Abstract
Rationale
The affective aspects of d-amphetamine (AMPH) may be mediated, in part, by cocaine- and amphetamine-regulated transcript (CART) peptides in the basolateral amygdala (BLA). The formation of context-drug associations produces either conditioned place preference (CPP) or conditioned place aversion (CPA).
Objectives
The aim of the present study was to determine whether intra-BLA infusions of CART 55–102 are either rewarding or aversive and modulate AMPH reward.
Materials and methods
Rats were implanted with bilateral cannulae in the BLA, were subjected to place conditioning, and were tested for CPP or CPA. Rats were conditioned with either intra-BLA infusions of artificial cerebral spinal fluid or one of three dose of CART 55–102 (1, 2, or 4 µg/side), intra-BLA infusions of a subrewarding dose of CART 55–102 (1 µg/side) plus injections of a subrewarding dose of AMPH (0.1 mg/kg, i.p.), or intra-BLA infusions of an aversive dose of CART 55–102 (4 µg/side) plus injections of a rewarding dose of AMPH (1.0 mg/kg, i.p.).
Results
Intra-BLA infusions of 2 µg/side CART 55–102 produced CPP, 4 µg/side produced CPA, and 1 µg/side produced neither CPP nor CPA. Intra-BLA infusions of a subrewarding dose of CART 55–102 (1 µg/side) plus injections of a subrewarding dose of AMPH (0.1 mg/kg, i.p.) produced CPP. Intra-BLA infusions of an aversive dose of CART 55–102 (4 µg/side) plus injections of a rewarding dose of AMPH (1.0 mg/kg, i.p.) produced neither CPP nor CPA.
Conclusions
Both the affective properties of intra-BLA CART 55–102 and its ability to either facilitate or block AMPH reward are dose dependent.
Keywords: Reward, Aversion, Amygdala, Associative learning, Peptide, Amphetamine
Introduction
Cocaine- and amphetamine-regulated transcript (CART) peptides function as neurotransmitters in the brain (Smith et al. 1997; Vicentic and Jones 2007). In the rat, the propeptide form of CART is processed into two biologically active fragments, 62–102 (CART 62–102) and 55–102 (CART 55– 102). CART peptides are widely distributed throughout the brain, and the CART signaling system plays multiple roles in the brain including the regulation of food intake, the stress response, anxiety and depression, and reward and addiction (see Rogge et al. 2008 for review).
The CART peptide signaling system plays a role in the rewarding and locomotor activating effects of psychostimulants. CART mRNA and peptide are expressed in brain areas involved in reward and locomotion such as the ventral tegmental area (VTA), the nucleus accumbens (NAc) shell, and the basolateral amygdala (BLA; Dallvechia-Adams et al. 2002; Fagergren and Hurd 1999; Koylu et al. 1998). Intra-VTA infusions of CART 55–102 increase locomotor activity and produce a conditioned place preference (CPP; Kimmel et al. 2000). Moreover, infusion of CART 55–102 into the NAc attenuated the locomotor activating effects of systemically administered cocaine and d-amphetamine (AMPH) yet had no effect on locomotor activity when administered alone (Jaworski et al. 2003). Finally, the findings that the acute locomotor activating effects of AMPH administration are attenuated and AMPH’s potency to produce a CPP was reduced in CART null mice suggest a modulatory role for CART peptides in the locomotor and affective properties of AMPH (Couceyro et al. 2005).
Place conditioning is a behavioral assay for measuring the rewarding or aversive aspects of natural stimuli and chemical agents (see Bardo and Bevins 2000; Tzschentke 1998 for review). In the place conditioning assay, an animal receives repeated pairings of a distinct contextual conditioned stimulus (CS) with a drug unconditioned stimulus (UCS). Intermixed with these CS–UCS pairings is similar exposure to another distinct contextual CS without the drug UCS (e.g., saline pairing). On the drug-free test day, exposure to the drug-paired contextual CS elicits approach or avoidance behavior. A significant increase in the time spent in the drug-paired context is referred to as a CPP and is interpreted as a rewarding effect of the drug. A significant decrease in the time spent in the drug-paired context is referred to as a conditioned place aversion (CPA) and is interpreted as an aversive effect of the drug. Place conditioning involves the formation of context-drug associations and requires memory consolidation. Repeated intermittent exposure to AMPH, such as that used to produce a CPP (Spyraki et al. 1982), also produces a progressive enhancement of motor behavior, termed motor sensitization (Segal and Mandell 1974). In the context of the present set of experiments, the place conditioning assay has two important limitations (see Bardo and Bevins 2000 for review). First, the application of the place conditioning procedure to pharmacological questions requiring full dose–effect curves is, at best, arduous. Second, it is not known if place conditioning is a valid procedure to measure reward or aversion in humans.
The BLA is involved in assigning affective value to stimuli (Cardinal et al. 2002; LeDoux 2000). Anatomically, the BLA is well positioned for appetitive and aversive associative learning. Afferents conveying information about the conditioned stimulus and the unconditioned stimulus from the VTA and substantia nigra (Asan 1997, 1998; Muller et al. 2009), cerebral cortex (Cechetto and Saper 1987; Fabri and Burton 1991; Mascagni et al. 1993; McDonald and Jackson 1987; Ottersen 1982; Turner and Herkenham 1991), thalamus (LeDoux et al. 1985, 1990, 1991; Turner and Herkenham 1991), and hippocampus (Canteras and Swanson 1992) converge on the BLA. Functional inactivation of the BLA disrupts contextual fear conditioning (Helmstetter and Bellgowan 1994; Muller et al. 1997) and blocks AMPH-induced CPP (Hsu et al. 2002). Thus, it appears that the BLA plays an important role in the formation of associations between environmental context and stimuli that produce either positive (e.g., the rewarding aspects of drugs) or negative (e.g., footshock) affect. In the present study, we used the place conditioning procedure to determine whether infusions of CART 55–102 into the BLA are either rewarding or aversive and whether they modulate AMPH reward.
Materials and methods
Subjects
Male Sprague Dawley rats (Harlan, Indianapolis, IN, USA), weighing 200–224 g (49–52 days old) at the start of the experiment, were housed in groups of three and allowed 1 week of habituation to the animal housing room prior to the beginning of the experiment. Animals were handled for 5 days before stereotaxic surgery. Food and water were available ad libitum in their home cages. Rats were maintained on a 12-h light–dark cycle with lights on at 0700 hours. All studies were carried out in accordance with the Declaration of Helsinki and with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) and were approved by the Institutional Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science.
Drugs
AMPH sulfate (Sigma, St. Louis, MO, USA) was dissolved in sterile 0.9% saline. Injections were administered at a volume of 1 ml/kg i.p. and doses refer to the drug base. Buprenorphine HCl (Buprenex, Bedford Laboratories, Bedford, OH, USA) was dissolved in sterile 0.9% saline and was administered s.c. at a volume of 1 ml/kg. CART 55–102 (American Peptide Company, Inc., Sunnyvale, CA, USA) was dissolved in artificial cerebral spinal fluid (aCSF; Harvard Apparatus, Holliston, MA, USA) as was used for intracranial delivery.
Place conditioning apparatus
Place conditioning studies were performed in a three-compartment apparatus (AccuScan Instruments, Inc., Columbus, OH, USA). The two larger, outer compartments (25×30×32 cm) were separated by a central compartment (10×25×32 cm) and differed in both visual and tactile cues. One outer compartment had white vertical lines on the walls and the other white horizontal lines. The compartments had floors with different textures. The central compartment had white walls and a Plexiglas floor and freely allowed movement between the two outer compartments unless barred by two white partitions, which restricted movement between compartments during conditioning sessions. Infrared sensors attached along all four sides of the place conditioning chamber recorded the movement and location of the animals. Conditioning and test sessions were conducted under conditions of dim illumination and in the presence of white noise.
Behavioral conditioning
Behavioral conditioning occurred according to published methods (Mucha et al. 1982; Rossi and Reid 1976) with modifications. Briefly, rats were placed into the center compartment and allowed free access to the entire apparatus for a 15-min pretest, approximately 72 h before conditioning. Four animals spent more than 70% of their time in either of the two outer compartments (i.e., exhibited a strong preexisting preference for one compartment over the other) and were excluded from the experiment. Animals that did not show a strong initial preference for one compartment over the other were randomly assigned to one of the treatment groups. Rats were randomly assigned to receive drug or vehicle in one or the other outer compartment. Approximately 72 h after the pretest, conditioning began and took place over a 5-day period (days 1–5). Conditioning sessions lasted for 45 min. In the first experiment, rats received an infusion of aCSF or one of three dose of CART 55–102 (1, 2, or 4 µg/side) into the BLA or the overlying caudate–putamen in one compartment on days 1, 3, and 5 and an infusion of aCSF (1 µl/side) into the BLA or overlying caudate–putamen in the other compartment on days 2 and 4. In the second experiment, animals received an intra-BLA infusion of CART 55–102 (1 µg/side) or aCSF (1 µl/side) and systemic administration of AMPH (0.1 mg/kg, i.p.) in one compartment on days 1, 3, and 5 and an intra-BLA infusion of aCSF (1 µl/side) and systemic administration of saline (1 ml/mg, i.p.) in the other compartment on days 2 and 4. In the third experiment, animals received an intra-BLA infusion of CART 55–102 (4 µg/side) and systemic administration of AMPH (1.0 mg/kg, i.p.) in one outer compartment on days 1, 3, and 5 and an intra-BLA infusion of aCSF (1 µl/side) and systemic administration of saline in the other outer compartment on days 2 and 4. In the third experiment, to determine whether the effects of intra-BLA infusions of CART 55–102 (4 µg/side) to modulate the rewarding effects of AMPH are due to the peptide acting on BLA circuitry rather than permanent damage to the BLA, rats that previously received intra-BLA infusions in the initial conditioning sessions were “reconditioned” (from days 9–13) and “retested” for CPP on day 16 in a drug- and vehicle-free state. These animals were randomly assigned to receive AMPH (1.0 mg/kg, i.p.) or saline (1 ml/kg, i.p.) in one or the other outer compartment. These animals received AMPH (1.0 mg/kg, i.p.) injections in one compartment on days 9, 11, and 13 without an intracerebral infusion. These same animals received saline (1 ml/kg, i.p.) injections in the other compartment on days 10 and 12 without an intracerebral infusion. In all experiments, the walls of the place conditioning apparatus were thoroughly washed and floors were replaced between each conditioning session. Approximately 72 h after the last conditioning session, drug-and vehicle-free animals were tested for CPP or CPA. For that test, rats were placed into the central compartment and the dividing partitions were removed; rats were then allowed to explore the entire place conditioning apparatus for 30 min. Time spent in each compartment was recorded and used to calculate CPP or CPA (i.e., time spent in the drug-paired compartment minus time spent in the vehicle-paired compartment during the place conditioning test). Horizontal locomotor activity was simultaneously recorded throughout conditioning and used to measure motor sensitization (i.e., locomotor activity on day 5 minus that on day 1 (initial conditioning); locomotor activity on day 13 minus that on day 9 (reconditioning)).
Surgical procedures for intracerebral implantation of guide cannulae
Surgical procedures occurred according to a published method (Shen et al. 2006) with modifications. Briefly, following 1 week of acclimation to the animal housing room, a total of 109 rats were anesthetized with equithesin (1% sodium pentobarbital, 4.25% chloral hydrate in 10% ethanol; 3 mg/kg, i.p.) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). After the skull was exposed, guide cannulae (26 gauge; Plastics One Inc., Roanoke, VA, USA) were lowered bilaterally into the BLA (2.8 mm posterior to bregma, 5.0 mm lateral to midline, 7.8 mm ventral to skull which is 1.0 mm above the BLA target; Paxinos and Watson 2007). For placement controls, cannulae were implanted dorsal to these stereotaxic coordinates (2.8 mm posterior to bregma, 5.0 mm lateral to midline, 5.0 mm ventral to skull which is 1.0 mm above the caudate– putamen target; Paxinos and Watson 2007). Stainless steel mounting screws and dental cement were used to secure the cannulae to the skull. The skin incision was closed with sutures and antibiotic ointment was applied. Animals received a postsurgery injection of buprenorphine (0.3 mg/kg, s.c.) to minimize postoperative discomfort. A dummy injector (33 gauge, which did not extend below the tip of the implanted cannulae) was inserted into each cannula. One week of recovery was allowed between the surgery and behavioral conditioning.
Intracerebral microinjection
Intracerebral microinjections occurred according to a published method (Shen et al. 2006) with modifications. During the last 3 days of the recovery period, the rats were acclimated to the intracerebral injection procedures as follows: The dummy cannulae were removed and 33-gauge infusion cannulae were inserted into the guide cannulae and left in place for 6 min. The infusion cannulae then were removed, dummy cannulae reinserted, and the rats returned to their home cage. For intracerebral injection, the infusion cannulae extended 1.0 mm below the BLA and caudate–putamen guide cannulae tips. The opposite end of each infusion cannula was connected to a 25-µl microsyringe (Stoelting Company, Wood Dale, IL, USA) via PE50 polyethylene tubing (Plastics One Inc., Roanoke, VA, USA). The microsyringes were placed into syringe pumps (Model 11 Pico Plus Syringe Pump, Harvard Apparatus, Holliston, MA, USA) set to deliver fluid at a rate of 0.2 µl/min. A total volume of 1.0 µl was delivered per side. One minute later (to allow for diffusion of the drug away from the tip of the infusion cannulae), the infusion cannulae were removed and dummy cannulae were replaced. In the first experiment, immediately after the intracerebral infusion, animals were placed into the conditioning apparatus. In the second and third experiments, the intracerebral infusion was followed by an i.p. injection of AMPH or saline. Following the i.p. injection, the rats were placed into the conditioning apparatus. Approximately 72 h after the last conditioning session, drug- and vehicle-free animals were tested for their preference or aversion.
Verification for cannulation site
After the completion of behavioral testing, rats were euthanized by carbon dioxide exposure. The brains were removed, fixed overnight in buffered 4% paraformaldehyde, sunk in 30% sucrose, and then cut in 40-µm coronal sections. Sections were mounted onto poly-l-lysine-coated slides (Polysciences, Inc., Warrington, PA, USA) then stained with cresyl violet. Intracerebral infusion sites (marked by a lesion produced by the infusion cannula) were verified using light microscopy and reconstructed onto a rat stereotaxic brain map (Paxinos and Watson 2007).
Statistics
Place conditioning and locomotor data were analyzed with two-way analysis of variance (ANOVA) with Bonferroni post hoc tests. The α level was set at 0.05. All data are represented as the mean±SEM.
Results
The place conditioning and locomotor effects of intra-BLA CART 55–102 administration
For animals that received intra-BLA infusions of aCSF (1 µl/side) or CART 55–102 (1 µg/side), there was no difference in the time spent in either compartment during the CPP test. Animals that received intra-BLA infusions of CART 55–102 (2 µg/side) spent more time in the CART 55–102-paired compartment than the aCSF-paired compartment during the place conditioning test (p<0.01; Fig. 1a). In contrast, animals that received intra-BLA infusions of CART 55–102 (4 µg/side) spent more time in the aCSF-paired compartment than the CART 55–102-paired compartment during the place conditioning test (p<0.01; Fig. 1a). Two-way ANOVA (drug treatment (aCSF, 1 µg/side CART 55–102, 2 µg/side CART 55–102, 4 µg/side CART 55–102)×conditioning compartment (aCSF-paired, CART 55–102-paired)) revealed a significant drug treatment×conditioning compartment interaction (F(3, 48)=15.580, p<0.0001). Infusions of CART 55–102 into the BLA prior to place conditioning had no effect on locomotor behavior on any of the conditioning days at any of the doses examined (Fig. 1b).
Fig. 1.
The effects of intra-BLA infusions of aCSF or CART (1, 2, 4 µg) on a time spent in each conditioning compartment during the place conditioning test and b locomotor activity on each of the conditioning days. Significant difference between time spent in the aCSF-paired and CART-paired compartment during the place conditioning test, **p< 0.01. Each bar in the graph represents the mean of six to nine rats; vertical lines represent SEM
The effects of intra-BLA administration of CART 55–102 on place conditioning and locomotor behavior are not due to effects in the overlying caudate–putamen
To determine the anatomical specificity of the effects of CART 55–102 to produce CPP or CPA, depending on dose, separate groups of animals received infusions of CART 55–102 (1, 2, 4 µg/side) into the overlying caudate–putamen (immediately dorsal to the BLA) on conditioning days 1, 3, and 5 and infusions of aCSF (1 µl/side) into the overlying caudate– putamen on conditioning days 2 and 4. For animals that received intracaudate–putamen infusions of CART 55–102, there was no difference in the amount of the time spent in either compartment during the place conditioning test. Infusions of CART 55–102 into the overlying caudate– putamen prior to place conditioning had no effect on locomotor behavior on any of the conditioning days at any of the doses examined.
The effects of intra-BLA administration of a dose of CART 55– 102 that does not produce CPP or CPA plus systemic administration of a dose of AMPH that does not produce CPP or CPA
To determine whether CART 55–102 has effects on BLA circuitry to facilitate AMPH reward, we first needed to identify a dose of AMPH that produced neither CPP nor CPA. Rats received intra-BLA infusions of aCSF prior to injections of a low dose of AMPH (0.1 mg/kg, i.p.). For these animals, there was no difference in the amount of the time spent in either compartment during the place conditioning test (Fig. 2a). A separate group of rats received intra-BLA infusions of a dose of CART 55–102 that produced neither CPP nor CPA (1 µg/side) prior to receiving injections of a dose of AMPH that produced neither CPP nor CPA (0.1 mg/kg, i.p.). Animals that received intra-BLA infusions of a dose of CART 55–102 that produced neither CPP nor CPA (1 µg/side) plus injections of a dose of AMPH that produced neither CPP nor CPA (0.1 mg/kg, i.p.) spent more time in the CART 55–102- and AMPH-paired compartment than the aCSF- and saline-paired compartment during the place conditioning test (p<0.01; Fig. 2a). For animals that received intra-BLA infusions of aCSF plus injections of saline (1 ml/kg, i.p.), there was no difference in the amount of the time spent in either compartment during the place conditioning test. Two-way ANOVA (drug treatment (aCSF+saline, CART 55–102+AMPH)×conditioning compartment (aCSF-and saline-paired, CART-, and AMPH-paired)) revealed a significant main effect of drug treatment (F(1, 48)=7.806, p<0.05). Intra-BLA infusions of aCSF prior to injections of a dose of AMPH that produced neither CPP nor CPA (0.1 mg/kg, i.p.) had no effect on locomotor behavior on any of the conditioning days (Fig. 2b). Intra-BLA infusions of a dose of CART 55–102 that produced neither CPP nor CPA (1µg/side) plus injections of a dose of AMPH that produced neither CPP nor CPA (0.1 mg/kg, i.p.) had no effect on locomotor behavior on any of the conditioning days (Fig. 2b). Finally, intra-BLA infusions of aCSF plus injections of saline (1 ml/kg, i.p.) had no effect on locomotor behavior on any of the conditioning days (Fig. 2b).
Fig. 2.
Effects of intra-BLA infusions either aCSF or a dose of CART 55–102 that does not produce CPP or CPA plus either saline or a dose of AMPH that does not produce CPP or CPA on a time spent in each conditioning compartment during the place conditioning test and b locomotor activity on each of the conditioning days. Significant difference between time spent in the aCSF- and saline-paired compartment and the CART 55–102- and AMPH-paired compartment during the place conditioning test, **p<0.01. Each bar in the graph represents the mean of seven to 12 rats; vertical lines represent SEM
Effects of intra-BLA administration of a dose of CART 55– 102 that produces a CPA plus systemic administration of a dose of AMPH that produces CPP
To determine whether CART 55–102 has effects on BLA circuitry to attenuate or block AMPH reward, rats received intra-BLA infusions of a dose of CART 55–102 that produced CPA (4 µg/side) prior to receiving injections of a dose of AMPH that produced CPP (1.0 mg/kg; a rewarding dose; Rademacher et al. 2006). For animals that received intra-BLA infusions of a dose CART 55–102 that produced CPA (4 µg/side) plus injections of a dose of AMPH that produced CPP (1.0 mg/kg, i.p.), there was no difference in the amount of the time spent in either compartment during the place conditioning test (Fig. 3a). For animals that received intra-BLA infusions of aCSF plus injections of saline, there was no difference in the amount of the time spent in either compartment during the place conditioning test. Intra-BLA infusions of CART 55–102 (4 µg/side) plus injections of AMPH (1.0 mg/kg, i.p.) prior to place conditioning increased locomotor behavior on conditioning days 1 (p<0.001), 3 (p<0.001), and 5 (p<0.001; Fig. 3b). For animals that received intra-BLA infusions of a dose of CART 55–102 that produced CPA (4 µg/side) plus injections of a dose of AMPH that produced CPP (1.0 mg/kg, i.p.), the number of infrared beam breaks was greater on conditioning day 5 than on conditioning day 1 (p<0.05). Two-way ANOVA (drug treatment (aCSF+saline, CART 55–102+AMPH) × conditioning days (days 1–5)) revealed a significant effect of drug treatment (F(1, 100)=84.590, p<0.0001), a significant effect of conditioning days (F(4, 100)= 13.260, p< 0.0001), and a significant drug treatment × conditioning days interaction (F(1, 100)= 13.900, p<0.0001).
Fig. 3.
Effects of intra-BLA infusions of a dose of CART 55–102 that produces CPA plus systemic administration of a dose of AMPH that produces CPP on a time spent in each conditioning compartment during the place conditioning test and b locomotor activity on each of the conditioning days. Significant difference in the number of infrared beam breaks between animals treated with aCSF plus saline compared to those treated with CART 55–102 (4 µg/side) plus AMPH (1.0 mg/kg, i.p.), ***p<0.001. Significant difference in the number of beam breaks on conditioning day 5 compared to conditioning day 1 for animals treated with CART 55–102 (4 µg/side) plus AMPH (1.0 mg/kg, i.p.), *p<0.05. Each bar in the graph represents the mean of nine to 13 rats; vertical lines represent SEM
The effect of a CPA-producing dose of CART 55–102 to block AMPH CPP is not due to permanent damage to the BLA
To determine whether the effect of intra-BLA infusions of an aversive dose of CART 55–102 to block AMPH CPP is due to the effects of the peptide acting on BLA circuitry rather than permanent damage to the BLA, rats that previously received intra-BLA infusions in the initial conditioning sessions were “reconditioned” (from days 9–13) and “retested” for CPP on day 16 in a drug- and vehicle-free state. These animals received AMPH (1.0 mg/kg, i.p.) injections in one compartment on days 9, 11, and 13 without an intracerebral infusion. These same animals received saline (1 ml/kg, i.p.) injections in the other compartment on days 10 and 12 without an intracerebral infusion. Animals that previously received intra-BLA infusions of a dose of CART 55–102 that produced CPA (4 µg/side) plus injections of a dose of AMPH that produced CPP (1.0 mg/kg, i.p.) and failed to express CPP on day 8 retained their capacity to form context-drug associations. After reconditioning, these animals spent more time in the AMPH-paired compartment than the saline-paired compartment during the place conditioning test (p<0.001; Fig. 4a). For animals that previously received intra-BLA infusions of aCSF plus injections of saline, there was no difference in the time spent in one or the other compartment during the place conditioning test after reconditioning with saline. Two-way ANOVA (drug treatment (saline, 1.0 mg/kg AMPH) × conditioning compartment (saline-paired, AMPH-paired)) revealed a significant effect of drug treatment (F(1, 28)= 14.540, p<0.001) and a significant drug treatment × conditioning compartment interaction (F(1, 28)=9.089, p<0.01). For animals with a history of intra-BLA infusions of aCSF or a dose of CART 55–102 that produced CPA (4 µg/side) plus either saline or a dose of AMPH that produced CPP (1.0 mg/kg, i.p.) that were reconditioned with a dose of AMPH that produced CPP (1.0 mg/kg, i.p.) or saline, injections of a dose of AMPH that produced CPP increased locomotor behavior on conditioning days 9 (p<0.001), 11 (p<0.001), and 13 (p< 0.001; Fig. 4b). For animals that received an injection of AMPH on days 9, 11, and 13, the number of infrared beam breaks was greater on conditioning day 13 than on conditioning day 9 (p<0.01). Two-way ANOVA (drug treatment (saline, AMPH) × conditioning days (days 9–13)) revealed a significant effect of drug treatment (F(1, 95)= 198.500, p<0.0001), a significant effect of conditioning days (F(4, 95)=21.790, p<0.0001), and a significant drug treatment× conditioning days interaction (F(4, 95)=28.720, p<0.0001).
Fig. 4.
The effect of injections of either saline or AMPH (1.0 mg/kg) on a time spent in each conditioning compartment during the place conditioning test and b locomotor activity on each of the conditioning days for rats that previously received intra-BLA infusions of a dose of CART 55–102 that produces CPA plus systemic administration of a dose of AMPH that produces CPP. Significant difference between the time spent in the saline-paired and the AMPH-paired compartment during the place conditioning test, ***p<0.001. Significant difference in the number of infrared beam breaks between animals reconditioned with a rewarding dose of AMPH (1.0 mg/kg, i.p.) compared to animals reconditioned with saline, ***p<0.001. Significant difference in the number of infrared beam breaks on conditioning day 13 compared to conditioning day 9 for animals reconditioned with AMPH (1.0 mg/kg, i.p.), **p<0.01. Each bar in the graph represents the mean of nine to 13 rats; vertical lines represent SEM
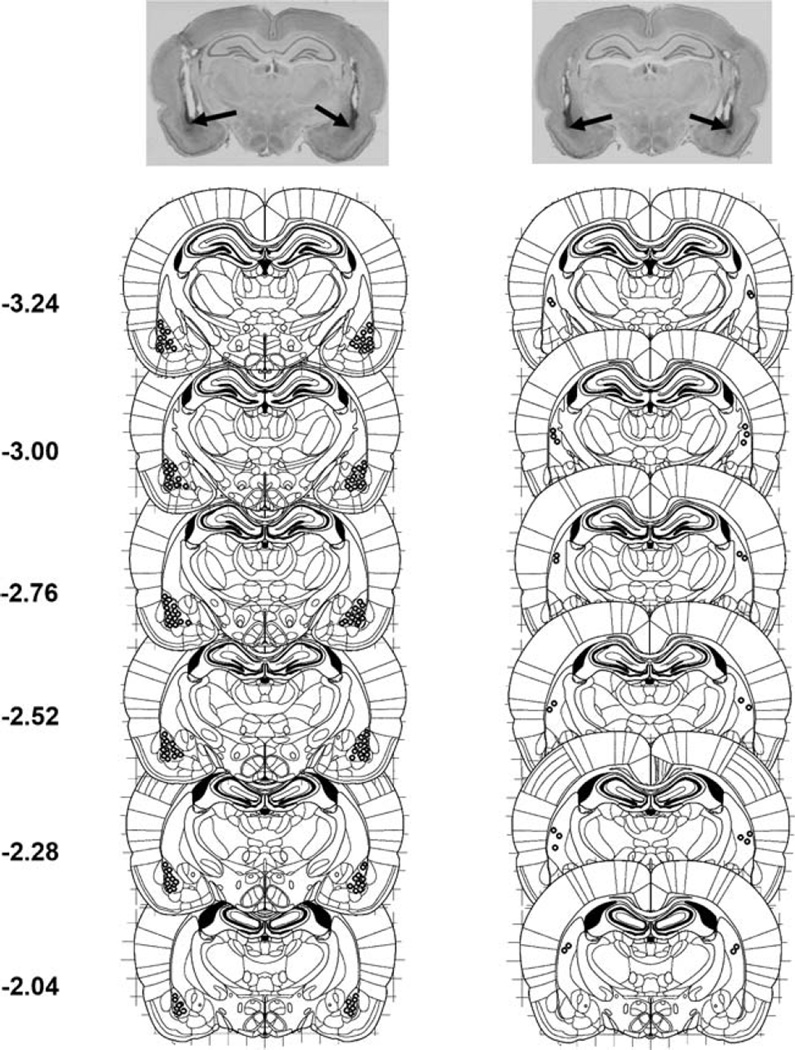
Histology
The location of the infusion sites (in the BLA or the overlying caudate–putamen) is shown for all subjects (Fig. 5). Numbers to the left of the panels indicate millimeters from the bregma.
Fig. 5.
Histological verification of the infusion sites. Photomicrographs of Nissl-stained coronal sections showing the representative placements for the bilateral BLA infusion sites (indicated by the arrows). Open circles mark the most ventral sites of the infusions for each targeted brain structure. Infusion sites in the BLA are illustrated in the column to the left; caudate–putamen sites are illustrated in the column to the right. Numbers to the left of the panels indicate millimeters from the bregma. Stereotaxic maps were reproduced, with permission from Elsevier Science, from a brain atlas (Paxinos and Watson 2007)
Discussion
In the present study, the place conditioning procedure was used to determine the affective aspects of intra-BLA infusions of CART 55–102. We found that whether intra-BLA infusions of CART 55–102 were rewarding, aversive, or neither rewarding nor aversive depended on dose. Intra-BLA infusions of 2 µg/side CART 55–102 produced CPP, 4 µg/side produced CPA, and 1 µg/side produced neither CPP nor CPA, findings consistent with the prevailing view that the BLA plays an important role in the formation of associations between environmental stimuli and both positive and negative affective states (Cardinal et al. 2002; LeDoux 2000). Importantly, the finding that intracaudate– putamen infusions of CART 55–102 (1, 2, 4 µg/side) produced neither CPP nor CPA excludes the possibility that the affective aspects of intra-BLA CART are due to effects in the overlying caudate–putamen and/or cerebral cortex. The findings that infusion of aCSF or CART 55–102 into the BLA prior to place conditioning had no effect on locomotor behavior on any of the conditioning days and at any of the doses, had no effect on the acute locomotor activating effects of AMPH, and had no effect on the ability of repeatedly administered AMPH to produce motor sensitization were anticipated since the BLA does not seem to play an important role in the locomotor activating properties of psychostimulants (Koob and Swerdlow 1988).
If the CART peptide signaling system mediates or modulates the rewarding effects of psychostimulants, as has been proposed (see Jaworski and Jones 2006 for review), then intra-BLA administration of CART 55–102 should facilitate, block, or attenuate AMPH reward. Intra-BLA administration of a subrewarding dose of CART 55– 102 (1 µg/side) combined with systemic administration of a subrewarding dose of AMPH (0.1 mg/kg, i.p.) produced CPP. Thus, CART 55–102, at a subrewarding dose, acted on BLA circuitry to facilitate AMPH reward. Additionally, intra-BLA administration of an aversive dose of CART 55– 102 (4 µg/side) combined with systemic administration of a rewarding dose of AMPH (1.0 mg/kg, i.p.) produced neither CPP nor CPA. Thus, CART 55–102, at an aversive dose, acted on BLA circuitry to block AMPH reward. Importantly, the effect of an aversive dose of CART 55–102 to block AMPH CPP cannot be due to permanent damage to the BLA since rats that had a history of receiving intra-BLA infusions in the initial conditioning sessions and were subsequently reconditioned and retested exhibited a robust AMPH-induced CPP.
Although the cellular mechanisms that underlie the ability of CART 55–102 to produce reward or aversion, depending on dose, and modulate AMPH reward are unknown, there is evidence for CART secreting neurons in the BLA (Koylu et al. 1998) as well as evidence that supports the existence of an inhibitory G protein-coupled CART receptor (Jones and Kuhar 2008; Lakatos et al. 2005; Yermolaieva et al. 2001). In addition, CART 55–102 has been shown to inhibit voltage-gated calcium channels in hippocampal neurons (Yermolaieva et al. 2001), increase the phosphorylation of cyclic AMP response element binding protein in hypothalamic neurons (Sarkar et al. 2004), and increase the phosphorylation of extracellular signal-regulated kinase (Lakatos et al. 2005). Finally, CART peptides have been found in dense core vesicles in axon terminals that formed symmetrical synapses, a finding that suggests that, at least for the primate NAc, CART peptides may be coreleased with GABA (Smith et al. 1997). Future research is aimed at determining which of these cellular mechanisms underlies the pattern of results reported herein.
The BLA is activated by emotionally salient stimuli (Cahill and McGaugh 1998; LeDoux 1993; Rosenkranz et al. 2003) and plays an important role in the formation of associations between environmental stimuli and both positive and negative affective states (Cardinal et al. 2002; LeDoux 2000). The BLA projects heavily to the NAc core, a brain region well positioned to integrate information derived from limbic structures (Mogenson et al. 1980; Brady and O’Donnell 2004) and facilitate the most appropriate motor output (e.g., approach or avoidance). Thus, the ability of intra-BLA infusions of CART 55–102 to produce either reward or aversion and either facilitate or block AMPH reward might be understood in terms of CART 55–102 acting on BLA circuitry to disrupt the balance between excitatory and inhibitory influences on NAc neurons. When interpreted in the context of the NAc activity hypothesis (Carlezon and Thomas 2009), intra-BLA infusions of CART 55–102 (2 µg/side) produced reward and intra-BLA infusions of a subrewarding dose of CART 55–102 (1 µg/side) interacted with a subrewarding dose of AMPH (0.1 mg/kg, i.p.) to produce reward because, in both cases, they decreased activity in the amygdalo-accumbens pathway, thereby reducing the inhibitory influence of the NAc on downstream reward pathways. In contrast, intra-BLA infusions of a higher dose of CART 55–102 (4 µg/side) produced aversion and blocked the rewarding effects of AMPH because, in both cases, they increased activity in the amygdalo-accumbens pathway, thereby increasing the inhibitory influence of the NAc on downstream reward pathways.
The results of the present study showing that intra-BLA infusions of CART 55–102 (4 µg/side) produced CPA and blocked AMPH CPP may have been due to the anxiogenic effects of CART 55–102. Although anxiety-like behaviors were not measured in the present study, our findings are consistent with a report that administration of CART 55– 102 i.c.v. produced anxiogenic effects in both the elevated plus maze task and social interaction test; these effects were attenuated by a corticotropin-releasing factor (CRF) 1 receptor antagonist (Chaki et al. 2003; Chaki et al., unpublished data). CRF neurons act as the primary mediator of anxiety-provoking stimuli in the mammalian brain. Although there is no evidence of colocalization of CART 55–102 and CRF, CART peptides are found in the BLA (Koylu et al. 1998), and its effects on BLA circuitry could regulate CRF release in the hypothalamus. Accordingly, it is known that the BLA projects to the major output nucleus of the amygdala, the central nucleus, via glutamatergic pyramidal cells (Paré et al. 1995). The central nucleus of the amygdala is able to control the neuroendrocrine zone of the hypothalamus via direct projections to the hypothalamus or indirectly through strong projections to the bed nucleus of the stria terminalis, which also innervates hypothalamic nuclei (see Sah et al. 2003 for review). Another mechanism by which CART 55–102 may mediate aversive states such as anxiety is through interactions with the endocannabinoid (eCB) signaling system in the BLA. There is a great deal of evidence implicating the eCB signaling system in aversive states such as anxiety. For example, cannabinoid receptors (CB1) are densely expressed in brain areas important for anxiety and emotional learning including the BLA (Katona et al. 2001) and stimuli that produce aversive states modify eCB signaling in the BLA (Rademacher et al. 2008). CB1 receptor null mice exhibit increased anxiety-like behaviors (Haller et al. 2002) and the inhibition of the catabolism of the eCB, anandamide, produces an anxiolytic effect (Kathuria et al. 2003). Moreover, CART has been implicated as a downstream mediator of eCBs. In support of this idea, CB1 null mice show reduced CART mRNA levels, and CB1 receptor mRNA is colocalized with CART (Cota et al. 2003, Osei-Hyiaman et al. 2005). Moreover, administration of CB1 receptor antagonists, which are known to inhibit food intake in wild-type mice, has no effect in CART-deficient mice (Osei-Hyiaman et al. 2005).
Although the results of the present study suggest that intra-BLA infusions of CART 55–102 produced either reward or aversion (depending on the dose) and either facilitated or blocked AMPH reward (depending on the dose), alternative explanations cannot be ruled out. For example, we cannot exclude the possibility that CART 55–102 and AMPH instigated different affective processes in different anatomical substrates. According to this view, the rats’ preferences are determined by the algebraic sum of these two processes. Moreover, it is known that the BLA is involved in the memory process required for the acquisition and expression of a place preference (Everitt et al. 1991; Fuchs et al. 2002; Hiroi and White 1991; Hsu et al. 2002; Schroeder and Packard 2002). Thus, it is possible that intra-BLA CART 55–102 modulated context-drug memories rather than producing an affective process that interacts with that of AMPH. In this view, intra-BLA infusions of CART 55–102 (1 µg/side) facilitated the expression of a CPP by strengthening a weak context-drug memory produced by systemic administration of a low dose of AMPH (0.1 mg/kg). In contrast, intra-BLA infusions of CART 55–102 (4 µg/side) impeded the expression of a CPP by interfering with the formation of a strong context-drug memory that regularly is produced by systemic administration of a higher dose of AMPH (1.0 mg/kg). Finally, we cannot exclude the possibility that intra-BLA CART 55–102 could have affective properties on its own and modulate the memory for a context paired with AMPH. Future research is aimed at determining if CART 55–102 has an effect on the formation of context-drug memories.
There are some important limitations to the present study. First, place measurements were the sole indices of reward and aversion. The results of the present study would be complemented by the use of other tests that reflect reward and aversion such as the combination of intracranial self-stimulation with a “curve-shift” analysis (Carlezon and Chartoff 2007). Second, it is difficult to apply the place conditioning procedure to produce full dose–effect curves (see Bardo and Bevins 2000 for review). Since each point on the dose–effect curve requires an independent group of animals, a large number of animals would be required to produce graded dose–effect curves. In contrast, the drug self-administration procedure is much more amenable to the production of graded dose–effect curves and could be used in future studies to fully characterize the relationship between the dose of CART 55–102 and affect. Third, the rats received three exposures to the drug-paired compartment and two exposures to the vehicle-paired compartment. Since these animals did not receive equal exposure to the two compartments, we cannot exclude the possibility that the results reported herein are due to differences in novelty and recency of exposure.
In summary, we have used the place conditioning procedure to demonstrate that intra-BLA infusions of CART 55–102 can be rewarding, aversive, or neither rewarding nor aversive depending on dose. In addition, intra-BLA infusions of CART 55–102 are able to either facilitate or block AMPH reward depending on dose. Importantly, these effects are not due to either CART 55–102 acting in the overlying caudate– putamen and/or cerebral cortex or permanent damage to the BLA. Future research is needed to elucidate the mechanism(s) underlying these effects.
Acknowledgments
The authors thank Philip Dougherty and IhteshamUr Rahman for technical assistance. This research was supported by National Institute on Drug Abuse grant DA015513.
Footnotes
Conflict of interest The authors have no actual or potential conflict of interest in relation to this article.
References
- Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288:449–469. doi: 10.1007/s004410050832. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Brady AM, O’Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chaki S, Kawashima N, Suzuki Y, Shimazaki T, Okuyama S. Cocaine- and amphetamine-regulated transcript peptide produces anxiety-like behavior in rodents. Eur J Pharmacol. 2003;464:49–54. doi: 10.1016/s0014-2999(03)01368-2. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, White FJ, Douglass J, Richards WG, Bannon AW. Cocaine- and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005;315:1091–1100. doi: 10.1124/jpet.105.091678. [DOI] [PubMed] [Google Scholar]
- Dallvechia-Adams S, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: colocalization with gamma-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. J Comp Neurol. 2002;448:360–372. doi: 10.1002/cne.10268. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Fabri M, Burton H. Ipsilateral cortical connections of primary somatic sensory cortex in rats. J Comp Neurol. 1991;311:405–424. doi: 10.1002/cne.903110310. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Hurd YL. Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport. 1999;10:3449–3452. doi: 10.1097/00001756-199911080-00034. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;19:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The lateral nucleus of the amygdala mediates the expression of the amphetamine-produced conditioned place preference. J Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu EH, Schroeder JP, Packard MP. The amygdala mediates memory consolidation for an amphetamine conditioned place preference. Behav Brain Res. 2002;129:93–100. doi: 10.1016/s0166-4328(01)00376-x. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Jones DC. The role of CART in the rewarding/reinforcing properties of psychostimulants. Peptides. 2006;27:1993–2004. doi: 10.1016/j.peptides.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jones DC, Kuhar MJ. CART receptor binding in primary cell cultures of the rat nucleus accumbens. Synapse. 2008;62:122–127. doi: 10.1002/syn.20476. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55–102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294(2):784–792. [PubMed] [Google Scholar]
- Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system. Ann N Y Acad Sci. 1988;537:216–227. doi: 10.1111/j.1749-6632.1988.tb42108.x. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384:198–202. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett. 1991;134:139–144. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1993;57:697–715. doi: 10.1016/0306-4522(93)90016-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of pyramidal cells in the rat basolateral amygdala. Brain Struct Funct. 2009;213:275–288. doi: 10.1007/s00429-008-0196-y. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Depetrillo M, Harvey-White J, Bannon AW, Cravatt BF, Kuhar MJ, Mackie K, Palkovits M, Kunos G. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81:273–282. doi: 10.1159/000087925. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y, Paré JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience. 1995;69:567–583. doi: 10.1016/0306-4522(95)00272-k. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th. New York: Academic; 2007. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith ME. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus– reward associations. Eur J Neurosci. 2006;24:2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi NA, Reid LD. Affective states associated with morphine injections. Physiol Psychol. 1976;4:535–538. [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Wittman G, Fekete C, Lechan RM. Central administration of cocaine- and amphetamine-regulated transcript increases phosphorylation of cAMP response element binding protein in corticotrophin-releasing hormone-producing neurons but not in prothyrotropin-releasing hormone-producing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;999:181–192. doi: 10.1016/j.brainres.2003.11.062. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Posttraining intra-basolateral amygdala scopolamine impairs food- and amphetamine-induced conditioned place preferences. Behav Neurosci. 2002;116:922–927. doi: 10.1037//0735-7044.116.5.922. [DOI] [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Shen F, Meredith GE, Napier TC. Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci. 2006;26:11041–11051. doi: 10.1523/JNEUROSCI.2898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Koylu EO, Couceyro P, Kuhar MJ. Ultrastructural localization of CART (cocaine- and amphetamine-regulated transcript) peptides in the nucleus accumbens of monkeys. Synapse. 1997;27:90–94. doi: 10.1002/(SICI)1098-2396(199709)27:1<90::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Dopaminergic substrates of amphetamine-induced place preference conditioning. Brain Res. 1982;253:185–193. doi: 10.1016/0006-8993(82)90685-0. [DOI] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Jones DC. The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J Pharmacol Exp Ther. 2007;320:499–506. doi: 10.1124/jpet.105.091512. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltagegated Ca2+ signaling in hippocampal neurons. J Neurosci. 2001;21:7474–7480. doi: 10.1523/JNEUROSCI.21-19-07474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]