Although infrequent, cases of colorectal cancer (CRC) during pregnancy have been reported in the last decade. With the trend in delayed child-bearing, CRC during pregnancy will likely rise in incidence. This review focuses on CRC antineoplastics and the challenges that are present when their use is considered during pregnancy.
Keywords: Pregnancy, Antineoplastics, Colorectal neoplasms, Monoclonal antibodies
Abstract
Cancer diagnosed during pregnancy has increased because of delayed child-bearing and the known occurrence of age-dependent malignancies. Cases of colorectal cancer (CRC) in pregnancy have recently been reported. With the expected rise in CRC diagnosed in young adults coupled with the current trend of delayed child-bearing, CRC during pregnancy is likely to increase. Treating pregnant women with CRC by using antineoplastics presents a dilemma because there are many unknowns to guide treatment decisions. We review the issues regarding the use of 10 CRC-approved agents in pregnancy.
Implications for Practice:
Colorectal cancer (CRC) in pregnancy is likely to become more common because of the current population trend in delayed child-bearing and the increase in CRC incidence expected among young adults. Practitioners should become familiar with the challenges associated with systemic treatment of a pregnant patient with CRC. This review addresses concerns surrounding the 10 systemic agents approved for CRC to help provide treatment guidance when such a case arises.
Abstract
摘要
在妊娠期间诊断为癌症的病例越来越多, 这是由于生育年龄推迟以及已知年龄依赖的恶性肿瘤的发生。近期有研究报告了妊娠期结直肠癌 (CRC) 病例。鉴于预期CRC诊断在较年轻的成年人中出现升高, 加之当前生育推迟的趋势, 妊娠期间CRC很可能会增加。对患有CRC的妊娠女性使用抗肿瘤治疗是一个两难问题, 因为在治疗决策指导中存在太多未知因素。我们对10种获批可用于妊娠期治疗的抗CRC药物的相关问题进行了回顾。The Oncologist 2016;21:563–570
对临床实践的提示: 由于目前人群的生育年龄有推迟趋势, 并且预期较年轻成年人中的结直肠癌 (CRC) 发生率会增加, 因此妊娠期间 CRC 很可能变得更为常见。临床医生应熟悉这一问题及妊娠期 CRC 患者系统治疗的相关挑战。本综述介绍了 10 种获批的 CRC 系统性治疗药物的相关问题, 可能有助于此类病例出现时的治疗指导。
Introduction
Cancer diagnosed during pregnancy has increased during the past several decades and currently has a reported incidence of 1 in 1,000 pregnancies [1–12]. This increase is linked to women delaying childbearing to the third and fourth decades of life, coupled with the known occurrence of age-dependent malignancies [1, 2, 6, 10–13]. Breast, ovarian, and cervical cancer; melanoma; and lymphoma are among the more common cancers diagnosed during pregnancy [1–3, 8, 9, 11, 12]. Colorectal cancer (CRC) during pregnancy is rare, with an incidence of 1 in 13,000 pregnancies [2, 10, 14]. Although infrequent, CRC cases during pregnancy have been reported in the past decade [15–31]. With the trend in delayed child-bearing, the rare situation of CRC during pregnancy will likely rise in incidence.
Ten systemic agents have been approved for CRC. Using these antineoplastics in a pregnant patient continues to be an undefined area, with many ethical, safety, and efficacy concerns. Physicians must weigh the risks to the fetus against the benefits of the mother’s treatment. Confounding treatment decisions are cancer stage, the mother’s outcomes, harm that can accompany treatment delays, treatment timing with regard to gestation, potential teratogenic effects, and, in the worst-case scenario, the consideration of termination of the pregnancy. Physicians making treatment decisions face many unknowns, thus further complicating an already sensitive discussion with the patient, the patient’s significant other, and the patient’s family. Unknown factors exist because of limited chemotherapy exposure in pregnancy, multiple agents used simultaneously, varying teratogenic potential, other teratogenic exposures (chemical, environmental, medication), data limited to retrospective cases, and pharmacokinetic changes that occur during pregnancy. Our review focuses on CRC antineoplastics and the challenges that are present when their use is considered during pregnancy.
Colorectal Cancer in the Young Adult Population
As mentioned previously, CRC during pregnancy may become more of a reality because of delayed child-bearing. Adding to this potential is the increase of young adults diagnosed with CRC. CRC is the third most common cancer diagnosed among women in the U.S. [32]. In women, it represents 8% of all new cancer cases, with 63,610 estimated new CRC cases expected in 2015. CRC is a malignancy that occurs in people at an older age; the median age of diagnosis is 68 years [33]. Although most CRC cases are diagnosed beyond age 45 years, 5.4% are diagnosed in patients younger than 45 years. Recent Surveillance, Epidemiology, and End Results (SEER) database reviews have revealed an increase in younger CRC patients [34–36]. Recently, a SEER database analysis from 1975 to 2010 found the overall age-adjusted CRC incidence rate has decreased; however, incidence has increased in patients age 20–49 years, with an approximate 2% annual increase in patients age 20–34 years [36]. In persons 20–34 years of age, the 2030 incidence rates for colon and rectal cancer are predicted to increase by 90% and 124.2%, respectively. In persons age 35–49 years, these rates are expected to increase by 27.7% for colon cancer and 46% for rectal cancer.
Unknown factors exist because of limited chemotherapy exposure in pregnancy, multiple agents used simultaneously, varying teratogenic potential, other teratogenic exposures (chemical, environmental, medication), data limited to retrospective cases, and pharmacokinetic changes that occur during pregnancy.
Overview of Pregnancy and Chemotherapy/Targeted Therapy
Drug Exposure During Pregnancy
Drug exposure to the fetus during pregnancy and teratogenic effects depend on medication characteristics that allow for placental transfer, the timing of gestation, dosing, administration route, and maternal drug metabolism. Most drugs cross the placenta by passive diffusion [6, 37–40]. Unionized, low-molecular-weight, low-protein-bound, and highly lipophilic agents will cross the placenta more readily [1, 38–40]. Drugs with a molecular weight of less than 500–600 cross the placenta, whereas those with molecular weights greater than 1,000 are reported to cross poorly [6, 11, 38, 39]. Most traditional antineoplastics have a weight below 400, resulting in potential chemotherapy exposure to the fetus [11]. Larger molecules, such as IgG, require specific receptor-mediated active transfer across the placenta [7, 8, 41, 42].
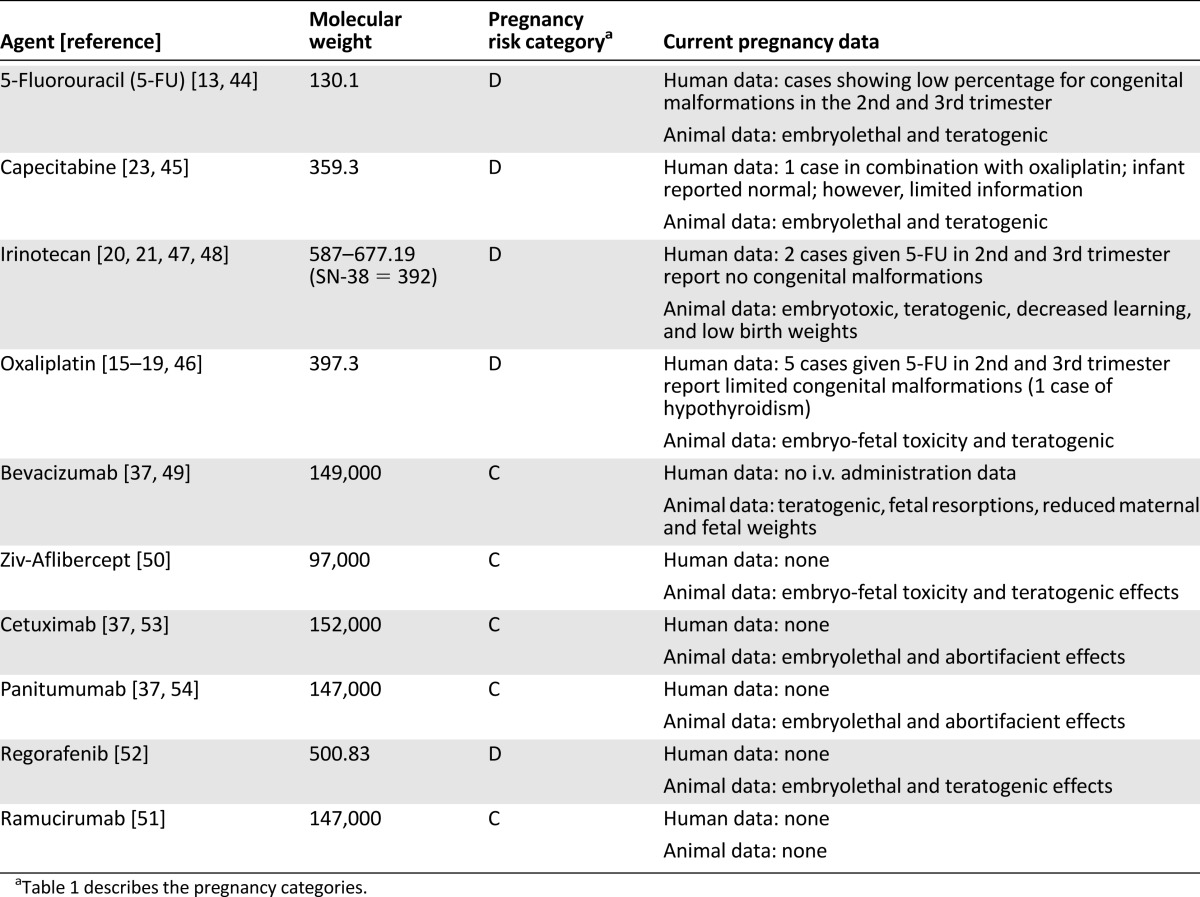
The U.S. Food and Drug Administration (FDA) has historically defined and classified medications into risk categories on the basis of known or potential effects on the fetus (Table 1) [43]. CRC antineoplastics are listed as category C and D because of a lack of human evidence, potential, or known harm [37, 44–54]. The FDA has recently put forth an initiative to transition from using these categories because of their simplicity [55]. Medication prescribing information will soon report an alternative and more descriptive method for pregnancy, lactation, and fertility statements. To date, however, these categories are still in place as this classification transitions into current practice with approved medications; therefore, practitioners must use caution and consider the limitations with this classification. Individual characteristics for CRC agents are summarized in Table 2 [13, 15–23, 37, 44–54].
Table 1.
Food and Drug Administration pregnancy risk categories
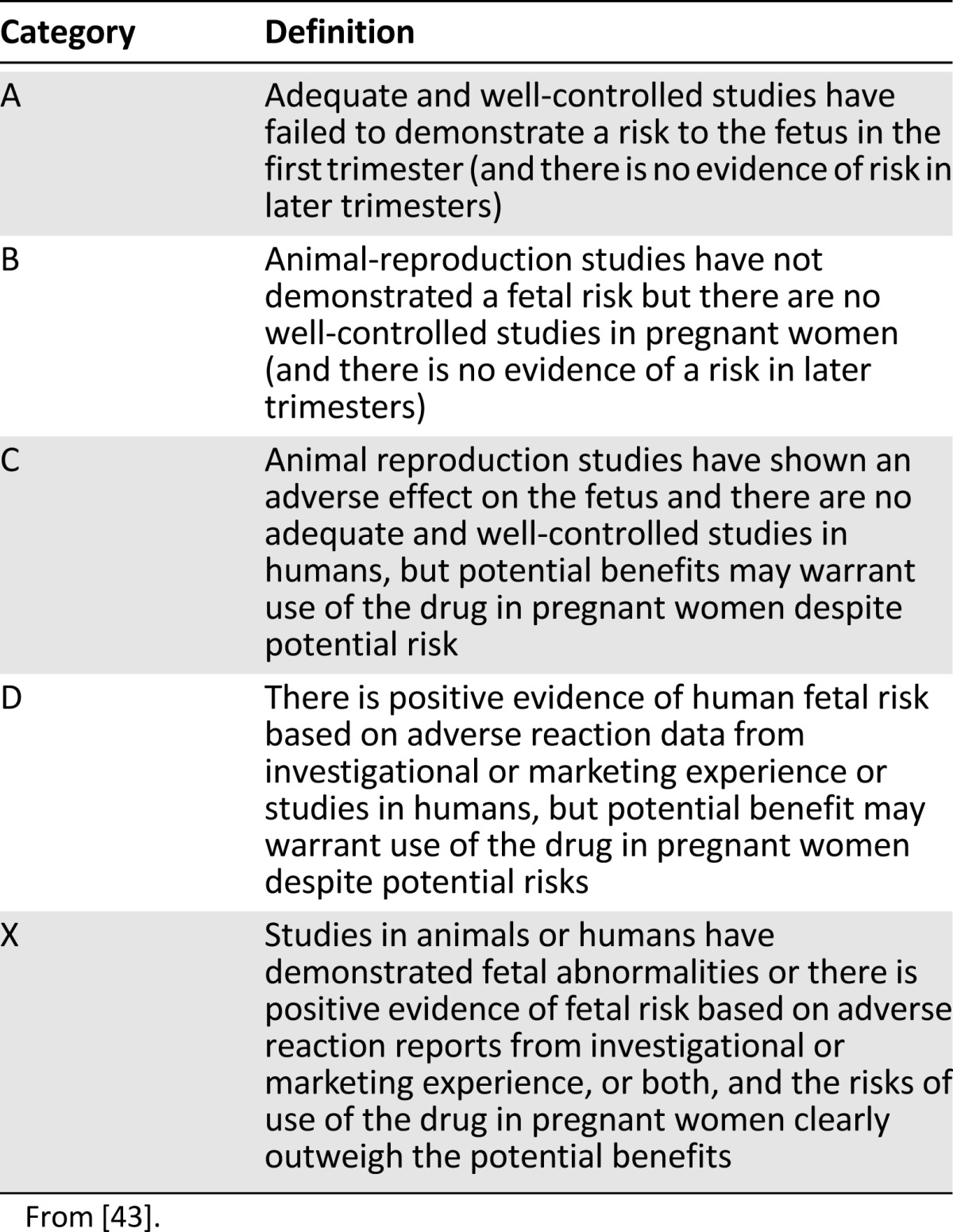
Table 2.
Characteristics of colorectal cancer therapies
Pharmacokinetic changes during pregnancy may affect chemotherapy metabolism and exposure [1, 39, 56–61]. During pregnancy, the volume of distribution increases, protein binding decreases, hepatic clearance changes, and renal elimination increases [1, 6, 56–58]. Medications that are excreted unchanged by the kidneys can be eliminated more rapidly because of an approximate 50% increase in creatinine clearance, with increases seen as early as 9–14 weeks’ gestation and peaking particularly in the second trimester [1, 39, 56, 60, 61]. A decrease in albumin allows for more unbound free drug; this is particularly concerning for those highly protein-bound antineoplastics, such as oxaliplatin, which is greater than 90% protein bound [39, 46, 56, 57, 61]. Hepatic alterations include increase in activity of certain hepatic enzymes, such as UGT, CYP3A4, CYP2C9, and CYP2A6, which can lead to changes in drug metabolism [56, 57, 61–63]. Irinotecan has a complex metabolism involving both conversions to its active metabolite (SN38) by carboxylesterase and to inactive metabolites via CYP3A4 [47]. SN38 is conjugated by UGT1A1 to SN38G, a much less active form. Irinotecan may follow an increased drug clearance pattern during pregnancy, similar to that of labetalol, that has increased clearance correlated to UGT1A1 upregulation [57, 62, 63]. Limited chemotherapy pharmacokinetic evaluations during pregnancy and the physiologic changes that occur throughout pregnancy make it difficult to extrapolate these alterations in determining dose, metabolism, and clearance.
Timing of Drug Administration
Major congenital malformations are reported at 3%–4% of the general population; approximately 2%–3% of these are correlated to drug administration [9, 13, 38–40, 64]. Congenital malformations rates related to chemotherapy administration inversely relate to gestational age. These malformations have been reported at 10%–20% in the first trimester, 8% in the second trimester, and 6% in the third trimester [2, 4, 11, 42]. Selig et al. found adverse pregnancy outcomes with chemotherapy to be 33%, 27%, and 25% with the first, second, and third trimester, respectively [4]. In this review, adverse pregnancy outcomes included congenital malformations, stillbirths, spontaneous abortions, functional defects, and blood or electrolyte abnormalities.
As evidenced by these citations, the most concerning time period for physical malformation from chemotherapy exposure is the first trimester [1, 2, 4, 9, 13]. During this stage of development, the embryo undergoes organogenesis at approximately weeks 2–8 after conception [1, 2, 9, 11, 38, 65]. Malformations correlate with the organ differentiation during the gestational time period with the neural tube, heart, limbs, and lips developing earlier followed by the ears and palate [1, 9, 65]. After organogenesis, the fetus continues to be vulnerable because of continued maturation of the eyes, teeth, ears, palate, genitalia, hematopoietic system, and central nervous system with growth and functional maturation continuing until term. Intrauterine growth restrictions, low birthweight, premature delivery, functional defects, and adverse effects of antineoplastics on the mother, such as myelosuppression, are risks to the fetus during the second and third trimesters [1, 8–11, 65]. Because of potential adverse effects, chemotherapy administration is not recommended 3 weeks before the expected delivery or beyond 35 weeks of gestational age; possible complications include myelosuppression, bleeding, and death during delivery [1, 2, 5, 8, 10, 11, 13]. Effects related to function, intellect, and behavior are difficult to correlate to a specific cause because they generally are not present at the time of birth and may present several years after the exposure [6, 11, 37, 40].
Recent reviews on monoclonal antibodies in pregnancy suggest an opposing time frame risk for administration [7, 8, 37, 41, 42]. Monoclonal antibodies are large hydrophilic compounds with molecular weights that well exceed placental diffusion transfer. To reach the fetus, active transport is required. During the first trimester, transfer of IgG is minimal; however, fetal IgG concentration begins to rise in the second trimester, indicating active transport across the placenta [7, 37, 41, 42]. Trastuzumab is an IgG subclass monoclonal antibody targeting human epidermal growth factor receptor 2. Trastuzumab in pregnancy develops in oligohydramnios, increasing the risk for preterm delivery and fetal mortality, particularly when given after the first trimester [7, 8, 13, 41, 42, 65]. Cetuximab, panitumumab, bevacizumab, ramucirumab, and ziv-aflibercept are IgG subclass monoclonal antibodies or fusion proteins and may follow this same fetal exposure pattern [7, 37, 49–51, 53, 54].
CRC Therapies and Pregnancy
Chemotherapy
The fluoropyrimidines, 5-fluorouracil (5-FU) and capecitabine, are the backbone for CRC treatment when used as monotherapy or in combination with additional chemotherapy, with or without targeted therapy. Both fluoropyrimidines have low molecular weights, and 5-FU has negligible protein binding; capecitabine, a prodrug to 5-FU, is moderately protein-bound (35% bound to albumin) [44, 45]. Drug exposure would likely be high given these drug properties, and animal studies in mice have demonstrated placental transfer [13, 37, 44, 45].
Many cases of human 5-FU exposure in pregnancy exist. According to the 2013 National Toxicology Program (NTP) monograph regarding chemotherapy and pregnancy, 178 cases with 5-FU have been reported [13]. A majority of these cases occurred in breast cancer, and in most (172 cases) the drug was given with additional therapy. Seventeen pregnant women received 5-FU in the first trimester; 161 did so in the second and third trimesters. The NTP monograph reports 4 cases out of 13 (31%) with major malformations when 5-FU was given in the first trimester; only 2 of 161 (1.2%) cases occurred in the second or third trimester. Malformations reported in the first trimester included microcephaly, low-set ears, hypertelorism, a right palmar simian crease, ventriculomegaly, colpocephaly, and skeletal deformities. These cases were coexposed to additional chemotherapy (methotrexate, cyclophosphamide, doxorubicin) or other teratogenic exposures (radiation or radiographic imaging). Clubfoot and hemihypertrophy of the lower extremity were malformations occurring in the second- and third-trimester group.
A follow-up of the children of 81 patients with breast cancer who had been given bolus 5-FU in combination with doxorubicin and cyclophosphamide (FAC) in the second and third trimester has been recently published; most patients (86%) had received >4 cycles of FAC [64]. Seventy-eight percent were available to answer the follow-up survey, and 3 cases of congenital malformations were reported. These abnormalities were Down syndrome, clubfoot, and ureteral reflux. The children were a median of 7 years (range, <1–21 years). Fifty patients responding to the survey answered questions about postneonatal outcomes; 98% considered the child healthy. Reported health concerns included developmental milestone delays, difficulties in school, asthma, vision, heart murmur, lazy eye, absence seizures, ear/nose/throat conditions, and gastroesophageal reflux disease; however, the authors correlated these percentages to the population prevalence. The rates of allergies and eczema were higher than those in the population, but the authors attributed this to over-reporting.
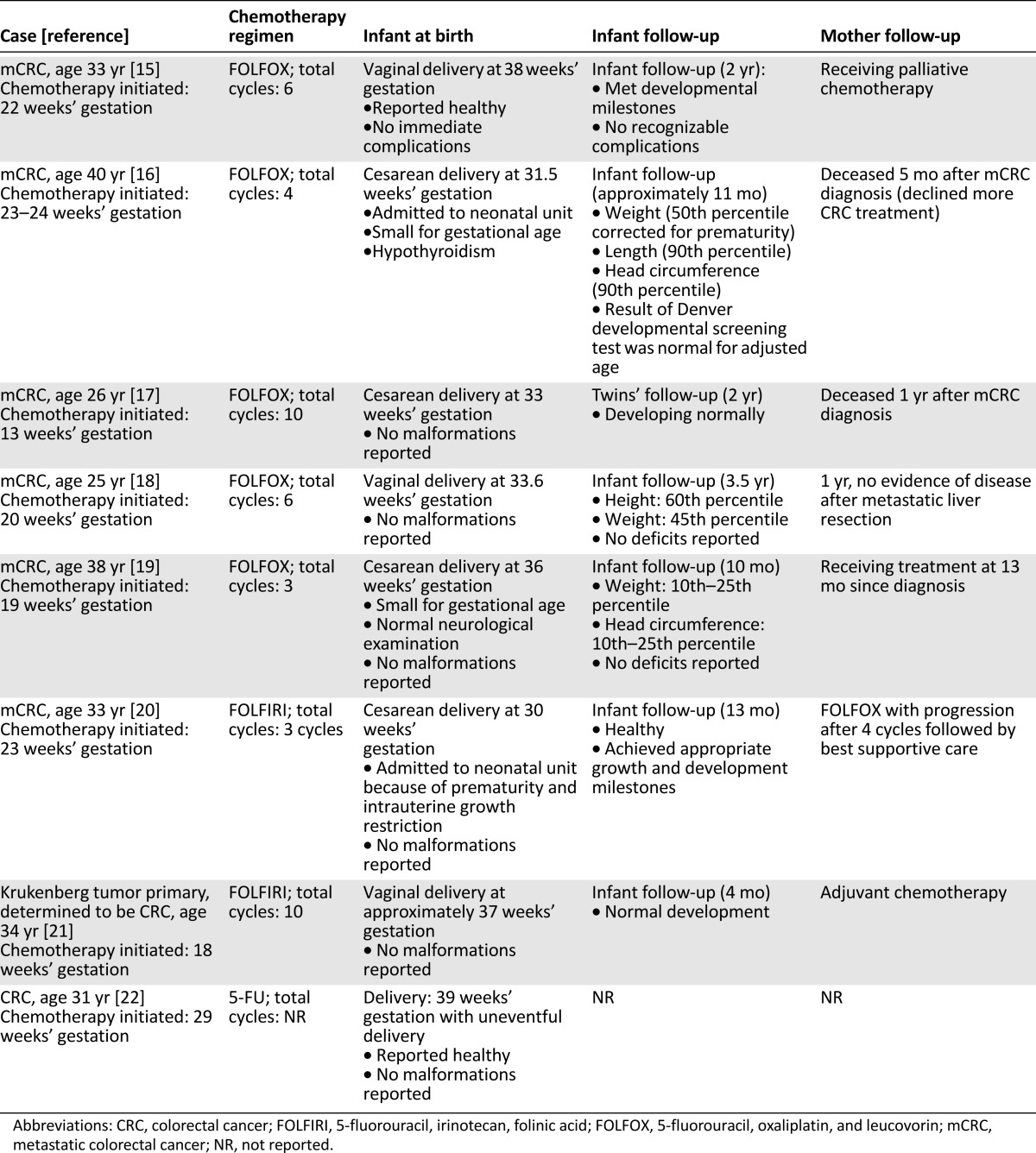
A concern with extrapolation from these cases is the route of administration; bolus dosing is used with FAC, whereas 5-FU in CRC is given as both a bolus and continuous infusion. No congenital abnormalities, except for hypothyroidism, were found in 8 pregnant women with CRC who received 5-FU alone or in combination (with oxaliplatin or irinotecan) after the first trimester (Table 3) [15–22]. Capecitabine was given in one case of CRC during pregnancy, along with oxaliplatin, in the first trimester [13, 23]. The authors report that the patient was exposed during the first trimester, but once the pregnancy was discovered treatment was stopped [23]. Infant follow-up at 2 years was reported as normal; however, there is limited information regarding the amount of exposure, the delivery, and infant follow-up.
Table 3.
Cases of CRC in pregnancy treated with chemotherapy
5-FU given in the second or third trimester appears to present a low concern for congenital malformations and has long-term follow-up in the bolus-dosing setting. Consideration for dihydropyrimidine dehydrogenase testing before administration should be considered for this population because significant adverse effects with 5-FU can occur when deficient in this enzyme. Literature regarding capecitabine is sparse; therefore, if a fluoropyrimidine is needed, 5-FU should be used.
Oxaliplatin, a third-generation platinum agent, is used in locally advanced and metastatic colorectal cancer (mCRC) in combination with a fluoropyrimidine, with or without targeted therapy. Oxaliplatin’s molecular weight is less than 400, indicating exposure to the fetus by placental transfer [46]. It is highly protein-bound (>90%); therefore, with lower albumin levels seen in pregnancy, this may indicate more free active drug. It is approximately 50% eliminated by the kidney and may have a higher clearance in pregnancy with standard dosing. Studies in pregnant rats given oxaliplatin from day 6 to 16 showed early resorptions, decreased fetal weight, and delayed ossification.
Seven humans receiving oxaliplatin in pregnancy have been described [15–19, 23, 66]. In the five CRC patients administered 5-FU, oxaliplatin, and leucovorin (FOLFOX), treatment was given after the first trimester (initiated at 13–23 weeks’ gestation) (Table 3) [15–19]. In one infant, hypothyroidism was reported, but none of the remaining infants had congenital malformations reported at birth. Two infants were described as small for gestational age (born at 31 and 36 weeks’ gestation, respectively) [16, 19]. No developmental deficits have been reported in the follow-up of these infants exposed to FOLFOX; however, height and weight for some of the children remain in a lower percentile [16, 18, 19]. One infant with congenital malformations, including a cleft lip, cleft palate, and esophageal atresia with a tracheoesophageal fistula, was exposed in the first trimester to a combination of vinorelbine, irinotecan, and oxaliplatin [13]. It is difficult to determine the exact agent that caused these malformations or whether the cause was the combination, the first-trimester administration, or genetic predisposition. Infants born without congenital malformations (with the exception of hypothyroidism) have been reported after administration of FOLFOX after the first trimester [15–19]. Although limited to six exposed infants, healthy infant follow-up has been reported, with the exception of lower growth percentiles in some children [16, 18, 19].
Irinotecan, a topoisomerase I inhibitor, is used in mCRC alone or in combination with targeted therapy with or without additional chemotherapy. Irinotecan, as mentioned previously, has a complex metabolism. Irinotecan is converted to SN38 (irinotecan’s active metabolite, which is responsible for most antitumor activity), and irinotecan is converted inactively via CYP3A4 [47]. SN38 elimination is through conjugation via the UGT1A1 enzyme. Pregnancy enzyme increases in CYP3A4, and UGT may lead to an increased conversion to inactive metabolites; this makes dosing and exposure variable [56, 57, 62, 63]. Irinotecan is moderately protein-bound (30%–68%), whereas SN38 is highly protein-bound (95%); therefore, more unbound SN38 is a potential concern [37, 47]. Both molecular weights are within the range that allows placental transfer [47, 48].
Human pregnancy exposure to irinotecan is limited to two cases in addition to the preceding case described in combination with oxaliplatin and vinorelbine [20, 21]. In both cases, irinotecan was combined with 5-FU and leucovorin and therapy was initiated after the first trimester (at 18 and 23 weeks’ gestation). No congenital malformations were reported; however, in one case intrauterine growth restriction at birth was reported. Although infant follow-up was not long-term, the authors reported healthy infants in both cases.
Animal cases are not as favorable. In rats and rabbits during organogenesis, a range of i.v. doses showed embryolethal and structural defects [37, 47]. Perhaps one reason for these differences in animal and human experience is the timing of administration; in the animals, the doses were given during organogenesis. However, in rats a dose given after organogenesis showed decreased learning ability and decreased female body weights. Effects of irinotecan and SN38 exposure to the human fetus are not known; however, given their pharmacokinetic properties, exposure is likely. Because there are only two human pregnancy cases, it would be premature to use this as a basis for recommending irinotecan use in pregnancy. If irinotecan must be used, practitioners should consider testing for UGT1A1 homozygous allele before administration because of increased neutropenia related to more exposure seen at standard dosing. If the patient is determined to be deficient in this enzyme or to have Gilbert’s disease, doses should be reduced.
If 5-FU, oxaliplatin, and irinotecan are used during pregnancy (after a thorough discussion with the family and after the patient provides consent), these agents should be given only in the second or third trimester; they should be held 3 weeks before delivery or at 35 weeks’ gestation, as recommended for other antineoplastics [1, 2, 5, 8, 10, 11, 13].
Angiogenesis Inhibitors
The complex placental vasculature is critical for fetal development and survival because it is responsible for the maternal-fetal blood supply, gas exchange, and nutrient supply [67, 68]. The vascular network requires vasculogenesis and angiogenesis, which begins 18–21 days after conception and continues through the gestational period. Vascular endothelial growth factor (VEGF) is involved in both processes. VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor are among the regulators of angiogenesis [42, 67, 68]. Inhibition and disruption of key regulators involved in this vascular network can produce pregnancy complications, including preeclampsia; intrauterine growth restriction; stillbirth; preterm delivery; miscarriage; and abnormal development of the heart, blood vessels, forelimbs, cranial region, and forebrain [42, 51, 52, 67, 68]. Thalidomide, an antineoplastic used primarily in multiple myeloma, is predominately known for its teratogenic effects and initial removal from the market in the early 1960s [7, 8]. Use by pregnant women for morning sickness resulted in an estimated 10,000 children having severe limb malformations [7, 8, 39]. Thalidomide’s mechanism of action is not fully elucidated; however, it is considered an immunomodulatory and antiangiogenic agent, and as a result agents with similar properties are particularly concerning [7].
Bevacizumab, ziv-aflibercept, ramucirumab, and regorafenib are angiogenesis inhibitors used in the mCRC setting. Bevacizumab, ziv-aflibercept, and ramucirumab are used in combination with chemotherapy, whereas regorafenib is currently approved as monotherapy. Bevacizumab, a humanized monoclonal antibody, targets VEGF-A ligand to inhibit angiogenesis [49]. There is no experience with intravenous bevacizumab administration in human pregnancy [7, 37, 41, 42, 49]. Data are limited to intravitreal administration cases [7, 41, 42]. Although normal fetal outcomes have been reported in some of these cases, outcomes cannot be extrapolated to the mCRC setting because of higher intravenous doses and systemic exposure (mCRC dosing is 5 mg/kg every 2 weeks compared with an intravitreal 1.25-mg flat dose) [41, 49]. Studies in rabbits revealed teratogenic effects with adverse fetal outcomes, including reduced maternal and fetal body weights and increased incidence of fetal skeletal alterations [37, 49]. Ziv-aflibercept is an IgG fusion protein that binds VEGF-A, VEGF-B, and placental growth factors 1 and 2, preventing their receptor binding [50]. As seen with bevacizumab, ziv-aflibercept experience is limited to animal studies. A rabbit model of ziv-aflibercept demonstrated external, visceral, and skeletal malformations. Ramucirumab, a fully human monoclonal antibody, blocks ligand stimulation of VEGF-A, VEGF-C, and VEGF-D by binding to VEGF receptor 2 [51]. To date, no human or animal studies have been reported with ramucirumab; however, effects would be expected to occur equally to those of other anti-VEGF class agents. Bevacizumab, ziv-aflibercept, and ramucirumab have high molecular weights, and transfer to the fetus is unknown; however, they are IgG monoclonal antibodies or fusion proteins and therefore exposure to the fetus may follow a process similar to that seen with IgG [7, 37, 41, 42, 50, 51].
Regorafenib, an oral multikinase inhibitor, targets VEGF-1, VEGF-2, VEGF-3, and platelet-derived growth factor, among other targets [52]. Regorafenib is highly protein-bound (>99%) and is metabolized by CYP3A4; thus, it may have pharmacokinetic alterations in pregnancy. It has a molecular weight of 500, making it a compound likely to cross the placenta. Rat and rabbit studies have shown cardiovascular, genitourinary, and skeletal malformations. Angiogenesis inhibitors should be considered contraindicated in pregnant CRC patients because of the significant lack of human experience, negative outcomes in animals, theoretical fetal exposure, and their high-risk angiogenesis target. Given the recent FDA approval of these agents, the literature lacks reports on their use in pregnant patients.
Epidermal Growth Factor Receptor Inhibitors
Cetuximab and panitumumab are epidermal growth receptor (EGFR) monoclonal antibodies, chimeric and fully human, respectively. They are used in combination with chemotherapy or as monotherapy for wild-type Kristen rat sarcoma viral oncogene (KRAS) mCRC. They are large molecules similar to other monoclonal antibodies; however, both may cross the placenta, as suggested with other IgG monoclonal antibodies [7, 37, 41, 42]. As with many targeted therapy agents, there is no human experience with these agents in pregnancy, and data are limited to animal studies. Cetuximab and panitumumab were given to monkeys during organogenesis, and in both evaluations embryolethal and abortifacient effects increased [37, 53, 54]. Two case reports in human pregnancy using erlotinib, a selective EGFR tyrosine kinase inhibitor, have been reported. In both of these cases, each patient became pregnant while taking erlotinib [69, 70]. One patient stopped therapy when the pregnancy was discovered (approximately 2 months’ gestation), and the other continued therapy during the pregnancy. The authors reported no congenital malformations in the infants. Two cases, however, cannot be extrapolated to a broader population or to the use of cetuximab and panitumumab in pregnancy. EGFR plays a role in placental development, embryonic growth, and endometrial function during early pregnancy [42, 53, 54, 71, 72]. Cetuximab and panitumumab should be avoided in pregnancy given the mechanism of inhibition on EGFR, a player in fetal growth and maintenance of pregnancy, teratogenic animal effects, and no human exposure. Furthermore, these agents are known to cause magnesium wasting, which may directly affect the fetus.
Cetuximab and panitumumab should be avoided in pregnancy given the mechanism of inhibition on EGFR, a player in fetal growth and maintenance of pregnancy, teratogenic animal effects, and no human exposure. Furthermore, these agents are known to cause magnesium wasting, which may directly affect the fetus.
Conclusion
CRC is accepted as one of the most common malignancies globally, but the rising incidence in young patients suggests the challenges that may lie ahead for a patient who is pregnant. CRC in young adults is aggressive [73]; therefore, postponing treatment until full pregnancy term in a pregnant CRC patient is unlikely to be feasible. The purpose of this review was to provide guidance to clinicians faced with difficult decisions when pregnancy arises in a patient with locally advanced or metastatic CRC. Because of organogenesis, chemotherapy should be avoided during the first trimester, as this is the most concerning time period for congenital malformations; chemotherapy administration should be reserved for the second and third trimesters. Patients and families should receive thorough counseling before any chemotherapy administration, including the consideration of fertility counseling. CRC monoclonal antibodies and multikinase inhibitors should be avoided in pregnancy because of a lack of human evidence and concerns regarding mechanism of action. Follow-up of infants who were exposed to chemotherapy in utero, particularly long-term follow-up regarding cognitive dysfunction, is an area that needs to be strengthened through reporting. Therefore, we stress the importance of reporting outcomes in all pregnant patients exposed to chemotherapy in order to obtain more information to make sound decisions and provide future guidance to other physicians.
Author Contributions
Conception/Design: Jane E. Rogers, Arvind Dasari, Cathy Eng
Manuscript writing: Jane E. Rogers, Arvind Dasari, Cathy Eng
Final approval of manuscript: Jane E. Rogers, Arvind Dasari, Cathy Eng
Disclosures
Cathy Eng: Roche, Bayer (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5:283–291. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]
- 2.Salani R, Billingsley CC, Crafton SM. Cancer and pregnancy: an overview for obstetricians and gynecologists. Am J Obstet Gynecol. 2014;211:7–14. doi: 10.1016/j.ajog.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Pavlidis N. Cancer and pregnancy: What should we know about the management with systemic treatment of pregnant women with cancer? Eur J Cancer. 2011;47(suppl 3):S348–S352. doi: 10.1016/S0959-8049(11)70199-X. [DOI] [PubMed] [Google Scholar]
- 4.Selig BP, Furr JR, Huey RW, et al. Cancer chemotherapeutic agents as human teratogens. Birth Defects Res A Clin Mol Teratol. 2012;94:626–650. doi: 10.1002/bdra.23063. [DOI] [PubMed] [Google Scholar]
- 5.Amant F, Han SN, Gziri MM, et al. Chemotherapy during pregnancy. Curr Opin Oncol. 2012;24:580–586. doi: 10.1097/CCO.0b013e328354e754. [DOI] [PubMed] [Google Scholar]
- 6.Van Calsteren K. Chemotherapy during pregnancy: pharmacokinetics and impact on foetal neurological development. Facts Views Vis ObGyn. 2010;2:278–286. [PMC free article] [PubMed] [Google Scholar]
- 7.Azim HA, Jr, Azim H, Peccatori FA. Treatment of cancer during pregnancy with monoclonal antibodies: A real challenge. Expert Rev Clin Immunol. 2010;6:821–826. doi: 10.1586/eci.10.77. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JM, Weber Schöndorfer C. Antineoplastic drugs. In: Schaefer C, Peters PWJ, Miller RK, editors. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 3rd ed. Amsterdam: Academic Press; 2015. pp. 374–399. [Google Scholar]
- 9.Koren G, Carey N, Gagnon R, et al. Cancer chemotherapy and pregnancy. J Obstet Gynaecol Can. 2013;35:263–280. doi: 10.1016/S1701-2163(15)30999-3. [DOI] [PubMed] [Google Scholar]
- 10.Pentheroudakis G, Orecchia R, Hoekstra HJ, et al. Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v266–v273. doi: 10.1093/annonc/mdq198. [DOI] [PubMed] [Google Scholar]
- 11.Surbone A, Peccatori F, Pavlidis N. Cancer and Pregnancy. Vol 178. New York, Berlin: Springer; 2007. [Google Scholar]
- 12.Lynch CD, Lee MJ, Priore GD. Chemotherapy and pregnancy. In: Mattison D, editor. Clinical Pharmacology During Pregnancy. London: Academic Press; 2013. pp. 201–215. [Google Scholar]
- 13.National Toxicology Program NTP Monograph: development effects and pregnancy outcomes associated with cancer chemotherapy use during pregnancy. NTP Monogr. 2013;(2):i-214. [PubMed] [Google Scholar]
- 14.Pavlidis NA. Coexistence of pregnancy and malignancy. The Oncologist. 2002;7:279–287. [PubMed] [Google Scholar]
- 15.Makoshi Z, Perrott C, Al-Khatani K, et al. Chemotherapeutic treatment of colorectal cancer in pregnancy: Case report. J Med Case Reports. 2015;9:140. doi: 10.1186/s13256-015-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanate AS, Auber ML, Higa GM. Priorities and uncertainties of administering chemotherapy in a pregnant woman with newly diagnosed colorectal cancer. J Oncol Pharm Pract. 2009;15:5–8. doi: 10.1177/1078155208094101. [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen JB, Østerlind K. Successful twin pregnancy outcome after in utero exposure to FOLFOX for metastatic colon cancer: A case report and review of the literature. Clin Colorectal Cancer. 2011;10:348–352. doi: 10.1016/j.clcc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Gensheimer M, Jones CA, Graves CR, et al. Administration of oxaliplatin to a pregnant woman with rectal cancer. Cancer Chemother Pharmacol. 2009;63:371–373. doi: 10.1007/s00280-008-0731-9. [DOI] [PubMed] [Google Scholar]
- 19.Dogan NU, Tastekin D, Kerimoglu OS, et al. Rectal cancer in pregnancy: A case report and review of the literature. J Gastrointest Cancer. 2013;44:354–356. doi: 10.1007/s12029-012-9463-5. [DOI] [PubMed] [Google Scholar]
- 20.Cirillo M, Musola M, Cassandrini PA, et al. Irinotecan during pregnancy in metastatic colon cancer. Tumori. 2012;98:155e–157e. doi: 10.1700/1217.13511. [DOI] [PubMed] [Google Scholar]
- 21.Taylor J, Amanze A, Di Federico E, et al. Irinotecan use during pregnancy. Obstet Gynecol. 2009;114:451–452. doi: 10.1097/AOG.0b013e3181a1d478. [DOI] [PubMed] [Google Scholar]
- 22.Kraljević M, Hoffmann H, Knipprath A, et al. Obstructing adenocarcinoma of the descending colon in a 31-year-old pregnant woman. Int J Surg Case Rep. 2014;5:958–960. doi: 10.1016/j.ijscr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: Results of an international registry. Am J Clin Oncol. 2010;33:221–228. doi: 10.1097/COC.0b013e3181a44ca9. [DOI] [PubMed] [Google Scholar]
- 24.Toosi M, Moaddabshoar L, Malek-Hosseini SA, et al. Rectal cancer in pregnancy: A diagnostic and therapeutic challenge. J Egypt Natl Canc Inst. 2014;26:175–179. doi: 10.1016/j.jnci.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Virgilio E, Costa G, Fransvea P, et al. Colorectal cancer in pregnancy: One disease, two patients. ANZ J Surg. 2013;83:595. doi: 10.1111/ans.12208. [DOI] [PubMed] [Google Scholar]
- 26.Khodaverdi S, Kord Valeshabad A, Khodaverdi M. A case of colorectal cancer during pregnancy: A brief review of the literature. Case Rep Ostet Gynecol. 2013;2013:Article ID 626393. doi: 10.1155/2013/626393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolusari A, Ugurluer G, Kotan C, et al. Rectal cancer and pregnancy: Report of two cases. Eur J Gynaecol Oncol. 2009;30:100–102. [PubMed] [Google Scholar]
- 28.Mechery J, Ikhena SE. Cancer of the descending colon during pregnancy. J Obstet Gynaecol. 2007;27:311–312. doi: 10.1080/01443610701241159. [DOI] [PubMed] [Google Scholar]
- 29.Lolis ED, Likoudis P, Voiniadis P, et al. Synchronous rectal and colon cancer caused by familial polyposis coli during pregnancy. J Obstet Gynaecol Res. 2007;33:199–202. doi: 10.1111/j.1447-0756.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 30.Minter A, Malik R, Ledbetter L, et al. Colon cancer in pregnancy. Cancer Contr. 2005;12:196–202. doi: 10.1177/107327480501200310. [DOI] [PubMed] [Google Scholar]
- 31.Vitoratos N, Salamalekis E, Makrakis E, et al. Sigmoid colon cancer during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002;104:70–72. doi: 10.1016/s0301-2115(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 32.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Colon and rectum cancer. Available at http://seer.cancer.gov/statfacts/html/colorect.html. Accessed May 31, 2015.
- 34.Hubbard JM, Grothey A. Adolescent and young adult colorectal cancer. J Natl Compr Canc Netw. 2013;11:1219–1225. doi: 10.6004/jnccn.2013.0144. [DOI] [PubMed] [Google Scholar]
- 35.Inra JA, Syngal S. Colorectal cancer in young adults. Dig Dis Sci. 2015;60:722–733. doi: 10.1007/s10620-014-3464-0. [DOI] [PubMed] [Google Scholar]
- 36.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briggs GG, Freeman RK, Yaffe SJ, et al. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 8th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 38.Sachdeva P, Patel BG, Patel BK. Drug use in pregnancy; a point to ponder! Indian J Pharm Sci. 2009;71:1–7. doi: 10.4103/0250-474X.51941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters P, Miller RK, Schaefer C. General commentary on drug therapy and drug risks in pregnancy. In: Schaefer C, Peters PWJ, Miller RK, editors. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 3rd ed. Amsterdam: Academic Press; 2015. pp. 1–23. [Google Scholar]
- 40.Leslie KK, Koil C, Rayburn WF. Chemotherapeutic drugs in pregnancy. Obstet Gynecol Clin North Am. 2005;32:627–640. doi: 10.1016/j.ogc.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Sarno MA, Mancari R, Azim HA, Jr., et al. Are monoclonal antibodies a safe treatment for cancer during pregnancy? Immunotherapy. 2013;5:733–741. doi: 10.2217/imt.13.64. [DOI] [PubMed] [Google Scholar]
- 42.Lambertini M, Peccatori FA, Azim HA., Jr Targeted agents for cancer treatment during pregnancy. Cancer Treat Rev. 2015;41:301–309. doi: 10.1016/j.ctrv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Regist 2008;73:30831–30868. [PubMed]
- 44.Adrucil (fluorouracil injection). Teva Parenteral Medicines, Inc. 2012. Available at http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0794add-67a7-4308-93e9-f889472716cc. Accessed May 31, 2015.
- 45.Xeloda (capecitabine). Genentech, Inc. 2015. Available at http://www.gene.com/download/pdf/xeloda_prescribing.pdf. Accessed August 15, 2015.
- 46.Eloxatin (oxaliplatin). Sanofi-Aventis. 2011. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021759s012lbl.pdf. Accessed August 17, 2015.
- 47.Camptosar (irinotecan). Pharmacia and Upjohn Co. 2014. Available at http://labeling.pfizer.com/ShowLabeling.aspx?id=533. Accessed May 31, 2015.
- 48.Sai K, Kaniwa N, Ozawa S, et al. A new metabolite of irinotecan in which formation is mediated by human hepatic cytochrome P-450 3A4. Drug Metab Dispos. 2001;29:1505–1513. [PubMed] [Google Scholar]
- 49.Avastin (bevacizumab). Genentech, Inc. 2009. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0169lbl.pdf. Accessed May 31, 2015.
- 50.Zaltrap (ziv-aflibercept). Sanofi-aventis. 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125418s000lbl.pdf. Accessed August 17, 2015.
- 51.Cyramza (ramucirumab). Eli Lilly and Co. 2015. Available at http://pi.lilly.com/us/cyramza-pi.pdf. Accessed August 17, 2015.
- 52.Stivarga (regorafenib). Bayer HealthCare Pharmaceuticals Inc. 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203085lbl.pdf. Accessed August 17, 2015.
- 53.Erbitux (cetuximab). Bristol-Myers Squibb Co. 2015. Available at http://pi.lilly.com/us/erbitux-uspi.pdf. Accessed August 17, 2015.
- 54.Vectibix (panitumumab). Amgen Inc. 2015. Available at http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf. Accessed August 17, 2015.
- 55.Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Regist 2014;79:72063–72103. [PubMed]
- 56.Matsui DM. Therapeutic drug monitoring in pregnancy. Ther Drug Monit. 2012;34:507–511. doi: 10.1097/FTD.0b013e318261c372. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Hebert MF, Venkataramanan R. Basic obstetric pharmacology. Semin Perinatol. 2014;38:475–486. doi: 10.1053/j.semperi.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koren G. Pharmacokinetics in pregnancy; clinical significance. J Popul Ther Clin Pharmacol. 2011;18:e523–e527. [PubMed] [Google Scholar]
- 60.Pacheco LD, Costantine MM, Hankins GDV. Physiologic changes during pregnancy. In: Mattison DR, editor. Clinical Pharmacology During Pregnancy. Amsterdam, Boston: Academic Press; 2013. pp. 5–16. [Google Scholar]
- 61.Hebert MF. Impact of pregnancy on maternal pharmacokinetics of medications. In: Mattison DR, editor. Clinical Pharmacology During Pregnancy. Amsterdam, Boston: Academic Press; 2013. pp. 17–39. [Google Scholar]
- 62.Jeong H, Choi S, Song JW, et al. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38:62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeong H. Altered drug metabolism during pregnancy: Hormonal regulation of drug-metabolizing enzymes. Expert Opin Drug Metab Toxicol. 2010;6:689–699. doi: 10.1517/17425251003677755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murthy RK, Theriault RL, Barnett CM, et al. Outcomes of children exposed in utero to chemotherapy for breast cancer. Breast Cancer Res. 2014;16:500. doi: 10.1186/s13058-014-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynch CD, Lee MJ, Del Priore G. Chemotherapy in pregnancy. In: Mattison DR, editor. Clinical Pharmacology During Pregnancy. Amsterdam, Boston: Academic Press; 2013. pp. 201–215. [Google Scholar]
- 66.National Toxicology Program. Appendices for NTP Monograph: developmental effects and pregnancy outcomes associated with cancer chemotherapy use during pregnancy. 2013. Available at https://ntp.niehs.nih.gov/pubhealth/hat/noms/chemo/index.html. Accessed August 17, 2015. [PubMed]
- 67.Zygmunt M, Herr F, Münstedt K, et al. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110(suppl 1):S10–S18. doi: 10.1016/s0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 68.Pereira RD, De Long NE, Wang RC, et al. Angiogenesis in the placenta: The role of reactive oxygen species signaling. BioMed Res Int. 2015;2015:814543. doi: 10.1155/2015/814543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zambelli A, Prada GA, Fregoni V, et al. Erlotinib administration for advanced non-small cell lung cancer during the first 2 months of unrecognized pregnancy. Lung Cancer. 2008;60:455–457. doi: 10.1016/j.lungcan.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 70.Rivas G, Llinás N, Bonilla C, et al. Use of erlotinib throughout pregnancy: A case-report of a patient with metastatic lung adenocarcinoma. Lung Cancer. 2012;77:469–472. doi: 10.1016/j.lungcan.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 71.Large MJ, Wetendorf M, Lanz RB, et al. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014;10:e1004451. doi: 10.1371/journal.pgen.1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forbes K, Westwood M. Maternal growth factor regulation of human placental development and fetal growth. J Endocrinol. 2010;207:1–16. doi: 10.1677/JOE-10-0174. [DOI] [PubMed] [Google Scholar]
- 73.Lieu CH, Renfro LA, de Gramont A, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. J Clin Oncol. 2014;32:2975–2984. doi: 10.1200/JCO.2013.54.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]