Abstract
In this issue of The Oncologist, Agarwal et al. report negative results from a phase I trial of LFA102. Although “negative” in terms of antitumor activity, the study provides useful pharmacokinetic and pharmacodynamic information. Future trials evaluating PRLR blockers alone and in combination with other agents may still be warranted in patients with breast and prostate cancer.
Introduction
In this issue, Agarwal et al. report negative results from a phase I trial of LFA102, a humanized monoclonal antibody (mAb) that binds to and inhibits prolactin receptor (PRLR) signaling, in patients with PRLR-positive metastatic breast cancer or metastatic castration-resistant prostate cancer (mCRPC) [1]. Although no anticancer activity was noted in the study, questions remain regarding the role of PRLR signaling in these and other cancers and whether further efforts to develop PRLR blockers are warranted. It should be noted that hormonal manipulation of the hypophyseal-pituitary axis has benefited patients through the use of gonadotropin-releasing hormone agonists in prostate and breast cancer and somatostatin analogs in neuroendocrine tumors [2]. Therefore, it may be possible to exploit the biology of these and other hypophyseal-pituitary hormones for the benefit of other cancer patients.
The authors are to be commended for publishing results of this carefully conducted study in the Clinical Trial Results section of The Oncologist (Panel 1). Although “negative” in terms of antitumor activity, the study provides useful pharmacokinetic and pharmacodynamic information. A rise in serum prolactin levels followed drug administration, possibly an indication of the effective blockade of the receptor by LFA102. However, a temporal delay was noted between pharmacokinetic parameters and pharmacodynamic response, possibly because of the time required for LFA102 to spread to peripheral tissues and inhibit peripheral PRLR, resulting in an elevation in serum prolactin levels as a compensatory feedback mechanism. The lack of response could have many explanations: a failure to maintain inhibition of the pathway throughout the treatment interval, an ineffective degree of inhibition of the receptor, or a failure to translate receptor inhibition into biologically important effects on tumor growth. The study in question did not biopsy tumor during the trial, so we have no information on these issues. Nor was there a selection strategy for tumors highly expressing the receptor, an approach that might have led to a more positive outcome.
Returning to the rationale for further work on inhibitors of the prolactin receptor, there is an obvious need to augment current hormonal therapies of breast and prostate cancers [3]. Antiestrogens and antiandrogens play key roles in the therapy of hormone-sensitive breast and prostate cancer, respectively, but metastatic tumors inevitably develop resistance to current endocrine-based therapies. Expression of prolactin in both breast and prostate tissue has been well-documented, and tumor production of prolactin has been implicated in the growth of breast and prostate cancer via autocrine or paracrine pathways [4, 5]. Wen et al. noted that G129R, an antagonist peptide of prolactin (PRL), blocked the tumoral PRL/PRLR axis, resulting in inhibition of tumor growth in orthotopic models of human ovarian cancer [6]. Prolonged treatment with G129R caused redundant autolysosomes in cancer spheroids, resulting in type II programmed cell death, or inducible autophagy [6]. Additionally, carboxypeptidase-D and nitric oxide levels are upregulated by PRL and testosterone in vitro, which favors survival of prostate cancer cells. However, inhibition of PRLR and the androgen receptor (AR) prevented carboxypeptidase-D and nitric oxide production in this setting, which implies that combined blockade of PRLR and AR may have a role in the treatment of prostate cancer [7]. It has been noted that human epidermal growth factor 2 (HER2)-positive breast cancers are more likely to proliferate and metastasize in the presence of autocrine PRL, which suggests that PRLR and HER2 interact to promote tumorigenesis and breast cancer progression. Therefore, investigators studied the combination of the monoclonal antibody trastuzumab and G129R in two PRL-expressing human breast cancer cell lines (T-47D and BT-474) with varying levels of HER2 and PRLR expression. The combination of trastuzumab and G129R additively inhibited cellular proliferation in vitro and in vivo as measured by inhibition of the growth of both cell line xenografts in athymic nude mice [8]. Therefore, targeting prolactin signaling appeared to be a logical strategy for further study in breast and prostate cancer.
The Biology of Prolactin and the PRLR
Prolactin is primarily produced by lactotrophs in the anterior pituitary gland of vertebrates [9]; however, it has also been found in many other extrapituitary sites, including reproductive, immune, neural, and integumentary tissues and other locations such as lacrimal glands, adipose tissue, blood endothelial cells, and kidney [10]. Prolactin is encoded by the PRL gene found on chromosome 6 in humans [11]. The mature 23-kDa protein is composed of 199 amino acids; however, isoforms of prolactin have been identified in the pituitary gland and plasma as a consequence of posttranslational modification of the 23-kDa protein [12]. There are several different isoforms of prolactin with regard to size. The shorter isoforms, such as the 23-kDa protein, are predominant and have greater biological activity than longer isoforms (≥48 kDa) [13]. Extrapituitary sites of secretion of a 16-kDa isoform, which has antiangiogenic and antitumor properties, have been identified in the myocardium, retina, chondrocytes, and mammary gland [14]. The 23-kDa prolactin isoform binds to the monomeric, transmembrane PRLR, initiating intracellular signaling through several distinct pathways: Jak/Stat, mitogen-activated protein kinases, Src, and phosphatidylinositol 3 phosphate kinase/Akt [15]. The wide distribution of PRLR on human tissues facilitates its role in various physiological processes, including cellular growth and differentiation, angiogenesis, hematopoiesis, reproduction, and lactation [16].
The Role of Prolactin and PRLR in Oncogenesis
The role of prolactin and PRLR in tumor growth remains controversial. In addition to prolactin produced by lactotrophs in the anterior pituitary, prolactin produced locally could promote tumorigenesis in an autocrine fashion in breast cancer [15]. However, cellular, molecular, and epidemiological studies investigating the role of prolactin in breast cancer have yielded conflicting results [17–20]. This observation could be because prolactin has a diverse role in the pathogenesis of breast cancer. For example, preclinical data suggest that prolactin may contribute to the development of breast cancer; in established cases, however, it may act instead to reduce the aggressiveness and spread of disease [21]. Interestingly, the long isoform of PRLR has been implicated in supporting the development of breast cancer metastases. In one study of two orthotopic and highly metastatic breast cancer cell lines (mouse 4T1 and human BT-474), long PRLR knockdown in three-dimensional culture resulted in apoptosis of 95% of tumor-initiating/cancer stem cells [22].
Autocrine and/or paracrine prolactin may also contribute to the pathogenesis of prostate cancer; for example, prostatic hyperplasia is seen in mice that overexpress prolactin in the prostate, and these changes may progress to intraepithelial neoplasia and even to adenocarcinoma of the prostate [23, 24]. In epidemiologic studies, the presence of prolactin and phosphorylated signal transducer and activator of transcription 5 (STAT5) in human prostate cancers is linked with high-grade tumors and more aggressive disease [25]; therefore, targeting autocrine and/or paracrine prolactin-mediated activation of STAT5 may represent a novel therapeutic target for patients with prostate cancer. For example, the efficacy of a Jak2 inhibitor, AZD1480, in preventing the growth of mCRPC via its effects on Jak2-dependent STAT5a/b signaling has been studied. Nine of 12 clinical prostate cancers responded to AZD1480 by profound cellular apoptosis, which was associated with reduced levels of nuclear STAT5a/b, suggesting that further clinical development of AZD1480 was warranted [26]. AZD1480 was also the subject of a phase I study in solid tumors conducted by Plimack et al.; however, development was halted given unusual dose-limiting toxicities, such as dizziness, anxiety, and ataxia, and limited clinical activity [27].
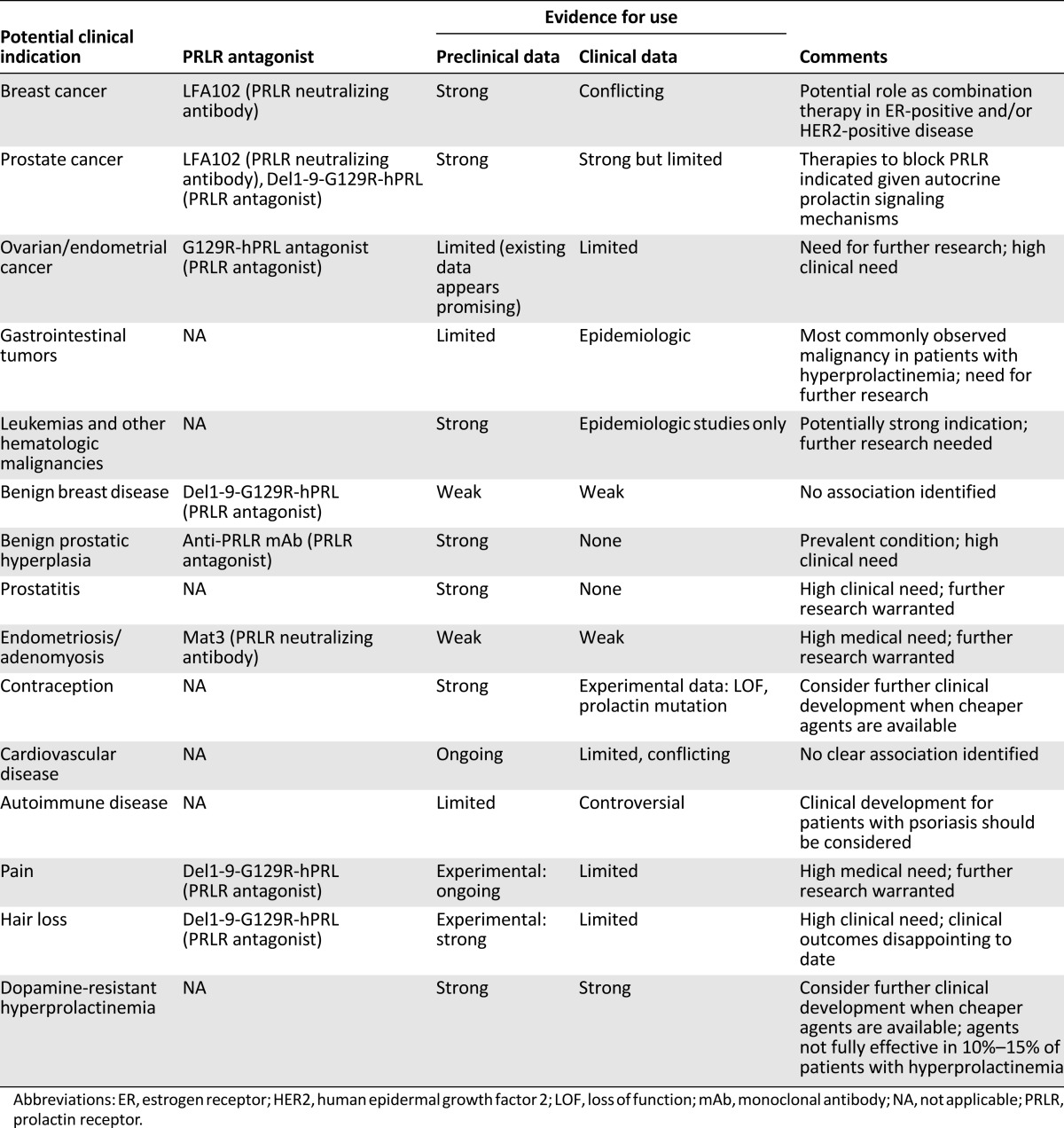
Prolactin analogs and anti-PRLR antibodies have already been developed as clinical candidates (Table 1), including LFA102 [28], as per the study by Agarwal et al. These compounds will potentially be developed to treat a variety of conditions apart from cancer, such as dopamine-resistant hyperprolactinemia, endometriosis, prostatitis, and autoimmune and cardiovascular diseases [28]. Both LFA102 and another neutralizing mAb, Mat3, effectively downregulate PRL-induced signaling both in vitro and in vivo, and two clinical trials of these compounds have been conducted to date (NCT01338831 and NCT01610050).
Table 1.
Potential clinical indications for PRLR inhibitors [28]
Discussion and Conclusion
Despite promising preclinical data, no clinical activity was observed in the phase I study by Agarwal et al., in which 73 patients were treated, including 53 at the highest dose level. Overall, LFA102 was safe and well-tolerated; the maximum tolerated dose was not reached, as there were no dose-limiting toxicities. Pharmacokinetic and pharmacodynamic data analyses confirmed that serum LFA102 maximum concentration and last area under the curve were approximately linearly dose-dependent. The recommended dose for phase II evaluation was 60 mg/kg LFA102; at that dose, the estimated half-life was 9 days, slightly lower than the typical half-life of mAbs, which is approximately 25 days. Therefore, the authors postulate that LFA102 administration may have been too infrequent, given the fact that LFA102 was administered only once every 4 weeks. Although in vitro studies showed that the drug had a high binding affinity for PRLR, the degree of inhibition of this target in human tissues was not assessed. Serum prolactin levels were used as a surrogate marker for PRLR inhibition, but this may not have been an optimal alternative biomarker of antitumor activity, as either the tumor or the pituitary gland could be responsible for increases in serum prolactin levels.
The potential agonistic and antagonistic effects of prolactin signaling and receptor blockade in breast cancer as discussed above illustrate the need for further studies to better inform the therapeutic potential of the prolactin axis. Moreover, as the prolactin signaling pathway intersects with other key pathways involved in breast cancer pathogenesis such as HER2 and estrogen, combination trials may be warranted [29]. Combination therapy with antiandrogens may also be beneficial in castration-resistant prostate cancer. Another challenge in clinical development is that detection of PRLR expression has not been not validated using currently available commercial antibodies, making it difficult to accurately identify a subgroup of patients who may benefit from PRLR blockers [28]. Additionally, single nucleotide polymorphisms of the PRLR have been reported, some of which may affect receptor function and response to receptor blockade [29]. Despite the lack of clinical efficacy observed in this phase I study, future trials evaluating PRLR blockers alone and in combination with other agents may still be warranted in patients with breast and prostate cancer. Future research should aim to clarify the importance and complexities of PRLR signaling and develop accurate assays for identifying patient subgroups that may benefit from PRLR blockade.
Disclosures
The authors indicated no financial relationships.
Footnotes
Editor's Note: See the related article, “Phase I Study of the Prolactin Receptor Antagonist LFA102 in Metastatic Breast and Castration-Resistant Prostate Cancer,” by Neeraj Agarwal et al. on page 535 of this issue.
References
- 1.Agarwal N, Machiels J-P, Suárez C, et al. Phase I study of the prolactin receptor antagonist LFA102 in metastatic breast and castration-resistant prostate cancer. The Oncologist. 2016;21:535–536. doi: 10.1634/theoncologist.2015-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limonta P, Montagnani Marelli M, Mai S, et al. GnRH receptors in cancer: From cell biology to novel targeted therapeutic strategies. Endocr Rev. 2012;33:784–811. doi: 10.1210/er.2012-1014. [DOI] [PubMed] [Google Scholar]
- 3.López-Otín C, Diamandis EP. Breast and prostate cancer: An analysis of common epidemiological, genetic, and biochemical features. Endocr Rev. 1998;19:365–396. doi: 10.1210/edrv.19.4.0337. [DOI] [PubMed] [Google Scholar]
- 4.Sethi BK, Chanukya GV, Nagesh VS. Prolactin and cancer: Has the orphan finally found a home? Indian J Endocrinol Metab. 2012;16(suppl 2):S195–S198. doi: 10.4103/2230-8210.104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goffin V, Touraine P, Culler MD, et al. Drug insight: Prolactin-receptor antagonists, a novel approach to treatment of unresolved systemic and local hyperprolactinemia? Nat Clin Pract Endocrinol Metab. 2006;2:571–581. doi: 10.1038/ncpendmet0270. [DOI] [PubMed] [Google Scholar]
- 6.Wen Y, Zand B, Ozpolat B, et al. Antagonism of tumoral prolactin receptor promotes autophagy-related cell death. Cell Reports. 2014;7:488–500. doi: 10.1016/j.celrep.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas LN, Morehouse TJ, Too CK. Testosterone and prolactin increase carboxypeptidase-D and nitric oxide levels to promote survival of prostate cancer cells. Prostate. 2012;72:450–460. doi: 10.1002/pros.21446. [DOI] [PubMed] [Google Scholar]
- 8.Scotti ML, Langenheim JF, Tomblyn S, et al. Additive effects of a prolactin receptor antagonist, G129R, and herceptin on inhibition of HER2-overexpressing breast cancer cells. Breast Cancer Res Treat. 2008;111:241–250. doi: 10.1007/s10549-007-9789-z. [DOI] [PubMed] [Google Scholar]
- 9.Chen WY. The many faces of prolactin in breast cancer. Adv Exp Med Biol. 2015;846:61–81. doi: 10.1007/978-3-319-12114-7_3. [DOI] [PubMed] [Google Scholar]
- 10.Harvey S, Martínez-Moreno CG, Luna M, et al. Autocrine/paracrine roles of extrapituitary growth hormone and prolactin in health and disease: An overview. Gen Comp Endocrinol. 2015;220:103–111. doi: 10.1016/j.ygcen.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Truong AT, Duez C, Belayew A, et al. Isolation and characterization of the human prolactin gene. EMBO J. 1984;3:429–437. doi: 10.1002/j.1460-2075.1984.tb01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker AM. S179D prolactin: Antagonistic agony! Mol Cell Endocrinol. 2007;276:1–9. doi: 10.1016/j.mce.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabharwal P, Glaser R, Lafuse W, et al. Prolactin synthesized and secreted by human peripheral blood mononuclear cells: An autocrine growth factor for lymphoproliferation. Proc Natl Acad Sci USA. 1992;89:7713–7716. doi: 10.1073/pnas.89.16.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard V, Young J, Chanson P, et al. New insights in prolactin: Pathological implications. Nat Rev Endocrinol. 2015;11:265–275. doi: 10.1038/nrendo.2015.36. [DOI] [PubMed] [Google Scholar]
- 15.Clevenger CV, Furth PA, Hankinson SE, et al. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bole-Feysot C, Goffin V, Edery M, et al. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 17.Das R, Vonderhaar BK. Prolactin as a mitogen in mammary cells. J Mammary Gland Biol Neoplasia. 1997;2:29–39. doi: 10.1023/a:1026369412612. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds C, Montone KT, Powell CM, et al. Expression of prolactin and its receptor in human breast carcinoma. Endocrinology. 1997;138:5555–5560. doi: 10.1210/endo.138.12.5605. [DOI] [PubMed] [Google Scholar]
- 19.Tworoger SS, Eliassen AH, Rosner B, et al. Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res. 2004;64:6814–6819. doi: 10.1158/0008-5472.CAN-04-1870. [DOI] [PubMed] [Google Scholar]
- 20.Tworoger SS, Eliassen AH, Zhang X, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013;73:4810–4819. doi: 10.1158/0008-5472.CAN-13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner KU, Rui H. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia. 2008;13:93–103. doi: 10.1007/s10911-008-9062-z. [DOI] [PubMed] [Google Scholar]
- 22.Yonezawa T, Chen KH, Ghosh MK, et al. Anti-metastatic outcome of isoform-specific prolactin receptor targeting in breast cancer. Cancer Lett. 2015;366:84–92. doi: 10.1016/j.canlet.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Wennbo H, Kindblom J, Isaksson OG, et al. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997;138:4410–4415. doi: 10.1210/endo.138.10.5461. [DOI] [PubMed] [Google Scholar]
- 24.Rouet V, Bogorad RL, Kayser C, et al. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc Natl Acad Sci USA. 2010;107:15199–15204. doi: 10.1073/pnas.0911651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sackmann-Sala L, Goffin V. Prolactin-induced prostate tumorigenesis. Adv Exp Med Biol. 2015;846:221–242. doi: 10.1007/978-3-319-12114-7_10. [DOI] [PubMed] [Google Scholar]
- 26.Gu L, Liao Z, Hoang DT, et al. Pharmacologic inhibition of Jak2-Stat5 signaling by Jak2 inhibitor AZD1480 potently suppresses growth of both primary and castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:5658–5674. doi: 10.1158/1078-0432.CCR-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plimack ER, Lorusso PM, McCoon P, et al. AZD1480: A phase I study of a novel JAK2 inhibitor in solid tumors. The Oncologist. 2013;18:819–820. doi: 10.1634/theoncologist.2013-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goffin V, Touraine P. The prolactin receptor as a therapeutic target in human diseases: Browsing new potential indications. Expert Opin Ther Targets. 2015;19:1229–1244. doi: 10.1517/14728222.2015.1053209. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson EM, Hugo ER, Borcherding DC, et al. Prolactin in breast and prostate cancer: Molecular and genetic perspectives. Discov Med. 2011;11:315–324. [PubMed] [Google Scholar]