Abstract
Foreign body-induced granuloma is an uncommon yet clinically significant cause of hypercalcemia. The molecular mechanisms are uncertain, although extrarenal calcitriol production has been proposed. We describe severe hypercalcemia associated with increased levels of plasma calcitriol in a patient with HIV and local granulomatous reaction five years after injection of polymethylmethacrylate (PMMA) as dermal filler for cosmetic body sculpting. Extensive evaluation revealed no identifiable cause of increased calcitriol levels. Nuclear imaging was remarkable for diffuse uptake in the subcutaneous tissues of the buttocks. Subsequent muscle biopsy and immunohistochemical staining showed strong local expression of CYP27B1 within histiocytes surrounding globules of PMMA. This case highlights an unfortunate complication of dermal fillers and shows that inflammatory cells can express high levels of CYP27B1 even without frank granulomas. The growing trend of body contour enhancement using injectable fillers should raise suspicion for this cause of hypercalcemia in clinical practice. Patients with HIV who receive this treatment for lipodystrophy or other cosmetic purposes may have increased susceptibility to hypercalcemia in the setting of underlying chronic inflammation. This may be a concern when changing anti-retroviral therapy, since alterations in levels of HIV viremia may initiate or contribute to worsening hypercalcemia.
Keywords: Hypercalcemia, CYP27B1, Calcitriol, Foreign-body Granuloma, Polymethylmethacrylate/adverse effects, HIV
Introduction
Hypercalcemia mediated by 1,25(OH)2 vitamin D (1,25(OH)2D or calcitriol) is an uncommon yet clinically significant cause of this metabolic disturbance. Foreign body reactions are reported to cause this form of hypercalcemia, but are only documented in a few case reports and clinical series. The unifying mechanism is presumed autonomous 25-OH vitamin D 1-α-hydroxylase (CYP27B1) activity within granulomata resulting in excessive circulating 1,25(OH)2D levels. However, evidence confirming this mechanism in non-sarcoid cases remains limited [1].
We evaluated a man with severe hypercalcemia and elevated 1,25(OH)2D levels after receiving polymethylmethacrylate (PMMA) injections for cosmetic soft tissue augmentation. We hypothesized that the hypercalcemia resulted from a foreign body reaction, producing increased levels of CYP27B1 within histiocytes surrounding an inflammatory response to PMMA. To confirm this, we performed muscle biopsy from sites corresponding to hypermetabolic uptake on PET-CT at locations correlating with PMMA injection sites.
Materials and Methods
The study was conducted with approval from the UCSF Committee for Human Research. For immunohistochemistry, sections were deparaffinized and rehydrated. Endogenous peroxidase was blocked with 3% hydrogen peroxide. Goat CYP27B1 antibody or normal goat serum (negative control) was applied to paraffin-embedded muscle sections (1:100, overnight, 4C) and was detected with biotinylated anti-goat secondary antibody followed by ABC peroxidase reagent (all antibodies from Santa Cruz Biotechnology, Inc.) [2]. Enzyme expression was visualized by diaminobenzidine substrate and hematoxylin counterstaining.
Case Report
A 52 year-old man with a history of controlled human immunodeficiency virus (HIV) and hepatitis B (HBV) infections presented to his internist with a 1-month history of fatigue, polyuria, and weight loss. Initial evaluation showed albumin-corrected serum calcium of 12.5 mg/dL (nl, 8.8–10.3) and creatinine of 1.9 mg/dL (nl, 0.77–1.22). His viral load was suppressed on tenofovir, emtricitabine, and rilpivirin, and his CD4+ T-cell count was 444 (nl, 410 – 1590 ×106/L). He had no history of opportunistic infection, and all prior routine laboratories were normal. The patient subsequently stopped all HIV medications because of concern for adverse drug reaction. He denied use of thiazide diuretics, lithium, calcium, and vitamin A or D supplements. Two months later he was admitted to University of California, San Francisco Medical Center (UCSF) for evaluation and management of persistent symptomatic hypercalcemia.
Review of systems was notable for myalgia. Physical examination showed prominent muscles diffusely tender to palpation. Laboratories revealed marked hypercalcemia and renal failure with albumin-corrected serum calcium of 14.6 mg/dL (nl, 8.5–10.2) and creatinine of 2.02 mg/dL (nl. 0.5–1.1). Further evaluation demonstrated elevated 1,25(OH)2D level [128 pg/ml (nl, 18–72; interassay coefficient of variation (CV) 7–10%; intraassay CV 5–7%]. Intact parathyroid hormone (iPTH) at < 3 pg/ml (nl, 12–65), PTH-related protein (PTHrP) at 0.2 pmol/L (nl < 2.0), and 25(OH)D at 17 ng/ml (nl, 30–100) were all low. Serum phosphate, TSH, and cortisol levels were normal. His CD4+ T-cell count was 378 with a viral load of 49415 copies/mL [nl, <40].
Workup for calcitriol-mediated hypercalcemia revealed elevated serum angiotensin-converting enzyme [ACE, 200 U/L (nl, 9–67)]. Non-contrast computed tomography (CT) imaging of the chest, abdomen, and pelvis showed nodular soft tissue densities in both buttocks but no evidence of sarcoid or malignancy. Whole-body 18-fluorodeoxyglucose (FDG) PET scan showed uptake in the subcutaneous regions of the proximal thighs, buttocks, and deltoids, without tracer-avid lymphadenopathy or masses (Figure 1A). Bone marrow biopsy, serum and urine protein electrophoreses with immunofixation, serum alpha-fetoprotein, and abdominal ultrasound were negative for malignancy.
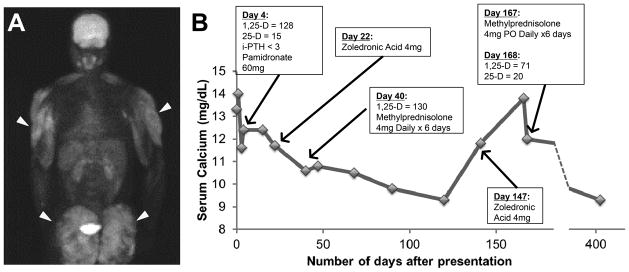
Figure 1.
Clinical evaluation. (A) FDG-PET scan shows diffuse fluorodeoxyglucose (FDG) uptake into subcutaneous tissues of proximal thighs and buttocks, corresponding to site of prior PMMA injection (arrows). (B) Serum calcium and treatment course. 1,25-D, 1,25-dihydroxyvitamin D; 25-D, 25(OH)D; iPTH, intact parathyroid hormone.
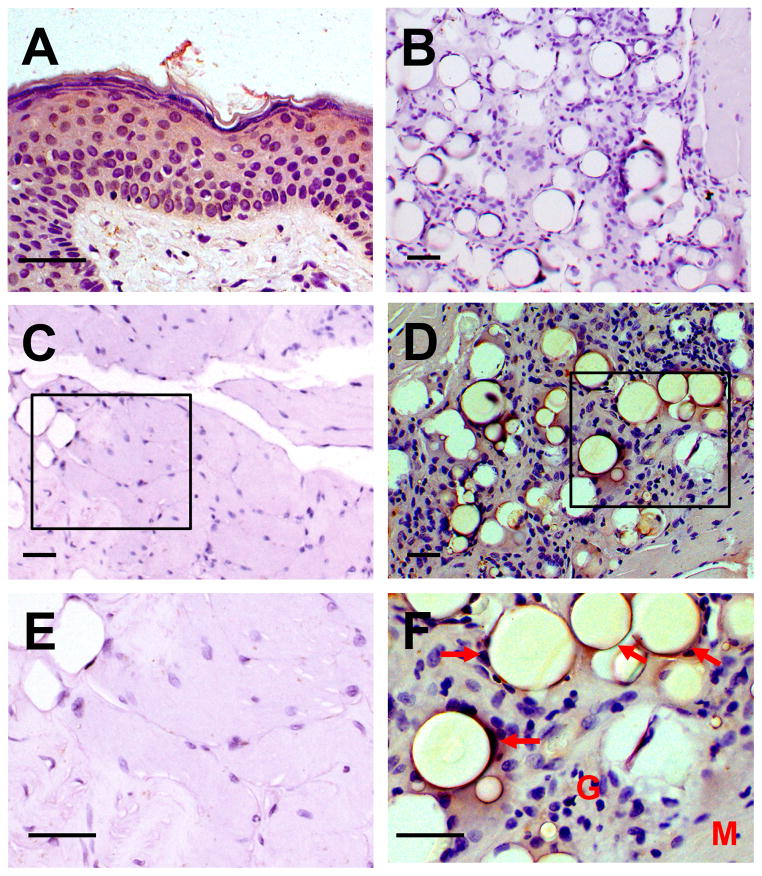
The patient later revealed a history of cosmetic injections with PMMA for HIV lipodystrophy, first in his buttocks five years previously and then his deltoids one month prior to symptom onset. Biopsies from both sites revealed florid giant cell reaction with histiocytic infiltration surrounding globules of amorphous material (PMMA) (Figure 2). Muscle sections stained with antibody to CYP27B1 revealed strong histiocytic expression of CYP27B1 (Figure 2).
Figure 2.
Histologic sections of muscle stained with antibody to CYP27B1 (25-OH vitamin D 1-α-hydroxylase). (A) Positive control (normal human skin biopsy), (B) Negative control (no CYP27B1 antibody, patient muscle), (C, E) Normal muscle (an unrelated patient), (D, F) Patient muscle (M) with strong CYP27B1 expression (brown) in the histiocytes surrounding presumed PMMA globules (arrows) in the inflammatory reaction (G). Scale bars = 50 μm.
The patient received intravenous (IV) fluids and pamidronate and resumed anti-retroviral therapy (ART). After 5 weeks, he received IV zoledronic acid and methylprednisolone for persistent hypercalcemia. Serum calcium levels subsequently normalized. Three months later, severe hypercalcemia recurred, which resolved after a second course of IV zoledronic acid and methylprednisolone. Eight months later, his serum calcium and creatinine remained normal (Figure 1B), and HIV viral load was undetectable. Serum ACE, measured as an adjunct for sarcoid work-up, was not trended since its utility is limited by poor specificity [3].
Discussion
This patient demonstrates calcitriol-mediated hypercalcemia secondary to elevated CYP27B1 expression within a foreign body reaction to cosmetic injections with PMMA.
A review of calcitriol-mediated hypercalcemia reported sarcoidosis as the most common etiology (49%), followed by hematological malignancy (17%), then systemic infections (8%). Only two cases of foreign body granulomatosis were reported [4]. Extensive evaluation of our patient was negative for common causes of hypercalcemia. In patients with HIV, granulomatous opportunistic infections are of concern since granulomatous reactivation can be associated with initiation of ART in patients with CD4+ T-cell counts < 100 cells/ml [5]. However, this was not compatible with our patient, as he maintained stable CD4+ T-cell counts (> 300 cells/ml) and had no history of opportunistic infections. Furthermore, tuberculin skin testing and chest X-ray were both negative, as were physical exam and imaging for lymphadenopathy.
An inflammatory reaction was evident from hypermetabolic uptake on PET-CT at anatomic locations correlating with prior PMMA injections. We hypothesized that the hypercalcemia was caused by calcitriol from activated histiocytes reacting to PMMA. Although muscle biopsies did not reveal discrete granulomas, they demonstrated a florid giant cell reaction with histiocytic infiltration surrounding PMMA globules. Immunostaining revealed strong histiocytic expression of CYP27B1, thus confirming our hypothesis.
We note the initial presentation occurred while the patient was on ART and progressed after he discontinued ART. It is well-established that ART suppresses systemic HIV-associated inflammation [6] and that treatment interruption increases systemic inflammation [7]. Therefore, while this patient’s treatment interruption did not initiate the inflammatory reaction, the correlation between detectable HIV viremia and worsening hypercalcemia suggests that uncontrolled viremia and resultant T-cell and macrophage activation may have contributed to PMMA-driven CYP27B1 activity. Restarting ART also correlated with resolution of the hypercalcemia. Therefore, blunting the immunologic response to PMMA may have helped resolve this patient’s hypercalcemia.
Our case highlights an important complication of dermal fillers, whose popularity has grown in recent decades such that there are currently over 160 products available worldwide [8]. Granulomatous reactions to these materials are reportedly rare (0.01 to 1% of cases used). Such complications can appear several months to years after injection [8].
Hypercalcemia resulting from a foreign body-induced granulomatous reaction to cosmetic injections with silicone was initially reported in 1984 [9]. The first report of hypercalcemia related to PMMA-induced granuloma emerged in 2014, with a series of four women [10] (Table 1). While it is presumed that the hypercalcemia from foreign body-induced granuloma is due to increased CYP27B1 activity in activated macrophages (as in sarcoidosis), evidence to confirm this is limited and has not been previously demonstrated with PMMA. In 2014, a case report described a young bodybuilder who developed hypercalcemia and elevated calcitriol after paraffin oil injection for muscle contouring. Immunohistochemical staining confirmed increased CYP27B1 expression by macrophages within multiple paraffinomas [11]. That case and ours support this mechanism of extrarenal calcitriol production by activated macrophages within a foreign body reaction to a dermal filler. However, our case establishes this scenario in the setting of PMMA, a novel filler with which providers have limited clinical experience. We also demonstrate that inflammatory cells can express high levels of CYP27B1 in the absence of discrete granuloma formation.
Table 1.
Comparison of reported cases of hypercalcemia in the setting of foreign body reaction to polymethylmethacrylate (PMMA) injected for cosmetic purposes. Ca, serum calcium; iPTH, intact parathyroid hormone; 25-D, 25(OH)D; 1,25-D, 1,25-dihydroxyvitamin D; Cr, serum creatinine; ACE, angiotensin-converting enzyme; CYP27B1, 25-OH vitamin D 1-α-hydroxylase; IV, intravenous; N/D, No data reported.
| Current Case | Case 1 (10) Negri et al. | Case 2 (10) Negri et al. | Case 3 (10) Negri et al. | Case 4 (10) Negri et al. | |
|---|---|---|---|---|---|
|
| |||||
| Age, Gender | 52, Male | 53, Female | 29, Female | 50, Female | 39, Female |
|
| |||||
| Pertinent History | HIV/HBV infection – controlled on ART | Mammary prostheses | Drug-induced CKD Stage III ; Mammary prostheses | Multiple prior implants and fillings | HIV infection – controlled on ART; Multiple prior implants and fillings |
|
| |||||
| Filler and Injection Location | PMMA to buttocks and deltoids | PMMA to gluteus | PMMA to gluteus, thighs, and buttocks | PMMA to legs | PMMA to lower extremities |
|
| |||||
| Time after PMMA injection to occurrence of hypercalcemia | 5 years (buttocks) 1 month (deltoids) |
4 months | 7 months | 6 months | Not specified, less than 14 months |
|
| |||||
| Baseline Labs: | |||||
| Serum Ca (mg/dL) | Ca = 14.6* (nl,8.5–10.2) | Ca = 15.2† | Ca = 12 | Ca = 13.2 | Ca = 12 |
| iPTH (pg/ml) | iPTH = 3.5 (nl, 12–65) | iPTH = 3.5† | iPTH = 11.6 | iPTH = 15 | iPTH = 16 |
| 25-D (ng/ml) | 25-D = 17 (nl, 30–100) | 25-D = N/D† | 25-D = 31.2 | 25-D = N/D | 25-D = 9 |
| 1,25D (pg/ml) | 1,25-D =128 (nl,18–72) | 1,25-D = 55.5† | 1,25-D = 88.3 | 1,25-D = 53.6 | 1,25-D = 94 (nl, 18–60) |
| ACE (U/L) | ACE = 200 (nl, 9–67) | ACE = N/D† | ACE = 91 (nl, <40) | ACE = N/D | ACE = N/D |
| Cr (mg/dL) | Cr = 2.02 (nl, 0.5–1.1) | Cr = N/D† | Cr = 5.62 | Cr = 2.2 | Cr = N/D |
| Alb = N/D† | Alb = N/D | Alb = N/D | Alb = N/D | ||
| *Albumin-corrected | †Normal ranges not indicated in case report | ||||
|
| |||||
| Relevant physical findings | Prominent muscles diffusely tender to palpation | N/D | N/D | N/D | N/D |
|
| |||||
| Relevant imaging | FDG-PET: Uptake in the subcutaneous regions of the proximal thighs, buttocks, and deltoids, without tracer avid lymphadenopathy or masses | N/D | FDG-PET: + metabolic activity at gluteus muscle, isquiotibial quadriceps and internal gemellus of both legs | MRI: nodular images in the subcutaneous tissue compatible with granulomas | FDG-PET: increased activity in the gluteus and thighs between muscles fibers suggesting infiltrates around methacrylate deposits |
|
| |||||
| Biopsy findings | Subcutaneous tissue of buttocks and deltoid: Florid giant cell reaction with histiocytic infiltration surrounding globules of amorphous material (PMMA) | Partial excision of gluteal filler: Methacrylate and silicone material associated with a foreign body granulomatous reaction | Sample insufficient | N/D | Muscle biopsy: Granulomas around methacrylate deposits |
| Immuno-histochemistry (IHC) | Strong histiocytic expression of CYP27B1 | N/D | N/D | N/D | N/D |
|
| |||||
| Treatment | Intravenous (IV) fluids, pamidronate, resumption of antiretroviral therapy; later zoledronic acid + methylprednisolone | Methylprednisone, followed by cyclophosphamide + steroids, then IV fluids, diuretics, calcitionin, then ketoconazole | IV pamidronate, then IV fluids, calcitonin, denosumab; then hemodialysis for progression of renal failure | Methylprednisone | Methylprednisone Intralesional injections of triamcinolone |
Because foreign body-induced hypercalcemia is rare, there are no standardized treatments. Standard management of hypercalcemia is employed (saline rehydration, IV bisphosphonates, and glucocorticoids). Glucocorticoids antagonize the actions of 1,25(OH)2D and suppress inflammatory mediators that stimulate CYP27B1 activity [12,4]. In patients who fail to respond to steroids, other options include chloroquine, hydroxychloroquine, and ketoconazole, on which there is less information [12,4,13]. In cases of intractable granulomas, surgical excision is recommended. Unfortunately, surgical removal of PMMA-induced granulomas can be difficult because PMMA diffuses into tissues [14]. Therefore, hypercalcemia may become chronic with the consequential risk of chronic renal dysfunction. In our case, bisphosphonates were selected because they are standard of care in acute hypercalcemia management. Further, as potent antiresorptive agents, they effectively inhibit ongoing bone resorption driven by calcitriol- and cytokine-mediated osteoclast activation. Glucocorticoids were subsequently employed to target the underlying inflammatory reaction and dysregulated calcitriol production.
In conclusion, granulomatous reaction to foreign substances is an important cause of hypercalcemia that can be chronic and refractory to treatment. Here, we demonstrate the mechanism of calcitriol-mediated hypercalcemia with elevated CYP27B1 expression by inflammatory cells, specifically without frank granuloma formation, in the setting of foreign body reaction to PMMA injections. The growing trend of body contour enhancement using injectable fillers should raise suspicion for this unusual cause of hypercalcemia in practice. Patients with HIV who receive injectable fillers for treatment of HIV lipodystrophy or other cosmetic purposes deserve particular consideration, especially in the setting of ART regimen changes, as HIV replication may initiate or contribute to worsening hypercalcemia. In such cases, suppression of HIV reduces abnormal T-cell and macrophage activation and may be critical in maintaining control of hypercalcemia.
Acknowledgments
We gratefully acknowledge the funding support for this project. SH is supported by an NIH T32 training grant (5T32DK007418-34; Michael S. German, MD, Program Director, Division of Diabetes, Endocrinology, and Metabolism) and from the Wilsey Family Fellowship to the UCSF Endocrinology, Diabetes, and Metabolism Training Program. JN is supported by NIH grant AI113143. YC is supported by a NIH F32 grant (5F32DK100084-02). DB is supported by grants from the VA and NIH AR050023. DS is supported by grants from the Research Service of the Department of Veterans Affairs and NIH RO1 AR AR055588. EH receives research grant support from the Doris Duke Charitable Fund (2014099) and the March of Dimes (1-FY14-211).
Footnotes
Authors’ roles: KJ contributed to the pathology analysis. YW and DB performed the immunohistochemistry. SH, EH, FN, and YC cared for the patient and provided detailed medical history. SH, EH, JN, and DS wrote the manuscript. All authors assisted with editing, approved the final version of the manuscript, and take responsibility for the integrity of the data analysis and presentation.
Conflict of Interest Statement: The authors have no conflicts of interest related to this work. UM and EH receive research funding support from Clementia Pharmaceuticals for projects unrelated to this work.
References
- 1.Jacobs TP, Bilezikian JP. Clinical review: Rare causes of hypercalcemia. The Journal of clinical endocrinology and metabolism. 2005;90(11):6316–6322. doi: 10.1210/jc.2005-0675. [DOI] [PubMed] [Google Scholar]
- 2.Lopes N, Sousa B, Martins D, Gomes M, Vieira D, Veronese LA, Milanezi F, Paredes J, Costa JL, Schmitt F. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 2010;10:483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shultz T, Miller WC, Bedrossian CW. Clinical application of measurement of angiotensin-converting enzyme level. JAMA : the journal of the American Medical Association. 1979;242(5):439–441. [PubMed] [Google Scholar]
- 4.Kallas M, Green F, Hewison M, White C, Kline G. Rare causes of calcitriol-mediated hypercalcemia: a case report and literature review. The Journal of clinical endocrinology and metabolism. 2010;95(7):3111–3117. doi: 10.1210/jc.2009-2673. [DOI] [PubMed] [Google Scholar]
- 5.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr. 2007;46(4):456–462. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 6.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9(2):139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 7.Hoy J, Grund B, Roediger M, Ensrud KE, Brar I, Colebunders R, Castro ND, Johnson M, Sharma A, Carr A. Interruption or deferral of antiretroviral therapy reduces markers of bone turnover compared with continuous therapy: The SMART body composition substudy. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(6):1264–1274. doi: 10.1002/jbmr.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi: 10.2147/CCID.S50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozeny GA, Barbato AL, Bansal VK, Vertuno LL, Hano JE. Hypercalcemia associated with silicone-induced granulomas. The New England journal of medicine. 1984;311(17):1103–1105. doi: 10.1056/NEJM198410253111707. [DOI] [PubMed] [Google Scholar]
- 10.Negri AL, Rosa Diez G, Del Valle E, Piulats E, Greloni G, Quevedo A, Varela F, Diehl M, Bevione P. Hypercalcemia secondary to granulomatous disease caused by the injection of methacrylate: a case series. Clin Cases Miner Bone Metab. 2014;11(1):44–48. [PMC free article] [PubMed] [Google Scholar]
- 11.Gyldenlove M, Rorvig S, Skov L, Hansen D. Severe hypercalcaemia, nephrocalcinosis, and multiple paraffinomas caused by paraffin oil injections in a young bodybuilder. Lancet. 2014;383(9934):2098. doi: 10.1016/S0140-6736(14)60806-0. [DOI] [PubMed] [Google Scholar]
- 12.Glass AR, Eil C. Ketoconazole-induced reduction in serum 1,25-dihydroxyvitamin D and total serum calcium in hypercalcemic patients. The Journal of clinical endocrinology and metabolism. 1988;66(5):934–938. doi: 10.1210/jcem-66-5-934. [DOI] [PubMed] [Google Scholar]
- 13.Sharma OP. Hypercalcemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med. 2000;6(5):442–447. doi: 10.1097/00063198-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Salles AG, Lotierzo PH, Gemperli R, Besteiro JM, Ishida LC, Gimenez RP, Menezes J, Ferreira MC. Complications after polymethylmethacrylate injections: report of 32 cases. Plast Reconstr Surg. 2008;121(5):1811–1820. doi: 10.1097/PRS.0b013e31816b1385. [DOI] [PubMed] [Google Scholar]