The U.S. Food and Drug Administration granted accelerated approval to pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. This work discusses the data supporting the approval decision, specifically highlighting the incorporation of a companion diagnostic in the key study and the optimal dose of pembrolizumab.
Keywords: Lung cancer, Pembrolizumab, Programmed death-ligand 1, U.S. Food and Drug Administration
Abstract
On October 2, 2015, the U.S. Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab, a breakthrough therapy-designated drug, for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors express programmed death-ligand 1 (PD-L1), as determined by an FDA-approved test, and who have disease progression on or after platinum-containing chemotherapy or targeted therapy against anaplastic lymphoma kinase or epidermal growth factor receptor, if appropriate. This indication was approved concurrently with the PD-L1 immunohistochemistry 22C3 pharmDx, a companion diagnostic test for patient selection based on PD-L1 tumor expression. The accelerated approval was granted based on durable objective response rate (ORR) and an acceptable toxicity profile demonstrated in a multicenter, open-label trial enrolling 550 patients with metastatic NSCLC. The efficacy population comprised 61 patients with tumors identified as strongly positive for PD-L1, and the confirmed ORR as determined by blinded independent central review was 41% (95% confidence interval: 28.6%, 54.3%); all were partial responses. At the time of the analysis, responses were ongoing in 21 of 25 patients (84%), with 11 patients (44%) having response duration of ≥6 months. The most commonly occurring (≥20%) adverse reactions included fatigue, decreased appetite, dyspnea, and cough. The most frequent (≥2%) serious adverse drug reactions were pleural effusion, pneumonia, dyspnea, pulmonary embolism, and pneumonitis. Immune-mediated adverse reactions occurred in 13% of patients and included pneumonitis, colitis, hypophysitis, and thyroid disorders. The accelerated approval regulations describe approval of drugs and biologic products for serious and life-threatening illnesses based on a surrogate endpoint likely to predict clinical benefit. Under these regulations, a confirmatory trial or trials is required to verify and describe the benefit of pembrolizumab for patients with metastatic NSCLC.
Implications for Practice:
This report presents key information on the U.S. Food and Drug Administration (FDA) accelerated approval of pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1, as determined by an FDA-approved test, and who have disease progression on or after platinum-containing chemotherapy or targeted therapy against anaplastic lymphoma kinase or epidermal growth factor receptor, if appropriate. The report discusses the data supporting the approval decision, specifically highlighting the incorporation of a companion diagnostic in the key study and the optimal dose of pembrolizumab.
Introduction
Lung cancer is the leading cause of cancer death in the U.S., and there will be an estimated 221,200 new cases and 158,040 deaths due to this disease in 2015 [1]. Approximately 85% of all lung cancers are classified as non-small cell lung cancer (NSCLC), which is subclassified by histologic subtype (squamous cell and nonsquamous). The majority of patients present with locally advanced or metastatic disease incurable with currently available therapies, with a median survival of 3–6 months with supportive care only [2]. Initial treatment of patients with metastatic NSCLC without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations is a platinum-based doublet chemotherapy regimen; in historic trials, the response rate ranged from 12% to 37%, median progression‐free survival (PFS) was from 4 to 7 months, median overall survival (OS) was from 8 to 13 months, and the estimated 1-year survival was 33% [2]. In second-line trials of unselected patients, objective response rate (ORR) with docetaxel, pemetrexed, and erlotinib ranged from 7% to 12%, median PFS from 2 to 3 months, and median OS from 8 to 9 months [3–5]. Nivolumab recently received traditional approval for the treatment of patients with metastatic squamous NSCLC with progressive disease on platinum-containing chemotherapy based on the results of a randomized trial of nivolumab versus docetaxel. Median OS was 9.2 months for patients randomized to receive nivolumab and 6.0 months for those randomized to receive docetaxel [hazard ratio = 0.59; 95% confidence interval (CI): 0.44-0.79; p < .001] [6].
Programmed cell death 1 (PD-1) is a transmembrane protein expressed primarily on the surface of activated T cells, whereas its ligands, programmed death-ligand 1 (PD-L1) and PD-L2, may be expressed on various tumor cells. PD-1 is an “immune checkpoint” receptor, and binding to its ligands leads to suppression of the immune system’s ability to reject tumor cells, primarily through inhibition of tumor cell apoptosis and cytotoxic T-cell activity [7]. Preclinical and clinical data suggest that PD-L1 upregulation by tumor cells appears to facilitate evasion of the immune system’s antitumor response.
Pembrolizumab (Keytruda; Merck, Sharp and Dohme Corp., Kenilworth, NJ, http://www.merck.com) is a humanized monoclonal IgG4-κ isotype antibody that binds to the PD-1 receptor, preventing interaction with PD-L1 and PD-L2. Pembrolizumab has received breakthrough therapy designation for both metastatic melanoma and advanced NSCLC. Whole-exome sequencing of NSCLC tumors treated with pembrolizumab has demonstrated that antitumor activity was associated with higher mutation burden in tumors thought to be partly related to exposure to carcinogens in tobacco; higher neo-antigen burden; and increased DNA repair pathway mutations [8]. In 2014, pembrolizumab received accelerated approval in the U.S. for treatment of patients with unresectable or metastatic melanoma and disease progression after ipilimumab, and, if BRAF V600 mutation positive, a BRAF inhibitor. Two doses of pembrolizumab were evaluated for this indication, with 2 or 10 mg/kg given every 3 weeks. The antitumor activity and safety profile appeared similar for both dosing regimens and supported the approved dose of 2 mg/kg every 3 weeks for the treatment of metastatic melanoma.
Trial Design
The U.S. Food and Drug Administration (FDA) reviewed data from a single, open-label, multicenter trial entitled, Phase I Study of Single Agent MK-3475 in Patients with Progressive Locally Advanced or Metastatic Carcinoma, Melanoma, and Non-Small Cell Lung Cancer (P001), also referred to as Keynote 001. Keynote 001 was designed to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of pembrolizumab in patients with melanoma or NSCLC. The study was initiated with a traditional 3+3 design for dose escalation in patients with advanced solid tumors, followed by multiple amendments that ultimately led to the addition of several cohorts and subgroups with differing patient characteristics, beginning with patients with melanoma and eventually expanding to patients with NSCLC. Eligibility criteria, objectives, and analysis plans were modified based on emerging data from Keynote 001 or from other studies in the pembrolizumab development program. Keynote 001 included multiple parts: Parts C and F enrolled patients with NSCLC exclusively and are the primary focus of this review (supplemental online Table 1 describes the different populations of patients evaluated in Keynote 001).
In parts C and F, patients were treated with intravenous pembrolizumab at a dose of 2 mg/kg every 3 weeks or 10 mg/kg every 3 or 2 weeks. Based on data from the pembrolizumab experience in melanoma that suggested the dosing regimen of 2 mg/kg every 3 weeks was comparable with the 10 mg/kg dosing regimens, part F3 was the last expansion cohort to be added, and patients enrolled were treated with 2 mg/kg pembrolizumab every 3 weeks. For all patients, study treatment was continued until disease progression as determined by investigator-assessed immune-related response criteria, unacceptable toxicity, withdrawal of consent, or investigator discretion. Patients with progressive disease (PD) were permitted to continue on study treatment if they did not exhibit decline in clinical symptoms/signs or PD at critical anatomic sites requiring urgent intervention. These patients were reimaged 4–6 weeks after initial progression and were eligible to remain on therapy and resume regularly scheduled imaging if the follow-up scan did not confirm PD.
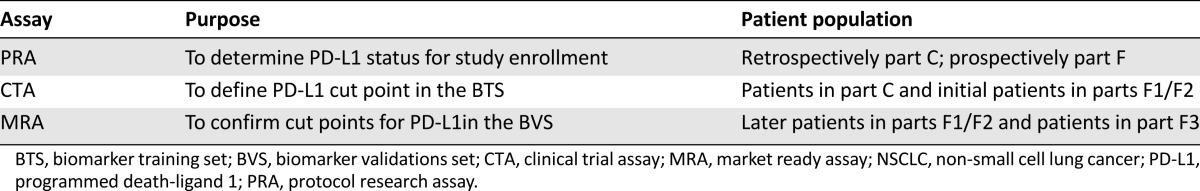
The primary endpoints were characterization of the tolerability and safety profile of single-agent pembrolizumab and evaluation of antitumor activity per Response Evaluation Criteria on Solid Tumors version 1.1 (RECIST 1.1) of pembrolizumab in patients with NSCLC with at least one prior systemic therapy, whose tumors express a high level of PD-L1. The trial also sought to investigate the relationship between the level of PD-L1 expression in tumors and antitumor activity of pembrolizumab. Patients with NSCLC on Keynote 001 had PD-L1 expression levels measured by immunohistochemistry (IHC) performed on formalin-fixed paraffin-embedded tumor samples. PD-L1 expression was tested by using several devices throughout the study. Patients were enrolled based on results from the prototype research assay (PRA) performed in a Clinical Laboratory Improvement Amendments laboratory. The PRA scoring method specified staining of the tumor cell membrane and mononuclear cell membrane with a cutoff of 1% or the presence of stromal staining of immune cells. The clinical trial assay (CTA) was the second version of the device and was used to determine the optimal cutoff for PD-L1 expression level and to refine the scoring method to specify tumor cell membrane staining only. The market ready assay (MRA) was the final version of the device and was used to prospectively assess clinical outcomes in three biomarker-defined populations; PD-L1 strong, PD-L1 weak, or PD-L1 negative. Table 1 summarizes the three assays for PD-L1 used in patients with NSCLC on Keynote 001.
Table 1.
PD-L1 assays for patients with NSCLC on Keynote 001
Results
A total of 1,236 patients with NSCLC signed informed consent for Keynote 001, and patients were enrolled at 47 sites in 10 countries. There were a high number of screen failures (54%), and most patients were ineligible because of tumors that were not PD-L1-positive by IHC (56%). Ultimately, there were 550 patients with NSCLC enrolled in Keynote 001; of those, 449 patients had received prior therapy for their disease, and 101 patients were treatment-naïve. In parts C, F1, and F2, there were 495 patients who received at least one dose of pembrolizumab (2 mg/kg every 3 weeks [n = 6] or 10 mg/kg every 2 weeks [n = 202] or every 3 weeks [n = 287]). Part F3 was the last expansion cohort to be added and enrolled 55 patients, who were treated with pembrolizumab at a dose of 2 mg/kg every 3 weeks.
A relationship between PD-L1 expression and pembrolizumab efficacy was observed in the first patients enrolled in part C; therefore, patients were further characterized by their tumor PD-L1 status. After testing with the PRA to determine at least 1% staining of tissue samples for inclusion in part F of the study, the CTA was used to retrospectively assess PD-L1 expression by using unstained sections from the same tumor sample used for the original PRA testing. A proportion score (PS) indicating the percentage of tumor cells exhibiting membranous staining was selected as the scoring method for the assay. With Amendment 7 of Trial P001, a biomarker training set (BTS) and biomarker validations set (BVS) were established. The BTS included patients from part C and initial patients enrolled in parts F1 and F2. The BTS defined the optimal cut point as PS ≥ 50% staining of tumor cells for PD-L1; tumors above this cut point were referred to as PD-L1 strongly positive, whereas tumors having a PS between 1% and 49% were designated as weakly positive and tumors with <1% were considered PD-L1 negative. To confirm these cut points, the remaining patients from parts F1 and F2 were included in the BVS. Patients in the BVS were not included in the BTS evaluation.
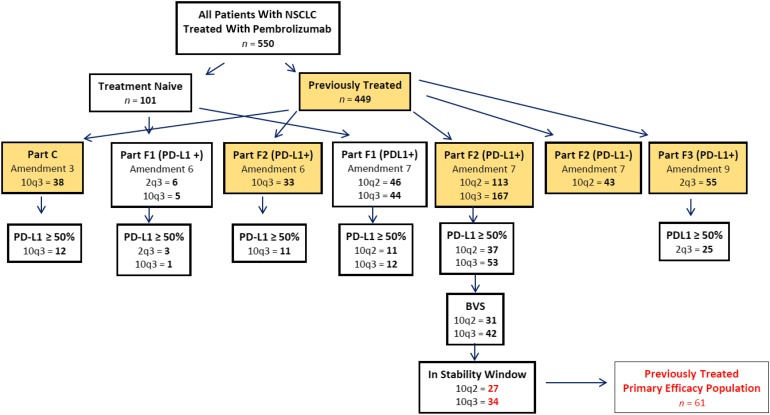
Patient selection for the primary efficacy analysis was determined based on results from the PD-L1 CTA and MRA. After all 550 patients with NSCLC had begun treatment with pembrolizumab, analytical validation data from the companion diagnostic indicated that the PD-L1 antigen was stable for up to 6 months once a tumor specimen had been fixed on a glass slide. For this reason, patient tumor samples were designated as being within the 6-month stability window or irrespective of the stability window. Figure 1 depicts how patients with NSCLC were allocated in Keynote 001.
Figure 1.
NSCLC patient allocation on Keynote 001.
Abbreviations: 10q3, 10 mg/kg every 3 weeks; 10q2, 10 mg/kg every 2 weeks; 2q3, 2 mg/kg every 3 weeks; BVS, biomarker validation set; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1.
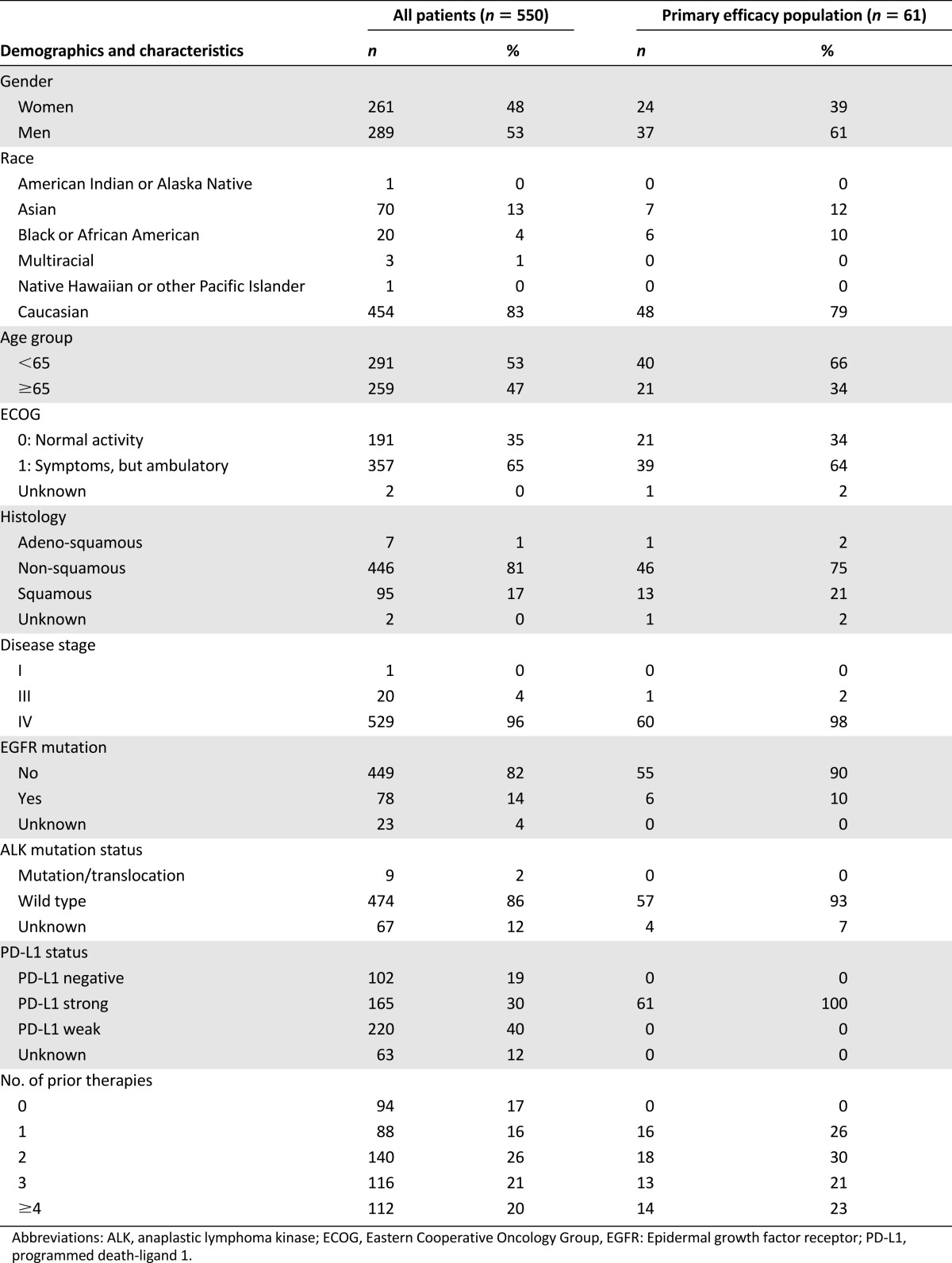
Baseline demographics and disease characteristics for all 550 patients with NSCLC and 61 patients included in the primary efficacy analysis are provided in Table 2. For the entire population of NSCLC patients, the median age was 64 years, and 47% were age ≥ 65 years old. The population was predominantly Caucasian (83%), and most had received ≥2 prior therapies (67%). Baseline Eastern Cooperative Oncology Group performance status was 0 (35%) or 1 (65%).
Table 2.
Baseline patient demographics and tumor characteristics
Efficacy
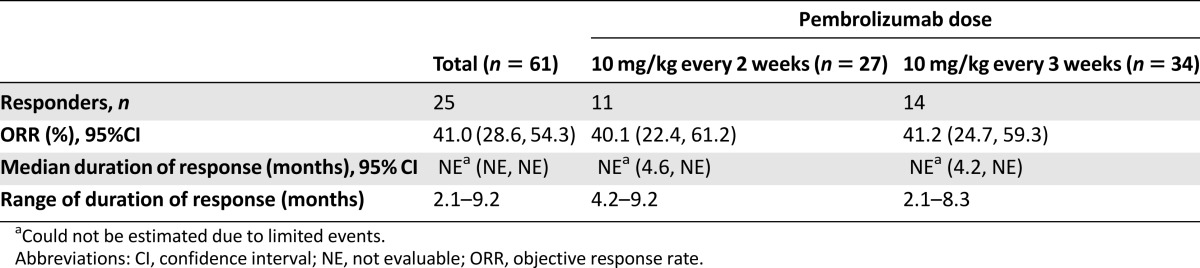
The primary efficacy population included 61 previously treated patients with NSCLC and PD-L1 PS ≥ 50% based on tumor samples that were within the 6-month stability window (Table 3). These patients were treated with a dose of 10 mg/kg every 2 weeks (n = 27) or every 3 weeks (n = 34). The blinded independent central review (BICR) assessed, confirmed ORR per RECIST 1.1 was 41% (95% CI: 28.6%, 54.3%). The median duration of response was not reached at the time of the analysis.
Table 3.
Efficacy results of primary efficacy population
Additional supportive efficacy data comes from part F3, which consisted of 25 patients with PD-L1 PS ≥ 50% treated with pembrolizumab at a dose of 2 mg/kg every 3 weeks. The BICR-assessed ORR was 28% (95% CI: 12.1%, 49.4%), and the median duration of response was not reached. Patients in part F3 were the last to enroll and had median follow-up for the independently reviewed imaging data of 7.7 months.
Additional exploratory analyses of subpopulations of the primary efficacy population were consistent with the results in the overall population of 61 patients. The ORR in patients with PS, 1%–49% (PD-L1 weakly positive), was similar to that with standard of care: 14.8% (95% CI: 7.9%, 24.4%). ORR was similar across histology (squamous and nonsquamous).
Safety
There were 550 patients with NSCLC on Keynote 001 who received at least one dose of pembrolizumab and were included in the safety analysis. The median duration of therapy was 2.8 months (1 day to 25.6 months). The incidence of adverse reactions, including serious adverse reactions, was similar between the two 10 mg/kg dosing schedules; therefore, these data were pooled. There were significantly fewer patients treated with the 2 mg/kg dose of pembrolizumab, and the majority had shorter follow-up compared with patients treated with the 10 mg/kg schedules; therefore, comparisons of adverse reactions between dosing regimens was not applicable.
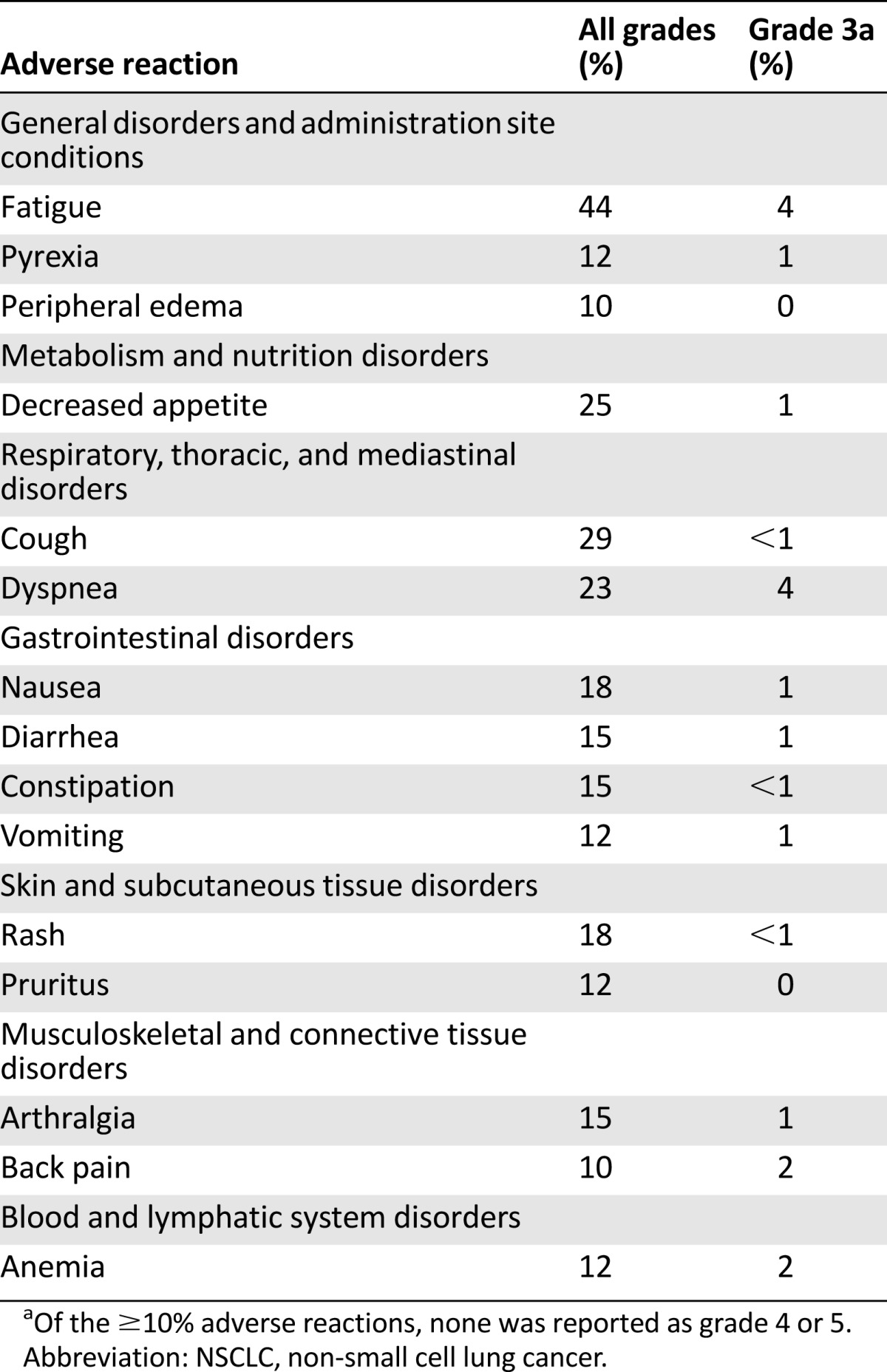
The most common adverse events, occurring in at least 10% of the population, are summarized in Table 4. Serious adverse events (SAEs) occurred in 208 (37.8%) of 550 patients with NSCLC. The most common SAEs were pleural effusion (4.5%), pneumonia (3.1%), dyspnea (2.9%), pulmonary embolism (2.4%), pneumonitis (2.2%), pyrexia (1.8%), respiratory failure 1.5%), pneumothorax (1.3%), pericardial effusion (1.1%), hypoxia (1.1%), and nausea (1.1%).
Table 4.
Adverse reactions occurring in ≥10% of patients with metastatic NSCLC on Keynote 001
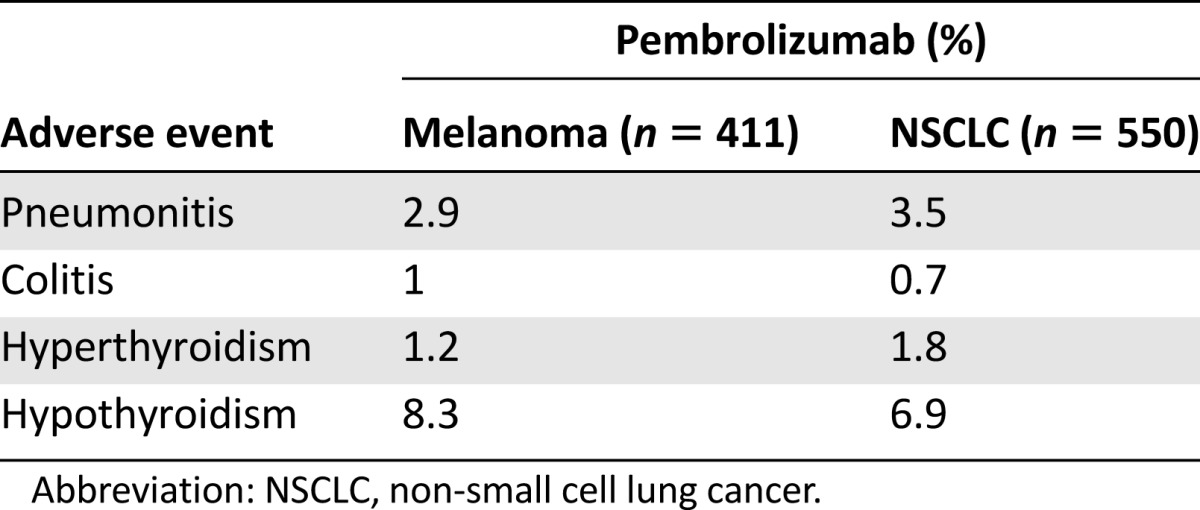
Immune-related adverse events (irAEs) occurred in 71 (13%) of 550 patients. Thyroid disorders were the most common irAEs observed, with hyperthyroidism occurring in 10 patients (1.8%) and hypothyroidism occurring in 38 patients (6.9%). Pneumonitis occurred in 19 (3.5%) patients, with one death attributed to pneumonitis. Pneumonitis occurred more frequently in patients with a history of asthma/chronic obstructive pulmonary disease than in those without (5.4% vs. 3.1%) and more frequently in patients with a history of prior thoracic radiation than in patients without (6.0% vs. 2.6%). Twelve patients (2.2%) discontinued pembrolizumab because of pneumonitis. Other irAEs included colitis occurring in four (0.9%) patients and hypophysitis occurring in one patient. There were 76 patients (14%) who discontinued pembrolizumab because of an adverse event, with 16 patients (2.9%) discontinuing because of an irAE. Table 5 displays the incidence of irAEs occurring in patients with metastatic melanoma and metastatic NSCLC treated with pembrolizumab on Keynote 001. The incidence of irAEs in these two patient populations appears similar.
Table 5.
Incidence of immune-related adverse events occurring in patients treated with pembrolizumab, by disease
Discussion
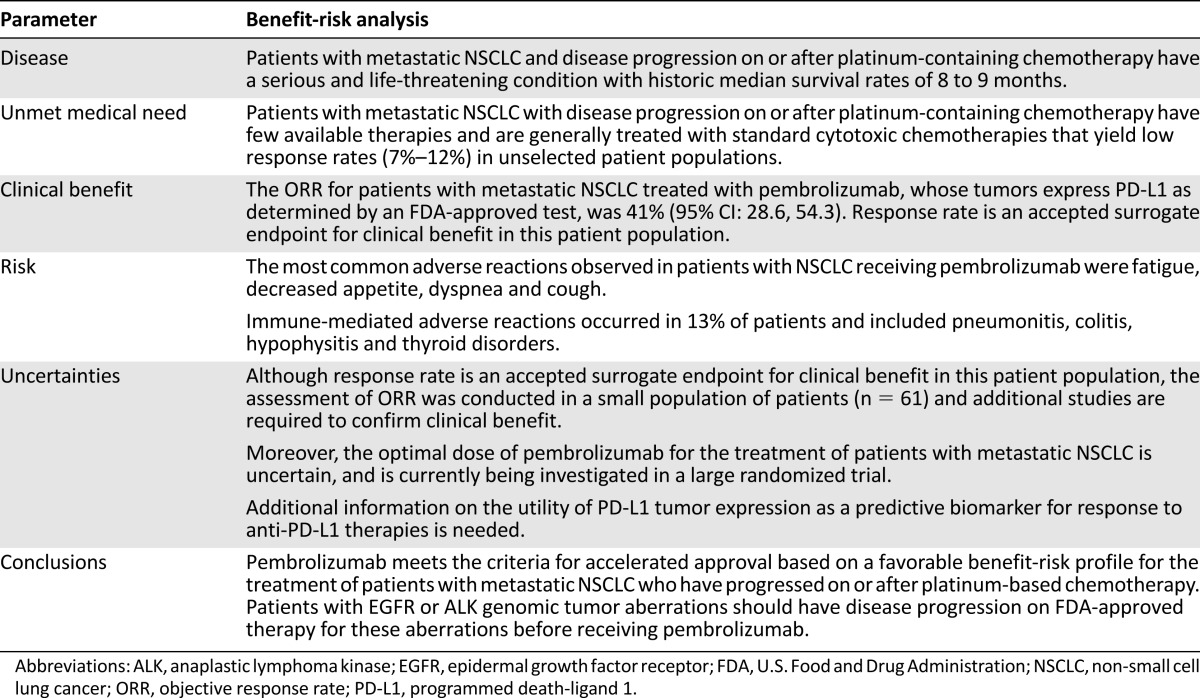
Metastatic NSCLC is a serious and life-threatening disease, with poor prognosis. The benefit-risk profile for pembrolizumab in the treatment of patients with metastatic NSCLC with progression after platinum-based therapy was considered favorable based on an ORR of significant magnitude and duration that exceeds that observed with available therapy for this disease (Table 6).
Table 6.
Benefit-risk analysis of pembrolizumab for patients with metastatic NSCLC whose tumors express PD-L1 and who have disease progression on or after platinum-containing chemotherapy
The accelerated approval regulations describe approval of drugs and biologic products for serious and life-threatening illnesses based on a surrogate endpoint or an effect on a clinical endpoint other than survival or irreversible morbidity that provides meaningful therapeutic benefit to patients over existing therapies [9]. Although there are several FDA-approved drugs for the treatment of metastatic NSCLC, with the exception of nivolumab (only approved for patients with squamous histology at the time of this pembrolizumab approval), the treatment effects on OS are modest.
Response rate is a recognized surrogate endpoint and has been the basis for accelerated approval of other oncology therapies. The FDA considered the ORR of 41% in 61 patients with metastatic NSCLC treated with pembrolizumab, whose tumors express PD-L1 as determined by an FDA-approved test, as evidence of a clinically meaningful magnitude of response. Moreover, the responses reported were notably durable, with 44% of patients having response duration of ≥6 months at the time of the analysis. This ORR exceeded the reported ORR for any of the approved drugs for second-line treatment of unselected patients with NSCLC at the time of this approval.
At the time of the data analysis, the FDA requested the interim analysis results from the ongoing Keynote 010 trial entitled, “A randomized, controlled, trial of pembrolizumab (2 mg/kg or 10 mg/kg every three weeks [Q3W]) vs docetaxel 75 mg/m2 in patients with NSCLC whose tumors express PD-L1 and who have experienced disease progression after platinum-containing systemic therapy.” These data were not available to Merck and were provided in a blinded fashion by a data safety monitoring committee. The results for the interim analysis of PFS and OS from this study were supportive of the activity of pembrolizumab in patients with metastatic NSCLC and were considered in the assessment of benefit-risk.
Uncertainty of Clinical Benefit
The primary efficacy population for the analysis of ORR in patients with metastatic NSCLC included a small number of patients with limited follow-up, and there remains uncertainty as to the correlation of ORR and duration of response to ultimate outcomes of clinical benefit, including OS. A recent analysis of 14 randomized, controlled trials including >12,000 patients with advanced NSCLC demonstrated an association between ORR and PFS, but not OS, based on a trial-level analysis. However, evaluation of the patient-level analysis did indicate that patients who achieved a response had improved PFS and OS compared with nonresponders [10].
Given the uncertainty of benefit to patients, traditional approval of pembrolizumab for patients with metastatic NSCLC requires confirmation of clinical benefit, as described in the accelerated approval regulations. Keynote 010 is an ongoing study in patients with metastatic NSCLC with PFS and OS as coprimary endpoints. Patients are randomized to receive docetaxel or pembrolizumab at a dose of 2 or 10 mg/kg every 3 weeks; therefore, the study aims to define the optimal dose of pembrolizumab in the metastatic NSCLC population in addition to demonstrating clinical benefit.
Uncertainty Regarding the Dose
Although the patients included in the primary efficacy analysis were treated with 10 mg/kg pembrolizumab every 2 or 3 weeks, the dose approved for use is 2 mg/kg every 3 weeks. There were several factors that were considered in the decision to approve this dose. (a) The exposure-response relationships for efficacy and safety are flat over the pembrolizumab dosing regimens studied in patients with NSCLC. (b) Given that pembrolizumab’s mechanism of action does not necessarily involve direct binding to cancer cells, the effective dose-response relationship is postulated to be the same across tumor types; therefore, there is biological plausibility that a lower dose of pembrolizumab has comparable antitumor activity (as was demonstrated in patients with metastatic melanoma). (c) Supportive data from patients treated with the approved dose in part F3 were considered. (d) Supplemental data available to FDA from the ongoing Keynote 010 trial at the time of the review were considered in the determination that the proposed dose of 2 mg/kg every 3 weeks provided an acceptable benefit-risk profile for patients. Recently, the sponsor reported that preliminary results from this trial demonstrated improved OS in patients treated with pembrolizumab at either dose compared with docetaxel.
Significance of PD-L1 Tumor Expression
In the trials used to support approval of nivolumab in patients with metastatic squamous NSCLC, there did not appear to be a correlation between outcomes and PD-L1 tumor status [11]. In contrast, the preliminary results from Keynote 001 suggested a correlation between high PD-L1 tumor expression and antitumor activity of pembrolizumab in patients with metastatic NSCLC [12]. Since the approval of pembrolizumab described in this paper, nivolumab received an expanded indication to include patients with metastatic nonsquamous NSCLC in October 2015. This expanded indication was granted based on the results of a single study that demonstrated a statistically robust and clinically meaningful improvement in OS in patients with nonsquamous NSCLC treated with nivolumab versus docetaxel. The treatment effect of nivolumab appeared to be largely restricted to patients with PD-L1 positive (PD-L1 expression in ≥1% of tumor cells) or PD-L1 strongly expressing (PD-L1 expression in ≥5% or 10%), with increasing magnitude of treatment effects on OS, PFS, and ORR with higher levels of PD-L1 expression.
It is worth noting that the PD-L1 assays utilized in the nivolumab and pembrolizumab studies were different, and results cannot be directly compared across studies. Early data from Keynote 010 indicates that patients with tumors that were designated as PD-L1 weakly positive (PD-L1 1%–49%) may also benefit from pembrolizumab treatment. Whether efficacy of anti-PD-L1 therapy varies with PD-L1 tumor expression and whether such a relationship is applicable for all histologies or subgroups (e.g., nonsquamous, squamous, EGFR+, etc.) remains unclear. Ongoing studies in various other cancers will likely provide additional insight.
Conclusion
The accelerated approval of pembrolizumab for patients with metastatic NSCLC whose tumors express PD-L1 and who have disease progression on or after platinum-containing chemotherapy is concurrent with approval of a companion diagnostic test for this patient population. Confirmation of clinical benefit is required for traditional approval, and results from Keynote 010 are pending. Further investigation to evaluate the role of PD-L1 tumor expression as a predictive biomarker is needed in lung cancer and other malignancies.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Footnotes
Editor's Note: See the related commentary, “Into the Clinic With Nivolumab and Pembrolizumab,” by Catherine A. Shu and Naiyer A. Rizvi, on page 527 of this issue.
Author Contributions
Conception/Design: Joohee Sul, Gideon M. Blumenthal
Provision of study material or patients: Joohee Sul
Collection and/or assembly of data: Joohee Sul, Xiaoping Jiang
Data analysis and interpretation: Joohee Sul, Gideon M. Blumenthal, Xiaoping Jiang, Kun He, Patricia Keegan
Manuscript writing: Joohee Sul, Gideon M. Blumenthal, Xiaoping Jiang, Kun He, Patricia Keegan, Richard Pazdur
Final approval of manuscript: Joohee Sul, Gideon M. Blumenthal, Xiaoping Jiang, Kun He, Patricia Keegan, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1.SEER Stat Fact Sheets: Lung and Bronchus Cancer. Available at http://seer.cancer.gov/statfacts/html/lungb.html. Accessed October 2015.
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 6.Kazandjian D, Khozin S, Blumenthal G, et al. Benefit-risk summary of nivolumab for patients with metastatic squamous cell lung cancer after platinum-based chemotherapy: A report from the US Food and Drug Administration. JAMA Oncol. 2012;2:118–122. doi: 10.1001/jamaoncol.2015.3934. [DOI] [PubMed] [Google Scholar]
- 7.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33:1008–1014. doi: 10.1200/JCO.2014.59.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.