The recognized incidence of intrahepatic cholangiocarcinoma in the U.S. continues to rise, whereas the incidence of extrahepatic cholangiocarcinoma is stable. The incidence of carcinoma of unknown primary has fallen dramatically during the same time period.
Keywords: Intrahepatic cholangiocarcinoma, Extrahepatic cholangiocarcinoma, Incidence, Epidemiology, Trend, Cancer of unknown primary
Abstract
Background.
Challenges in the diagnosis and classification of cholangiocarcinoma have made it difficult to quantify the true incidence of this highly aggressive malignancy.
Methods.
We analyzed the Surveillance, Epidemiology, and End Results data to assess long-term trends in the age-standardized incidence of intrahepatic and extrahepatic cholangiocarcinoma between 1973 and 2012, correcting for systematic coding errors. Because intrahepatic cholangiocarcinoma (ICC) may frequently be misdiagnosed as cancer of unknown primary (CUP), we also analyzed trends in the incidence of CUP.
Results.
Between 1973 and 2012, the reported U.S. incidence of ICC increased from 0.44 to 1.18 cases per 100,000, representing an annual percentage change (APC) of 2.30%; this trend has accelerated during the past decade to an APC of 4.36%. The incidence of extrahepatic cholangiocarcinoma increased modestly from 0.95 to 1.02 per 100,000 during the 40-year period (APC, 0.14%). The incidence of CUP with histologic features potentially consistent with cholangiocarcinoma decreased by 51% between 1973 and 2012 (APC, −1.87%), whereas the incidence of CUP with squamous or nonepithelial histologic features increased modestly (APC, 0.42%).
Conclusion.
The recognized incidence of ICC in the U.S. continues to rise, whereas the incidence of ECC is stable. The incidence of CUP has fallen dramatically during the same time period.
Implications for Practice:
Clinical distinctions between cholangiocarcinoma (particularly intrahepatic cholangiocarcinoma [ICC]) and cancer of unknown primary (CUP) can be challenging. Recent discoveries have identified recurrent and potentially targetable genomic abnormalities in ICC, highlighting the importance of improving diagnosis. This study demonstrates that the incidence of ICC is increasing in the U.S., whereas the incidence of extrahepatic cholangiocarcinoma is stable. Concomitantly, the incidence of CUP has declined dramatically, suggesting that improved distinction between ICC and CUP may be a major driver of the increasing recognized incidence of ICC. The increasing incidence of ICC warrants further study of prevention and treatment approaches.
Abstract
摘要
背景. 在胆管癌诊断和分类中存在的挑战使得这种高度侵袭性恶性肿瘤的真实发病率难以量化。
方法. 我们对监测、流行病学及预后 (SEER) 项目中的数据进行了分析, 评估1973-2012年间肝内和肝外胆管癌年龄标化发病率的长期变化趋势 (根据系统编码误差校正) 。由于肝内胆管癌 (ICC) 常可能被误诊为原发部位不明的癌症 (CUP), 我们还对 CUP 发病率的变化趋势进行了分析。
结果. 1973-2012 年间, 美国 ICC 发病率从 0.44/10 万人增长到 1.18/10 万人, 相当于年变化率 (APC) 为 2.30%。这一趋势在过去十年间进一步加剧, APC 提高至 4.36%。肝外胆管癌在 40 年间从 0.95/10 万人略增加到 1.02/10 万人 (APC: 0.14%)。 1973-2012 年间, 组织学特征可能与胆管癌一致的 CUP 发病率下降了 51% (APC: -1.87%), 而鳞癌或非上皮性组织学特征的 CUP 的发病率则略有升高 (APC: 0.42%)。
结论. 在美国, 确诊的ICC发病率持续升高, 而ECC发病率保持稳定。而相同时期的CUP发病率则呈现大幅下降。The Oncologist 2016;21:594–599
对临床实践的提示: 对胆管癌[尤其是肝内胆管癌 (ICC) ]与原发部位不明的癌症 (CUP) 进行临床鉴别富有挑战性。 ICC 中近期识别出来的的复发和潜在靶位基因组异常强调了改进诊断的重要性。本研究证实美国的 ICC 发病率在持续升高, 而肝外胆管癌的发病率保持稳定。同一时期, CUP 的发病率则大幅下降, 提示 ICC 与 CUP 鉴别的进步可能是确诊的 ICC 发病率升高的主要驱动因素。 ICC 持续升高的发病率提示有必要针对其预防和治疗手段开展进一步的研究。
Introduction
Cholangiocarcinoma is an aggressive epithelial malignancy of the bile ducts that often presents with locally advanced or metastatic disease and carries an extremely poor prognosis. Named after its presumed cell of origin, cholangiocarcinoma tumors can arise from anywhere in the biliary tract and may be difficult to identify on the basis of histopathologic analysis alone. Cholangiocarcinoma is subclassified anatomically, with intrahepatic cholangiocarcinoma (ICC) arising from within the liver and extrahepatic cholangiocarcinoma (ECC) arising from the extrahepatic bile ducts; however, ongoing challenges in tumor registry data have made it difficult to accurately estimate the true incidence of this disease.
During the past 15 years, many studies have raised the concern that the incidence of and mortality related to ICC are on the rise worldwide, whereas the incidence of ECC has remained relatively stable over time [1–8]. However, because most patients with ICC have no clearly identified risk factors for the disease, the rising incidence of ICC has not been linked to any specific exposure, risk factor, or demographic trend. Recent reports have suggested that at least some of the reported increase in ICC may be an artifact of changes in the World Health Organization’s International Classification of Diseases for Oncology (ICD-O) coding system, which is used for tumor registry reporting, rather than a true increase in the burden of disease [1, 7, 8]. Specifically, overdiagnosis of ICC is suspected to have occurred between 1992 and 2000—when ICD-O, version 2, was in use—related to the misclassification of perihilar (Klatskin) tumors as ICC instead of ECC. As a result, the magnitude of the observed worldwide increase in ICC and its underlying causes remains unclear.
In addition to discrepancies in its classification, the simple recognition and diagnosis of cholangiocarcinoma, particularly ICC, often prove challenging, and the condition remains a diagnosis of exclusion [9–11]. Immunohistochemistry (IHC) revealing positive staining for cytokeratin (CK) 7 and CK19 and negative staining for HepPar1 may help distinguish cholangiocarcinoma from hepatocellular carcinoma [12]. ECC usually presents with an obstructing tumor in the extrahepatic bile ducts [9]. However, ICC typically presents as adenocarcinoma within the liver and therefore must be distinguished from metastatic disease arising from several potential primary sites, including breast, lung, pancreas, and gastrointestinal tract [12]. Unfortunately, ICC may share a common IHC profile with many metastatic adenocarcinomas, characterized by positive staining for CK7 and CK19 and negative staining for CK20 [12]. Likewise, the serum markers often used to track ICC tumor burden, CEA and CA19-9, are not specific for this disease [13].
Thus, ICC may be frequently mistaken for metastatic disease from an occult primary site, or cancer of unknown primary (CUP) [14]. Indeed, a recent validated molecular profiling study asserted that biliary tract cancer is the most common tumor of origin in CUP, accounting for 21% of CUP diagnoses [15]. Although this study did not distinguish between biliary tract cancer subtypes, our group recently used a novel RNA in situ hybridization approach to show that as many as 22% of CUP cases involving the liver are actually ICC, specifically, rather than ECC or any other malignancy [16]. Given the relatively high incidence of CUP compared with biliary tract cancer, these data would suggest that the true incidence of ICC may be greatly underestimated.
The proper classification and diagnosis of ICC have recently become even more critical as specific and potent inhibitors are under clinical development for certain oncogenic mutations that are found at high frequencies in ICC but are rare or absent in other epithelial malignancies. These include “hot-spot” point mutations in IDH1/2, found in 10%–36% of tumors, and activating translocations of FGFR2, found in 11%–45% of ICC [17–27]. The significant variability in the frequency of these mutations across studies likely relates to differences in the patient populations examined and to the sequencing technique used to genotype these tumors.
To more accurately investigate long-term incidence trends of ICC and ECC, we interrogated Surveillance, Epidemiology, and End Results (SEER) Program datasets for the 40-year period between 1973 and 2012 and corrected for systematic coding errors that may lead to misclassification between ICC and ECC. We report on 40- and 10-year trends in the estimated U.S. incidence of ICC and ECC. Additionally, we compare salient demographic features of these diseases, including site-specific trends in relative incidence by gender, race, and ethnicity, as well as information on site-specific differences in age at diagnosis and disease stage. Given the strong evidence that ICC is frequently misdiagnosed as CUP, we also investigated incidence trends for CUP, focusing on histologic features that are potentially consistent with ICC, to provide new insight into the true burden of cholangiocarcinoma during the past 40 years.
Materials and Methods
Our study used data from the National Cancer Institute’s SEER program [28]. These data allow population-based estimates of cancer incidence in the U.S., covering the time period from 1973 through 2012. Cholangiocarcinoma was identified by diagnostic codes from the World Health Organization’s ICD-O coding system, using diagnostic coding criteria enumerated by Tyson et al. [7]. ICC was identified with a topography code of C22.0 (liver) and a histology code of 8140, 8160, 8161, 8480, 8481, or 8500 or with a topography code of C22.1 (intrahepatic bile ducts) and a histology code of 8000, 8010, 8020, 8140, 8160, 8161, 8260, 8480, 8481, 8490, or 8500. ECC was identified with a topography code of C24.1 (extrahepatic biliary ducts) and a histology code of 8000, 8010, 8020, 8140, 8160, 8161, 8260, 8480, 8481, 8490, or 8500, or for any case with a topography code of C22.0, C22.1, or C24.0 and a histology code of 8162 (Klatskin tumor). These histology codes identify adenocarcinomas as well as undifferentiated and unspecified histologic types (including “carcinoma, NOS [not otherwise specified]” and “neoplasm, malignant”) but exclude squamous histologic types and other confirmed histologic types that are generally inconsistent with cholangiocarcinoma. In a revision of the approach of Tyson et al., we included cancers with the topography-histology code pairing of C24.1 and 8020 (carcinoma, undifferentiated type, NOS); Tyson et al. included the histology code 8020 only when paired with topography code C22.1.
The approach avoids misclassification of Klatskin tumors as ICC, a problem that has been cited as potentially contributing to inflated estimates of ICC incidence between 1992 and 2000, when ICD-O, version 2, was in use [7]. We prospectively excluded cancer cases with topography codes of C24.8 (overlapping sites of the biliary tract) or C24.9 (malignant neoplasm of the biliary tract, unspecified) because these codes are infrequently used and cannot be reliably mapped to ICC, ECC, or other biliary tumor sites (such as the gallbladder or the ampulla of Vater).
Because the liver is a common site for metastatic cancer, ICC may be misdiagnosed as CUP [14, 15]. Therefore, it is likely that improvements in diagnostic technologies are contributing to the increased recognized incidence of ICC. To evaluate this possibility, we assessed long-term trends in the incidence of CUP, with stratification for histology codes that are potentially consistent with cholangiocarcinoma (i.e., ICD-O, version 3, histology codes included in our analysis for identification of cholangiocarcinoma) versus CUPs with histology codes that are not consistent with cholangiocarcinoma (e.g., CUPs with confirmed squamous or nonepithelial histology).
We calculated site-specific annual incidence rates using SEER*Stat [29], and we report 40- and 10-year incidence trends for ICC, ECC, and CUP. Data from the SEER 9 cancer registries were used for incidence rate estimates from 1973 to 1991, the SEER 13 registries provided data from 1992 to 1999, and the SEER 18 registries were used from 2000 to 2012 (all from the November 2014 data submission). This approach allowed us to use the most robust national estimates for disease incidence available for each calendar year in our analysis. All incidence rates are age-standardized to the 2000 U.S. population. Annual percentage change (APC) was calculated for 40- and 10-year periods by linear regression of log-transformed annual incidence rates [30]. Percentage change was calculated by using a 2-year average of the incidence rates observed at the beginning and end of the time period. In addition to overall population incidence, we also report relative incidence of ICC and ECC, stratified by gender, race (white vs. black or Asian), and ethnicity (non-Hispanic vs. Hispanic). Changes in relative incidence over time were calculated using linear regression of annual incidence rate ratio (IRR) estimates. Descriptively, IRR trends were reported with data aggregated over 5-year periods. Additional comparative data reported include the distribution of age and cancer stage among patients with incident ICC and ECC. Cancer stage information reported in the SEER database uses version 6 of the American Joint Committee on Cancer staging manual [31]. Statistical analyses were completed with SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
This study was completed with approval from the Dana-Farber/Harvard Cancer Center Institutional Review Board, protocol #15-296.
Results
Cholangiocarcinoma Incidence
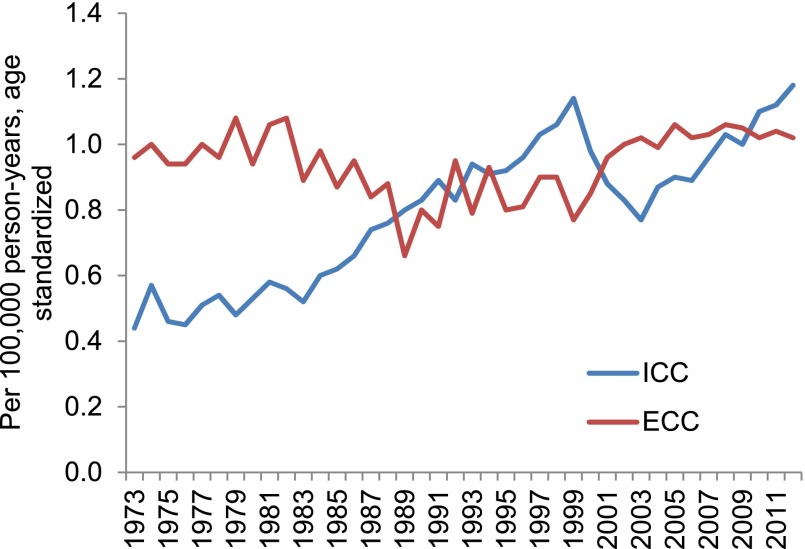
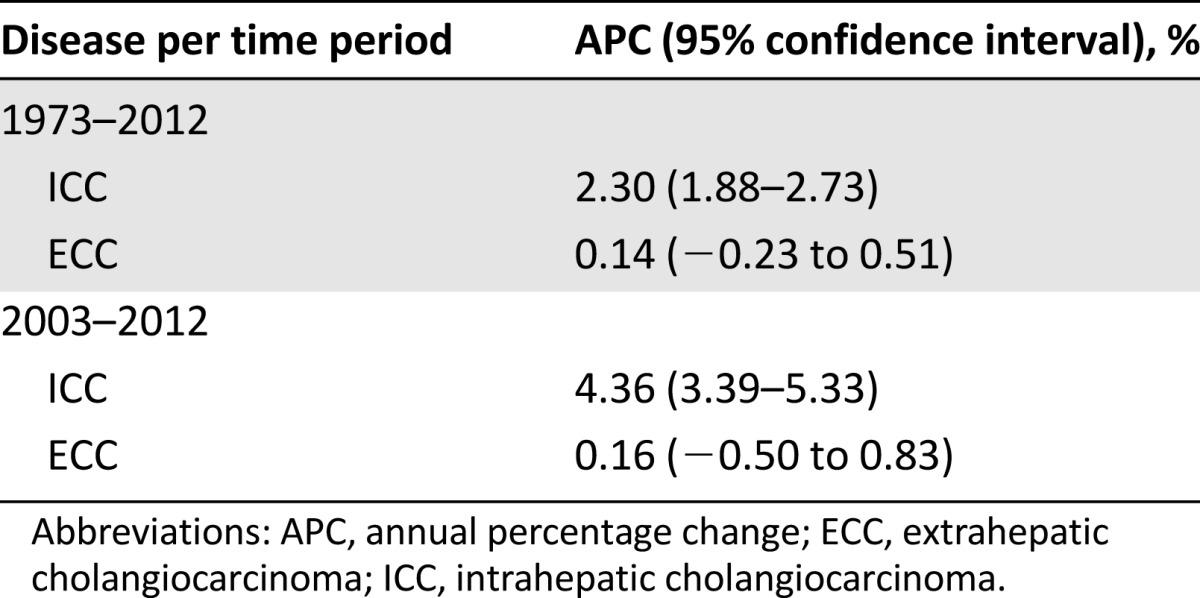
Forty-year incidence trends for ICC and ECC are shown in Figure 1. Between 1973 and 2012, the estimated U.S. incidence of ICC increased from 0.44 to 1.18 cases per 100,000 person-years. This change represents a 128% net increase and an APC of 2.30% (95% confidence interval [CI], 1.88%–2.73%). ECC increased from 0.96 to 1.02 per 100,000 over the same years, constituting a 5% net increase and an APC (annual percentage change) of 0.14% (95% CI, −0.23% to 0.51%) (Table 1). With restriction to the last 10 years of available data—a time period where tumor registry coding criteria for cholangiocarcinoma remained constant—ICC increased significantly at an APC of 4.36% (95% CI, 3.39%–5.33%) while ECC demonstrated a nonsignificant annual percentage change of 0.16% (95% CI, −0.50% to 0.83%).
Figure 1.
Age-adjusted incidence of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma, 1973–2012.
Abbreviations: ECC, extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma.
Table 1.
Annual percentage change in incidence of intrahepatic and extrahepatic cholangiocarcinoma over 40- and 10-year periods.
Age and Stage Distribution
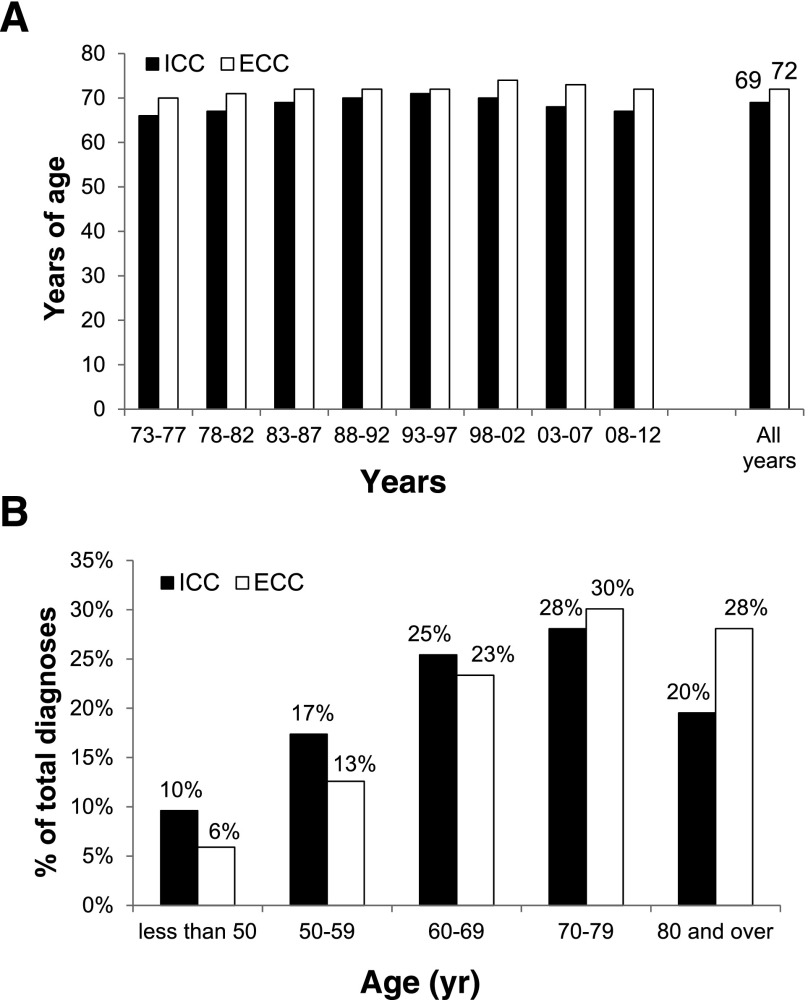
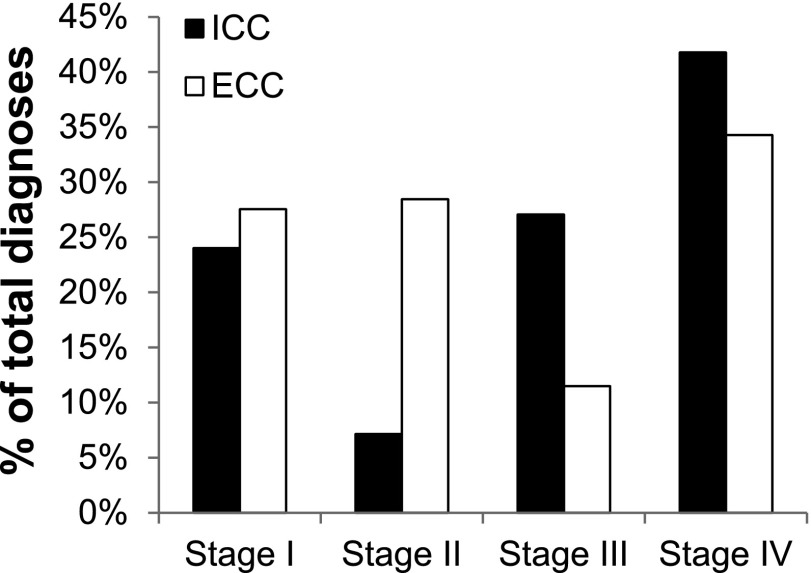
In addition to the age-standardized incidence trends of cholangiocarcinoma, we investigated trends in demographic and pathologic characteristics of patients diagnosed with ICC and ECC. The median age at diagnosis among patients diagnosed with cholangiocarcinoma between 2008 and 2012 was 67 years for ICC and 72 years for ECC. The median age at diagnosis was younger for ICC than for ECC for each 5-year period dating back to 1973 (Fig. 2A). Twenty-seven percent of ICC cases were diagnosed in individuals younger than 60 years of age, compared with 19% of ECC cases (Fig. 2B). ICC was also more frequently diagnosed at advanced stages when compared with ECC. For cases with available staging information (78% of diagnoses between 2004 and 2012), 69% of patients with ICC were diagnosed with stage III or stage IV disease, compared with 46% of ECC patients (Fig. 3). This finding is consistent with the lack of specific symptoms for early-stage ICC and symptomatic biliary obstruction leading to earlier detection of tumors within the extrahepatic biliary tract.
Figure 2.
Age distribution of ICC and ECC. (A): Median age at diagnosis by era. (B): Percentage of patients diagnosed with ICC or ECC within the indicated age range.
Abbreviations: ECC, extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma.
Figure 3.
Stage distribution of ICC and ECC, 2004–2010. Data do not include cases with missing data for cancer stage.
Abbreviations: ECC, extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma.
Gender, Race, and Ethnicity
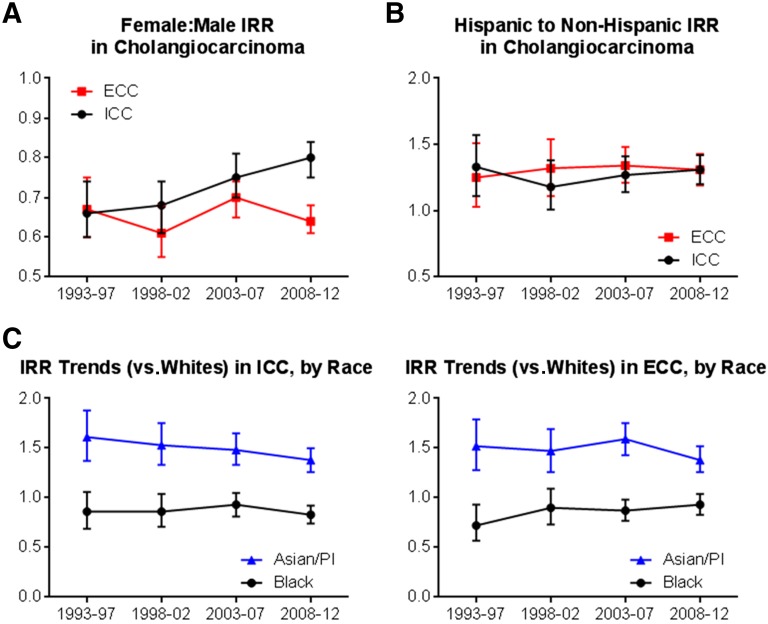
Because gender, race, and ethnicity have been previously identified as risk factors for cholangiocarcinoma, we calculated IRRs comparing the incidence of ICC and ECC by subgroups of race and ethnicity. We report trends in these IRRs for the last 20 years (1993–2012). The relative incidence of both ICC and ECC was lower in women than in men, with IRRs of 0.80 (95% CI, 0.75–0.84) for ICC and 0.64 (95% CI, 0.61–0.68) for ECC (both IRRs calculated for the most recent 5-year time period, 2008–2012) (Fig. 4A). The relative incidence of ICC in women (relative to men) increased significantly between 1993 and 2012 (p = .002); however, the relative incidence of ECC did not significantly change by gender during this time period.
Figure 4.
Relative incidence of ICC and ECC by gender, race, and ethnicity, 1993–2012. (A): Relative incidence (IRR), females relative to males. (B): Relative incidence, Hispanic relative to non-Hispanic ethnicity. (C): Relative incidence Asian/Pacific Islanders and blacks relative to whites.
Abbreviations: ECC, extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; IRR, incidence rate ratio; PI, Pacific Islander.
Trends in the relative incidence of ICC and ECC by race and ethnicity are shown in Figure 4B and 4C. Hispanic ethnicity (compared with non-Hispanic origin) was associated with an increased relative incidence of both ICC and ECC (IRR for ICC, 1.31 [95% CI, 1.20–1.42]; IRR for ECC, 1.31 [95% CI, 1.19–1.43]. The relative incidence of both ICC and ECC was unchanged from 1993 to 2012 in individuals of Hispanic versus non-Hispanic origin. Asian race was also associated with an increased relative incidence of both ICC (IRR for 2008–2012, 1.38; 95% CI, 1.26–1.50) and ECC (IRR, 1.38; 95% CI, 1.26–1.52) when compared with white race. The relative incidence of ICC in Asians versus whites declined modestly between 1993 and 2012 (p = .013) (Fig. 4C), whereas there was no significant change in the relative incidence of ECC between Asians and whites. In the most recent time period, black race was associated with a marginally significant reduction in the relative incidence of both ICC and ECC (IRR for ICC, 0.83 [95% CI, 0.74–0.92]; IRR for ECC, 0.93 [95% CI, 0.83–1.04]). The relative incidence of ICC in blacks and whites was stable between 1993 and 2012, while the relative incidence of ECC in blacks increased during this time period (p = .01).
Cholangiocarcinoma and Carcinoma of Unknown Primary
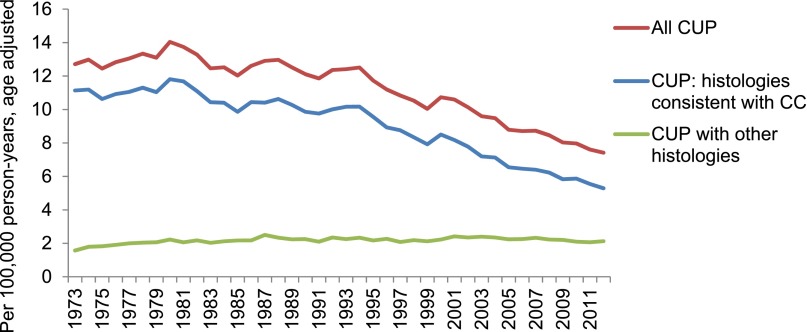
The overall age-standardized incidence of CUP decreased from 12.71 to 7.42 per 100,000 between 1973 and 2012 (APC, −1.41%; 95% CI, −1.65% to −1.17%) (Fig. 5). CUPs with a histology code potentially consistent with cholangiocarcinoma decreased from 11.14 to 5.29 cases per 100,000 (APC, −1.87%; 95% CI, −2.14% to −1.60%), while the incidence of other CUPs (e.g., those with confirmed squamous or nonepithelial histology) demonstrated a modest increase from 1.57 to 2.13 cases per 100,000 (APC, 0.42%; 95% CI, 0.18%–0.66%).
Figure 5.
Age-adjusted incidence of CUP, stratified by histology codes potentially consistent versus not consistent with with cholangiocarcinoma, 1973–2012.
Abbreviations: CC, cholangiocarcinoma; CUP, cancer of unknown primary.
Discussion
Although many epidemiologic studies have highlighted a worldwide increase in the incidence of ICC, recent reports have questioned the veracity of this finding. Multiple investigators have posited that at least part of the documented increase in the incidence of ICC is an artifact caused by systematic misclassification of perihilar (Klatskin) tumors as ICC instead of ECC [1, 7]. Indeed, these studies highlight a significant decrease in ICC incidence over a period from approximately 2000 to 2003, which likely corresponds to a shift in the classification of Klatskin tumors from ICC to ECC. Thus, these studies, which were limited to a relatively short period (less than 20 years), were heavily influenced by the reclassification period and did not observe an overall increase in ICC incidence.
Our study, analyzing SEER data over four decades (from 1973 to 2012) and extending the prior analysis of Tyson et al. with 5 years of additional data, provides an important update to this controversy. Our findings point to a 128% increase in the incidence of ICC over the past 40 years (for an APC of 2.30%), compared with a much more modest 5% increase in the incidence of ECC (APC of 0.14%). The increasing trend in ICC incidence is even more apparent during the past decade, a time period in which tumor registry procedures for classification of biliary cancers remained consistent, with an APC of 4.36% between 2003 and 2012 (compared with an APC of 0.16% for ECC). These findings confirm that that the reported U.S. incidence of ICC continues to rise, over both recent and long-term time periods, whereas the incidence of ECC remains stable to minimally increasing. Our study is also the first to highlight the likely contribution of the improved evaluation of CUP to the increasing recognized incidence of ICC.
Here, we present data showing that the U.S. incidence of CUP has been decreasing consistently over the last several decades, particularly in cases with a histologic type that is potentially consistent with cholangiocarcinoma. Although multiple factors may be contributing to the declining incidence of CUP, an improved ability of physicians to provide a specific diagnosis through advanced imaging, molecular diagnostics, and histopathologic techniques is likely an important cause of this decline. Concurrently, a substantial proportion of the observed increase in ICC may be due to improved diagnosis of cancers that would previously have been classified as CUP. The recent finding that 21% of CUP tumors share a common gene expression signature with biliary tract cancer suggests that we may continue to substantially underestimate the true disease burden of cholangiocarcinoma [15]—particularly ICC, which often resembles metastatic disease to the liver and may frequently be misclassified as CUP [14, 15]. If roughly 20% of CUP tumors reported in the SEER database are attributable to ICC (limited to CUP cases with histology that is potentially consistent with cholangiocarcinoma), the true U.S. incidence of ICC could still be nearly double the currently reported estimate. As the use of molecular diagnostics for the identification of targetable lesions, such as IDH1/2 mutations and FGFR2 fusions, increases, our ability to accurately diagnose these tumors may also improve, along with our understanding of the true incidence of ICC.
Beyond our improved ability to recognize and diagnose ICC, several epidemiologic or environmental trends may have contributed to the rise in ICC cases in the U.S. during this time period. Diabetes, obesity, alcohol use, and viral hepatitis have all been associated with an increased risk for ICC [32]. The degree to which other potential causes, including other diet factors, food preparation, environmental carcinogen exposure, and medication use (particularly medications that are excreted from the bile), are increasing the frequency of ICC remains poorly understood. Thus, a detailed investigation into the relative contribution of multiple potential factors, both known and unknown, that are contributing to this alarming trend is warranted.
Our study confirms previously reported associations of Asian race and Hispanic ethnicity with increased relative incidence of cholangiocarcinoma (both ICC and ECC) [33]. These associations were stable during the last 20 years, with the exception of moderate diminution of the increased risk for ICC associated with Asian race (the incidence rate ratio for ICC in Asians vs. whites declined from 1.61 in 1993–1997 to 1.38 in 2008–2012). Although the absolute incidence of cholangiocarcinoma is greater in men than in women (for both ICC and ECC), the difference in incidence rates between men and women has declined for ICC but not ECC. Although subtype-specific differences in incidence trends of cholangiocarcinoma may suggest differences in the cause of ICC versus ECC, our findings do not point to specific etiologic pathways.
Conclusion
We report an overall growth in the incidence of ICC in the U.S. over both 40- and 10-year time periods, with minimal change in the incidence of ECC. Regardless of whether these incidence data reflect a true increase in the burden of the disease, improved diagnosis, or a combination of both, the number of patients diagnosed with ICC may continue to rise for the foreseeable future. Therefore, it is imperative that improved mechanisms for ICC prevention, early detection, and management of advanced-stage disease are vigorously explored. Recent progress in molecular genetics and the development of pharmacological inhibitors of pathologic mutations specifically found in ICC, including drugs targeting mutations in IDH1/2 and FGFR2 fusions, provide one avenue to combat this rising challenge.
Acknowledgments
S.K.S. is supported by a National Cancer Institute Mentored Clinical Scientist Research Career Development Award (1K08CA194268-01) and a DF/HCC GI SPORE Career Development Project Award (P50CA127003). A.X.Z. is supported by a Research Grant from the V Foundation for Cancer Research. C.S.F. is supported by grants from the National Institutes of Health (5P50CA127003, 5R01CA124908), the Robert T. and Judith B. Hale Fund for Pancreatic Cancer Research, and the Lustgarten Foundation for Pancreatic Cancer Research. G.A.B. is supported by a program grant from the National Cancer Institute of the National Institutes of Health (R25CA09220).
Author Contributions
Conception/Design: Supriya K. Saha, Andrew X. Zhu, Charles S. Fuchs, Gabriel A. Brooks
Collection and/or assembly of data: Supriya K. Saha, Gabriel A. Brooks
Data analysis and interpretation: Supriya K. Saha, Andrew X. Zhu, Charles S. Fuchs, Gabriel A. Brooks
Manuscript writing: Supriya K. Saha, Andrew X. Zhu, Charles S. Fuchs, Gabriel A. Brooks
Final approval of manuscript: Supriya K. Saha, Andrew X. Zhu, Charles S. Fuchs, Gabriel A. Brooks
Disclosures
Charles S. Fuchs: Merck, Pfizer, Sanofi, Genentech, Medimmune, Eli Lilly (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
References
- 1.Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 3.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 4.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Taylor-Robinson SD, Toledano MB, Arora S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48:816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyson GL, Ilyas JA, Duan Z, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59:3103–3110. doi: 10.1007/s10620-014-3276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 9.Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 11.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 12.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Malaguarnera G, Paladina I, Giordano M, et al. Serum markers of intrahepatic cholangiocarcinoma. Dis Markers. 2013;34:219–228. doi: 10.3233/DMA-130964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371:757–765. doi: 10.1056/NEJMra1303917. [DOI] [PubMed] [Google Scholar]
- 15.Hainsworth JD, Rubin MS, Spigel DR, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol. 2013;31:217–223. doi: 10.1200/JCO.2012.43.3755. [DOI] [PubMed] [Google Scholar]
- 16.Ferrone CR, Ting DT, Shahid M, et al. The ability to diagnose intrahepatic cholangiocarcinoma definitively using novel branched DNA-enhanced albumin rna in situ hybridization technology. Ann Surg Oncol. 2016;23:290–296. doi: 10.1245/s10434-014-4247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 18.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. The Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan-On W, Nairismägi ML, Ong CK, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45:1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 20.Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1630–1638. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. The Oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sia D, Losic B, Moeini A, et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087. doi: 10.1038/ncomms7087. [DOI] [PubMed] [Google Scholar]
- 24.Simbolo M, Fassan M, Ruzzenente A, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5:2839–2852. doi: 10.18632/oncotarget.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voss JS, Holtegaard LM, Kerr SE, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44:1216–1222. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu AX, Borger DR, Kim Y, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol. 2014;21:3827–3834. doi: 10.1245/s10434-014-3828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute. Surveillance, Epidemiology and End Results. Overview of the SEER program. Available at http://seer.cancer.gov/about/overview.html. Accessed June 17, 2015.
- 29.National Cancer Institute. Surveillance Research Program, National Cancer Institute SEER*Stat software. Version 8.2.1. Available at http://www.seer.cancer.gov/seerstat. Accessed June 8, 2015.
- 30.National Cancer Institute. SEER*Stat trend algorithms. Available at http://seer.cancer.gov/seerstat/WebHelp/seerstat.htm#Trend_Algorithms.htm. Accessed June 8, 2015.
- 31.Green FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002. [Google Scholar]
- 32.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the united states. Liver Int. 2006;26:1047–1053. doi: 10.1111/j.1478-3231.2006.01350.x. [DOI] [PubMed] [Google Scholar]