Abstract
Reindeer (Rangifer tarandus tarandus) are large Holarctic herbivores whose heterogeneous diet has led to the development of a unique gastrointestinal microbiota, essential for the digestion of arctic flora, which may include a large proportion of lichens during winter. Lichens are rich in plant secondary metabolites, which may affect members of the gut microbial consortium, such as the methane-producing methanogenic archaea. Little is known about the effect of lichen consumption on the rumen and cecum microbiotas and how this may affect methanogenesis in reindeer. Here, we examined the effects of dietary lichens on the reindeer gut microbiota, especially methanogens. Samples from the rumen and cecum were collected from two groups of reindeer, fed either lichens (Ld: n = 4), or a standard pelleted feed (Pd: n = 3). Microbial densities (methanogens, bacteria and protozoa) were quantified using quantitative real-time PCR and methanogen and bacterial diversities were determined by 454 pyrosequencing of the 16S rRNA genes.
In general, the density of methanogens were not significantly affected (p>0.05) by the intake of lichens. Methanobrevibacter constituted the main archaeal genus (>95% of reads), with Mbr. thaueri CW as the dominant species in both groups of reindeer. Bacteria belonging to the uncharacterized Ruminococcaceae and the genus Prevotella were the dominant phylotypes in the rumen and cecum, in both diets (ranging between 16–38% total sequences). Bacteria belonging to the genus Ruminococcus (3.5% to 0.6%; p = 0.001) and uncharacterized phylotypes within the order Bacteroidales (8.4% to 1.3%; p = 0.027), were significantly decreased in the rumen of lichen-fed reindeer, but not in the cecum (p = 0.2 and p = 0.087, respectively). UniFrac-based analyses showed archaeal and bacterial libraries were significantly different between diets, in both the cecum and the rumen (vegan::Adonis: pseudo-F<0.05). Based upon previous literature, we suggest that the altered methanogen and bacterial profiles may account for expected lower methane emissions from lichen-fed reindeer.
Introduction
Reindeer are large ruminants, widespread across the Northern Hemisphere. There are approximately five million animals that are taxonomically divided into seven extant subspecies [1]. Our focus is on the Norwegian reindeer (Rangifer tarandus tarandus), which accounts for ~ 200,000 animals, which are mainly herded in a nomadic system by the Saami people. Reindeer husbandry constitutes an essential part of the economy for the Saami community. Thus, gaining more insights on the nutritional physiology of these ruminants would be of high interest. Reindeer are classified as intermediate ruminant feeders [2,3]. In winter, when food is scarce and plants with poor nutritional value are available, reindeer may include a large proportion of lichens as a valuable extra energy source due to their high content of easily degradable carbohydrates [4–8]. In addition, lichens also possess a highly heterogeneous chemical composition with high contents of antimicrobial plant secondary metabolites (PSM) [7,9]. Severe toxic, or even lethal, effects have been reported after the consumption of lichens by sheep and elk (Cervus canadensis) [10,11]. However, reindeer can tolerate large proportions of this foodstuff in their diet.
Like other ruminants, reindeer rely on a highly complex, specialized microbiota, shaped by co-evolution with their Arctic diet, such as lichens, to allow the symbiotic microbial fermentation of plants in their rumen. This microbial consortium includes anaerobic bacteria, ciliated protozoa, fungi, and methanogenic archaea. Several studies have addressed the goal of characterizing the rumen microbial consortia [8,12–14]. However, little data are available on the microbiome of the cecum. Most of the degradation and fermentation of carbohydrates takes place in the rumen, but some material can still remain undigested before reaching the cecum, where further microbial digestion occurs [15,16]. Like the rumen, cecal digestion is also performed by specialized consortia of microorganisms, which produce volatile fatty acids (VFAs) that are absorbed by the host. This additional site of fermentation provides an extra carbon and energy source, which may influence the animal’s metabolism [17]. Accordingly, diet may also influence the microbiota housed in the large intestine. The characterization of this microbiota together with the rumen would allow a better understanding on the effects produced by the feeding regime on some metabolic processes, such as methanogenesis.
Enteric methane emissions from ruminants may result in not only an energy loss for the individual animal, accounting 2–12% of the total gross energy intake (GEI) [18], but is also a source of atmospheric contamination [19]. Among the different microbial groups, methanogens are the only methane-producing microorganisms in the rumen and cecum. They produce methane mainly by the reduction of carbon dioxide (and also acetate) with electrons taken mostly from hydrogen, with also formate and methyl compounds as minority electron sources [20]. The action of this microbial group is important for the maintenance of optimal anaerobic digestion [21,22]. In particular, this is mostly achieved via intimate hydrogen exchange with the other microbial groups (mostly the rumen ciliated protozoa) in the gut as high partial pressures of hydrogen may disrupt the anaerobic fermentation of polysaccharides degradation end products [23,24]. Several in vitro studies demonstrated a methane-suppressing effect exerted by some PSM (e.g. condensed tannins), via either direct inhibition on methanogens, or to any of their syntrophic partners [25,26]. The intake of forage high in PSM, or the addition of some of these compounds, led to depressed methanogenesis, as well as changes to the archaeal community profiles in sheep, goat and deer [27–29]. Nevertheless, very few studies have focused on relating the intake of diets high in PSM, or how this may alter the diversity of methanogens and their relationship with predicted lower methane emissions.
In the current study, we characterized the rumen and cecum microbiota from two groups of captive Norwegian reindeer fed two different diets (lichens, high in PSM, or standard pelleted feed). Based upon the extensive literature indicating a methane-suppressing effect with diets high in PSM, and considering the outstanding tolerance of reindeer to the intake of lichens, our objectives were as follows: (1) obtain a detailed approximation on how the rumen and cecum microbiota were affected by the ingestion of lichens, comparable to other PSM-rich diets; and, (2) to investigate how the alteration of these microbial profiles, especially methanogens in both the rumen and cecum, with the intake of lichens may potentially account for predicted low methanogenesis. In summary, significant differences were observed in the bacterial and archaeal microbiota at rumen and cecum level between both feeding regimes, with some specific archaeal phylotypes being altered with a lichen-based diet.
Material and Methods
Ethics statement
Most reindeer in Norway are owned and herded in a nomadic system by the Saami people, and animals used in this study originated from a privately owned herd gathered about 30 minutes from the city of Tromsø, where the University of Tromsø –The Arctic University of Norway (UiT)–is located. Animals were bought directly from the owner, and transported to our research facilities at UiT. Reindeer (R. t. tarandus) are not an endangered or a protected species, and no specific permission was required to buy and transport the animals to UiT. This project does not include field studies. All animal experiments were conducted after arrival to UiT, in approved animal research facilities at Department of Arctic and Marine Biology (permit no. 089). In general, the animals were maintained in large outdoor pens and fed ad libitum a pelleted feed, a lichen-based diet, or a mix of both depending on the season. The animals were sacrificed in a laboratory facility appropriate for that purpose, following a method of euthanasia approved by the National Animal Research Authority (no. 5399) by stunning with a bolt pistol and subsequent bleeding.
Sampling
Whole rumen and cecum samples (n = 14; approx. 50 mL per sample) were collected from seven reindeer (NRruS = Norwegian Reindeer rumen Sample and NRceS = Norwegian Reindeer cecum Sample) immediately after slaughter, and kept at -80°C. Samples were divided into two groups based on diet composition: samples 1–3 were collected from reindeer feeding on grass-based pellet concentrate (Pd) (23.3% oats, 18.9% timothy grass, 16% wheat bran and 11.2% barley). Samples 4–7 were collected from reindeer fed on a mix of different species of lichens (Ld) (mainly Cladonia sterallis sp) collected in southern Norway. The animals were fed with the corresponding diet for four weeks before slaughter and sample collection. Detailed conditions for each animal, such as gender, date of birth, body mass, weight after slaughter, as well as, rumen and cecum pH values at sampling are listed in Table 1.
Table 1. Anatomical and physiological conditions in the rumen and cecum of Norwegian reindeer at sampling.
| Sample ID | Age | Sex | Body mass (kg) | Slaughter weight (kg) | Diet | Rumen pH | Cecum* pH |
|---|---|---|---|---|---|---|---|
| NRS1 | Born Spring 2012 | Female | 59.6 | 34 | Pellets/Conc. | 6.63 | 6.76 |
| NRS2 | Born Spring 2012 | Female | 52.0 | 29 | Pellets/Conc. | 6.54 | 6.85 |
| NRS3 | Born Spring 2012 | Female | 54.4 | 29 | Pellets/Conc. | 5.94 | 6.53 |
| NRS4 | Born Spring 2007 | Female | 71.4 | 37 | Lichen mix | 5.58 | 5.74 |
| NRS5 | Born Spring 2003 | Female | 77.2 | 36 | Lichen mix | 5.57 | 6.04 |
| NRS6 | Born Spring 2010 | Female | 61.8 | 30 | Lichen mix | 6.04 | 6.09 |
| NRS7 | Born Spring 2003 | Female | 64.2 | 28 | Lichen mix | 5.53 | 5.63 |
*Significant differences in the pH values between both feeding regimes based on a permutation Welch’s t-test statistical analysis (P<0.05). Conc. = Concentrate.
DNA extraction
Approximately 0.25 g aliquots from each partially thawed sample were used for the analyses. DNA extraction was performed following the Repeated Bead Beating plus Column (RBB+C) Method [30] with the following modifications. Stainless steel beads (Qiagen, Hilden, Germany) were used in place of sterile zirconia beads, and the incubation with DNase-free RNase was skipped (step 11). DNA was quantified using a NanoDrop 2000c spectrophotometer (Thermo Scientific, Waltham, MA, USA) and solutions were kept at -20°C until PCR amplification.
Quantitative real-time PCR
Cell densities for each microbial group present in the DNA extracts from the different samples were estimated using quantitative real-time PCR. External standards were used for each microbial group. External standards for methanogens consisted in a purchased mix of Methanobrevibacter smithii, Mbr. gottschalkii, Mbr. ruminantium and Mbr. millerae (LGC Standards, Teddington, UK). Bacterial external standards was performed as described by Denman and McSweeney [31], using serial log dilutions of Ruminococcus flavefaciens ranging from 9.53 x 105 to 9.53 x 108 cells per mL. The external standards for protozoa ciliates were also prepared as reported by Skillman et al. [32], where protozoa cells were counted microscopically and serial dilutions were carried out to concentrations ranging from 1.86 x 103 to 1.86 x 106. DNA extractions from the serial dilutions for each microbial group were subsequently used as standards for the reactions. The primers used for these analyses are listed in Table 2. Experiments were performed in a BioRad CFX Thermocycler system (BioRad, Hercules, CA, USA) at the Department of Animal Science at the University of Vermont (USA). A total reaction volume of 25 μL, consisted of 12.5 μL of SYBR Green mix (Quanti- Tect™ SYBR® Green PCR, Qiagen, Germany), 2.5 μL of each primer (400 μM) and 1 μL of DNA template (10 ng/ μL), and 6.5 μL of distilled water. PCR conditions were modified depending upon the microbial group tested (S1 File). A final melting curve analysis was performed after each experiment by continuously monitoring fluorescence signals from small increases of 0.5°C every 10s, in a temperature range from 60°C to 95°C, in order to check for primer specificity and discard DNA contamination. Calculations of threshold cycles (Ct) were automatically performed by the BioRad CFX manager software (v3.0). The logarithmic fraction of the resulting sigmoid-shaped curve after each reaction was used for calculations of the PCR efficiency, following the methods described by Liu and Saint [33]. Each DNA template was run in triplicate and Ct for only those reactions showing the highest efficiency (linear standard curve (R2) above 0.996) were included.
Table 2. List of the primers used in this study.
| Technique | Target microbe | Primer pair | Sequence (5’ to 3’ direction) | References |
|---|---|---|---|---|
| Quantitative real-time PCR | Methanogens | qmcra-F | TTCGGTGGATCDCARAGRG C | [34] |
| qmcra-R | GBARGTCGWAWCCGTAGAATCC | |||
| Bacteria | 1114F | CGGCAACGAGCGCAACCC | [35] | |
| 1275R | CCATTGTAGCACGTGTGTAGCC | |||
| Diversity | Protozoa | PSSU-316F | GCTTTCGWTGGTAGTGTATT | [36] |
| PSSU-539R | CTTGCCCTCYAATCGTWCT | |||
| Methanogens | 340F | CCCTAYGGGGYGCASCAG | [37] | |
| 1000R | GAGARGWRGTGCATGGCC | |||
| Bacteria | 27F | AGAGTTTGATCCTGG | [38] | |
| 519R | TTACCGCGGCTGCT |
16S rRNA amplicon library preparation
Bacterial and Archaeal PCR amplifications were performed in an Eppendorf Mastercycler Gradient (Eppendorf AG, Hamburg, Germany) in a total reaction volume of 25 μL. Reaction mixes consisted of 12.5 μL of iProof High-Fidelity Master Mix kit (BioRad, Hercules, CA, USA), containing 0.04 U/ μL of iProof DNA polymerase as well as 400 μM DNTPs. To each sample, 1.25 μL of Dimethyl sulfoxide (DMSO) was added in order to increase PCR efficiency, as well as 1 μL of each primer (400 nM) and 1 μL DNA template. 16S rRNA gene amplification was carried out using the bacterial primer set 27F and 515R, targeting the variable regions V1-V3 and yielding a 500-base pairs (bp) size amplicon product; and the archaeal primer set 340F and 1000R, producing a 660-bp size product (Table 2). Each primer contained one of the Life Sciences adaptors (adaptor A on the reverse primer and adaptor B on the forward primer). In addition, an 8-nucleotide (nts) multiplex identifier (MID) [39] was present downstream on the reverse primer to identify sequence reads for bioinformatics analyses.
Conditions for PCR reactions were as follows: an initial denaturation step at 98°C for 30 s; 25 or 35 cycles for bacterial or archaeal primer sets, respectively, consisting of denaturation at 98°C for 10 s; annealing at 60°C or 58°C for bacteria or archaea, respectively, for 30 s; and extension at 72°C for 45 s. A final extension step at 72°C for 7 min was run and samples were kept at 4°C until checked by 1% agarose gel electrophoresis. DNA concentration for each sample was quantified using a Qubit fluorometer (Invitrogen, Carlsbad, CA, USA), samples pooled in equimolar amounts and checked by 1% agarose gel electrophoresis. Resulting bands from the pooled samples were finally excised from gel and purified using the NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Düren, Germany), following manufacturer’s protocol. Purified amplicons were then stored at -20°C until sequencing. Sequencing was performed by 454/Roche GS FLX, LIB-L chemistry, at the Norwegian Sequencing Centre (NSC), in Oslo.
Sequence processing
Resulting bacterial and archaeal 16S rRNA sequences were analyzed using the Quantitative Insights Into Microbial Ecology (QIIME) pipeline [40]. A first filtering step was performed to assure quality. Sequences were discarded for the following reasons: total length fell out of 350–650 nts; homopolymer runs exceeded 6 bases; average quality score resulted less than 25; and a mismatch in the primer sequences occurred. Sequences were clustered as Operational Taxonomic Unit (OTU) based on a 97% similarity criterion with the QIIME-incorporated version of USEARCH [41] with a word length of 64. Any sequence flagged as putative chimera was identified with UCHIME [42], and finally discarded from the analysis.
Sequence analysis
OTU-representative sequences were chosen based on sequence abundance and subsequently aligned against a Greengenes core-set reference database [43], with the Python-based version of the Near Alignment Space Termination (NAST) algorithm [44] in QIIME. Taxonomic identification down to genus level for all the previously aligned sequences was performed using the RDP classifier [45], at a default 80% confidence cut-off, and where a Naïve-Bayesian algorithm is applied against the RDP-II reference database. Classification at species level for the Archaeal sequences was performed using the Basic Local Alignment Search Tool (BLAST) software [46] from the National Center for Biotechnology Information (NCBI) website. Alpha-diversity estimators assessing OTU richness (CHAO) [47], sample evenness (Shannon) [48], sample coverage (good’s coverage) [49] and total observed OTUs (i.e, observed_species) were calculated after random subsampling of the different datasets. Resulting rarefaction curves were generated with the make_rarefaction_plots.py script. In addition, pairwise sample dissimilarity analyses (beta-diversity) were performed using subsampled datasets adjusted to the one yielding the lowest counts in order to avoid any potential bias. Principal coordinate analysis (PCoA) plots were created using pre-calculated weighted UniFrac distance matrices. Analyses were performed separately based on microbial target (bacteria and archaea) and sampling site (rumen and cecum).
Functional prediction on metagenomes
Bacterial and archaeal gene contents of described metagenomes for each sample were predicted using PICRUSt [50] online version available in the online Galaxy platform (https://huttenhower.sph.harvard.edu/galaxy/). Firstly, a closed reference OTU table was created using sequence datasets obtained after quality check with QIIME (as previously described), with a Greengenes core set reference database. The resulting closed reference OTU table was then normalized based on 16S rRNA gene copy number prior to metagenome prediction, and subsequently categorized by function based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in Galaxy online. Statistical analysis and plot generation of the resulting biom file was performed with STAMP v2.0.9 [51]. In particular, pairwise comparison of the KEGG pathways between both types of diets was performed applying a Welcht’s (two-tailed) t-test with 95% confidence intervals. KEGG pathways displaying a p-value below 0.05 were considered as statistical significant.
Volatile fatty-acids chemical analysis
The concentrations of VFAs in reindeer rumen samples were analyzed using a high performance liquid chromatography (HPLC) (Dionex Ultimate 3000) equipped with a C-18 column (Agilent eclipse plus C-18; 3.5 μ; 2.1×150mm) and a UV detector set at 210 nm. The temperature of the column compartment was set at 40°C. The samples were loaded on a Dionex autosampler (Ultimate 3000). Before analysis, the samples were centrifuged at 14,000 rpm for 10 min and were then filtered through a 0.45 μm syringe filter. The pH of the samples was measured to assure the pH was less than 2.5. If not, then the pH was adjusted using sulfuric acid. Methanol (100%) and 2.5 mM sulfuric acid were used as eluents at a flow rate of 0.3 mL/min. VFAs including acetic, propionic, iso-butyric, n-butyric were measured during a 30-min run. Iso-butyric acid was not detected in any of the samples and thus it was finally discarded from the analysis.
Statistical analysis
Two-tailed Student’s t-test was used to check for significant differences between the results obtained with quantitative real-time PCR from the different feeding regimes. In addition, permutation Welch’s t-test [52] (9,999 permutations) analyses were performed to evaluate significance between similar phylotypes from different feeding regimes, using the ‘coin’ and ‘perm’ packages in ‘R’ (https://cran.r-project.org/mirrors.html). Significance for PCoA (beta-diversity) analyses was tested with multivariate permutation tests using the nonparametric method ‘Adonis’ (999 permutations) included in the package ‘vegan’ of the QIIME-incorporated version of ‘R’. Tests were performed after random subsampling of datasets from each sample taking a fixed number (depth) of sequences (2,000 sequences /sample). All the analyses were performed separately for bacteria and archaea as well as sampling site (rumen or cecum).
Results
Quantitative real-time PCR results in rumen and cecum samples
Population densities (cells per gram wet weight (cells/gww)) of the different microbial types (methanogens, bacteria and protozoa) found in rumen and cecum samples from both groups of reindeer were determined using quantitative real-time PCR (Tables 3 and 4). In summary, no significant differences were obtained comparing the number of any of the three microbial groups, regardless of feeding regime, or sampling site. Methanogens densities varied from a mean 1.7 x 107 to 8.86 x 106 cells/gww in rumen samples from lichen-fed reindeer (p = 0.481). In cecum samples these numbers constituted an average 2.56 x 105 and 2.32 x 106 cells per gram of wet weight with pellets and lichens, respectively (p = 0.06). Two cecal samples from the reindeer fed with pelleted feed (NRceS2 and NRceS3) yielded no signal for methanogens in the tests (Table 4). Similarly, one cecal sample from one of the reindeer, fed with pelleted feed (NRceS2), yielded no signal for protozoa ciliates. Rumen protozoa accounted for an average 3x107 and 4.92x106 cells per gram of wet weight for pellet and lichen fed reindeer, respectively. In cecum samples these numbers were 1.08x103 (pelleted diet) and 1.08x104 (lichen diet) cells/gww. As indicated, no significant differences were observed between the densities of protozoa from samples of reindeer fed with pellets, or lichens, in any of the sampling sites (rumen: p = 0.265; cecum: p = 0.095). Finally, bacterial counts remained practically unaltered between the two diets, and independently of the two sampling sites (Tables 3 and 4) (rumen: p = 0.731; cecum: p = 0.436). This microbial group showed the highest values in the rumen and cecum compared to the other two microbial groups (methanogens and protozoa), with average densities as high as x108cells/gww.
Table 3. Concentration of methanogens, bacteria and protozoa in the rumen of Norwegian reindeer.
| Animal | Methanogens | Bacteria | Protozoa | Diet |
|---|---|---|---|---|
| NRruS1 | 1.13x107 | 9.84x108 | 5.6x107 | Pellets |
| NRruS2 | 8.51x106 | 8.08x108 | 4.02x106 | Pellets |
| NRruS3 | 3.11x107 | 7.19x108 | 3x107 | Pellets |
| Mean(SE) | 1.7x107 (1.23x107) | 8.37x108 (1.35x108) | 3x107 (2.6x107) | Pellets |
| NRruS4 | 5.88x106 | 4.30x108 | 1.04x104 | Lichens |
| NRruS5 | 6.3x105 | 1.7x109 | 2.78x107 | Lichens |
| NRruS6 | 2.97x106 | 1.46x108 | 2.39x104 | Lichens |
| NRruS7 | 2.89x106 | 5.41x108 | 8.83x105 | Lichens |
| Mean(SE) | 8.86x106 (1.02 x107) | 3.84x108 (1.33x108) | 4.92x106 (1.12 x107) | Lichens |
Microbial populations were determined by qrtPCR. Total counts are given based on whole rumen contents (number of cells per gram wet weight).
SE = Standard error
Table 4. Concentration of methanogens, bacteria and protozoa in the cecum of Norwegian reindeer.
| Animal | Methanogens | Bacteria | Protozoa | Diet |
|---|---|---|---|---|
| NRceS1 | ND | 5.44x106 | ND | Pellets |
| NRceS2 | ND | 6.45x108 | 2.04x103 | Pellets |
| NRceS3 | 7.69x105 | 4.14x108 | 1.2x103 | Pellets |
| Mean (SE) | 2.56x105 (4.44x105) | 3.55x108 (3.24x108) | 1.08x103 (1.02x103) | Pellets |
| NRceS4 | 2.09x106 | 3.45x108 | 2.39x103 | Lichen |
| NRceS5 | 4.92x106 | 5.82x108 | 3.12x103 | Lichen |
| NRceS6 | 1.59x106 | 2.99x108 | 2.56x104 | Lichen |
| NRceS7 | 6.83 x106 | 3.11x108 | 1.21x104 | Lichen |
| Mean (SE) | 2.32x106 (1.83 x106) | 3.84x108 (1.33x108) | 1.08x104 (1.08x104) | Lichen |
Microbial populations were determined by qrtPCR. Total counts are given based on whole cecum contents (number of cells per gram wet weight).
SE = Standard error
ND = not detected. Ct values lower than 35 cycles.
Taxonomic identification
Bacterial 16S rRNA gene sequence analyses in rumen samples
In total, 117,774 bacterial 16S rRNA sequences were retrieved from the rumen samples collected in both groups of reindeer (fed pellets and lichens), with numbers varying from 14,528 to 19,055 sequences per sample. Quality check and trimming down to 500 bases in length resulted in 97,633 high quality sequences used for downstream analyses. OTU-clustering based on a 97% similarity criterion yielded 2,290 chimera-free OTUs, with 341 OTUs being shared between both feeding regimes. Identification at phylum level with RDP classifier tool showed Firmicutes and Bacteroidetes as the two most representative phyla in both groups (S2 and S3 Files). As much as 49.6% and 40.5% of total 35,012 sequences (on average) were assigned to Firmicutes and Bacteroidetes, respectively, in reindeer fed with pellets concentrate. Firmicutes-associated phylotypes constituted an average of 63.7% of 62,621 of total sequences in reindeer fed with lichens, whereas in reindeer fed pellets this group resulted in a 49.6%. In contrast, Bacteroidetes phylotypes accounted for an average of 40.5% of total sequences in reindeer fed pellets and 29.7% of total sequences in reindeer fed a lichen-based diet. Despite the differences observed in the relative abundance of these phyla between both groups of reindeer, statistically the difference was not significant for any of the samples (Permutation Welch’s t-test: Firmicutes: p = 0.196; Bacteroidetes: p = 0.344).
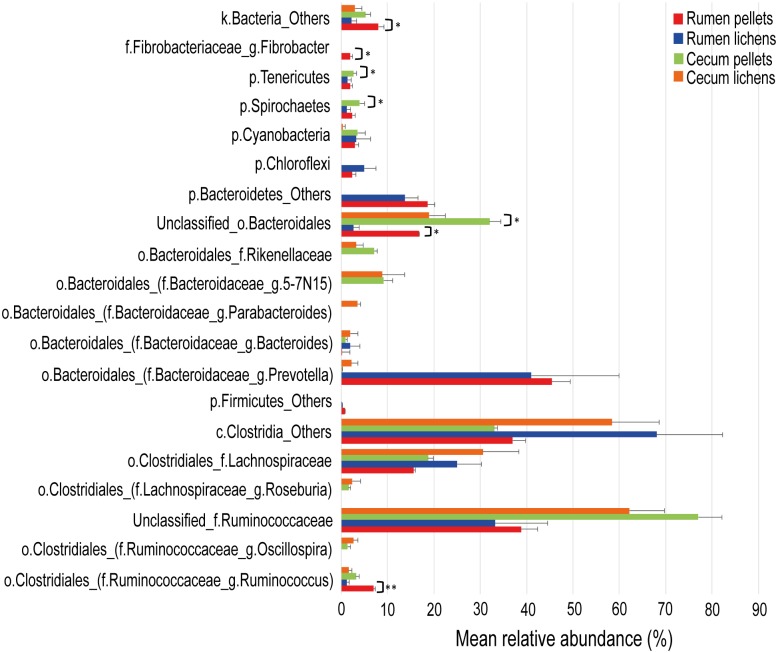
Classification down to genus level also agreed with the differences described above (Fig 1, S2 and S3 Files). Within the Firmicutes, the relative proportion of uncharacterized genera belonging to the class Clostridia represented an average 18.5% and 34.0% of total sequences with the intake of pellets or lichens, respectively (p = 0.198). Similarly, unclassified genera within the family Lachnospiraceae accounted for an average 7.8% of total sequences in reindeer fed with pellets and 12.5% with lichens (p = 0.2). Ruminococcus spp. (Pd: 3.5%; Ld: 0.6%) were significantly reduced (p = 0.001) by the intake of lichens and accounted for a 2.9% of the reads. Within the Bacteroidetes, only unclassified genera of the order Bacteroidales were significantly influenced (p = 0.027) by diet composition, decreasing from an average 8.4% to 1.3% of total sequences with lichen as the only sustenance. Members of the cellulose-degrading Fibrobacteres phylum were only present in reindeer fed pellets, which accounted 1% of the total sequences, on average.
Fig 1. Fluctuations on the rumen and cecum bacterial microbiota in lichen-fed Norwegian reindeer.
Mean values for the total 16S rRNA gene sequences assigned to each phylotype are displayed, with taxonomical classification down to genus level. Standard error (black lines) and statistical significance (asterisk symbol; p<0.05) obtained with permutation Welch’s t-test (9999 permutations) analysis are also included. Only samples from the same sampling site were used for statistical comparisons (i.e. rumen or cecum). Rumen sample from reindeer fed pellets (Rumen pellets) (red); Rumen-lichens (blue); Cecum pellets (green); Cecum lichens (orange).
Bacterial 16S rRNA sequence analyses in cecum samples
Cecum samples yielded 60,693 raw bacterial 16S rRNA gene sequences resulting in a final 46,845 high quality sequences after trimming to 500 bases. A total of 1,291 chimera-free OTUs were subsequently obtained with 210 OTUs shared between both groups. Similarly, as in rumen samples, Firmicutes and Bacteroidetes were the two major phyla, regardless of diet composition (S2 and S4 Files). The average percentage of total Firmicutes-related phylotypes were 67.3% and 79.2% of a total 12,648 and 34,197 sequences in pellet-fed and lichen-fed reindeer, respectively. Bacteroidetes represented an average 24.6% in reindeer fed pellets and 19.5% with lichens as the only sustenance. In both cases, the differences observed between both groups of reindeer, for these two phyla, were not significant (Firmicutes: p = 0.117; Bacteroidetes: p = 0.310).
Classification down to genus level showed uncharacterized phylotypes related to the family Ruminococcaceae as the major bacterial group in both groups of reindeer (Pd: 38.5%; Ld: 31.1% of total sequences on average), followed by phylotypes assigned to the order Clostridiales (Pd: 16.5%; Ld: 29.2% total sequences). Unclassified genera belonging to the family Lachnospiraceae resulted in an average 9.4% and 15.3% of total sequences in reindeer fed with pellets and lichens, respectively (p = 0.261). A similar trend was obtained for unclassified Clostridia-related phylotypes, which accounted for an average 16.5% of total sequences in pellets-fed reindeer and 29.2% in those fed with lichens (p = 0.09) (Fig 1, S2 and S4 Files). Instead, uncharacterized phylotypes belonging to the order Bacteroidetes experienced a decrease (p = 0.087) in lichen-fed reindeer (Pd: 16%; Ld: 9.5%). Phylotypes assigned to the phyla Spirochaetes (Pd: 2%; Ld: 0%) and Tenericutes (Pd: 1.3%; Ld: 0%) were significantly decreased in reindeer offered a lichen-based diet (Fig 1, S2 and S4 Files).
Archaeal 16S rRNA sequence analyses in rumen samples
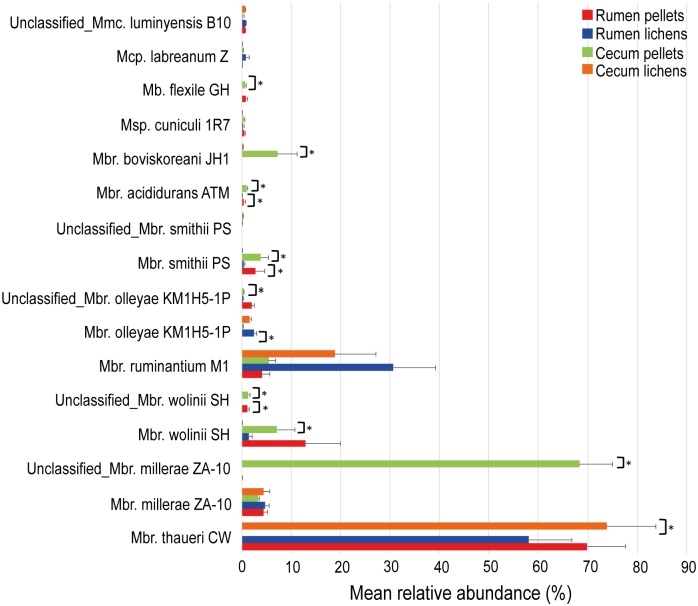
Overall, 78,201 Archaeal 16S rRNA sequences were retrieved from the seven rumen samples resulting in 75,739 after quality check and trimming down to 500 bases. Numbers of sequence per sample ranged 7,682 to 15,780 and yielded a total of 53 chimera-free OTUs, with as many as 49 OTUs shared by both groups of reindeer. In general, Euryarchaeota was the only phylum found in all the samples (S5 and S6 Files). At the genus level, Methanobrevibacter spp. accounted for the majority of the sequences, independent of the feeding regime (Pd: 97.8% of 32,144 total sequences; Ld: 98.1% of 43,595 total sequences). Taxonomical identification down to species/strain level (≥97% similarity with GenBank database representatives) showed phylotypes sharing a 98% similarity to Methanobrevibacter thaueri strain CW constituting the major archaeal taxa found in both groups of reindeer (Pd: 69.1%; Ld: 58.2% of total sequences, on average) (p = 0.35) (Fig 2, S5 and S6 Files). Phylotypes identified as Methanobrevibacter wolinii strain SH-related OTUs (98% similarity) represented the second most prevalent phylotype in the pellets-based group (13.4% total sequences), whereas they accounted for only an average 1.5% in reindeer fed with lichens. However, this variation was not significant (p = 0.092). A significant decrease was observed in members assigned to Methanobrevibacter smithii strain PS (97% similarity) in lichen-fed reindeer (Pd: 2.8%; Ld: 0.4%. p = 0.029). OTUs sharing a 99% similarity to Methanobrevibacter ruminantium strain M1 constituted the second most prevalent phylotype in reindeer fed with lichens (30.8% total sequences), but only an average 4.2% in pellets-fed reindeer. Despite the variation observed for this phylotype, between both groups of reindeer, no statistical significance was obtained (p = 0.054). Finally, phylotypes related to Methanobrevibacter olleyae strain KM1H5-1P (97% similarity) significantly increased in lichen-fed reindeer (Pd: 0.0%; Ld: 2.6%) (p = 0.030).
Fig 2. Fluctuations on the rumen and cecum archaeal microbiota in lichen-fed Norwegian reindeer.
Mean values for the total 16S rRNA sequences assigned to each phylotype are displayed, with taxonomical classification at species/strain level. Standard error (black lines) and statistical significance (asterisk symbol; p<0.05) with permutation Welch’s t-test (9999 permutations) analysis were also included. Only samples from the same sampling site were used for statistical comparisons (i.e. rumen or cecum). Rumen sample from reindeer fed pellets (Rumen pellets) (red); Rumen-lichen (blue); Cecum pellets (green); Cecum lichen (orange).
Archaeal 16S rRNA sequence analyses in cecum samples
A total of 73,977 Archaeal 16S rRNA sequences were generated from cecum samples, resulting in a final 71,498 sequences after quality filtering and trimming. Sequences were assigned to a total 47 OTUs with as much as 46 OTUs shared by all the samples. Similar to the rumen samples, phylotypes belonging to the phylum Euryarchaeota were the only microbes detected in all samples, with most of the OTUs belonging to the genus Methanobrevibacter (Pd: 96.7% of 32, 141 sequences; Ld: 99.2% of 39,357 sequences, on average) (Fig 2, S5 and S7 Files). At strain/species level, an average 68.1% of total sequences were identified as Methanobrevibacter millerae strain ZA-10 in reindeer fed with pellets, but sharing less than 97% identity with this methanogens. Instead, in lichen-fed reindeer, phylotypes sharing a 98% similarity to Methanobrevibacter thaueri strain CW constituted the major phylotype (73.8% total sequences, on average) (Fig 2, S5 and S7 Files). Other phylotypes, showing a significant decrease with a lichen-based diet, were Methanobrevibacter wolinii strain SH (98% similarity) (Pd: 7.1%; Ld: 0.1%; p = 0.027) and Methanobrevibacter boviskoreani strain JH1 (98% similarity) (Pd: 7.2%; Ld: 0.2%. p = 0.028). Phylotypes sharing a 99% similarity with Methanobrevibacter ruminantium strain M1 resulted in 5.4% and 18.8% of total sequences in reindeer fed with pellets and lichens, respectively (p = 0.258). (Fig 2, S5 and S7 Files). The same trend was observed for phylotypes identified as Mbr. olleyae strain KM1H5-1P (97% similarity) (Pd: 0.2%; Ld: 1.6% total sequences. p = 0.058).
Whole community comparisons
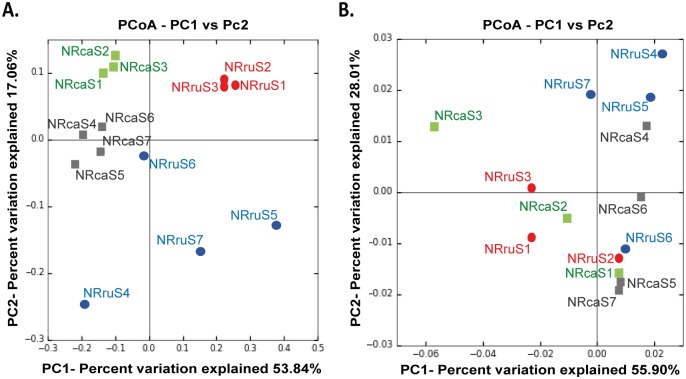
In general, the total number of unique bacterial OTUs, taken as an indicator of sample diversity, was significantly decreased (Monte Carlo distance-based t-test, p<0.05) when reindeer were fed with a lichen-based diet (Fig 3a, S1 Table). Between-sample comparisons of the different microbial libraries (beta diversity) illustrated by weighted UniFrac-based PCoA plots showed those samples belonging to the same feeding regime (pellets or lichens) grouping together in both sampling sites (rumen and cecum) (Fig 4a). Non-parametric Adonis tests (Rumen samples: pseudo-F = 0.037; Cecum samples: pseudo-F = 0.032) further corroborated these results, thus indicating that bacterial profiles from reindeer fed pellets or lichens were different, in both rumen and cecum.
Fig 3. Box-plots within-sample community diversity comparisons from rumen and cecum samples in Norwegian reindeer.
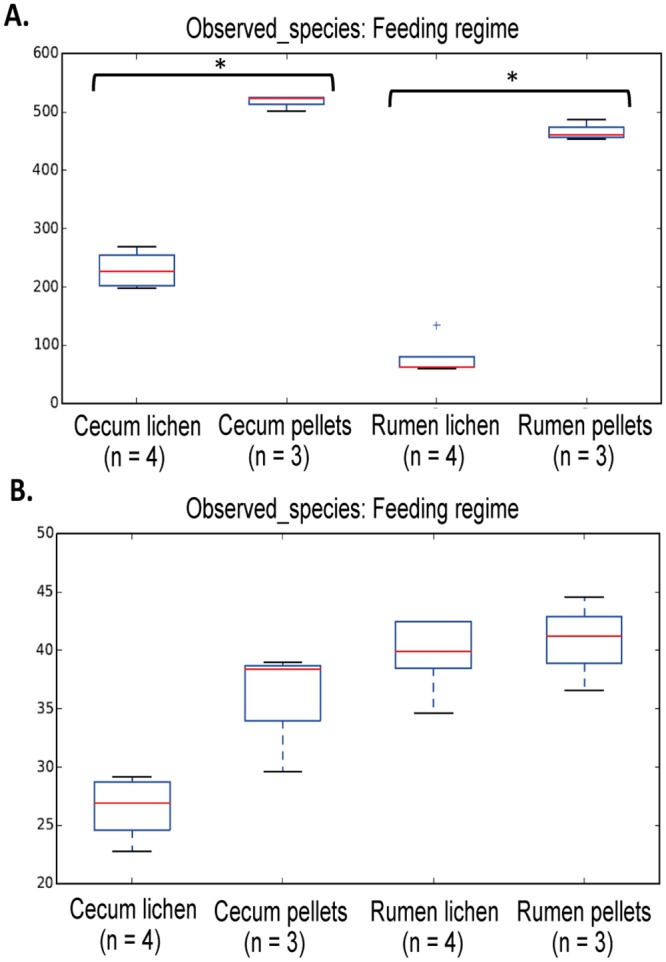
(A) Mean alpha diversity values for total unique OTUs (observed_species) calculated for the bacterial fraction of the microbiome. (B) Mean alpha diversity values for total unique OTUs (observed_species) calculated for the archaeal fraction of the microbiome. Box-plots were calculated using average values obtained from randomly subsampled datasets for each sample with a sample depth of 2000 sequences and 10 iterations at each subsampling step. Pairwise comparisons were performed only between samples from the same sample site (rumen or cecum) and statistical significance (asterisk symbol; p<0.05) was calculated with non-parametric t-test with Monte Carlo permutations (n = 999).
Fig 4. Principal coordinate analysis of the microbial community structure from rumen and cecum in Norwegian reindeer.
(A) PCoA plot illustrating the bacterial community structures. (B) PCoA plot illustrating the archaeal community composition. PCoA plots were generate based on weighted UniFrac distance matrices. Colour style and labelling refers to the type of diet provided and sample origin: rumen samples from reindeer fed pellets (rumen-pellets) (red dots); rumen-lichens (blue dots); cecum-pellets (green squares); cecum-lichens (dark-grey squares). Statistical comparisons were performed only between samples from the same sampling site (rumen or cecum).
No significant differences were observed on any alpha diversity parameter in the archaeal datasets (Fig 3b, S2 Table), except species richness (chao1), which was negatively influenced in cecum samples from reindeer fed lichens (Monte Carlo distance-based t-test, p = 0.04). PCoA plots showed a more scattered sample distribution than in bacteria (Fig 4b). However, UniFrac-based Adonis statistical tests (Rumen samples: pseudo-F = 0.029; Cecum samples: pseudo-F = 0.028) indicated significantly different archaeal profiles in rumen and cecum samples between both groups of reindeer.
Predicted microbiome function
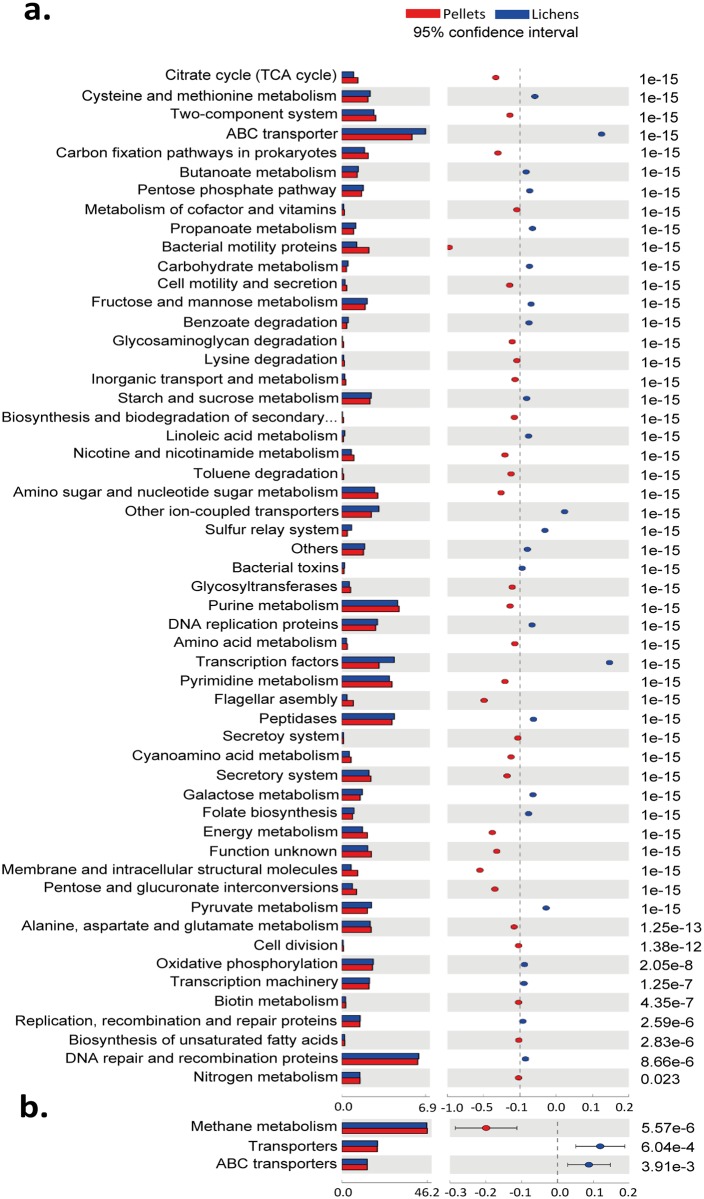
As observed, the different diets led to diverse microbial community structures in bacteria and archaea as well as in the two different digestive compartments. Accordingly, variations in the functional gene contents for each metagenome can also be assumed. In the absence of shotgun metagenomic sequencing data, we performed a first approximation to the gene content in our 16S rRNA libraries applying PICRUSt. Briefly, PICRUSt is used to predict present gene families from 16S rRNA gene data and a reference database applying evolutionary modelling. Relative abundances for imputed KEGG pathways were calculated so that any potential changes in the overall metabolic functions predicted to libraries from animals fed either pellets or lichens were assessed. Predicted genes related to several KEGG pathways, such as pyruvate and carbohydrate metabolism, as well as those pathways involved in fatty acid metabolism (propanoate and butanoate metabolism), showed a significantly higher relative abundance (p = 1.0e-15) with the consumption of lichens (Fig 5a). Gene predictions for starch and sucrose metabolism KEGG pathways involving metabolic routes for several polysaccharides like xylan, pectin, cellulose, and beta-glucan were also positively influenced on a lichen-based diet (p = 2.22e-15). In contrast, archaeal gene contents directly involved in methane metabolism were present in a significantly lower relative proportions in lichen fed reindeer (p = 1.0e-15) (Fig 5b).
Fig 5. Comparisons of imputed metagenome prediction of the bacterial and archaeal metagenomes in Norwegian reindeer.
Relative abundances for each KEGG metabolic pathway present in each metagenome were calculated and plotted with STAMP. KEGG pathways that were significantly different (IC: 95%. p-value <0.05) between diets for (A) bacterial and (B) archaeal predicted gene functions are showed. Color pattern is set based on diet composition: pellets concentrate (red); lichens (blue).
Volatile fatty acids chemical analysis
Total concentrations of some VFAs, such as acetate, propionate and n-Butyrate from rumen samples, in both groups of reindeer, were determined by HPLC. In summary, no statistical differences were observed in the average concentrations of acetate (Pd: 35.4 mg/mL; Ld: 30.8 mg/mL. p = 0.696) or n-Butyrate (Pd: 4 mg/mL; Ld: 4.9 mg/mL. p = 1) (S3 Table). Nonetheless, propionate displayed the highest fluctuation observed between diets with a considerable decrease from an average 48.2 mg/mL to 11.8 mg/mL in reindeer fed with lichens, although this difference was not statistically significant (p = 0.097).
Discussion
Fluctuations on microbial density by the ingestion of lichens
Rumen counts for the three microbial groups (bacteria, archaea and ciliate protozoa) tested in all samples were generally lower than those reported for free-ranging Norwegian reindeer [13]. This may likely be attributed to the nutritional constraints of a controlled diet in comparison to highly varied natural pastures, rich in proteins and minerals [5]. Both in vitro and in vivo studies indicated a high sensitivity to PSM by ciliates [25,53] and methanogens [26]. However, resistance has also been described in both microbial groups [54,55]. This resistance may potentially explain the lack of effect observed in the total numbers of methanogens and ciliates in the rumen and cecum of reindeer fed with lichens. Another potential explanation may be related to the PSM-degrading capacity reported for several rumen bacterial isolates from reindeer [56], which may attenuate the negative effects exerted by these compounds on the rumen and cecum microbiota in lichen-fed reindeer.
Bacterial diversity permutations with a lichen-based diet
In summary, Bacteroidetes and Firmicutes were the major phylotypes found in reindeer, offered either diet, and from both sampling sites. Although in general, Firmicutes was present at a higher proportion in cecum samples. These two phyla commonly dominated the gastrointestinal tract of free-ranging and captive ruminants [14,57,58]. The proportion of these two phyla depends on the type of substrates they are exposed to. For example, Bacteroidetes phylotypes are mostly associated with the presence of easily fermentable carbohydrates (i.e. starch), and proteins. Whereas, some Firmicutes-related members are involved in the degradation of recalcitrant substrates, such as cellulose [59]. Considering their divergent metabolic strategies, the proportion of these two phyla (i.e. Firmicutes to Bacteroidetes (F-B) ratio) will be affected by the polysaccharides found in the diet, which may partly affect the host metabolism via VFAs production from fermentation. No significant differences were observed between the F-B ratios obtained from each diet, in either the rumen (p = 0.143), or the cecum (p = 0.107) (Fig 1; S2 File). Nonetheless, UniFrac-based community analyses showed overall bacterial profiles significantly differed between both feeding regimes independently of the sampling site (pseudo-F<0.05). Reindeer in the present study were supplied with a mixture of lichens of the genera Cladonia and Cetraria, which are composed of several polysaccharides (hemicellulose, xylan, lichenin) structurally dissimilar to those commonly found in vascular plants [7]. Any existing disparity in the structural nature of these polysaccharides between both diets may partially account for the differences observed in their respective bacterial community from both anaerobic chambers.
PSM, which are found at a high proportion and variety in lichens, has extensively been reported to possess a disrupting effect on several bacterial phylotypes [26,54]. For instance, in vitro assays indicated a dose-dependent altered endoglucanase and proteolytic activities in the presence of condensed tannins for several Firmicutes species, in addition to growth inhibition at high concentrations in Ruminococcus albus [60]. This inhibitory effect on Ruminococcus spp. may explain the significant reduction in the relative proportion of this family obtained in the rumen of reindeer fed with lichens (Fig 1; S2 and S3 Files). Aagnes et al. [8] described the rumen microbiome in reindeer fed, ad libitum, a lichen-based diet and showed an increase in bacteria belonging to the phylum Firmicutes, like Clostridium and Streptococcus. Furthermore, in vitro assays with Clostridia-related isolates from the rumen of Norwegian reindeer also indicated degradation of some plant anti-nutrients (e.g. tannins), together with enhanced growth [56,61]. Nonetheless, a negative, or absent, response to the presence of tannins was also reported in in vitro studies, with members belonging to the class Clostridia [62,63], which may account for the lack of differences obtained between both groups of reindeer (rumen: p = 0.198; cecum: p = 0.090).
Prevotella spp. was also reported as the dominant genus within the phylum Bacteroidetes in the rumen microbiome of Chinese sika deer fed with an oak leaves-based diet, also high in PSM [29]. This genus may be able to tolerate and thrive under diets enriched in the antimicrobial PSM through an unknown mechanism, which may explain for their comparable relative proportions found in both diets. In contrast, a growth-suppressing effect has been described for some Bacteroides spp. in in vitro studies with phenolics and aromatic compounds extracted from tea [64]. This suppressing effect may explain for the significant decrease observed in unclassified Bacteroidales-related phylotypes in lichen-fed reindeer, in both anaerobic chambers (Fig 1; S2, S3 and S4 Files). In addition, a general decrease in diversity was observed in the rumen and cecum of reindeer fed with lichens. This observation agrees with decreased diversity reported in rumen samples from Chinese sika deer fed with a diet high in PSM [29].
Altered archaeal diversity and potential effects on methanogenesis
Similar to bacteria, the archaea were significantly different in reindeer from the two dietary groups (Fig 4b), with Methanobrevibacter spp. representing the major archaeal phylotypes in all the samples (Fig 2; S5 File). Methanobrevibacter was also found to be the dominant genus in free ranging reindeer (summer pastures) and cattle (high fiber diet) [13,65]. A two point five-fold increase in the abundance of members belonging to this genus has been described for cattle associated to high methane outputs compared to cattle yielding low-methane emissions [66]. In the present study, no significant differences were observed in the proportion of Methanobrevibacter spp. between reindeer fed with pellets, or lichens (S5 File; p = 0.9). Although these results may hint to unaltered methane emissions from reindeer, fed lichens, several in vivo and in vitro studies have described a strong negative effect on methanogenesis by PSM [25,26].
In some instances, the diversity of methanogens at strain level play key roles in affecting the methane production rather than the density of methanogens [67,68]. Methanobrevibacter thaueri is common in the rumen of several ruminants, such as reindeer and cattle with no diet specificity [13,69]. Up to now, only one study found that Mbr. thaueri was the dominant phylotype in the rumen of wild Impalas (Aepyceros melampus melampus) [70]. King et al. [71] suggested a classification method for methanogens based on phylogenetic distribution and representation, with Mbr. smithii, Mbr. gottschalkii, Mbr. millerae and Mbr. thaueri (SGMT-group) and Mbr. ruminantium and Mbr. olleyae (RO-group) divided in two separate clades. Although, Mbr. gottschalkii was not present in any samples, the SGMT:RO ratio was generally lower in reindeer fed with lichens, although these variations were not significant (Rumen: Pd = 12.1; Ld = 1.9. p = 0.129; Cecum: Pd = 12.4; Ld = 3.8. p = 0.9) (Fig 2, S5 File). Two of these methanogens, Mbr. smithii strain PS and Mbr. olleyae strain KM1H5-1P, were significantly altered with the intake of lichens, showing a decrease and an increase, respectively. A similar trend was obtained in cecum samples, although it was not statistically supported (p = 0.058). Terminal restriction fragment length polymorphism (t-RFLP) data, relating methanogenesis with methanogen community structure in Swedish dairy cows, suggested that methane emissions were positively correlated with dominance by the SGMT-clade over the RO-clade methanogens [72]. These results may suggest that increased relative proportion of RO-methanogens (or decreasing members of the SGMT clade) related to animals with lower methane yields. In conjunction with a significant increase in Mbr. olleyae strain KM1H5-1P, Mbr. ruminantium strain M1 constituted the second major phylotype in both the rumen and cecum of lichen-fed reindeer. The relative proportion of Mbr. ruminantium was, however, not observed to be significantly different between both groups of reindeer, maybe because of the big inter-individual variations observed in the lichen-fed group (Rumen: p = 0.054; cecum: p = 0.258). Considering that Mbr. smithii and Mbr. olleyae represented only a 2.9% and 2.6% of the total rumen archaeal sequences in reindeer fed pellets or lichens, respectively, it may seem difficult to explain predicted lower methane yields with the intake of lichens just based on the fluctuation of these two methanogens.
Mbr. wolinii and Mbr. boviskoreanis (≥97% similarity) were significantly reduced in the cecum of reindeer fed lichens (Fig 2; S5 and S7 Files). These two methanogens are phylogenetically related [73]; an aspect that may explain the similar effect on their relative proportions with lichens. Mbr. wolinii-related phylotypes markedly increased with the intake of a tannin-rich oak leaves-based diet in sika deer [74], which were hypothesized to emit less methane than Sika deer fed corn stalk. Alternatively, a higher proportion of Mbr. sp. AbM4, closely related to Mbr. wolinii, was also described in the rumen of cattle possessing low feed efficiencies, which were predicted to possess high methane yields [67]. Considering the disparity observed in both studies and the fact that cecum methanogenesis represents only a minor fraction of the total methane output from ruminants, the decrease in the relative proportion of these two methanogens would only account for a minor part of predicted reduced methane emissions with the intake of lichens. Nonetheless, the role played by these two methanogens on the overall methane output from ruminants would worth further investigation.
PICRUSt analysis showed dissimilarities in the relative abundance of genes involved in several KEGG pathways, which concurred with significant differences of bacterial and archaeal profiles between both groups of reindeer. Higher relative abundances for pyruvate and fatty acids (propionate and butyrate) metabolism-related genes were obtained in reindeer fed lichens (Fig 5a). Given that the concentration of rumen VFAs (propionate, acetate and n-butyrate) remained unaltered between both groups of reindeer (S3 Table), it was surprising to obtain such differences. In addition, significantly lower relative gene contents for ascribed genes involved in methane metabolism were found in reindeer fed lichens (Fig 5b). Deep metagenomics and metatranscriptomics studies, comparing microbial diversity and genetic profiles between high- and low-CH4 emitting sheep, demonstrated similar gene contents for KEGG pathways involved in methanogenesis [75]. Instead, higher expression levels for some of these genes were reported for the animals with high methane yields. Based on these findings, the differences observed between reindeer fed pellets or lichens may not necessarily predict for lower methane yields with a lichen-based diet. It would demand the use of more specific approaches (metatranscriptomics) in order to complement the results obtained in our study and assess for differences in the expression level of these genes involved in methanogenesis, which would allow elucidating potential changes in the methane output with the ingestion of lichens. The PICRUSt analysis represent a valuable first look at the respective genetic profiles from both groups of reindeer in order to guide future analysis.
In conclusion, the present study demonstrated that both the bacterial and archaeal microbiomes housed in the rumen and cecum of Norwegian reindeer was altered in response to the intake of a mixed lichen diet. As discussed, several factors might lead to such fluctuations, though reported high PSM contents in lichens is hypothesized to be the main driving force. These results are a valuable complement to previous findings addressing the use of diets high in PSM, or extracted compounds, envisaged as a potential strategy for enteric methane mitigation. The outstanding tolerance demonstrated by Norwegian reindeer to a PSM-rich diet certainly stands for its potential as a candidate to conduct more research focused on understanding the relationship between the effects exerted by these plant anti-nutrients on the archaeal community structure, and how this is linked with predicted lower methanogenesis. Nonetheless, a direct estimation on the methane yields from reindeer fed lichens would be the best way to determine differences in the methane output between both feeding regimes, and it would be a valuable support to the findings presented in this study. More research is paramount to pinpoint the community structures displayed by the other syntrophic partners of methanogens co-habiting the rumen and cecum, such as ciliate protozoa or fungi, under similar feeding regimes. This would certainly allow for a broader view on the metagenomic profiles specific for those conditions when low methane outcomes are obtained.
Supporting Information
(XLSX)
(XLSX)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(XLSX)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This study is linked to the framework of the International Polar Year (IPY) as part of the consortium IPY#399 EALAT: Climate change and reindeer husbandry. Funding was provided by the Reindeer Husbandry Research Fund and the University of Tromsø –The Arctic University of Norway. We are grateful to the staff of animal caretakers, Hans Lian and Hans Arne Solvang, for their help feeding and sampling the animals. PBP is supported by a European Research Commission Starting Grant Fellowship (336355 –MicroDE).
Data Availability
All fastq files are available from the Sequence Read Archive (SRA) database (accession number SRP063699).
Funding Statement
Funding was provided by the Reindeer Husbandry Research Fund (http://www.reindrift.no/?id=943&subid=0; Project account A36010) and the University of Tromsø (UiT), The Arctic University of Norway. PBP is supported by a European Research Commission Starting Grant Fellowship (336355 – MicroDE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Banfield AWF. A revision of the reindeer and caribou, genus Rangifer. National Museum of Canada, Bulletin; 1961;177: 1–137. [Google Scholar]
- 2.Turi JM. The world reindeer livelihood—current situation: threats and possibilities In: In: Kankaanpää S, Wüller-Wille L, Susiluoto P, Sutinen ML (eds). Northern Timberline Forests: Environmental and Socio-Economic Issues and Concerns, The Finnish Forest Res Inst; 2002; 70–75. [Google Scholar]
- 3.Mathiesen SD, Sørmo W, Haga ØE, Norberg HJ, Utsi THA, Tyler NJC. The oral anatomy of arctic ruminants: coping with seasonal changes. J Zool Lond. 2000;251: 119–128. [Google Scholar]
- 4.Mathiesen SD, Aagnes TH, Sørmo W. Forage chemistry and the digestive system in reindeer (Rangifer tarandus tarandus) in northern Norway and on South Georgia. Rangifer. 1999;19: 91–101. [Google Scholar]
- 5.Mathiesen SD, Haga ØE, Kaino T, Tyler NJC. Diet composition, rumen papillation and maintenance of carcass mass in female Norwegian reindeer (Rangifer tarandus tarandus) in winter. J Zool Lond. 2000;251: 129–138. [Google Scholar]
- 6.Skogland T. Wild reindeer foraging-niche organization. Holarct Ecol. 1984;7: 345–379. [Google Scholar]
- 7.Storeheier PV, Mathiesen SD, Tyler NJC, Olsen MA. Nutritive values of terricolous lichens for reindeer in winter. Linechologist. 2002;34: 247–257. [Google Scholar]
- 8.Aagnes TH, Sørmo W, Mathiesen SD. Ruminal microbial digestion in free-living, in captive lichen-fed, and in starved reindeer (Rangifer tarandus tarandus) in winter. Appl Environ Microbiol. 1995;61: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svihus B, Holand Ø. Lichen polysaccharides and their relation to reindeer/caribou nutrition. J Range Manage. 2000;53: 642–648. [Google Scholar]
- 10.Dailey RN, Montgomery DL, Ingram JT, Siemion R, Vasquez M, Raisbeck MF. Toxicity of lichen secondary metabolite (+)-Usnic acid in domestic sheep. Vet Pathol. 2008;45: 19–25. 10.1354/vp.45-1-19 [DOI] [PubMed] [Google Scholar]
- 11.Cook WE, Raisbeck MF, Cornish TE, Williams ES, Brown B, Hiatt G, Kreeger TJ. Paresis and death in elk (Cervus elaphus) due to lichen intoxication in Wyoming. J Wildl Dis. 2007;43: 498–503. [DOI] [PubMed] [Google Scholar]
- 12.Sundset MA, Praesteng KE, Cann IK, Mathiesen SD, Mackie RI. Novel rumen bacterial diversity in two geographically separated sub-species of reindeer. Microb Ecol. 2007;54: 424–438. [DOI] [PubMed] [Google Scholar]
- 13.Sundset MA, Edwards JE, Cheng YF, Senosiain RS, Fraile MN, Northwood KS, et al. Molecular diversity of the rumen microbiome of Norwegian reindeer on natural summer pasture. Microb Ecol. 2009;57: 335–348. 10.1007/s00248-008-9414-7 [DOI] [PubMed] [Google Scholar]
- 14.Ishaq SL, Wright AD. High-throughput DNA sequencing of the ruminal bacteria from moose (Alces alces) in Vermont, Alaska, and Norway. Microb Ecol. 2014;68: 185–195. 10.1007/s00248-014-0399-0 [DOI] [PubMed] [Google Scholar]
- 15.Orskov ER, Fraser C, Mason VC, Mann SO. Influence of starch digestion in the large intestine of sheep on caecal fermentation, caecal microflora and faecal nitrogen excretion. Br J Nutr. 1970;24: 671–682. [DOI] [PubMed] [Google Scholar]
- 16.Mathiesen SD, Orpin CG, Greenwood Y, Blix AS. Seasonal changes in the cecal microflora of the high-arctic Svalbard reindeer (Rangifer tarandus platyrhynchus). 1987;53: 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harfoot CG. Anatomy, physiology and microbiology of the ruminant digestive tract. Prog Lipid Res. 1978;17: 1–19. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DE, Ward GM. Estimates of animal methane emissions. Environ Mon and Asses. 1996;42: 133–141. [DOI] [PubMed] [Google Scholar]
- 19.Moss AR, Jouany JP, Newbold J. Methane production by ruminants: in contribution to global warming. Ann Zootech. 2000;49: 231–253. [Google Scholar]
- 20.Rouvière PE, Wolfe RS. Novel biochemistry of methanogenesis. J Biol Chem. 1988;263: 7913–7916. [PubMed] [Google Scholar]
- 21.Blaxter KL, Clapperton JL. Prediction of the amount of methane produced by ruminants. Br J Nutr. 1965;19: 511–522. [DOI] [PubMed] [Google Scholar]
- 22.Holter JB, Young AJ. Methane prediction in dry and lactating Holstein cows. J Dairy Sci. 1992;75: 2165–2175. [DOI] [PubMed] [Google Scholar]
- 23.Vogels GD, Hoppe WF, Stumm CK. Association of methanogenic bacteria with rumen ciliates. Appl Environ Microbiol. 1980;40: 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ushida K, Newbold CJ, Jouany JP. Interspecies hydrogen transfer between the rumen ciliate Polyplastron multivesiculatum and Methanosarcina barkeri. J Gen Appl Microbiol. 1997;43: 129–131. [DOI] [PubMed] [Google Scholar]
- 25.Tan HY, Sieo CC, Abdullah N, Liang JB, Huang XD, Ho YW. Effect of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Animal Feed Science and Technology. 2011;169: 185–193. [Google Scholar]
- 26.Bodas R, Prieto N, García-González R, Andrés S, Giráldez FJ, López S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Animal Feed Science and Technology. 2012;176: 78–93. [Google Scholar]
- 27.Carulla JE, Kreuzer M, Machmüller A, Hess HD. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Australian Journal of Agricultural Research. 2005;56: 961–970. [Google Scholar]
- 28.Puchala R, Min BR, Goetsch AL, Sahlu T. The effect of a condensed tannin-containing forage on methane emission by goats. J Anim Sci. 2005;83: 182–186. [DOI] [PubMed] [Google Scholar]
- 29.Li ZP, Liu HL, Guang YL, Bao K, Wang KY, Xu C, Yang YF, Yang HF, Wright ADG. Molecular diversity of rumen bacterial communities from tannin-rich and fiber-rich forage fed domestic Sika deer (Cervus nippon) in China. BMC Microbiol. 2013;13: 151 10.1186/1471-2180-13-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36: 808–812. [DOI] [PubMed] [Google Scholar]
- 31.Denman SE, McSweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006;58: 572–582. [DOI] [PubMed] [Google Scholar]
- 32.Skillman LC, Toovey AF, Williams AJ, Wright AD. Development and validation of a real-time PCR method to quantify rumen protozoa and examination of variability between Entodinium populations in sheep offered a hay-based diet. Appl Environ Microbiol. 2006;72: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Saint DA. Validation of a quantitative method for real time PCR kinetics. Biochem Biophys Res Commun. 2002;294: 347–353. [DOI] [PubMed] [Google Scholar]
- 34.Denman SE, Tomkins NW, McSweeney CS. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol Ecol. 2007;62: 313–322. [DOI] [PubMed] [Google Scholar]
- 35.Denman SE, McSweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006;58: 572–582. [DOI] [PubMed] [Google Scholar]
- 36.Sylvester JT, Karnati SKR, Yu Z, Morrison M, Firkins JL. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J Nutr. 2004;134: 3378–3384. [DOI] [PubMed] [Google Scholar]
- 37.Gantner S, Andersson AF, Alonso-Sáez L, Bertilsson S. Novel primers for 16S rRNA-based Archaeal community analyses in environmental samples. J Microbiol Methods. 2011;84: 12–18. 10.1016/j.mimet.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 38.Pope PB, Mackenzie AK, Gregor I, Smith W, Sundset MA, McHardy AC, et al. Metagenomics of the Svalbard reindeer rumen microbiome reveals abundance of polysaccharide utilization loci. PLoS One. 2012;7: e38571 10.1371/journal.pone.0038571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 2008;5: 235–237. 10.1038/nmeth.1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7: 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26: 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 42.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27: 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72: 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26: 266–267. 10.1093/bioinformatics/btp636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, et al. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 2003;31: 442–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215: 403–410. [DOI] [PubMed] [Google Scholar]
- 47.Chao A. Nonparametric estimation of the number of classes in a population. Scand J Statist. 1984;11: 265–270. [Google Scholar]
- 48.Shannon CE. A mathematical theory of communication. The Bell System Technical Journal. 1948;27: 379–423, 623–656. [Google Scholar]
- 49.Good IJ. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40: 237–264. [Google Scholar]
- 50.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes J, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30: 3123–3124. 10.1093/bioinformatics/btu494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welch BL. The generalisation of student's problems when several different population variances are involved. Biometrika. 1947;34: 28–35. [DOI] [PubMed] [Google Scholar]
- 53.Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88: 587–605. [DOI] [PubMed] [Google Scholar]
- 54.Patra AK, Saxena J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev. 2009;22: 204–219. 10.1017/S0954422409990163 [DOI] [PubMed] [Google Scholar]
- 55.Sliwinski BJ, Soliva CR, Machmüller A, Kreuzen M. Efficacy of plant extracts rich in secondary constituents to modify rumen fermentation. Animal Feed Science and Technology. 2002;101: 101–114. [Google Scholar]
- 56.Glad T, Falk A, Barboza P, Kohn A, Brusetti L, Mathiesen SD, et al. Fate and effect of usnic acid in lichen on the bacterial population in the reindeer rumen. Microb Ecol. 2009;57: 570–571. [Google Scholar]
- 57.Sundset MA, Barboza PS, Green TK, Folkow LP, Blix AS, Mathiesen SD. Microbial degradation of usnic acid in the reindeer rumen. Naturwissenschaften. 2010;97: 273–278. 10.1007/s00114-009-0639-1 [DOI] [PubMed] [Google Scholar]
- 58.de Oliveira MN, Jewell KA, Freitas FS, Benjamin LA, Tótola MR, Borges AC, et al. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet Microbiol. 2013;164: 307–314. 10.1016/j.vetmic.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 59.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6: 121–131. 10.1038/nrmicro1817 [DOI] [PubMed] [Google Scholar]
- 60.Min BR, Attwood GT, McNabb WC, Molan AL, Barry TN. The effect of condensed tannins from Lotus corniculatus of the proteolytic activities and growth of rumen bacteria. Animal Feed Science and Technology. 2005;121: 45–58. [Google Scholar]
- 61.Sundset MA, Kohn A, Mathiesen SD, Praesteng KE. Eubacterium rangiferina, a novel usnic acid-resistant bacterium from the reindeer rumen. Naturwissenschaften. 2008;95: 741–749. 10.1007/s00114-008-0381-0 [DOI] [PubMed] [Google Scholar]
- 62.Ephraim E, Odenyo A, Ashenafi M. Isolation and characterization of tannin-degrading bacteria from fecal samples of some wild ruminants in Ethiopia. Animal Feed Science and Technology. 2005;118: 243–253. [Google Scholar]
- 63.Jones GA, McAllister TA, Muir AD, Cheng KJ. Effects of Sainfoin (Onobrychis viciifolia Scop.) Condensed Tannins on Growth and Proteolysis by Four Strains of Ruminal Bacteria. Appl Environ Microbiol. 1994;60: 1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol. 2006;157: 876–884. [DOI] [PubMed] [Google Scholar]
- 65.Sirohi SK, Chaudhary PP, Singh N, Singh D, Puniya AK. The 16S rRNA and mcrA gene based comparative diversity of methanogens in cattle fed on a high fiber based diet. Gene. 2013;523: 161–166. 10.1016/j.gene.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 66.Wallace RJ, Rooke JA, McKain N, Duthie CA, Hyslop JJ, Ross DW, et al. The rumen microbial metagenome associated with high methane production in cattle. BMC Genomics. 2015;16: 839 10.1186/s12864-015-2032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou M, Hernandez-Sanabria E, Guan LL. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. Appl Environ Microbiol. 2009;75: 6524–6533. 10.1128/AEM.02815-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou M, Chung YH, Beauchemin KA, Holtshausen L, Oba M, McAllister TA, et al. Relationship between rumen methanogens and methane production in dairy cows fed diets supplemented with a feed enzyme additive. J Appl Microbiol. 2011;111: 1148–1158. 10.1111/j.1365-2672.2011.05126.x [DOI] [PubMed] [Google Scholar]
- 69.Wright AD, Auckland CH, Lynn DH. Molecular diversity of methanogens in feedlot cattle from Ontario and Prince Edward Island, Canada. Appl Environ Microbiol. 2007;73: 4206–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cersosimo LM, Lachance H, St-Pierre B, van Hoven W, Wright AD. Examination of the rumen bacteria and methanogenic archaea of wild Impalas (Aepyceros melampus melampus) from Pongola, South Africa. Microbio Ecol. 2015;69: 577–585. [DOI] [PubMed] [Google Scholar]
- 71.King EE, Smith RP, St-Pierre B, Wright AD. Differences in the rumen methanogen populations of lactating Jersey and Holstein dairy cows under the same diet regimen. Appl Environ Microbiol. 2011;77: 5682–5687. 10.1128/AEM.05130-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danielsson R, Schnürer A, Arthurson V, Bertilsson J. Methanogenic population and CH4 production in Swedish dairy cows fed different levels of forage. Appl Environ Microbiol. 2012;78: 6172–6179. 10.1128/AEM.00675-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JH, Kumar S, Lee GH, Chang DH, Rhee MS, Yoon MH, et al. Methanobrevibacter boviskoreani sp. nov., isolated from the rumen of Korean native cattle. Int J Syst Evol Microbiol. 2013;63: 4196–4201. 10.1099/ijs.0.054056-0 [DOI] [PubMed] [Google Scholar]
- 74.Li ZP, Liu HL, Li GY, Bao K, Wang KY, Xu C, et al. Molecular diversity of rumen bacterial communities from tannin-rich and fiber-rich forage fed domestic Sika deer (Cervus nippon) in China. BMC Microbiol. 2013;13: 151 10.1186/1471-2180-13-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi W, Moon CD, Leahy SC, Kang D, Froula J, Kittelmann S, et al. Methane yield phenotypes linked to differential gene expression in the sheep rumen microbiome. Genome Res. 2014;24: 1517–1525. 10.1101/gr.168245.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(XLSX)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(A) Classification of bacteria at phylum level. (B) Classification of bacteria up to genus level.
(PDF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All fastq files are available from the Sequence Read Archive (SRA) database (accession number SRP063699).