Dear Editors,
Despite the high prevalence of endometriosis, little is known about the underlying disease mechanisms [1]. The development and maintenance of endometriosis lesions are linked to the process of Epithelial-Mesenchymal Transition (EMT), which describes the transformation of stationary epithelial cells into proliferative and invasive mesenchymal cells [2]. Since the canonical Wnt-signaling system is an important driver for EMT [3], we hypothesized that Wnt signaling might be altered in different types of endometriosis lesions [4] leading to changes in related EMT processes. Hence, this study's objective was to quantify the expression changes of (1) Wnt-signaling pathway genes such as Wnt agonists, antagonists and receptors and (2) Wnt-target genes (e.g. E-Cadherin and S100A4), which play a role in EMT, in endometrium from women with endometriosis (cases) and without (controls) as well as endometriosis lesions at different stages of the cycle.
A total of 77 tissue samples were collected from women of reproductive age (71 cases and 6 controls). All subjects in the age group between 23 and 49 yr (34.2 ± 6.9 years) reported regular menstrual cycles, were not pregnant, and had not been on hormonal treatment. In cases, the diagnosis of endometriosis was based on previous pathological reports. Endometrial pathology was excluded in healthy controls. The exact stage of the menstrual cycle was determined by the patient's menstrual history, and tissues were grouped into follicular (day 5- 14) versus luteal phase (day 15 – 30) samples [5]. RNA expression changes for all Wnt-signaling pathway and target genes were quantified in all samples by quantitative real-time RT-PCR. RNA samples were extracted and reverse-transcribed. PCR mixture contained forward and reverse primers, SYBR PCR mix, and 1μl cDNA. An ABI 7900HT Fast Real-Time PCR system was used under standard conditions. Data were analyzed using the ΔΔCt relative quantification method. Per PCR run, multiple endometriosis tissues were compared to a control sample, which was matched regarding the menstrual cycle phase. To compare changes in gene expression, Mann-Whitney tests were performed. p-Levels <0.05 were regarded as statistically significant.
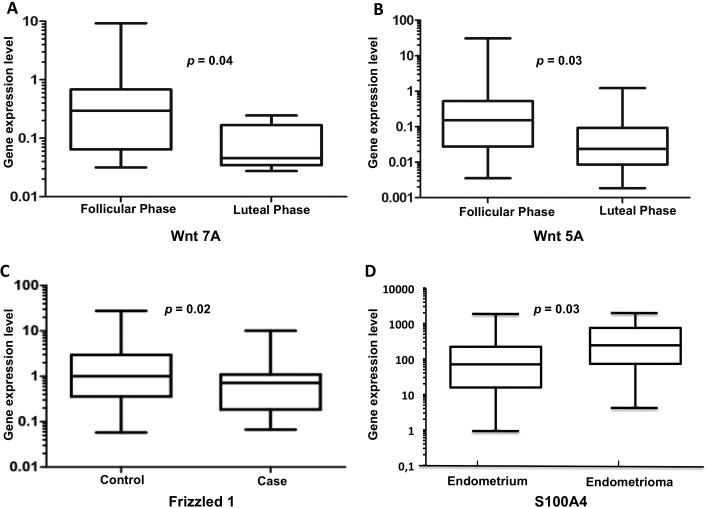
We found cycle-specific changes of Wnt signaling genes in the endometrium of cases and controls: Expression of Wnt 7A was decreased in control (p=0.04, Fig. 1A) and case samples (p=0.003) in the luteal phase when compared to the follicular phase. Case endometrium also showed a decrease in Wnt 5A expression (p=0.03) in the luteal phase (Fig. 1B). When disease specific gene expression changes were analyzed, a downregulation of Frizzled 1 (p=0.02, Fig. 1C), LRP 5 (p=0.003) and 6 (p=0.05) receptors was present in case endometrium versus controls. In ovarian endometriosis, an upregulated expression of Frizzled 1 (p=0.002) and 7 (p=0.058) as well S100A4 (p=0.03, Fig. 1D) and a downregulated expression of Wnt 7A (p=0.02), Dkk 1 (p=0.02) and E-Cadherin (p=0.02) was documented when compared with matching endometrial samples of patients with endometriosis.
Figure 1.
Expression patterns of Wnt-signaling and Wnt-target genes were cycle- and disease- as well as lesion-specific. (A) Cycle dependent change in Wnt 7A gene expression in control endometrium. (B) Cycle dependent change in Wnt 5A gene expression in case endometrium. (C) Change in Frizzled 1 expression in case endometrium in comparison to control tissue. (D) Expression change of S100A4 in endometrioma tissue compared to endometrium obtained from an endometriosis patient.
Overall, our analysis identified expression patterns of Wnt-signaling as well as Wnt-target genes that were cycle-, disease- as well as lesion-specific. Alteration of Wnt-signaling in endometriosis as exemplified by the gene expression changes of Frizzled 1 can be related either to disease-related changes of the tissue itself or be associated with the introduction of cells into a specific microenvironment such as the ovary. These specific Wnt-signaling expression patterns might play a role in regulating physiologic and pathologic processes in the endometrium or support EMT in ovarian lesions facilitating the transition to ovarian cancer. Hence, our findings provide evidence that the Wnt-signaling pathway might be a promising target for novel endometriosis treatments and underline the need to conduct more related research in the future.
Acknowledgment
We thank Kate Lawrenson, PhD for her assistance with the Prism by Graphpad software. The study was supported by departmental funds. Alice Lee received funding from the National Institute of Environmental Health Sciences (T32 ES013678).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farquhar C. Endometriosis. Bmj. 2007;334:249–53. doi: 10.1136/bmj.39073.736829.BE. doi:10.1136/bmj.39073.736829.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartley J, Jülicher A, Hotz B, Mechsner S, Hotz H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch Gynecol Obstet. 2014;289:871–81. doi: 10.1007/s00404-013-3040-4. doi:10.1007/s00404-013-3040-4. [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J, et al. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–79. doi: 10.1172/JCI65871. doi:10.1172/JCI65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68:585–96. doi: 10.1016/s0015-0282(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Alonso M, Blesa D, Simón C. The genomics of the human endometrium. Biochim Biophys Acta. 2012;1822:1931–42. doi: 10.1016/j.bbadis.2012.05.004. doi:10.1016/j.bbadis.2012.05.004. [DOI] [PubMed] [Google Scholar]