Testing for mismatch repair (MMR) status in colorectal cancer (CRC) may provide useful prognostic and predictive information. The impact of such testing on real-world practices regarding adjuvant chemotherapy for resected CRC was evaluated. This study found that knowledge of MMR status influences treatment decisions for patients with stage II CRC, in an era when clear recommendations as to how these findings should influence practice were lacking.
Keywords: Microsatellite instability, Mismatch repair deficiency, Colorectal cancer, Adjuvant chemotherapy, Treatment decisions
Abstract
Background.
Testing for mismatch repair (MMR) status in colorectal cancer (CRC) may provide useful prognostic and predictive information. We evaluated the impact of such testing on real-world practice regarding adjuvant chemotherapy for patients with resected CRC.
Patients and Methods.
A total of 175 patients with stage II and III mismatch repair-deficient (MMRD) CRC were identified from an Australian population-based study of incident CRCs. Their treatment decisions were compared with those for a cohort of 773 stage-matched patients with mismatch repair-proficient (MMRP) CRCs. The effect of MMR status, age, and pathologic characteristics on treatment decisions was determined using multiple regression analysis.
Results.
Overall, 32% of patients in stage II and 71% of patients in stage III received adjuvant chemotherapy. Among the stage II patients, those with MMRD cancer were less likely to receive chemotherapy than were MMRP cases (15% vs. 38%; p < .0001). In this group, the treatment decision was influenced by age, tumor location, and T stage. MMR status influenced the treatment decision such that its impact diminished with increasing patient age. Among patients with stage III tumors, no difference was found in the chemotherapy rates between the MMRD and MMRP cases. In this group, age was the only significant predictor of the treatment decision.
Conclusion.
The findings of this study suggest that knowledge of the MMR status of sporadic CRC influences treatment decisions for stage II patients, in an era when clear recommendations as to how these findings should influence practice are lacking.
Implications for Practice:
Microsatellite instability (MSI) is a molecular marker of defective DNA mismatch repair found in 15% of sporadic colorectal cancers. Until recently, expert guidelines on the role of MSI as a valid biomarker in the selection of stage II patients for adjuvant chemotherapy were lacking. Conducted at a time when the clinical utility of routine MSI testing was unclear, this study found that clinicians were influenced by MSI status in selecting stage II patients for chemotherapy. Furthermore, the impact of MSI on treatment decisions was greatest in younger patients and declined progressively until age 80 years, when no effect was found.
Abstract
摘要
背景. 对结直肠癌 (CRC) 进行错配修复 (MMR) 状态检验可能提供有用的预后和预测信息。我们评价了这种检验在真实世界临床实践中对手术切除后 CRC 患者辅助化疗的影响。
患者与方法. 我们从澳大利亚基于人群的偶发性 CRC 研究中, 共鉴别出 175 例有错配修复缺陷 (MMRD) 的 II 期和 III 期 CRC 患者。将他们的治疗决策与 773 例分期匹配的错配修复功能完善的 CRC 患者队列进行比较。使用多元回归分析确定 MMR 状态、年龄和病理特征对治疗决策的影响。
结果. 总体而言, 32%的 II 期患者和 71%的 III 期患者接受了辅助化疗。 II 期患者中, MMRD 癌症患者接受化疗的概率低于 MMRP 病例 (15% vs 38%, P<0.0001)。该患者组的治疗决策受年龄、肿瘤部位和 T 分期的影响。 MMR 状态对治疗决策的影响随着患者年龄增长而削弱。在 III 期患者中, 未发现 MMRD 和 MMRP 患者的化疗率存在差异。年龄是该患者组治疗决策的唯一显著预测因素。
结论. 本研究的结果提示, 对偶发性CRC MMR状态的了解影响了II期患者的治疗决策, 但是有关这些发现会如何对临床实践产生影响, 当前尚无明晰的建议。The Oncologist 2016;21:618–625
对临床实践的提示: 微卫星不稳定性 (MSI) 是 DNA 错配修复缺失的分子标记物, 见于 15% 的偶发性结直肠癌。直到最近, 关于 MSI 作为有效的生物标记物在经选择的 II 期患者辅助化疗中的作用, 在专家指南里仍然是缺失的。我们的研究开展于 MSI 常规检验在临床上的用途仍不明确的时代, 却发现临床医生在经选择的 II 期患者的化疗决策中受到了 MSI 状态的影响。此外, 受 MSI 对治疗决策影响程度最大的患者是较年轻的患者, 影响的程度则随着年龄增长而不断下降, 直至 80 岁时不再有任何影响。
Introduction
Microsatellite instability (MSI) is the molecular hallmark of colorectal cancers (CRCs) that have evolved as a result of inactivation of the DNA mismatch repair (MMR) system. It occurs either in the setting of Lynch syndrome, underpinned by germline mutation of a mismatch repair gene (MLH1, MSH2, MSH6, or PMS2), or more commonly because of biallelic epigenetic inactivation of the MLH1 gene. Mismatch repair-deficient (MMRD) tumors have distinctive features, including a predilection for the proximal colon, poor differentiation, and increased numbers of tumor-infiltrating lymphocytes [1]. Patients with these tumors have a better stage-adjusted prognosis than those with MMR-proficient (MMRP) tumors [2–5]. MMRP tumors are predictive of a poor response to 5-fluorouracil (5-FU)-based chemotherapy regimens [6–8] and might respond differently to regimens containing irinotecan [9, 10].
Although universal MMR testing in CRC has clear benefits in terms of cost-effective detection of Lynch syndrome [11], the clinical utility of MMR testing in sporadic cases of CRC has only recently been recognized [12–14]. The latest version of the National Comprehensive Cancer Network guidelines has recommended the inclusion of MSI in the evaluation of patients with stage II CRC to assist in the decision-making process regarding adjuvant chemotherapy [15]. However, at the time our study was conducted, consensus among expert groups was lacking regarding the incorporation of MMR status into risk-stratification panels or decision-making processes despite evidence to indicate that MMR status has prognostic value in patients with stage II and III CRC [16–18]. Given this discrepancy between evidence and expert opinion at the time, it is of interest to consider the extent to which MMR status was used by oncologists in real-world practice.
We previously undertook a multicenter prospective study of the implementation of universal MMR testing of all incident CRCs in the South-East Sydney Illawarra Area Health Service (SESIAHS), a health care region serving 1.2 million Australian residents [19]. The results of MMR testing were incorporated into standard pathology reports and made available to all treating surgeons and oncologists. The present study builds directly on this previous work. Conducted at a time when the evidence for MMRD as a favorable prognostic marker had only recently emerged, the present study analyzed the rates of adjuvant chemotherapy treatment in a population-based cohort of individuals with resected CRC to determine whether the tumor MMR status had an impact on the use of adjuvant chemotherapy in those with stage II and III disease. We also evaluated a range of other factors known to influence the decisions regarding adjuvant therapy, including patient age, T stage, and tumor location.
Patients and Methods
Patient Selection
The South Eastern Sydney Local Health District human research ethics committee approved the study (13/150). As previously reported, 245 patients with MMRD tumors were recruited from a population-based molecular screening program of all incident cases of CRC diagnosed between July 1, 2006 and October 31, 2009 in the SESIAHS [19]. Of these, 175 patients with stage II or III CRC were selected as the MMRD cohort for the present study. A control cohort of patients with stage II or III MMRP CRC from the same region and period was derived from the New South Wales Clinical Cancer Registry (ClinCR). ClinCR is a population-based registry that collects information pertaining to the diagnosis, treatment, and care of all patients diagnosed with primary cancer within state-run public health care facilities. All incident cases of stage II or III CRC diagnosed in SESIAHS during the study period were identified from ClinCR using the following selection criteria: (a) definitive surgical resection for CRC between July 1, 2006 and October 31, 2009; and/or (b) the initiation of adjuvant chemotherapy for CRC between September 1, 2006 and January 1, 2010. The second selection criterion was designed to identify potentially eligible subjects who did not have surgical treatment records in ClinCR because they had undergone surgical resection at a private sector institution. It also considered a time window of 8 weeks from the date of definitive surgery to the initiation of adjuvant chemotherapy. Although ClinCR does not capture the MMR status of tumors, the information gained from our previous study allowed us to identify all MMRD cases from within the ClinCR cohort and thereby derive a cohort of 773 MMRP cases.
Data Extraction
The patient demographic details (e.g., sex, age at diagnosis) and tumor characteristics (e.g., primary site, histopathological grade, and TNM stage [20] at diagnosis) were determined for both cohorts from their respective databases. The key determinants of the treatment decision that were not captured in the study included the presence of lymphovascular or perineural invasion and tumor presentation with obstruction or perforation. Data on adjuvant chemotherapy for the MMRP cohort were extracted from the ClinCR database. Data relating to the oncological review and adjuvant chemotherapy for the MMRD cohort were collected by a review of the patient medical records as a part of the present study.
Statistical Analysis
As treatment decisions can be influenced by factors such as age, tumor location, degree of differentiation, or T stage, these variables were assessed for potential confounding of the study factor, MMR status. Patients were stratified by disease stage (II–III). For each disease stage, we performed univariate logistic regression to assess for any associations between the treatment decisions and the variables of MMR status (MMRD or MMRP), age (modeled as a continuous variable), tumor location (colonic or rectal), tumor differentiation (moderately well- and well-differentiated or poorly differentiated and undifferentiated/anaplastic), and T stage (T1–T4 stage). Using a p value of ≤.1, the variables associated with the treatment decision were selected for multiple regression analysis. We also investigated for possible interactions between variables, such that the effect of one of the variables differs depending on the level of the other variable. A backward elimination approach was then used during logistical regression to obtain a final model containing only variables with p < .05. Statistical analysis was performed using STATA, version 13.0 (StataCorp, College Station, TX, http://www.stata.com).
Results
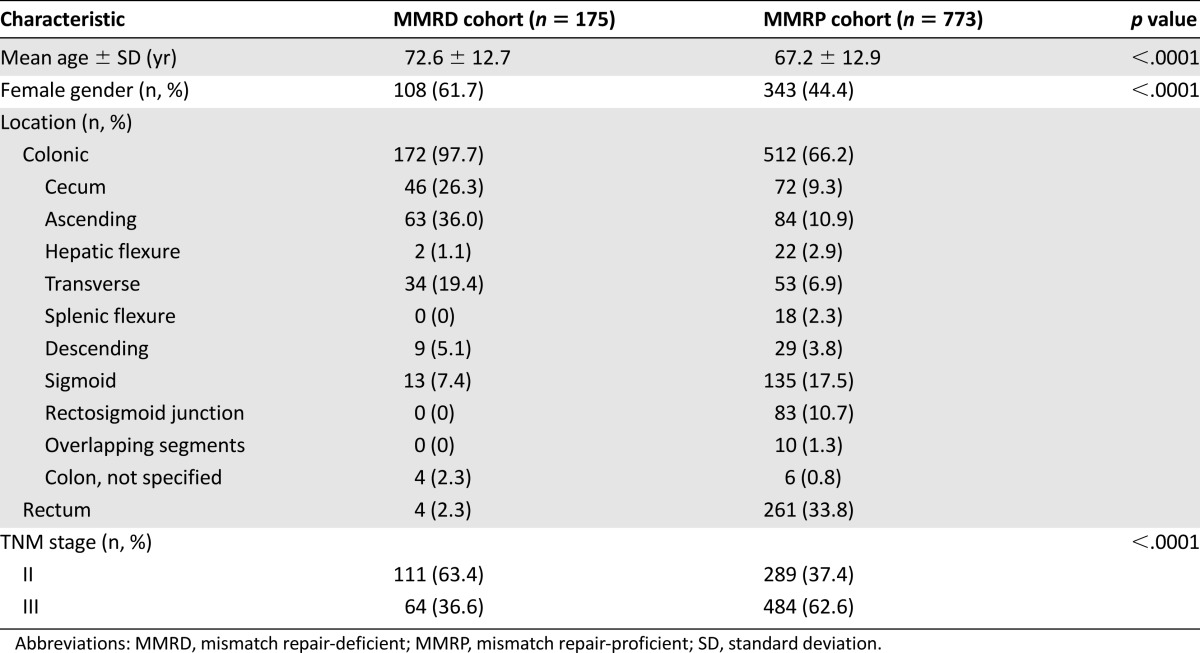
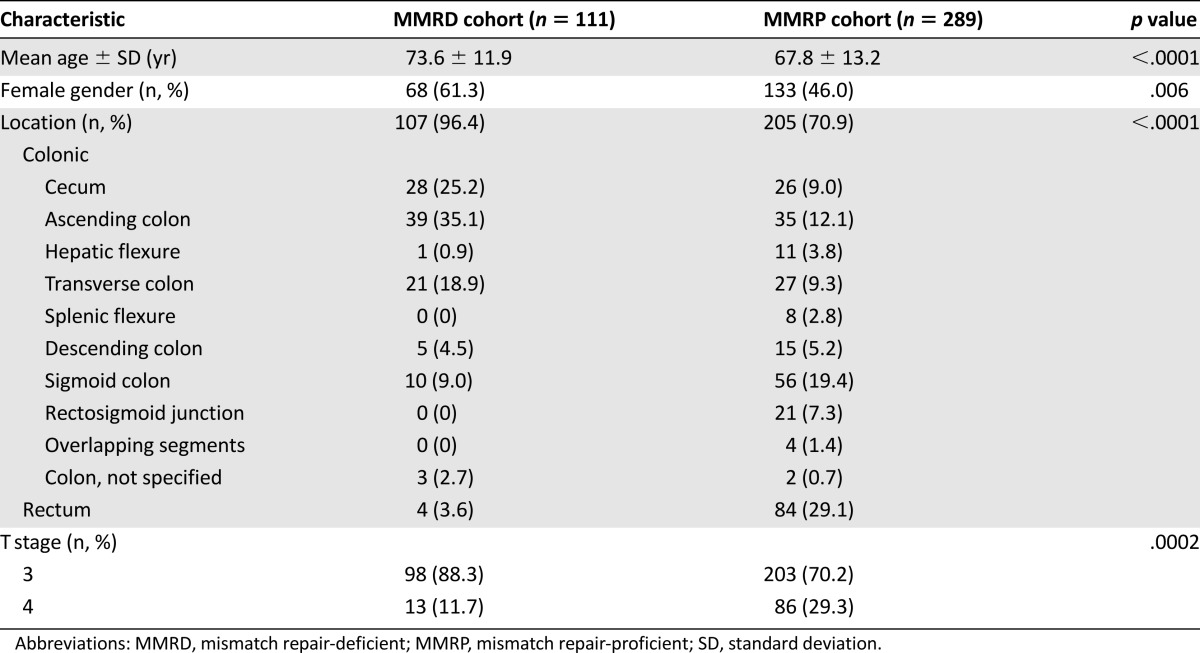
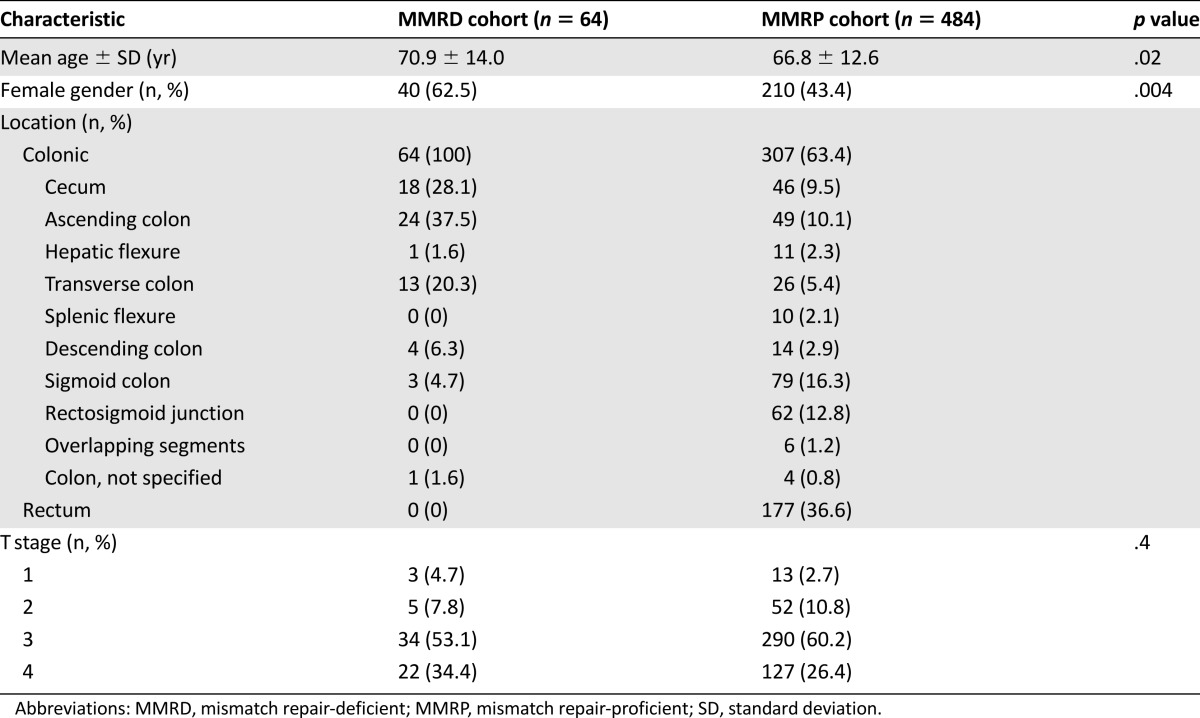
The patients and tumor characteristics of the entire study cohort and the MMRD and MMRP cohorts are listed in Table 1. Patients with MMRD tumors were more likely to be female and older at diagnosis compared with their MMRP counterparts. MMRD tumors were more likely to be colonic rather than rectal in location and have lower T and TNM stages. The associations between MMRD tumors and older age, female gender, and colonic tumor site were maintained when the subgroup analysis was performed according to disease stage (Tables 2, 3). However, the association between MMRD tumors and lower T stage was only seen in those with stage II disease.
Table 1.
Characteristics of study sample
Table 2.
Characteristics of stage II patients
Table 3.
Characteristics of stage III patients
Adjuvant chemotherapy was received by 32% of all patients with stage II disease and 72% of those with stage III disease. In patients with stage II disease, significantly fewer patients in the MMRD cohort received adjuvant chemotherapy compared with those in the MMRP cohort (17 of 111 [15%] in the MMRD group vs. 109 of 289 [38%] in the MMRP group; p < .0001). In those with stage III disease, no significant differences were found in the proportion receiving chemotherapy between the two cohorts (44 of 64 [69%] in the MMRD group vs. 348 of 484 [72%] in the MMRP group; p = .6).
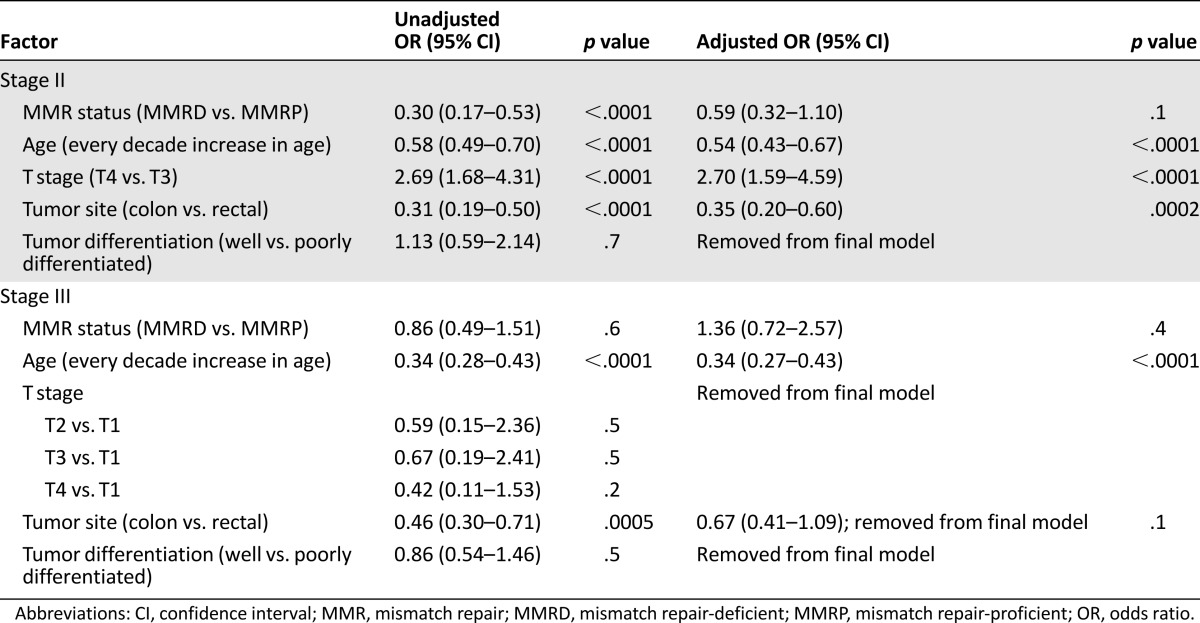
Multiple regression analyses were conducted to examine the apparent relationship between the treatment decision and MMR status and the relative influence of potential confounding factors such as age, tumor site, and T stage. The results are listed in Table 4 and described further below.
Table 4.
Multivariate analysis of factors associated with receiving adjuvant chemotherapy for colorectal cancer, stratified by stage of disease
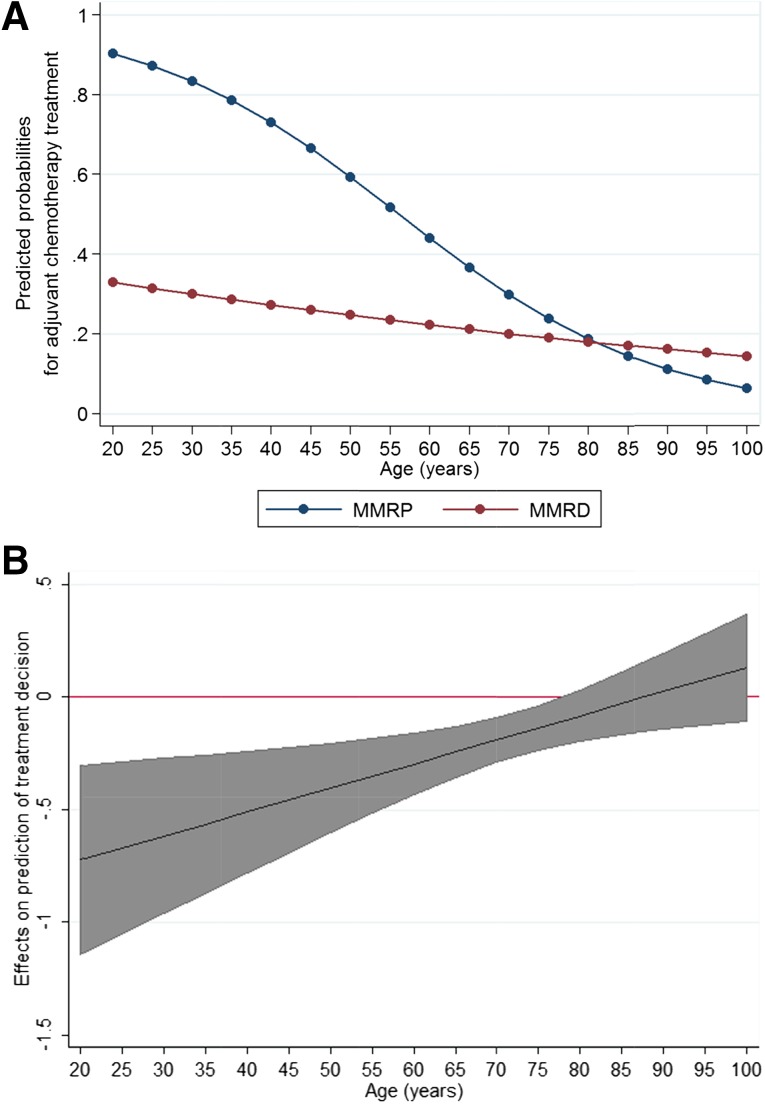
In stage II, univariate analysis showed that a clear association of treatment with MMR status (χ2 = 20.37; 1 degree of freedom [DF]; p < .0001) and with the potential confounders: age (χ2 = 39.96; 1DF; p < .0001), tumor site (χ2 = 22.30; 1DF; p < .0001), and T stage (χ2 = 16.85; 1DF; p < .0001). No association was found with tumor differentiation (χ2 = 0.13; 1DF; p = .7). A significant interaction was found between age and MMR status; thus, the effect of age on the treatment decision was dependent on the MMR status of the tumor. As illustrated in Figure 1A, increasing age caused only a very modest reduction in the chemotherapy administration rates among patients with MMRD tumors. In contrast, a steep decline was found in the treatment rates with increasing age for patients with MMRP tumors. Figure 1B shows that the difference between the MMRP and MMRD groups in the likelihood of receiving chemotherapy was greatest in young patients and declined progressively with age. For patients who are 80 years old and older, MMR status did not have a statistically significant impact on treatment likelihood.
Figure 1.
Predicted probabilities of treatment for 5-year increments of age in stage II. (A): Age-related predicted probabilities of treatment for the MMRD and MMRP groups. (B): Marginal conditional effects of MMR status on prediction of treatment decisions in stage II showing the difference, with 95% confidence intervals, in the predicted probabilities of adjuvant chemotherapy between the MMRD and MMRP groups. The MMRD/MMRP difference decreased with age. The 95% confidence intervals included 0 at approximately 80 years of age; thus, this difference was not significant after 80 years of age.
Abbreviations: MMR, mismatch repair; MMRD, mismatch repair deficient; MMRP, mismatch repair proficient.
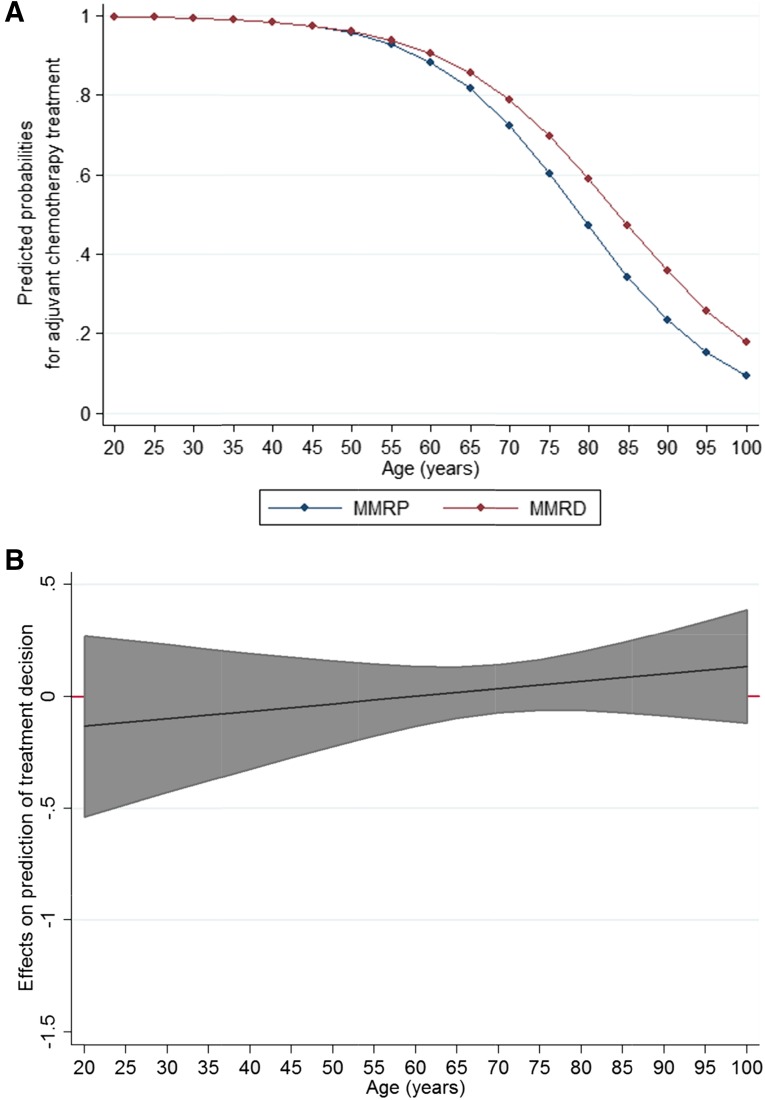
In stage III, univariate analysis showed no significant association of treatment with MMR status (χ2 = 0.28; 1DF; p = .6). However, treatment was associated with the confounders of age (χ2 = 129.80; 1DF; p < .0001) and tumor site (χ2 = 12.06; 1DF; p = .0005). No association was apparent with T stage (χ2 = 5.67; 3DF; p = .1) or tumor differentiation (χ2 = 0.43; 1DF; p = .5). No interaction was found between the variables. As illustrated in Figure 2, age-related changes in the predicted probabilities of treatment were similar for the two groups, and no significant difference between the two groups was seen across all ages. After adjusting for confounders, the odds ratio of receiving adjuvant chemotherapy for those with MMRD tumors compared with those with MMRP tumors was 0.34 (95% confidence interval, 0.72–2.57; p = .4).
Figure 2.
Predicted probabilities of treatment for 5-year increments of age in stage III. (A): Age-related predicted probabilities of treatment for the MMRD and MMRP groups. (B): Marginal conditional effects of MMR status on prediction of treatment decisions in stage III showing that the MMRD and MMRP difference, with 95% confidence intervals, is non-significant across all ages because the 95% confidence intervals included 0.
Abbreviations: MMR, mismatch repair; MMRD, mismatch repair deficient; MMRP, mismatch repair proficient.
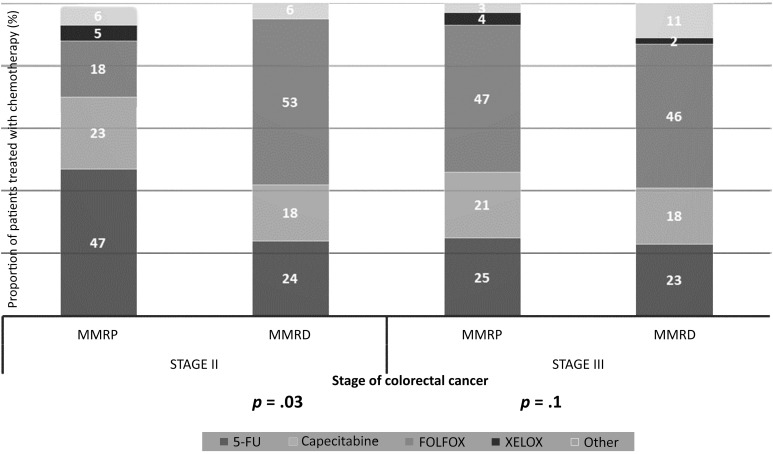
In patients receiving adjuvant chemotherapy for stage II disease, a statistically significant difference was found in the choice of chemotherapeutic agents between the MMRD and MMRP cohorts. Single-agent 5-FU-based chemotherapy was the preferred regimen for those with MMRP tumors (51 of 109 [47%] in the MMRP group vs. 4 of 17 [24%] in the MMRD group; p = .03). In contrast, FOLFOX (folinic acid, 5-fluorouracil, oxaliplatin) was favored for those with MMRD tumors (9 of 17 [53%] in the MMRD group vs. 20 of 109 [18%] in the MMRP group; p = .03). For stage III patients, no statistically significant differences were found in the choice of chemotherapy regimen between the two cohorts. FOLFOX was the most commonly prescribed regimen for both cohorts and was used in 46% (20 of 44) and 47% (162 of 384) of the MMRD and MMRP cohorts, respectively (Fig. 3).
Figure 3.
Choice of chemotherapy regimen for stage II and III colorectal cancer, stratified by tumor MMR status. For each stage, the proportion of patients treated with different chemotherapy agents is shown, according to mismatch repair status. For patients with stage II disease, a significantly greater proportion of patients with MMRD tumors were treated with the FOLFOX regimen compared with patients with MMRP tumors (53% vs. 18%; p = .03). For patients with stage III disease, no statistically significant difference was found between the two cohorts regarding the choice of chemotherapeutic agents.
Abbreviations: 5-FU, 5-fluorouracil; FOLFOX, folinic acid, 5-FU, oxaliplatin; MMR, mismatch repair; MMRD, mismatch repair deficient; MMRP, mismatch repair proficient; XELOX, capecitabine plus oxaliplatin.
Discussion
Our study showed that the effect of MMR status on adjuvant chemotherapy varied according to the stage of disease. The finding that MMR status did not influence use of adjuvant chemotherapy in stage III disease is consistent with current practice guidelines [16–18], which consider this treatment the standard of care for all medically fit patients. For patients with stage II disease, our study showed that those with MMRD tumors were less likely to receive adjuvant chemotherapy than those with MMRP tumors. Although adjuvant chemotherapy is not the standard practice for stage II CRC [17, 18], it is nevertheless considered for those cases with pathologic characteristics that predict for a poor prognosis, because those individuals might derive greater benefit from treatment. These “high-risk” features have traditionally included T4 stage, inadequately sampled lymph nodes, poor tumor differentiation, lymphovascular invasion, bowel obstruction, and bowel perforation. Although the National Comprehensive Cancer Network guidelines had been recently updated to incorporate MSI into its risk assessment panel, no clear agreement had been reached among expert groups concerning the role of MSI as a valid biomarker at the time the present study was conducted [16].
In our study, clinicians relied mostly on well-accepted pathological risk factors in deciding on adjuvant chemotherapy. We found that both T4 stage and rectal tumor location are associated with an increased likelihood of receiving adjuvant chemotherapy. No significant association between tumor differentiation and chemotherapy administration was found, perhaps because many MMRD tumors are paradoxically “high grade” in terms of differentiation.
On multiple regression analysis, we were unable to show that MMR status influenced the treatment choice in stage II disease, despite a clear trend in the direction of reduced treatment for these tumors. The interaction between the variables MMR status and age is a possible reason for this. In younger patients with stage II disease, knowledge of tumor MMR status appeared to have had a significant impact on the treatment decision-making process regarding the use of adjuvant chemotherapy. However, in elderly patients, age seemed to become the primary factor in the decision regarding chemotherapy. This might reflect the trend, observed in our study and previous epidemiologic studies [21, 22], that the rates of chemotherapy use decline with increasing patient age. Although comorbidity is an important factor in treatment decision-making, population-based analyses have shown that the use of chemotherapy declines with age independent of the presence of comorbidities [21, 22], possibly from a perception that elderly patients will derive less benefit. When the overall inclination to offer chemotherapy diminishes with patient age, other factors that influence treatment decisions could have a lesser impact.
Beyond the simple decision to treat, it has also been suggested that MMR status might influence the response to specific chemotherapy regimens, although this has been an area of considerable controversy. In contrast to earlier studies [23], Ribic et al. reported in 2003 that although patients with MMRD tumors had a modestly better prognosis than those with MMRP tumors, they did not benefit from 5-FU-based adjuvant chemotherapy [7]. These findings were further corroborated in a pooled analysis of 1,027 patients with stage II and III colon cancer, which showed that adjuvant 5-FU-based chemotherapy significantly improved disease-free survival for patients with MMRP tumors, although no improvement was found for those with MMRD tumors [24]. This resistance of MMRD tumors to 5-FU, thought to result from the incorporation of 5-FU metabolites into the DNA [25, 26], might possibly be overcome by combination therapy with oxaliplatin, because oxaliplatin chemosensitivity is independent of the MMR system [27]. Although no prospective trials have compared oxaliplatin versus non-oxaliplatin-containing adjuvant regimens in patients with MMRD tumors, evidence from retrospective studies has suggested that stage III patients with MMRD tumors might have improved outcomes with FOLFOX compared with single-agent 5-FU [28]. Thus, it is noteworthy that FOLFOX was preferred over single-agent 5-FU-based chemotherapy in patients with stage II MMRD tumors. Despite the uncertainty of the data regarding the choice of regimen for these patients, clinicians appear to have been influenced by the existing data regarding the impact of MMR status on chemotherapy responsiveness. In contrast, in stage III CRC, for which chemotherapy is the standard treatment of choice, MMR status did not appear to influence the choice of agent.
The present study had some limitations, the most important of which was the range of information captured in the cancer registry. Data relating to lymphovascular invasion, tumor presentation with obstruction or perforation, comorbidities, or performance status were not captured in the databases, because these were not systematically recorded in the pathology reports, operation reports, or clinical notes. Thus, we were unable to account for some factors recognized to influence the treatment choice. The other limitations of the present study were those present for all population-based cancer registries, including inadvertent misclassification of data, duplicate reports, and reporting delays [29]. Also, the present study was conducted in 2006–2009, and practices could have evolved in light of emerging data.
Conclusion
Our study showed that significantly fewer patients in the MMRD cohort received chemotherapy compared with those in the MMRP cohort for stage II disease. In contrast, no significant differences were shown in the rates of chemotherapy between the MMRD and MMRP cohorts for stage III disease. The relationship between MMR status and treatment decision was modified by age, such that the impact of MMR status on treatment diminished with increasing patient age. The treatment decisions for stage II patients were also influenced by age, T stage, and tumor location. These findings suggest that although clinicians place a strong emphasis on the traditional markers of “high-risk” disease in selecting stage II patients for adjuvant chemotherapy, MMR status also has a significant bearing on treatment decisions, especially for younger patients. Conducted at a time when the expert guidelines were still unclear regarding the role of MSI in clinical management, the results of the present study highlight the importance of incorporating MSI into risk stratification tools used to support clinicians in making decisions regarding the use of adjuvant therapy for patients with stage II CRC.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank Dr. Ali Tafreshi for his assistance in collecting the data. This research was supported by the Strategic Research Partnership Grant SRP 06-06 from the New South Wales Cancer Council. The study sponsor played no part in the study design, analysis, interpretation, writing, or decision to submit for publication. E.Y.H. is a recipient of an NHMRC Postgraduate Scholarship (Grant GNT1093776).
Author Contributions
Conception/Design: Nicholas J. Hawkins, Robyn L. Ward
Provision of study material or patients: Nicholas J. Hawkins, David Goldstein, Winston Liauw, Philip Clingan, Melvin Chin, Robyn L. Ward
Collection and/or assembly of data: Emily Y. He, Gabriel Mak, Felicia Roncolato
Data analysis and interpretation: Emily Y. He
Manuscript writing: Emily Y. He
Final approval of manuscript: Nicholas J. Hawkins, David Goldstein, Winston Liauw, Philip Clingan, Melvin Chin, Robyn L. Ward
Disclosures
Emily Y. He: NHMRC Postgraduate Scholarship (Grant GNT1093776) (RF); David Goldstein: Celgene, Pfizer, Bayer (C/A), Celgene (RF); Robyn L. Ward: Strategic Research Partnership Grant No. SRP 06-06 from the New South Wales Cancer Council (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 3.Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131:729–737. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward RL, Cheong K, Ku SL, et al. Adverse prognostic effect of methylation in colorectal cancer is reversed by microsatellite instability. J Clin Oncol. 2003;21:3729–3736. doi: 10.1200/JCO.2003.03.123. [DOI] [PubMed] [Google Scholar]
- 6.Jover R, Zapater P, Castells A, et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 2006;55:848–855. doi: 10.1136/gut.2005.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol. 2009;27:1814–1821. doi: 10.1200/JCO.2008.18.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tejpar S, Bosman F, Delorenzi M, et al. Microsatellite instability (MSI) in stage II and III colon cancer treated with 5FU-LV or 5FU-LV and irinotecan (PETACC 3-EORTC 40993-SAKK 60/00 trial) J Clin Oncol. 2009;27(suppl):15s. [Google Scholar]
- 11.Snowsill T, Huxley N, Hoyle M, et al. A systematic review and economic evaluation of diagnostic strategies for Lynch syndrome. Health Technol Assess. 2014;18:1–406. doi: 10.3310/hta18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: A National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25:767–772. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16:30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boland CR. Clinical uses of microsatellite instability testing in colorectal cancer: An ongoing challenge. J Clin Oncol. 2007;25:754–756. doi: 10.1200/JCO.2006.09.4607. [DOI] [PubMed] [Google Scholar]
- 15.NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. V.2.2016. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed February 2, 2016.
- 16.Benson AB, III, Venook AP, Bekaii-Saab T, et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw. 2014;12:1028–1059. doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 17.Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi64–vi72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 18.Benson AB, III, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 19.Ward RL, Hicks S, Hawkins NJ. Population-based molecular screening for Lynch syndrome: Implications for personalized medicine. J Clin Oncol. 2013;31:2554–2562. doi: 10.1200/JCO.2012.46.8454. [DOI] [PubMed] [Google Scholar]
- 20.Edge SBBD, Compton CC, Fritz AG, et al., editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 21.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 22.Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 23.Elsaleh H, Joseph D, Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 24.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003;106:66–73. doi: 10.1002/ijc.11176. [DOI] [PubMed] [Google Scholar]
- 26.Tajima A, Hess MT, Cabrera BL, et al. The mismatch repair complex hMutS alpha recognizes 5-fluorouracil-modified DNA: Implications for chemosensitivity and resistance. Gastroenterology. 2004;127:1678–1684. doi: 10.1053/j.gastro.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Fink D, Nebel S, Aebi S, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–4886. [PubMed] [Google Scholar]
- 28.Zaanan A, Cuilliere-Dartigues P, Guilloux A, et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann Oncol. 2010;21:772–780. doi: 10.1093/annonc/mdp383. [DOI] [PubMed] [Google Scholar]
- 29.Izquierdo JN, Schoenbach VJ. The potential and limitations of data from population-based state cancer registries. Am J Public Health. 2000;90:695–698. doi: 10.2105/ajph.90.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]