Abstract
Objectives.
The present study examined the role of enactment in source memory in a cognitively impaired population. As seen in healthy older adults, it was predicted that source memory in people with mild cognitive impairment due to Alzheimer’s disease (MCI-AD) would benefit from the self-reference aspect of enactment.
Method.
Seventeen participants with MCI-AD and 18 controls worked in small groups to pack a picnic basket and suitcase and were later tested for their source memory for each item.
Results.
For item memory, self-referencing improved corrected recognition scores for both MCI-AD and control participants. The MCI-AD group did not demonstrate the same benefit as controls in correct source memory for self-related items. However, those with MCI-AD were relatively less likely to misattribute new items to the self and more likely to misattribute new items to others when committing errors, compared with controls.
Discussion.
The enactment effect and self-referencing did not enhance accurate source memory more than other referencing for patients with MCI-AD. However, people with MCI-AD benefited in item memory and source memory, being less likely to falsely claim new items as their own, indicating some self-reference benefit occurs for people with MCI-AD.
Key Words: Alzheimer’s disease, Enactment effect, Mild cognitive impairment, Self-reference, Source memory.
Source monitoring, the ability to distinguish in memory who said or did something (Johnson, Hashtroudi, & Lindsay, 1993), can be challenging for healthy older adults. Consistent with the widespread memory impairment in people who are experiencing mild cognitive impairment suggestive of the early signs of the Alzheimer’s disease (AD) pathophysiologic process (MCI-AD; Budson & Solomon, 2012), people with MCI-AD demonstrate greater source memory impairment than healthy controls (Dodson et al., 2011; Multhaup & Balota, 1997; Pierce, Sullivan, Schacter, & Budson, 2005; Schacter, Harbluk, & McLachlan, 1984). However, despite the memory loss associated with MCI-AD, it is possible that source memory may be enhanced in some conditions (Goldman, Winograd, Goldstein, O’Jile, & Green, 1994), such as when information is related to the self. For example, people with more severe memory loss associated with dementia due to AD have demonstrated a generation effect, better memory for self-generated items (Multhaup & Balota, 1997). Self-performed tasks (SPT) can improve memory compared with strictly verbal tasks for older and younger adults (Cohen, 1989; Engelkamp & Cohen, 1991). However, research on this enactment effect in people with dementia has yielded mixed results, with some finding benefits (Herlitz, Adolfsson, Bäckman, & Nilsson, 1991; Karlsson et al., 1989; Lekeu, Van der Linden, Moonen, & Salmon, 2002; Masumoto, Takai, Tsuneto, & Kashiwagi, 2004) and others finding no benefits (Dick, Kean, & Sands, 1989; Mack, Eberle, Frölich, & Knopf, 2005). These differing findings may reflect differences in study design with benefits emerging in more structured tasks that require more goal-directed action, (as in Hutton, Sheppard, Rusted, & Ratner, 1996). The goal-directed nature of the tasks may have allowed for deeper encoding, facilitating memory. Therefore, SPT could improve source memory in both healthy and cognitively impaired older adults due to involvement of the self.
The self-referencing effect, better memory for information connected to the self, may contribute to the enactment effect through the role of the self in performing actions. Recent work has shown that people with MCI-AD benefit from memory strategies (Hutchens et al., 2013) such as self-reference (Kalenzaga, Bugaïska, & Clarys, 2013; Kalenzaga & Clarys, 2013; Lalanne, Grolleau, & Piolino, 2010), as do younger and older adults (Glisky & Marquine, 2009; Gutchess, Kensinger, Yoon, Schacter, 2007; Mueller, Wonderlich, & Dugan, 1986) despite potential for increases in false alarms for self-related information (Rosa & Gutchess, 2013). Memory for self-relevant information also improves for people with other types of severe memory impairments (Heatherton, Krendl, Macrae, & Kelley, 2007) when utilizing self-imagination (Grilli & Glisky, 2010, 2011; Grilli & McFarland, 2011). The present project extended these findings to assess whether enacting actions oneself benefits memory in people with MCI-AD.
We predicted that although healthy older adults would demonstrate better overall item and source memory than people with MCI-AD, self-performing actions would reduce memory impairments such that MCI-AD patients would perform more like controls. This decrease in impairment may occur because the self condition should benefit item and source memory in people with MCI-AD, as found previously for healthy older adults, relative to other performed actions (Rosa & Gutchess, 2011). Finally, there should be more source errors among non-self conditions, particularly for participants with MCI-AD, whereas “self” should be associated with more accurate attributions and fewer misattributions.
Method
Participants
Forty participants were recruited through the Center for Translational Cognitive Neuroscience, Veterans Affairs Boston Healthcare System, and Brandeis University. Twenty participants with a clinical diagnosis of MCI due to the AD pathophysiologic process (Albert et al., 2011) and 20 healthy older adults were tested in their homes or the lab. Three participants were excluded from analyses due to Mini-Mental Status Exam (Folstein, Folstein, & McHugh, 1975) scores that indicated moderate AD rather than mild cognitive impairment (<20; Reisberg et al., 2010). Two controls were also excluded from analyses due to age (<60 years, and thus not age-matched), leaving 18 control (6 males/12 females) and 17 MCI-AD (9 males/8 females) participants. Participants provided written informed consent and were compensated for participation.
Procedure
Participants took part in the study with a close other (e.g., a spouse or friend) and with a confederate who was unknown to the participants. As a group, participants simulated two everyday activities: packing a picnic basket and packing a suitcase. Items were based on a previous study (e.g., tablecloth, utensils, clothing, personal care items; Rosa & Gutchess, 2011), were counterbalanced across old (items used during the experiment) and new (items not used during the experiment) conditions, and were presented in one of four predetermined orders.
In each scenario, participants, seated around a table, took turns placing items into the picnic basket and suitcase (Hashtroudi, Johnson, & Chrosniak, 1990). Rotating around the table, the experimenter named the item aloud, displayed the item name on a printed notecard, and handed the item to the participant to place into the basket or suitcase. Each participant placed 16 items and observed the placement of 32 items.
Following a 10-min retention interval during which participants completed the Shipley Vocabulary inventory (Shipley, 1986), there was a surprise, self-paced source recognition test. The memory test included the 48 items placed in the suitcase/picnic basket as well as 16 new plausible lure items. Participants were asked to indicate the source of each item by circling one of four listed sources (self, close other, unknown other, new). Finally, participants completed the cognitive battery (see Table 1; Adjutant General’s Office, 1944; Balota et al., 1999; Baumeister & Leary, 1995; Buckner, 2004; Mack, Freed, Williams, & Henderson, 1992; Monsch et al., 1992; Morris et al., 1989).
Table 1.
Demographic Information by Patient Status
| Control | MCI-AD | p value | Cohen’s d | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 77.50 | 4.13 | 79.71 | 6.22 | ns | 0.42 |
| Education* | 16.19 | 2.78 | 14.12 | 2.47 | .03 | 0.79 |
| Years close other known | 50.50 | 19.60 | 54.06 | 16.05 | ns | 0.20 |
| Inclusion of close other in self (out of 7) | 6.17 | 1.30 | 5.76 | 1.72 | ns | 0.27 |
| Shipley (out of 40) | 35.17 | 4.74 | 32.06 | 5.18 | ns | 0.63 |
| MMSE (out of 30)*** | 28.89 | 1.18 | 26.47 | 2.32 | .001 | 1.31 |
| CERAD | ||||||
| Immediate (out of 30)** | 16.67 | 3.58 | 13.18 | 2.98 | .004 | 1.06 |
| Delayed (out of 10)* | 5.22 | 2.49 | 3.56 | 1.97 | .04 | 0.74 |
| Recognition (out of 10)** | 9.67 | 0.59 | 8.38 | 1.50 | .004 | 1.13 |
| Word fluency | ||||||
| FAS* | 48.61 | 15.57 | 37.94 | 14.77 | .05 | 0.70 |
| CAT** | 45.50 | 9.46 | 33.18 | 12.67 | .003 | 1.10 |
| Boston naming (out of 15)** | 14.11 | 1.32 | 12.12 | 2.50 | .007 | 0.99 |
| Trails A | 41.12 | 15.35 | 51.25 | 27.45 | ns | 0.46 |
| Trails B** | 76.24 | 22.63 | 129.67 | 68.62 | .01 | 1.05 |
Notes. CAT = categories; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; MCI-AD = mild cognitive impairment due to Alzheimer’s disease; MMSE = Mini-Mental State Examination.
*Significant difference at p ≤ .05. **Significant difference at p ≤ .01. ***Siginificant difference at p ≤ .001.
Results
Participant Characteristics
There were no significant differences between controls and MCI-AD on age, vocabulary (Shipley, 1986), or number of years participants knew their close other, but the groups differed on most cognitive tests as well as on years of education (see Table 1).
Item Memory
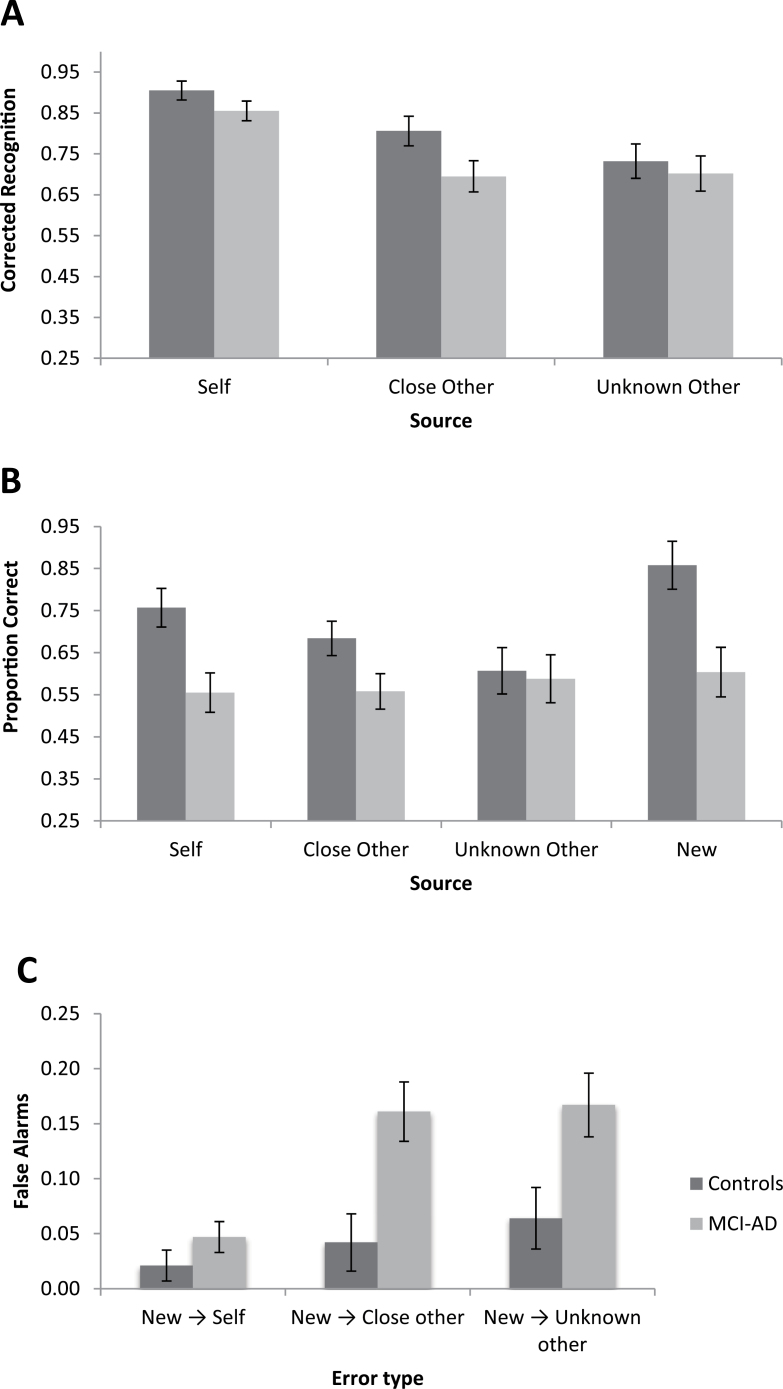
In order to compare effects to previous reports in the literature, we first assessed item memory for self and other. Corrected item recognition for self was calculated by subtracting the proportion of new items assigned “self” from the proportion of old self items recognized as being old (called “self,” “close other,” or “unknown other”). Corrected item recognition for close and unknown other were calculated in the same manner. Using education as a covariate, we conducted a 2×3 analysis of variance (ANOVA) with group (MCI-AD/controls) as the between subjects variable and the encoding condition (self/close other/unknown other) as the within subject variable. This analysis revealed a main effect of encoding condition, F(2,64) = 5.17, p = .01, η2 p = .14, with items in the self condition (M = .88, SD = 0.10, 95% confidence interval [CI] [0.85, 0.91]) leading to better memory than items in the close other (M = 0.75, SD = 0.15, 95% CI [0.70, 0.80]; F(1,33) = 33.22, p < .001, η2 p = .50) or unknown other (M = 0.72, SD = 0.18, 95% CI [0.66, 0.78]; F(1,33) = 34.39, p < .001, η2 p = .51) conditions. There was no main effect of group (p = .09) and no interaction across conditions (p > .10), with MCI-AD and controls demonstrating similar patterns of performance across the three conditions (see Figure 1A).
Figure 1.
Pattern of item memory showing corrected recognition is better for self than others for both MCI-AD and controls (A). Correct source recognition for controls and MCI-AD illustrates that while controls receive a benefit from self-reference, the performance of the MCI-AD group is flat across all conditions (B). Participants are disproportionately more likely to misattribute new items to others rather than the self (C). Error bars represent standard error of the mean. MCI-AD, mild cognitive impairment due to Alzheimer’s disease.
Correct Source Recognition
Source recognition scores equal the number of sources correctly remembered in each condition (e.g., self items correctly recognized as “self”) divided by the total number of items in the condition (i.e., 16). To assess the effect of self-referencing on source memory, a 2 (MCI-AD/controls) × 4 (self/close other/unknown other/new) mixed design ANOVA with group as the between subject variable and encoding condition as the within subject variable using education as a covariate indicated a main effect of group, F(1,32) = 7.37, p = .01, η2 p = .19, with controls performing better (M = 0.73, SD = 0.22, 95% CI [0.65, 0.80]) than MCI-ADs (M = 0.58, SD = 0.22, 95% CI [0.50, 0.65]). There was also a significant interaction between condition and group, F(3,96) = 3.10, p = .03, η2 p = .09. For controls, planned comparisons indicate that self items were remembered significantly better than close (F(1,17) = 6.06, p = .03, η2 p = .26, 95% CI [0.01, 0.15]) and unknown other referenced items, (F(1,17) = 7.81, p = 0.01, η2 p = .32, 95% CI [0.04, 0.26]), as seen in Figure 1B. There was also a marginal difference between memory for close and unknown other items, F(1,17) = 4.21, p = .06, η2 p = .20, 95% CI (−0.002, −0.13). For MCI-AD, there were no significant differences between self, close, or unknown other, p’s > .10.
Source Memory Errors
Previous work revealed higher rates of source confusion between close and unknown others (Rosa & Gutchess, 2011); we predicted that the MCI-AD group would have more pronounced error rates in these conditions, relative to the self. Errors are based on the proportion of old items attributed to an incorrect source (e.g., self items called “close other”). A 2 (MCI-AD/controls) × 6 (type of memory error) mixed design ANOVA controlling for education indicated a main effect of group, F(1,32) = 8.02, p = .008, η2 p = .20, with MCI-ADs committing more errors (M = 0.15, SD = 0.09, 95% CI [0.12, 0.19]) than controls (M = 0.09, SD = 0.09, 95% CI [0.06, 0.12]). There was no effect of condition and no interaction, p’s > .10.
To test the prediction that MCI-AD would be more susceptible to false alarms, misattributing new items to an incorrect source (thus endorsing it as previously seen), we conducted a 2 (MCI-AD/controls) × 3 (self/close other/unknown other) mixed design ANOVA controlling for education with group as the between subjects variable and error type as the within subject variable. This analysis indicated a main effect of group, F(1,32) = 9.84, p = .004, η2 p = .24, with MCI-ADs committing more false alarms (M = 0.13, SD = 0.11, 95% CI [0.09, 0.16]) than controls (M = 0.04, SD = 0.11, 95% CI [0.01, 0.08]), and a main effect of error type, F(2,64) = 3.68, p = .03, η2 p = .10, with more new items called “close” (M = 0.10, SD = 0.12, 95% CI [0.06, 0.14]) and “unknown other” (M = 0.11, SD = 0.13, 95% CI [0.08, 0.16]) than “self” (M = 0.03, SD = .06, 95% CI [0.01, 0.05]). There was also a marginal interaction between group and error type, F(2,64) = 2.95, p = .06, η2 p = .08. Within subject contrasts of new items called “self” versus new items called “close other” and “unknown other” indicated an interaction between error type and group, F(1,32) = 5.98, p = .02, η2 p = .16. Looking at controls and MCI-ADs separately, specific contrasts indicated that both controls (F(1,17) = 4.85, p = .04, η2 p = .22, 95% CI [−0.06, −0.001]) and MCI-ADs (F(1,16) = 12.48, p = 0.003, η2 p = .44, 95% CI [−0.21, −0.05]), are more likely to falsely call a new item “unknown other” than to call a new item “self.” MCI-ADs are more likely to call a new item “close other” than “self” (F(1,16) = 9.25, p = .008, η2 p = .37, 95% CI [−0.18, −0.03]) while the same is not true of controls (p > .05). Finally, there is no difference in new items called “close other” or “unknown other” for controls or MCI-ADs, p > .10 (see Figure 1C).
Discussion
The present study extended research demonstrating that for healthy younger and older adults, enactment improves memory accuracy and reduces source misattributions to the self, as opposed to others (Rosa & Gutchess, 2011). As shown in previous work (Kalenzaga et al., 2013; Kalenzaga & Clarys, 2013; Lalanne et al., 2010; Multhaup & Balota, 1997), in the present study MCI-AD patients demonstrated better item memory, based on corrected recognition scores, for items associated with the self, much like older adult controls. This result may represent an extension of the enactment effect, previously demonstrated to benefit people with dementia (Herlitz et al., 1991; Karlsson et al., 1989; Lekeu et al., 2002; Masumoto et al., 2004); this study emphasizes the mnemonic benefits to source memory when actions are performed by the self, as opposed to others. While healthy older adults demonstrated better source memory for self compared with close and unknown other items (replicating Rosa & Gutchess, 2011), enactment did not benefit source memory for MCI-AD participants, who did not differ across conditions. Declines in specific memory commonly seen in AD (Ally, Gold, & Budson, 2009; Budson, Daffner, Desikan, & Schacter, 2000) may have contributed to the MCI-AD groups’ poor source memory, and lack of benefits for self-performed tasks. Therefore, while enactment improved item memory in people with MCI-AD, the supports to memory (McDonald-Miszczak, Hubley, & Hultsch, 1996) were not strong enough to overcome declines in specific memory associated with MCI-AD.
The pattern of memory errors was equally of interest to accurate memory. As expected, people with MCI-AD tended to endorse items as previously seen, committing more source memory errors than controls. Although enactment did not provide the expected memory benefit to correct source recognition for people with MCI-AD, it did affect the pattern of memory errors such that relative to older controls, new items were misattributed disproportionately less often to the self than to another person. These data may suggest that when people with MCI-AD lack the source details to correctly identify the item’s owner (Hodges & Greene, 1998), enactment and self-referencing may serve a protective function in memory, preventing participants from misattributing the source of items to the self.
The present project is limited in that participants were assigned items and thus did not benefit from a sense of ownership over the items. Allowing participants to select their own items could increase the accuracy of source memory for self-performed tasks. Despite this limitation, our findings illustrate that the enactment effect and self-referencing benefit item memory and decrease self-related source errors. These findings illustrate aspects of memory that may be preserved early in the AD pathophysiologic process, potentially revealing a promising approach to improve memory more broadly in people with MCI-AD.
Funding
This research was supported by National Institute on Aging grants R01 AG025815 (A. E. Budson) and P30 AG13846 (A. E. Budson), by Department of Veterans Affairs, Veterans Health Administration, VISN 1 Early Career Development Award (R. G. Deason), and by the Psychology Department at Brandeis University (N. M. Rosa). This material is also the result of work supported with resources and the use of facilities at the VA Boston Healthcare System.
Acknowledgments
The authors thank Peter Millar, Sean Flannery, Dr. Margeaux Auslander, and Raymond Bombardier for their research assistance and acknowledge the helpful comments and suggestions of Dr. Leslie Zebrowitz, Dr. Joseph Cunningham, Dr. Alice Cronin-Golomb, and Brittany Cassidy.
References
- Adjutant General’s Office. (1944). Army individual test battery: Manual of directions and scoring. Washington, DC. [Google Scholar]
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C, … Phelps C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 270–279. doi:10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally B. A., Gold C. A., Budson A. E. (2009). An evaluation of recollection and familiarity in Alzheimer’s disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition, 69, 504–513. doi:10.1016/j.bandc.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota D. A., Cortese M. J., Duchek D., Roediger H. L., McDermott K. B., Yerys B. E. (1999). Verdical and false memories in healthy older adults and in dementia of the Alzheimer’s type. Cognitive Neuropsychology, 16, 361–384. doi:10.1080/026432999380834 [Google Scholar]
- Baumeister R. F., Leary M. R. (1995). The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117, 497–529. doi:0033-2909/95 [PubMed] [Google Scholar]
- Buckner R. L. (2004). Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44, 195–208. doi:10.1016/j.neuron.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Budson A. E., Daffner K. R., Desikan R., Schacter D. L. (2000). When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer’s disease. Neuropsychology, 14, 277–287. doi:10.1037/0894-4105.14.2.277 [DOI] [PubMed] [Google Scholar]
- Budson A. E., Solomon P. R. (2012). New diagnostic criteria for Alzheimer’s disease and mild cognitive impairment for the practical neurologist. Practical Neurology, 12, 88–96. doi:10.1136/practneurol-2011-000145 [DOI] [PubMed] [Google Scholar]
- Cohen R. L. (1989). Memory for action events: The power of enactment. Educational Psychology Review, 1, 57–80. doi:1040-726x/89/0300-0057$06.00/0 [Google Scholar]
- Dick M. B., Kean M.-L., Sands D. (1989). Memory for action events in Alzheimer’s type dementia: Further evidence of an encoding failure. Brain and Cognition, 9, 71–89. doi:10.1016/0278-2626(89)90045–6 [DOI] [PubMed] [Google Scholar]
- Dodson C. S., Spaniol M., O’Connor M. K., Deason R. G., Ally B. A., Budson A. E. (2011). Alzheimer’s disease and memory-monitoring impairment: Alzheimer’s patients show a monitoring deficit that is greater than their accuracy deficit. Neuropsychologia, 49, 2609–2618. doi:10.1016/j.neuropsychologia.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkamp J., Cohen R. L. (1991). Current issues in memory of action events. Psychological Research, 53, 175–182. doi:10.1007/BF00941384 [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Glisky E. L., Marquine M. J. (2009). Semantic and self-referential processing of positive and negative trait adjectives in older adults. Memory (Hove, England), 17, 144–157. doi:10.1080/09658210802077405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. P., Winograd E., Goldstein F. C., O’Jile J., Green R. C. (1994). Source memory in mild to moderate Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 16, 105–116. doi:10.1080/01688639408402621 [DOI] [PubMed] [Google Scholar]
- Grilli M. D., Glisky E. L. (2010). Self-imagining enhances recognition memory in memory-impaired individuals with neurological damage. Neuropsychology, 24, 698–710. doi:10.1037/a0020318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M. D., Glisky E. L. (2011). The self-imagination effect: Benefits of a self-referential encoding strategy on cued recall in memory-impaired individuals with neurological damage. Journal of the International Neuropsychological Society, 17, 1–5. doi:10.1017/S1355617711000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M. D., McFarland C. P. (2011). Imagine that: Self-imagination improves prospective memory in memory-impaired individuals with neurological damage. Neuropsychological Rehabilitation, 21, 847–859. doi:10.1080/09602011.2011.627263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess A. H., Kensinger E. A., Yoon C., Schacter D. L. (2007). Ageing and the self-reference effect in memory. Memory (Hove, England), 15, 822–837. doi:783624081 [pii] 10.1080/09658210701701394 [DOI] [PubMed] [Google Scholar]
- Hashtroudi S., Johnson M. K., Chrosniak L. D. (1990). Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychology and Aging, 5, 119–126. doi:10.1037/0882-7974.5.1.119 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Krendl A. C., Macrae C. N., Kelley W. M. (2007). A social brain sciences approach to understanding self. In Sedikides C., Spencer S. (Eds.), The self. New York: Psychology Press. [Google Scholar]
- Herlitz A., Adolfsson R., Bäckman L., Nilsson L. G. (1991). Cue utilization following different forms of encoding in mildly, moderately, and severely demented patients with Alzheimer’s disease. Brain and Cognition, 15, 119–130. doi:0278-2626/91 [DOI] [PubMed] [Google Scholar]
- Hodges J. D. W., Greene J. R. W. (1998). Knowing about people and naming them: Can Alzheimer’s disease patients do one without the other? Quarterly Journal of Experimental Psychology: Section A, 51, 121–134. doi:10.1080/027249898391783 [DOI] [PubMed] [Google Scholar]
- Hutchens R. L., Kinsella G. J., Ong B., Pike K. E., Clare L., Ames D, … Parsons S. (2013). Relationship between control beliefs, strategy use, and memory performance in amnestic mild cognitive impairment and healthy aging. The Journals of Gerontology. Series B, Psychological sciences and Social Sciences, 68, 862–871. doi:10.1093/geronb/gbt016 [DOI] [PubMed] [Google Scholar]
- Hutton S., Sheppard L., Rusted J. M., Ratner H. H. (1996). Structuring the acquisition and retrieval environment to facilitate learning in individuals with dementia of the Alzheimer type. Memory (Hove, England), 4, 113–130. doi:10.1080/096582196388997 [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Hashtroudi S., Lindsay D. S. (1993). Source monitoring. Psychological Bulletin, 114, 3–28. doi:10.1037/0033-2909.114.1.3 [DOI] [PubMed] [Google Scholar]
- Kalenzaga S., Bugaïska A., Clarys D. (2013). Self-reference effect and autonoetic consciousness in Alzheimer disease: Evidence for a persistent affective self in dementia patients. Alzheimer Disease and Associated Disorders, 27, 116–122. doi:10.1097/WAD.0b013e318257dc31 [DOI] [PubMed] [Google Scholar]
- Kalenzaga S., Clarys D. (2013). Self-referential processing in Alzheimer’s disease: Two different ways of processing self-knowledge? Journal of Clinical and Experimental Neuropsychology, 35, 455–471. doi:10.1080/13803395.2013.789485 [DOI] [PubMed] [Google Scholar]
- Karlsson T., Bäckman L., Herlitz A., Nilsson L. G., Winblad B., Osterlind P. O. (1989). Memory improvement at different stages of Alzheimer’s disease. Neuropsychologia, 27, 737–742. doi:0028-3932/89 [DOI] [PubMed] [Google Scholar]
- Lalanne J., Grolleau P., Piolino P. (2010). [Self-reference effect and episodic memory in normal aging and Alzheimer’s disease: myth or reality?]. Psychologie & Neuropsychiatrie Du Vieillissement, 8, 277–294. doi:10.1684/pnv.2010.0231 [DOI] [PubMed] [Google Scholar]
- Lekeu F., Van der Linden M., Moonen G., Salmon E. (2002). Exploring the effect of action familiarity on SPTs recall performance in Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 24, 1057–1069. doi:1380–3395/02/2408-1057 [DOI] [PubMed] [Google Scholar]
- Mack W., Eberle C., Frölich L., Knopf M. (2005). Memory for performed actions in dementia of Alzheimer type: Further evidence for a global semantic memory deficit. Dementia and Geriatric Cognitive Disorders, 20, 381–387. doi:10.1159/000089135 [DOI] [PubMed] [Google Scholar]
- Mack W. J., Freed D. M., Williams B. W., Henderson V. W. (1992). Boston Naming Test: Shortened versions for use in Alzheimer’s disease. Journal of Gerontology, 47, P154–P158. doi:10.1093/geronj/47.3.P154 [DOI] [PubMed] [Google Scholar]
- Masumoto K., Takai T., Tsuneto S., Kashiwagi T. (2004). Influence of motoric encoding on forgetting function of memory for action sentences in patients with Alzheimer’s disease. Perceptual and Motor Skills, 98, 299–306. doi:10.246/pms.98.1.299-306 [DOI] [PubMed] [Google Scholar]
- McDonald-Miszczak L., Hubley A. M., Hultsch D. F. (1996). Age differences in recall and predicting recall of action events and words. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 51, P81–P90. doi:10.1093/geron/51B.2.P81 [DOI] [PubMed] [Google Scholar]
- Monsch A. U., Bondi M. W., Butters N., Salmon D. P., Katzman R., Thal L. J. (1992). Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology, 49, 1253–1258. doi:10.1001/archneur.1992.00530360051017 [DOI] [PubMed] [Google Scholar]
- Morris J. C., Heyman A., Mohs R. C., Hughes J. P., van Belle G., Fillenbaum G, … Clark C. (1989). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 39, 1159–1165. [DOI] [PubMed] [Google Scholar]
- Mueller J. H., Wonderlich S., Dugan K. (1986). Self-referent processing of age-specific material. Psychology and Aging, 1, 293–299. doi:10.1037/0882-7974.1.4.293 [DOI] [PubMed] [Google Scholar]
- Multhaup K. S., Balota D. A. (1997). Generation effects and source memory in healthy older adults and in adults with dementia of the Alzheimer type. Neuropsychology, 11, 382–391. doi: 10.1037/0894-4105.11.3.382 [DOI] [PubMed] [Google Scholar]
- Pierce B. H., Sullivan A. L., Schacter D. L., Budson A. E. (2005). Comparing source-based and gist-based false recognition in aging and Alzheimer’s disease. Neuropsychology, 19, 411–419. doi:10.1037/0894-4105.19.4.411 [DOI] [PubMed] [Google Scholar]
- Reisberg B., Jamil I.A., Khan S., Monteiro I., Torossian C., Ferris S, … Wegiel J. (2010). Staging dementia. In Abou-Saleh M. T., Katona C., Kumar A. (Eds.) Principles and practice of geriatric psychiatry (pp. 162–169). Chichester, England: Wiley-Blackwell. doi:10.1002/9780470669600.ch31 [Google Scholar]
- Rosa N. M., Gutchess A. H. (2013). False memory in aging resulting from self-referential processing. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 68, 882–892. doi:10.1093/geronb/gbt018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa N. M., Gutchess A. H. (2011). Source memory for action in young and older adults: Self vs. close or unknown others. Psychology and Aging, 26, 625–630. doi:10.1037/a0022827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D. L., Harbluk J. L., McLachlan D. R. (1984). Retrieval without recollection: An experimental analysis of source amnesia. Journal Of Verbal Learning & Verbal Behavior, 23, 593–611. doi:10.1016/S0022-5371(84)90373–6 [Google Scholar]
- Shipley W. C. (1986). Shipley Institute of Living Scale. Los Angeles: Western Psychological Services. doi:10.1080/00223980.1940.9917704 [Google Scholar]