In the prospective Exemestane and Letrozole Pharmacogenetics trial of adjuvant aromatase inhibitor (AI) therapy for early-stage breast cancer, worsening of multiple treatment-related symptoms during AI therapy predicted AI early discontinuation. If these findings are confirmed in independent trials, early detection of changes in PRO measures could be used clinically to target interventions in patients at high risk for early discontinuation.
Keywords: Aromatase inhibitors, Patient-reported outcomes, Early discontinuation, Quality of life
Abstract
Background.
Early discontinuation of aromatase inhibitors (AIs) is common and leads to poor outcomes but is challenging to predict. In the Exemestane and Letrozole Pharmacogenetics trial, a high rate of early discontinuation due to intolerance was observed. We hypothesized that early changes in patient-reported outcomes (PROs) predict AI discontinuation and that biochemical factors are associated with changes in PROs.
Patients and Methods.
Postmenopausal women with early-stage breast cancer enrolled in a prospective randomized trial of exemestane versus letrozole completed questionnaires at baseline and serially over 24 months to assess overall quality of life (EuroQOL Visual Analog Scale [VAS]); mood; and multiple symptoms, including a musculoskeletal symptom cluster. A joint mixed-effects/survival model was used to estimate the effect of the change in PROs on AI discontinuation. Associations between biochemical factors and change in PROs were examined.
Results.
A total of 490 patients were analyzed. Worsening of EuroQOL VAS and the musculoskeletal cluster were associated with the highest risk for early discontinuation (hazard ratio [HR], 2.77 [95% confidence interval (CI), 2.72–2.81; p = .015]; HR, 4.39 [95% CI, 2.40–8.02; p < .0001], respectively). Pharmacokinetics and estrogen metabolism were not consistently associated with change in PRO measures. No clinically significant differences in any PRO between AIs were observed.
Conclusion.
Changes in PROs early during AI therapy were associated with treatment discontinuation. Identification of these changes could be used to target interventions in patients at high risk for early discontinuation.
Implications for Practice:
Early changes in patient-reported outcomes (PROs) can predict nonpersistence to aromatase inhibitor therapy. If used in clinical practice, PROs might identify women at highest risk for early discontinuation and allow for interventions to improve tolerance before significant toxicities develop. Further research is needed to improve capturing PROs in routine clinical practice.
Abstract
摘要
背景. 芳香化酶抑制剂 (AI) 早期停药很常见, 可导致转归不良, 但又难以预测。在依西美坦和来曲唑的药物遗传学研究中, 观察到很高比例的早期停药是由不耐受引起。我们假设患者报告转归 (PRO) 的早期改变可预测 AI 停药, 而且生化因素与 PRO 的改变有关。
患者与方法. 一项比较依西美坦与来曲唑的前瞻性随机临床试验纳入了绝经后早期乳腺癌女性患者, 其中患者在基线以及 24 个月期间连续完成问卷以评估总体生活质量[EuroQOL视觉模拟量表 (VAS) ]、心境, 以及包括肌肉骨骼症状簇在内的多种症状。使用联合混合效应/生存模型估算 PRO 改变对 AI 停药的影响。对生化因素与 PRO 改变之间的相关性进行检验。
结果. 共对 490 例患者进行了分析。 EuroQOL VAS 分数变差及肌肉骨骼症状簇与最高等级的早期停药风险相关, 风险比 (HR) 分别为 2.77 [95%置信区间 (CI): 2.72∼2.81) P=0.015]和 4.39 (95%CI: 2.40∼8.02, P<0.0001)。药代动力学及雌激素代谢与 PRO 测值变化之间不存在一贯的相关性。未观察到不同 AI 的各种 PRO 之间存在具有临床意义的差异。
结论. AI治疗期间的PRO早期改变与停药相关。鉴别出这些改变有助于对早期停药高危患者采取有针对性的干预措施。The Oncologist 2016;21:539–546
对临床实践的提示: 患者报告转归 (PRO) 的早期改变可预测患者对芳香化酶抑制剂治疗的不依从。如将 PRO 用于临床实践, 也许能鉴别出早期停药的最高危患者, 使得医生能够在出现明显的毒性之前对其进行干预以改善耐受性。有必要开展进一步的研究以改进 PRO 在日常临床工作中的采集。
Introduction
Aromatase inhibitors (AIs) improve survival compared with tamoxifen and are the preferred adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer [1]. Previous large adjuvant endocrine trials have reported no significant decrease in overall health-related quality of life (HRQOL) during adjuvant AI therapy [2–4]. Despite these data, in multiple research and clinical practice settings early discontinuation is common, ranging from 30% to 70% [5]. Although reasons for early discontinuation are multifactorial, data suggest that up to 30% of patients discontinue AI therapy because of adverse symptoms, most commonly arthralgias [6, 7].
Multiple studies have explored predictors of early discontinuation on the basis of baseline demographic and/or clinicopathologic features; however, inconsistent results between studies have made it difficult for clinicians to accurately predict which patients are at greatest risk for early discontinuation. Interestingly, one report found that although a baseline history of anxiety or depression was not associated with early discontinuation, treatment for anxiety that developed after initiation of endocrine therapy was associated with early discontinuation [8]. This latter observation suggests that a change in symptom burden early in the course of therapy could be a predictor for discontinuation and that early identification could possibly allow for earlier intervention.
The Exemestane and Letrozole Pharmacogenetics (ELPh) trial was a randomized study to investigate the pharmacogenetic influences on the effects of AI therapy; it included prospective patient-reported outcome (PRO) measures throughout the initial 24-months of AI therapy. Measures included were for global HRQOL, depression, anxiety, and symptom burden. We previously reported a statistically significant difference in time to treatment discontinuation in this cohort, with a shorter time to discontinuation for those treated with exemestane compared with letrozole [6]. The primary objective of this exploratory analysis was to determine whether early changes in PRO measures predicted early discontinuation and compare longitudinal changes in PROs by two distinct AIs. To examine the mechanism underlying development of PROs during AI therapy, we also examined the association between changes in PROs and various biochemical factors. On the basis of previous data [9], hypotheses were as follows: (a) there would be no significant difference in PRO measures between AIs, (b) negative early changes in PROs would predict early discontinuation, and (c) greater suppression of estrogen metabolites would be associated with greater negative changes in PROs.
Materials and Methods
Study Participants
Postmenopausal women with stage 0–III hormone receptor-positive breast cancer who were initiating treatment with an AI were eligible for enrollment on the ELPh trial. Details of the trial have been previously published (ClinicalTrials.gov NCT00228956) [6, 10]. In brief, all indicated surgery, chemotherapy, and/or radiation therapy were completed before enrollment. Prior tamoxifen therapy was permitted. No patients could have previously received AI therapy for any reason. Supportive care as directed by the clinical team was permitted for management of any treatment-emergent toxicity and was not protocol driven except for offering patients the option to cross over to the alternative AI. The institutional review boards at all three participating sites (Johns Hopkins University, Indiana University, University of Michigan) approved the clinical trial. Patients were required to provide written informed consent before undergoing study-related procedures.
Study Procedures
Patients were randomly assigned in a 1:1 ratio to treatment with exemestane (Aromasin; Pfizer, New York, NY), 25 mg orally daily, or letrozole (Femara; Novartis, Basel, Switzerland), 2.5 mg orally daily. Randomization was stratified by prior chemotherapy, prior tamoxifen, and bisphosphonate use. At baseline and after 1, 3, 6, 12, and 24 months, patients were clinically evaluated and completed PRO questionnaires (see next section). Blood samples were collected at baseline and after 3 months of therapy for evaluation of estrogen metabolites and pharmacokinetics (PK).
PRO Questionnaires
Validated tools for HRQOL, depression, anxiety, and symptom burden were used. Specifically, HRQOL was assessed by using the EuroQOL Visual Analog Scale (VAS). The EuroQOL VAS is one component of the EQ-5D; this 20-cm, Cantril-like ladder scale ranging from 0 (death) to 100 (best health) has demonstrated reliability and validity in populations with cancer [11]. The minimally important difference for the EuroQOL VAS is 7–12 [12]. Depression was assessed with the Center for Epidemiologic Studies–Depression (CESD) tool, a 20-item self-report tool that evaluates for the presence and severity of depressive symptoms. Scores range from 0 to 60, with scores of 16 or higher indicating high depressive symptoms [13]. Anxiety was assessed with the anxiety subscale of the Hospital Anxiety and Depression Scale (HADSA). This is a brief 7-item tool, and scores range from 0 to 21; scores of 8 or higher indicate anxiety [14]. A 47-item tool, composed largely of items from the Breast Cancer Prevention Trial Symptom Checklist (BCPT-SCL) [15], assessed general symptom burden (supplemental online Table 1). Scores for each item range from 0 to 4, with a higher score indicating worse symptom burden. Of the 47 items assessed, the current analysis of general symptom burden used 34 items analyzed as six separate symptom clusters (weight/body image, vasomotor, vulvovaginal, musculoskeletal, cognitive, and mood) developed by using methods described later in the text.
Laboratory Studies
As previously described, serum samples obtained at baseline and at 3 months were assayed for estradiol (E2), estrone-1-sulfate (E1S), and estrone (E1) by using an ultrasensitive gas chromatography/tandem mass spectroscopy assay [16]. The lower limits of quantification were 0.625 pg/mL for E2, 2.88 pg/mL for E1S, and 1.56 pg/mL for E1. Serum concentrations of exemestane and letrozole were measured at baseline and follow-up at 3 months (Z. Desta, personal communication) [17].
Statistical Analysis
In this exploratory analysis, the first objective was to compare early changes in PRO mean scores from baseline to months 1, 3, and 6 among patients who continued therapy through 24 months (persistent group) compared with those who discontinued before 24 months (nonpersistent) by using the Wilcoxon rank-sum test. In addition, we also analyzed whether the PRO measures predicted for time to discontinuation, where discontinuation of the initial AI therapy before 24 months was due to the development of intolerable toxicity (as reported by the patient as the primary cause of treatment discontinuation). To account for nonrandom dropout, a joint mixed-effects/survival model was used to estimate the effect of the change in PROs over 24 months on AI discontinuation. All PROs were modeled as continuous variables. Natural cubic splines were used in modeling time for all models with two to four knots (polynomial in time to allow for nonlinearity of association between time and PRO).
For the symptom clusters, we ran a confirmatory factor analysis at baseline to confirm the grouping of items based on the BCPT-SCL eight-symptom scale [18] and found high levels of goodness of fit for all clusters except the vulvovaginal cluster. Specifically, for the five other symptom clusters, the root mean square error of approximation was 0.043 (acceptable models use <0.06) and the comparative fit index was 0.93 (acceptable, >0.90). For each symptom cluster, the outcome is the square root (for distributional purposes in our mixed models) of the sum of the questions in each cluster and the average score per cluster for each time point. If a woman did not answer every question in the cluster, we used the average of those questions that she did answer.
For the second objective, we analyzed the changes from baseline in HRQOL, psychological distress, and symptom burden during the 24-month study period between AIs. PRO evaluation was missing at any time point after the patient had discontinued the AI. Mean change scores from baseline to each follow-up time point for those who had not yet discontinued were compared between treatment groups for all PRO domains and analyzed by using the Wilcoxon rank-sum test. A negative mean change in PRO indicates the score decreased from baseline and a positive mean change in PRO indicates the score increased from baseline. Associations between baseline variables of interest, including age, race, body mass index (BMI), prior use of tamoxifen, prior use of chemotherapy (yes vs. no and taxane vs. no taxane) and changes in PROs were assessed by using Spearman correlation for continuous variables and Wilcoxon rank-sum test for categorical variables.
Estrogen levels were measured at baseline and 3 months by using estrone sulfate, estradiol, and estrone. The correlations between the absolute estrogen level at 3 months and the change in estrogen from baseline to 3 months with the change in EuroQOL VAS, CESD, HADSA, and symptom clusters were examined by using Spearman correlation. Only one measure of PK data was available (taken at 3 months), so the drug PK level at 3 months was correlated to the change in PRO from baseline to 3 months using Spearman correlation. Multiple comparisons were not controlled for because of the exploratory nature of the analysis. Statistical analyses were conducted using SAS v9.3 (SAS Institute, Inc, Cary, NC, http://www.sas.com).
Results
Patient Characteristics
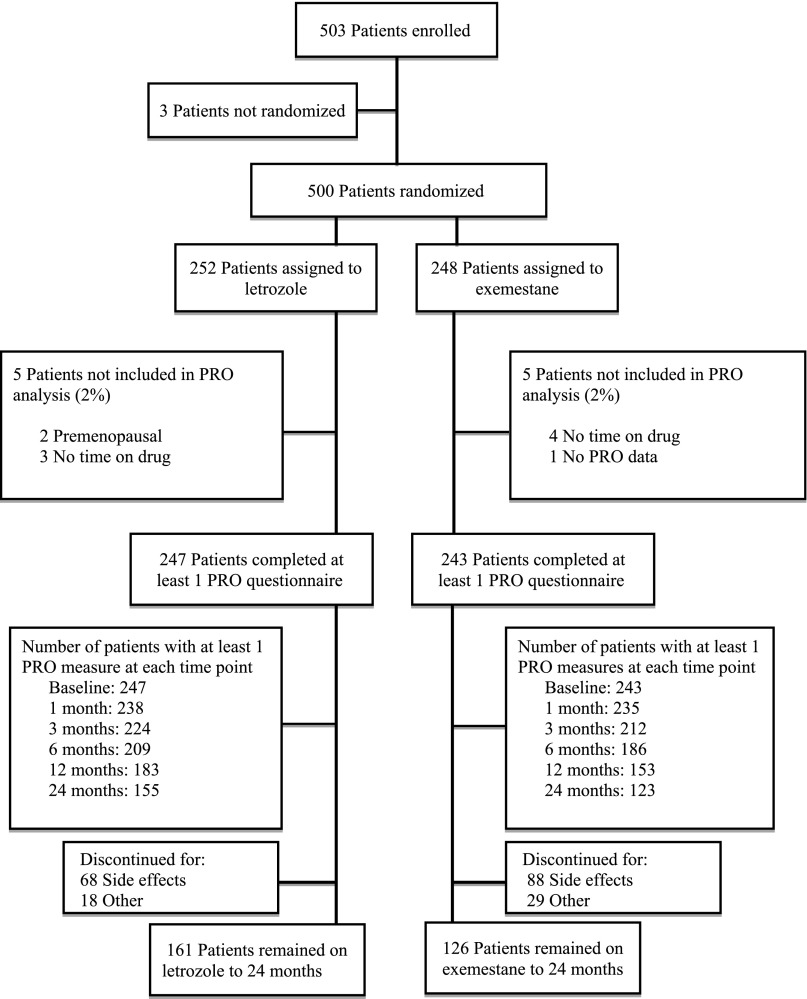
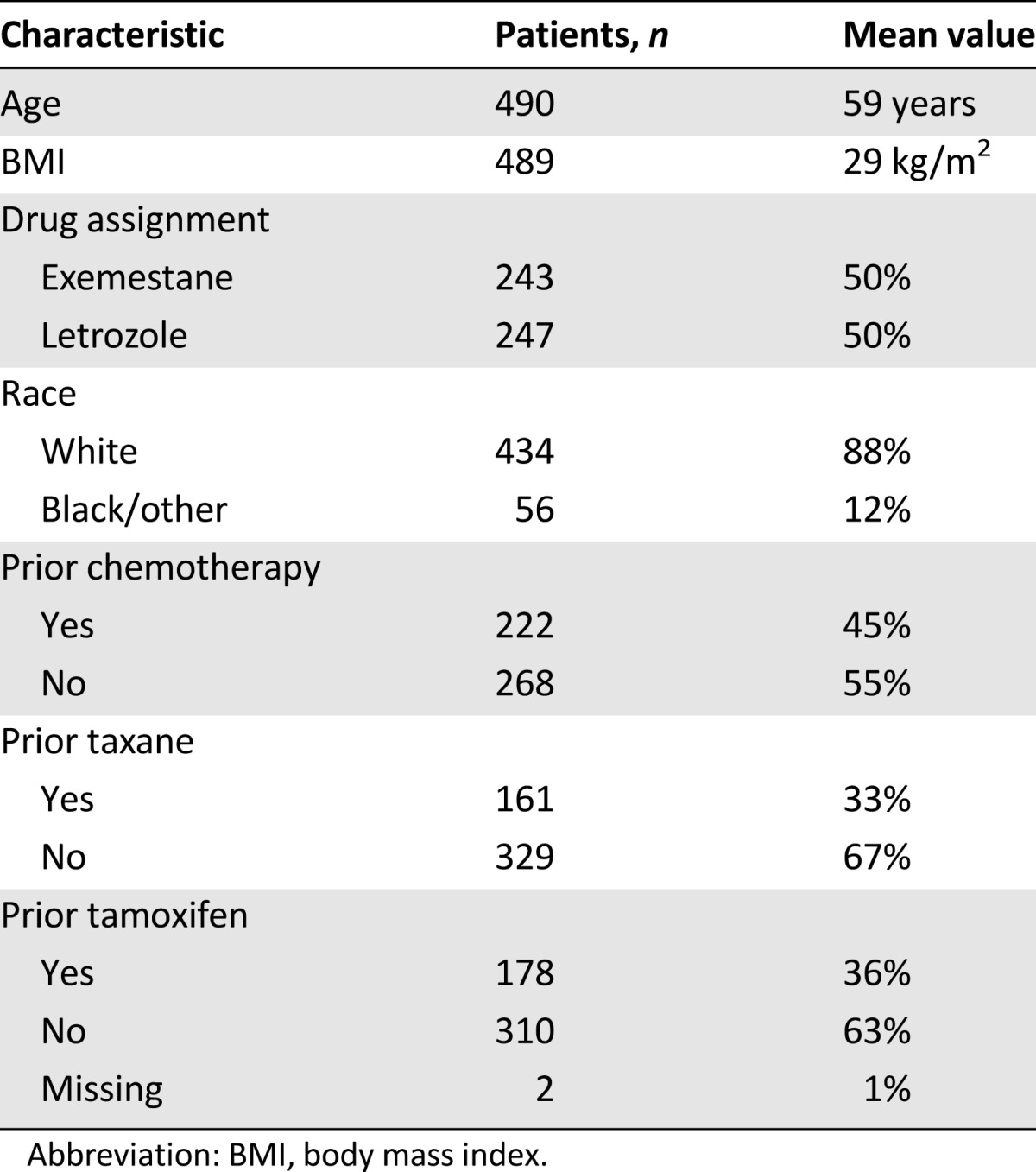
Five hundred three patients signed informed consent. Three patients withdrew from study participation before randomization (Fig. 1). Of the 500 eligible patients, 252 (50.4%) were randomly assigned to letrozole and 248 (49.6%) to exemestane. Ten patients (4%) were not included in the current analysis: 5 in each treatment arm because of no time on drug (n = 7), premenopausal status (n = 2), and no completed PRO data (n = 1). Baseline characteristics for the 490 patients in the current analysis are listed in Table 1. Most patients were white (n = 434 [88%]), nearly half had received adjuvant chemotherapy (n = 222 [45%]), and 178 (36%) had been treated with tamoxifen. Baseline scores for all PROs were not significantly different between treatment arms, as shown in supplemental online Table 2.
Figure 1.
Consolidated Standards of Reporting Trials diagram.
Abbreviation: PRO, patient-reported outcome.
Table 1.
Baseline demographic characteristics (n = 490)
Longitudinal Effects of AI Therapy on PROs
Mean change by drug from baseline to each time point for EuroQOL VAS, CESD, and HADSA are shown in supplemental online Figure 1A–1C, respectively. Although statistically significant differences between AIs were observed in mean change from baseline on the EuroQOL VAS at 6 months (−3.27 for exemestane vs. −0.38 for letrozole; p = .03) and CESD at 3 months (1.28 for exemestane vs. −0.14 for letrozole; p = .01), these were isolated findings and not consistent over time or symptom domain. No other significant differences in mean changes from baseline to any time point during the 24-month study period were found between treatment arms for these measures. Mean change from baseline to each time point for the six symptom clusters are shown in supplemental online Table 3. Significant differences between drugs were found in the weight/body image, musculoskeletal, and cognitive symptom clusters; however, the differences were uncommonly and inconsistently observed during the study period.
Changes in PRO and AI Early Discontinuation
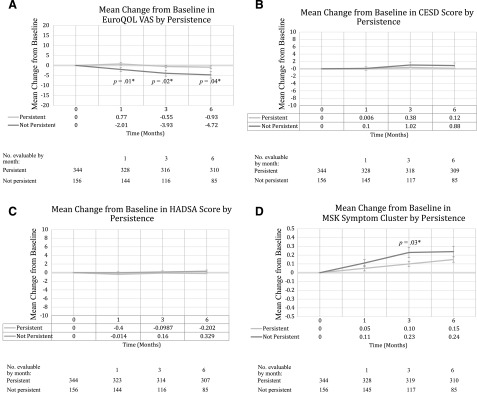
Of the 490 patients analyzed, 156 (32%) patients discontinued AI treatment before 24 months because of toxicity. Of those, 88 (36.2%) randomly assigned to exemestane and 68 (27.5%) randomly assigned to letrozole discontinued therapy (p = .039). Forty-seven additional patients discontinued for reasons other than toxicity, such as recurrence, nonadherence with study procedures, and ovarian function recovery. Early changes in PRO mean scores (EuroQOL VAS, CESD, HADSA, and musculoskeletal [MSK] symptom cluster) from baseline to months 1, 3, and 6 by those who continued therapy until 24 months (persistent group) compared with those who discontinued before 24 months (nonpersistent) are shown in Figure 2A–2D. Women who were nonpersistent were more likely to have worse Euro QOL VAS and MSK symptom cluster scores at all early time points compared with those that were persistent. No early differences in CESD or HADSA were observed.
Figure 2.
Mean changes from baseline in outcome measures by persistence. (A): EuroQOL VAS. Positive mean change in quality of life indicates improvement from baseline. Mean (±SD) baseline EuroQOL scores: persistent, 83.9 ± 12.14; not persistent, 84.8 ± 12.58 (p = .21). p value by Wilcoxon test. ∗, Statistically significant. QOL VAS range, 0–100 (100 indicating best imaginable health state). (B): CESD. Positive mean change in CES-D indicates improvement from baseline. Mean baseline CES-D scores: persistent, 7.5 ± 6.73; not persistent, 9.2 ± 8.10; p = .036. p value by Wilcoxon test; ∗, Statistically significant. CESD range, 0–60 (≥16 suggests clinical depression). (C): HADSA. Positive mean change in HADSA indicates improvement from baseline. Mean baseline HADS-A scores: persistent, 3.90 ± 3.03; not persistent, 4.4 ± 3.28; p = .11. p value by Wilcoxon test. HADSA range, 0–21 (≥8 suggests clinical anxiety). (D): MSK symptom cluster. Positive mean change in MSK symptom cluster indicates worse from baseline. Mean baseline MSK symptom cluster scores: persistent, 0.51 ± 0.049; not persistent, 0.62 ± 0.53; p = .012. p value by Wilcoxon test. ∗, Statistically significant. MSK symptom cluster range, 0–4.
Abbreviations: CESD, Centers for Epidemiologic Studies–Depression; HADSA, anxiety scale of the Hospital Anxiety and Depression Scale; MSK, musculoskeletal; VAS, Visual Analog Scale.
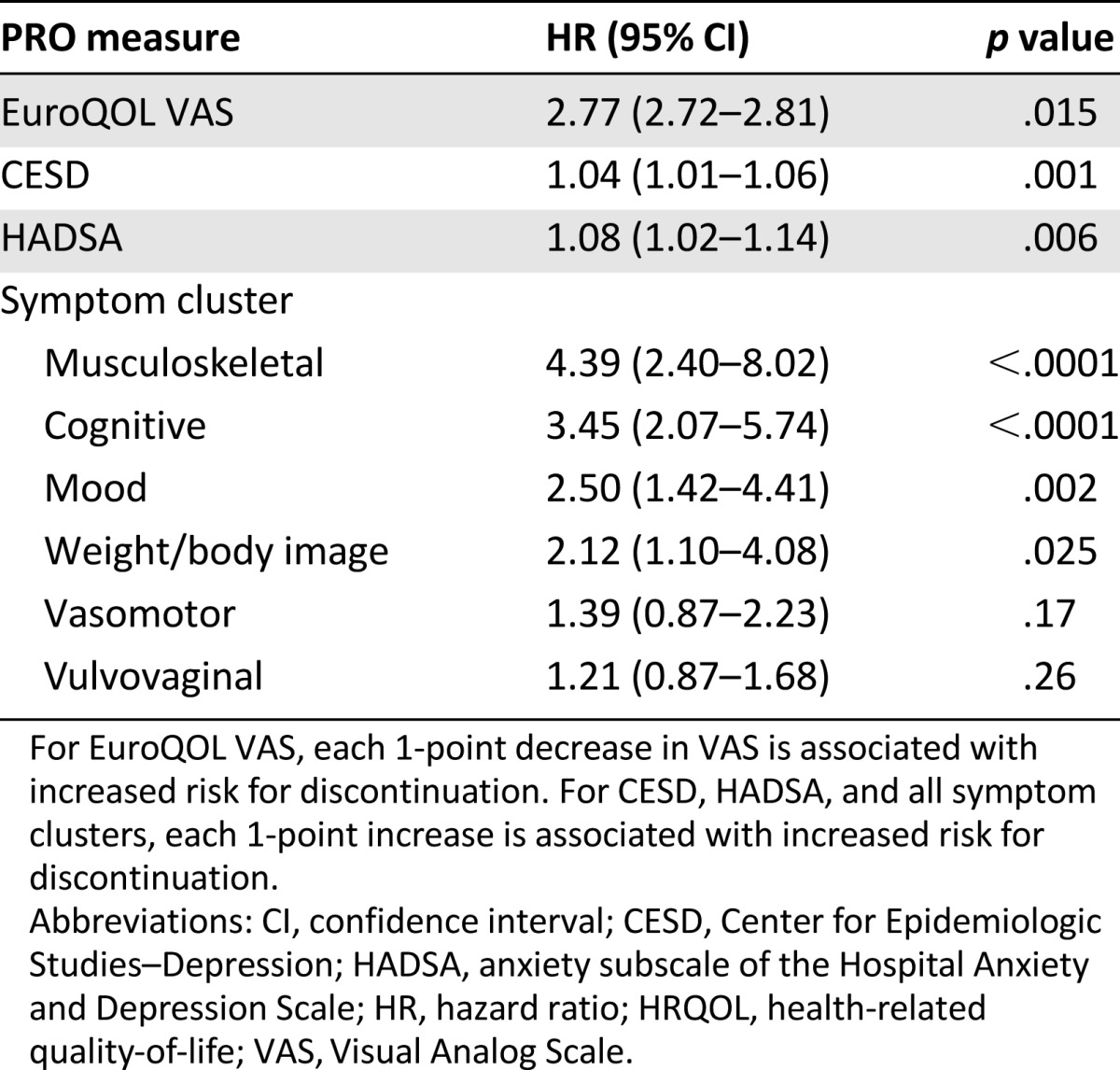
Table 2 demonstrates the findings of the joint mixed-effects model used to estimate the effect of change in PROs on early discontinuation of AIs. Worsening in EuroQOL VAS was associated with increased risk for early discontinuation (hazard ratio [HR], 2.77; 95% confidence interval [CI], 2.72–2.81; p = .015). Worsening in depression (CESD) and anxiety (HADSA) scores was weakly associated with early discontinuation (HR, 1.04 [95% CI, 1.01–1.06; p = .001] and 1.04 [95% CI, 1.01–1.06; p = .006], respectively). Worsening in the musculoskeletal, cognitive, mood, and weight/body image clusters was also associated with increased risk for early discontinuation. Of these, the musculoskeletal symptom cluster was most highly associated with increased risk for early discontinuation (HR, 4.39; 95% CI, 2.4–8.02; p < .0001).
Table 2.
Effect of the change in patient-reported outcome scores on early discontinuation of aromatase inhibitor treatment
Predictors of Changes in PROs During AI Therapy
Prior use of tamoxifen was not associated with any significant difference in EuroQOL VAS, CESD, or HADSA throughout the study period except EuroQOL VAS at 3 months (−3.1 for prior tamoxifen vs. −0.54 for no tamoxifen; p = .032) (data not shown). Compared with no prior tamoxifen use, prior tamoxifen use was associated with greater worsening in the musculoskeletal symptom cluster, lesser worsening at 3 months in the mood symptom cluster, and lesser worsening in the vasomotor symptom cluster throughout the study period. Prior exposure to chemotherapy or taxane compared with no such exposure was associated with a lesser worsening in the vasomotor symptom cluster and a greater worsening in the vulvovaginal symptom cluster (data not shown). Neither prior chemotherapy nor taxane exposure was associated with a significant change in EuroQOL VAS, CESD, or HADSA. No difference in the musculoskeletal symptom cluster was observed in patients with prior taxane exposure. No statistically significant associations were identified between change in any PRO measure during the 24-month study period and race, age, or BMI (data not shown).
Effect of Estrogen Metabolism and AI Pharmacokinetics on PROs
We examined correlations between change in PROs and change in both serum estrogen metabolite concentrations (estradiol, estrone, estrogen sulfate) and AI concentration. No clinically relevant associations were found (supplemental online Table 4).
Discussion
Compared with tamoxifen, upfront or sequential therapy with an AI is associated with improved disease-free and overall survival in postmenopausal women with hormone receptor-positive breast cancer [1, 19]. In the ELPh clinical trial, one third of patients discontinued AI treatment because of patient-reported intolerable symptom burden. We were able to identify early changes in PROs in the cohort that ultimately stopped as a result of treatment toxicity. These findings are consistent with a recent retrospective cohort study that found joint pain predicted for early AI discontinuation [20]. The ability to identify patients with high likelihood of developing intolerable adverse effects could have clinical implications for proactive symptom management strategies to minimize early discontinuation and improve adherence to life-prolonging therapies. In a feature unique to this analysis, we also explored biochemical changes and demographic variables that affected changes in PRO measures and could provide a mechanistic explanation for the development of the changes; however, none showed a consistent effect.
One challenge of studies of change in PROs during therapy is missing data associated with early treatment discontinuation. This is particularly true with AI therapy, for which a high rate of early discontinuation with therapy due to toxicity has been reported [6]. Bias can occur when women with the greatest symptom burden discontinue therapy early and do not contribute to the longitudinal PRO analyses. In the current study, most of the 32% who discontinued did so because of adverse symptoms at a median time of 6.1 months and therefore failed to contribute to the subsequent PRO analyses.
Although we observed a greater worsening in EuroQOL VAS and musculoskeletal symptom burden early in the treatment course (baseline to months 1, 3, and 6) in those who subsequently discontinued therapy compared with those who remained on therapy, nonrandom dropout and multiple comparisons limit these analyses. We attempted to account for the effect of missing data by using a joint mixed-effects/survival model. Our exploratory analysis suggests that the progressive development of symptoms over time is associated with early discontinuation of AI therapy. Most notably, increasing scores in the musculoskeletal symptom cluster over time were associated with a substantially increased risk for discontinuing AI therapy early.
Despite the prevalence and negative effect of arthralgias on HRQOL, the true mechanism for these symptoms remains to be fully understood. One hypothesis is based on the influence of estrogen depletion. This is, in part, supported by findings from a survey study finding that shorter interval since the onset of menopause was associated with higher AI-associated arthralgia burden, suggesting that these women might have had a larger decrease in absolute estrogen levels [21]. Although this is an interesting finding, our current data and prior work from our group [22] have not observed an association with absolute change in estrogen or estrogen metabolites following AI therapy and the onset of arthralgias. However, the current study was underpowered for a definitive analysis, and larger adequately powered studies are needed. More recently, an inflammatory basis for arthralgia and other AI-associated symptoms has been postulated [23]. Unfortunately, a recently reported targeted anti-inflammatory therapy using omega-3 fatty acids showed no difference over placebo [24]. Overall, these data, along with the current study, suggest that successful interventions to improve overall HRQOL and decrease early discontinuation will likely have to target multiple domains.
If similar findings are validated in larger prospective studies, this information could help inform studies of interventions to improve tolerance of and adherence to therapy. For example, inclusion of patients who develop treatment-emergent symptoms early in the course of AI therapy might enrich for a more “at risk” population that is more likely to benefit from the adherence intervention, thereby increasing the power of the study. To date, prospective trials, such as those evaluating patient educational materials and/or supportive services, have failed to demonstrate improvements in persistence rates of adjuvant endocrine therapy [25–27]. The reason for the lack of improvement observed in these trials could be due to the inclusion of a general cohort of breast cancer patients, many of whom are unlikely to discontinue treatment, rather than a prespecified group at highest risk for nonpersistence.
Another issue that can result in decreased statistical power to detect changes in PRO measures is the enrollment of a cohort of women with excellent baseline HRQOL and low levels of depression and symptom burden. This is highlighted by the consistent findings in trials of tamoxifen [28] and recent adjuvant [29, 30] and neoadjuvant [31] trials of AI therapy that show little change in psychological distress during the study period. Similarly, the ELPh study participants as a group had no significant change in validated measures of depression or anxiety. As in the larger studies discussed earlier, most patients in ELPh also had low rates of depression and anxiety both at baseline and during therapy. Patients with a high pre-existing symptom burden may have more difficulty tolerating therapy compared with those without symptoms, but they are generally not represented in large numbers in clinical trials. Concomitant use of medications for depression and anxiety during study participation might affect these measures over time. However, in the ELPh trial analysis, because of limitations on available data regarding indications for use of concomitant medications as well as low numbers of patients who reported mood issues, analyses on concurrent psychotropic medications use were not performed.
Despite the high incidence of early discontinuation due to patient-reported symptom burden, analyses of the data from the entire cohort and by drug are consistent with the available body of literature describing minimal change in PROs over time in large randomized adjuvant AI trials [2–4, 29, 30, 32–34]. What then accounts for the high rate of nonadherence and early discontinuation observed in clinical practice even though the available studies overwhelmingly suggest that overall PROs are minimally affected? To answer this, it is important to understand the difference between commonly used general HRQOL versus symptom-focused PROs. Tools such as the Functional Assessment of Cancer Therapy (FACT)-B and Short Form-36 are psychometrically reliable and valid and measure a variety of domains. Although these commonly used HRQOL tools are able to capture differences between various populations over time (i.e., advanced cancer vs. minimal disease) their ability to capture clinically important differences in trials involving well-balanced populations that are comparing agents with similar toxicity profiles (i.e., adjuvant endocrine therapy) appears limited. In contrast, symptom-specific measures, such as the select items of the endocrine subscale of FACT, are focused on a limited set of adverse symptoms that might allow patients to respond more precisely to symptom severity and allow investigators to discern meaningful differences over time [35]. The large AI adjuvant trials (such as the Arimidex, Tamoxifen Alone or in Combination study; the Intergroup Exemestane Study; and National Cancer Institute of Canada MA.17) exemplify this phenomenon because they found no meaningful difference in overall HRQOL between treatment arms while observing some distinct treatment-specific adverse endocrine symptoms.
Conclusion
In the prospective ELPh trial of adjuvant AI therapy for early-stage breast cancer, worsening of multiple treatment-related symptoms during AI therapy predicted AI early discontinuation. If these findings are confirmed in independent trials, early detection of changes in PRO measures could be used clinically to target interventions in patients at high risk for early discontinuation.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study was supported in part by Pharmacogenetics Research Network Grant U01-GM61373 (to D.A.F.) and Clinical Pharmacology Training Grant 5T32-GM08425 (to D.A.F.) from the National Institute of General Medical Sciences, National Institutes of Health (NIH), Bethesda, MD, and by Grant M01-RR00042 (University of Michigan), M01-RR00750 (Indiana University), and M01-RR00052 (Johns Hopkins University) from the National Center for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. N.J.S. was supported (in part) by Cancer Center Biostatistics training grant 5T32-CA083654 (to J.T.). N.L.H. is a Damon Runyon-Lilly Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (Grant CI-53-10) and was also supported (in part) the National Cancer Institute Clinical Cancer Investigator Team Leadership Award (supplement to 3-P30-CA046592) (to M.W.). In addition, the ELPh trial was supported by grants from Pfizer, Inc., and Novartis Pharma AG; additional support was provided by Fashion Footwear Association of New York/QVC Presents Shoes on Sale. Study medication was provided by Pfizer (exemestane) and Novartis (letrozole).
Author Contributions
Conception/Design: Kunal C. Kadakia, Claire F. Snyder, Vered Stearns, N. Lynn Henry
Provision of study material or patients: N. Lynn Henry
Collection and/or assembly of data: Kunal C. Kadakia, Kelley M. Kidwell, Nicholas J. Seewald, N. Lynn Henry
Data analysis and interpretation: Kunal C. Kadakia, Claire F. Snyder, Kelley M. Kidwell, Nicholas J. Seewald, David A. Flockhart, Todd C. Skaar, Zereunesay Desta, James M. Rae, Julie L. Otte, Janet S. Carpenter, Anna M. Storniolo, Daniel F. Hayes, Vered Stearns, N. Lynn Henry
Manuscript writing: Kunal C. Kadakia, Claire F. Snyder, Kelley M. Kidwell, Nicholas J. Seewald, David A. Flockhart, Todd C. Skaar, Julie L. Otte, Janet S. Carpenter, Anna M. Storniolo, Daniel F. Hayes, Vered Stearns, N. Lynn Henry
Final approval of manuscript: Kunal C. Kadakia, Claire F. Snyder, Kelley M. Kidwell, Nicholas J. Seewald, David A. Flockhart, Todd C. Skaar, Zereunesay Desta, James M. Rae, Julie L. Otte, Janet S. Carpenter, Anna M. Storniolo, Daniel F. Hayes, Vered Stearns, N. Lynn Henry
Disclosures
Claire F. Snyder: Pfizer Oncology, Walgreens (C/A), Genentech, WellPoint/Anthem (RF), Immunomedics, Oncolytics Biotech, Express Scripts, Merck (OI); Daniel F. Hayes: Janssen (C/A), Janssen, Astra Zeneca, Pfizer, Eli Lilly (RF), Eli Lilly (H), Inbiomotion, Oncimmune (OI), Janssen Diagnostics (IP); Vered Stearns: AbbVie, Merck, Celgene, Novartis, Medimmune, Pfizer, Puma (RF); N. Lynn Henry: Celldex Pharmaceuticals, BioMarin (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Early Breast Cancer Trialists’ Collaborative Group Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 2.Fallowfield L, Cella D, Cuzick J, et al. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol. 2004;22:4261–4271. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Fallowfield LJ, Bliss JM, Porter LS, et al. Quality of life in the intergroup exemestane study: A randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol. 2006;24:910–917. doi: 10.1200/JCO.2005.03.3654. [DOI] [PubMed] [Google Scholar]
- 4.Whelan TJ, Goss PE, Ingle JN, et al. Assessment of quality of life in MA.17: A randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005;23:6931–6940. doi: 10.1200/JCO.2005.11.181. [DOI] [PubMed] [Google Scholar]
- 5.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 8.Kemp A, Preen DB, Saunders C, et al. Early discontinuation of endocrine therapy for breast cancer: Who is at risk in clinical practice? Springerplus. 2014;3:282. doi: 10.1186/2193-1801-3-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398–1404. doi: 10.1200/JCO.2012.44.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry NL, Chan HP, Dantzer J, et al. Aromatase inhibitor-induced modulation of breast density: Clinical and genetic effects. Br J Cancer. 2013;109:2331–2339. doi: 10.1038/bjc.2013.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickard AS, Wilke CT, Lin HW, et al. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–384. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter JS, Andrykowski MA, Wilson J, et al. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues Ment Health Nurs. 1998;19:481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- 14.Stafford L, Judd F, Gibson P, et al. Comparison of the hospital anxiety and depression scale and the center for epidemiological studies depression scale for detecting depression in women with breast or gynecologic cancer. Gen Hosp Psychiatry. 2014;36:74–80. doi: 10.1016/j.genhosppsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Ganz PA, Day R, Ware JE, Jr, et al. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 16.Santen RJ, Demers L, Ohorodnik S, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Desta Z, Kreutz Y, Nguyen AT, et al. Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther. 2011;90:693–700. doi: 10.1038/clpt.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella D, Land SR, Chang CH, et al. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): Psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2008;109:515–526. doi: 10.1007/s10549-007-9682-9. [DOI] [PubMed] [Google Scholar]
- 19.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chim K, Xie SX, Stricker CT, et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer. 2013;13:401. doi: 10.1186/1471-2407-13-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry NL, Conlon A, Kidwell KM, et al. Effect of estrogen depletion on pain sensitivity in aromatase inhibitor-treated women with early-stage breast cancer. J Pain. 2014;15:468–475. doi: 10.1016/j.jpain.2014.01.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauml J, Chen L, Chen J, et al. Arthralgia among women taking aromatase inhibitors: Is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res. 2015;17:89. doi: 10.1186/s13058-015-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershman DL, Unger JM, Crew KD, et al. Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol. 2015;33:1910–1917. doi: 10.1200/JCO.2014.59.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadji P, Blettner M, Harbeck N, et al. The Patient’s Anastrozole Compliance to Therapy (PACT) Program: A randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol. 2013;24:1505–1512. doi: 10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 26.Neven P, Markopoulos C, Tanner M, et al. The impact of educational materials on compliance and persistence rates with adjuvant aromatase inhibitor treatment: First-year results from the compliance of aromatase inhibitors assessment in daily practice through educational approach (CARIATIDE) study. Breast. 2014;23:393–399. doi: 10.1016/j.breast.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Yu KD, Zhou Y, Liu GY, et al. A prospective, multicenter, controlled, observational study to evaluate the efficacy of a patient support program in improving patients’ persistence to adjuvant aromatase inhibitor medication for postmenopausal, early stage breast cancer. Breast Cancer Res Treat. 2012;134:307–313. doi: 10.1007/s10549-012-2059-8. [DOI] [PubMed] [Google Scholar]
- 28.Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93:1615–1623. doi: 10.1093/jnci/93.21.1615. [DOI] [PubMed] [Google Scholar]
- 29.Ohsumi S, Shimozuma K, Ohashi Y, et al. Health-related quality of life and psychological distress of breast cancer patients after surgery during a phase III randomized trial comparing continuation of tamoxifen with switching to anastrozole after adjuvant tamoxifen for 1-4 years: N-SAS BC 03. Breast Cancer Res Treat. 2011;127:143–152. doi: 10.1007/s10549-011-1400-y. [DOI] [PubMed] [Google Scholar]
- 30.Takei H, Ohsumi S, Shimozuma K, et al. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04) Breast Cancer Res Treat. 2012;133:227–236. doi: 10.1007/s10549-011-1943-y. [DOI] [PubMed] [Google Scholar]
- 31.Taira N, Iwata H, Hasegawa Y, et al. Health-related quality of life and psychological distress during neoadjuvant endocrine therapy with letrozole to determine endocrine responsiveness in postmenopausal breast cancer. Breast Cancer Res Treat. 2014;145:155–164. doi: 10.1007/s10549-014-2935-5. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Fallowfield L, Barker P, et al. Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat. 2006;100:273–284. doi: 10.1007/s10549-006-9260-6. [DOI] [PubMed] [Google Scholar]
- 33.Muss HB, Tu D, Ingle JN, et al. Efficacy, toxicity, and quality of life in older women with early-stage breast cancer treated with letrozole or placebo after 5 years of tamoxifen: NCIC CTG intergroup trial MA.17. J Clin Oncol. 2008;26:1956–1964. doi: 10.1200/JCO.2007.12.6334. [DOI] [PubMed] [Google Scholar]
- 34.Jones SE, Cantrell J, Vukelja S, et al. Comparison of menopausal symptoms during the first year of adjuvant therapy with either exemestane or tamoxifen in early breast cancer: Report of a Tamoxifen Exemestane Adjuvant Multicenter trial substudy. J Clin Oncol. 2007;25:4765–4771. doi: 10.1200/JCO.2007.10.8274. [DOI] [PubMed] [Google Scholar]
- 35.Ganz PA. Assessing the quality and value of quality-of-life measurement in breast cancer clinical trials. J Natl Cancer Inst. 2011;103:196–199. doi: 10.1093/jnci/djq542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.