To correctly inform therapeutic decisions, this review gives a critical update on how molecular response levels are incorporated in clinical decision algorithms and how detection of BCR-ABL1 mutations should be interpreted. The review provides a practical guide for molecular testing and the interpretation and contextualization of the results.
Keywords: Leukemia, chronic myeloid; BCR-ABL1; Tyrosine kinase inhibitor; Minimal residual disease; Molecular testing
Abstract
Optimal use of current therapeutic opportunities for chronic myeloid leukemia patients requires integration of clinical and laboratory monitoring. Assessment of molecular response (MR) by real-time quantitative polymerase chain reaction is the most sensitive way to monitor tyrosine kinase inhibitor (TKI) treatment efficacy. Besides major molecular response, which has emerged as a safe haven for survival since the initial studies of first-line imatinib treatment, two additional MR milestones have recently been defined: early molecular response and deep molecular response. The achievement of such MR milestones within defined time points during therapy is thought to draw the ideal trajectory toward optimal long-term outcome and, possibly, successful treatment discontinuation. Sensitive and reproducible MR measurement and proper interpretation of MR results are therefore critical to correctly inform therapeutic decisions. In patients who do not achieve an optimal response to TKI therapy, BCR-ABL1 mutation screening should also be performed, because it may deliver useful information for TKI choice. This review aims to help clinicians apply and translate the latest response definitions and clinical recommendations into practice. We provide a critical update on how these recommendations have incorporated MR levels in the clinical decision algorithms and how detection of BCR-ABL1 mutations should be interpreted. We also include a practical guide for pathologists and molecular biologists to best perform molecular testing and for hematologists and oncologists to best integrate it into routine practice.
Implications for Practice:
Ever-more-potent therapeutic strategies have been developed for chronic myeloid leukemia (CML) in parallel with the evolution of therapeutic goals and the refinement of response definitions and monitoring schemes and procedures. Terminology and methodology continue to evolve rapidly, making it difficult for busy hematology/oncology professionals to keep abreast of the newest developments. Optimal CML patient management results from the timely and rational use of molecular testing, the critical assessment of the power and pitfalls of current technology, and the appropriate interpretation and contextualization of results.
Current Options for the Treatment of Chronic Myeloid Leukemia
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that accounts for 15%–20% of all cases of leukemia in adults [1]. The landmark discovery of the Philadelphia chromosome (Ph) in 1960 [2] and the t(9;22)(q34;q11) chromosomal translocation from which it originates in 1973 [3] provided the first demonstration of a chromosomal abnormality being consistently associated with the development of cancer. The t(9;22) translocation and the resulting BCR-ABL1 gene rearrangement are detectable in virtually all CML patients. CML is also one of the first malignancies for which the principle of targeted therapy has found successful application. In the late 1980s, several seminal studies contributed to unravel the molecular pathogenesis of CML, showing the central role of the deregulated tyrosine kinase activity of BCR-ABL1 in the initiation and maintenance of the disease [4–8]. It soon became evident that turning BCR-ABL1 off would selectively eliminate leukemic cells while sparing the normal ones. Imatinib mesylate [9], the first tyrosine kinase inhibitor (TKI) to enter clinical evaluation as an anticancer drug in 1998, emerged serendipitously from a time-consuming process of random screening of a large number of compounds created using the structure of the adenosine triphosphate binding site [10]. After clinical trials whose success surpassed almost everyone’s expectations, in 2002 imatinib became the gold standard for front-line treatment of all newly diagnosed CML patients [11].
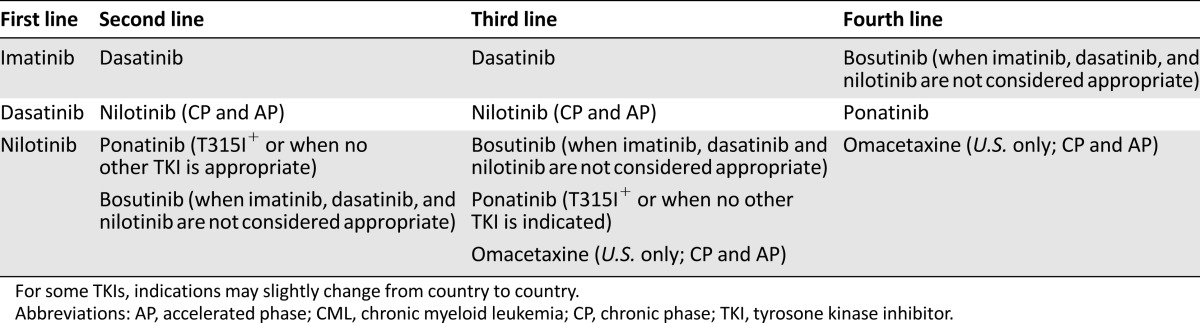
After more than 15 years of clinical use, the safety and efficacy of imatinib are well established. However, imatinib has an Achilles heel that Ph+ cells may exploit. Several point mutations in the kinase domain, where imatinib binds, have been observed in patients with recurrent disease [12]. This prompted pharmaceutical companies to rationally develop and test second-generation TKIs with greater potency and/or improved binding modalities. Such second-generation TKIs include dasatinib [13] and nilotinib [14], available in many countries for first-line, second-line, or subsequent use, and bosutinib [15], currently approved only for patients with resistance or intolerance to prior therapy. First-line use of dasatinib and nilotinib has been shown to induce faster and deeper responses, with a lower percentage of cases developing drug resistance and progressing to advanced phase [16–19]. However, the shorter follow-up of patients on nilotinib and dasatinib, together with the occurrence of some severe adverse events (especially cardiovascular and pulmonary) that were never reported in patients treated with imatinib, currently poses a question about the long-term safety of second-generation TKIs [20].
The shorter follow-up of patients on nilotinib and dasatinib, together with the occurrence of some severe adverse events (especially cardiovascular and pulmonary) that were never reported in patients treated with imatinib, currently poses a question about the long-term safety of second-generation TKIs.
The unmet need represented by patients harboring the T315I BCR-ABL1 mutation (which is cross-resistant to imatinib, dasatinib, nilotinib, and bosutinib) has recently been addressed, to some extent, by omacetaxine [21–25] as well as by the development of the third-generation TKI ponatinib [26–29]. Omacetaxine (formerly homoharringtonine; approved in the U.S. only) is a plant alkaloid long known to have some degree of activity in CML, with a mechanism of action that is unclear but is independent of BCR-ABL1 binding and inhibition [30–32]. Ponatinib is a TKI active against several kinases, with a binding mode that is less susceptible to disruption by single point mutations [26, 33]. Although safety concerns deriving from serious vascular occlusive events reported in the phase 2 study have halted its clinical development in the first-line setting, ponatinib is regarded as a precious option for patients who have developed the T315I mutation or have failed two lines of TKI therapy. The very recent report that a vascular endothelial growth factor receptor inhibitor approved for renal cancer, axitinib, is also effective, in vitro and in vivo, against T315I-positive CML [34, 35] suggests that among existing or emerging compounds developed for other indications, some might turn out to be useful for TKI-resistant CML as well. Thus, the pharmacologic scenario is likely to evolve further.
Allogeneic stem cell transplantation (aSCT) had an important role in the pre-TKI era [36] and remains the only proven curative option for CML. However, because of the high morbidity and mortality still associated with aSCT, it is now confined to patients who are diagnosed in advanced phase and is kept as a salvage option after multiple TKI failure in all the others [37]. An exception exists in those countries where, because of financial limitations or other bureaucratic/infrastructure restrictions, TKI therapy is difficult to obtain or sustain, and aSCT, if feasible, is still the preferred first-line option [37].
Hematologists and oncologists treating CML patients these days can thus rely on a wide spectrum of treatment opportunities (Table 1). The best clinical outcomes will result from the best use of these opportunities, which in turn requires optimal integration of expert clinical and laboratory monitoring.
Table 1.
Overview of the available TKI options in CML
Monitoring Response in CML
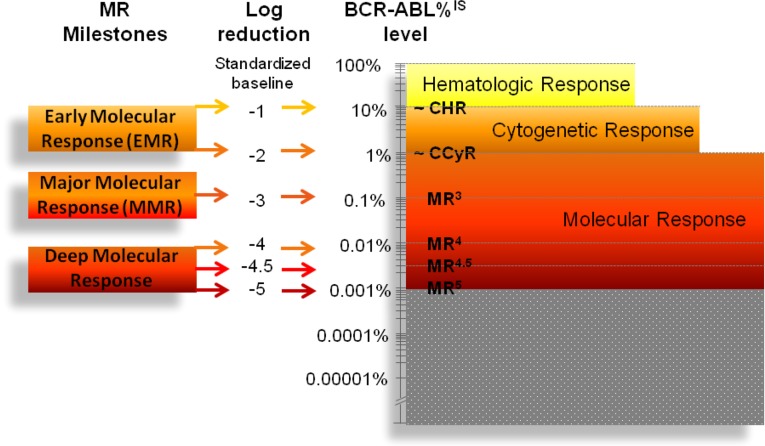
Three levels of response to therapy can be defined in CML patients: hematologic, cytogenetic, and molecular (Fig. 1) [38–40].
Figure 1.
Levels of hematologic, cytogenetic, and molecular response in CML and the novel concepts of early molecular response and deep molecular response. The gray area indicates that RQ-PCR cannot measure MRD below MR5 (BCR-ABL1 <0.001%); nevertheless, residual leukemia cells may still be present. Novel technologies (such as digital PCR) and approaches (such as assessing genomic DNA rather than RNA), might, in the future, extend the dynamic range of MR detection below MR5.
Abbreviations: CCyR, complete cytogenetic response; CHR; complete hematologic response; CML, chronic myeloid leukemia; IS, International Scale; MMR, major molecular response; MR, molecular response; MRD, minimal residual disease; RQ-PCR, real-time quantitative reverse-transcription polymerase chain reaction.
Hematologic response refers to the normalization of blood cell count. The utility of hematologic monitoring is limited to the fırst few months of therapy, when dose adjustments for hematologic toxicity may be needed.
Cytogenetic response (CyR) denotes the percentage of residual bone marrow metaphases showing evidence of Ph, as assessed by chromosome G banding analysis (CBA). Fluorescence in situ hybridization (FISH) on interphase nuclei from peripheral blood can also be used, as an alternative, to monitor CyR. CBA was the main tool to monitor residual disease burden in the pre-TKI era. CBA or FISH is still used now if molecular monitoring is not available; however, they do not allow stratification of responders below the level of complete CyR (CCyR), which is achieved by more than 80% of patients receiving TKI therapy.
Molecular response (MR) measures the reduction of BCR-ABL1 fusion transcripts and, as such, has the greatest sensitivity. Once CCyR is achieved, only molecular methods make it possible to follow the dynamics of minimal residual disease (MRD) over time. Currently, the gold standard for MR monitoring is real-time quantitative reverse-transcription polymerase chain reaction (RQ-PCR), which was introduced with the very first clinical trials of imatinib [41].
Clinical Value of Molecular Monitoring
Over the years, dozens of clinical studies with imatinib, dasatinib, and nilotinib worldwide have soundly demonstrated the clinical relevance of MR monitoring (recently reviewed by Hanfstein et al. [42]). The results have been used to establish key MR milestones to be achieved at specific time points during therapy.
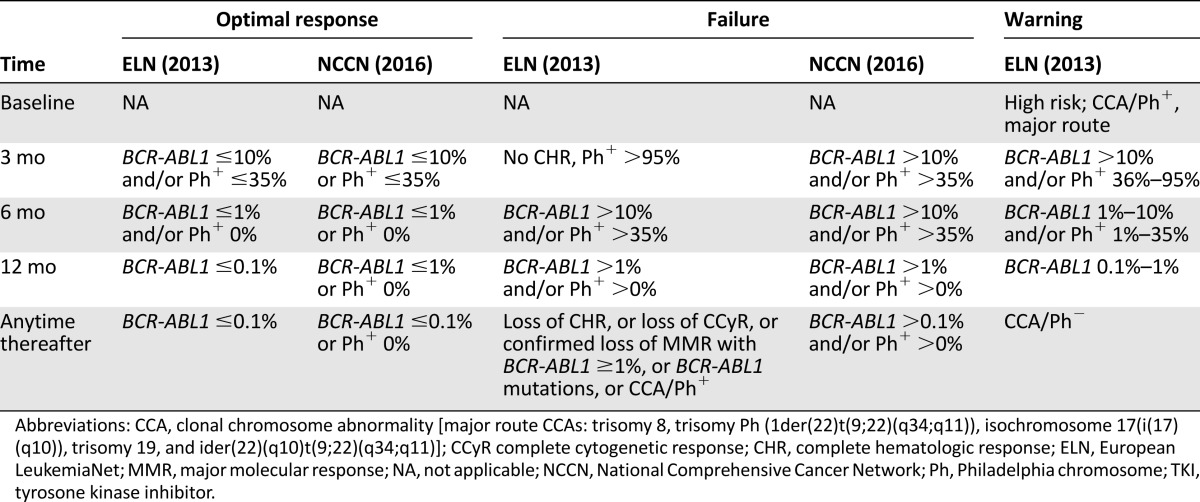
BCR-ABL1 levels ≤10% at 3 months and ≤1% at 6 months represent the so-called early molecular response. Early molecular response has been shown to predict the rate and depth of any subsequent response (CCyR and major molecular response [MMR]) and to correlate with significantly improved long-term outcomes (progression-free survival [PFS] and overall survival [OS]). BCR-ABL1 ≤0.1% is MMR, the “safe haven” for survival, to be achieved within 12 months of therapy. Finally, BCR-ABL1 ≤0.01%, down to 0.001%, defines the so-called deep or deeper MR that has also been shown to predict significantly better long-term outcomes (failure-free survival, transformation-free survival, PFS, and OS).
Besides predicting optimal outcome, deep or deeper MR is currently considered to be the gateway to treatment-free remission, which is becoming a high-priority goal of CML treatment, from both a patient perspective and a health-economics perspective [43]. Permanent TKI treatment discontinuation (often referred to as functional cure) is particularly desirable in younger patients with CML, since it would allow safe conception and pregnancy and dissipate concerns of potential late, off-target complications. The relevance of discontinuation to the health care system is also not negligible. Interim analysis of the first 200 patients enrolled in the European Stop Tyrosine Kinase Inhibitor (EURO-SKI) trial, presented at the December 2014 ASH meeting in San Francisco, estimated drug-related savings for the eight participating countries to be more than 7 million euros just in the first year of the study [44].
Best Practices in Molecular Monitoring of CML Patients: What Clinicians Need to Know
As mentioned above, MR is assessed by RQ-PCR: after total RNA isolation from peripheral blood leukocytes and reverse transcription (RT) of RNA to cDNA, the absolute copy number of the target transcript together with that of a control gene transcript (necessary to correct for differences in RNA quantity and quality and RT efficacy across samples) are determined using plasmid calibration curves [45–47]. Results are expressed as a percentage ratio between the sum of BCR-ABL1 copies and the sum of control gene (CG) copies across replicates (typically two replicates, with some laboratories using three replicates and a few using a single measurement) [48]. Recommended CGs are ABL, BCR, or GUSB [48]. ABL and BCR historically were, and continue to be, the most widely used. However, they both may introduce biases in quantitation at very high and very low levels of residual disease, respectively (with use of ABL resulting in an underestimation and use of BCR resulting in an overestimation of MRD), because the assay cannot discriminate between sequences of the nontranslocated versus the translocated allele [49]. For this reason, and given the ever-increasing importance of both early and deep MR, GUSB (another CG transcript shown, in initial studies [47], to be a suitable candidate for its stability over time irrespective of treatment and for its half-life, comparable to that of BCR-ABL1) is currently being considered as an alternative [48].
To be used in clinical decision making, MR has to be measured reliably and reproducibly. Sources of technical and experimental variability intervening at each analytical step include (a) sample quantity, quality, and delivery time; (b) RNA extraction method, yield, and purity; (c) starting quantity and quality of the RNA that is reverse-transcribed to cDNA and efficiency of the reverse transcriptase enzyme/kit; and (d) type of RQ-PCR instrument and chemistry, protocol (homebrew assay vs commercial kit), and type of CG used. Indeed, soon after the introduction of RQ-PCR for molecular monitoring of CML patients, it was realized that results were not comparable, even among expert laboratories. International standardization efforts were thus undertaken. It was decided that each laboratory could maintain local instruments and protocols provided that (a) results be comparable and (b) adequate levels of sensitivity be routinely achieved [49–51]. This led, in 2005, to the introduction of the International Scale (IS) [49]. On the IS, MR is traditionally defined in terms of log-reduction from a standardized baseline, set to 100% (Fig. 1). According to the depth of MR, patients can be stratified into those who are in MR4, MR4.5, and MR5, with the superscript indicating the log-reduction (Fig. 1). To be used in clinical decision making according to the international (European LeukemiaNet [ELN] or National Comprehensive Cancer Network [NCCN]) treatment recommendations [40, 52], MR has to be reported on the IS. The depth of response that can be measured by the assays (i.e., sensitivity) may vary from sample to sample and from laboratory to laboratory, because it is subordinated to the CG copy number. According to the latest recommendations, ABL CG copy number must be ≥10,000 to define MR4, ≥32,000 to define MR4.5, and ≥100,000 to define MR5; GUSB CG copy number must be ≥24,000 to define MR4, ≥77,000 to define MR4.5, and ≥240,000 to define MR5 [48]. If, even in a single replicate of two or three, ABL copy number is <10,000 or GUSB copy number is <24,000, the sample must be considered inevaluable for MR regardless of what the BCR-ABL1 copy number is [48].
The main challenges facing laboratories performing molecular monitoring of CML patients are, therefore, the following: (a) all the analytical steps from sampling to performing RQ-PCR must be optimized, to maximize the limit of detection (sensitivity) of BCR-ABL1 transcripts and ensure reliable measurements; (b) established criteria for quality control of each run (intercept, slope, and correlation coefficient of the plasmid standard curves) and definitions of acceptable/unacceptable results and detectable/undetectable disease must be followed strictly [48]; and (c) results must be reported on the IS, to allow comparability of results and adoption of ELN or NCCN molecular checkpoints for the definition of response. This requires the testing laboratory either to obtain (and periodically revalidate) a conversion factor by sample exchange with an established reference laboratory (the BCR-ABL1/CG ratio percentage will have to be multiplied by this factor) or to use kits and reagents that have been calibrated to the World Health Organization International Genetic Reference Panel, recently made available [53, 54].
Hematologists and oncologists sending samples for MR assessment should verify that the testing laboratory is producing and scoring results according to the latest international recommendations [48].
What is the optimal timing of molecular monitoring? In all patients at diagnosis, a qualitative multiplex RT-PCR should be performed, to assess the transcript type [40]. The great majority of newly diagnosed CML patients test positive for the e13a2 (also known as b2a2) or e14a2 (also known as b3a2) BCR-ABL1 rearrangement, but some may rather harbor atypical transcripts such as e13a3, e14a3, e1a2, e1a3, e19a2, e19a3, e6a2, e8a2, or e18a2 [55]. If multiplex RT-PCR is not performed at diagnosis, detecting atypical transcripts later on may not be possible. Qualitative RT-PCR is also useful for diagnostic confirmation of CML in all cases (up to 5%) with a cryptic translocation [55]. Afterward, during TKI therapy, all patients should be monitored by RQ-PCR on a regular basis: every 3 months until MMR has been achieved, then every 3–6 months [40].
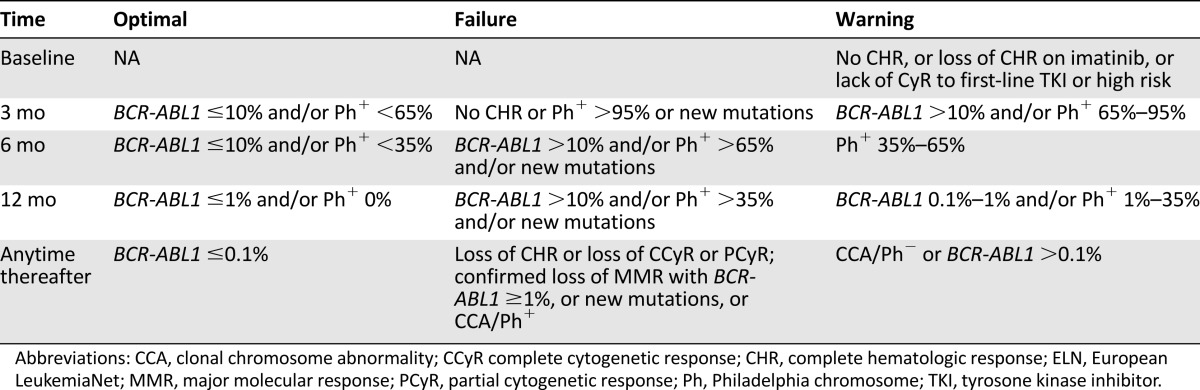
ELN treatment recommendations have established critical checkpoints at 3, 6, and 12 months, so that, depending on MR levels, more careful monitoring or treatment change can be undertaken as appropriate [40]. Namely, the ELN recommendations categorize response into optimal (no change of therapy is indicated); warning (formerly, “suboptimal”) (more frequent monitoring is recommended to permit timely change in case of subsequent treatment failure); and failure (the patient should receive a different treatment) (Table 2). NCCN guidelines may also be followed, as an alternative (Table 2) [52]. Updated annually, the NCCN guidelines provide algorithms for decision making at 3, 6, and 12 months, based on the response level. The main difference between the ELN recommendations and the NCCN guidelines lies in the clinical implications of the 3-month monitoring results. At 3 months, the ELN considers a BCR-ABL1 transcript level greater than 10% a warning, irrespective of the TKI. In contrast, the NCCN mandates a dose increase or a change to an alternate TKI if the primary treatment is imatinib, whereas treatment continuation at the same dose or change to an alternate TKI is possible if the primary treatment is dasatinib or nilotinib. At 6 months, both ELN and NCCN are concordant in mandating a change of therapy in all patients with a BCR-ABL1 level persistently above 10%. At 12 months, BCR-ABL1 levels lower than 0.1% (MMR or better) are an optimal response, and BCR-ABL1 levels greater than 1% are a failure according to both ELN and NCCN. The 2013 ELN recommendations also established definitions of optimal response, warning, and failure to be used in patients receiving second-line TKI therapy after imatinib failure (Table 3). Again, key MR levels appear at every time point [40].
Table 2.
Definitions of response to first-line TKI therapy according to ELN recommendations and comparison with the NCCN guidelines
Table 3.
Definitions of response to second-line TKI therapy after imatinib failure according to ELN recommendations
In addition to the correct timing of MR evaluations, the importance of correct sampling and delivery modalities should not be overlooked. It is now well established that a peripheral blood sample is sufficient for MR response assessment. Bone marrow is not necessary, and alternating bone marrow and peripheral blood samples should be avoided. To facilitate achieving sensitivity levels necessary to score MR4.5 and MR5, sample quantity is important. Ten milliliters of blood may be sufficient, but more (up to 20 mL) would be preferable. Because progressive degradation of RNA starting soon after blood collection is an inevitable physiological phenomenon [56], samples should be delivered to the laboratory and processed within 24 hours, which mandates avoiding sampling on Fridays and holiday eves unless agreed on with the laboratory. Whole blood can be shipped at room temperature or refrigerated, but it must never be frozen unless specific vials containing RNA stabilizing solutions [57–59] are used.
Drug Resistance and BCR-ABL1 Mutation Analysis
Resistance can be defined using the ELN or NCCN criteria for failure. Patients with failure on first-line imatinib treatment have been shown to have decreased PFS and OS compared with patients with optimal responses [60]. Resistance is thus associated with a greater risk of disease progression. Biologically, it is believed that the increased BCR-ABL1 expression and its functional reactivation, associated with resistance [61–63], are responsible for the enhanced genomic instability and perturbed differentiation [64–66] that are intrinsic features of blast crisis (BC). Even in the TKI era, treatment of BC remains a challenge, and patients who progress have a dismal outcome [67, 68]; hence, preventing disease progression from CP to BC must be the main aim of every clinician treating CML.
Although drug resistance mechanisms are likely to be many and not necessarily mutually exclusive, point mutations in the BCR-ABL1 kinase domain (detectable at frequencies ranging from 25% to 70%, depending on whether the patient is in chronic or advanced phase) [12, 69] are the only ones whose assessment is currently recommended by both ELN and NCCN. More than 60 different mutations are known to be associated with resistance to imatinib, and not all of them are equally sensitive to other TKIs [70]. In case of failure (once low compliance and inadequate dosing are excluded), mutational analysis should be performed [52, 71]. ELN also recommends mutational analysis in case of suboptimal response (warning) [40, 71]: although mutation frequency seems to be low in patients with warning, detection of a mutation would definitively establish the need of a treatment change and, in some cases, provide indications about which alternative TKI is most likely to be effective. BCR-ABL1 mutation analysis should not be performed at diagnosis (except in those rare patients who are diagnosed in accelerated or blastic phase) or in patients who have an optimal response to therapy.
Biologically, it is believed that the increased BCR-ABL1 expression and its functional reactivation, associated with resistance, are responsible for the enhanced genomic instability and perturbed differentiation that are intrinsic features of blast crisis.
Generally, an aliquot of the same RNA (or even cDNA) used for RQ-PCR is sufficient to perform BCR-ABL1 mutation analysis. If neither RNA nor cDNA are available, sampling has to be done following exactly the same recommendations in terms of source of material, quantity, and delivery requirements as for RQ-PCR samples [72].
The recommended method for BCR-ABL1 mutation screening is conventional Sanger sequencing [71]. Hematologists and oncologists should base their clinical decisions on conventional sequencing results and treat results coming from other techniques (e.g., allele-specific PCR assays such as allele-specific oligonucleotide or amplification-refractory mutation system PCR) for research use only [71]. Although next-generation sequencing (NGS) has recently been shown to be a promising candidate alternative to conventional sequencing [73–75], international standardization efforts are still at the very beginning, and their progress is hampered by the continuous evolution of chemistries and platforms. In addition, software programs for alignment to mRNA sequences and reliable mutation calling still need to be optimized, and common definitions of quality control metrics and acceptable performances are still in progress.
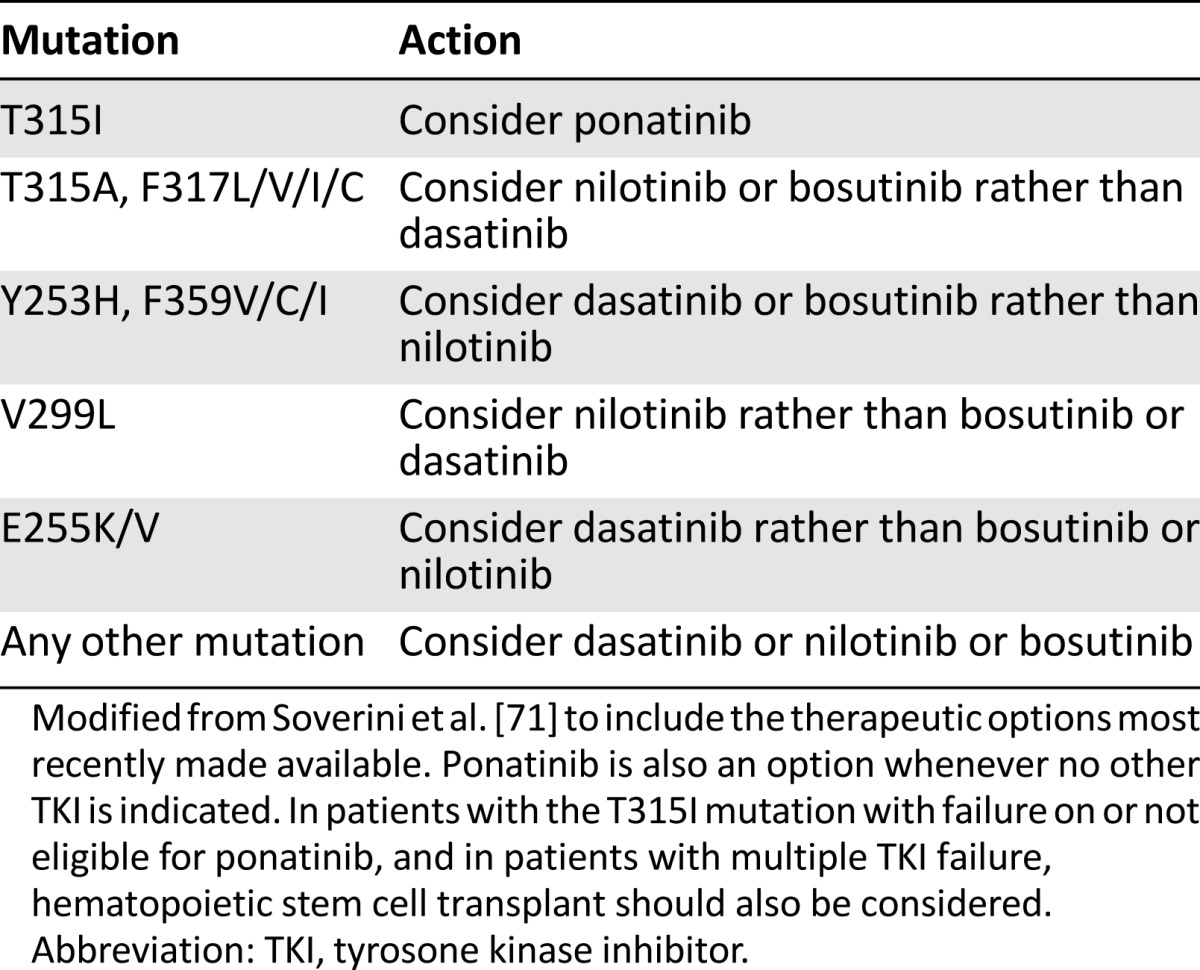
Table 4 reports updated recommendations on how to interpret BCR-ABL1 mutation results in light of the recent availability of bosutinib and ponatinib in many countries. For nilotinib and dasatinib, these recommendations come from the integration of in vitro observations (IC50, the intracellular drug concentration needed to inhibit by 50% the growth of a cell line transfected to express a specific mutated BCR-ABL1 isoform) [71, 76] and in vivo evidence (the BCR-ABL1 mutations that have been found to be selected in patients who relapsed on dasatinib or nilotinib) [77–83]. For bosutinib, in vitro data are available [84, 85], but clinical data are still scarce and mainly come from company-sponsored phase 2 and 3 trials [86–90].
Table 4.
Updated indications on how to choose the TKI according to BCR-ABL1 mutation status
Optimal Management of CML Patients: Teamwork
Optimal management of CML patients requires peer dialog and synergy between pathologists/molecular biologists and hematologists/oncologists. The former bring a high degree of expertise and have an essential role in communicating results and their implications to the latter.
In follow-up MR reports, the pathologist or molecular biologist should explicitly indicate whether the sample is evaluable for MR and whether the MR level indicates an optimal response, a warning, or a failure according to ELN recommendations or NCCN guidelines. If the sample is inevaluable, the pathologist should comment on whether sample quality or quantity were adequate and recommend timely resampling. If the sample is evaluable, the pathologist or molecular biologist should indicate what level of MR can be defined. In case of inconsistencies with MR results at previous time points, BCR-ABL1 transcript level fluctuations in the absence of MMR loss, and borderline results, the pathologist or molecular biologist should also recommended resampling and reconfirmation of results before any clinical decision is taken.
The pathologist and molecular biologist should also suggest (or recommend) a mutation analysis in case BCR-ABL1 transcript levels indicate warning (or failure). If the patient tests positive for a mutation, the pathologist should suggest more thorough monitoring of the patient, who will have a significantly higher probability of developing additional mutations under second- or subsequent-line TKI therapy. If specific mutations (Y253H, E255K/V, V299L, T315I/A, F317L/V/I/C, F359V/I/C) are detected, the pathologist should also indicate which TKI or TKIs are more likely to be effective against that specific mutant (Table 4). The clinician will thus integrate BCR-ABL1 mutation status with comorbidities and risk factors, bearing in mind that detection of any of the mutations mentioned above is a stronger predictor of TKI inefficacy than comorbidities and risk factors are for the occurrence of adverse events.
Conclusion
Although the advent of TKIs has dramatically improved patient outcomes, CML is not easy to manage. Both the therapeutic arsenal and the technologic solutions for molecular testing are still evolving. The NCCN guidelines for patient treatment are updated on an yearly basis, and the next ELN recommendations are expected by the end of 2016. In such a rapidly changing scenario, education and constant update of clinicians involved in patient management about MR definitions, monitoring schemes, decision algorithms, and technological advances—as well as perfect synergy between hematology/oncology and laboratory professionals—are of utmost importance.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Simona Soverini, Caterina De Benedittis, Manuela Mancini, Giovanni Martinelli
Collection and/or assembly of data: Simona Soverini, Caterina De Benedittis, Manuela Mancini, Giovanni Martinelli
Data analysis and interpretation: Simona Soverini, Caterina De Benedittis, Manuela Mancini, Giovanni Martinelli
Manuscript writing: Simona Soverini, Caterina De Benedittis, Manuela Mancini, Giovanni Martinelli
Final approval of manuscript: Simona Soverini, Caterina De Benedittis, Manuela Mancini, Giovanni Martinelli
Disclosures
Simona Soverini: Ariad Pharma, Novartis, Bristol-Myers Squibb (C/A); Giovanni Martinelli: Ariad, Amgen, Pfizer (C/A), Novartis, Bristol-Myers Squibb (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 2.Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. [PubMed] [Google Scholar]
- 3.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 4.Lugo TG, Pendergast AM, Muller AJ, et al. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin J, Chianese E, Witte ON. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci USA. 1987;84:6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 7.Kelliher MA, McLaughlin J, Witte ON, et al. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci USA. 1990;87:6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heisterkamp N, Jenster G, ten Hoeve J, et al. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990;344:251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- 9.Soverini S, Martinelli G, Iacobucci I, et al. Imatinib mesylate for the treatment of chronic myeloid leukemia. Expert Rev Anticancer Ther. 2008;8:853–864. doi: 10.1586/14737140.8.6.853. [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JR, Bross P, Cohen M, et al. Approval summary: Imatinib mesylate capsules for treatment of adult patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase. Clin Cancer Res. 2003;9:1972–1979. [PubMed] [Google Scholar]
- 12.Soverini S, Branford S, Nicolini FE, et al. Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leuk Res. 2014;38:10–20. doi: 10.1016/j.leukres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Lindauer M, Hochhaus A. Dasatinib. Recent Results Cancer Res. 2014;201:27–65. doi: 10.1007/978-3-642-54490-3_2. [DOI] [PubMed] [Google Scholar]
- 14.Ostendorf BN, le Coutre P, Kim TD, et al. Nilotinib. Recent Results Cancer Res. 2014;201:67–80. doi: 10.1007/978-3-642-54490-3_3. [DOI] [PubMed] [Google Scholar]
- 15.Isfort S, Keller-v Amsberg G, Schafhausen P, et al. Bosutinib: A novel second-generation tyrosine kinase inhibitor. Recent Results Cancer Res. 2014;201:81–97. doi: 10.1007/978-3-642-54490-3_4. [DOI] [PubMed] [Google Scholar]
- 16.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Shen ZX, Saglio G, et al. Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina. Blood. 2015;125:2771–2778. doi: 10.1182/blood-2014-09-601674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hjorth-Hansen H, Stenke L, Söderlund S, et al. Dasatinib induces fast and deep responses in newly diagnosed chronic myeloid leukaemia patients in chronic phase: Clinical results from a randomised phase-2 study (NordCML006) Eur J Haematol. 2015;94:243–250. doi: 10.1111/ejh.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gugliotta G, Castagnetti F, Breccia M, et al. Long-term outcome of a phase 2 trial with nilotinib 400 mg twice daily in first-line treatment of chronic myeloid leukemia. Haematologica. 2015;100:1146–1150. doi: 10.3324/haematol.2015.129221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Hematol. 2015;94(suppl 2):S149–S158. doi: 10.1007/s00277-015-2318-y. [DOI] [PubMed] [Google Scholar]
- 21.Nicolini FE, Chomel JC, Roy L, et al. The durable clearance of the T315I BCR-ABL mutated clone in chronic phase chronic myelogenous leukemia patients on omacetaxine allows tyrosine kinase inhibitor rechallenge. Clin Lymphoma Myeloma Leuk. 2010;10:394–399. doi: 10.3816/CLML.2010.n.073. [DOI] [PubMed] [Google Scholar]
- 22.Cortes J, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–2580. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortes J, Digumarti R, Parikh PM, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate for chronic-phase chronic myeloid leukemia patients resistant to or intolerant of tyrosine kinase inhibitors. Am J Hematol. 2013;88:350–354. doi: 10.1002/ajh.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvandi F, Kwitkowski VE, Ko CW, et al. U.S. Food and Drug Administration approval summary: Omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. The Oncologist. 2014;19:94–99. doi: 10.1634/theoncologist.2013-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes JE, Kantarjian HM, Rea D, et al. Final analysis of the efficacy and safety of omacetaxine mepesuccinate in patients with chronic- or accelerated-phase chronic myeloid leukemia: Results with 24 months of follow-up. Cancer. 2015;121:1637–1644. doi: 10.1002/cncr.29240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehrle J, Pahl HL, von Bubnoff N. Ponatinib: A third-generation inhibitor for the treatment of CML. Recent Results Cancer Res. 2014;201:99–107. doi: 10.1007/978-3-642-54490-3_5. [DOI] [PubMed] [Google Scholar]
- 29.Cortes JE, Talpaz M, Kantarjian H. Ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2014;370:577. doi: 10.1056/NEJMc1315234. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien S, Kantarjian H, Keating M, et al. Homoharringtonine therapy induces responses in patients with chronic myelogenous leukemia in late chronic phase. Blood. 1995;86:3322–3326. [PubMed] [Google Scholar]
- 31.O’Brien S, Kantarjian H, Koller C, et al. Sequential homoharringtonine and interferon-alpha in the treatment of early chronic phase chronic myelogenous leukemia. Blood. 1999;93:4149–4153. [PubMed] [Google Scholar]
- 32.Kantarjian HM, Talpaz M, Smith TL, et al. Homoharringtonine and low-dose cytarabine in the management of late chronic-phase chronic myelogenous leukemia. J Clin Oncol. 2000;18:3513–3521. doi: 10.1200/JCO.2000.18.20.3513. [DOI] [PubMed] [Google Scholar]
- 33.Zhou T, Commodore L, Huang WS, et al. Structural mechanism of the Pan-BCR-ABL inhibitor ponatinib (AP24534): Lessons for overcoming kinase inhibitor resistance. Chem Biol Drug Des. 2011;77:1–11. doi: 10.1111/j.1747-0285.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 34.Okabe S, Tauchi T, Tanaka Y, et al. Anti-leukemic activity of axitinib against cells harboring the BCR-ABL T315I point mutation. J Hematol Oncol. 2015;8:97. doi: 10.1186/s13045-015-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pemovska T, Johnson E, Kontro M, et al. Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature. 2015;519:102–105. doi: 10.1038/nature14119. [DOI] [PubMed] [Google Scholar]
- 36.Horowitz MM, Rowlings PA, Passweg JR. Allogeneic bone marrow transplantation for CML: A report from the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1996;17(suppl 3):S5–S6. [PubMed] [Google Scholar]
- 37.Radich J. Stem cell transplant for chronic myeloid leukemia in the imatinib era. Semin Hematol. 2010;47:354–361. doi: 10.1053/j.seminhematol.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 39.Baccarani M, Castagnetti F, Gugliotta G, et al. Response definitions and European Leukemianet Management recommendations. Best Pract Res Clin Haematol. 2009;22:331–341. doi: 10.1016/j.beha.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes T, Branford S. Molecular monitoring of chronic myeloid leukemia. Semin Hematol. 2003;40(suppl 2):62–68. doi: 10.1053/shem.2003.50044. [DOI] [PubMed] [Google Scholar]
- 42.Hanfstein B, Müller MC, Hochhaus A. Response-related predictors of survival in CML. Ann Hematol. 2015;94(suppl 2):S227–S239. doi: 10.1007/s00277-015-2327-x. [DOI] [PubMed] [Google Scholar]
- 43.Mahon FX, Etienne G. Deep molecular response in chronic myeloid leukemia: The new goal of therapy? Clin Cancer Res. 2014;20:310–322. doi: 10.1158/1078-0432.CCR-13-1988. [DOI] [PubMed] [Google Scholar]
- 44.Mahon FX, Richter J, Guilhot J et al. Interim analysis of a pan European stop tyrosine kinase inhibitor trial in chronic myeloid leukemia: The EURO-SKI study. Paper presented at: 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, CA. [Google Scholar]
- 45.Preudhomme C, Chams-Eddine L, Roumier C, et al. Detection of BCR-ABL transcripts in chronic myeloid leukemia (CML) using an in situ RT-PCR assay. Leukemia. 1999;13:818–823. doi: 10.1038/sj.leu.2401393. [DOI] [PubMed] [Google Scholar]
- 46.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17:2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 47.Beillard E, Pallisgaard N, van der Velden VH, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe against cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 48.Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003. doi: 10.1038/leu.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cross NC. Standardisation of molecular monitoring for chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009;22:355–365. doi: 10.1016/j.beha.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Müller MC, Cross NC, Erben P, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23:1957–1963. doi: 10.1038/leu.2009.168. [DOI] [PubMed] [Google Scholar]
- 52.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Chronic Myelogenous Leukemia. Version 1. 2016. Available at http://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. Accessed October 10, 2015.
- 53.White HE, Hedges J, Bendit I, et al. Establishment and validation of analytical reference panels for the standardization of quantitative BCR-ABL1 measurements on the international scale. Clin Chem. 2013;59:938–948. doi: 10.1373/clinchem.2012.196477. [DOI] [PubMed] [Google Scholar]
- 54.White HE, Matejtschuk P, Rigsby P, et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL mRNA. Blood. 2010;116:e111–e117. doi: 10.1182/blood-2010-06-291641. [DOI] [PubMed] [Google Scholar]
- 55.Foroni L, Gerrard G, Nna E, et al. Technical aspects and clinical applications of measuring BCR-ABL1 transcripts number in chronic myeloid leukemia. Am J Hematol. 2009;84:517–522. doi: 10.1002/ajh.21457. [DOI] [PubMed] [Google Scholar]
- 56.Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48:1647–1653. [PubMed] [Google Scholar]
- 57.Müller MC, Merx K, Weisser A, et al. Improvement of molecular monitoring of residual disease in leukemias by bedside RNA stabilization. Leukemia. 2002;16:2395–2399. doi: 10.1038/sj.leu.2402734. [DOI] [PubMed] [Google Scholar]
- 58.Thörn I, Olsson-Strömberg U, Ohlsen C, et al. The impact of RNA stabilization on minimal residual disease assessment in chronic myeloid leukemia. Haematologica. 2005;90:1471–1476. [PubMed] [Google Scholar]
- 59.Prezeau N, Silvy M, Gabert J, et al. Assessment of a new RNA stabilizing reagent (Tempus Blood RNA) for minimal residual disease in onco-hematology using the EAC protocol. Leuk Res. 2006;30:569–574. doi: 10.1016/j.leukres.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 60.Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112:4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnes DJ, Palaiologou D, Panousopoulou E, et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 2005;65:8912–8919. doi: 10.1158/0008-5472.CAN-05-0076. [DOI] [PubMed] [Google Scholar]
- 62.Gaiger A, Henn T, Hörth E, et al. Increase of bcr-abl chimeric mRNA expression in tumor cells of patients with chronic myeloid leukemia precedes disease progression. Blood. 1995;86:2371–2378. [PubMed] [Google Scholar]
- 63.Marega M, Piazza RG, Pirola A, et al. BCR and BCR-ABL regulation during myeloid differentiation in healthy donors and in chronic phase/blast crisis CML patients. Leukemia. 2010;24:1445–1449. doi: 10.1038/leu.2010.101. [DOI] [PubMed] [Google Scholar]
- 64.Neviani P, Santhanam R, Trotta R, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Skorski T. Genetic mechanisms of chronic myeloid leukemia blastic transformation. Curr Hematol Malig Rep. 2012;7:87–93. doi: 10.1007/s11899-012-0114-5. [DOI] [PubMed] [Google Scholar]
- 66.Chang JS, Santhanam R, Trotta R, et al. High levels of the BCR/ABL oncoprotein are required for the MAPK-hnRNP-E2 dependent suppression of C/EBPalpha-driven myeloid differentiation. Blood. 2007;110:994–1003. doi: 10.1182/blood-2007-03-078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hehlmann R, Saussele S. Treatment of chronic myeloid leukemia in blast crisis. Haematologica. 2008;93:1765–1769. doi: 10.3324/haematol.2008.001214. [DOI] [PubMed] [Google Scholar]
- 68.Silver RT, Cortes J, Waltzman R, et al. Sustained durability of responses and improved progression-free and overall survival with imatinib treatment for accelerated phase and blast crisis chronic myeloid leukemia: Long-term follow-up of the STI571 0102 and 0109 trials. Haematologica. 2009;94:743–744. doi: 10.3324/haematol.2009.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: By the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 70.Soverini S, Martinelli G, Rosti G, et al. Advances in treatment of chronic myeloid leukemia with tyrosine kinase inhibitors: The evolving role of Bcr-Abl mutations and mutational analysis. Pharmacogenomics. 2012;13:1271–1284. doi: 10.2217/pgs.12.103. [DOI] [PubMed] [Google Scholar]
- 71.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–1215. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 72.Alikian M, Gerrard G, Subramanian PG, et al. BCR-ABL1 kinase domain mutations: Methodology and clinical evaluation. Am J Hematol. 2012;87:298–304. doi: 10.1002/ajh.22272. [DOI] [PubMed] [Google Scholar]
- 73.Soverini S, De Benedittis C, Machova Polakova K, et al. Unraveling the complexity of tyrosine kinase inhibitor-resistant populations by ultra-deep sequencing of the BCR-ABL kinase domain. Blood. 2013;122:1634–1648. doi: 10.1182/blood-2013-03-487728. [DOI] [PubMed] [Google Scholar]
- 74.Machova Polakova K, Kulvait V, Benesova A, et al. Next-generation deep sequencing improves detection of BCR-ABL1 kinase domain mutations emerging under tyrosine kinase inhibitor treatment of chronic myeloid leukemia patients in chronic phase. J Cancer Res Clin Oncol. 2015;141:887–899. doi: 10.1007/s00432-014-1845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kastner R, Zopf A, Preuner S, et al. Rapid identification of compound mutations in patients with Philadelphia-positive leukaemias by long-range next generation sequencing. Eur J Cancer. 2014;50:793–800. doi: 10.1016/j.ejca.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soverini S, Rosti G, Iacobucci I, et al. Choosing the best second-line tyrosine kinase inhibitor in imatinib-resistant chronic myeloid leukemia patients harboring Bcr-Abl kinase domain mutations: How reliable is the IC50? The Oncologist. 2011;16:868–876. doi: 10.1634/theoncologist.2010-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soverini S, Martinelli G, Colarossi S, et al. Presence or the emergence of a F317L BCR-ABL mutation may be associated with resistance to dasatinib in Philadelphia chromosome-positive leukemia. J Clin Oncol. 2006;24:e51–e52. doi: 10.1200/JCO.2006.08.9128. [DOI] [PubMed] [Google Scholar]
- 78.Soverini S, Colarossi S, Gnani A, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Haematologica. 2007;92:401–404. doi: 10.3324/haematol.10822. [DOI] [PubMed] [Google Scholar]
- 79.Soverini S, Martinelli G, Colarossi S, et al. Second-line treatment with dasatinib in patients resistant to imatinib can select novel inhibitor-specific BCR-ABL mutants in Ph+ ALL. Lancet Oncol. 2007;8:273–274. doi: 10.1016/S1470-2045(07)70078-5. [DOI] [PubMed] [Google Scholar]
- 80.Jabbour E, Jones D, Kantarjian HM, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations. Blood. 2009;114:2037–2043. doi: 10.1182/blood-2009-01-197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27:4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Müller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: Analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114:4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khorashad JS, Milojkovic D, Mehta P, et al. In vivo kinetics of kinase domain mutations in CML patients treated with dasatinib after failing imatinib. Blood. 2008;111:2378–2381. doi: 10.1182/blood-2007-06-096396. [DOI] [PubMed] [Google Scholar]
- 84.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27:469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 85.Zabriskie MS, Eide CA, Tantravahi SK, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26:428–442. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cortes JE, Kantarjian HM, Brümmendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–4576. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. J Clin Oncol. 2012;30:3486–3492. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119:3403–3412. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gambacorti-Passerini C, Brümmendorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: Minimum 24-month follow-up. Am J Hematol. 2014;89:732–742. doi: 10.1002/ajh.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gambacorti-Passerini C, Kantarjian HM, Kim DW, et al. Long-term efficacy and safety of bosutinib in patients with advanced leukemia following resistance/intolerance to imatinib and other tyrosine kinase inhibitors. Am J Hematol. 2015;90:755–768. doi: 10.1002/ajh.24034. [DOI] [PMC free article] [PubMed] [Google Scholar]