After the launch of Rwanda’s first public cancer referral center and breast clinic, cancer detection rates were high among patients presenting with an undiagnosed breast concern. These findings will provide initial data to allow monitoring of changes in the distribution of benign and malignant disease and of cancer stage as cancer awareness and services expand nationally.
Keywords: Breast cancer, Benign breast disease, Africa, Rwanda
Abstract
Background.
Breast cancer incidence is rising in low- and middle-income countries. Understanding the distribution of breast disease seen in clinical practice in such settings can guide early detection efforts and clinical algorithms, as well as support future monitoring of cancer detection rates and stage.
Patients and Methods.
We conducted a retrospective medical record review of 353 patients who presented to Butaro Cancer Center of Excellence in Rwanda with an undiagnosed breast concern during the first 18 months of the cancer program.
Results.
Eighty-two percent of patients presented with a breast mass. Of these, 55% were diagnosed with breast cancer and 36% were diagnosed with benign disease. Cancer rates were highest among women 50 years and older. Among all patients diagnosed with breast cancer, 20% had stage I or II disease at diagnosis, 46% had locally advanced (stage III) disease, and 31% had metastatic disease.
Conclusion.
After the launch of Rwanda’s first public cancer referral center and breast clinic, cancer detection rates were high among patients presenting with an undiagnosed breast concern. These findings will provide initial data to allow monitoring of changes in the distribution of benign and malignant disease and of cancer stage as cancer awareness and services expand nationally.
Implications for Practice:
The numbers of cases and deaths from breast cancer are rising in low-income countries. In many of these settings, health care systems to address breast problems and efficiently refer patients with symptoms concerning for cancer are rudimentary. Understanding the distribution of breast disease seen in such settings can guide early detection efforts and clinical algorithms. This study describes the characteristics of patients who came with a breast concern to Rwanda’s first public cancer referral center during its first 18 months. More than half of patients with a breast mass were diagnosed with cancer; most had late-stage disease. Monitoring changes in the types of breast disease and cancer stages seen in Rwanda will be critical as breast cancer awareness and services grow.
Introduction
Breast cancer incidence is rising in low- and middle-income countries (LMIC) because of longer life expectancies, the decreased burden of infectious diseases, and changes in reproductive risk factors [1, 2]. Patients with breast cancer in LMIC experience longer diagnostic delays than patients in high-income countries, leading to later-stage presentations [3]. Combined with limited access to effective treatments, these delays lead to high case fatality rates [2,3]. Globally, interest is thus growing in the development of breast cancer screening and early detection programs in LMIC [4]. However, optimal early detection strategies are not well-characterized in settings where population-based mammography screening is not yet available, primary care services are limited, and pathology and treatment services are nascent [4]. Particularly when health care resources are highly constrained, the ideal of population-based screening must be balanced with the need to focus resources where they will make the most important difference, for example, through targeting early cancer detection among symptomatic women [4]. Understanding the current spectrum of breast diseases seen in primary care and referral facilities in LMIC can help countries and their health care facilities prepare for early detection programs, including developing clinical algorithms for initial evaluation and referral of patients with a breast problem. Such initial data can also facilitate monitoring shifts in distribution of disease diagnosed before, during, and after implementation of early detection programs.
Rwanda, a small, population-dense country of 11 million in East Africa, has recently embarked on an ambitious national plan to address noncommunicable diseases, including cancer [5, 6]. Before 2012, select cancer therapies were available on a small scale to patients with the means to afford it at Rwanda’s two main teaching hospitals and a semiprivate hospital in Kigali. Chemotherapy was provided free of charge to a small number of patients at Rwinkwavu Hospital, a rural district hospital in the eastern province. In July 2012, with financial and technical support from the non-governmental organization Partners in Health and from Dana-Farber Cancer Institute in Boston, Massachusetts, Rwanda’s Ministry of Health opened a public cancer referral center at Butaro Hospital, with a dedicated cancer clinic, ward, anatomic and clinical pathology laboratory, and staff trained in cancer care. The Butaro Cancer Center of Excellence (BCCOE) was the first public facility in the country to provide cancer diagnosis and care (especially chemotherapy) on a significant scale using standardized protocols [6, 7]. BCCOE was also the first facility providing large-scale cancer services that were affordable to the general population; because of charitable support, chemotherapy, pathology, and other cancer diagnostic and care services are provided free of charge [6]. BCCOE currently serves the largest volume of cancer patients in the country.
Breast cancer is the most common malignancy diagnosed and treated at BCCOE [8]. Although some patients are referred to BCCOE from other facilities with an existing diagnosis of breast cancer, most present with an undiagnosed breast concern. To meet the growing demand for breast-specific care, the oncology clinic at BCCOE became functionally the first comprehensive breast clinic in Rwanda (in mid-2014, a breast clinic opened at Rwanda Military Hospital, a Kigali referral hospital). Most palpable masses were biopsied, with diagnostic ultrasonography conducted on a limited basis. At the start of the program in 2012, all pathology specimens were sent to the Department of Pathology at Brigham and Women’s Hospital (Boston, MA). Local anatomic pathology resources were and continue to be developed such that pathology technicians in Butaro process tissue specimens, and microscopic diagnoses are increasingly made via telepathology by volunteer pathologists [9]. Patients diagnosed with cancer were staged by using physical examination, chest radiography, and abdominal ultrasonography.
No formal programs for early detection or screening of breast cancer yet exist in Rwanda. To support planning for emerging early detection strategies and initially evaluate breast disease encountered, we assessed the number and types of breast problems seen among patients who were evaluated for a previously undiagnosed breast concern at BCCOE during the first 18 months of the program.
Patients and Methods
Patients
We identified all 454 patients presenting with a breast concern to the BCCOE oncology program from July 1, 2012, through December 31, 2013, by using the electronic medical record, identifying women who participated in a questionnaire about delays in breast diagnosis and care, and reviewing paper medical records. Of these patients, 5 were excluded because their paper medical records could not be located, and 96 were excluded because they had an established diagnosis of breast cancer at another institution and had been subsequently referred to Butaro for treatment. This resulted in a final cohort of 353 patients. This study received ethical approval from the Rwanda National Ethics Committee and the Brigham and Women’s Hospital Institutional Review Board.
Data Collection and Key Variables
Paper medical records for each patient were abstracted by one reviewer (L.E.P.) using a standardized paper abstraction form. Abstracted information included patient demographic characteristics (age, sex, HIV status, address, referring facility), physical examination findings (presence of mass, lymphadenopathy, skin changes, nipple discharge), whether a biopsy was done, biopsy results (from printed pathology reports), clinical diagnosis, and cancer stage.
Statistical Analysis
We conducted descriptive statistical analyses on patient demographic characteristics, descriptive information about final diagnoses, and pathologic characteristics of benign lesions. For women with cancer, we described stage of disease and tumor characteristics. Data were analyzed by using SAS software, version 9.3 (SAS Institute, Cary, NC, USA, https://www.sas.com).
Results
Demographic and clinical characteristics of the 353 women who presented to BCCOE with a previously undiagnosed breast concern are shown in Table 1. Eighty-two percent of patients (n = 289) presented with a palpable mass on examination. Among the 289 patients with palpable breast masses, 154 (53%) were given a pathologic diagnosis of breast cancer and 6 (2%) were given a clinical diagnosis of breast cancer with no documented pathological confirmation. Considered by age, this included 12 of the 65 women younger than 30 years presenting with a breast mass (18.5%), 31 of 59 women (52.5%) aged 30–39 years, 45 of 73 women (61.6%) aged 40–49 years, and 72 of 92 women (78.3%) aged 50 years and older presenting with a mass. Among all women with breast masses, 6 (2%) were diagnosed with neoplasia that was not breast carcinoma, 82 (28%) were given a pathological diagnosis of benign disease, 23 (8%) were given a clinical diagnosis of benign disease, and 18 (6%) were not given a clear diagnosis at their last documented encounter (Table 2).
Table 1.
Demographic and clinical characteristics of patients presenting with a breast complaint and no previous diagnosis from July 1, 2012, to December 31, 2013 (n = 353)
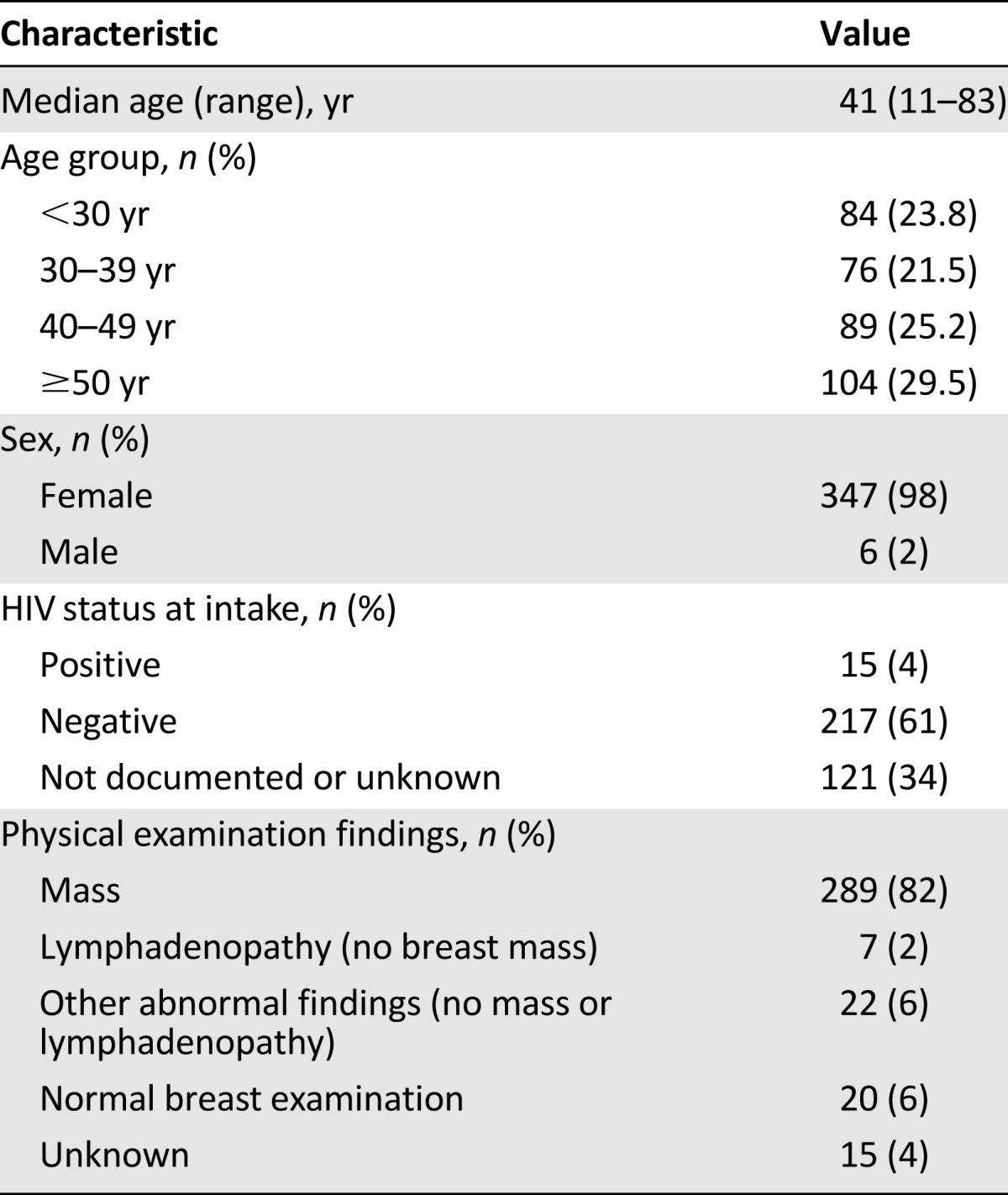
Table 2.
Diagnoses among patients with breast masses (n = 289)
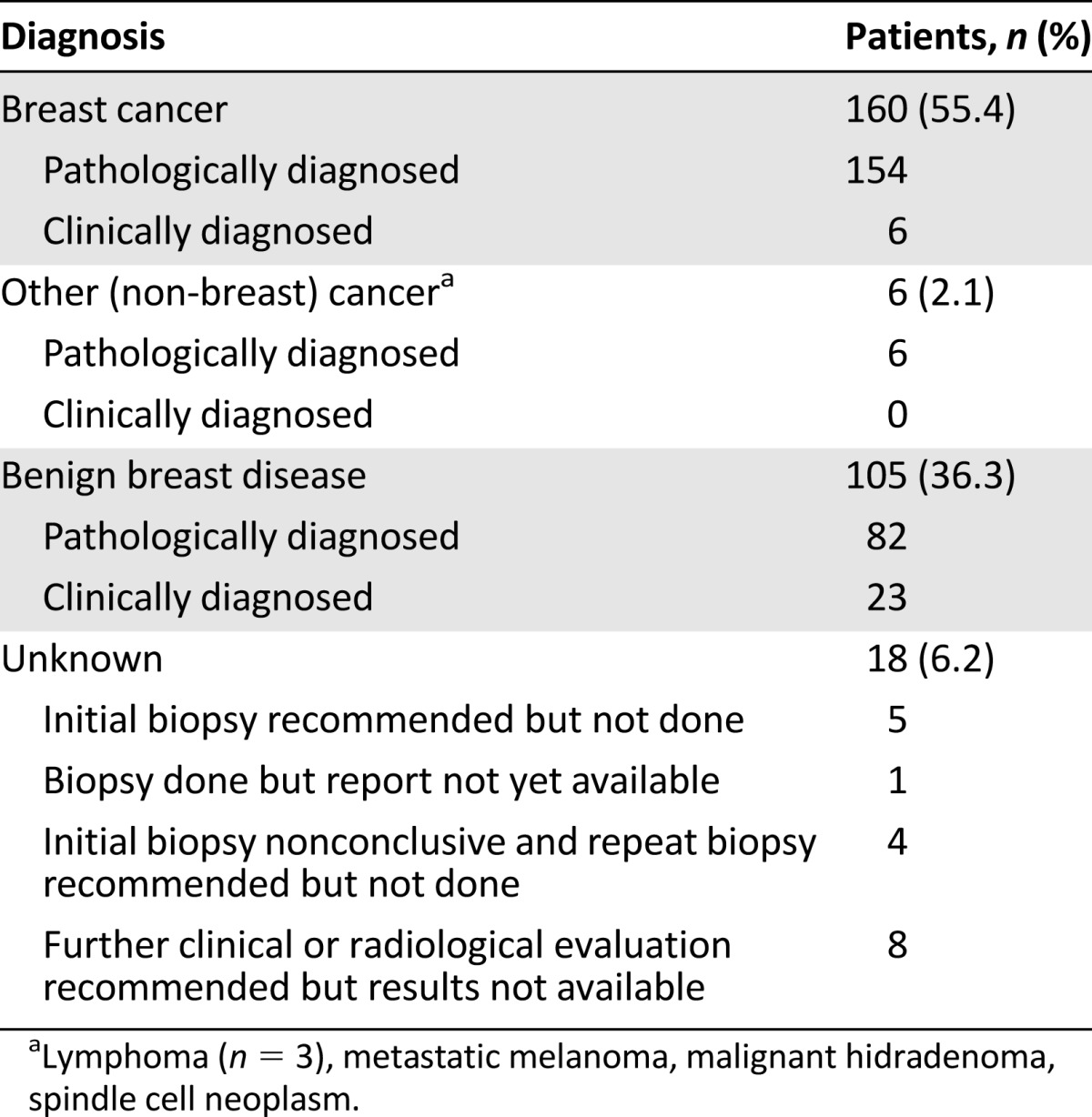
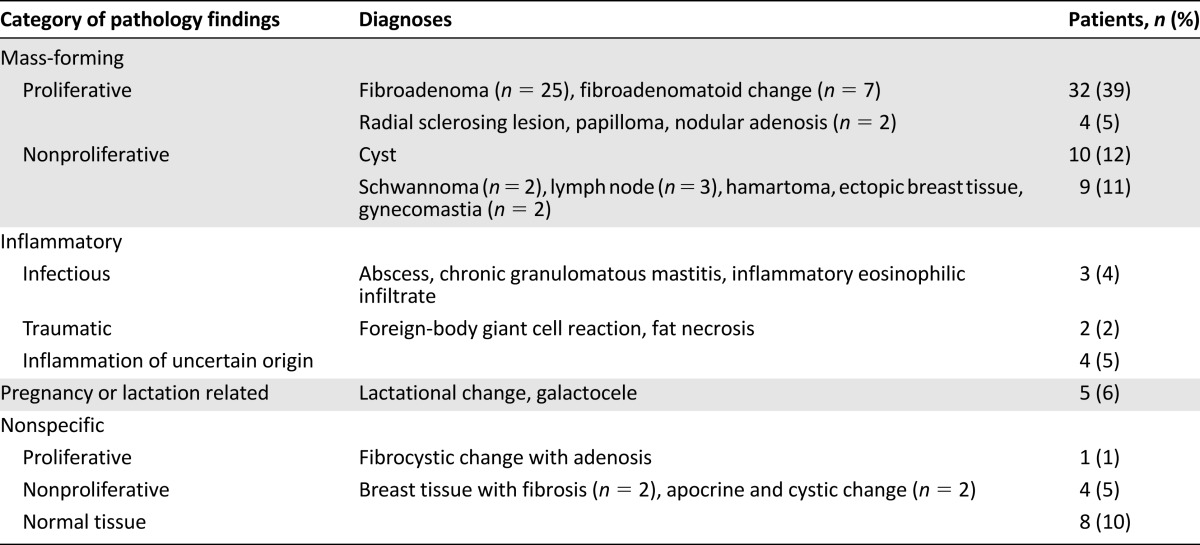
The 6 patients who were diagnosed with breast cancer with no documented pathological confirmation were admitted to the program during its first 5 months (between July and November 2012). Of these 6 patients, 4 had clinical or radiologic evidence of metastatic disease, 1 patient was treated for stage II breast cancer (but the medical record notes that the pathology specimen was lost), and 1 was treated for stage III breast cancer (but pathological diagnosis from a biopsy was not documented and results on surgical pathology were indeterminate). Among the 18 patients with unknown diagnoses, 5 had been recommended to have a biopsy but did not have one; 1 had a biopsy but the result was never found; 4 had an inconclusive biopsy but a repeat was never done; and 8 were recommended to have further clinical follow-up or were referred for mammography but never returned. Among patients with breast masses who had benign biopsy results, 67% of results were consistent with mass-forming lesions (with fibroadenoma being the most common), 11% reflected inflammatory processes, 6% reflected pregnancy- or lactation-related changes, and 16% were nonspecific, including normal breast tissue in 10% (Table 3).
Table 3.
Pathologic categories of benign breast disease among patients with breast masses who had a biopsy and no cancer diagnosis (n = 82)
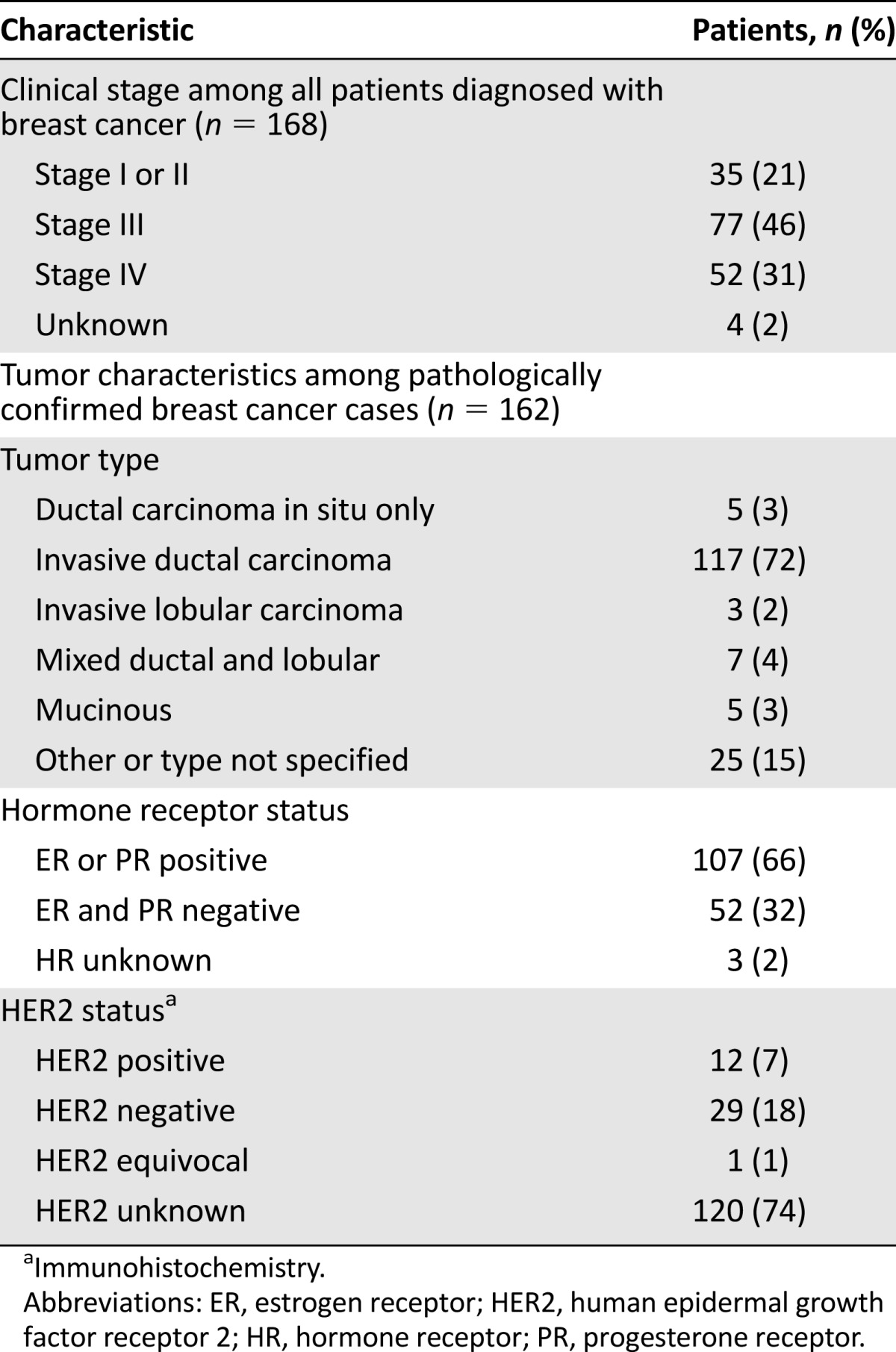
Among the 353 patients presenting with any type of breast symptom, a total of 168 (48%) were diagnosed with breast cancer. Of these, 20% had stage I or II disease at diagnosis, 46% had locally advanced (stage III) disease, and 31% had metastatic disease (Table 4). Invasive ductal carcinoma was the most common histological type (72%). Sixty-six percent of pathologically confirmed breast cancers were hormone receptor-positive. Human epidermal growth factor receptor 2 (HER2) status was not routinely provided in pathology reports during the study period given the unavailability of HER2-targeted therapies.
Table 4.
Clinical characteristics of breast cancer diagnosed at Butaro Cancer Center of Excellence
Discussion
Among the 353 patients who presented with an undiagnosed breast concern to Rwanda’s first public cancer facility during its first 18 months, about half were ultimately diagnosed with breast cancer, and most of these women presented with stage III or IV disease. About two thirds of these cancers were hormone receptor-positive, which is similar to rates of hormone receptor-positive cancers in the United States [10]. Although more detailed analysis of these findings is underway, these results are consistent with emerging reports suggesting that when testing conditions are optimized, most breast tumors in Africa are estrogen receptor-positive [11]. The proportion of patients diagnosed with breast cancer was higher than published reports from other breast clinics in sub-Saharan Africa [12–15], Europe [16], and North America [17, 18]. Among patients with benign masses who had biopsies, the distribution of disease was not markedly different from that of benign conditions seen in breast clinics in developed and developing countries [16, 18].
Several case series have described the outcomes of breast biopsies in sub-Saharan African health facilities. A small number of these cases series are from community facilities [19], but most are from referral centers [12, 20]. Our study is unique in capturing the patient population referred to a national breast clinic at a pivotal moment: the initial launch of accessible cancer services in a small, low-income sub-Saharan African country. Our findings may be of use to other inaugural national breast cancer programs and will be vital for monitoring in Rwanda.
The most striking of our findings is the high prevalence of cancer among all patients and especially among those presenting with a breast mass. Although rates were highest among women 50 years of age or older (of whom 78.3% with a palpable mass were diagnosed with breast cancer), the rates among younger women with masses were considerable (18.5%, 52.5%, and 61.6% for women younger than 30, 30–39, and 40–49 years, respectively). It is important to note that our rates do not reflect the cancer rate in a screened general population but rather the rate among a group of women referred to a specialty clinic for a breast concern. However, these rates are still higher than those in other settings; for example, in a Canadian study, rates of cancer diagnosis among women presenting to a breast clinic with palpable masses were 10% overall, 1% among women younger than 40, 9% among women 41–55, and 37% among women older than 55 years [18]. Our rates are also higher than those in a pilot breast cancer screening study in Sudan, where 17 (14.4%) of 118 women with positive findings on screening clinical breast examinations were diagnosed with ductal carcinoma in situ or invasive cancer [21].
Our clinic’s high cancer detection rate has several possible explanations. First, referring providers may have sent to BCCOE only patients whom they perceived to be at highest risk for cancer. Particularly during the period of the study, however, the availability of pathology services in other Rwandan facilities was minimal. Thus, if the high cancer detection rate reflected referral decisions only, this would suggest that many women with benign and malignant breast conditions were not getting comprehensive evaluation at other facilities. Second, consistent with a study we conducted that assessed women’s delays in seeking and obtaining breast cancer care [8], the high prevalence of cancer, and stage III/IV cancer in particular, also suggest that women themselves are not presenting until their breast masses are large and/or other symptoms are significant [8]. No national breast cancer awareness-raising campaigns had occurred in Rwanda during this study period, and women with more minor (and more likely benign) findings may not have sought care. Finally, our high cancer detection rate likely reflects the large number of women with prevalent cancer who did not have access to diagnostic or treatment services (or were not aware of available cancer treatment in Rwanda) until the opening of BCCOE. We anticipate that this cancer detection rate will fall as breast cancer awareness rises among patients, breast evaluation capacity grows among providers, the pool of prevalent but undiagnosed cancers declines, and a larger proportion of women with breast masses seek evaluation.
Most patients presenting to BCCOE with breast concerns had a mass, in contrast to some other sub-Saharan African settings, where pain (with no abnormalities on exam) was a predominant symptom [22]. This again may reflect referral decisions, although notably in most primary and secondary care centers in Rwanda, clinicians have minimal training in clinical breast examination. Among patients with benign masses, the distribution of diagnoses is not markedly different from that in European [16] and North American [18] settings and some other African reports [20], with fibroadenomas being the most common benign mass-forming lesion. As cancer detection rates fall and rates of women presenting with benign breast disease rise, ultrasonography could become a valuable tool in identifying masses that do not require a biopsy.
Of note, 6% of our patients did not receive any documented clinical or pathologic diagnosis after evaluation, and although one third of those were considered to need biopsy, that procedure was not performed. These data reveal the need for improved patient tracking systems to reduce loss to follow-up, a major issue in low-resource settings. Reasons for loss to follow-up in our and similar settings should be examined to identify other barriers, such as stigma and transport costs, because psychosocial and economic support for patients may be a key part of effective diagnostic and treatment programs in LMIC. In addition, early in the program, six patients were diagnosed with breast cancer without documented pathological confirmation. As clinical skills and experience grew, all subsequent breast cancers during the next 15 months were pathologically diagnosed.
Conclusion
During the 18 months after the opening of Rwanda’s first public cancer facility and first multidisciplinary breast clinic, a large number of patients presented with breast concerns, and 55% of those with breast masses were ultimately diagnosed with cancer. Our findings will provide initial data to allow monitoring of changes in the distribution of benign and malignant disease and of cancer stage at BCCOE as cancer services evolve and expand nationally, as more and more providers are trained in cancer care, and as community awareness of breast disease and breast cancer increases. In turn, this monitoring will enable appropriate allocation of resources for capacity-building, diagnosis, and treatment to ensure that accessible and affordable services are available to meet patient demand.
Acknowledgments
The efforts of Lydia E. Pace were funded by the Mary Ann Tynan Fellowship in Women’s Health.
Author Contributions
Conception/Design: Lydia E. Pace, Neo Tapela, Egide Mpanumusingo, Nancy L. Keating, Lawrence N. Shulman, Tharcisse Mpunga
Provision of study material or patients: Jean Bosco Bigirimana, Cadet Mutumbira, Alain Uwumugambi, Tharcisse Mpunga
Collection and/or assembly of data: Lydia E. Pace, Jean-Marie V. Dusengimana, Vedaste Hategekimana, Hamissy Habineza, Jean Bosco Bigirimana, Cadet Mutumbira, Egide Mpanumusingo, Alain Uwumugambi
Data analysis and interpretation: Lydia E. Pace, Jean-Marie V. Dusengimana, Vedaste Hategekimana, Jean Bosco Bigirimana, Jane E. Brock, Emily Meserve, Deborah Dillon, Nancy L. Keating, Lawrence N. Shulman, Tharcisse Mpunga
Manuscript writing: Lydia E. Pace, Jean-Marie V. Dusengimana, Neo Tapela, Jane E. Brock, Emily Meserve, Alain Uwumugambi, Nancy L. Keating, Lawrence N. Shulman, Tharcisse Mpunga
Final approval of manuscript: Lydia E. Pace, Jean-Marie V. Dusengimana, Vedaste Hategekimana, Hamissy Habineza, Jean Bosco Bigirimana, Neo Tapela, Cadet Mutumbira, Egide Mpanumusingo, Jane E. Brock, Emily Meserve, Alain Uwumugambi, Deborah Dillon, Nancy L. Keating, Lawrence N. Shulman, Tharcisse Mpunga
Disclosures
Deborah Dillon: Siemens (C/A [spouse]). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Shulman LN, Willett W, Sievers A, et al. Breast cancer in developing countries: Opportunities for improved survival. J Oncol. 2010;2010:595167. doi: 10.1155/2010/595167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 3.Unger-Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol. 2014;5:465–477. doi: 10.5306/wjco.v5.i3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yip CH, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer. 2008;113(suppl):2244–2256. doi: 10.1002/cncr.23842. [DOI] [PubMed] [Google Scholar]
- 5.Binagwaho A, Muhimpundu MA, Bukhman G. 80 under 40 by 2020: An equity agenda for NCDs and injuries. Lancet. 2014;383:3–4. doi: 10.1016/S0140-6736(13)62423-X. [DOI] [PubMed] [Google Scholar]
- 6.Shulman LN, Mpunga T, Tapela N, et al. Bringing cancer care to the poor: Experiences from Rwanda. Nat Rev Cancer. 2014;14:815–821. doi: 10.1038/nrc3848. [DOI] [PubMed] [Google Scholar]
- 7.Stulac S, Binagwaho A, Tapela N, et al. Capacity building for oncology programmes in sub-Saharan Africa: The Rwanda experience. Lancet Oncol. 2015;16:e405–e413. doi: 10.1016/S1470-2045(15)00161-8. [DOI] [PubMed] [Google Scholar]
- 8.Pace LE, Mpunga T, Hategekimana V, et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centers in Rwanda. The Oncologist. 2015;20:780–788. doi: 10.1634/theoncologist.2014-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mpunga T, Tapela N, Hedt-Gauthier BL, et al. Diagnosis of cancer in rural Rwanda: Early outcomes of a phased approach to implement anatomic pathology services in resource-limited settings. Am J Clin Pathol. 2014;142:541–545. doi: 10.1309/AJCPYPDES6Z8ELEY. [DOI] [PubMed] [Google Scholar]
- 10.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: A systematic review and meta-analysis. PLoS Med. 2014;11:e1001720. doi: 10.1371/journal.pmed.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeje EA, Mofikoya BO, Oku YE. Pattern of breast masses in Lagos: A private health facility review of 189 consecutive patients. Nig Q J Hosp Med. 2010;20:38–41. doi: 10.4314/nqjhm.v20i1.58015. [DOI] [PubMed] [Google Scholar]
- 13.Njeze GE. Breast lumps: A 21-year single-center clinical and histological analysis. Niger J Surg. 2014;20:38–41. doi: 10.4103/1117-6806.127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed HG, Ali AS, Almobarak AO. Frequency of breast cancer among Sudanese patients with breast palpable lumps. Indian J Cancer. 2010;47:23–26. doi: 10.4103/0019-509X.58854. [DOI] [PubMed] [Google Scholar]
- 15.Irabor DO, Okolo CA. Outcome of one hundred and forty-nine consecutive breast biopsies in Ibadan, Nigeria. Breast Dis. 2011;33:109–114. doi: 10.3233/BD-2010-0329. [DOI] [PubMed] [Google Scholar]
- 16.Ellis H, Cox PJ. Breast problems in 1,000 consecutive referrals to surgical out-patients. Postgrad Med J. 1984;60:653–656. doi: 10.1136/pgmj.60.708.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerlikowske K, Smith-Bindman R, Ljung BM, et al. Evaluation of abnormal mammography results and palpable breast abnormalities. Ann Intern Med. 2003;139:274–284. doi: 10.7326/0003-4819-139-4-200308190-00010. [DOI] [PubMed] [Google Scholar]
- 18.Sterns EE. Age-related breast diagnosis. Can J Surg. 1992;35:41–45. [PubMed] [Google Scholar]
- 19.Ohene-Yeboah MO. An audit of excised breast lumps in Ghanaian women. West Afr J Med. 2005;24:252–255. doi: 10.4314/wajm.v24i3.28208. [DOI] [PubMed] [Google Scholar]
- 20.Okoth C, Galukande M, Jombwe J, et al. Benign proliferative breast diseases among female patients at a sub-Saharan Africa tertiary hospital: A cross sectional study. BMC Surg. 2013;13:9. doi: 10.1186/1471-2482-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abuidris DO, Elsheikh A, Ali M, et al. Breast-cancer screening with trained volunteers in a rural area of Sudan: A pilot study. Lancet Oncol. 2013;14:363–370. doi: 10.1016/S1470-2045(12)70583-1. [DOI] [PubMed] [Google Scholar]
- 22.Ohene-Yeboah M, Amaning E. Spectrum of complaints presented at a specialist breast clinic in Kumasi, Ghana. Ghana Med J. 2008;42:110–113. doi: 10.4314/gmj.v42i3.43612. [DOI] [PMC free article] [PubMed] [Google Scholar]