Abstract
The purpose of this meta-analysis was to analyze the available evidence concerning the effects of depression on non-adherence to adjuvant endocrine therapy (AET) in women with breast cancer. MEDLINE and PsycInfo databases from inception through May 1, 2015 were searched using terms related to AET adherence. Articles were reviewed and selected based on predetermined selection criteria, and effect sizes from included studies were extracted. Pooled effect estimates were obtained using random-effects meta-analyses. Of the 312 articles identified, 9 met the inclusion criteria. Overall, depression was significantly associated with non-adherence to AET (Cohen’s d = 0.35, 95 % CI 0.19–0.52). This effect was not significantly moderated by patient age (<65 vs ≥65 years), length of study follow-up (<18 months vs ≥18 months), or method of assessing adherence (objective vs self-report). However, within these subgroups, significant effects of depression were found only for younger patients (d = 0.46; 95 % CI 0.19–0.72) and in studies of shorter duration (<18 months) (d = 0.49; 95 % CI 0.22–0.76). These results suggest that AET adherence may be lower among women with greater depressive symptoms, and this effect may be exacerbated in younger women during the early phases (<18 months) of AET. Management of depressive symptoms in women with breast cancer may help in enhancing adherence to AET and improve cancer treatment outcomes.
Keywords: Mood, Tamoxifen, Aromatase inhibitor, Mental illness, Estrogen therapy, Outcomes
Introduction
Clinical depression affects 5–6 % of the world population annually [1]. Among patients with cancer, however, the overall prevalence ranges from 8 to 24 % [2]. Among those with breast cancer, meta-analytic studies suggest that approximately 20 % suffer from significant depressive symptoms [2], with some studies reporting rates as high as 57 % [3]. While depression itself is a serious, potentially fatal, medical condition, it also has distal impacts on health outcomes in patients with cancer. Specifically, elevated symptoms of depression have been associated with a reduced 5-year chance of survival, even when controlling for histopathological grade, number of positive lymph nodes, tumor size, type of operation received, chemotherapy and/or endocrine therapy, estrogen receptor status, and age [4]. In a meta-analysis of 76 prospective studies encompassing 176,863 patients with multiple cancers, Pinquart and Duberstein [5] reported a significant increase in mortality risk in patients with elevated depressive symptoms. Importantly, the elevated mortality risk conferred by depression was independent of disease stage, disease location (e.g., breast, lung, brain), and whether depression was assessed prior to or after a cancer diagnosis.
One potential pathway by which depression is associated with increased mortality in women with breast cancer is through its effect on adherence to adjuvant endocrine therapies. Most women with invasive breast cancer first complete treatments such as chemotherapy, radiation, and/or surgery, and then are often recommended to begin adjuvant endocrine therapy. These medications have been critical to preventing recurrence of cancer [6] and for long-term survival [7, 8] of women with breast cancer. However, long-term adherence to oral anticancer treatments is a growing concern among healthcare providers. Several studies examining adherence to adjuvant endocrine therapy for breast cancer have found adherence rates hovering between 50 and 75 % [9–11]. One study of nearly 2400 patients with breast cancer found a high rate of adherence during the first year of follow-up, but rates dropped precipitously over time, with only 50 % of patients adherent by 4 years of treatment [9]. Other studies show short-term non-adherence to oral chemotherapeutic agents, with one study showing only 53 % of patients were adherent over a 6-month follow-up period [12].
The aim of the current meta-analysis was twofold. The first aim was to assess whether depression was significantly associated with lower adherence to adjuvant endocrine therapy (AET) in women with breast cancer. The second aim was to examine the effect of the following moderators: (a) age, (b) study duration, and (c) method of assessing adherence. It was predicted that depression would be associated with lower adherence to AET, and that this effect would be stronger in (a) younger women, (b) studies of shorter duration (i.e., depression has stronger acute effects), and (c) studies that used subjective (self-report) methods of assessing medication adherence.
Method
Search criteria
MEDLINE and PsycINFO search engines were used to identify relevant studies published before May 1, 2015. Search terms were set broadly to maximize potential hits, and the following terms were used to identify studies meeting inclusion criteria. The first stem group focused on adjuvant endocrine therapies and included the terms “adjuvant estrogen therapy” or “adjuvant endocrine therapy” or “estrogen therapy” or “endocrine therapy” or “tamoxifen” or “aromatase inhibitor” or “anastrozole” or “letrozole” or “exemestane”. The second stem group simply consisted of the term “adherence”. To identify further studies, we also used the ancestry method, which involves reviewing references of those articles included in the search results for other relevant articles not identified in the original search.
Inclusion and exclusion criteria
We included studies that: (a) included as an outcome the discontinuation of, non-adherence to, or lack of persistence in taking AET medications among women with breast cancer, (b) assessed depression by semi-structured or structured diagnostic interviews, by ICD-9 or ICD-10 diagnostic codes, or by self-report instruments, (c) reported statistical information that would allow for the calculation of an effect size, and (d) were written in English. We excluded studies that assessed adherence to other cancer treatments (e.g., radiation and chemotherapy).
Study selection
Three hundred and twelve unique articles were identified through the search strategy. The titles and abstracts of the articles were reviewed and a total of 276 were excluded because they did not assess predictors of AET adherence, did not collect data (e.g., review article or commentary), or were not written in English. The full text of the remaining 36 articles was reviewed for their relevance to the research question of which 27 did not meet full inclusion criteria. Of these, 20 were excluded because they did not assess depressive symptoms as a predictor of AET adherence, and the remaining 7 articles did not provide enough information to calculate an effect size. In total, 9 articles were retained for inclusion in the meta-analysis. The process of study selection is shown in Fig. 1.
Fig. 1.
Search and study selection process
Statistical analysis
To determine if depression was associated with AET adherence, meta-analytic procedures were used. The following data were extracted to answer the research questions: (a) sample size; (b) mean/median age; (c) class of medication (e.g., aromatase inhibitor), (d) length of follow-up, (e) method of assessing non-adherence (e.g., medication possession ratio and self-report), and (f) data needed to compute effect sizes. When needed, attempts were made to contact study authors for additional data.
Effect sizes were converted to Cohen’s d [13]. For studies reporting means and standard deviations of depression scores for adherent and non-adherent groups, Cohen’s d was calculated as the difference between the means of the two groups divided by the pooled standard deviation. If studies did not provide the means and standard deviations, we calculated Cohen’s d from r, (log) odds ratios, and t tests [14]. All effect sizes were converted such that a positive sign indicated that depression was associated with greater non-adherence to AET.
Random effect models with inverse variance weights were used to aggregate individual effect sizes into pooled effect estimates with 95 % confidence limits (CI), using the software program MIX 2.0 [15]. Heterogeneity was examined using I2 and the Q-statistic. The ‘fail-safe n’ was calculated to assess the robustness of the resulting effect size [16]. To assess for publication bias, we performed an Eggers regression test (standard normal deviates regressed on precision) [17].
Subgroup analyses were conducted to examine whether potential moderator variables could account for the variability among effect sizes. Moderators included (a) age (<65 years vs ≥65 years); (b) length of follow-up period (<18 months vs ≥18 months), and (c) method of assessing non-adherence (objective vs self-report). Moderator analyses were conducted using mixed effects using the method of moments via the SPSS macros published by Wilson [18].
Results
Characteristics of the studies
Characteristics of the studies are presented in Table 1. A total of 9 studies consisting of 17,735 patients with breast cancer were included. The articles were published over an 11-year period from 2004 to 2014. The median sample size of the studies was 270 (range 91–13,593). Five studies had a mean or median age <65 years, and three had samples with mean/median age ≥65 years, and one study did not report mean or median age. The median study duration was 18 months (range 12 months to 5 years). Four studies examined adherence to selective estrogen receptor modulator (SERMs), two examined aromatase inhibitor (AI) adherence, and the remaining three included a mix of SERMs and AIs. Five studies used objective methods of capturing medication adherence (e.g., MEMS and medication possession ratio) and the remaining four measured adherence via patient self-report.
Table 1.
Characteristics of studies included in the meta-analysis
| Author (year) | N | Age (years) Mean (SD) [Median] |
Study duration (months) |
Endocrine therapy |
Adherence method | Depression measure |
Depression severity |
|---|---|---|---|---|---|---|---|
| Bender et al. [39] | 91 | 56.7 (9.7) | 18 | Mix | Objective (MEMS) | BDI-II | Mean = 5.7 ± 5.0 |
| Cluze et al. [40] | 154 | 37.0 (3.3) | 28 | Tamoxifen | Objective (Pharm Refill) |
CESD | 14.9 % > 23 |
| Fink et al. [41] | 287 | [>65] | 24 | Tamoxifen | Self-report | MHI-5 | 44.6 % ≤ 71.9 |
| Kidwell et al. [42] |
448 | [59] | 12 | Aromatase Inhibitors |
Objective (discontinue from clinical trial) |
CESD | 15 % ≥ 16 |
| Klepin et al. [43] | 1328 | 67.2 (4.3) | 60 | SERMs | Objective (pill count; drop from trial) |
GDS | Mean = 1.3 ± 1.9 |
| Lash et al. [44] | 253 | [>65] | 60 | Tamoxifen | Self-report | MHI-5 | 38.7 % ≤ 71.4 |
| Manning and Bettencourt [45] |
165 | – | 13 | Mix | Self-report | CESD | Mean = 1.95 ± 0.30 |
| Sedjo and Devine [46] |
13,593 | 55.5 (5.7) | 12 | Aromatase Inhibitors |
Objective (MPR) | ICD-9 | 1.4 % depressed |
| Stanton et al. [47] |
1416 | 56.0 (8.7) | 12 | Mix | Self-report | HADS | Mean = 12.6 ± 3.3 |
Abbreviations for endocrine therapy SERM selective estrogen receptor modulator
Abbreviations for adherence method MEMS medication event monitoring system; MPR medication possession ratio. Abbreviations for depression measure BDI-II Beck Depression Inventory–II; CESD Center for Epidemiologic Studies Depression scale; MHI-5 Mental Health Inventory-5; GDS Geriatric Depression Scale; ICD-9 International Classification of Diseases-9; HADS Hospital Anxiety and Depression Scale
Overall effect of depression on AET adherence
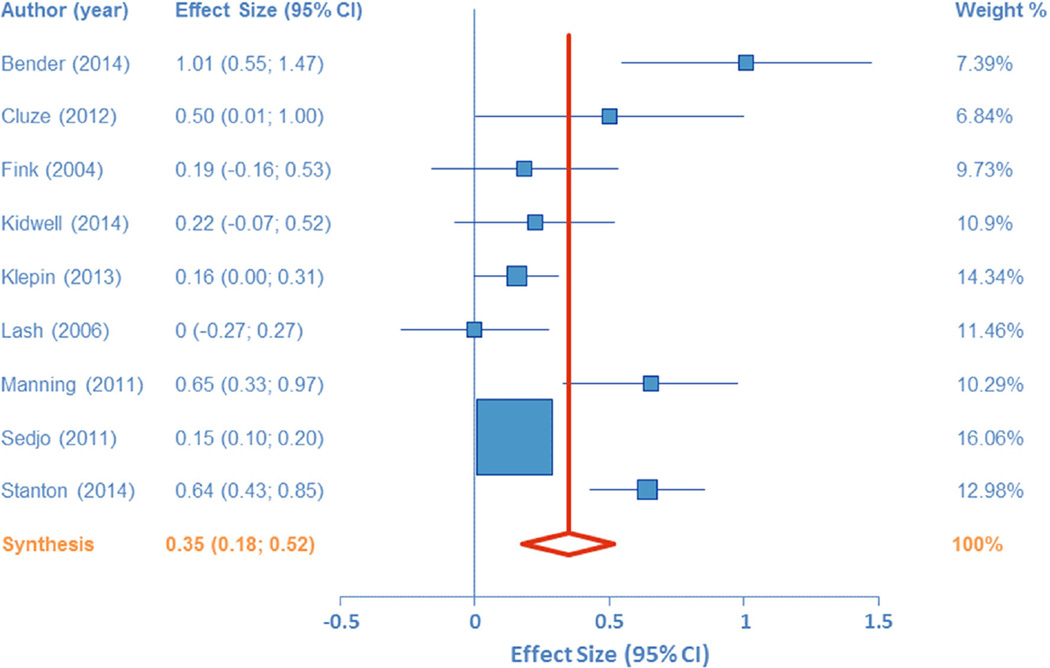
Figure 2 synthesizes the overall effect of depression on AET adherence. The pooled effect size (Cohen’s d) for the 9 studies was 0.35 (95 % CI 0.18–0.52; Z = 4.00, p <0.001), whereby greater depressive symptoms were associated with lower adherence. Using Cohen’s recommended values for small (d = 0.20), medium (d = 0.50), and large (0.80) effects, these results indicate a small-to-medium effect of depression on adherence. Conversion of Cohen’s d to an odds ratio allows one to evaluate the odds of a depressed patient being non-adherent relative to the odds of a non-depressed patient. Using the formulae provided by Borenstein et al. [19], the odds ratio for these data is 1.89 (95 % CI 1.38–2.57). The fail-safe n for this meta-analysis was 247, indicating 247 studies would need to have been conducted in which the effect was zero in order to increase the p value to above 0.05. Heterogeneity analyses indicated a large amount of variability within the effect sizes (Q = 43.21, df = 8, p < 0.001; I2 = 81.49 %, 95 % CI 65.94–89.94 %). Egger’s test was not significant (B = 1.92 ±0.93, t = 2.06, p = 0.08).
Fig. 2.
Forest plot of the effect size for the relations between depression and adherence to adjuvant endocrine therapy
Moderator analyses
Age
Eight studies were included in the analysis of the moderating effect of age on the relations between depression and adherence. Results indicated no between group differences (<65 years vs ≥65 years) in effect sizes (Q = 2.54, df = 1, p = 0.11), although examination of the within group effect sizes indicated a pooled effect of 0.46 for younger patients (95 % CI 0.19–0.72; Z = 3.38, p < 0.001) and 0.11 for older patients (95 % CI −0.21 to 0.44; Z = 0.68, p = 0.50).
Duration of follow-up
Results indicated that variability in effect sizes was not significantly explained by length of study follow-up (<18 months vs ≥18 months). However, examination of within-group effects indicated that studies of shorter duration had a pooled effect size of 0.49 (95 % CI 0.22–0.76, Z = 3.58, p < 0.001), whereas studies of longer duration had a pooled effect size of 0.18 (95 % CI −0.13 to 0.49, Z = 1.16, p = 0.25).
Adherence method
The moderator analysis of method of assessing adherence indicated no between group differences in effect sizes (Q = 0.12, df = 1, p = 0.73). The pooled effect for objective methods was 0.32 (95 % CI 0.10–0.54, Z = 2.81, p = 0.005), and for self-report was 0.38 (95 % CI 0.13–0.63, Z = 2.96, p = 0.003).
Discussion
The overall aim of this meta-analysis was to determine if depression was associated with reduced adherence to AET in women with breast cancer. Results indicate that individuals with depression have greater non-adherence relative to patients without depression. These results extend existing research demonstrating the role of depression in predicting medication adherence in patients with various medical conditions. For example, elevations in depressive symptoms have been implicated in non-adherence to antiretroviral therapy (ART) in people with HIV [20], antihypertensive medications in community-dwelling older adults [21], and hypoglycemic and lipid-lowering medications in people with type 2 diabetes [22]. While not the focus of this study, it is worth noting that depression has also been linked to lower adherence to non-AET cancer treatments [12, 23, 24].
Age, duration of follow-up, and method of assessing adherence were also investigated for moderating the relationship between depression and AET adherence. While these moderator analyses did not reach statistical significance, this is likely attributable to low power from relatively few published studies (k = 9). However, examination of the effects provide some promising leads on potential moderator effects. For example, the effect of depression on adherence was particularly strong in younger women with breast cancer (d = 0.46; 95 % CI 0.19–0.72), and in studies of shorter duration (<18 months) (d = 0.49; 95 % CI 0.22–0.76). These results might suggest that depression’s effect on adherence may be strongest in younger women in the earlier phases of AET therapy (i.e., within the first 18 months of beginning therapy), and once patients pass this early phase they may “stick with” their therapy for the long-run. However, more studies are needed to confirm this effect. As to the trend toward an age effect, this seems consistent with prior research showing that younger women appear to be less adherent to a variety of cancer-related health behaviors. For example, younger women appear less likely to adhere to AET [25, 26] and surveillance mammography [27] relative to older women. Although not assessed in those studies, future research should examine if the effect of age on adherence is exacerbated by elevations in depressive symptoms.
One important implication of the present findings is that evidence is provided for a potential mechanism explaining the link between depression and cancer mortality [5]. When depressed patients fail to take adequate doses of AET, or choose to outright discontinue their treatment, they expose themselves to increased risk for recurrence of cancer and mortality [28]. What still remains unanswered is how depression specifically acts to reduce AET adherence. One possible mechanism is the role of depression in enhancing somatic experiences. Specifically, it has been suggested that depression is associated with high rates of somatization, symptom amplification, and heightened awareness of bodily sensation [29]. Other reports indicate that depressed patients are significantly more likely to seek medical care for nonspecific complaints such as fatigue, dizziness, headache, abdominal pain, and back pain [30]. As AET is not without side effects, it is plausible that depression serves to amplify these effects [31], ultimately raising the risk for non-adherence. Also, depressive thoughts that often accompany depression may act to alter patients’ expectations on the usefulness or value of adhering to medications [32] or their motivation for doing so.
Depression is a treatable condition via both psychological [33, 34] and pharmacologic [35] interventions, often with better results when used in combination. The efficacy of these interventions has also been demonstrated in patients with cancer [36, 37]. The results of this meta-analysis suggest that effective identification and management of depressive symptoms in patients with cancer may aid in promoting long-term adherence to AET, thus improving overall cancer outcomes and mortality. There is preliminary evidence that patients who receive a greater number of psychotherapy consultations have higher rates of adherence than those with fewer consults [10]. However, it is unclear if reduction in depressive symptoms was the key mechanism underlying this relationship. Future studies are needed to examine whether interventions for depression can successfully enhance AET adherence via their effects on depressive symptoms.
The value of promoting adherence to AET cannot be understated. In addition to clear benefits to patient health outcomes (i.e., reduced risk for recurrence and mortality), adherence to AET has potential economic implications. In a cost-effectiveness study of 1263 women with breast cancer taking Tamoxifen, McCowan and colleagues [38] determined the economic value of changing a patient from low to high adherence. In other words, women with low adherence were found to have a shorter time to recurrence, increased medical costs, and a worse quality of life. They conclude that interventions that encourage patients to continue taking their treatment on a daily basis for the recommended 5-year period may be highly cost-effective. Thus, in addition to possible benefits to cancer-related health outcomes, there are also economic implications should reducing depressive symptoms in women taking AET prove effective for improving adherence.
There are a number of limitations to the current study. As noted above, the limited number of studies included (k = 9) limited the power to detect significant moderator effects. A second limitation was nearly all of the studies included participants exhibiting mild symptoms of depression, and only one study examined whether a clinical diagnosis of depression, via ICD-9 codes, was a predictor of adherence to AET. While it is noteworthy that a significant effect of mild depression was found, there is a potential that the true effects of more severe depression on adherence rates may actually be higher. Thus, more research is needed focusing on individuals with varying severities of depression (e.g., mild, moderate, and severe) to parse out the effects of greater symptoms on adherence. A third, related limitation is that none of the studies focused exclusively on examining the effects of depression on AET adherence. Rather, the majority were devoted to examining patient and disease characteristics that predicted adherence (e.g., age, medication beliefs, and primary tumor therapy) or that retroactively examined differences between adherent and non-adherent patients on psychosocial factors. The inclusion or exclusion of depression in the published results may have been decided based on the significance of its effect, resulting in a trend toward publication bias. This again underscores the importance of future prospective studies specifically examining the role of depression on patient adherence.
In conclusion, we found that AET adherence was lower among those with depressive symptoms compared to those without, and this association did not significantly differ by patient age, length of follow-up period (<18 months vs ≥18 months), or method of assessing non-adherence (objective vs self-report). However, within-group analyses indicated that depression was significantly related to adherence in younger but not older patients and may be stronger during the beginning phases of taking AET (i.e., first 18 months). These findings provide one mechanism by which depression may be associated with increased mortality in cancer, and suggest that early identification and management of even mild depressive symptoms may contribute to greater adherence to AET, an essential treatment to prevent the recurrence of some breast cancers.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krebber AM, Buffart LM, Kleijn G, Riepma IC, de Bree R, Leemans CR, Becker A, Brug J, van Straten A, Cuijpers P, Verdonckde Leeuw IM. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23:121–130. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachs G, Rasoul-Rockenschaub S, Aschauer H, Spiess K, Gober I, Staffen A, Zielinski C. Lytic effector cell activity and major depressive disorder in patients with breast cancer: a prospective study. J Neuroimmunol. 1995;59:83–89. doi: 10.1016/0165-5728(95)00029-2. [DOI] [PubMed] [Google Scholar]
- 4.Watson M, Haviland JS, Greer S, Davidson J, Bliss JM. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999;354:1331–1336. doi: 10.1016/s0140-6736(98)11392-2. [DOI] [PubMed] [Google Scholar]
- 5.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Muller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrutia G, Valentini M, Wang Y, Peto R. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma AM, Barone J, Wallis AE, Wu NJ, Garcia LB, Estabrook A, Rosenbaum-Smith SM, Tartter PI. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am J Surg. 2008;196:500–504. doi: 10.1016/j.amjsurg.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 10.Brito C, Portela MC, de Vasconcellos MT. Adherence to hormone therapy among women with breast cancer. BMC Cancer. 2014;14:397. doi: 10.1186/1471-2407-14-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemp A, Preen DB, Saunders C, Boyle F, Bulsara M, Malacova E, Roughead EE. Early discontinuation of endocrine therapy for breast cancer: who is at risk in clinical practice? SpringerPlus. 2014;3:282. doi: 10.1186/2193-1801-3-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebovits AH, Strain JJ, Schleifer SJ, Tanaka JS, Bhardwaj S, Messe MR. Patient noncompliance with self-administered chemotherapy. Cancer. 1990;65:17–22. doi: 10.1002/1097-0142(19900101)65:1<17::aid-cncr2820650106>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Hedges LV, Olkin I. Statistical methods for meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- 14.Lipsey MW, Wilson DB. Practical meta analysis. Thousand Oaks: Sage; 2001. [Google Scholar]
- 15.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. [Accessed 21 Aug 2010];2010 http://mason.gmu.edu/~dwilsonb/ma.html. [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- 20.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11:291–307. doi: 10.1007/s11904-014-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentil L, Vasiliadis HM, Preville M, Bosse C, Berbiche D. Association between depressive and anxiety disorders and adherence to antihypertensive medication in community-living elderly adults. J Am Geriatr Soc. 2012;60:2297–2301. doi: 10.1111/j.1532-5415.2012.04239.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, Ciechanowski P, Ludman EJ, Bush T, Young B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 23.Wells JS, Strickland OL, Dalton JA, Freeman S. Adherence to intravenous chemotherapy in African American and white women with early-stage breast cancer. Cancer Nurs. 2015;38:89–98. doi: 10.1097/NCC.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbar O, De-Nour AK. Adjustment to illness and dropout of chemotherapy. J Psychosom Res. 1989;33:1–5. doi: 10.1016/0022-3999(89)90100-1. [DOI] [PubMed] [Google Scholar]
- 25.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 26.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirtz HS, Boudreau DM, Gralow JR, Barlow WE, Gray S, Bowles EJ, Buist DS. Factors associated with long-term adherence to annual surveillance mammography among breast cancer survivors. Breast Cancer Res Treat. 2014;143:541–550. doi: 10.1007/s10549-013-2816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. Int J Methods Psychiatr Res. 2003;12:34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luber MP, Meyers BS, Williams-Russo PG, Hollenberg JP, DiDomenico TN, Charlson ME, Alexopoulos GS. Depression and service utilization in elderly primary care patients. Am J Geriatr Psychiatry. 2001;9:169–176. [PubMed] [Google Scholar]
- 31.Badger TA, Braden CJ, Mishel MH. Depression burden, self-help interventions, and side effect experience in women receiving treatment for breast cancer. Oncol Nurs Forum. 2001;28:567–574. [PubMed] [Google Scholar]
- 32.Weidenbacher HJ, Beadles CA, Maciejewski ML, Reeve BB, Voils CI. Extent and reasons for nonadherence to antihypertensive, cholesterol, and diabetes medications: the association with depressive symptom burden in a sample of American veterans. Patient Prefer Adherence. 2015;9:327–336. doi: 10.2147/PPA.S74531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang AX, Delucchi K, Dunn LB, Nelson JC. A systematic review and meta-analysis of psychotherapy for late-life depression. Am J Geriatr Psychiatry. 2015;23:261–273. doi: 10.1016/j.jagp.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry. 2013;58:376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- 35.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 36.Hart SL, Hoyt MA, Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, Steel JL, Cuijpers P, Mohr DC, Berendsen M, Spring B, Stanton AL. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst. 2012;104:990–1004. doi: 10.1093/jnci/djs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140. doi: 10.1186/1471-244X-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCowan C, Wang S, Thompson AM, Makubate B, Petrie DJ. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer. 2013;109:1172–1180. doi: 10.1038/bjc.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bender CM, Gentry AL, Brufsky AM, Casillo FE, Cohen SM, Dailey MM, Donovan HS, Dunbar-Jacob J, Jankowitz RC, Rosenzweig MQ, Sherwood PR, Sereika SM. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum. 2014;41:274–285. doi: 10.1188/14.ONF.274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, Carrieri MP, Giorgi R. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23:882–890. doi: 10.1093/annonc/mdr330. [DOI] [PubMed] [Google Scholar]
- 41.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor–positive breast cancer. J Clin Oncol. 2004;22:3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 42.Kidwell KM, Harte SE, Hayes DF, Storniolo AM, Carpenter J, Flockhart DA, Stearns V, Clauw DJ, Williams DA, Henry NL. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. 2014;120:2403–2411. doi: 10.1002/cncr.28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klepin HD, Geiger AM, Bandos H, Costantino JP, Rapp SR, Sink KM, Lawrence JA, Atkinson HH, Espeland MA. Cognitive factors associated with adherence to oral antiestrogen therapy: results from the cognition in the study of tamoxifen and raloxifene (Co-STAR) study. Cancer Prev Res. 2014;7:161–168. doi: 10.1158/1940-6207.CAPR-13-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 45.Manning M, Bettencourt BA. Depression and medication adherence among breast cancer survivors: bridging the gap with the theory of planned behaviour. Psychol Health. 2011;26:1173–1187. doi: 10.1080/08870446.2010.542815. [DOI] [PubMed] [Google Scholar]
- 46.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 47.Stanton AL, Petrie KJ, Partridge AH. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat. 2014;145:525–534. doi: 10.1007/s10549-014-2961-3. [DOI] [PubMed] [Google Scholar]