The development of antiangiogenic therapies and their subsequent analysis in rigorous therapeutic trials have redefined current management strategies for metastatic, persistent, or recurrent cervical cancer. They remain an exciting area of current exploration.
Keywords: Uterine cervical neoplasms, Angiogenesis inhibitors, Biological markers, Clinical trials, Cervical cancer, Clinical trials, Antiangiogenesis therapy, Biomarkers
Abstract
Background.
Treatment options for women with metastatic, persistent, or recurrent cervical cancer are limited and thus the disease portends a poor prognosis. It is critical to understand the pathophysiology of cervical cancer to better delineate therapeutic targets. The development of antiangiogenic therapies and their subsequent analysis in rigorous therapeutic trials have redefined current management strategies and is an exciting area of current exploration.
Results.
Translational trials have furthered the understanding of molecular determinants of angiogenesis. Phase II trials have shown promising trends with developing antiangiogenic therapies. A practice-changing phase III trial has recently been published. Given the potential benefits and different toxicity spectrum compared with standard cytotoxic chemotherapy, antiangiogenic options are under active investigation for this vulnerable patient population. Emerging data are promising for other antiangiogenic-directed therapeutics, as well as cervical cancer molecular biomarkers to guide diagnosis and treatment.
Conclusion.
Antiangiogenic therapies have evolved during the past 20 years and remain an exciting area of current exploration.
Implications for Practice:
Understanding of the angiogenic microenvironment has furthered understanding of tumor biology and management. Antiangiogenic therapies show promise for women with advanced cervical cancer. A review of the evolution of these biologic agents shows them to be an effective and tolerable management strategy for many patients in this vulnerable population, with exciting future potential.
Introduction
On the global scale, numerically, cervical cancer remains the most lethal gynecologic malignancy, with 529,800 new cases and 275,100 deaths in 2011 [1]. Because of effective screening and early detection through the Papanicolaou test and improving dissemination of the human papillomavirus (HPV) vaccine, cervical cancer is less prevalent in the U.S.; however, it continues to be a severe burden, with 12,990 new cases diagnosed and 4,120 deaths in 2016 alone [2]. A subset of these women will present with metastatic disease or develop recurrent disease after initial therapy. Unfortunately, treatment strategies for these patients are limited to palliation of symptoms, with most efforts to control disease progression and prolong life meeting with relatively poor success [3]. On the basis of several clinical trials, the backbone of therapy was established as cisplatin and paclitaxel [4]. Poor outcomes in this population despite chemotherapy have necessitated continued exploration into new therapeutic strategies.
Cervical Cancer Pathogenic Molecular Cascade
It is well-established that squamous cell carcinoma of the cervix is strongly associated with infection by high-risk subtypes of HPV [5]. Of these subtypes, HPV-16 and HPV-18 are responsible for 70% of invasive cervical cancer and 50% of high-grade cervical intraepithelial neoplasia [6, 7]. Specifically, it is dysregulation of the HPV E6 and E7 oncogenes that facilitates transformation and maintenance of a dysplastic and subsequently malignant phenotype [8, 9].
Dysregulation of oncogene expression induces chromosomal instability, promoting integration of the HPV genome into cellular chromosomes [10]. This results in disruption of the E2 transcription regression factor, thereby causing enhanced E6 and E7 activity. E6 degrades the cellular tumor suppressor gene product, p53, leading to an arrest of DNA repair and apoptosis and thus continued cellular proliferation [11]. Conversely, E7 inactivates another cellular tumor suppressor gene product, pRb, which results in upregulation of p53 and potentiation of apoptosis [12]. These alterations on cell cycle progression induce several changes in the angiogenic pathway.
Through modulation of p53 expression, E6 and E7 modify transcription factor regulation, thereby altering gene expression, protein function, and tumor development. One example includes hypoxia inducible factor (HIF)-1α, which controls the expression of various cytokines and growth factors. HPV E6 represses HIF-1α expression via p53, whereas HPV E7 increases transcription [13, 14]. Both thrombospondin-1 (TSP-1) and maspin are regulated by p53 and are decreased in cells expressing E6 and E7 [15–17]. These seemingly contrasting effects allow tumor to differentially express a desired phenotype to respond to the changing microenvironment. In the setting of tumor growth beyond the confines of the existing blood supply, new blood vessel formation is essential.
Angiogenesis and Cervical Cancer
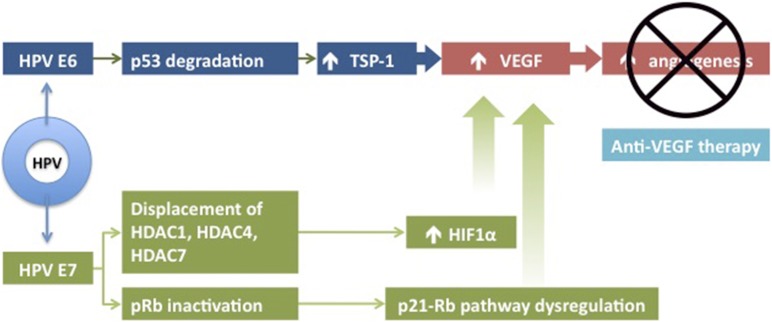
Angiogenesis is the process of new blood vessel formation, a necessary function for embryogenesis, new tissue growth, and healing. New vessel growth, or neovascularization, is essential for tumor proliferation, growth, invasion, and metastasis [18]. Vascular aberrations are characteristic of cervical dysplasia and neovascularization in cervical tumors can predict aggressive clinical behavior and poor prognosis [19]. The process of new vessel growth is induced, in part, by vascular endothelial growth factor (VEGF), which induces endothelial cell activation and proliferation and facilitates remodeling [20–22]. VEGF subtypes A–E bind to three tyrosine kinase membrane receptors: VEGF receptor (VEGFR)-1 (or Flt-1), VEGFR-2 (or Flk-1), or VEGFR-3 (or Flt-4). VEGF subtypes A and B bind to VEGFR-1; VEGF subtypes A, C, and E bind to VEGFR-2; and VEGF subtypes C and D bind to VEGFR-3 [23]. VEGFR-2 is the primary receptor mediating VEGF-induced angiogenesis [24]. Elevated HIF-1α expression, as well as displaced histone deacetylase, elevated TSP-1, and dysregulated p-21 retinoblastoma pathways result in elevations of VEGF [13, 25, 26]. The E6 and E7 oncogenic pathways described in the preceding section, and shown in Figure 1, can mediate upregulation of VEGF.
Figure 1.
Oncogenic regulation of angiogenesis. Reprinted from [63] with permission.
Abbreviations: HDAC, histone deacetylase; HIF, hypoxia inducible factor; HPV, human papillomavirus; Rb, retinoblastoma; TSP, thrombospondin; VEGF, vascular endothelial growth factor.
Determinants of Angiogenic Microenvironment
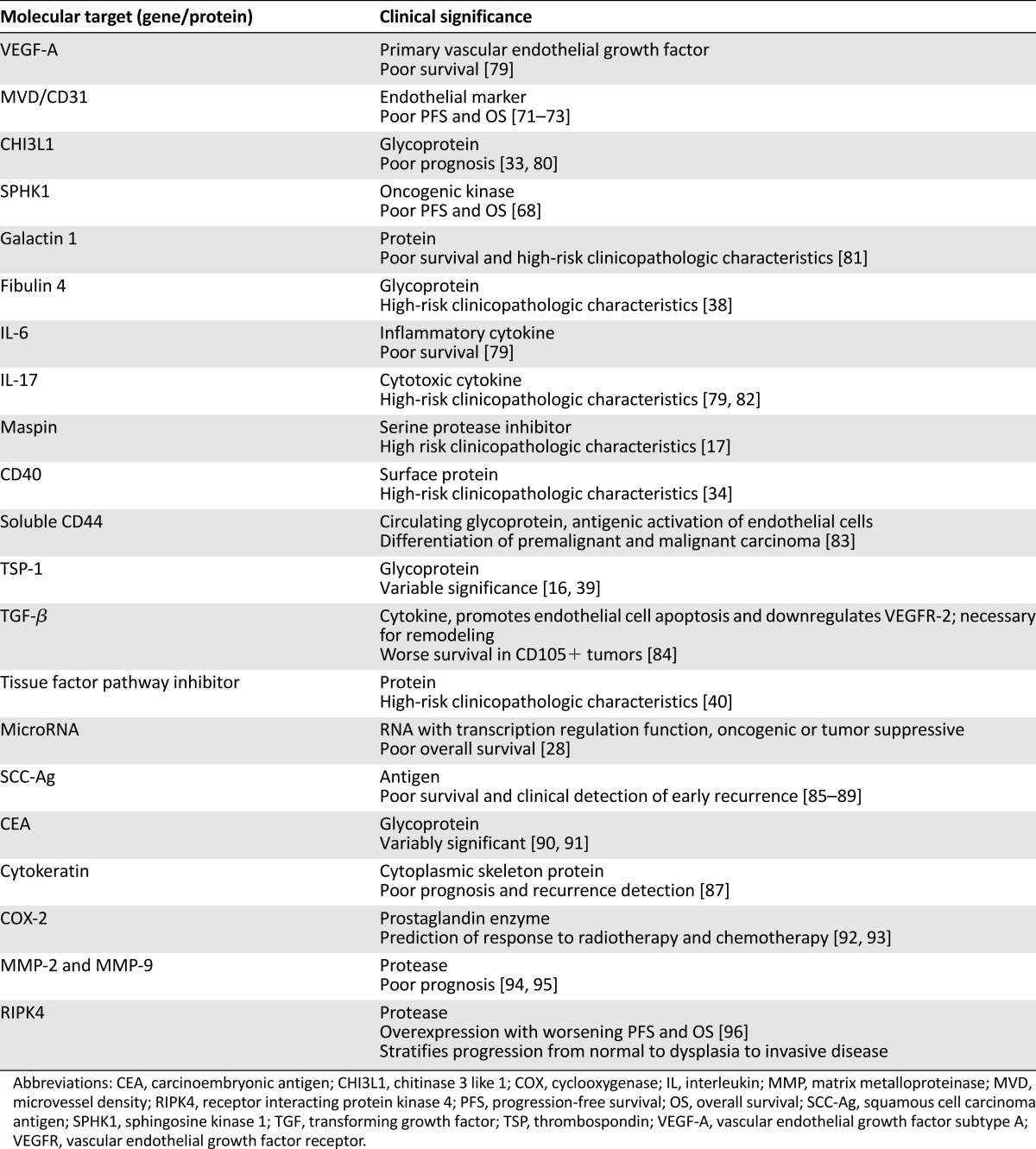
In addition to E6 and E7 oncogenic modulation of VEGF, several other factors contribute to the shift from dysplasia to invasive cervical cancer [27–41]. Some factors affecting angiogenesis are summarized in Table 1. Angiogenesis may be enhanced by upregulation of surrounding specific transcription factors and increased protein and cytokine expression. Several gene regulatory factors are briefly reviewed. Octamer-binding transcription factor (OCT)-4 is an established transcription factor critical for maintaining stem cell pluripotency and is overexpressed in cervical cancer. In vivo evaluation by Li et al. showed that nuclear OCT4A was responsible for the self-renewal of cervical cancer stem cells, whereas cytoplasmic OCT4B enhanced angiogenesis and promoted tumor mobility. Angiogenesis was induced through upregulation of CD34, VEGF, HIF-1α, and IL-6 [27]. MicroRNA (miRNA) is also closely tied with oncogenic and tumor suppressive phenotype expression and can enhance angiogenesis through VEGF-dependent and -independent pathways [28, 29].
Table 1.
Molecular determinants of angiogenesis
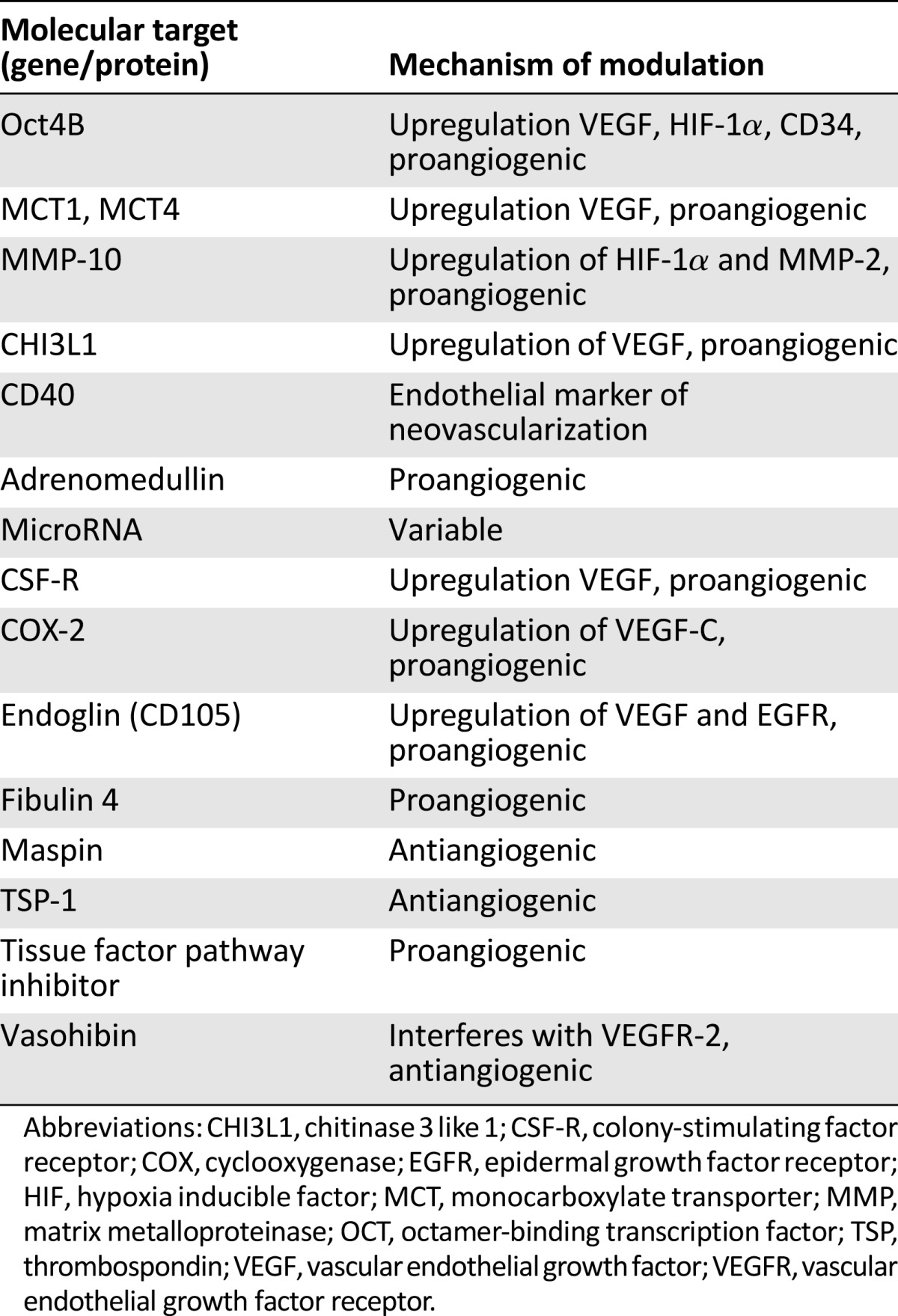
Alterations in protein and cytokine expression also regulate angiogenesis. For example, monocarboxylate transporter (MCT) is a surface protein critical to the transport of lactic acid. A 2015 study showed that MCT4 expression was unregulated after HPV transfection and that increasing MCT4 correlated with progression to malignancy. Additionally, an inverse correlation was noted between VEGF subtype A and MCT1 expression. This suggests an association between lactate transport, VEGF regulation, and angiogenesis [30]. Further evaluation of an alternative pathway by Zhang et al. showed that matrix metalloproteinase (MMP)-10 was linked to tumor cell migration, invasion, endothelial development, and resistance to apoptosis. Specifically, elevated MMP-10 expression stimulates HIF-1α and MMP-2, resulting in a proangiogenic environment. Additional data obtained by using synthetic RNA targeting MMP-10 in vivo showed diminished tumor growth and reduction of angiogenesis [31]. This may prove an exciting area of future study.
Another regulatory factor is chitinase 3-like 1 (CHI3L1), a secreted glycoprotein that functions as a cytokine and as a mediator in antiapoptotic pathways. It has been strongly correlated with stage and outcome in several solid tumors, and previous studies have established that this glycoprotein promotes angiogenesis through VEGF-dependent and -independent pathways [32]. CHI3L1 is elevated in women with cervical cancer and is associated with poor prognosis [33]. It promotes endothelial cell migration and tube formation in vitro and was associated with VEGF expression and microvessel density via immunohistochemistry. This establishes a role as a prognostic biomarker as well as possible therapeutic target. Table 1 briefly summarizes the many proteins modulating angiogenesis and gives insight into the complexity of this process and the numerous pathways that may serve as future targets for therapy.
Development of TNP-470
On the basis of the critical role of angiogenesis in the carcinogenesis of cervical cancer, disruption of this pathway via targeted therapeutics should disrupt tumor growth and progression. In the early 1990s, scientists began exploring the antiangiogenic compound fumagillin, an antibiotic secreted by Aspergillus fumigatus [42]. The more potent antiangiogenic analog, TNP-470, was then developed. In vitro studies showed inhibition of endothelial cell migration and proliferation [43]. Further in vivo and preclinical studies confirmed inhibition of growth in several human tumor xenografts, including colon, prostate, and breast cancer; choriocarcinoma; and neurofibrosarcoma [44–47]. Given its promise, a phase I trial for women with cervical cancer was undertaken.
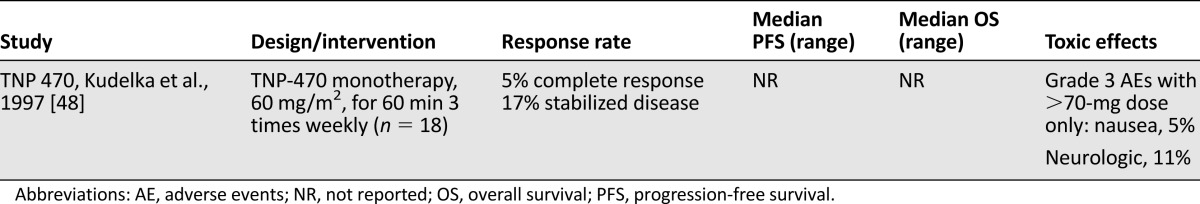
The phase I trial of the novel angiogenesis inhibitor TNP-470 in women with recurrent or metastatic squamous cell cancer of the cervix was published in 1997 [48]. Eighteen patients were evaluated and treated with TNP-470 at 60 mg/m2 dosed for 60 minutes three times weekly. Antitumor activity was seen in four patients. One experienced a complete response [49], and three had stabilization of previously progressive disease. This regimen was well-tolerated, with adverse events limited to grade 1–2 nausea and vomiting and only one patient experiencing grade 3 symptoms. Neurologic toxicity was the dose-limiting adverse effect and was found to be dose related and reversible. This trial is summarized in Table 2. As the first example of the efficacy of antiangiogenesis therapy in this disease, this trial served as the foundation of subsequent clinical trials with antiangiogenics for women with advanced cervical cancer.
Table 2.
Phase I trials of antiangiogenesis in advanced cervical cancer
Evolution of Bevacizumab
Antiangiogenic therapy developed further throughout the 1990s. Bevacizumab is a humanized monoclonal antibody directed against VEGF, with the goal of neutralizing VEGF subtype A and directly blocking signal transduction through VEGF receptor 1 and 2 [20, 50]. The pharmacokinetics and tolerability were established in 1997, and U.S. Food and Drug Administration (FDA) approved the drug for several malignancies, including metastatic colorectal cancer, metastatic non-small cell lung cancer, recurrent glioblastoma multiforme, and renal cell carcinoma [51–55]. A retrospective case series of six heavily pretreated women (median of three prior regimens) with advanced cervical cancer was reported in 2006 by Wright et al. [56]. They showed a 67% overall response rate with the use of bevacizumab in combination with cytotoxic regimens. Both this and the promising results of TNP-470 catalyzed phase II clinical trials using antiangiogenesis therapy.
Investigating Bevacizumab in Cervical Cancer
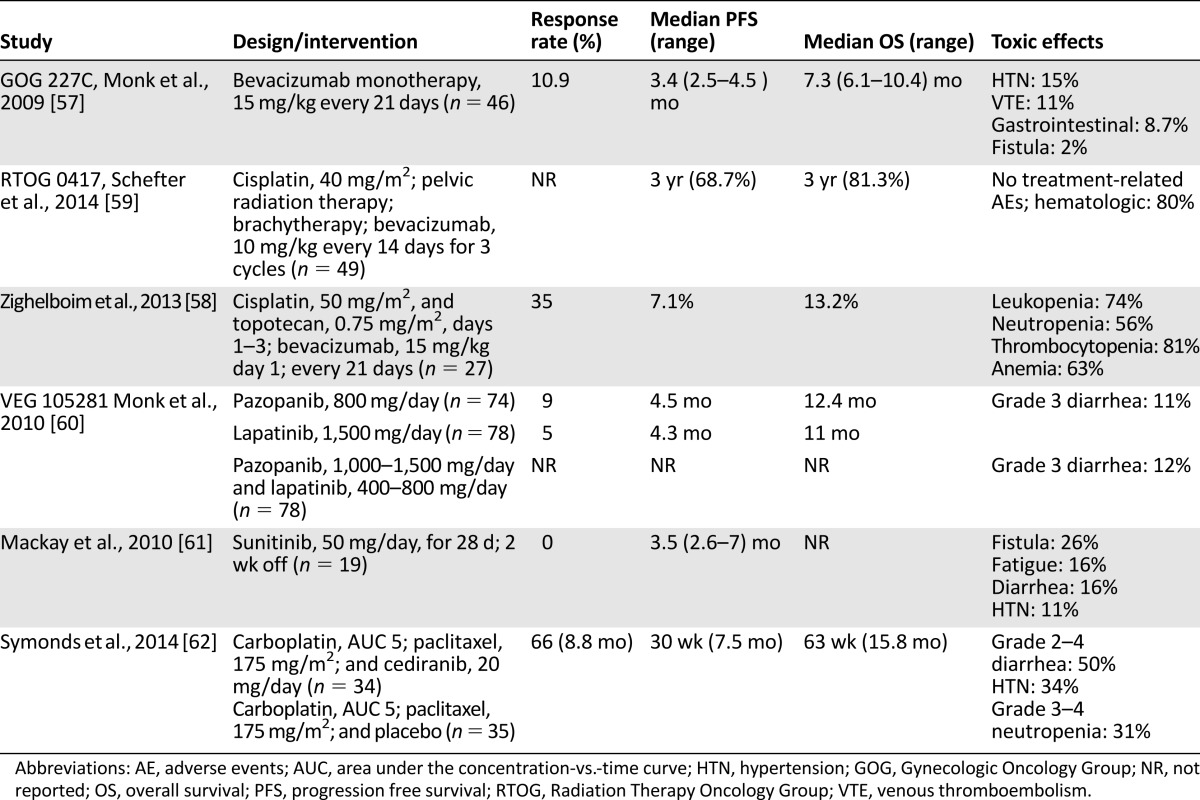
The first phase II evaluation of bevacizumab with advanced cervical cancer was performed by Monk et al. in 2009 under the auspices of the Gynecology Oncology Group (GOG) in GOG protocol 227C [57]. This study included 46 women treated with bevacizumab at 15 mg/kg every 21 days. Of these women, 38 (82.6%) had prior pelvic radiation therapy as well one cytotoxic regimen (n = 34 [73.9%]) or two cytotoxic regimens (n = 12 [26.1%]). A therapeutic response was seen, with 5 patients (10.9%; two-sided 90% confidence interval [CI], 4%–22%) experiencing partial response and 11 patients (23.9%; two-sided 90% CI, 14%–37%) experiencing progression-free survival for at least 6 months. For all patients, the median response duration was 6.21 months (range, 2.83–8.28 months). The median progression-free survival was 3.4 months (95% CI, 2.53–4.53), and the overall survival was 7.29 months (95% CI, 6.11–10.41). In addition to showing that bevacizumab was biologically active in this subset of women with cervical cancer, GOG 227C also showed that it was well-tolerated. Notable grade 3 or 4 adverse events included neutropenia (n = 1), anemia (n = 2), gastrointestinal effects (n = 4), hypertension (n = 7), thromboembolism (n = 5), vaginal bleeding (n = 1), fistula (n = 1), and cardiovascular effects (n = 2). Only 1 grade 5 infection was identified. These observations suggested acceptable safety and improved tolerability with use of bevacizumab.
Another phase II clinical trial was performed in 2013 to evaluate the combination of cisplatin, 50 mg/m2, with topotecan, 0.75 mg/m2, on days 1–3 and bevacizumab, 15 mg/kg, on day 1 [58]. Eligible women had no prior chemotherapy for recurrence. A total of 27 patients were enrolled. Results showed a median progression-free survival (PFS) of 7.1 months (80% CI, 4.7–10.1 months) and overall survival (OS) of 13.2 months (80% CI, 8.0–15.4 months). Grade 3 or 4 hematologic toxicity was common (thrombocytopenia, 82%; leukopenia, 74%; anemia, 63%; and neutropenia, 56%), and 78% of patients required an unanticipated hospital admission for toxicity management or supportive care. This regimen appears to be active but has unacceptable toxicity.
A third phase II study in 2014, by Schefter et al. through the Radiation Therapy Oncology Group (RTOG) protocol 0417, evaluated 49 women with stage IB–IIIB disease [59]. These women were treated with cisplatin, 40 mg/m2; whole pelvic radiation therapy; brachytherapy; and bevacizumab, 10 mg/kg every 2 weeks for 3 cycles. The 3-year OS was 81.3% (95% CI, 67.2%–89.8%) and 3-year PFS was 68.7% (95% CI, 53.5%–79.8%). No significant treatment-related adverse events occurred. This trial suggested that bevacizumab in combination with standard pelvic chemoradiation was efficacious and warrants further investigation.
Alternative Antiangiogenic Therapies
In 2010, Monk et al. evaluated alternative strategies, including pazopanib and lapatinib, both as single agents and in combination [60]. Pazopanib is a selective multitargeted receptor tyrosine kinase inhibitor that targets the VEGF receptor, platelet-derived growth factor receptor, and c-Kit. Conversely, lapatinib is a dual tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR) and Her2/neu. This study targeted women with stage IVB persistent or recurrent disease and at least one prior cytotoxic chemotherapy regimen exposure for metastases. Of the 230 women enrolled, 152 were randomly assigned to monotherapy with lapatinib, 800 mg orally daily, or lapatinib, 1,500 mg orally daily; 78 were randomly assigned to combination therapy with pazopanib, 1,000 mg or 1,500 mg orally daily, and lapatinib, 400 mg or 800 mg daily. The futility boundary was crossed at a planned interim analysis and the combination arm was terminated.
Pazopanib monotherapy showed significantly improved PFS (hazard ratio [HR], 0.66; 90% CI, 0.48–0.91; p = .013) and OS (HR, 0.67; 90% CI, 0.46–0.99; p = .045). Median survival was 50.7 weeks for pazopanib and 39.1 weeks for lapatinib. Adverse events included grade 3 diarrhea in 11% of patients treated with pazopanib and 12% of those treated with lapatinib. This study demonstrated improved progression-free survival and improved tolerability of this alternative antiangiogenic agent in women with advanced cervical cancer.
A related oral agent, sunitinib, has also been evaluated [61]. Sunitinib is a multityrosine kinase inhibitor with antiangiogenic action at the VEGF receptor 1, 2, and 3 as well as PDGF and c-Kit. This phase II trial enrolled 19 patients with unresectable locally advanced or metastatic cervical cancer who had received up to one prior chemotherapy regimen. Women were dosed at 50 mg/day for 4 weeks, followed by 2 weeks off. This 6-week cycle was repeated for a maximum of six times for stable disease or two times if a response was obtained. There were no documented responses to therapy, with median PFS of 3.5 months (95% CI, 2.6–7 months). Additionally, morbidity was significant; 26% of women developed a fistula. Given the drug’s morbidity and insufficient evidence of activity as a single agent, it was determined that sunitinib did not warrant further study. All phase II trials addressing antiangiogenic therapies are summarized in Table 3 [57–62].
Table 3.
Phase II trials of vascular endothelial growth factor axis-based antiangiogenesis therapies in locally advanced and advanced cervical cancer
Establishing a Survival Advantage With Bevacizumab
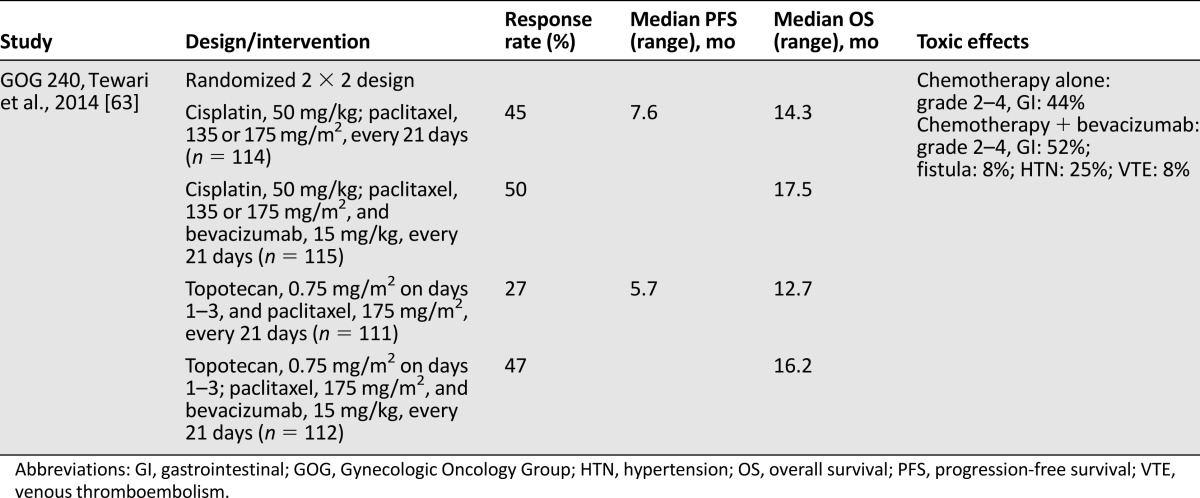
Given its demonstrated therapeutic potential, bevacizumab was evaluated in a phase III trial by Tewari et al. under GOG 240 [63]. This multicenter international trial included women with metastatic, recurrent, or persistent squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Women were randomly assigned to one of four treatment arms, using paclitaxel, 135 or 175 mg/m2, on day 1 plus cisplatin, 50 mg/m2, on day 2, with or without bevacizumab, 15 mg/kg, on day 2 in comparison with paclitaxel, 175 mg/m2, on day 1 plus topotecan, 0.75 mg/m2, on days 1–3 with or without bevacizumab, 15 mg/kg on day 1. Women were treated every 21 days until disease progression or unacceptable toxicity.
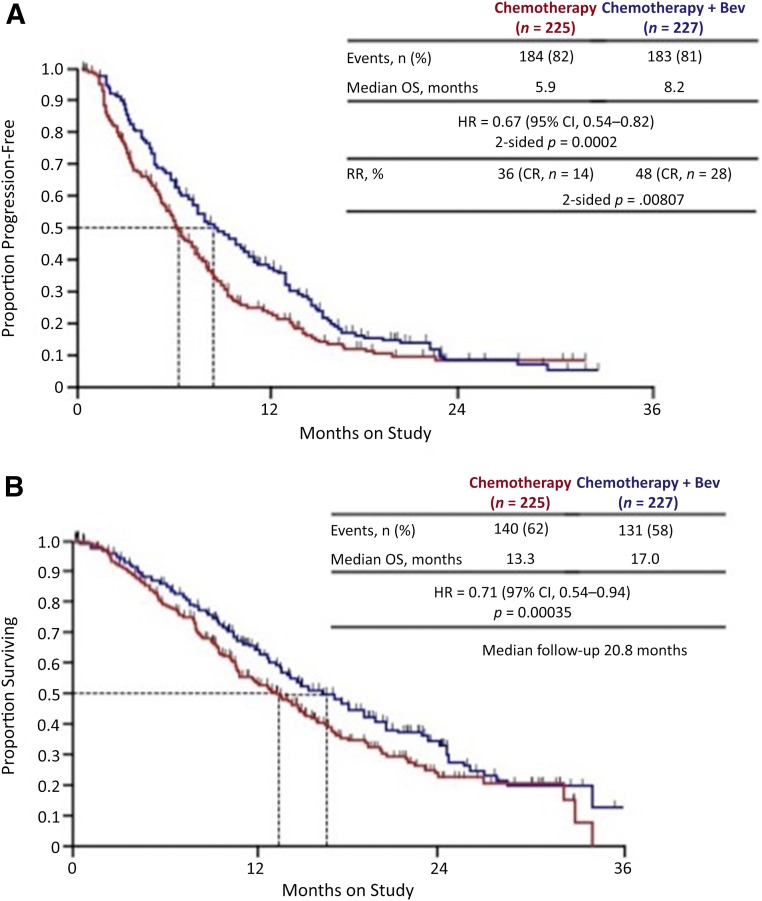
An initial interim analysis of 173 patients in 2012 revealed that the topotecan plus paclitaxel doublet was not superior to the cisplatin and paclitaxel doublet. After a second analysis in late 2012, the National Cancer Institute’s Data Safety Monitoring Board recommended ending the trial at 20.8 months’ median follow-up. This was in response to a statistically significant therapeutic advantage demonstrated in women treated with bevacizumab, regardless of the cytotoxic therapy combination (Fig. 2). This included overall survival of 17 months (vs. 13.3 months) and progression-free survival of 8.2 months (vs. 5.9 months) (Fig. 2). There was no significant deterioration of patient-reported outcomes, and the adverse events were similar to those previously observed, including fistula (8%), thromboembolism (8%), and manageable hypertension (25%). The design and results are summarized in Table 4.
Figure 2.
Gynecology Oncology Group 240 results. Progression-free survival (A) and overall survival (B) Kaplan-Meier curves. Reprinted from [63]. Copyright 2014 The Massachusetts Medical Society. Used with permission.
Abbreviations: CI, confidence interval; CP, cisplatin-paclitaxel; TP, topotecan-paclitaxel.
Table 4.
Phase III trial of vascular endothelial growth factor axis-based antiangiogenesis therapy in advanced cervical cancer
Given this significant improvement in OS and PFS with the addition of bevacizumab to cisplatin and paclitaxel, both bevacizumab-containing triplet regimens studied are listed as category 1 in the National Comprehensive Cancer Network clinical practice guidelines for cervical cancer. GOG 240 led directly to regulatory approval by the FDA of bevacizumab for advanced cervical cancer on August 14, 2014 [64, 65]. This is the first time a targeted agent has significantly improved overall survival in women struggling with a gynecologic malignancy.
Future Areas of Investigation
The landmark trial proving the therapeutic benefits of bevacizumab sparked renewed vigor into the investigation of alternative antiangiogenic therapies. Most recently, Symonds et al. performed the Cediranib in Recurrent Cervical Cancer trial, reported in 2014 (Table 3) [62]. Cediranib is a tyrosine kinase inhibitor of VEGF receptors 1, 2, and 3. This was a randomized, double-blind, phase II trial of women with recurrent or metastatic cervical cancer with no previous exposure to cisplatin, except for radiosensitizing cisplatin. Women were dosed with cediranib, 20 mg orally daily, or matched placebo, in combination with carboplatin AUC5 and paclitaxel, 175 mg/m2 three times weekly. This was continued until documented disease progression or inability to tolerate adverse events. A total of 69 patients were randomly assigned; 79% completed 6 cycles of chemotherapy. Median PFS for the cediranib and placebo arms was 35 weeks and 30 weeks, respectively (HR, 0.61; 80% CI, 0.41–0.89; p = .046). No significant difference in OS was demonstrated. The overall response rate with cediranib was 66% (80% CI, 53%–77%) compared with 42% with placebo (80% CI, 30%–55%). Cediranib showed significantly more grade 3 or 4 neutropenia than placebo (31% vs. 9%; p = .019). Additionally, 50% experienced grade 2, 3, or 4 diarrhea and 34% experienced grade 2, 3, or 4 hypertension. Given this agent’s manageable toxicity but lack of demonstrated improvement in overall survival, further assessment of this agent may be warranted.
On the basis of translational studies defining the molecular components influencing angiogenesis, there are many promising targets for upcoming research. Although this list is far from complete given the rapid advancement in this field, we briefly summarize here the use of RNA, growth factor suppression, cytotoxic therapy adjuncts, and kinase inhibitors.
As described earlier in this review, matrix metalloproteinases and CHI3L1 are intriguing targets. Additionally, forkhead box protein (FOX)-M1 is a proto-oncogene critical to cell cycle progression, and it has been well studied in several solid tumors. Li et al. established in 2014 that FOXM1 is unregulated in cervical cancer tissues and that micro-RNA (miRNA), specifically miR-342-3p, may counteract these actions. It serves to inhibit cell proliferation, migration, and invasion and may be a possible therapeutic target [66]. In another variation of RNA utilization, VEGF small hairpin RNA (shRNA) plasmids can affect angiogenesis. A combination of shRNA and radiotherapy as a combined therapy showed inhibition of VEGF expression (p < .05), induced apoptosis (p > .05), downregulation of HIF-1α and overall reduced angiogenesis when compared in a mouse xenograft model to bank control and either monotherapy with shRNA or radiotherapy. This enhanced radiosensitivity offers promise for further clinical evaluation [67]. An additional proposed target is sphingosine kinase (SPHK) 1, an oncogenic kinase that, when upregulated, has an established role in solid tumor development and progression. Its expression is significantly increased in cervical cancer via immunohistochemistry and is associated with advanced clinical features, as indicated by greater invasion depth, worse International Federation of Gynecology and Obstetrics stage, increased tumor size, and lymphovascular invasion. This translates into lower overall survival and recurrence-free survival. In vivo studies with SPHK inhibitors significantly decreased tumor burden. These findings support the use of SPHK as a prognostic marker as well as targeted molecular therapy [68].
Monotherapy with sunitinib was evaluated by Mackay et al. as described previously in this review and had insufficient activity and high morbidity in a phase II trial [61]. In 2014, Abdel-Aziz et al. proposed that chloroquine augmented sunitinib cytotoxicity on the basis of studies of cervical cancer cell lines. Specifically, apoptosis was enhanced and angiogenic pathways were inhibited. Although data are not available regarding improved tolerability, this intriguing adjunctive therapy may merit additional evaluation [69].
In vivo models of cervical cancer have been exposed to gallic acid, a well-established Chinese medicinal herb. Gallic acid resulted in suppression of EGFR, among other signaling pathways, and caused a significant decrease in cervical cancer cell proliferation as well as endothelial cell tube formation. Further exploration may be warranted on the basis of this apparent inhibition of growth and angiogenesis [70].
Prognostic Biomarkers
As our understanding of the tumor environment continues to unfold, it is increasingly apparent that expression of certain markers is associated with more aggressive tumor phenotypes and with worse survival outcomes. Table 5 summarizes proposed molecular targets and their translational significance. Many of these genes, proteins, or biomarkers are under investigation or have been evaluated in translational settings only. Currently, there is no clinical standard for prediction of meaningful clinical information given a lack of marker specificity.
Table 5.
Prognostic biomarkers for invasive cervical carcinoma
Several previous studies have established that microvessel density (MVD) as a representation of neovascularization is a hallmark of progression from normal to cervical dysplasia and malignancy [71]. Additionally, increased MVD is associated with worse survival [72, 73]. A large body of evidence is also accruing regarding miRNA because upregulation of oncogenic miRNA and downregulation of tumor suppressive miRNA appear to be integral to tumor development and clinicopathologic features [28]. Additional protein biomarkers may include squamous cell carcinoma antigen, serum fragments of cytokeratin, carcinoembryonic antigen, matrix metalloproteinases, or specific cytokines [74, 75]. This area of study is in its early stages; however, as we further clarify the complex pathways facilitating carcinogenesis and angiogenesis, we will be able to target critical pathways and expand the use of biomarkers for diagnostic and prognostic purposes [76–78].
Several previous studies have established that microvessel density (MVD) as a representation of neovascularization is a hallmark of progression from normal to cervical dysplasia and malignancy. Additionally, increased MVD is associated with worse survival.
Conclusion
The evolution of biologic agents in women with recurrent or metastatic cervical malignancy has spanned two decades. Given the poor prognosis attributed to advanced cervical cancer, the demonstrated improvement in survival with GOG 240 has practice-changing implications. Exciting alternative antiangiogenic therapies show initial promise in phase II trials, and as our understanding of tumor microenvironment and molecular determinants of angiogenesis continues to expand, additional targets for therapy and prognostication will become possibilities. It is a scientific priority to further develop effective and well-tolerated management strategies for this vulnerable population.
Acknowledgment
This work was supported by an NCI T32 Training Grant in Gynecologic Oncology (the Ruth L. Kirschstein Institutional Training Research Grant, 2T32 CA06039611) awarded to the University of California, Irvine.
Author Contributions
Conception/Design: Jill K. Alldredge, Krishnansu S. Tewari
Provision of study material or patients: Krishnansu S. Tewari
Manuscript writing: Jill K. Alldredge, Krishnansu S. Tewari
Final approval of manuscript: Jill K. Alldredge, Krishnansu S. Tewari
Disclosures
Krishnansu S. Tewari: Roche/Genentech, Advaxis (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Greer BE, Koh WJ, Abu-Rustum NR, et al. Cervical cancer. J Natl Compr Canc Netw. 2010;8:1388–1416. doi: 10.6004/jnccn.2010.0104. [DOI] [PubMed] [Google Scholar]
- 4.Thigpen T, Shingleton H, Homesley H, et al. Cis-platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: A phase II study of the Gynecologic Oncology Group. Cancer. 1981;48:899–903. doi: 10.1002/1097-0142(19810815)48:4<899::aid-cncr2820480406>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 6.Frazer IH. Development and implementation of papillomavirus prophylactic vaccines. J Immunol. 2014;192:4007–4011. doi: 10.4049/jimmunol.1490012. [DOI] [PubMed] [Google Scholar]
- 7.Dochez C, Bogers JJ, Verhelst R, et al. HPV vaccines to prevent cervical cancer and genital warts: an update. Vaccine. 2014;32:1595–1601. doi: 10.1016/j.vaccine.2013.10.081. [DOI] [PubMed] [Google Scholar]
- 8.Melsheimer P, Vinokurova S, Wentzensen N, et al. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin Cancer Res. 2004;10:3059–3063. doi: 10.1158/1078-0432.ccr-03-0565. [DOI] [PubMed] [Google Scholar]
- 9.Baker CC, Phelps WC, Lindgren V, et al. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987;61:962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krill LS, Tewari KS. Exploring the therapeutic rationale for angiogenesis blockade in cervical cancer. Clin Ther. 2015;37:9–19. doi: 10.1016/j.clinthera.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Ocejo O, Viloria-Petit A, Bequet-Romero M, et al. Oncogenes and tumor angiogenesis: The HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19:4611–4620. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 12.Toussaint-Smith E, Donner DB, Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. 2004;23:2988–2995. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Bodily JM, Beglin M, et al. Hypoxia-specific stabilization of HIF-1α by human papillomaviruses. Virology. 2009;387:442–448. doi: 10.1016/j.virol.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodily JM, Mehta KP, Laimins LA. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer Res. 2011;71:1187–1195. doi: 10.1158/0008-5472.CAN-10-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu MP, Tzeng CC, Wu LW, et al. Thrombospondin-1 acts as a fence to inhibit angiogenesis that occurs during cervical carcinogenesis. Cancer J. 2004;10:27–32. doi: 10.1097/00130404-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kodama J, Hashimoto I, Seki N, et al. Thrombospondin-1 and -2 messenger RNA expression in invasive cervical cancer: Correlation with angiogenesis and prognosis. Clin Cancer Res. 2001;7:2826–2831. [PubMed] [Google Scholar]
- 17.Liu Z, Shi Y, Meng W, et al. Expression and localization of maspin in cervical cancer and its role in tumor progression and lymphangiogenesis. Arch Gynecol Obstet. 2014;289:373–382. doi: 10.1007/s00404-013-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tewari KS, Monk BJ. New strategies in advanced cervical cancer: From angiogenesis blockade to immunotherapy. Clin Cancer Res. 2014;20:5349–5358. doi: 10.1158/1078-0432.CCR-14-1099. [DOI] [PubMed] [Google Scholar]
- 20.Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 21.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 22.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 23.Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 24.Millauer B, Longhi MP, Plate KH, et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- 25.Choi KS, Bae MK, Jeong JW, et al. Hypoxia-induced angiogenesis during carcinogenesis. J Biochem Mol Biol. 2003;36:120–127. doi: 10.5483/bmbrep.2003.36.1.120. [DOI] [PubMed] [Google Scholar]
- 26.Eskander RN, Tewari KS. Targeting angiogenesis in advanced cervical cancer. Ther Adv Med Oncol. 2014;6:280–292. doi: 10.1177/1758834014543794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li SW, Wu XL, Dong CL, et al. The differential expression of OCT4 isoforms in cervical carcinoma. PLoS One. 2015;10:e0118033. doi: 10.1371/journal.pone.0118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Li B, Xie C. The roles and clinical significance of microRNAs in cervical cancer. Histol Histopathol. 2016;31:131–9. doi: 10.14670/HH-11-666. [DOI] [PubMed] [Google Scholar]
- 29.Huang TH, Chu TY. Repression of miR-126 and upregulation of adrenomedullin in the stromal endothelium by cancer-stromal cross talks confers angiogenesis of cervical cancer. Oncogene. 2014;33:3636–3647. doi: 10.1038/onc.2013.335. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro C, Garcia EA, Morais-Santos F, et al. Lactate transporters and vascular factors in HPV-induced squamous cell carcinoma of the uterine cervix. BMC Cancer. 2014;14:751. doi: 10.1186/1471-2407-14-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Miyake M, Lawton A, et al. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14:310. doi: 10.1186/1471-2407-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francescone RA, Scully S, Faibish M, et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286:15332–15343. doi: 10.1074/jbc.M110.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngernyuang N, Francescone RA, Jearanaikoon P, et al. Chitinase 3 like 1 is associated with tumor angiogenesis in cervical cancer. Int J Biochem Cell Biol. 2014;51:45–52. doi: 10.1016/j.biocel.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Huang Q, Qu QX, Xie F, et al. CD40 is overexpressed by HPV16/18-E6 positive cervical carcinoma and correlated with clinical parameters and vascular density. Cancer Epidemiol. 2011;35:388–392. doi: 10.1016/j.canep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Hammes LS, Tekmal RR, Naud P, et al. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol Oncol. 2008;110:445–451. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Xiao J, Yang Y, et al. COX-2 expression is correlated with VEGF-C, lymphangiogenesis and lymph node metastasis in human cervical cancer. Microvasc Res. 2011;82:131–140. doi: 10.1016/j.mvr.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Barbu I, Crăiţoiu S, Simionescu CE, et al. CD105 microvessels density, VEGF, EGFR-1 and c-erbB-2 and their prognostic correlation in different subtypes of cervical adenocarcinoma. Rom J Morphol Embryol. 2013;54:519–530. [PubMed] [Google Scholar]
- 38.Chen J, Zhang J, Liu X, et al. Overexpression of fibulin-4 is associated with tumor progression and poor prognosis in patients with cervical carcinoma. Oncol Rep. 2014;31:2601–2610. doi: 10.3892/or.2014.3139. [DOI] [PubMed] [Google Scholar]
- 39.Randall LM, Monk BJ, Darcy KM, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:583–589. doi: 10.1016/j.ygyno.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Zhang Y, Wang SZ, et al. Reduced expression of tissue factor pathway inhibitor-2 contributes to apoptosis and angiogenesis in cervical cancer. J Exp Clin Cancer Res. 2012;31:1. doi: 10.1186/1756-9966-31-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshinaga K, Ito K, Moriya T, et al. Roles of intrinsic angiogenesis inhibitor, vasohibin, in cervical carcinomas. Cancer Sci. 2011;102:446–451. doi: 10.1111/j.1349-7006.2010.01812.x. [DOI] [PubMed] [Google Scholar]
- 42.Killough JH, Magill GB, Smith RC. The treatment of amebiasis with fumagillin. Science. 1952;115:71–72. doi: 10.1126/science.115.2977.71. [DOI] [PubMed] [Google Scholar]
- 43.Brem H, Ingber D, Blood CH, et al. Suppression of tumor metastasis by angiogenesis inhibition. Surg Forum. 1991;42:439–441. [Google Scholar]
- 44.Tanaka T, Konno H, Matsuda I, et al. Prevention of hepatic metastasis of human colon cancer by angiogenesis inhibitor TNP-470. Cancer Res. 1995;55:836–839. [PubMed] [Google Scholar]
- 45.Yamaoka M, Yamamoto T, Ikeyama S, et al. Angiogenesis inhibitor TNP-470 (AGM-1470) potently inhibits the tumor growth of hormone-independent human breast and prostate carcinoma cell lines. Cancer Res. 1993;53:5233–5236. [PubMed] [Google Scholar]
- 46.Yanase T, Tamura M, Fujita K, et al. Inhibitory effect of angiogenesis inhibitor TNP-470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res. 1993;53:2566–2570. [PubMed] [Google Scholar]
- 47.Takamiya Y, Friedlander RM, Brem H, et al. Inhibition of angiogenesis and growth of human nerve-sheath tumors by AGM-1470. J Neurosurg. 1993;78:470–476. doi: 10.3171/jns.1993.78.3.0470. [DOI] [PubMed] [Google Scholar]
- 48.Kudelka AP, Levy T, Verschraegen CF, et al. A phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin Cancer Res. 1997;3:1501–1505. [PubMed] [Google Scholar]
- 49.Kudelka AP, Verschraegen CF, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N Engl J Med. 1998;338:991–992. doi: 10.1056/NEJM199804023381412. [DOI] [PubMed] [Google Scholar]
- 50.Eskander RN, Tewari KS. Development of bevacizumab in advanced cervical cancer: Pharmacodynamic modeling, survival impact and toxicology. Future Oncol. 2015;11:909–922. doi: 10.2217/fon.14.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 52.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 53.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 54.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 55.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 56.Wright JD, Viviano D, Powell MA, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol. 2006;103:489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Monk BJ, Sill MW, Burger RA, et al. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: A gynecologic oncology group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zighelboim I, Wright JD, Gao F, et al. Multicenter phase II trial of topotecan, cisplatin and bevacizumab for recurrent or persistent cervical cancer. Gynecol Oncol. 2013;130:64–68. doi: 10.1016/j.ygyno.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schefter T, Winter K, Kwon JS, et al. RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:101–105. doi: 10.1016/j.ijrobp.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 60.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 61.Mackay HJ, Tinker A, Winquist E, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol. 2010;116:163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Symonds P, Gourley C, Davidson S et al. LBA25_PR-CIRCCa: A randomized double blind phase II trial of carboplatin-paclitaxel plus cediranib versus carboplatin-paclitaxel plus placebo in metastatic/recurrent cervical cancer. Presented at European Society for Medical Oncology 2014 Congress; September 28, 2014; Madrid, Spain. [Google Scholar]
- 63.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cervical Cancer. Version 1/2015. NCCN.org. Available at https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed January 21, 2016.
- 65.U.S. Food and Drug Administration. FDA news release. FDA approves Avastin to treat patients with aggressive and late-stage cervical cancer. August 14, 2014. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm410121.htm. Accessed January 15, 2016.
- 66.Li KR, Chu HJ, Lv T, et al. miR-342-3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett. 2014;588:3298–3307. doi: 10.1016/j.febslet.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 67.Qi L, Xing LN, Wei X, et al. Effects of VEGF suppression by small hairpin RNA interference combined with radiotherapy on the growth of cervical cancer. Genet Mol Res. 2014;13:5094–5106. doi: 10.4238/2014.July.7.2. [DOI] [PubMed] [Google Scholar]
- 68.Kim HS, Yoon G, Ryu JY, et al. Sphingosine kinase 1 is a reliable prognostic factor and a novel therapeutic target for uterine cervical cancer. Oncotarget. 2015;6:26746–26756. doi: 10.18632/oncotarget.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdel-Aziz AK, Shouman S, El-Demerdash E, et al. Chloroquine synergizes sunitinib cytotoxicity via modulating autophagic, apoptotic and angiogenic machineries. Chem Biol Interact. 2014;217:28–40. doi: 10.1016/j.cbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Zhao B, Hu M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol Lett. 2013;6:1749–1755. doi: 10.3892/ol.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper RA, Wilks DP, Logue JP, et al. High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clin Cancer Res. 1998;4:2795–2800. [PubMed] [Google Scholar]
- 72.Wiggins DL, Granai CO, Steinhoff MM, et al. Tumor angiogenesis as a prognostic factor in cervical carcinoma. Gynecol Oncol. 1995;56:353–356. doi: 10.1006/gyno.1995.1062. [DOI] [PubMed] [Google Scholar]
- 73.Sharma B, Singh N, Gupta N, et al. Diagnostic modalities of precancerous and cancerous cervical lesions with special emphasis on CD31 angiogenesis factor as a marker. Pathol Res Int. 2013;2013:243168. doi: 10.1155/2013/243168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dasari S, Wudayagiri R, Valluru L. Cervical cancer: Biomarkers for diagnosis and treatment. Clin Chim Acta. 2015;445:7–11. doi: 10.1016/j.cca.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Gadducci A, Guerrieri ME, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Crit Rev Oncol Hematol. 2013;86:104–129. doi: 10.1016/j.critrevonc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Eskander RN, Tewari KS. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr Opin Obstet Gynecol. 2014;26:314–321. doi: 10.1097/GCO.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 77.Monk BJ, Tewari KS. Evidence-based therapy for recurrent cervical cancer. J Clin Oncol. 2014;32:2687–2690. doi: 10.1200/JCO.2014.56.8733. [DOI] [PubMed] [Google Scholar]
- 78.Tewari KS, Monk BJ. Development of a platform for systemic antiangiogenesis therapy for advanced cervical cancer. Clin Adv Hematol Oncol. 2014;12:737–748. [PubMed] [Google Scholar]
- 79.Punt S, Houwing-Duistermaat JJ, Schulkens IA, et al. Correlations between immune response and vascularization qRT-PCR gene expression clusters in squamous cervical cancer. Mol Cancer. 2015;14:71. doi: 10.1186/s12943-015-0350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitsuhashi A, Matsui H, Usui H, et al. Serum YKL-40 as a marker for cervical adenocarcinoma. Ann Oncol. 2009;20:71–77. doi: 10.1093/annonc/mdn552. [DOI] [PubMed] [Google Scholar]
- 81.Punt S, Thijssen VL, Vrolijk J, et al. Galectin-1, -3, and -9 expression and clinical significance in squamous cervical cancer. PLoS One. 2015;10:e0129119. doi: 10.1371/journal.pone.0129119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Hou F, Liu X, et al. Tc17 cells in patients with uterine cervical cancer. PLoS One. 2014;9:e86812. doi: 10.1371/journal.pone.0086812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Subramanyam D, Rajendra W, Lokantha V. Evaluation of soluble CD44 protein marker to distinguish the benign and squamous cell carcinoma cases in cervical cancer patients. Med Oncol. 2014;31:1–7. doi: 10.1007/s12032-014-0139-9. [DOI] [PubMed] [Google Scholar]
- 84.Lin H, Huang CC, Ou YC, et al. High immunohistochemical expression of TGF-β1 predicts a poor prognosis in cervical cancer patients who harbor enriched endoglin microvessel density. Int J Gynecol Pathol. 2012;31:482–489. doi: 10.1097/PGP.0b013e31824c23a4. [DOI] [PubMed] [Google Scholar]
- 85.Ohara K, Tanaka Y, Tsunoda H, et al. Assessment of cervical cancer radioresponse by serum squamous cell carcinoma antigen and magnetic resonance imaging. Obstet Gynecol. 2002;100:781–787. doi: 10.1016/s0029-7844(02)02204-4. [DOI] [PubMed] [Google Scholar]
- 86.Takeda M, Sakuragi N, Okamoto K, et al. Preoperative serum SCC, CA125, and CA19-9 levels and lymph node status in squamous cell carcinoma of the uterine cervix. Acta Obstet Gynecol Scand. 2002;81:451–457. doi: 10.1034/j.1600-0412.2002.810513.x. [DOI] [PubMed] [Google Scholar]
- 87.Gaarenstroom KN, Kenter GG, Bonfrer JMG, et al. Can initial serum cyfra 21-1, SCC antigen, and TPA levels in squamous cell cervical cancer predict lymph node metastases or prognosis? Gynecol Oncol. 2000;77:164–170. doi: 10.1006/gyno.2000.5732. [DOI] [PubMed] [Google Scholar]
- 88.Chou CY, Wang ST, Kuo HC, et al. Serum level of squamous cell carcinoma antigen and tumor size are useful to identify preoperatively patients at high risk of cervical cancer. Cancer. 1994;74:2497–2501. doi: 10.1002/1097-0142(19941101)74:9<2497::aid-cncr2820740917>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 89.Bolli JA, Doering DL, Bosscher JR, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–173. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- 90.Disaia P, Morrow C, Haverback B, et al. Carcinoembryonic antigen in cancer of the female reproductive system. Serial plasma values correlated with disease state. Cancer. 1977;39:2365–2370. doi: 10.1002/1097-0142(197706)39:6<2365::aid-cncr2820390609>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 91.Borras G, Molina R, Xercavins J, et al. Tumor antigens CA 19.9, CA 125, and CEA in carcinoma of the uterine cervix. Gynecol Oncol. 1995;57:205–211. doi: 10.1006/gyno.1995.1126. [DOI] [PubMed] [Google Scholar]
- 92.Ryu HS, Chang KH, Yang HW, et al. High cyclooxygenase-2 expression in stage IB cervical cancer with lymph node metastasis or parametrial invasion. Gynecol Oncol. 2000;76:320–325. doi: 10.1006/gyno.1999.5690. [DOI] [PubMed] [Google Scholar]
- 93.Jung YW, Kim SW, Kim S, et al. Prevalence and clinical relevance of cyclooxygenase-1 and -2 expression in stage IIB cervical adenocarcinoma. Eur J Obstet Gynecol Reprod Biol. 2010;148:62–66. doi: 10.1016/j.ejogrb.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 94.Lein M, Jung K, Laube C, et al. Matrix-metalloproteinases and their inhibitors in plasma and tumor tissue of patients with renal cell carcinoma. Int J Cancer. 2000;85:801–804. doi: 10.1002/(sici)1097-0215(20000315)85:6<801::aid-ijc11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Wu T, Zhang B, et al. Matrix metalloproteinase-9 is a prognostic marker for patients with cervical cancer. Med Oncol. 2012;29:3394–3399. doi: 10.1007/s12032-012-0283-z. [DOI] [PubMed] [Google Scholar]
- 96.Liu DQ, Li FF, Zhang JB, et al. Increased RIPK4 expression is associated with progression and poor prognosis in cervical squamous cell carcinoma patients. Sci Rep. 2015;5:11955. doi: 10.1038/srep11955. [DOI] [PMC free article] [PubMed] [Google Scholar]