Abstract
Lessons Learned
Despite evidence for a role for prolactin signaling in breast and prostate tumorigenesis, a prolactin receptor-binding monoclonal antibody has not produced clinical efficacy.
Increased serum prolactin levels may be a biomarker for prolactin receptor inhibition.
Results from the pharmacokinetic and pharmacodynamics (PD) studies suggest that inappropriately long dosing intervals and insufficient exposure to LFA102 may have resulted in lack of antitumor efficacy.
Based on preclinical data, combination therapy of LFA102 with those novel agents targeting hormonal pathways in metastatic castration-resistant prostate cancer and metastatic breast cancer is promising.
Given the PD evidence of prolactin receptor blockade by LFA102, this drug has the potential to be used in conditions such as hyperprolactinemia that are associated with high prolactin levels.
Background.
Prolactin receptor (PRLR) signaling is implicated in breast and prostate cancer. LFA102, a humanized monoclonal antibody (mAb) that binds to and inhibits the PRLR, has exhibited promising preclinical antitumor activity.
Methods.
Patients with PRLR-positive metastatic breast cancer (MBC) or metastatic castration-resistant prostate cancer (mCRPC) received doses of LFA102 at 3–60 mg/kg intravenously once every 4 weeks. Objectives were to determine the maximum tolerated dose (MTD) and/or recommended dose for expansion (RDE) to investigate the safety/tolerability of LFA102 and to assess pharmacokinetics (PK), pharmacodynamics (PD), and antitumor activity.
Results.
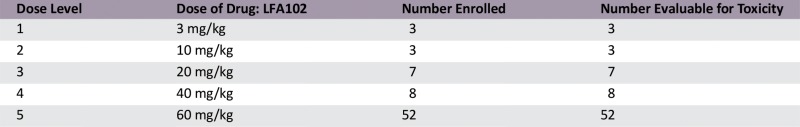
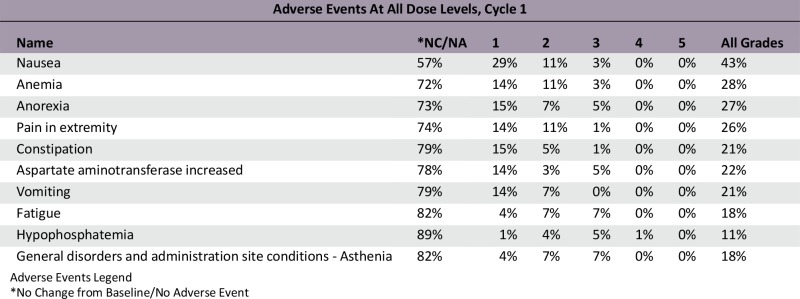
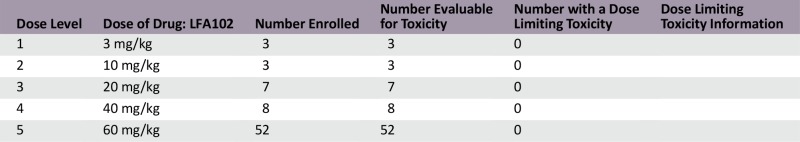
A total of 73 patients were enrolled at 5 dose levels. The MTD was not reached because of lack of dose-limiting toxicities. The RDE was established at 60 mg/kg based on PK and PD analysis and safety data. The most common all-cause adverse events (AEs) were fatigue (44%) and nausea (33%) regardless of relationship. Grade 3/4 AEs reported to be related to LFA102 occurred in 4% of patients. LFA102 exposure increased approximately dose proportionally across the doses tested. Serum prolactin levels increased in response to LFA102 administration, suggesting its potential as a biomarker for PRLR inhibition. No antitumor activity was detected.
Conclusion.
Treatment with LFA102 was safe and well tolerated, but did not show antitumor activity as monotherapy at the doses tested.
Abstract
作者总结
经验
• 尽管有证据显示催乳素信号转导在乳腺和前列腺的肿瘤发生中发挥了一定作用, 但与催乳素受体结合的单克隆抗体尚未产生临床有效性。
• 血清催乳素水平升高可能作为催乳素受体抑制的生物标记物。
• 药代动力学和药效学 (PD) 研究提示, 不适当的长给药间歇及暴露剂量不足可能导致 LFA102 缺乏抗肿瘤有效性。
• 基于临床前数据, 采用 LFA102 与激素通路靶向新药联合方案用于治疗转移性去势抵抗性前列腺癌和转移性乳腺癌, 可望产生一定疗效。
鉴于催乳素受体受 LFA102 阻断的 PD 证据, 该药在诸如与高催乳素水平相关的高催乳素血症等情况下有一定应用潜力。
摘要
背景. 催乳素受体 (PRLR) 信号转导与乳腺和前列腺癌有关。人源化单克隆抗体 (mAb) LFA102 可与 PRLR 结合并抑制 PRLR, 其显示出的临床前抗肿瘤活性具有一定前景。
方法. 给予 PRLR 阳性的转移性乳腺癌 (MBC) 或转移性去势抵抗性前列腺癌 (mCRPC) 患者 LFA102 3∼60 mg/kg 静脉注射, 每 4 周一次。研究旨在确定最大耐受剂量 (MTD) 和 (或) 扩展阶段建议剂量 (RDE) 以调查 LFA102 的安全性/耐受性, 以及评估药代动力学 (PK) 、药效学 (PD) 和抗肿瘤活性。
结果. 共纳入 73 例患者, 接受了 5 个水平的给药剂量治疗。由于未发生剂量限制毒性, 因此未达到 MTD。依据 PK 和 PD 分析以及安全性数据, 确定 RDE 为 60 mg/kg。最常见的全因不良事件 (AE) 为乏力 (44%) 和恶心 (33%) , 无论是否与研究药物相关。 6%的患者发生了与 LFA102 相关的 3/4 级 AE。LA102 的暴露水平大致随检验剂量的增加而升高。血清催乳素水平随 LFA102 的给药而升高, 提示其具有作为 PRLR 抑制的生物标记物的潜力。研究未发现抗肿瘤活性。
结论. LFA102治疗安全且可耐受, 但在本研究检验剂量水平上单药使用未显示出抗肿瘤活性。The Oncologist 2016;21:535–536i
Discussion
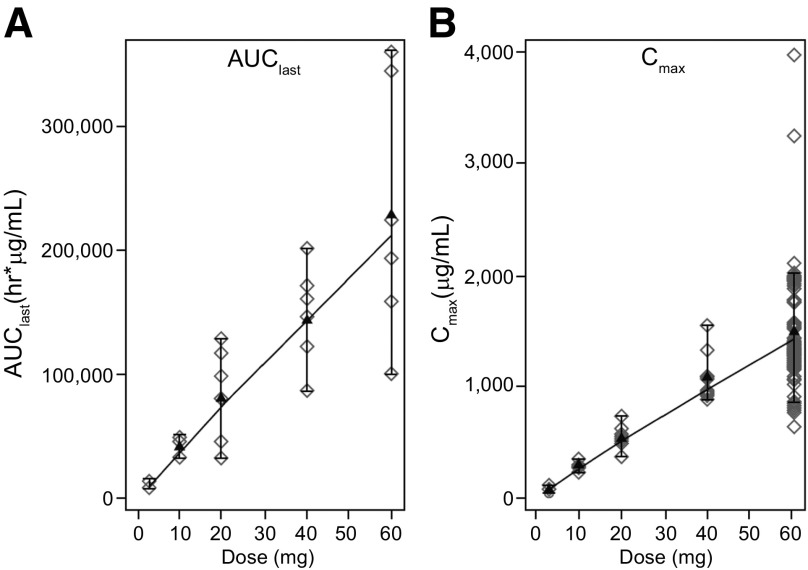
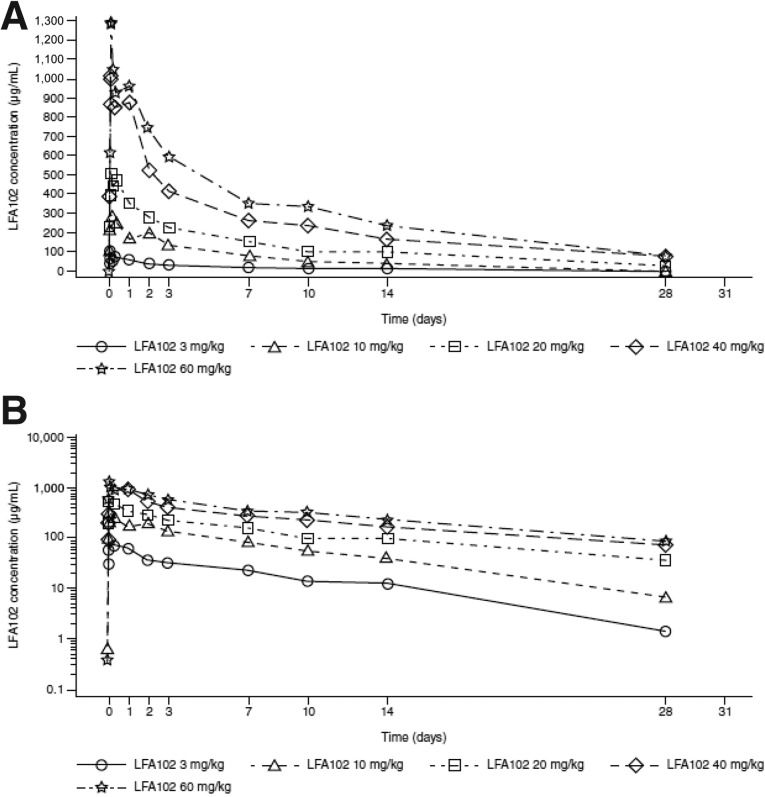
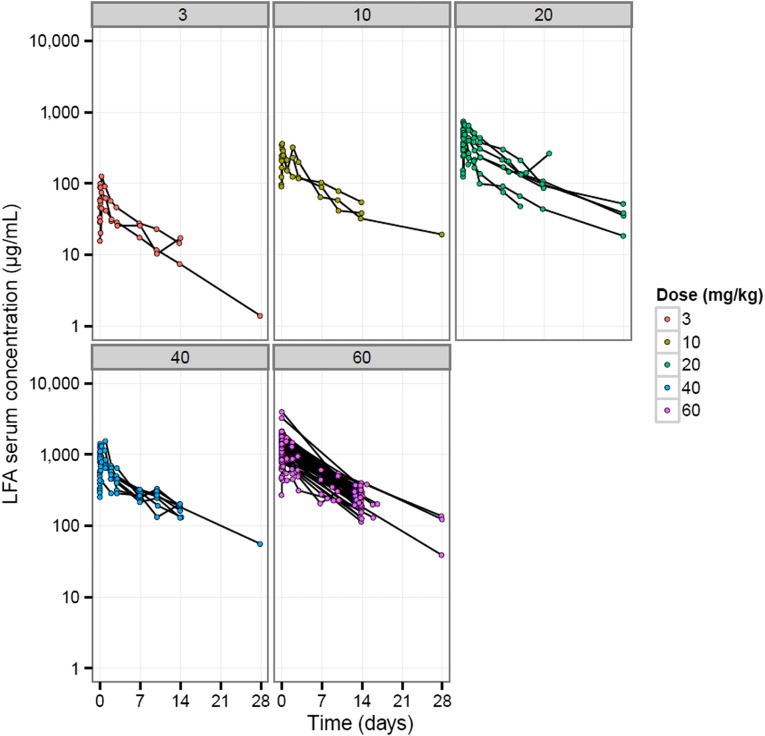
Prolactin, a pituitary-derived polypeptide hormone, is implicated in breast and prostate tumorigenesis. Expression of the PRLR has been confirmed in breast and prostate cancers. This phase I study evaluated LFA102 in 73 patients with PRLR-positive MBC or mCRPC, treated at doses of 3–60 mg/kg. During dose escalation, LFA102 demonstrated favorable safety and tolerability at all doses. No dose-limiting toxicities (DLTs) occurred; therefore, the MTD was not reached, although the RDE was established at 60 mg/kg based on safety, PK, and PD data supported by Bayesian logistic regression modeling. Dose proportionality analysis showed that serum LFA102 maximum concentration observed (Cmax) and area under the last measurable concentration (AUClast) were approximately linearly dose dependent (Fig. 1) and should provide sufficient exposure to achieve efficacy. However, no objective responses were observed in patients with MBC, and in patients with mCRPC, there were no prostate-specific antigen (PSA) responses.
Figure 1.
AUClast and Cmax increase with LFA102 dose in a relatively proportional manner. AUClast (A) and Cmax (B) results for individual patients in cycle 1. For each dose, parameter values (open symbols), least-square mean (black triangles), and 90% least-square means confidence interval (vertical bars) are shown. Serum LFA102 concentrations were measured up to day 28 of cycle 1 via dense sampling followed by trough concentration measurement in subsequent cycles. Concentration-time profiles show biexponential disposition typical for monoclonal antibodies. Cmax and AUClast increased in a relatively proportional manner with increasing LFA102 doses.
Abbreviations: AUClast, area under the last measurable concentration; Cmax, maximum concentration observed.
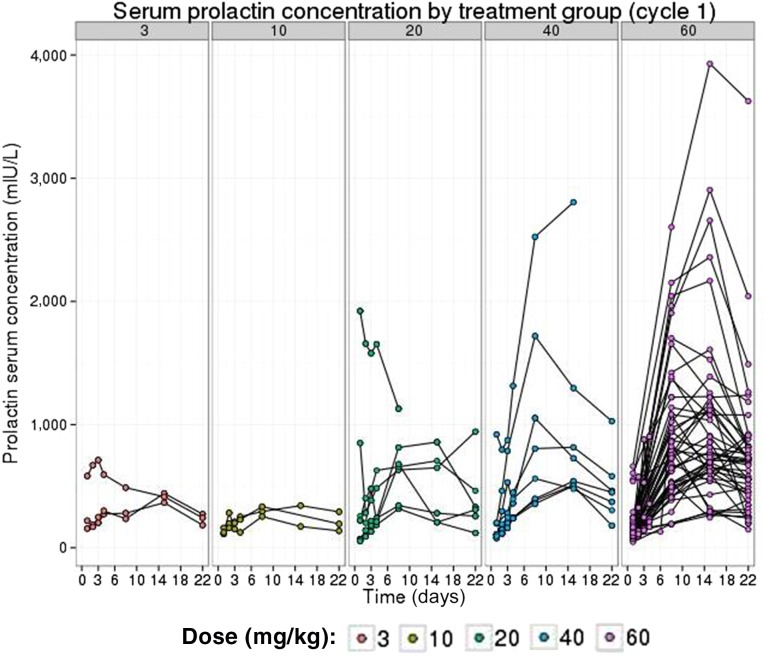
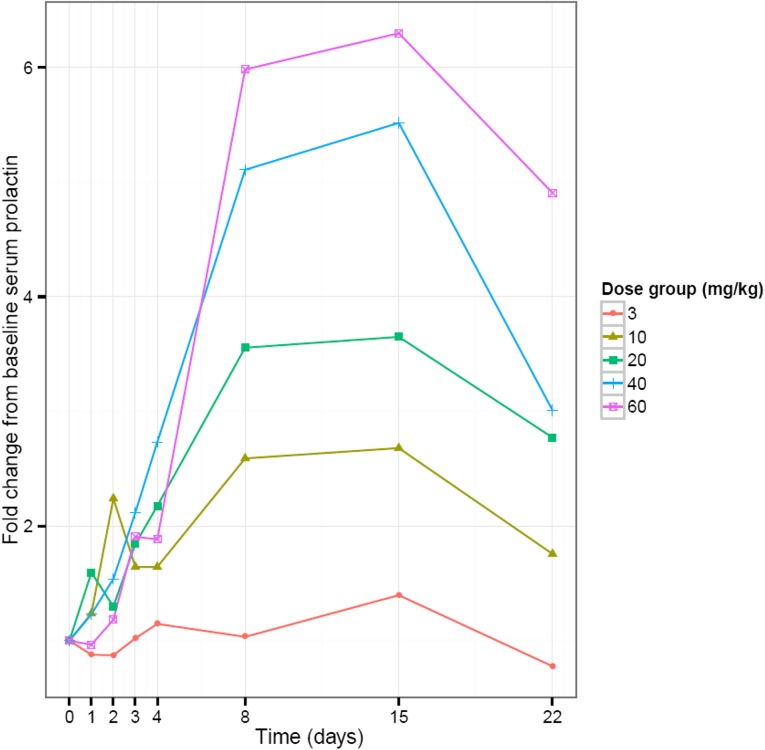
In vitro data have shown a high binding affinity of LFA102 to PRLR, but because assessing LFA102 binding within tumors is impractical in patients, our study used serum prolactin levels as a surrogate marker for PRLR inhibition. A sixfold change in serum prolactin levels from baseline was observed in patients treated with LFA102 60 mg/kg, indicative of inhibition of PRLR and ruling out poor target binding as causing lack of efficacy (Fig. 2). Other potential explanations for the lack of LFA102 efficacy include that prolactin may not be an oncogenic driver in breast and prostate cancer in humans, unforeseen compensatory modulation of downstream signaling pathways in response to PRLR inhibition, or upregulation of other tumorigenic signaling pathways that compensate for PRLR inhibition. Nevertheless, preclinical data show that letrozole potentiates the efficacy of LFA102 when administered in combination in a rat mammary cancer model. Therefore, although LFA102 monotherapy may not show antitumor activity, it may have potential for treating prolactin-dependent tumors in combination with other recently approved, novel hormonal pathway targeting agents in MBC and mCRPC. Furthermore, given the PD evidence of prolactin receptor blockade by LFA102, this drug has the potential to be used in conditions such as hyperprolactinemia that are associated with high prolactin levels.
Figure 2.
Serum prolactin levels rise with increasing doses of LFA102. Linear views of individual serum prolactin concentration-time profiles grouped by LFA102 dose group are shown. Individual patient serum prolactin increased after LFA102 administration.
Trial Information
- Disease
Breast cancer
- Disease
Prostate cancer
- Stage of disease / treatment
Metastatic / Advanced
- Prior Therapy
1 prior regimen
- Type of study - 1
Phase I
- Type of study - 2
Adaptive Design
- Primary Endpoint
Recommended Phase II Dose
- Primary Endpoint
Maximum Tolerated Dose
- Primary Endpoint
Safety
- Primary Endpoint
Tolerability
- Secondary Endpoint
Pharmacokinetics
- Secondary Endpoint
Pharmacodynamic
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
Exploratory: Effects of LFA102 on serum prolactin levels.
- Investigator’s Analysis
Evidence of target inhibition but no or minimal antitumor activity
Drug Information
- Drug 1
- Generic/Working name
LFA102
- Drug type
Antibody
- Dose
mg/kg
- Route
IV
- Schedule of Administration
10 mg/kg once every 4 weeks.
Dose Escalation Table
Patient Characteristics
- Number of patients, male
39
- Number of patients, female
34
- Stage
Locally advanced or metastatic disease.
- Age
Median (range): 66.0 years (41.0–89.0 years)
- Number of prior systemic therapies
Median (range): Not Collected
- Performance Status: ECOG
0 — 30
1 — 38
2 — 5
3 — 0
unknown —
- Cancer Types or Histologic Subtypes
Breast and prostate, 73
Primary Assessment Method
Control Arm: Breast And Prostate
- Number of patients screened
73
- Number of patients enrolled
73
- Number of patients evaluable for toxicity
73
- Number of patients evaluated for efficacy
73
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 0 (0%)
- Response assessment SD
n = 13 (18%)
- Response assessment PD
n = 41 (56%)
- Response assessment OTHER
n = 19 (26%)
Control Arm: Total Patient Population
- Number of patients screened
73
- Number of patients enrolled
73
- Number of patients evaluable for toxicity
73
- Number of patients evaluated for efficacy
73
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 0 (0%)
- Response assessment SD
n = 13 (18%)
- Response assessment PD
n = 41 (56%)
- Response assessment OTHER
n = 19 (26%)
Adverse Events
Serious Adverse Events
Dose Limiting Toxicities
Pharmacokinetics/Pharmacodynamics
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator’s Assessment
Evidence of target inhibition but no or minimal antitumor activity
Prolactin is a pituitary-derived polypeptide hormone implicated in breast and prostate tumorigenesis [1–3]. Prolactin is also expressed in several extrapituitary sites, in addition to breast and prostate tumors themselves [1, 4–7]. Expression of PRLR has been confirmed in various cancers, including breast and prostate [8–13]. Data suggest that increased serum prolactin levels may increase breast cancer risk and correlate with worse prognosis [14–16]. Overexpression of prolactin in murine mammary glands leads to tumor formation, and transplanted PRLR-negative tumors exhibit delays in tumor expansion compared with PRLR-positive tumors in mice [17, 18]. Although prolactin is expressed in normal human prostate, high expression in prostate tumors is associated with high-grade prostate cancer and worse prognosis [4, 19]. Overexpression of prolactin in mouse prostate causes hyperplasia and tumorigenesis [20, 21]. Therefore, blocking prolactin signal transduction is an attractive target in breast and prostate cancers.
Attempts made to inhibit PRLR signaling in vivo have been unsuccessful [22–27]. LFA102 is a humanized mAb that binds to the extracellular domain of PRLR. LFA102 inhibits PRLR signal transduction and cell proliferation in human breast cancer cells and causes tumor regression in animal xenograft models. Rats treated with LFA102 showed increased serum prolactin levels, suggesting this may be a potential biomarker for PRLR inhibition [28]. These data suggest that LFA102 has the potential to be an effective therapeutic agent in patients with breast or prostate cancer.
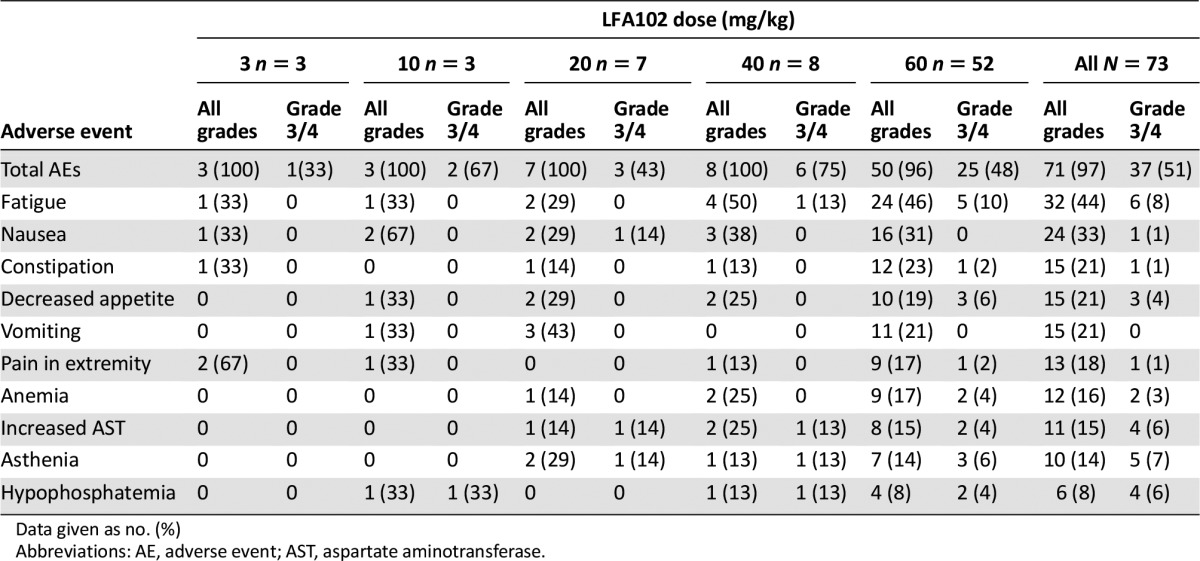
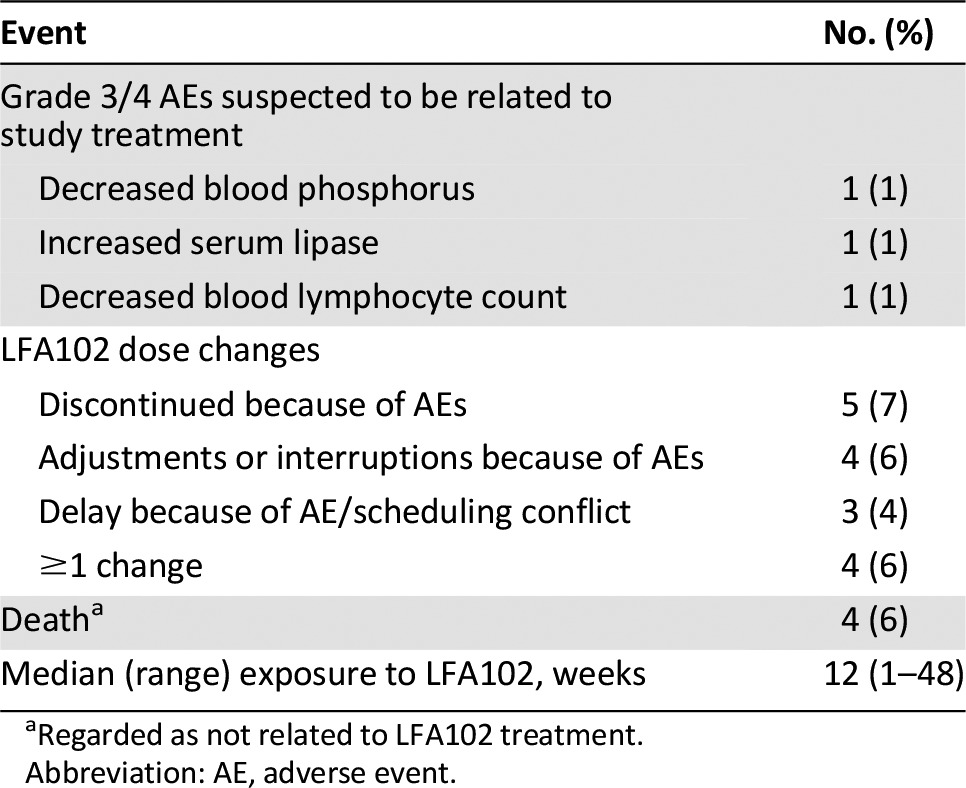
This phase I study evaluated LFA102 in patients with PRLR-positive MBC or mCRPC. Between September 2011 and March 2014, 73 patients were treated with LFA102 at doses of 3–60 mg/kg. During dose escalation, no DLTs occurred and the MTD was not reached. The RDE was established at 60 mg/kg, the highest tested dose level. The most common AEs, regardless of study drug relationship, were fatigue (44%), nausea (33%), constipation, decreased appetite, and vomiting (21% each). Of the 73 patients treated, 3 patients (4%) had grade 3 or 4 AEs suspected to be related to the study drug: decreased blood phosphorus, increased serum lipase, and decreased blood lymphocyte count, each in 1 patient (1%).
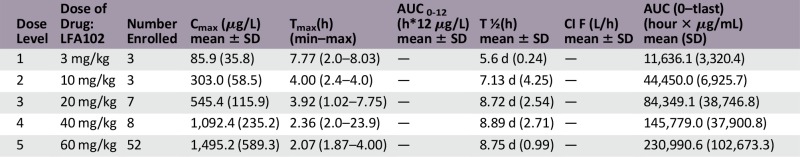
The serum LFA102 concentration-time profiles showed biexponential disposition typical for mAbs. Cmax and AUClast increased in a relatively proportional manner with increasing LFA102 doses (Fig. 1). The geometric mean apparent volume of distribution at steady state (Vss) and clearance across the treatment groups were similar, indicating linear PK. The geometric mean of Vss for doses of 3–60 mg/kg ranged from 4 to 6 L. The geometric mean half-life ranged from 6 to 9 days. At the RDE of 60 mg/kg, the mean (± SD) Cmax was 1,495 ± 589 µg/mL (coefficient of variation [CV%]: 39) and mean (± SD) AUClast was 230,991 ± 102,673 hour × µg/mL (CV% = 45), indicating moderate interindividual variability. No antidrug-antibody-positive samples were detected.
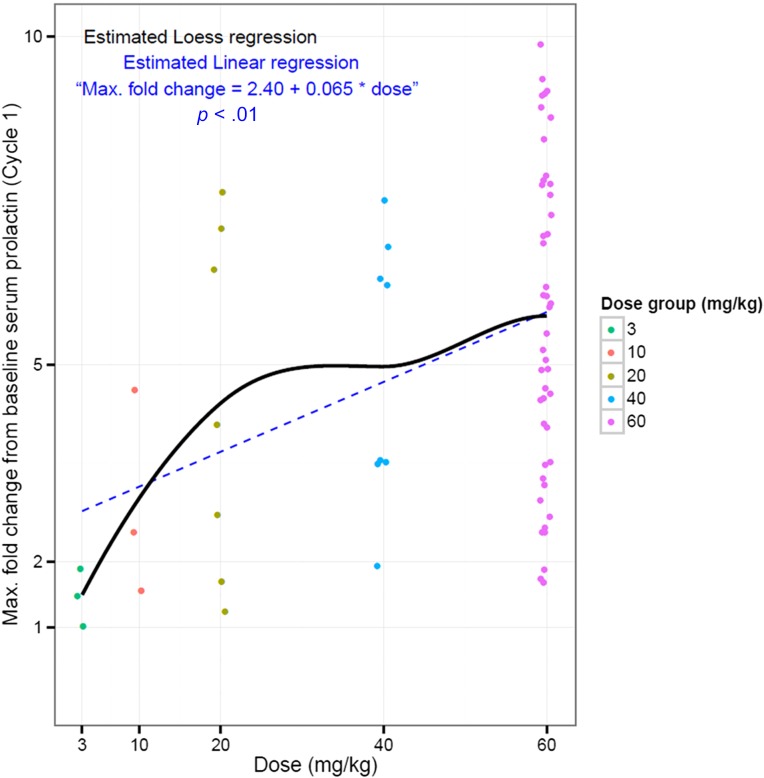
An exploratory objective of the study was to determine the effect of LFA102 treatment on serum prolactin levels in patients. The fold change from baseline increased in a dose-dependent manner, reached a maximum between days 8 and 15, and declined after day 15. The maximum fold-change in serum prolactin levels increased with doses up to 20 mg/kg and reached a plateau between 40 and 60 mg/kg. The temporal delay between PK and PD response is suspected to reflect the time needed for LFA102 to distribute to peripheral tissues, inhibit peripheral PRLR, and, consequently, lead to increased serum prolactin as a compensatory feedback mechanism.
The primary objective of this study was to determine the MTD and/or RDE of LFA102 in patients with MBC or mCRPC patients. An RDE of 60 mg/kg was established based on safety, PK, and PD, supported by the Bayesian logistic regression model. LFA102 demonstrated a favorable safety profile and tolerability at all doses tested. Dose proportionality analysis showed that serum LFA102 Cmax and AUClast were approximately linearly dose-dependent. LFA102 Vss was close to the volume of plasma, suggesting limited peripheral distribution typical of mAbs. At 60 mg/kg, the LFA102 half-life was 9 days, which, although within the reported range of mAbs, is slightly lower than the typical immunoglobulin G (IgG) with a half-life of approximately 25 days [29]. A possible explanation for this might be a lower affinity for the neonatal Fc receptor for IgG, which protects IgG from proteolytic degradation, leading to faster clearance.
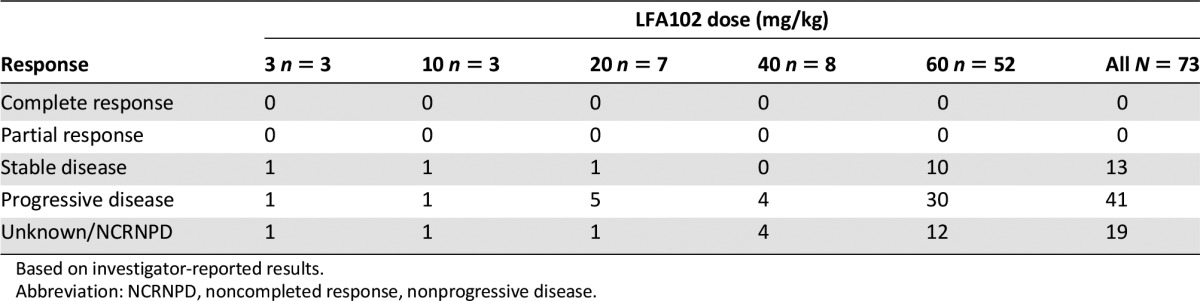
No objective responses were observed in patients with MBC during this study. In patients with mCRPC, there were no PSA responses. Thirteen of 73 patients (18%) experienced stable disease as their best response to LFA102 treatment. The majority of patients (67 of 73 patients; 92%) discontinued the study because of disease progression. One explanation for the lack of antitumor activity is the possibility of insufficient exposure. After a single dose of LFA102 10 mg/kg by i.v., serum LFA102 Cmax values were comparable between rodent and human subjects (268 µg/mL and 303 µg/mL, respectively; data not shown). Administration of a single dose of LFA102 10 mg/kg showed antitumor activity in a prolactin-dependent mouse tumor xenograft model (Nb2-11-luc) [28]. Consequently, the 60 mg/kg LFA102 dose in patients, which resulted in a mean Cmax of 1,495 ± 589 µg/mL and a mean steady-state trough concentration of 106 ± 34 µg/mL, would be anticipated to provide sufficient LFA102 exposure to achieve efficacy.
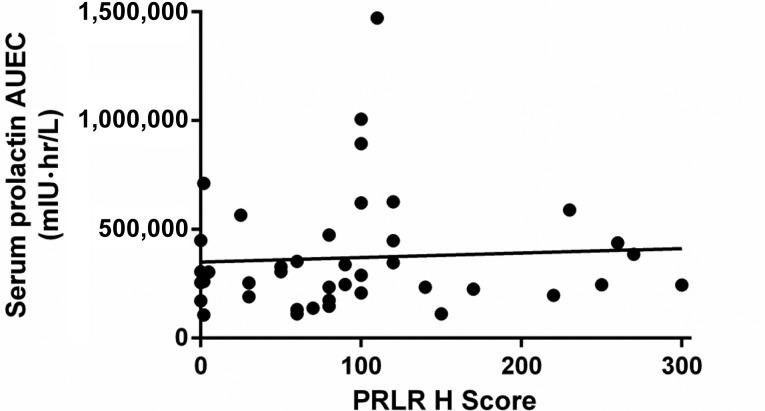
In vitro data showed a high binding affinity of LFA102 to PRLR [28]. Assessing LFA102 binding to PRLR directly within tumors is impractical in patients; therefore, serum prolactin levels were used as a surrogate marker for PRLR inhibition. A sixfold change in serum prolactin levels from baseline was observed in patients treated with LFA102 60 mg/kg, indicative of inhibition of PRLR. The compensatory increase in serum prolactin indicates that LFA102 binds to PRLR in patients, ruling out poor target binding as causative of lack of efficacy. However, the source of serum prolactin increase could either be the tumor or the pituitary gland. No correlation between tumor PRLR expression and serum prolactin response was observed. Therefore, the observed increase in serum prolactin is more likely to be a pituitary-driven feedback to LFA102 as a result of peripheral, nontumoral PRLR inhibition rather than a tumor-specific process. Furthermore, the increase in serum prolactin was transient; it was maintained up to 15 days following LFA102 administration (supplemental online Fig. 3). Based on this observation, more frequent LFA102 dosing (e.g., every 2 weeks) could have resulted in sustained PRLR inhibition and perhaps a better efficacy profile.
Another potential explanation for the lack of LFA102 efficacy is that prolactin may not be an oncogenic driver in breast and prostate cancer in humans. Prolactin activity as an oncogenic driver in human tumors has been difficult to assess directly in preclinical models of human breast and prostate cancers [28]. Mouse prolactin does not activate human PRLR; therefore, human breast or prostate cancer cells or primary tumors cannot be used for xenograft models in mice to assess the requirement for PRLR signaling in driving oncogenesis [30]. Other explanations for the lack of LFA102 efficacy include unforeseen compensatory modulation of downstream signaling pathways in response to PRLR inhibition, or upregulation of other compensatory tumorigenic signaling pathways.
Finally, letrozole potentiates the efficacy of LFA102 when administered in combination in a rat mammary cancer model [28]. These preclinical results raise the possibility that although LFA102 monotherapy may not show antitumor activity, it may still have the potential to treat prolactin-dependent tumors in combination with other agents, such as novel hormonal pathway targeting agents in MBC and mCRPC. Furthermore, given the PD evidence of prolactin receptor blockade by LFA102, this drug has the potential to be used in conditions such as hyperprolactinemia that are associated with high prolactin levels.
Supplementary Material
Supplemental Figure 1.
(A): Linear view. (B): Semilogarithmic view.
Supplemental Figure 2.
Individual LFA102 concentration-time profiles by treatment group: semi-logarithmic view (cycle 1).
Supplemental Figure 3.
Geometric mean for fold change from baseline for serum prolactin versus time profiles by treatment group (cycle 1).
Supplemental Figure 4.
Correlation of maximum fold change from baseline serum prolactin with dose.
Supplemental Figure 5.
Correlation of serum prolactin exposure with baseline prolactin receptor expression, 60 mg/kg dose group. r2 = .003; p = .7.
Abbreviations: AUEC, area under the effect curve; PRLR, prolactin receptor.
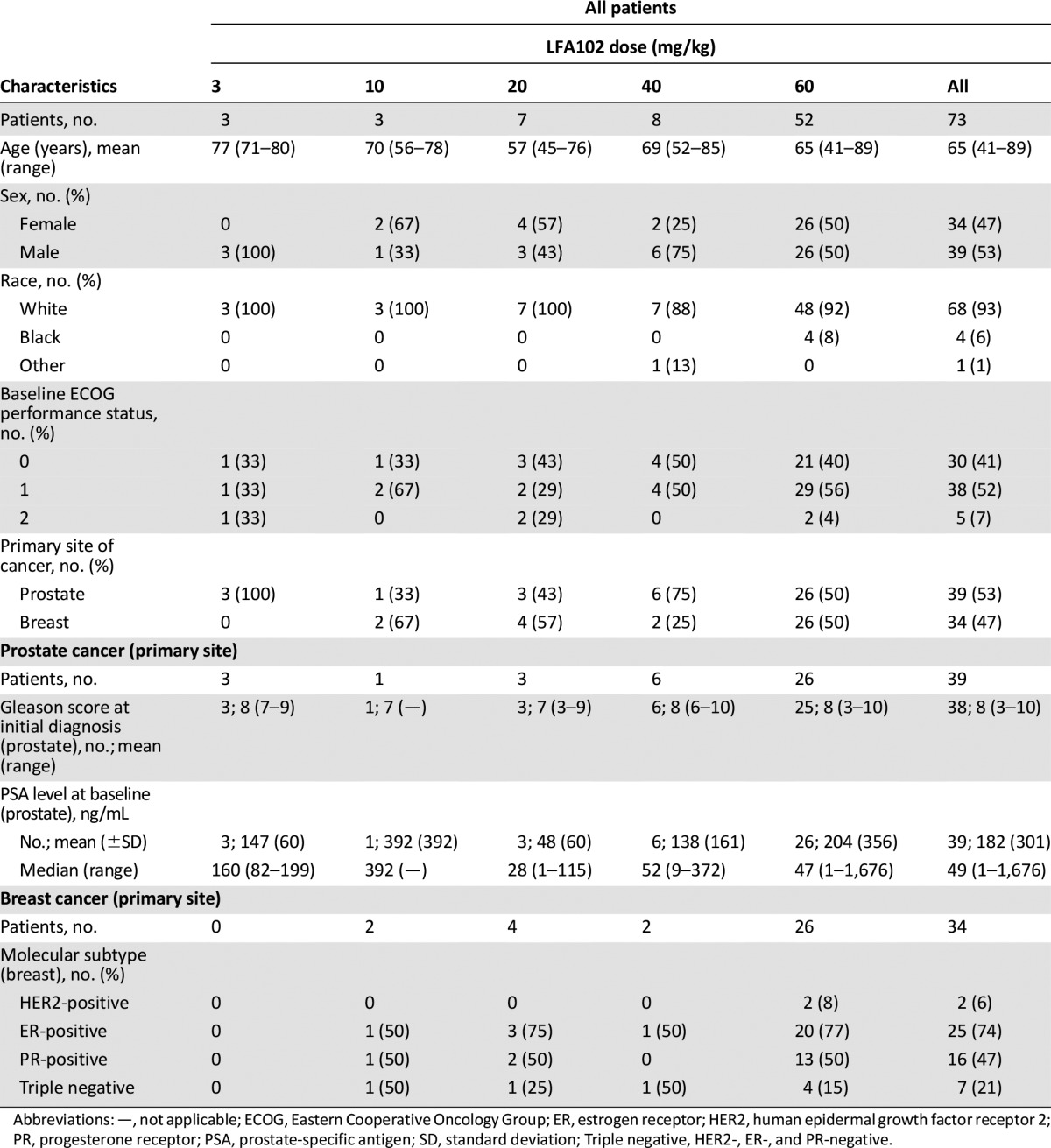
Table 1.
Patients’ characteristics
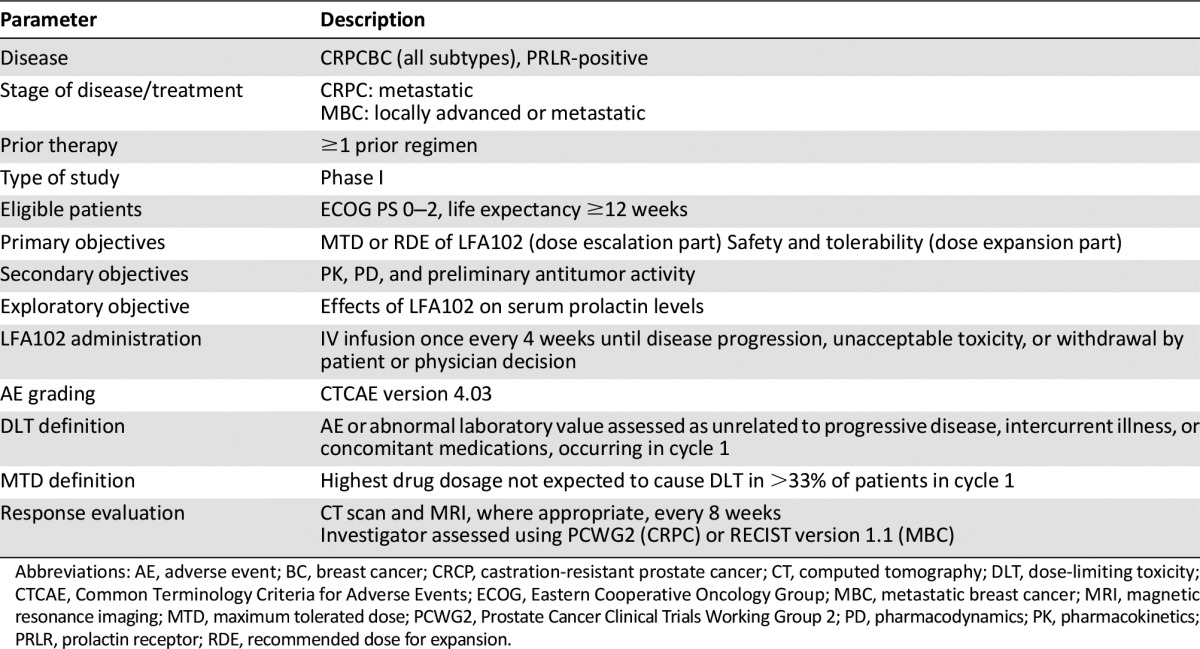
Supplemental Table 1.
Trial information
Supplemental Table 2.
Most common AEs (≥15% for all grades or ≥5% for grade 3/4 in all patients) regardless of study drug relationship
Supplemental Table 3.
Safety, tolerability, dose changes, and exposure to LFA102
Supplemental Table 4.
Best overall response to LFA102 treatment
Acknowledgments
We thank the patients who took part in the trial and their families, as well as the staff who assisted with the study at each site. This study was sponsored by Novartis Pharmaceuticals, which also provided financial support for medical editorial assistance. We thank Karen Beckett for medical editorial assistance with the manuscript.
Footnotes
ClinicalTrials.gov Identifier: NCT01338831
Sponsor: Novartis Pharmaceuticals Corporation
Principal Investigator: Neeraj Agarwal
IRB Approved: Yes
Click here to access other published clinical trials.
Disclosures
Jean-Pascal Machiels: Novartis (RF); Nancy Lewis: Novartis, Jefferson (E); Ahmad Awada: Roche, Pfizer (C/A); Mark Stein: Jansen, Medivation, Oncoceutics, Amgen, Advaxis, Merck (RF); Rebecca Mosher: Novartis (E); Ernesto Wasserman: Novartis (E); Mohamed Elmeliegy: Novartis (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ben-Jonathan N, Liby K, McFarland M, et al. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol Metab. 2002;13:245–250. doi: 10.1016/s1043-2760(02)00603-3. [DOI] [PubMed] [Google Scholar]
- 2.Ormandy CJ, Camus A, Barra J, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 3.Damiano JS, Wasserman E. Molecular pathways: Blockade of the PRLR signaling pathway as a novel antihormonal approach for the treatment of breast and prostate cancer. Clin Cancer Res. 2013;19:1644–1650. doi: 10.1158/1078-0432.CCR-12-0138. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Ahonen TJ, Alanen K, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–4782. doi: 10.1158/0008-5472.CAN-03-3499. [DOI] [PubMed] [Google Scholar]
- 5.Clevenger CV, Plank TL. Prolactin as an autocrine/paracrine factor in breast tissue. J Mammary Gland Biol Neoplasia. 1997;2:59–68. doi: 10.1023/a:1026325630359. [DOI] [PubMed] [Google Scholar]
- 6.Hugo ER, Borcherding DC, Gersin KS, et al. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J Clin Endocrinol Metab. 2008;93:4006–4012. doi: 10.1210/jc.2008-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milewicz T, Ryś J, Wójtowicz A, et al. Overexpression of P53 protein and local hGH, IGF-I, IGFBP-3, IGFBP-2 and PRL secretion by human breast cancer explants. Neuroendocrinol Lett. 2011;32:328–333. [PubMed] [Google Scholar]
- 8.Touraine P, Martini JF, Zafrani B, et al. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab. 1998;83:667–674. doi: 10.1210/jcem.83.2.4564. [DOI] [PubMed] [Google Scholar]
- 9.Gill S, Peston D, Vonderhaar BK, et al. Expression of prolactin receptors in normal, benign, and malignant breast tissue: An immunohistological study. J Clin Pathol. 2001;54:956–960. doi: 10.1136/jcp.54.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leav I, Merk FB, Lee KF, et al. Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am J Pathol. 1999;154:863–870. doi: 10.1016/S0002-9440(10)65333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levina VV, Nolen B, Su Y, et al. Biological significance of prolactin in gynecologic cancers. Cancer Res. 2009;69:5226–5233. doi: 10.1158/0008-5472.CAN-08-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauernhofer T, Pichler M, Wieckowski E, et al. Prolactin receptor is a negative prognostic factor in patients with squamous cell carcinoma of the head and neck. Br J Cancer. 2011;104:1641–1648. doi: 10.1038/bjc.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbaum L, Pollheimer MJ, Bauernhofer T, et al. Clinicopathological significance of prolactin receptor expression in colorectal carcinoma and corresponding metastases. Mod Pathol. 2010;23:961–971. doi: 10.1038/modpathol.2010.83. [DOI] [PubMed] [Google Scholar]
- 14.Tworoger SS, Eliassen AH, Sluss P, et al. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol. 2007;25:1482–1488. doi: 10.1200/JCO.2006.07.6356. [DOI] [PubMed] [Google Scholar]
- 15.Wu ZS, Yang K, Wan Y, et al. Tumor expression of human growth hormone and human prolactin predict a worse survival outcome in patients with mammary or endometrial carcinoma. J Clin Endocrinol Metab. 2011;96:E1619–E1629. doi: 10.1210/jc.2011-1245. [DOI] [PubMed] [Google Scholar]
- 16.Tworoger SS, Eliassen AH, Zhang X, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013;73:4810–4819. doi: 10.1158/0008-5472.CAN-13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose-Hellekant TA, Arendt LM, Schroeder MD, et al. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–4674. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakes SR, Robertson FG, Kench JG, et al. Loss of mammary epithelial prolactin receptor delays tumor formation by reducing cell proliferation in low-grade preinvasive lesions. Oncogene. 2007;26:543–553. doi: 10.1038/sj.onc.1209838. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhang Y, Glass A, et al. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res. 2005;11:5863–5868. doi: 10.1158/1078-0432.CCR-05-0562. [DOI] [PubMed] [Google Scholar]
- 20.Kindblom J, Dillner K, Sahlin L, et al. Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology. 2003;144:2269–2278. doi: 10.1210/en.2002-0187. [DOI] [PubMed] [Google Scholar]
- 21.Rouet V, Bogorad RL, Kayser C, et al. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc Natl Acad Sci USA. 2010;107:15199–15204. doi: 10.1073/pnas.0911651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goffin V, Bernichtein S, Touraine P, et al. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev. 2005;26:400–422. doi: 10.1210/er.2004-0016. [DOI] [PubMed] [Google Scholar]
- 23.Holtkamp W, Nagel GA. [Bromocriptine in chemotherapy-resistant, metastatic breast cancer. Results of the GO-MC-BROMO 2/82 AIO Study] Onkologie. 1988;11:121–127. doi: 10.1159/000216502. [DOI] [PubMed] [Google Scholar]
- 24.Bonneterre J, Mauriac L, Weber B, et al. Tamoxifen plus bromocriptine versus tamoxifen plus placebo in advanced breast cancer: Results of a double blind multicentre clinical trial. Eur J Cancer Clin Oncol. 1988;24:1851–1853. doi: 10.1016/0277-5379(88)90097-1. [DOI] [PubMed] [Google Scholar]
- 25.Bontenbal M, Foekens JA, Lamberts SWJ, et al. Feasibility, endocrine and anti-tumour effects of a triple endocrine therapy with tamoxifen, a somatostatin analogue and an antiprolactin in post-menopausal metastatic breast cancer: A randomized study with long-term follow-up. Br J Cancer. 1998;77:115–122. doi: 10.1038/bjc.1998.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clevenger CV, Zheng J, Jablonski EM, et al. From bench to bedside: Future potential for the translation of prolactin inhibitors as breast cancer therapeutics. J Mammary Gland Biol Neoplasia. 2008;13:147–156. doi: 10.1007/s10911-008-9074-8. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson EM, Hugo ER, Tuttle TR, et al. Unexploited therapies in breast and prostate cancer: blockade of the prolactin receptor. Trends Endocrinol Metab. 2010;21:691–698. doi: 10.1016/j.tem.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damiano JS, Rendahl KG, Karim C, et al. Neutralization of prolactin receptor function by monoclonal antibody LFA102, a novel potential therapeutic for the treatment of breast cancer. Mol Cancer Ther. 2013;12:295–305. doi: 10.1158/1535-7163.MCT-12-0886. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 30.Utama FE, LeBaron MJ, Neilson LM, et al. Human prolactin receptors are insensitive to mouse prolactin: implications for xenotransplant modeling of human breast cancer in mice. J Endocrinol. 2006;188:589–601. doi: 10.1677/joe.1.06560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.