The incidence and timing of first cardiac events, impact on trastuzumab prescription, and role of left ventricular ejection fraction (LVEF) monitoring in trastuzumab-treated patients with HER2-positive early breast cancer was assessed. Trastuzumab was discontinued in 36 of 230 patients because of cardiotoxicity; 15 of these had significantly reduced LVEF, and 84.8% stopped taking the drug within the first 6 months of initiating the therapy.
Keywords: Cardiotoxicity, Cardiac monitoring, Adjuvant trastuzumab, Daily practice
Abstract
Introduction.
We assessed the incidence and timing of first cardiac events, impact on trastuzumab prescription, and role of left ventricular ejection fraction (LVEF) monitoring in daily practice of trastuzumab-treated patients with human epidermal growth receptor 2 (HER2)-positive early breast cancer.
Methods.
We included all patients with stage I–III breast cancer diagnosed in the early years (2005–2007) after the introduction of adjuvant trastuzumab in five hospitals in Southeast Netherlands. We studied the incidence and timing of cardiotoxicity in patients treated with adjuvant trastuzumab, using similar cardiac endpoints as in the Herceptin Adjuvant (HERA) trial.
Results.
Of 2,684 included patients, 476 (17.7%) had a HER2-positive tumor. Of these, 269 (56.9%) were treated with adjuvant chemotherapy, and of these, 230 (85.5%) also received trastuzumab. Cardiotoxicity was observed in 29 of 230 patients (12.6%). Twenty of the 230 patients (8.7%) had symptomatic cardiotoxicity, defined as a drop in LVEF of at least 10 percentage points and to below 50%, accompanied by symptoms of congestive heart failure. Trastuzumab was definitely discontinued because of supposed cardiotoxicity in 36 patients (15.6%), of whom only 15 (6.5%) had a significant LVEF drop. Of the 36 patients who prematurely discontinued trastuzumab (including the 29 in whom cardiotoxicity was observed), 84.8% stopped in the first 6 months. No cardiac deaths were seen.
Conclusion.
In the first years after implementation of trastuzumab for treatment of early breast cancer, physicians frequently based their decision to discontinue treatment on patient symptoms apart from LVEF outcome. We suggest that focusing LVEF monitoring on the first 6 months might be more cost-effective without compromising patient safety. Nonetheless, further research is needed.
Implications for Practice:
Knowledge of when cardiotoxicity occurs in daily practice will help shape the best follow-up method for cardiac monitoring in trastuzumab-treated patients with human epidermal growth receptor 2-positive early breast cancer. In the first years after implementation of trastuzumab for treatment of early breast cancer, physicians frequently based their decision to discontinue treatment on patient symptoms apart from left ventricular ejection fraction (LVEF) outcome. When cardiotoxicity was found in daily practice, it occurred mainly in the first 6 months after start of trastuzumab. This study suggests that focusing LVEF monitoring on the first 6 months might be more cost-effective without compromising patient safety. This insight stresses the relevance of performing real-world analyses.
Abstract
摘要
引言. 对于临床上接受曲妥珠单抗治疗的人类表皮生长因子受体 2 (HER2) 阳性的早期乳腺癌患者, 我们对首次心脏事件的发生率和发生时机、对曲妥珠单抗处方的影响, 以及左心室射血分数 (LVEF) 监测的作用进行了评估。
方法. 本研究在荷兰东南部的 5 家医院中, 入选了在曲妥珠单抗辅助治疗用于临床实践后初期 (2005-2007 年 ) 诊断的所有 I∼III 期乳腺癌患者。我们对接受曲妥珠单抗辅助治疗患者的心脏毒性发生率和发生时机进行了研究, 所用的心脏终点与赫赛汀辅助治疗 (HERA) 试验相同。
结果. 在入选的 2684 例患者中, 476 例 (17.7%) 为 HER2 阳性。其中 269 例 (56.9%) 接受辅助化疗, 这些患者中又有 230 例 (85.5%) 还接受了曲妥珠单抗治疗。在 29/230 例 (12.6%) 的患者中观察到心脏毒性。 20/230 例 (8.7%) 存在症状性心脏毒性 (定义为 LVEF 下降≥10% 且 LVEF<50%), 并伴有充血性心力衰竭症状。 36 例 (15.6%) 患者因推断发生心脏毒性而明确停止曲妥珠单抗治疗, 其中仅有 15 例 (6.5%) 出现显著的 LVEF 下降。在 36 例永久停用曲妥珠单抗 (包括 29 例观察到心脏毒性) 的患者中, 84.8%在治疗最初 6 个月内停药。研究未观察到心脏性死亡。
结论. 在曲妥珠单抗应用于早期乳腺癌治疗的最初数年间, 医生常根据患者症状 (而非LVEF转归) 决定停药。我们建议在治疗的最初6个月集中进行LVEF监测, 这样做可能经济效益更好, 而不会影响患者的安全性。但还是就此需要开展进一步的研究。The Oncologist 2016;21:555–562
对临床实践的提示: 在使用曲妥珠单抗对人类表皮生长因子受体 2 阳性早期乳腺癌患者进行治疗的日常实践中, 了解心脏毒性会在什么时候发生有助于对心脏监测的随访策略作出最佳调整。在曲妥珠单抗应用于早期乳腺癌治疗的最初数年中, 医生常根据患者症状, 而不是左心室射血分数 (LVEF) 转归决定停药。本研究建议在治疗的最初 6 个月集中进行 LVEF 监测, 这样做可能经济效益更好, 而不会影响患者的安全性。本文的观点强调了开展真实世界分析的现实意义。
Introduction
Breast cancer is the most frequently diagnosed cancer in women in The Netherlands and worldwide [1, 2]. Overexpression of the human epidermal growth receptor 2 (HER2) protein and amplification of the HER2 gene is seen in 15%–20% of breast cancers. These result in a more aggressive course of disease [3, 4], although recent analyses indicate that breast cancer molecular subtypes may be more relevant for prognosis than clinical HER2 status [5].
Trastuzumab is a humanized monoclonal antibody against the extracellular domain of the HER2 tyrosine kinase receptor. Trastuzumab trials in early breast cancer showed a reduction of the recurrence risk of about 50% and a 30% reduction in mortality [3, 6–13], which next led to an almost worldwide implementation of trastuzumab in daily practice [14, 15].
Yet, myocardial cells also express HER2, which induces a cardioprotective effect on the heart in response to stress and explains why the inhibition of HER2 by trastuzumab causes an increased susceptibility for cardiotoxicity [16–18]. Early studies in advanced breast cancer showed that cardiac events were seen in 27% of patients when trastuzumab was concurrently administered with an anthracycline and in 13% when combined with paclitaxel [19, 20].
In adjuvant trials, the cardiac endpoints used and the method and frequency of monitoring patients varied and different cutoff points for the decrease of left ventricular ejection fraction (LVEF) were used [7, 9, 10, 12, 13, 21]. A cardiac event was reported in approximately 5%–10% of patients in these randomized controlled trials and persisted in about 20% of these. Although trastuzumab-induced cardiotoxicity seems reversible in most patients in a strictly monitored clinical trial setting, these results may be different from what is seen in daily practice. In daily practice, cardiomyopathy with LVEF levels of 20%–30% have been observed in patients treated with adjuvant trastuzumab, leading to a higher level of temporary or permanent discontinuation of therapy, as compared with the clinical trial setting [22–25].
Although LVEF measurements once every 3 months throughout trastuzumab treatment have been the norm, an ideal cardiac monitoring strategy has not been clearly established. Focusing the monitoring on the first 6 months might be more cost-effective without compromising patient safety.
In our real-world study, we identified patients with HER2-positive early breast cancer who were treated with chemotherapy and trastuzumab, and assessed the incidence and timing of first cardiac events, their impact on trastuzumab prescription, and the clinical relevance of LVEF monitoring over time.
Patients and Methods
Study Design
This study was part of a large observational study of all patients (N = 2,684) diagnosed with a stage I–III breast cancer in five hospitals in Southeast Netherlands between January 2005 and December 2007. The results of real-life use and effectiveness of adjuvant trastuzumab in this cohort have been reported elsewhere [26]. Reimbursement of adjuvant trastuzumab in The Netherlands was available as of September 2005; therefore, patients with HER2-positive early breast cancer diagnosed in 2005 were eligible for treatment with trastuzumab following initial adjuvant chemotherapy. These patients were identified through The Netherlands Cancer Registry. For this analysis, all patients with HER2-positive disease treated with trastuzumab were included. In total, 476 patients (17.7%) had HER2-positive disease; from this group, 269 received chemotherapy and 230 (48.3%) received neo-adjuvant trastuzumab.
Data Collection
Between 2009 and 2011, data were collected from the patients’ files by trained data managers under direct supervision of a medical specialist and researchers. We retrospectively collected the LVEF data of all patients. According to the Dutch guidelines, LVEF measurements had to be performed before start of trastuzumab and at least every 3 months until end of trastuzumab treatment. Before start of trastuzumab treatment, the LVEF had to be higher than 55% [27]. In addition to the LVEF data, information on actual duration of trastuzumab treatment was also collected.
Definitions of Endpoints
In this study, we used the cardiac outcome of the Herceptin Adjuvant (HERA) trial to define cardiotoxicity in daily practice. In the HERA trial, the New York Heart Association (NYHA) functional classification was used to define the level of heart failure. A clinically relevant LVEF drop is defined by an absolute LVEF decline of at least 10 percentage points from baseline to a level below 50%. LVEF had to be determined by multigated acquisition scan or ultrasound. A reduced LVEF level had to be confirmed by repeated LVEF assessment approximately 3–5 weeks after the first documented drop [13]. An asymptomatic LVEF drop was defined as NYHA class I, a mild symptomatic drop as NYHA class II, and a severe drop as NYHA class III or IV, of which the latter two had to be confirmed by a cardiologist [4, 13].
Statistical Analysis
We compared the patient and tumor characteristics between the patients who experienced a cardiotoxic event and the patients who did not following treatment with trastuzumab for HER2-positive disease. We assessed the incidence and timing of cardiac events, including those in all patients who stopped treatment with trastuzumab. Then we made a distinction between those who definitely stopped trastuzumab treatment and those who temporarily stopped trastuzumab treatment. Next, we analyzed the role of LVEF monitoring over time, and we determined the impact on trastuzumab prescription.
Time on treatment was defined as time from the start of treatment until definite cessation of treatment. When the treatment was temporarily stopped, the time on treatment was obtained with the Kaplan-Meier method. We obtained data on when and if treatment was definitively stopped. The median and interquartile range (IQR) of the time on treatment were derived from this curve.
Six months after start of trastuzumab was considered a landmark. At that time, every patient was still at risk of dying of breast cancer. Residual overall survival time was defined as time from this landmark to date of death. All patients still alive were censored at the date of their last follow-up. The difference in residual survival between patients who stopped taking trastuzumab before the landmark and patients who stopped after this point was addressed with the log-rank test.
At the landmark, three patients already had a disease progression and were no longer at risk for progression. Residual progression-free survival time was defined from the landmark to date of progression. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC, https://www.sas.com) [28].
Results
Patients
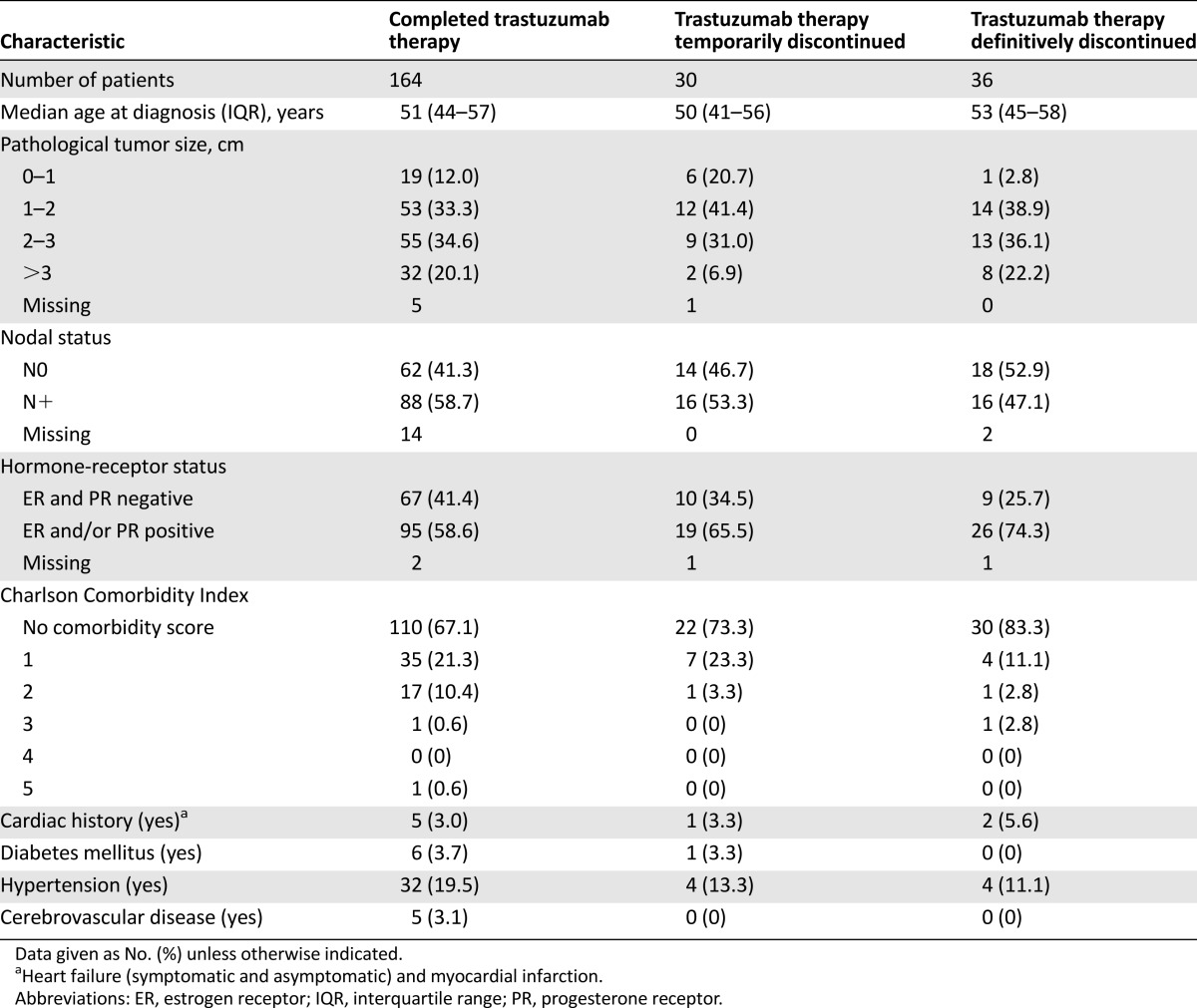
The baseline patient and tumor characteristics of the 230 patients treated with neo-adjuvant trastuzumab in daily practice are shown in Table 1. The chemotherapy regimens of patients receiving adjuvant trastuzumab consisted of anthracycline (37%), anthracycline and taxane (60%), taxane (2%) and therapy containing no anthracyclines and no taxanes (0.9%). Of these 230 patients, 164 completed trastuzumab treatment, 30 patients temporarily discontinued treatment, and 36 patients permanently discontinued treatment. There were no patients reported who did not start trastuzumab because of a low LVEF. There were 20 patients who had an LVEF below 55% at the first LVEF measurement; of these patients, 2 had LVEF below 50%. The median age in the group that permanently discontinued treatment was slightly higher than that of the group that temporarily discontinued treatment. A tendency toward more N0 tumors and more estrogen-receptor (ER)-positive tumors in patients permanently stopping trastuzumab treatment was seen. Patients who permanently discontinued treatment had also slightly smaller tumors. The presence of comorbidities, more specifically, history of cardiac or cerebrovascular diseases, diabetes, and hypertension, did not differ between those who continued and those who discontinued trastuzumab because of supposed cardiotoxicity. In daily practice, trastuzumab was most often initiated after finishing treatment with chemotherapy (n = 187; 80%), according to the treatment protocol of the HERA trial [3], especially in the first 2 years after introduction of trastuzumab in The Netherlands.
Table 1.
Baseline characteristics of patients categorized by actually delivered trastuzumab therapy
Incidence of Cardiotoxicity
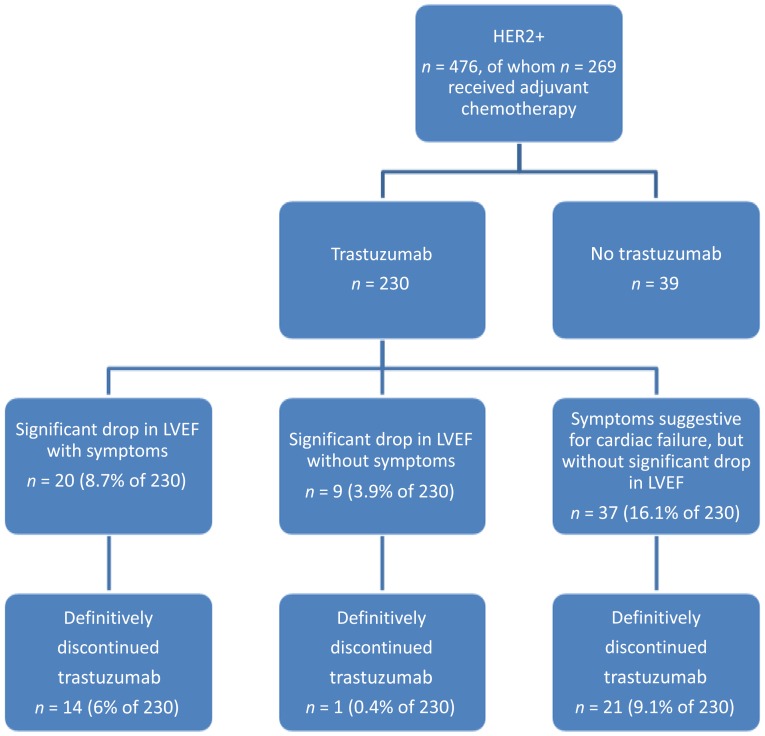
Of the 230 patients who were treated with trastuzumab, none died of a cardiac event. Median follow-up was 5.0 years (range: 0.1–6.7 years). Severe congestive heart failure, defined as symptomatic cardiotoxicity (defined as NYHA class III and IV) with significant drop in LVEF (absolute decline of at least 10 percentage points from baseline LVEF and a reduction in LVEF to below 50%), was observed in 20 of 230 patients (8.7%). A confirmed significant drop in LVEF without symptoms was observed in 9 of 230 patients (3.9%). Therefore, cardiotoxicity, as defined in the HERA trial, was observed in 29 of 230 patients (12.6%) in this real-world study. Of the total 230 patients treated with trastuzumab, 15 (6.5%) definitely stopped trastuzumab treatment at around 11 months (range: 10.5–11.8 months). In our daily-practice study, another 37 patients were thought to have cardiotoxicity because of symptomatology but without significant LVEF drop. Figure 1 shows an overview of the patients in this study and the incidence of cardiac symptoms events.
Figure 1.
Overview of the patient population in this study.
Abbreviations: HER2+, human epidermal growth receptor 2-positive; LVEF, left ventricular ejection fraction.
LVEF Monitoring and Timing of Cardiac Events
In 81% of the patients in our study, LVEF measurements were carried out before the start of trastuzumab therapy. During treatment with trastuzumab, LVEF measurements were carried out in 97% of patients.
Of all 230 patients included in the study, 36 patients permanently discontinued treatment. Of these 36 patients, only 15 had a LVEF drop that required discontinuation of the treatment according to the guidelines. In these patients, the first LVEF drop was seen at a median of 2.5 months (range: 0.7–10.3 months). Twelve of these 15 patients (80%) had a permanent drop in LVEF during the first 6 months after start of trastuzumab treatment. Most of the treatment discontinuations because of (presumed) cardiotoxicity, including patients with and without a significant LVEF drop, occurred within the first 6 months after start of treatment.
Trastuzumab Prescription and Survival Outcomes
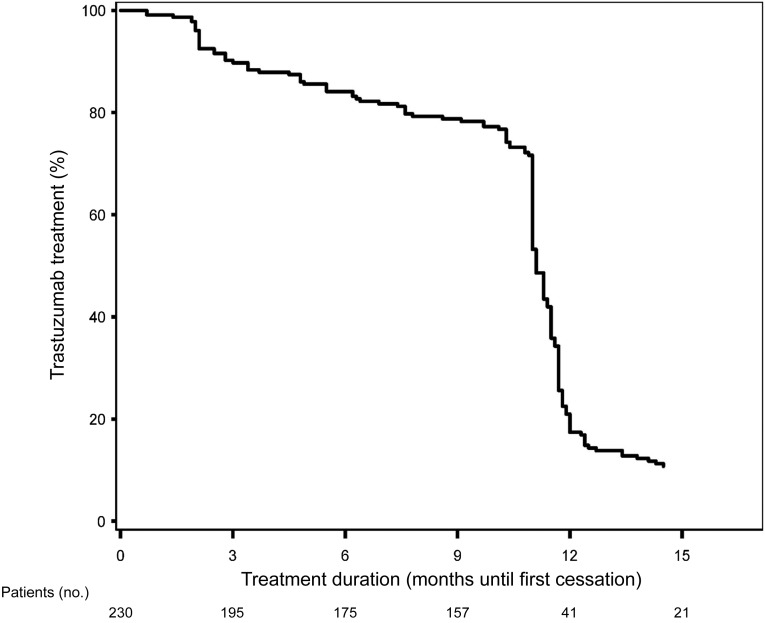
Trastuzumab was delivered for a median period of 49 weeks (IQR: 45–52 weeks). Figure 2 shows the percentage of patients on trastuzumab treatment over time. Fifty-six of the 66 patients (84.6%) who temporarily (n = 30) or permanently (n = 36) discontinued trastuzumab did so within the first 6 months of treatment.
Figure 2.
Kaplan-Meier curve showing percentage of patients receiving adjuvant trastuzumab treatment.
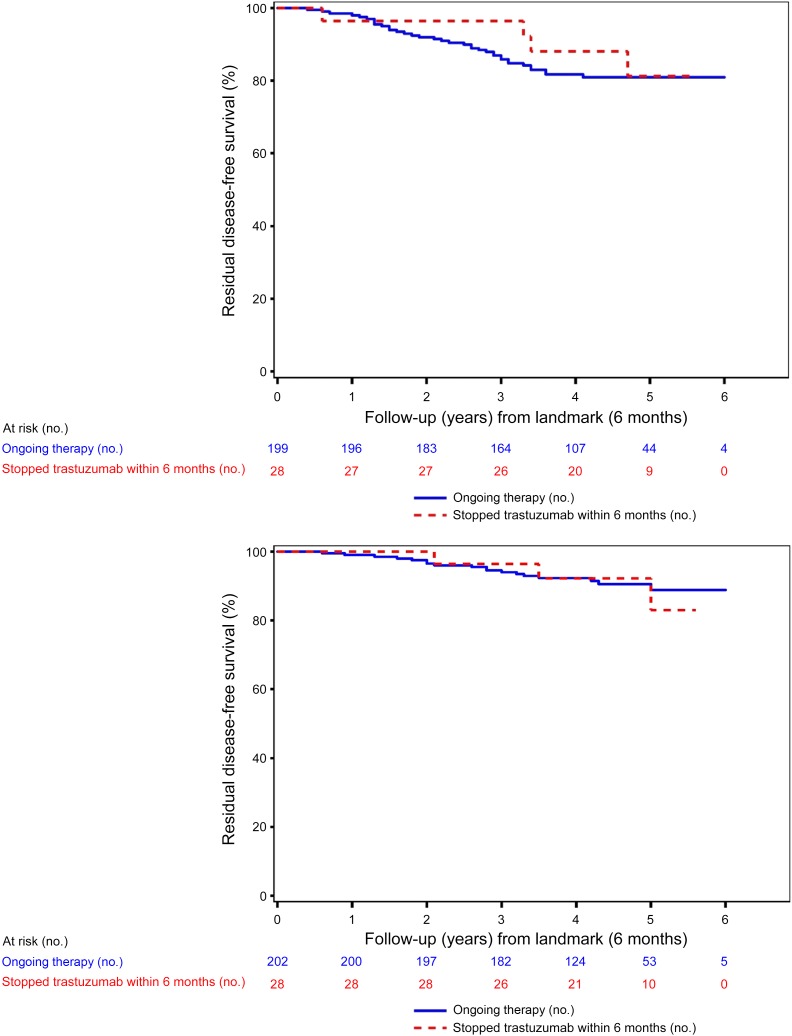
Permanent premature discontinuation of treatment within 6 months after the start did not have a significant effect on survival of the patients. The 5-year residual overall survival (OS) in patients who prematurely discontinued treatment versus those who completed trastuzumab was 83.7% versus 89.0% (p = .84) (Fig. 3).
Figure 3.
Survival of patients with human epidermal growth receptor 2-positive disease who stopped trastuzumab treatment before and after 6 months.
Discussion
In this study, we determined the incidence and timing of cardiotoxicity in patients with HER2-positive breast cancer treated with neo-adjuvant trastuzumab. We also assessed the impact of cardiotoxicity on the temporary or permanent discontinuation of trastuzumab treatment in daily practice and identified the impact of cardiac events on trastuzumab prescription. Finally, we assessed the clinical significance of LVEF monitoring over time.
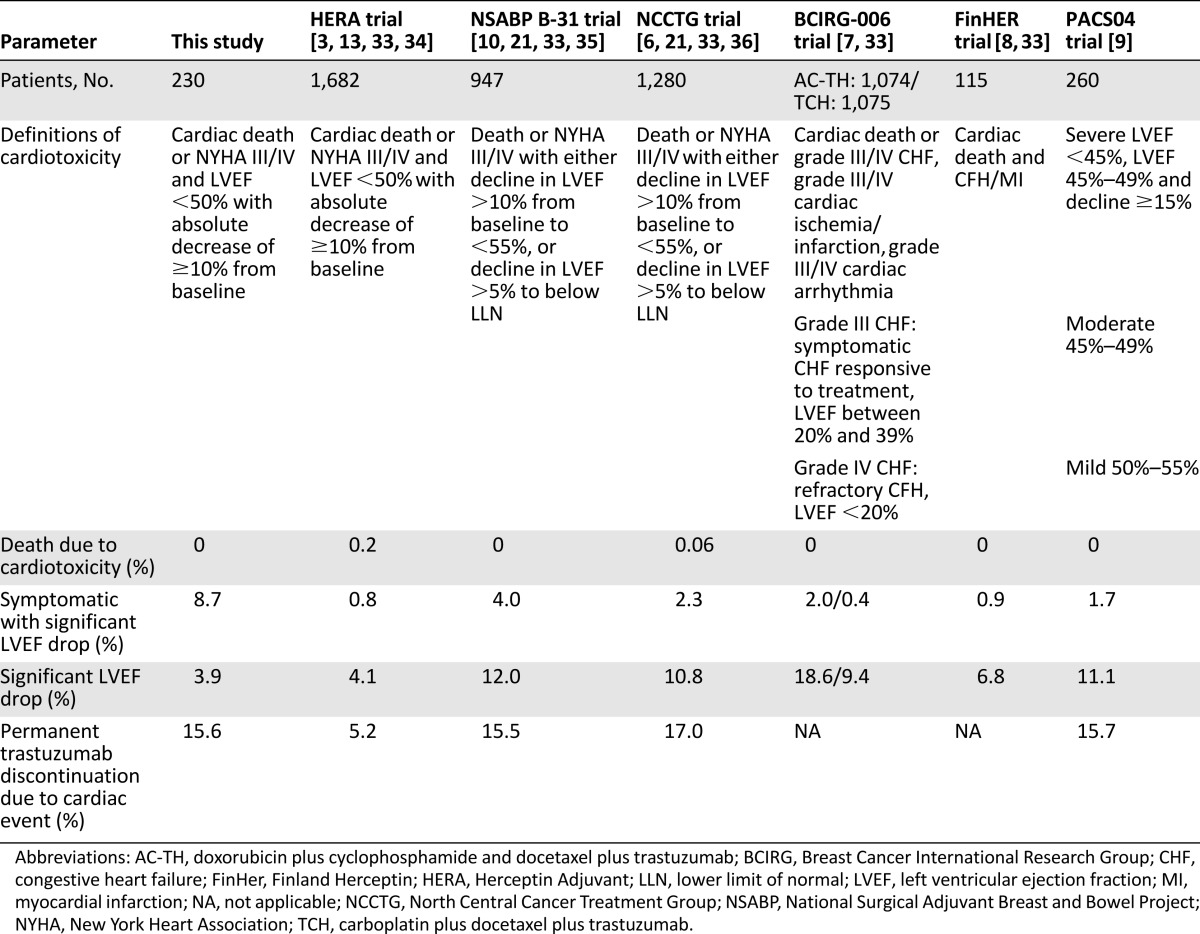
We found that the frequency of trastuzumab-related cardiotoxicity in our real-world study (12.6%) was higher than in the HERA study and other randomized controlled trials (Table 2). Other real-world studies occasionally have reported higher incidences of cardiotoxicity than in our study, ranging from 14% to 31% [22–24, 29]. In clinical practice, most cardiotoxicity has been proven to be temporary [29]. The incidence of death due to cardiotoxicity was comparably very low in both our and other studies.
Table 2.
Cardiac safety results of adjuvant trastuzumab trials
The presence of symptomatic cardiotoxicity in our study was not always accompanied by a significant decrease in LVEF, and a decrease in LVEF did not always indicate symptomatic cardiotoxicity. The phase III adjuvant clinical trials have demonstrated that the risk of developing asymptomatic LVEF drops after receiving trastuzumab was as high as 10%, compared with only 1% of the patients who actually developed symptomatic cardiotoxicity [24]. In the first years after introduction of adjuvant trastuzumab, we observed that physicians frequently based their decision to discontinue treatment on patient “symptoms” apart from LVEF outcome. Symptoms were considered heart failure described by a doctor, defined as NYHA III or IV. However, symptomatic cardiac failure without a drop in LVEF is very uncommon. Perhaps physicians were not familiar with trastuzumab and its side effects; cardiotoxicity was suspected in patients with symptoms that could also have another explanation. Patients’ preferences and concerns on long-term outcome may also have played a role.
The European Society for Medical Oncology guidelines recommend using an algorithm for cardiac monitoring in patients who receive anthracyclines and trastuzumab in the adjuvant setting, to be assessed at baseline, 3, 6, and 9 months during treatment, and then at 12 and 18 months after the initiation of treatment. Monitoring should be repeated during or following treatment as clinically indicated [30]. The European Society of Cardiology guidelines suggested that in cancer therapeutics-related cardiac toxicity, LVEF should be confirmed by repeated cardiac imaging performed 2–3 weeks after the baseline diagnosis [31]. In our study, 84.8% of patients who temporary or permanently discontinued trastuzumab did so within the first 6 months of treatment. In previous studies, more attention was given to the discontinuation and restarting of trastuzumab, and the reversibility cardiotoxicity, rather than to the timing of the onset of cardiotoxicity. Other studies that assessed the timing of cardiotoxicity suggest that the first 3 months of trastuzumab treatment are the most precarious ones, counting for most of the cardiotoxic events, and that cardiotoxicity occurring more than 6 months after start of trastuzumab is rare [22, 23]. These findings suggest that focusing LVEF monitoring on the first 6 months might be more cost-effective without compromising patient safety. Nonetheless, further research is needed.
It may also explain why we and others noticed that in daily practice, follow-up was not always very strictly performed [32]. Based on these new findings, we think the current guidelines need to be updated regarding the most optimal LVEF monitoring algorithm outside the clinical trial setting.
In the majority of cases, LVEF values stabilized or returned toward baseline following cessation of trastuzumab treatment. Data from the N9831 trial, on the other hand, suggest that higher rates of severe cardiac disorder were seen when trastuzumab treatment was administered during chemotherapy (3.5% vs. 2.5% when administered after completion of chemotherapy). Among those diagnosed with a cardiac event (3.5%) in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial, two-thirds of patients continued to receive cardiac medications and 71% had a decrease in LVEF relative to baseline on follow-up, highlighting persistent cardiac dysfunction [6]. The Finland Herceptin (FinHER) study, in which patients received a short course of trastuzumab, reported no cardiotoxicity [8]. In the update data, a 0.9% incidence of congestive heart failure (CHF) was seen. Comparing our study to the prior trials gives insight about cardiotoxicity in daily practice. Despite a much higher percentage of “cardiac symptoms” in our study compared with the randomized controlled trials, the percentage of discontinued treatment (6.5%), death due to cardiotoxicity (0%), and LVEF drop (12.6%) were comparable. Most importantly, the majority of cardiac events occurred in the first treatment months.
We observed that the median age in the group that permanently discontinued treatment was slightly higher than that of the group that temporarily discontinued treatment. Patients permanently stopping trastuzumab treatment also tended to have smaller tumors, more node-negative disease, and, more often, ER-positive tumors. It could be that in these patients with a more favorable risk profile, a lower threshold to discontinue treatment was accepted by both physicians and patients. Remarkably, presence of a number of comorbidities did not differ between those who continued or discontinued trastuzumab because of supposed cardiotoxicity. In other observational studies, age, anthracycline exposure, the presence of cardiovascular risk factors, and borderline LVEF before treatment did also not always have a significant impact on the onset of symptomatic cardiotoxicity [22, 23]. Data from the randomized controlled trials mostly excluded patients who did not meet the strict eligibility criteria. Potential participants who had a low LVEF before treatment, elderly patients, or patients with certain cardiac risk factors were excluded. In daily practice, patients were not so strictly selected; this might explain the higher prevalence of cardiotoxicity in daily practice compared with the trial setting [20].
Permanent premature discontinuation of treatment within 6 months did not appear to have a significant effect on survival of the patients. However, a trend toward a better overall survival existed in patients who completed the treatment, especially when considering that patients who prematurely discontinued treatment overall had a better breast cancer prognostic profile. As far as we know, there have been no studies that have examined this specific effect on disease-free survival. However, there are studies that tested for a shorter treatment period [12]. In the study performed by the Hellenic Oncology Research Group, 6 and 12 months of adjuvant trastuzumab had comparable outcomes, but a more recent study showed that the 3-year disease-free survival was in favor of the 12-month treatment group in comparison with the 6-month treatment group [35].
Some limitations of our study should be mentioned, including the relatively small sample size and the study’s retrospective nature. Because of the latter, reasons for discontinuation of trastuzumab could not always be retrieved. Also, due to inconsistent monitoring in daily practice, it was not possible to obtain complete data on LVEF. The follow-up LVEF was not performed in 3.4% of our patients. Nevertheless, other authors in real-world metastatic settings reported that follow-up LVEF data were missing in almost half of the patients [22]. Likewise, in one adjuvant study, adequate cardiac monitoring was identified in about two-thirds of the patients [32].
Conclusion
Trastuzumab was implemented rapidly but also cautiously in The Netherlands. In the first years after the introduction of trastuzumab, physicians based their decision to discontinue treatment on patients’ symptoms apart from LVEF outcome. This stresses the relevance of performing real-world analyses. Probably, physicians had to become familiar with trastuzumab, the indications for its use, and the monitoring of their patients. Moreover, it is important to realize that patients treated in routine clinical practice are less selected than trial populations and may have a different cardiac risk profile. A multidisciplinary approach consisting of increasing the knowledge of when cardiotoxicity occurs in daily practice will help shape the best follow-up method for cardiac monitoring. In addition, the whole field of cardio-oncology reflects the need for oncologists, cardiologists, pharmacists, nurses, and others to work together. In the future, advances in technologies such as three-dimensional echocardiography and cardiac MRI, in combination with biomarkers and clinical risk prediction models, may help move this field forward.
Uniform cardiac monitoring and evaluating periodic LVEF assessment in daily practice also may improve quality and lower costs of LVEF assessment in daily practice. Cardiotoxicity occurred mainly in the first 6 months after starting trastuzumab, indicating that the most cost-effective LVEF monitoring strategy would be to limit its use to the first 6 months of trastuzumab treatment. For the future, use of cardiac molecular markers for prediction or early detection of cardiotoxicity might be a solution for preventing permanent cardiotoxicity of HER2-targeted therapy.
Acknowledgments
We thank Wim A.J.G. Lemmens for his assistance with statistical analysis. This work was supported by Netherlands Organization for Health Research and Development (ZonMw: 80-82500-98-9056) and Roche Netherlands B.V.
Author Contributions
Conception/Design: Shanly C. Seferina, Petronella G.M. Peer, Adri C. Voogd, Vivianne C.G. Tjan-Heijnen
Provision of study material or patients: M. Wouter Derksen, Franchette van Den Berkmortel, Roel J.W. van Kampen, Agnès J. van de Wouw, Vivianne C.G. Tjan-Heijnen
Collection and/or assembly of data: Shanly C. Seferina, Vivianne C.G. Tjan-Heijnen
Data analysis and interpretation: Shanly C. Seferina, Maaike de Boer, Manuela Joore, Petronella G.M. Peer, Adri C. Voogd, Vivianne C.G. Tjan-Heijnen
Manuscript writing: Shanly C. Seferina, Maaike de Boer, M. Wouter Derksen, Franchette van Den Berkmortel, Roel J.W. van Kampen, Agnès J. van de Wouw, Manuela Joore, Petronella G.M. Peer, Adri C. Voogd, Vivianne C.G. Tjan-Heijnen
Final approval of manuscript: Shanly C. Seferina, Maaike de Boer, M. Wouter Derksen, Franchette van Den Berkmortel, Roel J.W. van Kampen, Agnès J. van de Wouw, Manuela Joore, Petronella G.M. Peer, Adri C. Voogd, Vivianne C.G. Tjan-Heijnen
Disclosures
The authors indicated no financial relationships.
References
- 1.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–318. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Integraal Kankercentrum Nederland. [Incidence and mortality of cancer.] Available at http://www.cijfersoverkanker.nl/incidentie-sterfte-50.html. Accessed August 16, 2014.
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 5.Prat A, Carey LA, Adamo B, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014;106:dju152. doi: 10.1093/jnci/dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 9.Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 10.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: Final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 13.de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32:2159–2165. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. FDA approval for trastuzumab. Available at http://www.cancer.gov/about-cancer/treatment/drugs/fda-trastuzumab. Accessed March 7, 2013.
- 15.European Medicines Agency. Herceptin. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000278/human_med_000818.jsp&mid=WC0b01ac058001d124. Accessed August 16, 2014.
- 16.Lee KF, Simon H, Chen H, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 17.Erickson SL, O’Shea KS, Ghaboosi N, et al. ErbB3 is required for normal cerebellar and cardiac development: A comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 18.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 19.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 20.Onitilo AA, Engel JM, Stankowski RV. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: Prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf. 2014;5:154–166. doi: 10.1177/2042098614529603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guglin M, Cutro R, Mishkin JD. Trastuzumab-induced cardiomyopathy. J Card Fail. 2008;14:437–444. doi: 10.1016/j.cardfail.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Tarantini L, Cioffi G, Gori S, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Card Fail. 2012;18:113–119. doi: 10.1016/j.cardfail.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Wadhwa D, Fallah-Rad N, Grenier D, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: A retrospective study. Breast Cancer Res Treat. 2009;117:357–364. doi: 10.1007/s10549-008-0260-6. [DOI] [PubMed] [Google Scholar]
- 25.Tarantini L, Gori S, Faggiano P, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: A multicenter cohort analysis. Ann Oncol. 2012;23:3058–3063. doi: 10.1093/annonc/mds127. [DOI] [PubMed] [Google Scholar]
- 26.Seferina SC, Lobbezoo DJA, de Boer M, et al. Real-life use and effectiveness of adjuvant trastuzumab in early breast cancer patients: A study of the Southeast Netherlands Breast Cancer Consortium. The Oncologist. 2015;20:856–863. doi: 10.1634/theoncologist.2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Integraal Kankercentrum Nederland. Richtlijn mammacarcinoom versie 2.0 Consensus based 2012-02-13. Available at http://www.oncoline.nl./mammacarcinoom. Accessed July 24, 2014.
- 28.SAS Institute Inc . SAS/STAT user’s guide, version 9.2. 2nd ed. Cary, NC: SAS Institute Inc.; 2009. [Google Scholar]
- 29.McArthur HL, Chia S. Cardiotoxicity of trastuzumab in clinical practice. N Engl J Med. 2007;357:94–95. doi: 10.1056/NEJMc070065. [DOI] [PubMed] [Google Scholar]
- 30.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 7):vii155–vii166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 31.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez-MacGregor M, Niu J, Zhang N, et al. Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol. 2015;33:2176–2183. doi: 10.1200/JCO.2014.58.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: Overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47:2273–2281. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Telli ML, Hunt SA, Carlson RW, et al. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 35.Mavroudis D, Saloustros E, Malamos N, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG) Ann Oncol. 2015;26:1333–1340. doi: 10.1093/annonc/mdv213. [DOI] [PubMed] [Google Scholar]
- 36.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]