This meta-analysis showed that adjuvant trastuzumab therapy was strongly associated with an increased risk of significant congestive heart failure in patients with early breast cancer, particularly after 2 years of use.
Keywords: Trastuzumab, Adjuvant, Congestive heart failure, Breast cancer, HER2, Meta-analysis
Abstract
Background.
The use of trastuzumab has proven to be a successful strategy in patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer; however, it is associated with an increased risk of cardiac dysfunction. We performed an up-to-date, comprehensive meta-analysis to clarify the risk of congestive heart failure (CHF) in patients with early breast cancer receiving different durations of adjuvant trastuzumab with the longest-term follow-up.
Methods.
Eligible studies included randomized control trials of HER2-positive early breast cancer patients with or without trastuzumab in adjuvant chemotherapy. Adequate reporting of CHF data were required for inclusion. Statistical analyses were conducted to calculate the overall incidence, relative risk (RR), and 95% confidence interval (CI) by use of a fixed-effects model.
Results.
Six randomized control trials including 18,111 patients were identified. The overall incidence of high-grade CHF in patients treated with trastuzumab versus placebo was 1.44% (95% CI, 0.79%–2.64%) and the RR was 3.19 (95% CI, 2.03–5.02; p < .00001). In subgroup analysis, the difference in CHF incidence failed to achieve significance. The RR for 8 mg/kg trastuzumab (high dose) was greater than that for 4 mg/kg (low dose) (RR, 6.79, 95% CI, 2.03–22.72, p = .0001; versus RR, 2.64; 95% CI, 1.61–4.32; p = .002). Additionally, higher RRs were observed for patients receiving trastuzumab for 1 year (RR, 3.29; 95% CI, 2.07–5.25) and 2 years (RR, 9.54; 95%CI, 2.19–41.43), but not 9 weeks (RR, 0.50; 95% CI, 0.05–5.49) compared with control groups. No evidence of publication bias was observed.
Conclusion.
Adjuvant trastuzumab therapy was strongly associated with an increased risk of significant CHF in patients with early breast cancer, particularly in 2-year use.
Implications for Practice:
This comprehensive meta-analysis evaluated the risk of congestive heart failure with a usage profile of adjuvant trastuzumab in patients with early breast cancer. Before initiating treatment with trastuzumab, a risk-benefit analysis for individual patients should be critically evaluated, considering that the prognosis is closely related to drug dose and duration of use. Cardiac function should be monitored throughout the treatment period and also during follow-up. Thus, early identification of trastuzumab-related cardiac dysfunction can allow effective medical intervention, elimination of symptoms, recovery of function, and continuation of trastuzumab therapy.
Abstract
摘要
背景. 已证实曲妥珠单抗用于治疗人类表皮生长因子受体 2 (HER2) 阳性乳腺癌患者是成功的策略, 但该药与心功能不全发生风险升高有关。我们开展了一项最新的全面 meta 分析, 旨在明确接受不同疗程的曲妥珠单抗辅助治疗的早期乳腺癌患者在最长时间的随访中发生充血性心力衰竭 (CHF) 的风险。
方法. 符合标准的研究包括在 HER2 阳性早期乳腺癌患者中开展的辅助化疗联合或不联合曲妥珠单抗治疗的随机对照临床试验。入选标准要求研究充分报告 CHF 数据。统计学分析中, 使用固定效应模型计算总发生率、相对危险度 (RR) 和95%置信区间 (CI) 。
结果. 共识别出 6 项随机对照临床试验, 涉及 18111 例患者。曲妥珠单抗与安慰剂相比, 高等级 CHF 的总发生率为 1.44%(95%CI: 0.79%∼2.64%), RR 为 3.19(95%CI: 2.03∼5.02, P<0.00001)。在亚组分析中, CHF 发生率差异未能达到具有统计学意义的水平。曲妥珠单抗 8 mg/kg (高剂量) 的 RR 大于 4 mg/kg (低剂量), RR 分别为 6.79 (95%CI: 2.03∼22.72, P=0.0001) 和 2.64 (95%CI: 1.61∼4.32, P=0.002)。此外, 与对照组患者相比, 接受曲妥珠单抗 1 年和 2 年治疗的患者 RR 较高, 1 年 RR 为 3.29 (95%CI: 2.07∼5.25), 2 年 RR 为 9.54(95%CI: 2.19∼41.43), 而接受 9 周治疗的 RR 则较低 (0.50, 95%CI: 0.05∼5.49)。没有证据显示存在发表偏倚。
结论. 曲妥珠单抗辅助治疗与早期乳腺癌患者中有意义的CHF风险升高具有强相关性, 尤其是接受2年治疗的患者。The Oncologist 2016;21:547–554
对临床实践的提示: 本项全面 meta 分析在早期乳腺癌患者中评价了曲妥珠单抗辅助治疗在不同用量下的充血性心力衰竭发生风险。考虑到曲妥珠单抗给药剂量和疗程与预后密切相关, 因此在开始曲妥珠单抗治疗前, 应严格评价每名患者的风险获益分析。在整个治疗阶段和随访期间应密切监测心功能。因此, 早期鉴别出曲妥珠单抗相关的心功能不全可以进行有效的医学干预, 以减少症状和恢复功能, 并且继续曲妥珠单抗治疗。
Introduction
The use of trastuzumab has proven to be a successful strategy in patients with human epidermal growth factor receptor 2 (HER2)-positive early-stage and metastatic breast cancer [1, 2]. It has also been to shown to improve clinical outcomes in several malignancies, including gastric cancer [3, 4], urothelial carcinoma [5], advanced bladder cancer [6], and non-small-cell lung cancer [7], and it is currently approved by the U.S. Food and Drug Administration for the treatment of these malignancies.
However, the treatment is associated with an increased risk of cardiac dysfunction, which may be attributed to the blockage of HER2 signaling in cardiac myocytes [8]. In particular, congestive heart failure (CHF) is the most serious adverse effect of trastuzumab.
Three previous meta-analyses [9–11] have reported the cardiac risk associated with trastuzumab in several trials of trastuzumab in breast cancer. However, those studies were limited to shorter-duration trastuzumab use, and the CHF profile of 2-year trastuzumab was not reported. Patients with early-stage breast cancer may be treated with adjuvant trastuzumab over long periods; hence this topic is of special importance in these patients. We sought to investigate the incidence and risk of CHF in the most up-to-date meta-analysis of randomized controlled trials (RCTs) of adjuvant trastuzumab in early breast cancer (EBC) patients.
Methods
Data Sources and Selection of the Studies
We reviewed PubMed citations from January 1966 to July 2015. The search criteria included only randomized controlled trials published in the English language and the keywords trastuzumab (as well as its commercial name, Herceptin) and breast cancer or adenocarcinoma of the breast. We also searched abstracts and virtual meeting presentations from American Society of Clinical Oncology (ASCO) conferences from January 2004 to July 2015 and abstracts from the San Antonio Breast Cancer Symposium (SABCS) from 2007 to 2015. When more than one publication was identified from the same clinical trial, we used the most recent or complete report of that trial. An independent search using the citation database Web of Science (developed by the Institute for Scientific Information) was conducted to ensure that no clinical trials were missed. The most recent trastuzumab package insert was accessed to identify relevant information [12].
Data Extraction and Clinical Endpoints
Data abstraction was conducted independently by three investigators (H.-D. Long, Y.-E. Lin, and J.-J. Zhang) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13], and any discrepancies among reviewers were resolved by consensus. For each study, we extracted the following information: study characteristics, year of publication, patient characteristics, trastuzumab treatment information, median follow-up duration, and reported CHF events. The primary outcomes were high-grade CHF, including symptomatic CHF, definite cardiac death (due to CHF, myocardial infarction, or primary arrhythmia), or probable cardiac death (sudden death without documented etiology). High-grade CHF (namely symptomatic CHF) was defined as New York Heart Association class III or IV.
Statistical Analysis
For the calculation of incidence, the number of patients with CHF events and patients treated with trastuzumab were extracted from the safety profiles of selected RCTs. The incidence of patients with CHF and 95% confidence intervals (CIs) were derived from each trial. We also calculated relative risk (RR) and 95% CI of CHF events in patients assigned to trastuzumab versus controls in the same trial.
Statistical heterogeneity among trials was estimated by using the Cochran Q statistic [14]. Heterogeneity was considered statistically significant at pheterogeneity <.1. If heterogeneity existed, data were analyzed using a random-effects model. In the absence of heterogeneity, a fixed-effects model was used. A two-tailed p value of less than .05 was considered statistically significant. We also conducted subgroup analysis for trastuzumab loading dose and duration of treatment. Finally, publication bias was evaluated by using Begg’s and Egger’s tests [15, 16]. Analyses were conducted using Review Manager version 5.2 (Cochrane Collaboration, Oxford, U.K., http://www.cochrane.org), Stata/SE version 12.0 (Stata Corp., College Station, TX, http://www.stata.com), and R statistical software, version 2.15.0 (https://www.r-project.org).
Results
Search Result
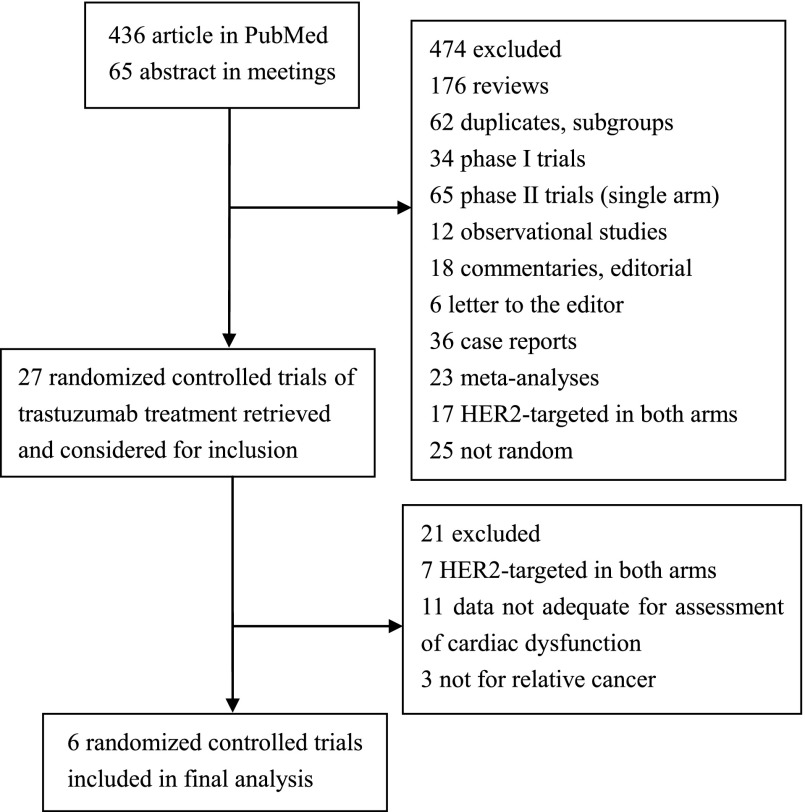
Our search yielded a total of 601 trastuzumab studies: 536 abstracts from PubMed and 65 from ASCO or SABCS meetings. After evaluating each study, 543 were initially excluded. We carefully screened each of the remaining 27 trials and excluded an additional 20 trials for being duplicates, subgroup analysis only, or combined analysis. In the FinXX trial, CHF events were not reported [17]. Finally, six trials were selected for inclusion in the meta-analysis [18–24]. HERA [23] and BIG1-01 [24] were the same trial, and reports of CHF events were the same. A detailed selection process is represented in Figure 1.
Figure 1.
Flow diagram of studies identified, included, and excluded.
Abbreviation: HER2, human epidermal growth factor receptor 2.
Trial Characteristics
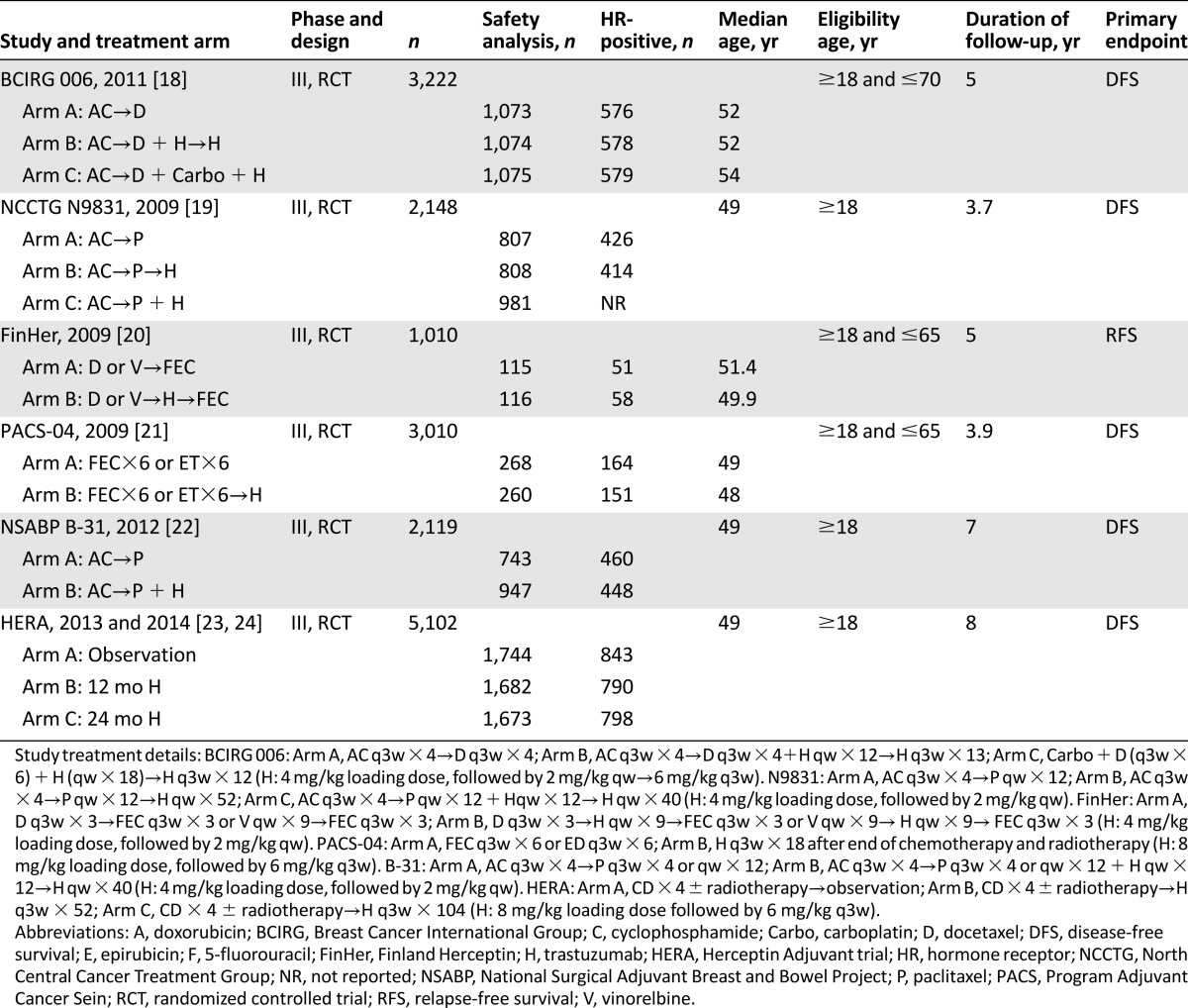
The baseline characteristics of each trial are listed in Table 1. Six RCTs (five large [18, 19, 21, 22, 24] and one small [20]) were open, phase III, prospective, multicenter trials. Participants were randomly assigned to receive treatment with or without trastuzumab.
Table 1.
Characteristics of randomized controlled clinical trials included in the meta-analysis
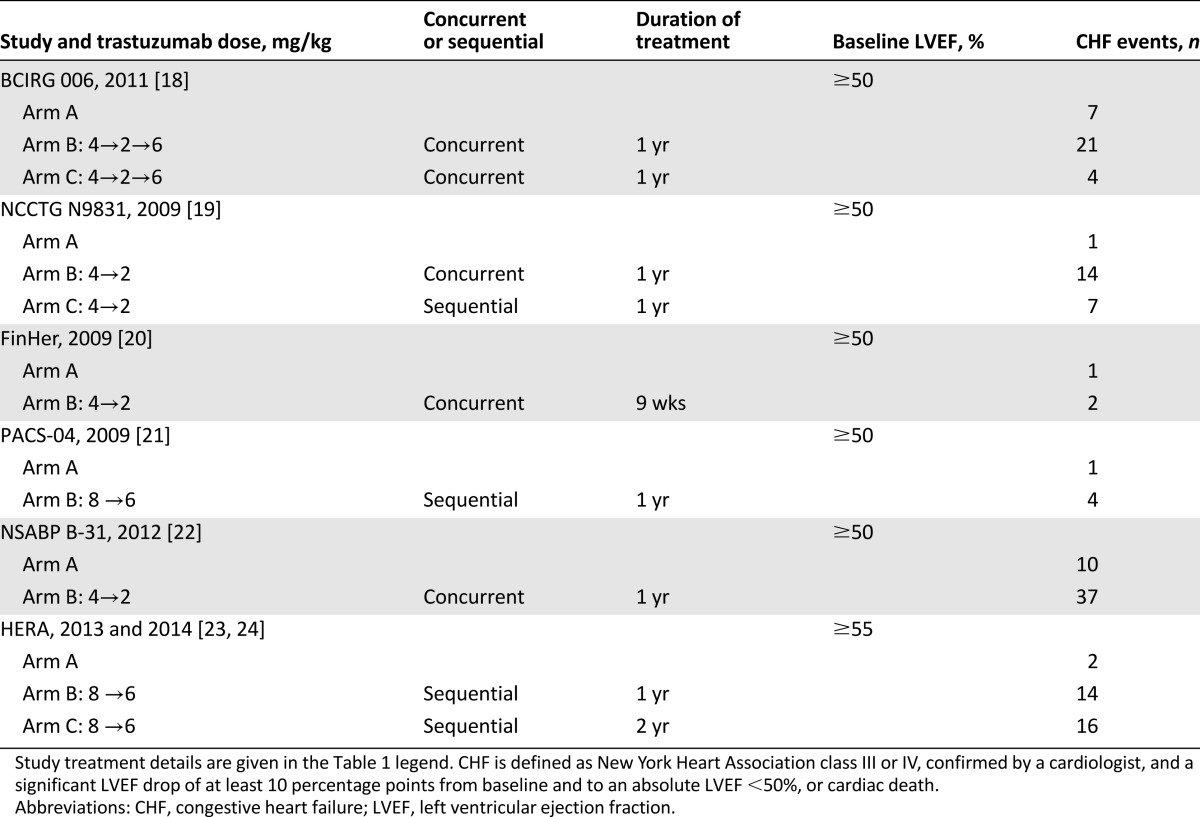
Data from 18,111 patients were available for the meta-analysis. All selected trials included early-stage patients with breast cancer, with adequate organ function, coagulation, and hematologic function. All the trials typically excluded patients with uncontrolled hypertension, clinically significant congestive heart failure, cerebrovascular disease, peripheral vascular disease, unstable angina, or recent history of myocardial infarction. All trials allowed inclusion of patients with prior anthracycline exposure; moreover, all trials treated patients with concomitant anthracycline and patients with HER2-positive breast disease. CHF events were reported in each trial (Table 2).
Table 2.
Summary of CHF events with trastuzumab
The longest median follow-up was 8 years in the HERA trial, which was the only one with a 2-year duration of trastuzumab, followed by the NASBP B-31, with a median follow-up of 7 years. FinHer and BCIRG 006 both had 5-year median follow-up times. In short, two-thirds of the trials had a median follow-up of ≥5 years.
Incidence of CHF
The overall incidence of CHF among 8,615 patients receiving trastuzumab from six trials was 1.44% (95% CI 0.79%–2.64%) according to the random-effects model (Q = 45.19; p < .0001; I2 =89%) (Table 3).
Table 3.
Incidence and RR of CHF for patients with breast cancer who were treated with trastuzumab, stratified by trastuzumab loading dose and duration of treatment
RR of CHF
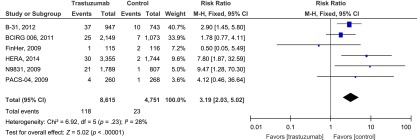
To define the particular contribution of trastuzumab to the development of CHF and exclude the influence of confounding factors, we determined the overall RR of CHF from the six RCTs. The overall RR of high-grade CHF with trastuzumab was 3.04-fold higher (95% CI 1.12- to 7.85-fold; p < .00001) than for patients who did not receive trastuzumab. No significant heterogeneity among the included trials was observed (Q = 6.92; p = .23; I2 =28%) (Fig. 2).
Figure 2.
Relative risk of high-grade congestive heart failure events associated with trastuzumab versus control among patients with breast cancer.
Abbreviations: BCIRG, Breast Cancer International Group; CI, confidence interval; FinHer, Finland Herceptin; HERA, Herceptin Adjuvant trial; M-H, Mantel-Haenszel; PACS, Program Adjuvant Cancer Sein.
Influence of Trastuzumab Dose
There are two approved doses for trastuzumab, 4.0 mg/kg (low loading dose) [18–20, 22] and 8.0 mg/kg (high loading dose) [21, 24]. Therefore, we attempted to look at incidence and RR stratified by trastuzumab dose. The incidence rates of CHF were 0.95% (95% CI, 0.68%–1.33%) and 1.64% (95% CI, 0.74%–3.57%) (Table 2) for the high and low doses, respectively, with no statistically significant difference (p = .23). Nevertheless, the overall RRs of CHF for the high and low doses were 6.79 (95% CI, 2.03–22.73) and 2.64 (95% CI, 1.62–4.33), respectively, for which a significant difference was found (p < .00001) (Table 3).
Influence of Duration of Trastuzumab
The duration of trastuzumab treatment was 1 year [18, 19, 22], 2 years [23, 24], or 9 weeks [21], with CHF incidence of 1.45% (95% CI 0.78%–2.66%), 1.09% (95% CI, 0.67%–1.78%), and 0.94% (95% CI, 0.19%–4.51%), respectively. The respective RRs were 3.29 (95% CI, 2.07–5.25), 9.54 (95% CI, 2.19–41.43), and 0.50 (95% CI, 0.05–5.49). The difference in incidence was not statistically significant (p = .75), but the RR was (p < .00001) (Table 3).
Sensitivity Analysis
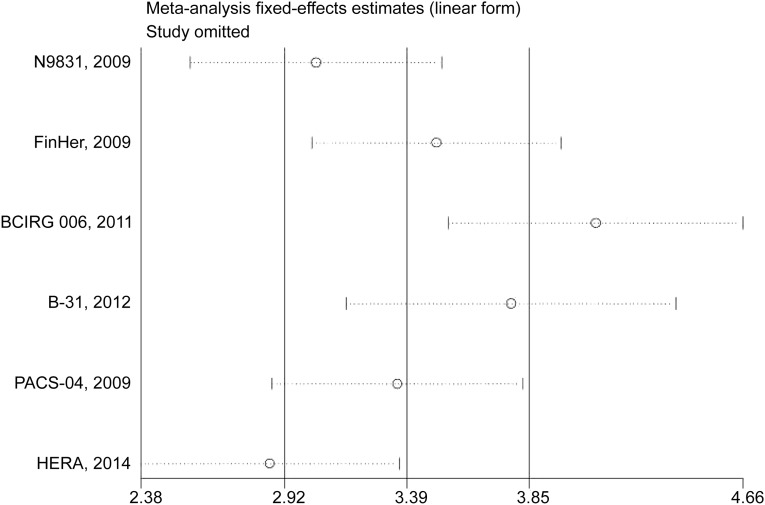
We deleted each study one at a time from the overall pooled analysis to check the influence of the removed data set to the overall RR. Two studies (BCIRG 006 [18] and HERA [24]) materially changed the between-study heterogeneities in trastuzumab dose and duration of trastuzumab, respectively. After the deletion of either of the two studies, the heterogeneity vanished, whereas the conclusion stayed significant (Fig. 3).
Figure 3.
Sensitivity analysis for the influence of individual studies on the overall RR for the association between trastuzumab and congestive heart failure events. The vertical midline indicates the overall RR; the lateral vertical lines indicate its 95% CI; and circles indicate the pooled RR when the left study is omitted in this meta-analysis. The two ends of every dotted line represent the respective 95% CI.
Abbreviations: BCIRG, Breast Cancer International Group; CI, confidence interval; FinHer, Finland Herceptin; HERA, Herceptin Adjuvant trial; PACS, Program Adjuvant Cancer Sein; RR, relative risk.
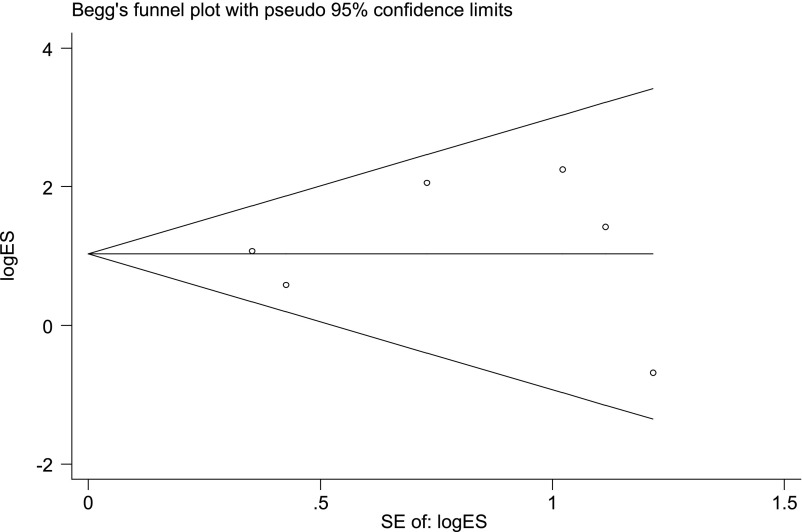
Publication Bias
No obvious publication bias was detected for the RR of CHF in trastuzumab-treated patients with breast cancer by either Egger’s (p = .711) or Begg’s (p = .707) test (Fig. 4).
Figure 4.
Funnel plot studies evaluating the relative risk of high-grade congestive heart failure events associated with trastuzumab versus control patients with breast cancer. Egger’s test, p = .711; Begg’s test, p = .707.
Discussion
To our knowledge, this is the first long-follow-up report to show a significant increase in the risk of CHF in EBC patients treated with adjuvant trastuzumab. The overall incidence of clinically significant (high-grade) CHF was 1.44%. Despite the reasonably low absolute incidence, our results demonstrate an overall increase of threefold in the CHF risk of trastuzumab-treated patients compared with controls. Patients with EBC may be at an especially increased risk of CHF because of their trastuzumab use profile or other exposure factors in the adjuvant setting.
Stratified analyses were performed to evaluate subgroups with potentially higher incidence of CHF. No different incidence rate was found between high and low doses of trastuzumab, but sevenfold and threefold higher RRs were observed with high and low doses versus no trastuzumab, indicating that dose is closely related to risk of CHF. This also suggests that the low dose may already be reaching the saturation level to impair cardiac function. Other crucial findings include a 3.29-fold higher risk when using trastuzumab for 1 year and a 9.54-fold higher risk for 2 years compared with patients who did not receive trastuzumab. However, caution should be taken when analyzing these subgroups; because of the RCT setting, we are not able to directly determine whether the analysis is more significant or simply equivalent.
Consonant with previous meta-analysis [11], the incidence of CHF was still low among patients receiving trastuzumab, but the risk of CHF was increased by approximately three to four times compared with controls. Unlike previous studies limited to shorter-term follow-up that did not simultaneously quantify heterogeneity according to trastuzumab dose and duration of use, our study has updated currently available data of trastuzumab-associated CHF in EBC patients with longer-term follow-up, for example 5 years [18, 20], 7 years [22], and 8 years [24]. In addition, the incidence of CHF in the 2-year use duration of the HERA trial was also low; intriguingly, CHF events tended to become more uncommon at the later 5-year follow-up.
Unlike that of anthracycline, trastuzumab-related cardiac dysfunction is not dose dependent and is generally reversible [25]. As recommended in the package insert [12], trastuzumab is administered in an initial loading dose of 4 mg/kg and a once-weekly maintenance dose of 2 mg/kg until disease progression or metastasis. Nonetheless, in the adjuvant setting, trastuzumab can be given with an initial loading dose of 8 mg/kg and 6 mg/kg every 3 weeks in addition to this weekly dosing. From our available data for analysis (only HERA and PACS-04 trials used 8 mg loading dose and 6 mg maintenance; the other trials [NCCTG N9831, FinHer, BCIRG 006, and NSABP B-31] used the 4-mg dose), the higher dose carried a 6.79-fold higher risk for CHF, compared with 2.64-fold for the lower dose, versus the observation group. This difference between the two dose regimens is significant (p < .00001), suggesting that dose may be closely associated with the risk of CHF. Because of the RCT setting, CHF data could have been recorded more strictly than in general practice. As a result, our study provides evidence that high-dose trastuzumab indirectly causes a greater risk of CHF than low-dose therapy.
To determine whether duration of adjuvant trastuzumab treatment can affect the risk of CHF, we carried out a subgroup analysis. Similar improvements in progression-free and overall survival were found for for 2 years versus 1 year of trastuzumab use [18, 22–24]. The 2-year arm of HERA showed the highest RR for CHF during adjuvant trastuzumab; 1-year treatment demonstrated that the cumulative RR of CHF tends to plateau after treatment completion; and the 9-week duration in FinHer failed achieve statistically different RRs for CHF, with no benefit to overall survival. These data suggest that 1 year might be the ideal use of trastuzumab for early-stage breast cancer patients (supplemental online Fig. 1).
Because the patients enrolled in clinical trials had already been screened for cardiac function, and age has been regarded as a risk factor of cardiac toxicity [26], our meta-analysis might underreport the cardiac toxicity, because some older patients with cancer might be excluded owing to suboptimal cardiac function or other complications, leading to a lower observable incidence of CHF. Moreover, all of these studies excluded patients with any cardiac disease, and this is partly why the incidence seems lower than in clinical experience. Recently proposed guidelines for patients receiving trastuzumab recommend a monitoring schedule that assesses baseline and on-treatment cardiac function and intervention strategies with cardiovascular medication to improve cardiac status before, during, and after treatment. If severe CHF occurs, it is strongly recommended to stop trastuzumab therapy [27, 28].
Recently proposed guidelines for patients receiving trastuzumab recommend a monitoring schedule that assesses baseline and on-treatment cardiac function and intervention strategies with cardiovascular medication to improve cardiac status before, during, and after treatment. If severe CHF occurs, it is strongly recommended to stop trastuzumab therapy.
The strengths of our pooled analysis are the large sample, the assessment of CHF risk according to trastuzumab use, and the consistency of the primary findings and the internal validation study. The principal limitation of our analysis is the unavailability of individual patient information. Therefore, establishment of risk factors for the development of CHF (including prior exposure to cardiotoxic agents) or of potentially contributing cocomorbidities (including prior cardiovascular disease) is not possible in this analysis. In contrast, in a retrospective cohort study of trastuzumab- and anthracycline-based breast cancer therapy in 12,500 patients, an increased rate of CHF (12.1%) was observed in a community-based setting, in which a higher prevalence of cardiac risk factors might exist [29]. It is important to point out that expanded access programs are usually designed to include confounding factors, so the incidence of CHF might not truly reflect the actual trastuzumab-related CHF risk among EBC patients, whether consistent with the clinical trials or not. In a recent report, trastuzumab treatment was found to be associated with a significant increase in the risk of severe hypertension [30]. Therefore, an increase in the risk of CHF may have been secondary to increased incidence of severe hypertension. However, we could not relate the incidence of CHF to secondary hypertension, as neither causality nor association was reported in any trial. It is also important to note that death, significant disability from high-grade CHF, and timing of CHF were not captured in the studies analyzed; therefore, the impact of trastuzumab on these aspects cannot be determined. These are typical disadvantages inherent to meta-analysis of literature-based studies, and only individual patient data will be able to answer these questions and establish clinical risk factors for trastuzumab-induced CHF. Nevertheless, meta-analysis from individual patient data can also carry significant bias, as data are available to limited numbers of research groups and for only a few types of studies that have high public health priority; consequently, when an opportunity arises, pooled analysis may be possible in the future.
Conclusion
Trastuzumab significantly increases risk of high-grade CHF in the longest-term follow-up of HER2-positive patients with EBC compared with control groups. A higher risk of trastuzumab-associated CHF compared with no trastuzumab was seen with a loading dose of 8 mg/kg and treatment duration of 2 years, irrespective of other benefits. The findings have important implications for physicians and investigators to optimize the balance between oncologic clinical benefit and life-threatening adverse events; this adverse effect should be closely monitored. Importantly, more optimal therapeutic strategies need to be investigated.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by grants from the Medical Science and Technology Project of Guangzhou City in China (20142A011024, 20152A011023, and 2016A013010049).
Footnotes
For Further Reading: Graeme J. Koelwyn, Nia C. Lewis, Susan L. Ellard et al. Ventricular-Arterial Coupling in Breast Cancer Patients After Treatment With Anthracycline-Containing Adjuvant Chemotherapy. The Oncologist 2016;21:141–149.
Implications for Practice: Anthracycline-induced cardiotoxicity results in significantly impaired ventricular-arterial coupling in the years following chemotherapy, owing specifically to decreased left ventricular contractility. This subclinical dysfunction was identified only under exercise stress. A comprehensive evaluation of vascular structure and function yielded no differences between those treated with anthracyclines and controls. Combined with a stress stimulus, ventricular-arterial coupling might hold significant value beyond characterization of integrative cardiovascular function, in particular as a part of a risk-stratification strategy after anthracycline-containing chemotherapy. Although vascular function and structure were not different in this cohort, this does not undermine the importance of identifying vascular (dys)function in this population, because increases in net arterial load during exercise might amplify the effect of reductions in contractility on cardiovascular function after anthracycline-containing chemotherapy.
Author Contributions
Conception/Design: Hui-Dong Long
Provision of study material or patients: Hui-Dong Long
Collection and/or assembly of data: Hui-Dong Long, Yun-En Lin, Rui-Nian Zheng
Data analysis and interpretation: Juan-Juan Zhang, Rui-Nian Zheng
Manuscript writing: Hui-Dong Long, Wen-Zhao Zhong
Final approval of manuscript: Hui-Dong Long
Disclosures
The authors indicated no financial relationships.
References
- 1.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Fujita T. Trastuzumab for gastric cancer treatment. Lancet. 2010;376:1735–; author reply 1735–1736. doi: 10.1016/S0140-6736(10)62125-3. [DOI] [PubMed] [Google Scholar]
- 5.Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: Results of a multicenter phase II National Cancer Institute trial. J Clin Oncol. 2007;25:2218–2224. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 7.Altundag K, Altundag O, Morandi P, et al. Targeted therapy for targeted patients: trastuzumab in adjuvant treatment of non-small-cell lung cancer. J Clin Oncol. 2005;23:1325–; author reply 1326–1327. doi: 10.1200/JCO.2005.05.192. [DOI] [PubMed] [Google Scholar]
- 8.Tarantini L, Gori S, Faggiano P, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: A multicenter cohort analysis. Ann Oncol. 2012;23:3058–3063. doi: 10.1093/annonc/mds127. [DOI] [PubMed] [Google Scholar]
- 9.Viani GA, Afonso SL, Stefano EJ, et al. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahabreh IJ, Linardou H, Siannis F, et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: A systematic review and meta-analysis of randomized controlled trials. The Oncologist. 2008;13:620–630. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 11.Chen T, Xu T, Li Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: A meta-analysis. Cancer Treat Rev. 2011;37:312–320. doi: 10.1016/j.ctrv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Genentech. Package Insert: Herceptin (Trastuzumab). Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/trasgen020900lb.htm. Accessed February 29, 2016.
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269, W264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Outcome of patients with HER2-positive breast cancer treated with or without adjuvant trastuzumab in the Finland Capecitabine Trial (FinXX) Acta Oncol. 2014;53:186–194. doi: 10.3109/0284186X.2013.820840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halyard MY, Pisansky TM, Dueck AC, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. 2009;27:2638–2644. doi: 10.1200/JCO.2008.17.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: Final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 21.Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 22.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 24.de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32:2159–2165. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 25.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 26.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 27.Carver JR. Management of trastuzumab-related cardiac dysfunction. Prog Cardiovasc Dis. 2010;53:130–139. doi: 10.1016/j.pcad.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Jones AL, Barlow M, Barrett-Lee PJ, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: Updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100:684–692. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrmann J, Herrmann SM, Haddad TC. New-onset heart failure in association with severe hypertension during trastuzumab therapy. Mayo Clin Proc. 2014;89:1734–1739. doi: 10.1016/j.mayocp.2014.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.