Introduction
Atherosclerosis is a systemic disease of the arterial vessel wall. While the mortality due to cardiovascular events is decreasing, the prevalence of atherosclerosis and its comorbidities, and the consequent heath care costs are expected to rise sharply in the near future1.
Since the precise etiology and pathogenesis of this complex, multi-factorial disease are still not fully understood, the clinical assessment of cardiovascular risk has been traditionally based on population risk factors (https://www.framinghamheartstudy.org2). However, this approach still largely fails to capture the individual's cardiovascular risk: most cardiovascular events occur in patients with 1 or few traditional risk factors, while individuals classified as high-risk may never experience clinical events3.
The past 10 years have seen a significant paradigm shift in our understanding of the mechanisms of atherogenesis. From being considered the mere result of passive lipid accumulation in the vessel wall, atherosclerosis is now classified as an active inflammatory condition4, 5. The presence of abundant, active inflammatory cells is a known hallmark of high-risk, vulnerable atherosclerotic plaques4, 5. Many studies have identified several systemic pro-inflammatory conditions (such as lupus6, rheumatoid arthritis7-9, and primary cardiovascular events themselves10) as emerging, independent risk factors for atherosclerosis. New evidence suggests that atherosclerosis arises from the complex influence of genetic, environmental, and behavioural variables on systemic and local inflammation through a complex network of molecules, cells and organs.
Thanks to the recent technological advancements of high throughput ‘-omics’, a plethora of these genes, proteins and cells involved in the atherosclerotic cascade have already been identified. However, many steps still need to be taken to fully exploit this information, and improve patients' risk stratification and anti-atherosclerotic therapies. The mutual relationship between genetic and molecular “key drivers”, and their interplay in peripheral blood, atherosclerotic plaques and other organs still need to be established. Furthermore, quantitative methods to non-invasively measure these markers in the vessel wall and other tissues need to be developed and validated before they can be routinely used in the clinical practice.
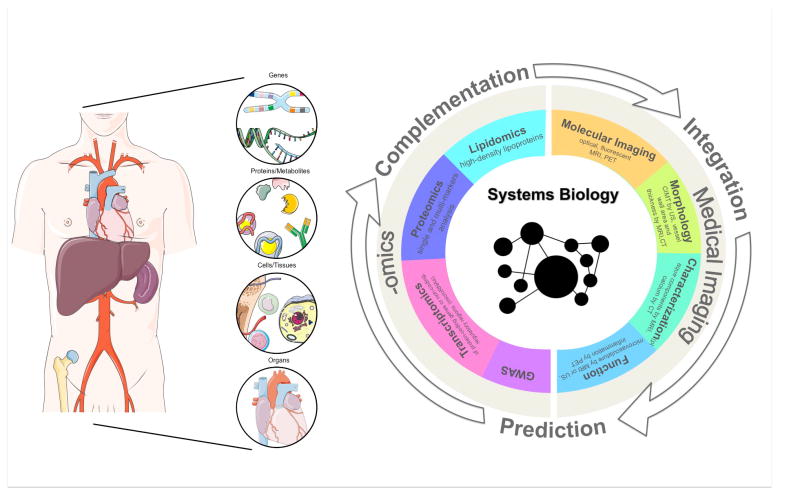
In this review, we highlight novel work in high-throughput ‘-omics’, systems biology and non-invasive quantitative imaging of atherosclerosis, with specific emphasis on articles recently featured in ATVB. New advancements in these disciplines are discussed separately, as well as in their complementary applications, to showcase how these fields may be successfully integrated to improve cardiovascular risk prediction and patients' stratification in the future clinical practice (Figure 1).
Figure 1.
Schematic representation of the integration between data from high-throughput ‘-omics’ and imaging phenotypes in a systems biology approach, focused on building biological networks from genes and molecules, up to tissues and organs to study disease mechanisms.
Systems Biology and high-throughput ‘-omics’ of atherosclerosis
Systems biology can be broadly defined as the combination of experimental and computational research used to understand complex biological systems11. It involves the integration of data derived from high throughput ‘-omics’ with computational/statistical tools to build comprehensive networks, and predictive physiological models12 (Figure 1).
In recent years high throughput ‘-omics’ have been intensely applied to the study of atherosclerosis13, with the aim to deepen our knowledge of this disease and refine our tools for cardiovascular risk assessment. For example, recent data14 from the Erasmus Rucphen Family and Rotterdam Study have shown that common genetic variants for total cholesterol and LDL-C are, in combination, significantly associated with subclinical and clinical outcomes of atherosclerosis. Other investigations have suggested association between soluble interleukin (IL) -2 receptor subunit α (sIL-2R α, regulating lymphocytes activation) and cardiovascular disease (CVD), and also uncovered the genetic determinants of its levels15. These results substantiate our existing knowledge on the impact of long-life, cumulative exposure to modifiable and non-modifiable risk factors on cardiovascular risk16, 17. While previous genome wide association studies (GWAS)18 have reported only marginal improvements19, 20 in risk stratification compared to the Framingham Risk Score3, 21, 22, more recent investigations23, 24 found that genetic risk scores (GRS) from validated GWAS significantly improved prediction of cardiovascular events over traditional risk factors, from 4-5%23 up to 12%24. Extensive bioinformatics analyses25 of loci known to be associated with coronary artery disease (CAD) from GWAS, suggest that sequence variations mainly occur in noncoding regions of the genome and promote CAD risk by either affecting gene expression or by leading to amino acid changes. By extending the list of candidate genes likely linked to CAD, these studies suggest that bioinformatics analysis of GWAS may be beneficial in the context of atherosclerosis as well as other diseases.
By quantifying the expression levels of protein-coding genes, transcriptomic studies 26,27,28,29 identified several promising biomarkers for cardiovascular disease26 and vulnerable plaques30. The Systems Approach to Biomarker Research (SABRe) study (launched as part of the Framingham Heart Study) recently found that 35 genes and 3 gene clusters (metagenes) were differentially expressed in cases with CHD versus controls, while GUK1 38 and other genes were differentially spliced in the two populations31. More recently, transcriptomics has been extended to study non-coding, regulatory gene transcripts, such as microRNAs. Several types of circulating microRNAs have recently been implicated in CVD3, 32-45. Using transcriptomics the prospective Bruneck study found that 3 miRNAs (miR-126, miR-223 and miR-197) originating from platelets improved patients' risk classification for coronary heart disease compared to the Framingham Risk Score46. Transcriptomics analyses have also directly implicated several miRNAs in the development of vascular inflammation as mediated by wall sheer stress (WSS)47. Numerous flow-sensitive miRNAs (miR-10a, miR-633, miR-21, miR-92a) have already been identified48.
Proteomic studies49, 50,51-53,54,55,56 collectively identified more than 150 potential single biomarkers of cardiovascular disease49, 57, 58. More recent studies have seen a shift from the analysis of single protein markers to simultaneously quantifying the combination of the levels of several proteins in multi-marker panel analyses. A recent study in 135 myocardial infarction (MI) cases and matched controls59 found that both single and multi-marker analysis of plasma proteins were associated with incidence of MI, with multi-markers analysis providing higher discrimination. Similar results were found in a prospective study, where multi-markers analysis in 336 patients was found to be predictive of cardiovascular disease (CVD, p<0.0001), with moderate improvement over traditional risk factors (C-statistic of 0.69 versus 0.73) 59.
While these and previous studies offer invaluable insights on the genetic, molecular and metabolic basis of atherosclerosis, they still do not provide a cohesive, integrated approach to the study of this disease. Recent approaches have tried to overcome this obstacle by applying a combination of ‘-omics’. For example a combination of GWAS and transcriptomics is being used to investigate the recently suggested association between the haplogroup 1 of the Y male chromosome and an increased risk of coronary artery disease (CAD)60. New studies at the interface between metabolomics (lipidomics61-63) and proteomics have shed light on the complexity of the human lipoproteome 64, 65, and helped characterizing more than 90 forms of high-density liproproteins (HDL) particles associated with different lipoproteins, with a diverse range of anti-atherosclerotic properties 66, beyond the known effects on cholesterol efflux. A combined approach67 using adipose tissue transcriptomics, HDL lipidomics, and genotyping found a “shift” towards inflammatory HDL particle types in individuals with low HDL cholesterol, mirrored by an increase in inflammatory markers in adipose tissue and in the peripheral blood.
By taking these approaches a step forward, Shang et al68, 69 employ gene subnetworks profiling of the Stockholm Atherosclerosis Gene Expression (STAGE) study to find a candidate gene strongly correlated to leucocyte migration, and assess its association with clinical manifestation of disease (coronary angiography, carotid intima-media thickness by ultrasound). This work offers an example of how a more integrated systems biology approach may be used to better understand the process of atherogenesis, from the genetic determinants to the phenotypic manifestations of systemic and vascular inflammation. As part of the SABRe study mentioned above, Huan et al70 also employ an integrated systems biology approach to find differential gene coexpression modules (DMs) in the blood of subjects with coronary heart disease (CHD) and matched controls. By integrating these results with previous GWAS and single nucleotide polymorphisms (SNPs), the authors are able to draw a causal relationship between the DMs and CHD in this cohort. With the further integration of Bayesian networks and protein-protein interaction networks they also identify key drivers (KD), regulatory genes important for the DMs stability and therefore potential targets for novel drugs. This network driven, integrated approach not only identifies genes related to CHD, but also strives to build a network structure that informs on the molecular interactions of genes associated with CHD risk.
Despite the enormous potential of a panomic/systems biology approach to atherosclerosis, several obstacles have to be overcome so that ‘-omics’ can be successfully used in future clinical practice. Firstly, a causal relationship between biomarkers and disease mechanisms has to be solidly established. Dissecting causal effects from confounders can prove very challenging in cross-sectional studies, while prospective, causal studies with cardiovascular events as endpoints are costly and lengthy to perform. Furthermore, performing ‘-omics’ analyses on direct tissue samples may not be always feasible, while peripheral blood analyses may only reflect transient changes in metabolites that do not necessarily inform on the overall disease activity.
Imaging
In recent years medical imaging has made great strides in the evaluation of virtually every organ in the body, including atherosclerotic plaques. Modality specific imaging traits (imaging “phenotypes”) emerge from the combination of tissues structure, physiology and function, and inform on organs physiology and pathology71, 72.
Several imaging modalities have already found widespread use in the clinical practice to evaluate atherosclerotic burden. Carotid intima-media thickness (CIMT) by surface ultrasound (US)73, 74 is one such technique, although its clinical usefulness to significantly improve risk prediction over traditional risk factors has recently been questioned75, 76. Recently, CIMT was found to decrease in subjects consuming a Mediterranean diet (MedDiet) supplemented with 30 g/d of mixed nuts, compared with a control, low fat diet, thereby corroborating the results form the Primary Prevention of Cardiovascular Disease with a Mediterranean Diet (PREDIMED) trial77. Other than CIMT, surface US can be used to measure other parameters related to plaque vulnerability, such as vascular strain or the extent of plaque microvasculature using non targeted micro bubbles78. US with micro bubbles targeted to vascular cell adhesion molecule 1 (VCAM-1) and platelet glycoprotein Ibα was recently validated in genetically modified mice as being able to assess the anti-inflammatory properties of apocynin, before detectable changes in macrophages burden79.
More invasive procedures involving intravascular (IVUS) or transesophageal US78 can also be performed. For example, transesophageal US was recently employed in mongrel dogs to quantify changes in aortic area and elastic properties from velocity-vector imaging (VVI) with aging80. Another study used a combination of multi-vessel IVUS and novel near infra-red spectroscopy (NIRS)81, 82 to evaluate features of vulnerability in fibroatheromas of diabetic/hypercholesterolemic pigs83. Longitudinally, IVUS demonstrated a progressive increase in plaque and media areas, with the appearance of necrotic cores and regions of positive vascular remodeling. Compared with histological samples, NIRS positive lesions exhibited features of high-risk fibroatheromas, such as large plaque size, necrotic cores, thin fibrous cap, and abundant presence of inflammatory cells. A more recent study using IVUS demonstrated a greater progression in CAD patients classified as statin hyporesponders, compared to individuals that exhibited LDL-C reductions of more than 15% from baseline84.
Coronary calcium score (CAC) evaluated by non contrast enhanced computed tomography (CT)85, 86, is another non-invasive measure of overall atherosclerotic burden, which has been described to predict the risk of future clinical events87, 88. A recent follow-up study of the Multi-Ethnic Study of Atherosclerosis (MESA) trial found that abdominal aortic calcium (AAC) and CAC were predictors of coronary heart disease and cardiovascular events independent of one another, with only AAC being independently associated with cardiovascular mortality, and showing a stronger association than CAC with overall mortality89. Recent studies 90 suggest that this measure could be complemented by cardiac computed tomography angiography (CCTA)91 with the use of a iodinated contrast agent to provide additional information on the degree and distribution of coronary plaque stenosis, vessel wall positive remodeling, and plaque composition (such as presence of low-attenuation plaques and spotty calcification), which have been identified as markers of vulnerability92.
Other modalities, such as magnetic resonance imaging (MRI), and positron emission tomography (PET) are actively being investigated in both the pre-clinical and clinical research arenas for their potential translation into clinical practice. With its superior soft tissue contrast compared to CT, and the possibility to image large segments of the vasculature with high spatial resolution, non-contrast enhanced MRI has been extensively investigated as a method to characterize atherosclerotic plaques components, such as lipid core, fibrous cap, intraplaque hemorrhage and presence of thrombi93-95. A recent study96 performed on 1016 individuals from the Framingham Heart Study Offspring cohort studied the prevalence and risk factor (RF) correlates for aortic plaque (AP) detected by MRI and CT. The study found that while AP by both imaging modalities is associated with smoking and increasing age, the association with other risk factors differs between calcified plaques detected by CT, and non-calcified lesions detected by MRI. The study postulates that the relative predictive value of AP detected by MRI and CT still needs to be investigated. Combined with the use of non-specific gadolinium (Gd) based contrast agents, MRI has been also use to interrogate plaque physiology and quantify the extent of microvascular permeability97-99, another important hallmark of plaque vulnerability. Other physiological parameters, such as carotid arterial strain and distensibility calculated from MRI, have been shown to predict the future incidence of cerebral microbleeds (CMBs) in 2512 patients recruited as part of the prospective, population-based Age, Gene/Environment Susceptibility (AGES)-Reykjavik study100. PET, combined with the anatomical information from CT101 or, more recently, MRI88, has been extensively validated to quantify vascular inflammation itself and its changes upon therapeutic intervention, both in humans and animals using the tracer 18F fluorodeoxyglucose (FDG)102, 103. Recently, 18F-FDG PET was used to demonstrate a decrease in vascular inflammation in Ldlr-/- atherosclerotic mice after treatment with melanocortin peptides104. Other PET tracers are now being investigated, such as sodium fluoride (NaF)105 targeting micro-calcification, or tracers targeted to specific molecules, such as αvβ3, VCAM-1102, 68Ga-Fucoidan for P-selectin106 (abundantly expressed in vulnerable, but not stable plaques), 64Cu-FBP8 for thrombus detection and fibrin quantification107, and 64Cu-DOTATATE to selectively quantify plaque macrophages via the somatostatin receptor subtype-2108.
Among emerging imaging modalities, optical coherence tomography (OCT) is gaining increasing interest because of its ability to provide high-resolution images of tissues microstructure. Recently, OCT was first validated in atherosclerotic rabbits and then used in a prospective study in patients to evaluate vascular healing after the implantation of drug-eluting stents, where it was found to be able to discriminate between immature and mature (healed) neointimal tissue109. Another study used OCT in 40 patients with mild coronary atherosclerosis to study the composition of coronary segments after stimulus with acetylcholine. The study found that the segments showing presence of macrophages and microchannels (microvasculature), exhibited a more prominent change in the diameter of coronary arteries, indicating higher endothelial dysfunction110. Recently, novel, in vivo multiphoton laser scanning microscopy was used to study plaque microvasculature and confirmed that plaque-associated vasa vasorum exhibit increased permeability, and increased leukocyte adhesion and extravasation111.
Imaging as a tool for systems biology
From the account above, it emerges that, through the non-invasive characterization of tissues anatomy and physiology, medical imaging may be an ideal complement to ‘-omics’ technologies for a comprehensive systems biology approach to cardiovascular disease. Several approaches are currently being explored to successfully integrate imaging in this framework.
Although confined to pre-clinical investigations, molecular imaging already reports on specific biological processes (optical imaging, fluorescence imaging), and can even directly quantify gene expression (bioluminescence)1 or cells (macrophages) development, migration112, 113 and presence in tissues throughout the body114. Among translatable modalities, molecular imaging with MRI and PET can also similarly be used for this purpose. Aside from the increasing number of MRI contrast agents being developed to target specific biomarkers93, MRI with ultra small superparamagnetic iron particles (USPIO) has been widely validated as a tool to detect plaque macrophages content in atherosclerotic plaques in both animals115 and patients116. Recent studies in mice have shown the successful integration of proteomics, metabolomics and quantitative and anatomical MR imaging to phenotype transgenic mice in regards to creatinine and phosphocreatinine cardiac metabolism117. In addition to the quantification of plaque local inflammation, the use of 18F FDG PET was recently extended to study the interplay between local and systemic inflammation and to substantiate the existence of a cardiosplenic axis in humans (implicated from animal models10 in the high incidence of secondary cardiovascular events in patients with previous MI) 118. Similarly, another study has recently demonstrated increased vascular inflammation by 18F FDG PET in patients with psoriasis, independent of cardiovascular risk factors119. These studies shows an example of the integration of 18F FDG PET in a ‘systems physiology’ approach. Similarly, several clinical imaging modalities are currently being investigated to quantify non-invasively the extent (CT, MRI) and metabolic activity (18F FDG PET) of visceral and subcutaneous body fat, regarded as a potential marker and risk predictor of cardiovascular disease120.
Some studies have already focused on investigating the genetic and molecular correlates of imaging traits. A recent study identified the genetic variations influencing the effect of smoking on CIMT, thereby exemplifying how the study of gene-environment interactions may explain the interindividual variation in both cardiovascular events and surrogate measures of cardiovascular risk121. The Genetic Loci and the Burden of Atherosclerotic Lesions (GLOBAL) study122 (NCT01738828) brings this concept to a different level by aiming to comprehensively integrate plaque phenotype by cardiovascular imaging, with a panomic approach including genomic, transcriptomic, proteomic, metabolomics and lipidomic in a systems biology framework. The study plans to examine single-omic and multi-omic associations with each imaging phenotype evaluated (CAC and CT angiography) in training and validation datasets.
Final remarks
In this report we have reviewed the most recent advances in ‘-omics’/systems biology and non-invasive medical imaging applied to atherosclerosis and cardiovascular disease, with specific focus on papers recently published in ATVB.
The combination of ‘-omics’ and systems biology is nowadays used more and more frequently to elucidate mechanisms of disease, and is also being investigated as a complement to clinical data to improve patients risk stratification. Examples of these approaches are GWAS23, 24, the study of coding and non-coding RNAs using transcriptomics31, 32-45, as well as tissue and blood proteomics49, 50,51-53,54,55,56,57,58,59. More recent, sophisticated analyses68, 69,70 aim to integrate this information in a comprehensive, systems biology approach to build a network structure of the molecular interactions in cardiovascular disease.
Medical imaging is also making tremendous advancements in the diagnosis and characterization of cardiovascular disease. Modalities such as US, CT and MRI already allow for the accurate quantification of plaque burden and lesion characterization. Other approaches, such as PET and MRI with contrast report on relevant physiological parameters, i.e. plaque permeability and inflammation, while molecular imaging techniques can shed light on specific molecular/cellular processes.
While taken separately both ‘-omics’ and medical imaging can already tremendously contribute to our understanding of cardiovascular disease and to our ability to stratify patients' risk, their successful integration may bring additional, significant benefits.
Similarly to what is being recently proposed in oncology123-129, the first step in integrating these two disciplines will be establishing the association130 between “imaging phenotypes” and specific genetic, molecular and cellular signatures in atherosclerotic plaques and other organs involved in atherogenesis. Once these correlations will be robustly established, the use of imaging phenotypes may be extended to function as predictors124, 125 of plaques genetic and molecular makeup123-129 in both the pre-clinical and clinical arenas. In this scenario, the integration of imaging and ‘-omics’ in a systems biology framework may be better positioned to improve risk stratification and assessment of therapeutic response of atherosclerotic patients in the future clinical practice (Figure 1).
Contributor Information
Claudia Calcagno, Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY.
Willem J.M. Mulder, Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, Department of Medical Biochemistry, Academic Medical Center, Amsterdam, The Netherlands
Matthias Nahrendorf, Center for Systems Biology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Zahi A. Fayad, Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY
References
- 1.Nahrendorf M, Frantz S, Swirski FK, Mulder WJ, Randolph G, Ertl G, Ntziachristos V, Piek JJ, Stroes ES, Schwaiger M, Mann DL, Fayad ZA. Imaging systemic inflammatory networks in ischemic heart disease. Journal of the American College of Cardiology. 2015;65:1583–1591. doi: 10.1016/j.jacc.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Https://www.Framinghamheartstudy.Org.
- 3.Mayr M, Zampetaki A, Willeit P, Willeit J, Kiechl S. Micrornas within the continuum of postgenomics biomarker discovery. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:206–214. doi: 10.1161/ATVBAHA.112.300141. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Ladich ER, Burke AP, Kolodgie FD. Histopathology of carotid atherosclerotic disease. Neurosurgery. 2006;59:S219–227. doi: 10.1227/01.NEU.0000239895.00373.E4. discussion S213-213. [DOI] [PubMed] [Google Scholar]
- 5.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 6.van Leuven SI, Mendez-Fernandez YV, Wilhelm AJ, Wade NS, Gabriel CL, Kastelein JJ, Stroes ES, Tak PP, Major AS. Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus-prone ldlr(-/-) mice. Annals of the rheumatic diseases. 2012;71:408–414. doi: 10.1136/annrheumdis-2011-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon DH, Peters MJ, Nurmohamed MT, Dixon W. Unresolved questions in rheumatology: Motion for debate: The data support evidence-based management recommendations for cardiovascular disease in rheumatoid arthritis. Arthritis and rheumatism. 2013;65:1675–1683. doi: 10.1002/art.37975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He M, Liang X, He L, Wen W, Zhao S, Wen L, Liu Y, Shyy JY, Yuan Z. Endothelial dysfunction in rheumatoid arthritis: The role of monocyte chemotactic protein-1-induced protein. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1384–1391. doi: 10.1161/ATVBAHA.113.301490. [DOI] [PubMed] [Google Scholar]
- 9.Furer V, Fayad ZA, Mani V, Calcagno C, Farkouh ME, Greenberg JD. Noninvasive cardiovascular imaging in rheumatoid arthritis: Current modalities and the emerging role of magnetic resonance and positron emission tomography imaging. Seminars in arthritis and rheumatism. 2012;41:676–688. doi: 10.1016/j.semarthrit.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitano H. Computational systems biology. Nature. 2002;420:206–210. doi: 10.1038/nature01254. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey SA, Gold ES, Aderem A. A systems biology approach to understanding atherosclerosis. EMBO molecular medicine. 2010;2:79–89. doi: 10.1002/emmm.201000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorkegren JL, Kovacic JC, Dudley JT, Schadt EE. Genome-wide significant loci: How important are they? Systems genetics to understand heritability of coronary artery disease and other common complex disorders. Journal of the American College of Cardiology. 2015;65:830–845. doi: 10.1016/j.jacc.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanassoulis G, Peloso GM, O'Donnell CJ. Genomic medicine for improved prediction and primordial prevention of cardiovascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2049–2050. doi: 10.1161/ATVBAHA.113.301814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durda P, Sabourin J, Lange EM, et al. Plasma levels of soluble interleukin-2 receptor alpha: Associations with clinical cardiovascular events and genome-wide association scan. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2246–2253. doi: 10.1161/ATVBAHA.115.305289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isaacs A, Willems SM, Bos D, Dehghan A, Hofman A, Ikram MA, Uitterlinden AG, Oostra BA, Franco OH, Witteman JC, van Duijn CM. Risk scores of common genetic variants for lipid levels influence atherosclerosis and incident coronary heart disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2233–2239. doi: 10.1161/ATVBAHA.113.301236. [DOI] [PubMed] [Google Scholar]
- 17.Gebreab SY, Riestra P, Khan RJ, Xu R, Musani SK, Tekola-Ayele F, Correa A, Wilson JG, Rotimi CN, Davis SK. Genetic ancestry is associated with measures of subclinical atherosclerosis in african americans: The jackson heart study. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1271–1278. doi: 10.1161/ATVBAHA.114.304855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Huffman JE, Yamakuchi M, et al. Genome-wide association study for circulating tissue plasminogen activator levels and functional follow-up implicates endothelial stxbp5 and stx2. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1093–1101. doi: 10.1161/ATVBAHA.113.302088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripatti S, Tikkanen E, Orho-Melander M, et al. A multilocus genetic risk score for coronary heart disease: Case-control and prospective cohort analyses. Lancet. 2010;376:1393–1400. doi: 10.1016/S0140-6736(10)61267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thanassoulis G, Peloso GM, Pencina MJ, Hoffmann U, Fox CS, Cupples LA, Levy D, D'Agostino RB, Hwang SJ, O'Donnell CJ. A genetic risk score is associated with incident cardiovascular disease and coronary artery calcium: The framingham heart study. Circulation Cardiovascular genetics. 2012;5:113–121. doi: 10.1161/CIRCGENETICS.111.961342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casas JP, Cooper J, Miller GJ, Hingorani AD, Humphries SE. Investigating the genetic determinants of cardiovascular disease using candidate genes and meta-analysis of association studies. Annals of human genetics. 2006;70:145–169. doi: 10.1111/j.1469-1809.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 22.Greenland P, Alpert JS, Beller GA, et al. 2010 accf/aha guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Journal of the American College of Cardiology. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Ganna A, Magnusson PK, Pedersen NL, de Faire U, Reilly M, Arnlov J, Sundstrom J, Hamsten A, Ingelsson E. Multilocus genetic risk scores for coronary heart disease prediction. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2267–2272. doi: 10.1161/ATVBAHA.113.301218. [DOI] [PubMed] [Google Scholar]
- 24.Tikkanen E, Havulinna AS, Palotie A, Salomaa V, Ripatti S. Genetic risk prediction and a 2-stage risk screening strategy for coronary heart disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2261–2266. doi: 10.1161/ATVBAHA.112.301120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braenne I, Civelek M, Vilne B, et al. Prediction of causal candidate genes in coronary artery disease loci. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2207–2217. doi: 10.1161/ATVBAHA.115.306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siemelink MA, Zeller T. Biomarkers of coronary artery disease: The promise of the transcriptome. Current cardiology reports. 2014;16:513. doi: 10.1007/s11886-014-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedrotty DM, Morley MP, Cappola TP. Transcriptomic biomarkers of cardiovascular disease. Progress in cardiovascular diseases. 2012;55:64–69. doi: 10.1016/j.pcad.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bijnens AP, Lutgens E, Ayoubi T, Kuiper J, Horrevoets AJ, Daemen MJ. Genome-wide expression studies of atherosclerosis: Critical issues in methodology, analysis, interpretation of transcriptomics data. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1226–1235. doi: 10.1161/01.ATV.0000219289.06529.f1. [DOI] [PubMed] [Google Scholar]
- 29.Erbilgin A, Siemers N, Kayne P, Yang WP, Berliner J, Lusis AJ. Gene expression analyses of mouse aortic endothelium in response to atherogenic stimuli. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2509–2517. doi: 10.1161/ATVBAHA.113.301989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perisic L, Hedin E, Razuvaev A, Lengquist M, Osterholm C, Folkersen L, Gillgren P, Paulsson-Berne G, Ponten F, Odeberg J, Hedin U. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals pcsk6 as a novel protease in unstable carotid atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2432–2443. doi: 10.1161/ATVBAHA.113.301743. [DOI] [PubMed] [Google Scholar]
- 31.Joehanes R, Ying S, Huan T, Johnson AD, Raghavachari N, Wang R, Liu P, Woodhouse KA, Sen SK, Tanriverdi K, Courchesne P, Freedman JE, O'Donnell CJ, Levy D, Munson PJ. Gene expression signatures of coronary heart disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1418–1426. doi: 10.1161/ATVBAHA.112.301169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tijsen AJ, Pinto YM, Creemers EE. Circulating micrornas as diagnostic biomarkers for cardiovascular diseases. American journal of physiology Heart and circulatory physiology. 2012;303:H1085–1095. doi: 10.1152/ajpheart.00191.2012. [DOI] [PubMed] [Google Scholar]
- 33.Deddens JC, Colijn JM, Oerlemans MI, Pasterkamp G, Chamuleau SA, Doevendans PA, Sluijter JP. Circulating micrornas as novel biomarkers for the early diagnosis of acute coronary syndrome. Journal of cardiovascular translational research. 2013;6:884–898. doi: 10.1007/s12265-013-9493-9. [DOI] [PubMed] [Google Scholar]
- 34.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T. Increased microrna-1 and microrna-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circulation Cardiovascular genetics. 2011;4:446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 35.Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, Doevendans PA, Hoes AW, Sluijter JP. Early assessment of acute coronary syndromes in the emergency department: The potential diagnostic value of circulating micrornas. EMBO molecular medicine. 2012;4:1176–1185. doi: 10.1002/emmm.201201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM. Mir423-5p as a circulating biomarker for heart failure. Circulation research. 2010;106:1035–1039. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 37.De Guire V, Robitaille R, Tetreault N, Guerin R, Menard C, Bambace N, Sapieha P. Circulating mirnas as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: Promises and challenges. Clinical biochemistry. 2013;46:846–860. doi: 10.1016/j.clinbiochem.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Abraham E. Micrornas in immune response and macrophage polarization. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:170–177. doi: 10.1161/ATVBAHA.112.300068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. Micrornas in metabolic disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boon RA, Vickers KC. Intercellular transport of micrornas. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dang LT, Lawson ND, Fish JE. Microrna control of vascular endothelial growth factor signaling output during vascular development. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:193–200. doi: 10.1161/ATVBAHA.112.300142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiedler J, Thum T. Micrornas in myocardial infarction. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:201–205. doi: 10.1161/ATVBAHA.112.300137. [DOI] [PubMed] [Google Scholar]
- 43.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T, Zeiher AM, Landmesser U, Dimmeler S. Characterization of levels and cellular transfer of circulating lipoprotein-bound micrornas. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1392–1400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 44.Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A. Microrna-126, -145, and -155: A therapeutic triad in atherosclerosis? Arteriosclerosis, thrombosis, and vascular biology. 2013;33:449–454. doi: 10.1161/ATVBAHA.112.300279. [DOI] [PubMed] [Google Scholar]
- 45.Huan T, Rong J, Tanriverdi K, et al. Dissecting the roles of micrornas in coronary heart disease via integrative genomic analyses. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1011–1021. doi: 10.1161/ATVBAHA.114.305176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM, Mayr A, Weger S, Schett G, Shah A, Boulanger CM, Willeit J, Chowienczyk PJ, Kiechl S, Mayr M. Prospective study on circulating micrornas and risk of myocardial infarction. Journal of the American College of Cardiology. 2012;60:290–299. doi: 10.1016/j.jacc.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 47.Bryan MT, Duckles H, Feng S, Hsiao ST, Kim HR, Serbanovic-Canic J, Evans PC. Mechanoresponsive networks controlling vascular inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2199–2205. doi: 10.1161/ATVBAHA.114.303424. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S, Kim CW, Simmons RD, Jo H. Role of flow-sensitive micrornas in endothelial dysfunction and atherosclerosis: Mechanosensitive athero-mirs. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2206–2216. doi: 10.1161/ATVBAHA.114.303425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindsey ML, Mayr M, Gomes AV, Delles C, Arrell DK, Murphy AM, Lange RA, Costello CE, Jin YF, Laskowitz DT, Sam F, Terzic A, Van Eyk J, Srinivas PR. Transformative impact of proteomics on cardiovascular health and disease: A scientific statement from the american heart association. Circulation. 2015 doi: 10.1161/CIR.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 50.Didangelos A, Yin X, Mandal K, Saje A, Smith A, Xu Q, Jahangiri M, Mayr M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: A proteomics approach. Molecular & cellular proteomics : MCP. 2011;(10):M111. doi: 10.1074/mcp.M111.008128. 008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmerli LU, Schiffer E, Zurbig P, Good DM, Kellmann M, Mouls L, Pitt AR, Coon JJ, Schmieder RE, Peter KH, Mischak H, Kolch W, Delles C, Dominiczak AF. Urinary proteomic biomarkers in coronary artery disease. Molecular & cellular proteomics : MCP. 2008;7:290–298. doi: 10.1074/mcp.M700394-MCP200. [DOI] [PubMed] [Google Scholar]
- 52.Brown CE, McCarthy NS, Hughes AD, Sever P, Stalmach A, Mullen W, Dominiczak AF, Sattar N, Mischak H, Thom S, Mayet J, Stanton AV, Delles C. Urinary proteomic biomarkers to predict cardiovascular events. Proteomics Clinical applications. 2015;9:610–617. doi: 10.1002/prca.201400195. [DOI] [PubMed] [Google Scholar]
- 53.Zhang ZY, Thijs L, Petit T, Gu YM, Jacobs L, Yang WY, Liu YP, Koeck T, Zurbig P, Jin Y, Verhamme P, Voigt JU, Kuznetsova T, Mischak H, Staessen JA. Urinary proteome and systolic blood pressure as predictors of 5-year cardiovascular and cardiac outcomes in a general population. Hypertension. 2015;66:52–60. doi: 10.1161/HYPERTENSIONAHA.115.05296. [DOI] [PubMed] [Google Scholar]
- 54.Gerszten RE, Asnani A, Carr SA. Status and prospects for discovery and verification of new biomarkers of cardiovascular disease by proteomics. Circulation research. 2011;109:463–474. doi: 10.1161/CIRCRESAHA.110.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melander O, Modrego J, Zamorano-Leon JJ, Santos-Sancho JM, Lahera V, Lopez-Farre AJ. New circulating biomarkers for predicting cardiovascular death in healthy population. Journal of cellular and molecular medicine. 2015 doi: 10.1111/jcmm.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagnato C, Thumar J, Mayya V, Hwang SI, Zebroski H, Claffey KP, Haudenschild C, Eng JK, Lundgren DH, Han DK. Proteomics analysis of human coronary atherosclerotic plaque: A feasibility study of direct tissue proteomics by liquid chromatography and tandem mass spectrometry. Molecular & cellular proteomics : MCP. 2007;6:1088–1102. doi: 10.1074/mcp.M600259-MCP200. [DOI] [PubMed] [Google Scholar]
- 57.Basak T, Varshney S, Akhtar S, Sengupta S. Understanding different facets of cardiovascular diseases based on model systems to human studies: A proteomic and metabolomic perspective. Journal of proteomics. 2015 doi: 10.1016/j.jprot.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Pinna R, Madrigal-Matute J, Tarin C, Burillo E, Esteban-Salan M, Pastor-Vargas C, Lindholt JS, Lopez JA, Calvo E, de Ceniga MV, Meilhac O, Egido J, Blanco-Colio LM, Michel JB, Martin-Ventura JL. Proteomic analysis of intraluminal thrombus highlights complement activation in human abdominal aortic aneurysms. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2013–2020. doi: 10.1161/ATVBAHA.112.301191. [DOI] [PubMed] [Google Scholar]
- 59.Yin X, Subramanian S, Hwang SJ, O'Donnell CJ, Fox CS, Courchesne P, Muntendam P, Gordon N, Adourian A, Juhasz P, Larson MG, Levy D. Protein biomarkers of new-onset cardiovascular disease: Prospective study from the systems approach to biomarker research in cardiovascular disease initiative. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:939–945. doi: 10.1161/ATVBAHA.113.302918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bloomer LD, Nelson CP, Eales J, Denniff M, Christofidou P, Debiec R, Moore J, Consortium C, Zukowska-Szczechowska E, Goodall AH, Thompson J, Samani NJ, Charchar FJ, Tomaszewski M. Male-specific region of the y chromosome and cardiovascular risk: Phylogenetic analysis and gene expression studies. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1722–1727. doi: 10.1161/ATVBAHA.113.301608. [DOI] [PubMed] [Google Scholar]
- 61.Meikle PJ, Wong G, Barlow CK, Kingwell BA. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacology & therapeutics. 2014;143:12–23. doi: 10.1016/j.pharmthera.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Hinterwirth H, Stegemann C, Mayr M. Lipidomics: Quest for molecular lipid biomarkers in cardiovascular disease. Circulation Cardiovascular genetics. 2014;7:941–954. doi: 10.1161/CIRCGENETICS.114.000550. [DOI] [PubMed] [Google Scholar]
- 63.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, Spector TD, Willeit J, Kiechl S, Mayr M. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based bruneck study. Circulation. 2014;129:1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. [DOI] [PubMed] [Google Scholar]
- 64.Gordon SM, Li H, Zhu X, Shah AS, Lu LJ, Davidson WS. A comparison of the mouse and human lipoproteome: Suitability of the mouse model for studies of human lipoproteins. Journal of proteome research. 2015;14:2686–2695. doi: 10.1021/acs.jproteome.5b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, Gordon SM, Zhu X, Deng J, Swertfeger DK, Davidson WS, Lu LJ. Network-based analysis on orthogonal separation of human plasma uncovers distinct high density lipoprotein complexes. Journal of proteome research. 2015;14:3082–3094. doi: 10.1021/acs.jproteome.5b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of hdl. The Journal of clinical investigation. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laurila PP, Surakka I, Sarin AP, et al. Genomic, transcriptomic, and lipidomic profiling highlights the role of inflammation in individuals with low high-density lipoprotein cholesterol. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:847–857. doi: 10.1161/ATVBAHA.112.300733. [DOI] [PubMed] [Google Scholar]
- 68.Shang MM, Talukdar HA, Hofmann JJ, et al. Lim domain binding 2: A key driver of transendothelial migration of leukocytes and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2068–2077. doi: 10.1161/ATVBAHA.113.302709. [DOI] [PubMed] [Google Scholar]
- 69.Civelek M, Lusis AJ. From hairballs to an understanding of transendothelial migration of monocytes in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1809–1810. doi: 10.1161/ATVBAHA.114.304151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huan T, Zhang B, Wang Z, et al. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1427–1434. doi: 10.1161/ATVBAHA.112.300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez-Friera L, Ibanez B, Fuster V. Imaging subclinical atherosclerosis: Is it ready for prime time? A review. Journal of cardiovascular translational research. 2014;7:623–634. doi: 10.1007/s12265-014-9582-4. [DOI] [PubMed] [Google Scholar]
- 72.Fuster V, Lois F, Franco M. Early identification of atherosclerotic disease by noninvasive imaging. Nature reviews Cardiology. 2010;7:327–333. doi: 10.1038/nrcardio.2010.54. [DOI] [PubMed] [Google Scholar]
- 73.Katakami N, Kaneto H, Shimomura I. Carotid ultrasonography: A potent tool for better clinical practice in diagnosis of atherosclerosis in diabetic patients. Journal of diabetes investigation. 2014;5:3–13. doi: 10.1111/jdi.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sillesen H, Fuster V. Predicting coronary heart disease: From framingham risk score to ultrasound bioimaging. The Mount Sinai journal of medicine, New York. 2012;79:654–663. doi: 10.1002/msj.21343. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1341–1345. doi: 10.1161/ATVBAHA.113.302075. [DOI] [PubMed] [Google Scholar]
- 76.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 77.Sala-Vila A, Romero-Mamani ES, Gilabert R, Nunez I, de la Torre R, Corella D, Ruiz-Gutierrez V, Lopez-Sabater MC, Pinto X, Rekondo J, Martinez-Gonzalez MA, Estruch R, Ros E. Changes in ultrasound-assessed carotid intima-media thickness and plaque with a mediterranean diet: A substudy of the predimed trial. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:439–445. doi: 10.1161/ATVBAHA.113.302327. [DOI] [PubMed] [Google Scholar]
- 78.de Korte CL, Hansen HH, van der Steen AF. Vascular ultrasound for atherosclerosis imaging. Interface focus. 2011;1:565–575. doi: 10.1098/rsfs.2011.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khanicheh E, Qi Y, Xie A, Mitterhuber M, Xu L, Mochizuki M, Daali Y, Jaquet V, Krause KH, Ruggeri ZM, Kuster GM, Lindner JR, Kaufmann BA. Molecular imaging reveals rapid reduction of endothelial activation in early atherosclerosis with apocynin independent of antioxidative properties. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2187–2192. doi: 10.1161/ATVBAHA.113.301710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim SA, Lee KH, Won HY, Park S, Chung JH, Jang Y, Ha JW. Quantitative assessment of aortic elasticity with aging using velocity-vector imaging and its histologic correlation. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1306–1312. doi: 10.1161/ATVBAHA.113.301312. [DOI] [PubMed] [Google Scholar]
- 81.Sanon S, Dao T, Sanon VP, Chilton R. Imaging of vulnerable plaques using near-infrared spectroscopy for risk stratification of atherosclerosis. Current atherosclerosis reports. 2013;15:304. doi: 10.1007/s11883-012-0304-6. [DOI] [PubMed] [Google Scholar]
- 82.Jaguszewski M, Klingenberg R, Landmesser U. Intracoronary near-infrared spectroscopy (nirs) imaging for detection of lipid content of coronary plaques: Current experience and future perspectives. Current cardiovascular imaging reports. 2013;6:426–430. doi: 10.1007/s12410-013-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel D, Hamamdzic D, Llano R, Patel D, Cheng L, Fenning RS, Bannan K, Wilensky RL. Subsequent development of fibroatheromas with inflamed fibrous caps can be predicted by intracoronary near infrared spectroscopy. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:347–353. doi: 10.1161/ATVBAHA.112.300710. [DOI] [PubMed] [Google Scholar]
- 84.Kataoka Y, St John J, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Atheroma progression in hyporesponders to statin therapy. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:990–995. doi: 10.1161/ATVBAHA.114.304477. [DOI] [PubMed] [Google Scholar]
- 85.Youssef G, Kalia N, Darabian S, Budoff MJ. Coronary calcium: New insights, recent data, and clinical role. Current cardiology reports. 2013;15:325. doi: 10.1007/s11886-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Danad I, Min JK. Computed tomography: The optimal imaging method for differentiation of ischemic vs non-ischemic cardiomyopathy. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2015 doi: 10.1007/s12350-015-0146-z. [DOI] [PubMed] [Google Scholar]
- 87.Nasir K, Michos ED, Blumenthal RS, Raggi P. Detection of high-risk young adults and women by coronary calcium and national cholesterol education program panel iii guidelines. Journal of the American College of Cardiology. 2005;46:1931–1936. doi: 10.1016/j.jacc.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 88.Raggi P, Khan A, Arepali C, Stillman AE. Coronary artery calcium scoring in the age of ct angiography: What is its role? Current atherosclerosis reports. 2008;10:438–443. doi: 10.1007/s11883-008-0067-2. [DOI] [PubMed] [Google Scholar]
- 89.Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, Cohoon KP, Srikanthan P, Watson KE, Wong ND. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the multi-ethnic study of atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1574–1579. doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalra DK, Heo R, Valenti V, Nakazato R, Min JK. Role of computed tomography for diagnosis and risk stratification of patients with suspected or known coronary artery disease. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1144–1154. doi: 10.1161/ATVBAHA.113.302074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Min JK, Hachamovitch R, Rozanski A, Shaw LJ, Berman DS, Gibbons R. Clinical benefits of noninvasive testing: Coronary computed tomography angiography as a test case. JACC Cardiovascular imaging. 2010;3:305–315. doi: 10.1016/j.jcmg.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 92.Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C, Belur P, Hulten E, Villines TC. Coronary atherosclerosis imaging by coronary ct angiography: Current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovascular imaging. 2011;4:537–548. doi: 10.1016/j.jcmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Bakermans AJ, Abdurrachim D, Moonen RP, Motaal AG, Prompers JJ, Strijkers GJ, Vandoorne K, Nicolay K. Small animal cardiovascular mr imaging and spectroscopy. Progress in nuclear magnetic resonance spectroscopy. 2015;88-89:1–47. doi: 10.1016/j.pnmrs.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 94.Singh N, Moody AR, Roifman I, Bluemke DA, Zavodni AE. Advanced mri for carotid plaque imaging. The international journal of cardiovascular imaging. 2015 doi: 10.1007/s10554-015-0743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Usman A, Sadat U, Graves MJ, Gillard JH. Magnetic resonance imaging of atherothrombotic plaques. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2015 doi: 10.1016/j.jocn.2015.03.060. [DOI] [PubMed] [Google Scholar]
- 96.Chuang ML, Gona P, Oyama-Manabe N, Manders ES, Salton CJ, Hoffmann U, Manning WJ, O'Donnell CJ. Risk factor differences in calcified and noncalcified aortic plaque: The framingham heart study. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1580–1586. doi: 10.1161/ATVBAHA.114.303600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wasserman BA. Advanced contrast-enhanced mri for looking beyond the lumen to predict stroke: Building a risk profile for carotid plaque. Stroke; a journal of cerebral circulation. 2010;41:S12–16. doi: 10.1161/STROKEAHA.110.596288. [DOI] [PubMed] [Google Scholar]
- 98.Calcagno C, Cornily JC, Hyafil F, Rudd JH, Briley-Saebo KC, Mani V, Goldschlager G, Machac J, Fuster V, Fayad ZA. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced mri and 18f-fdg pet. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1311–1317. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kerwin W, Hooker A, Spilker M, Vicini P, Ferguson M, Hatsukami T, Yuan C. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107:851–856. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 100.Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: The age, gene/environment susceptibility (ages)-reykjavik study. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1889–1895. doi: 10.1161/ATVBAHA.115.305451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alie N, Eldib M, Fayad ZA, Mani V. Inflammation, atherosclerosis, and coronary artery disease: Pet/ct for the evaluation of atherosclerosis and inflammation. Clinical Medicine Insights Cardiology. 2014;8:13–21. doi: 10.4137/CMC.S17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orbay H, Hong H, Zhang Y, Cai W. Positron emission tomography imaging of atherosclerosis. Theranostics. 2013;3:894–902. doi: 10.7150/thno.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tawakol A, Singh P, Mojena M, Pimentel-Santillana M, Emami H, MacNabb M, Rudd JH, Narula J, Enriquez JA, Traves PG, Fernandez-Velasco M, Bartrons R, Martin-Sanz P, Fayad ZA, Tejedor A, Bosca L. Hif-1alpha and pfkfb3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1463–1471. doi: 10.1161/ATVBAHA.115.305551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rinne P, Silvola JM, Hellberg S, Stahle M, Liljenback H, Salomaki H, Koskinen E, Nuutinen S, Saukko P, Knuuti J, Saraste A, Roivainen A, Savontaus E. Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1346–1354. doi: 10.1161/ATVBAHA.113.302963. [DOI] [PubMed] [Google Scholar]
- 105.Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, Richardson H, White A, McKillop G, van Beek EJ, Boon NA, Rudd JH, Newby DE. Coronary arterial 18f-sodium fluoride uptake: A novel marker of plaque biology. Journal of the American College of Cardiology. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 106.Li X, Bauer W, Israel I, Kreissl MC, Weirather J, Richter D, Bauer E, Herold V, Jakob P, Buck A, Frantz S, Samnick S. Targeting p-selectin by gallium-68-labeled fucoidan positron emission tomography for noninvasive characterization of vulnerable plaques: Correlation with in vivo 17.6t mri. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1661–1667. doi: 10.1161/ATVBAHA.114.303485. [DOI] [PubMed] [Google Scholar]
- 107.Blasi F, Oliveira BL, Rietz TA, Rotile NJ, Naha PC, Cormode DP, Izquierdo-Garcia D, Catana C, Caravan P. Multisite thrombus imaging and fibrin content estimation with a single whole-body pet scan in rats. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2114–2121. doi: 10.1161/ATVBAHA.115.306055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, Hojgaard L, Ripa RS, Kjaer A. 64cu-dotatate pet/mri for detection of activated macrophages in carotid atherosclerotic plaques: Studies in patients undergoing endarterectomy. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1696–1703. doi: 10.1161/ATVBAHA.114.305067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malle C, Tada T, Steigerwald K, Ughi GJ, Schuster T, Nakano M, Massberg S, Jehle J, Guagliumi G, Kastrati A, Virmani R, Byrne RA, Joner M. Tissue characterization after drug-eluting stent implantation using optical coherence tomography. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1376–1383. doi: 10.1161/ATVBAHA.113.301227. [DOI] [PubMed] [Google Scholar]
- 110.Choi BJ, Matsuo Y, Aoki T, Kwon TG, Prasad A, Gulati R, Lennon RJ, Lerman LO, Lerman A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2473–2477. doi: 10.1161/ATVBAHA.114.304445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rademakers T, Douma K, Hackeng TM, Post MJ, Sluimer JC, Daemen MJ, Biessen EA, Heeneman S, van Zandvoort MA. Plaque-associated vasa vasorum in aged apolipoprotein e-deficient mice exhibit proatherogenic functional features in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:249–256. doi: 10.1161/ATVBAHA.112.300087. [DOI] [PubMed] [Google Scholar]
- 112.Taqueti VR, Nahrendorf M, Di Carli MF. Translational molecular imaging: Repurposing an old technique to track cell migration into human atheroma. Journal of the American College of Cardiology. 2014;64:1030–1032. doi: 10.1016/j.jacc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 113.van der Valk FM, Kroon J, Potters WV, Thurlings RM, Bennink RJ, Verberne HJ, Nederveen AJ, Nieuwdorp M, Mulder WJ, Fayad ZA, van Buul JD, Stroes ES. In vivo imaging of enhanced leukocyte accumulation in atherosclerotic lesions in humans. Journal of the American College of Cardiology. 2014;64:1019–1029. doi: 10.1016/j.jacc.2014.06.1171. [DOI] [PubMed] [Google Scholar]
- 114.Swirski FK, Nahrendorf M. Imaging macrophage development and fate in atherosclerosis and myocardial infarction. Immunology and cell biology. 2013;91:297–303. doi: 10.1038/icb.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sosnovik DE, Nahrendorf M. Cells and iron oxide nanoparticles on the move: Magnetic resonance imaging of monocyte homing and myocardial inflammation in patients with st-elevation myocardial infarction. Circulation Cardiovascular imaging. 2012;5:551–554. doi: 10.1161/CIRCIMAGING.112.978932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18f-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. Journal of the American College of Cardiology. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 117.Phillips D, Ten Hove M, Schneider JE, Wu CO, Sebag-Montefiore L, Aponte AM, Lygate CA, Wallis J, Clarke K, Watkins H, Balaban RS, Neubauer S. Mice over-expressing the myocardial creatine transporter develop progressive heart failure and show decreased glycolytic capacity. Journal of molecular and cellular cardiology. 2010;48:582–590. doi: 10.1016/j.yjmcc.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: Demonstration of a cardiosplenic axis in humans. JACC Cardiovascular imaging. 2015;8:121–130. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, Ng Q, Joshi AA, Krishnamoorthy P, Dave J, Rose SM, Doveikis J, Playford MP, Prussick RB, Ehrlich A, Kaplan MJ, Lockshin BN, Gelfand JM, Mehta NN. Severity of psoriasis associates with aortic vascular inflammation detected by fdg pet/ct and neutrophil activation in a prospective observational study. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang H, Chen YE, Eitzman DT. Imaging body fat: Techniques and cardiometabolic implications. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2217–2223. doi: 10.1161/ATVBAHA.114.303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L, Rundek T, Beecham A, Hudson B, Blanton SH, Zhao H, Sacco RL, Dong C. Genome-wide interaction study identifies rcbtb1 as a modifier for smoking effect on carotid intima-media thickness. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:219–225. doi: 10.1161/ATVBAHA.113.302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Voros S, Maurovich-Horvat P, Marvasty IB, Bansal AT, Barnes MR, Vazquez G, Murray SS, Voros V, Merkely B, Brown BO, Warnick GR. Precision phenotyping, panomics, and system-level bioinformatics to delineate complex biologies of atherosclerosis: Rationale and design of the “genetic loci and the burden of atherosclerotic lesions” study. Journal of cardiovascular computed tomography. 2014;8:442–451. doi: 10.1016/j.jcct.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 123.Golugula A, Lee G, Master SR, Feldman MD, Tomaszewski JE, Madabhushi A. Supervised regularized canonical correlation analysis: Integrating histologic and proteomic data for predicting biochemical failures. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2011;2011:6434–6437. doi: 10.1109/IEMBS.2011.6091588. [DOI] [PubMed] [Google Scholar]
- 124.Gevaert O, Xu J, Hoang CD, Leung AN, Xu Y, Quon A, Rubin DL, Napel S, Plevritis SK. Non-small cell lung cancer: Identifying prognostic imaging biomarkers by leveraging public gene expression microarray data--methods and preliminary results. Radiology. 2012;264:387–396. doi: 10.1148/radiol.12111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nair VS, Gevaert O, Davidzon G, Napel S, Graves EE, Hoang CD, Shrager JB, Quon A, Rubin DL, Plevritis SK. Prognostic pet 18f-fdg uptake imaging features are associated with major oncogenomic alterations in patients with resected non-small cell lung cancer. Cancer research. 2012;72:3725–3734. doi: 10.1158/0008-5472.CAN-11-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Colen R, Foster I, Gatenby R, Giger ME, Gillies R, Gutman D, Heller M, Jain R, Madabhushi A, Madhavan S, Napel S, Rao A, Saltz J, Tatum J, Verhaak R, Whitman G. Nci workshop report: Clinical and computational requirements for correlating imaging phenotypes with genomics signatures. Translational oncology. 2014;7:556–569. doi: 10.1016/j.tranon.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zinn PO, Mahajan B, Sathyan P, Singh SK, Majumder S, Jolesz FA, Colen RR. Radiogenomic mapping of edema/cellular invasion mri-phenotypes in glioblastoma multiforme. PLoS One. 2011;6:e25451. doi: 10.1371/journal.pone.0025451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, Chan BK, Matcuk GR, Barry CT, Chang HY, Kuo MD. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nature biotechnology. 2007;25:675–680. doi: 10.1038/nbt1306. [DOI] [PubMed] [Google Scholar]
- 129.Diehn M, Nardini C, Wang DS, McGovern S, Jayaraman M, Liang Y, Aldape K, Cha S, Kuo MD. Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci U S A. 2008;105:5213–5218. doi: 10.1073/pnas.0801279105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: Transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. Journal of the American College of Cardiology. 2015;65:846–855. doi: 10.1016/j.jacc.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]