blocks VTV–Golgi docking and SNARE complex formation.
(A) VTVs (150 μg of protein) containing [14C]TAG were incubated at 4 °C with 10 μl of pre-immune IgG (bar 1) or 10 μl of anti-Sec22b antibodies (bar 2) for 1 h at 4 °C. Hepatic cis-Golgi (300 μg of protein) was incubated at 4 °C with 10 μl of anti-GOS28 antibodies (bar 3). In each case, the excess antibodies were removed by washing. After antibody treatment, non-radiolabelled cis-Golgi was added to tubes containing IgG-treated VTV (bar 1), Sec22b antibody-treated VTV (bar 2) and untreated [14C]TAG-loaded VTVs were added to tubes containing antibody-treated cis-Golgi (bar 3). The VTV–Golgi docking reaction was carried out and the post-docking Golgi membranes were separated, and Golgi-associated [14C]TAG levels were measured. Results are means + S.E.M. (n = 4). (B) Post-docking Golgi membranes from (A) were solubilized in Laemmli buffer, and proteins were separated by SDS/PAGE and probed with anti-Sec22b antibodies. Lanes 1–3 represent the same docking conditions as applied in bars 1–3 in (A) respectively. (C) Post-docking Golgi membranes from (A) (bars 1 and 2) were solubilized in Laemmli buffer and proteins were separated by SDS/PAGE and probed with anti-GOS28 antibodies. Lanes 1 and 2 represents the same docking conditions as used in bars 1 and 2 in (A) respectively.
Role of SNARE proteins in fusion of VTVs to cis-Golgi
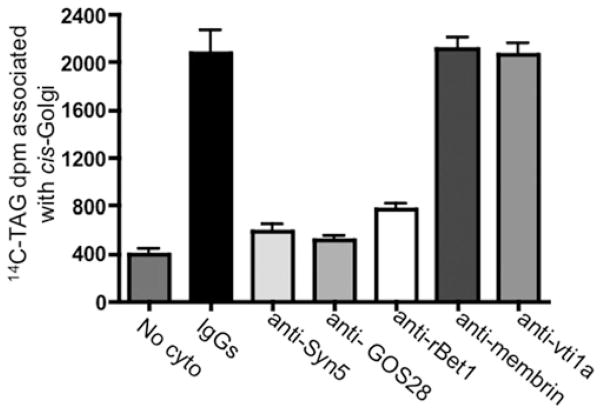
To establish the functional role of each component of the SNARE complex in ER–Golgi transport of VLDL, either VTVs containing [14C]TAG were treated with anti-Sec22b antibodies or hepatic cis-Golgi membranes were incubated with antibodies against Syn5, GOS28 or rBet1 for 1 h at 4°C. After removing the unbound antibodies by washing, either the treated VTVs were incubated with non-radiolabelled hepatic cis-Golgi or the treated cis-Golgi were incubated with VTVs containing [14C]TAG under conditions that allow VTV–Golgi fusion. As shown in Figures 2 and 8, blocking of SNARE proteins with antibodies against Sec22b (Figure 2), Syn5, GOS28 or rBet1 (Figure 8) greatly reduced the VLDL delivery to the Golgi. Treatment of Golgi with pre-immune IgG or antibodies against membrin and vti1a did not affect VTV–Golgi fusion (Figure 8), suggesting that the observed reduction in fusion activity was specific. Taken together, these results demonstrate that Sec22b, Syn5, GOS28 and rBet1 have an important physiological role in VTV–Golgi fusion and thus in VLDL delivery to the hepatic cis-Golgi.
Figure 8. Effect of antibodies against cis-Golgi SNARE proteins on VTV–Golgi fusion.
Hepatic cis-Golgi (300 μg of protein) was incubated with either pre-immune IgG, or antibodies against Syn5, GOS28, rBet1, membrin and vti1a for 1 h at 4 °C. The cis-Golgi membranes were then washed to remove unbound antibody. After antibody treatment, native [14C]TAG-loaded VTVs were added to tubes containing antibody-treated cis-Golgi. The VTV and cis-Golgi were allowed to fuse by incubating them for 30 min at 37 °C with hepatic cytosol (500 μg of protein) and ATP. No cyto represents a negative control where untreated VTVs and Golgi membranes were used without cytosol in the fusion assay. After incubation, the cis-Golgi proteins were isolated on a sucrose step gradient and the Golgi-associated [14C]TAG levels were counted. Results are means + S.E.M. (n = 4).
DISCUSSION
Subsequent to their biogenesis in the ER lumen, newly synthesized VLDL particles are directed towards the Golgi where their structural protein apoB100 gets further glycosylated and phosphorylated [8–11]. This ER–Golgi movement of VLDL is prerequisite for their ultimate secretion from hepatocytes. We have previously identified and characterized a new ER-derived vesicle (the VLDL-transport vesicle or VTV), which is exclusively engaged in the targeted delivery of the VLDL to the Golgi lumen. Although VTVs egress from hepatic ER membranes and utilize COPII machinery, their biogenesis is different from the protein-transport vesicles [21,22]. Since VTVs carry a unique cargo and have emerged to be distinct from the protein-transport vesicles in their morphology and biochemical composition, it is possible that they utilize different machinery for their targeting to and fusion with hepatic cis-Golgi. However, the underlying mechanism of the VTV–Golgi fusion process has not been scrutinized at the molecular level. The present study describes a specific composition of SNARE proteins, which form a functional SNARE complex implicated in the docking and fusion of the VTV with hepatic cis-Golgi.
Our previous published results suggested that VTVs concentrate Sec22b as compared with its parent membranes, the ER [21], which led us to examine whether this protein serves as a functional v-SNARE for the VTVs. In the present paper, we made extensive efforts to provide biochemical and immunoelectron microscopic evidences to show that Sec22b is concentrated and localized on to the VTV surface. Because Sec22b has been shown to be exclusively engaged in the ER–Golgi protein trafficking step [40,41], its co-localization on VTVs with p58 and Sar1, two classical markers for ER-derived vesicles [15,42,43], further supports our findings that VTVs are derived from the ER membranes. Interestingly, Sec22b binds to apoB100 at the ER level (S. A. Siddiqi, unpublished work), which is consistent with proteomic results from Chuck’s group [49]. Blocking of Sec22b on VTVs using specific antibodies resulted in great inhibition of the VTV–Golgi fusion process and thus reduced VLDL delivery to the Golgi lumen. These results strongly support our notion that Sec22b serves as a functional v-SNARE for VTVs.
That the ER–Golgi transport of nascent proteins and lipoproteins involves several combinations of SNARE proteins to form a four-member α-helix coiled-coil structure necessary for fusion [28] strongly supports the thesis that the composition of a SNARE complex varies with the type of ER-derived vesicles. There are at least three different proteins (i.e. Sec22b, VAMP7 and Ykt6) which serve as v-SNAREs for different kinds of ER-derived vesicles in various mammalian cells [30,39,40]. All of these v-SNAREs form distinct complexes with their cognate t-SNAREs present on the surface of cis-Golgi. Sec22b forms a SNARE complex with membrin, Syn5 and rBet1 in NRK (normal rat kidney) cells [50], whereas Ykt6 interacts with GOS28, Syn5 and rBet1 to form a functional SNARE complex [39]. Both of these complexes play a central role in the ER–Golgi transport of newly synthesized proteins in mammalian systems. Our results show that VTVs do not contain Ykt6, although it is present in rat hepatic ER. However, Hasegawa et al. [51] have shown that Ykt6 protein is localized primarily to a unique lysosome-like compartment in rat neurons and functions as a neuronal SNARE. Our previous studies have shown that VAMP7 forms a special physiologically active SNARE complex with Syn5, rBet1 and vti1a [30,31]. This unique SNARE complex is required for the fusion of the PCTV with intestinal cis-Golgi [30,31]. Interestingly, we did not find any VAMP7 in hepatic ER, albeit both hepatic and intestinal ER membranes share a common feature in that they both produce TAG-rich lipoproteins. Taken together, these observations manifest that a specific ER-derived vesicle utilizes a specific composition of SNARE complex to fuse with Golgi. Since VTVs are different from other ER-derived vesicles, i.e. PCTVs and protein-transport vesicles, we hypothesize that these unique vesicles would use a specific set of SNARE proteins to form a specific complex essential for their fusion with hepatic cis-Golgi.
To demonstrate the SNARE complex formation between VTV and cis-Golgi membranes, we established an in vitro docking assay that allowed the interactions between VTVs and cis-Golgi membranes, but restricted the actual fusion of two membranes. Because the fusion process of the two membranes is ATP-driven, removal of Mg2+-ATP from the reaction and incubating the VTVs with Golgi at 4°C enabled SNARE complex formation via protein–protein interactions between VTVs and the Golgi. However, the actual fusion of the VTVs and the Golgi membranes does not occur under these conditions. The elimination of Mg2+-ATP was required to stabilize the protein–protein interactions because, even at 4°C, Mg2+-ATP is able to dissociate the SNARE complex, which is consistent with other published reports [30,46]. Using specific antibodies against Sec22b, we were able to pull-down a large protein complex from solubilized post-docking VTV–Golgi membranes. Our immunoprecipitation results coupled with the identification using MALDI–TOF and Western blotting suggest that Sec22b, Syn5, GOS28 and rBet1 form a SNARE complex that migrated at ~ 110 kDa in SDS/PAGE. The collective molecular mass of these proteins is 111 kDa which is very close to the observed molecular mass at which the SNARE complex migrated. Because all four proteins (Sec22b, Syn5, GOS28 and rBet1) possess a C-terminal membrane-binding domain and are exposed to cytosol, they are able to constitute a four-membered coiled-coil structure, which is the minimal requirement for docking/fusion to occur.
Using purified recombinant SNARE proteins and a combinatorial approach, a variety of possible SNARE compositions have been identified; however, the physiological relevance of these complexes remains to be determined. In primary mammalian cells, so far only one functional SNARE complex had been identified by our group [30], which facilitates the PCTV–Golgi fusion in small intestinal epithelial cells. In the present paper, we report another physiologically active SNARE complex in hepatic cells that participates in ER–Golgi export of VLDL. Our results pertaining to blocking each SNARE component before VTV–Golgi fusion strongly suggest their physiological role in VLDL export from the ER to the Golgi in primary rat hepatocytes (Figures 2 and 8).
In summary, our results reveal a previously unidentified physiological role for Sec22b in the primary mammalian system. The present study strongly suggests that the VTV is able to dock and fuse with hepatic cis-Golgi and can deliver its cargo, VLDL, to the Golgi lumen. Our results confirm that the VTV utilizes Sec22b as a v-SNARE for its targeting to and docking/fusion with the hepatic cis-Golgi. Furthermore, we have shown that Sec22b on VTVs forms a functional ~ 110 kDa SNARE complex with hepatic cis-Golgi proteins (Syn5, rBet1 and GOS28) which is required for the VTV–Golgi fusion process to deliver VLDL particles to the Golgi lumen.
Acknowledgments
We sincerely thank Dr Charles Mansbach II (University of Tennessee, Memphis, TN, U.S.A.) for sharing the reagents.
FUNDING
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [grant number DK-81413 (to S.A.S.)] by the University of Central Florida start-up package (to S.A.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Abbreviations used
- apoB
apolipoprotein B
- COPII
coat complex II
- DTT
dithiothreitol
- ECL
enhanced chemiluminescence
- endo H
endoglycosidase H
- ER
endoplasmic reticulum
- MALDI–TOF
matrix-assisted laser-desorption ionization–time-of-flight
- NEFA
non-esterified (‘free’) fatty acid
- PBS-T
PBS containing 0.05 % Tween 20
- PCTV
pre-chylomicron transport vesicle
- SNARE
soluble N-ethylmaleimide-sensitive factor-attachment protein receptor
- Syn5
syntaxin 5
- TAG
triacylglycerol
- TFA
trifluoroacetic acid
- t-SNARE
target SNARE
- VAMP7
vesicle-associated membrane protein 7
- VLDL
very-low-density lipoprotein
- v-SNARE
vesicle SNARE
- VTV
VLDL-transport vesicle
Footnotes
AUTHOR CONTRIBUTION
Shaila Siddiqi carried out most of the experiments and analysed the results. Arul Mani performed some experiments and helped to prepare the References list. Shadab Siddiqi conceived, designed and performed the experiments, analysed the results and wrote the paper.
References
- 1.Shelness GS, Ingram MF, Huang XF, DeLozier JA. Apolipoprotein B in the rough endoplasmic reticulum: translation, translocation and the initiation of lipoprotein assembly. J Nutr. 1999;129:456S–462S. doi: 10.1093/jn/129.2.456S. [DOI] [PubMed] [Google Scholar]
- 2.Jamil H, Gordon DA, Eustice DC, Brooks CM, Dickson JK, Jr, Chen Y, Ricci B, Chu CH, Harrity TW, Ciosek CP, Jr, et al. An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc Natl Acad Sci USA. 1996;93:11991–11995. doi: 10.1073/pnas.93.21.11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakillah A, Nayak N, Saxena U, Medford RM, Hussain MM. Decreased secretion of ApoB follows inhibition of ApoB–MTP binding by a novel antagonist. Biochemistry. 2000;39:4892–4899. doi: 10.1021/bi9924009. [DOI] [PubMed] [Google Scholar]
- 4.Shelness GS, Ledford AS. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr Opin Lipidol. 2005;16:325–332. doi: 10.1097/01.mol.0000169353.12772.eb. [DOI] [PubMed] [Google Scholar]
- 5.Olofsson SO, Asp L, Boren J. The assembly and secretion of apolipoprotein B-containing lipoproteins. Curr Opin Lipidol. 1999;10:341–346. doi: 10.1097/00041433-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Borchardt RA, Davis RA. Intrahepatic assembly of very low density lipoproteins: rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987;262:16394–16402. [PubMed] [Google Scholar]
- 7.Rusinol A, Verkade H, Vance JE. Assembly of rat hepatic very low density lipoproteins in the endoplasmic reticulum. J Biol Chem. 1993;268:3555–3562. [PubMed] [Google Scholar]
- 8.Tran K, Thorne-Tjomsland G, DeLong CJ, Cui Z, Shan J, Burton L, Jamieson JC, Yao Z. Intracellular assembly of very low density lipoproteins containing apolipoprotein B100 in rat hepatoma McA-RH7777 cells. J Biol Chem. 2002;277:31187–31200. doi: 10.1074/jbc.M200249200. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg HN. Synthesis and secretion of apolipoprotein B from cultured liver cells. Curr Opin Lipidol. 1995;6:275–280. doi: 10.1097/00041433-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Gusarova V, Seo J, Sullivan ML, Watkins SC, Brodsky JL, Fisher EA. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J Biol Chem. 2007;282:19453–19462. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- 11.Macri J, Adeli K. Conformational changes in apolipoprotein B modulate intracellular assembly and degradation of Apo B containing lipoprotein particles in HepG2 cells. Arterioscler Thromb Vasc Biol. 1997;17:2982–2994. doi: 10.1161/01.atv.17.11.2982. [DOI] [PubMed] [Google Scholar]
- 12.Bamberger MJ, Lane MD. Possible role of the Golgi apparatus in the assembly of very low density lipoprotein. Proc Natl Acad Sci USA. 1990;87:2390–2394. doi: 10.1073/pnas.87.7.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JA. Evidence that during very low density lipoprotein assembly in rat hepatocytes most of the triacylglycerol and phospholipid are packaged with apolipoprotein B in the Golgi complex. FEBS Lett. 1988;232:405–408. doi: 10.1016/0014-5793(88)80780-4. [DOI] [PubMed] [Google Scholar]
- 14.Barlowe C. COPII and selective export from the endoplasmic reticulum. Biochim Biophys Acta. 1998;1404:67–76. doi: 10.1016/s0167-4889(98)00047-0. [DOI] [PubMed] [Google Scholar]
- 15.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 16.Hughes H, Stephens DJ. Assembly, organization, and function of the COPII coat. Histochem Cell Biol. 2008;129:129–151. doi: 10.1007/s00418-007-0363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang BL, Wang Y, Ong YS, Hong W. COPII and exit from the endoplasmic reticulum. Biochim Biophys Acta. 2005;1744:293–303. doi: 10.1016/j.bbamcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Sato K, Nakano A. Mechanisms of COPII vesicle formation and protein sorting. FEBS Lett. 2007;581:2076–2082. doi: 10.1016/j.febslet.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 19.Gürkan C, Stagg SM, Lapointe P, Balch WE. The COPII cage: unifying principles of vesicles coat assembly. Nat Rev Mol Cell Biol. 2006;7:727–738. doi: 10.1038/nrm2025. [DOI] [PubMed] [Google Scholar]
- 20.Fromme JC, Orci L, Schekman R. Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol. 2008;18:330–336. doi: 10.1016/j.tcb.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqi SA. VLDL exits from the endoplasmic reticulum in a specialized vesicle, the VLDL transport vesicle, in rat primary hepatocytes. Biochem J. 2008;413:333–342. doi: 10.1042/BJ20071469. [DOI] [PubMed] [Google Scholar]
- 22.Gusarova V, Brodsky JL, Fisher EA. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. J Biol Chem. 2003;278:48051–48058. doi: 10.1074/jbc.M306898200. [DOI] [PubMed] [Google Scholar]
- 23.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 24.Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- 25.Jahn R, Scheller RH. SNAREs: engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 26.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell. 2007;18:3463–3471. doi: 10.1091/mbc.E07-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqi SA, Siddiqi S, Mahan J, Peggs K, Gorelick FS, Mansbach CM., II The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J Biol Chem. 2006;281:20974–20982. doi: 10.1074/jbc.M601401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqi SA, Mahan J, Siddiqi S, Gorelick FS, Mansbach CM., II Vesicle-associated membrane protein 7 is expressed in intestinal ER. J Cell Sci. 2006;119:943–950. doi: 10.1242/jcs.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar NS, Mansbach CM., II Determinants of triacylglycerol transport from the endoplasmic reticulum to the Golgi in intestine. Am J Physiol. 1997;273:G18–G30. doi: 10.1152/ajpgi.1997.273.1.G18. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqi SA, Gorelick FS, Mahan JT, Mansbach CM., II COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J Cell Sci. 2003;116:415–427. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- 34.Wong DM, Webb JP, Malinowski PM, Macri J, Adeli K. Proteomic profiling of the prechylomicron transport vesicle involved in the assembly and secretion of apoB-48 containing chylomicrons in the intestinal enterocytes. Proteomics. 2009;9:3698–3711. doi: 10.1002/pmic.200800914. [DOI] [PubMed] [Google Scholar]
- 35.Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM., II Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem. 2007;282:17974–17984. doi: 10.1074/jbc.M610765200. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqi SA, Mansbach CM., II PKCζ-mediated phosphorylation controls budding of the prechylomicron transport vesicle. J Cell Sci. 2008;121:2327–2338. doi: 10.1242/jcs.022780. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqi S, Siddiqi SA, Mansbach CM., II Sec24C is required for the docking of the prechylomicron transport vesicle with the Golgi. J Lipid Res. 2009;51:1093–1100. doi: 10.1194/jlr.M002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee K, Siddiqi SA, Hashim S, Raje M, Basu SK, Mukhopadhyay A. Live Salmonella recruits N-ethylmaleimide sensitive fusion protein on phagosomal membrane and promotes fusion with early endosome. J Cell Biol. 2000;148:741–753. doi: 10.1083/jcb.148.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Hong W. Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum–Golgi transport. J Biol Chem. 2001;276:27480–27487. doi: 10.1074/jbc.M102786200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Wong SH, Tang BL, Xu Y, Hong W. Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment. Mol Biol Cell. 1999;10:435–453. doi: 10.1091/mbc.10.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hay JC, Chao DS, Kuo CS, Scheller RH. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell. 1997;89:149–158. doi: 10.1016/s0092-8674(00)80191-9. [DOI] [PubMed] [Google Scholar]
- 42.Klumperman J, Schweizer A, Clausen H, Tang BL, Hong W, Oorschot V, Hauri HP. The recycling pathway of protein ERGIC-53 and dynamics of the ER–Golgi intermediate compartment. J Cell Sci. 1998;111:3411–3425. doi: 10.1242/jcs.111.22.3411. [DOI] [PubMed] [Google Scholar]
- 43.Ying M, Flatmark T, Saraste J. The p58-positive pre-Golgi intermediates consist of distinct subpopulations of particles that show differential binding of COPI and COPII coats and contain vacuolar H+-ATPase. J Cell Sci. 2000;113:3623–3638. doi: 10.1242/jcs.113.20.3623. [DOI] [PubMed] [Google Scholar]
- 44.Rothman JE. Mechanism of intracellular transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 45.Wilson DW, Whiteheart SW, Wiedmann M, Bunner M, Rothman J. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992;117:531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sollner T, Bennett MK, Whiteheart S, Scheller RH, Rothman JE. A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 47.Johnson PA, Sudhof TC. The multisubunit structure of synaptophysin: relationship between disulfide bonding and homo-oligomerization. J Biol Chem. 1990;265:8869–8873. [PubMed] [Google Scholar]
- 48.Yang B, Gonzales L, Jr, Prekeris R, Steegmaier M, Advani RJ, Scheller RH. SNARE interactions are not selective: implications for membrane fusion specificity. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- 49.Rashid KA, Hevi S, Chen Y, Le Caherec F, Chuck SL. A proteomic approach identifies proteins in hepatocytes that bind nascent apolipoproteins B. J Biol Chem. 2002;277:22010–22017. doi: 10.1074/jbc.M112448200. [DOI] [PubMed] [Google Scholar]
- 50.Xu D, Joglekar AP, Williams AL, Hay JC. Subunit structure of a mammalian ER/Golgi SNARE complex. J Biol Chem. 2000;275:39631–39639. doi: 10.1074/jbc.M007684200. [DOI] [PubMed] [Google Scholar]
- 51.Hasegawa H, Zinsser S, Rhee Y, Vik-Mo EO, Davanger S, Hay JC. Mammalian ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain. Mol Biol Cell. 2003;14:698–720. doi: 10.1091/mbc.E02-09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]