Abstract
The cysteine protease cathepsin C (CatC) activates granule-associated proinflammatory serine proteases in hematopoietic precursor cells. Its early inhibition in the bone marrow is regarded as a new therapeutic strategy for treating proteolysis-driven chronic inflammatory diseases, but its complete inhibition is elusive in vivo. Controlling the activity of CatC may be achieved by directly inhibiting its activity with a specific inhibitor or/and by preventing its maturation. We have investigated immunochemically and kinetically the occurrence of CatC and its proform in human hematopoietic precursor cells and in differentiated mature immune cells in lung secretions. The maturation of proCatC obeys a multistep mechanism that can be entirely managed by CatS in neutrophilic precursor cells. CatS inhibition by a cell-permeable inhibitor abrogated the release of the heavy and light chains from proCatC and blocked ∼80% of CatC activity. Under these conditions the activity of neutrophil serine proteases, however, was not abolished in precursor cell cultures. In patients with neutrophilic lung inflammation, mature CatC is found in large amounts in sputa. It is secreted by activated neutrophils as confirmed through lipopolysaccharide administration in a nonhuman primate model. CatS inhibitors currently in clinical trials are expected to decrease the activity of neutrophilic CatC without affecting those of elastase-like serine proteases.
Keywords: cysteine protease, inflammation, neutrophil, protease, protease inhibitor, serine protease
Introduction
Cathepsin C (CatC)3 (EC 3.4.14.1), also known as dipeptidyl peptidase I, is a member of cysteine cathepsins, which are lysosomal proteases belonging to the C1 family of papain-like cysteine peptidases (1–3). Cysteine cathepsins are synthetized as proteolytically inactive zymogens bearing a N-terminal removable propeptide (4, 5). CatC is synthesized as a 60-kDa zymogen that undergoes proteolysis at several sites to release an internal propeptide. The resulting mature CatC monomer is made up of 3 tightly linked subunits, a 16-kDa N-terminal exclusion domain, a 23-kDa catalytic heavy chain, and a 7.5-kDa light chain that are held together by non-covalent interactions (2). The relevant protease(s) responsible for the physiological maturation of proCatC have not yet been identified in humans. Human recombinant cathepsins L (CatL) and S (CatS) activate purified proCatC (6) in vitro, whereas mature CatC is still produced in CatL/S double-deficient mice (7).
The crystal structure of mature human CatC is a compact tetramer (2). Each monomer bears an active site and consists of a heavy and a light chain with a papain-like structure (2). The exclusion domain built on the papain-like structure is responsible for the aminopeptidase activity of CatC, as it blocks the protease active site beyond the S2 pocket (2). CatC has wide substrate specificity (8). It is most active at a slightly acidic pH (9) and is activated by Cl− ions in a pH-dependent manner (10). Gly-Phe-AMC and Gly-Arg-AMC are the substrates most commonly used to measure its activity in biological samples.
High concentrations of CatC and its mRNA are found in the spleen, liver, kidneys, and lungs (11) and in hematopoietic cells (12). Mast cells and cytotoxic T lymphocytes chemically activated with a calcium ionophore in vitro secrete CatC into the extracellular milieu, suggesting that the enzyme is also released from intracellular stores together with other granule-associated proteins (13, 14). The secretion of CatC by neutrophils and macrophages in response to external signals has not been experimentally demonstrated (14).
The best characterized physiological function of CatC is the processing of granule-associated serine proteases including human neutrophil elastase, proteinase 3 (PR3), cathepsin G (CatG), mast cell chymase, and lymphocyte granzymes A and B (1, 15) in immune defense cells. These serine proteases from immune cells can also participate in the progression of a variety of inflammatory diseases including those that affect the respiratory system. The removal of a N-terminal dipeptide by CatC has been confirmed in vivo using CatC−/− mice (15). In humans, the loss of CatC function is responsible for the Papillon-Lefèvre syndrome (16). This disorder is characterized by hyperkeratosis of the palms of the hands and soles of the feet together with chronic inflammation of the gingiva, which impairs the development of permanent teeth (17, 18). The lack of CatC activity in these patients blocks almost completely the activity of neutrophil serine proteases (NSPs) and reduces the level of their zymogen in neutrophils (19, 20). Neutrophils are retained in the blood, but their bactericidal activity is not affected (19–22).
Several attempts have been made to design and synthesize selective and non-toxic inhibitors of CatC. Most of the reported inhibitors are based on the preferred dipeptide substrates bearing either irreversible or reversible electrophilic groups. In the past years dipeptidyl nitriles have attracted great attention due to their strong inhibitory activity against human CatC (23, 24). Inactivation of CatC in the bone marrow of rats by treating them with a reversible cyclopropyl nitrile inhibitor for 2 weeks at doses that resulted in the nearly complete inhibition of CatC only partially prevents activation of NSPs (25). Thus, a continuous very high degree of CatC inhibition is required to effectively block the maturation of downstream elastase-like NSPs in vivo (25, 26). These genetic and pharmacological data support the hypothesis that CatC is a therapeutic target for the treatment of several inflammatory and autoimmune diseases affecting the respiratory systems (23, 27).
The inhibition of CatC and its impact on NSP activity have not been investigated in human neutrophilic precursors although NSPs are produced during the myeloblastic and promyelocytic stages of neutrophil maturation (28). In this work we have investigated the maturation of the CatC zymogen in the human neutrophilic precursors PLB-985 (human myelomonoblastic precursor cells) and HL-60 (human promyelocytic precursor cells) to identify the protease(s) involved in this process and evaluate the degree of CatC inhibition required to achieve a near complete inhibition of elastase-like proteases in human neutrophilic precursors. We then looked for the presence of CatC in the lung secretions of nonhuman primates with lung inflammation and in patients with neutrophilic inflammation.
Experimental Procedures
Materials
Mature human CatC and PR3 were from Unizyme Laboratories (Hørsholm, Denmark) and Athens Research & Technology (Athens, GA), respectively. Murine anti-human CatC (Ab1) directed against the heavy chain of CatC, rabbit anti-human golgin-84, and murine anti-His6-tag antibodies were provided by Santa Cruz Biotechnology (Heidelberg, Germany). Goat anti-CatC (Ab2) directed against the propeptide was obtained from R&D Systems Europe (Lille, France). The mouse anti-human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was obtained from GeneTex (Irvine, CA). Human CatS was from Millipore (Molsheim, France). Human CatL and polyclonal anti-CatL and anti-CatS antibodies were obtained from R&D Systems Europe. Rabbit anti-PR3 was supplied by Abcam (Cambridge, UK). The fluorogenic substrate Gly-Phe-AMC and the inhibitor Gly-Phe-CHN2 were from MP Biomedicals (Illkirch, France). E64c ((2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane) and E64d (2S,3S-trans-(ethoxycarbonyloxirane-2-carbonyl)-l-leucine-(3-methylbutyl) amide) were from Santa Cruz Biotechnology. The fluorogenic substrates Abz-VADnVADYQ-EDDnp/Abz-TPFSGQ-EDDnp were from Genecust (Dudelange, Luxembourg). The calcium ionophore A23187 was supplied by Sigma.
In Vitro Processing of ProCatC by CatS
Human proCatC was produced by transient transfection of human embryonic kidney (HEK293) cells using a synthetic cDNA for CatC and was purified from serum-free culture supernatants by nickel affinity chromatography. Purified human recombinant proCatC-His6 (1–5 μm) was incubated with human CatS (0.2 μm) at 37 °C for increasing time periods in the presence or absence of a CatC inhibitor (ICatC, (S)-2-amino-N-((1R,2R)-1-cyano-2-(4′-(4-methylpiperazin-1-ylsulfonyl) biphenyl-4-yl)cyclopropyl)butanamide, from J. Pedersen) (2 μm). The reaction was made in 20 mm acetic acid, 150 mm NaCl, 10 mm DTT, and 1 mm EDTA, pH 4.5. The progress of the maturation reaction was analyzed by immunochemical detection of CatC.
Cell Culture and Induction of Cell Differentiation
Human myeloid leukemia cells (PLB-985; a generous gift from M. A. Gougerot-Pocidalo and E. Pedruzzi, IB2M INSERM, CHU Bichat, Paris, France) were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 50 units/ml penicillin, and 50 μg/ml streptomycin at 37 °C until the cell density reached 2 × 105/ml. They were then transferred to RPMI 1640 medium supplemented with 0.5% dimethylformamide (DMF) and 5% FCS where they underwent granulocytic differentiation. This medium was changed every 3 days. Granulocytic differentiation was checked on day 6 by analyzing the CD11b on the cell surface by flow cytometry (Coriwa Epics Elite ESP flow cytometer equipped with a 488-nm argon laser). Cells (3 × 106 in 100 μl) were incubated with 20 μl of anti-CD11b phycoerythrin mAbs (BD Biosciences) for 30 min at 4 °C, and cells were collected by centrifugation at 500 × g for 5 min. The resulting pellets were washed twice and suspended in phosphate-buffered saline (PBS) for flow cytometry. The forward and side scattering of each sample was measured for at least 10,000 events. Controls were cells incubated with 20 μl of mAbs anti-IgG1 phycoerythrin.
HL-60 cells (a generous gift from Dr S. Chollet-Martin, INSERM UMRS-996, CHU Bichat, Paris, France) were cultured in RPMI 1640 medium supplemented with 10% FCS, 50 units/ml penicillin, and 50 μg/ml streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
A549 human lung epithelial cells from a bronchioloalveolar carcinoma were cultured in RPMI-Glutamax I containing 0% or 10% heat-inactivated FCS, 0.1 mg/ml streptomycin, and 100 units/ml penicillin at 37 °C in a humidified atmosphere containing 5% CO2. BEAS-2B cells, isolated from normal human epithelium after autopsy of non-cancerous individuals and immortalized by viral transformation (CRL-9609) (ATCC, LGC Promochem, Molsheim, France), were maintained in F12-K nutrient mixture supplemented with 10% heat-inactivated FCS, 1% l-glutamine, 10 mm HEPES (all from Gibco), 0.1 mg/ml streptomycin, and 100 units/ml penicillin at 37 °C in a humidified atmosphere containing 5% CO2. Cell viability was evaluated by the MTS cell proliferation colorimetric assay (Promega, Charbonnières-les-Bains, France) following the manufacturer's instructions.
Inhibition of Intracellular Proteases
PLB-985 and HL-60 cells (3 × 106 cells) were cultured for 48 h and treated as required with E64c (100 μm) or E64d (100 μm). Continuous inhibition of CatS/CatC was maintained in 1-week cell cultures (starting at 2 × 105 cells) with or without the CatS inhibitor (29) (ICatS, N-(1-cyanocyclopropyl)-3-((2,3-difluorobenzyl)sulfonyl)-2-((2,2,2-trifluoro-1-(4-fluorophenyl)ethyl)amino)propanamide) (1–10 μm) and/or the CatC inhibitor (ICatC) (0.01 to 2 μm), changing the culture medium every day. The precursor cells were incubated with non-cytotoxic nitrile inhibitors for up to 1 week to allow a complete turnover of stored and premade active proteases and de novo protease synthesis (26).
Confocal Microscopy
PLB-985 cells were fixed with 3.7% formaldehyde and permeabilized by incubation in PBS, 0.05% Tween for 15 min. They were then incubated for 30 min in PBS, 10% bovine serum albumin (BSA) at room temperature and then for 30 min with murine anti-human CatC mAb (Ab1 diluted 1:40 in PBS, 10% BSA) or rabbit anti-human golgin-84 mAb (diluted 1:200 in PBS, 10% BSA). They were then washed in PBS and incubated with Alexa Fluor 647-conjugated goat anti-rabbit or fluorescein-goat anti-mouse IgGs for 30 min at room temperature. Finally, cells were layered on a slide using a Cytospin 4 centrifuge (Thermo Fisher Scientific, IL) and analyzed using a Olympus Fluoview 500 confocal laser scanning microscope.
Activation of PLB-985 Cells and Purified Human Blood Neutrophils
Suspensions of undifferentiated or differentiated PLB-985 cells (10 × 106 in 1 ml of 10 mm PBS, 1 mm MgCl2, and 1 mm CaCl2, pH 7.4) were activated by incubation with calcium ionophore A23187 (5 μm final) for 30 min at 37 °C. The activity of CatC in the cell-free supernatants was monitored spectrofluorometrically at 460 nm using the Gly-Phe-AMC substrate.
Neutrophils were purified from fresh whole blood taken from healthy volunteers, and the cell purity was checked by flow cytometry (30). The monocyte/lymphocyte fraction was also recovered. All cells were counted and kept in PBS at room temperature. Purified neutrophils (4 × 106 in 500 μl of 10 mm PBS, 1 mm MgCl2, and 1 mm CaCl2) were activated by incubating them with calcium ionophore A23187 (1 μm final) for 15 min at 37 °C. These activated cells were collected by centrifugation at 500 × g for 10 min; the CatC in the supernatants was analyzed by Western blots (WB), and the CatC activity in the cells was analyzed by enzyme assay.
Collection and Treatment of Bronchoalveolar Lavage Fluids and Sputum Samples
The samples of bronchoalveolar lavage fluids (BALF) and sputa were the residual material collected from patients hospitalized at the Department of Pneumology of the Centre Hospitalier Régional Universitaire of Tours during the course of their clinical investigation program. All patients gave their informed consent, in accordance with the Helsinki Declaration (2000) of the World Medical Association.
BALF were centrifuged at 1000 × g for 10 min and then at 3500 × g for 15 min. Supernatants were concentrated (30×) on Vivaspin (filtration threshold: 10 kDa, Sartorius Stadium, Aubagne, France) and then stored at −80 °C. Homogenized sputa from patients with cystic fibrosis (CF) or asthma were centrifuged at 10,000 × g for 10 min, and the supernatants were concentrated 20-fold.
Synthesis of the Nitrile Inhibitors l-Thi-l-Phe-CN, l-Thi-d-Phe-CN, and d-Thi-l-Phe-CN
All diastereoisomers of β-(2-thienyl)-alanyl-phenylalanine nitrile were synthesized by the coupling of either R- or S-2-amino-3-phenyl-propionitrile to Boc-protected d- or l-β-(2-thienyl)-alanine (Thi). The synthesis was accomplished by applying in solution coupling of Boc-Thi or Boc-d-Thi (0.8 eq) with the aminonitrile (1 eq) in either l or d configuration in the presence of the coupling agent TBTU (O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate) (0.96 eq) and DIPEA (N,N-diisopropylethylamine) (3.6 eq) in 5 ml of dichloromethane. It was followed by removal of the Boc protection group using 50% trifluoroacetic acid (TFA) in dichloromethane. The final products were purified by reverse-phase high performance liquid chromatography (RP-HPLC) equipped with a semipreparative column C8 Supelco Wide Pore (Sigma) using the linear gradient 25–45% B in 25 min and then lyophilized from tert-butanol to yield a white solid. TFA·l-Thi-l-Phe-CN: yield, 30%; MS-electrospray ionization, [M+H]+ = 300.1 (calculated, 300.4), [M+Na]+ = 322.1 (calculated 322.4); HPLC, tR = 18.0 min. TFA·l-Thi-d-Phe-CN: yield, 47%; MS-MALDI-TOF, [M+H]+ = 300.1 (calculated, 300.4); HPLC, tR = 18.9 min. TFA·d-Thi-l-Phe-CN: yield, 56%; MS-MALDI-TOF, [M+H]+ = 300.1 (calculated, 300.4); HPLC, tR = 18.6 min.
Measurements of Protease Activities in Cell Lysates and Lung Secretions
PLB-985, HL-60, A549, and BEAS-2B cells were lysed in PBS containing 0.5% Nonidet P-40. Purified neutrophils were lysed in 50 mm HEPES buffer, 750 mm NaCl, and 0.05% Nonidet P-40, pH 7.4. Soluble fractions were separated from cell debris by centrifugation at 10,000 × g for 10 min. Soluble fractions were concentrated by ultrafiltration (Vivaspin (filtration threshold, 10 kDa)) in some experiments. Proteins were assayed with a bicinchoninic acid assay (BCA) (Thermo Fisher Scientific, Villebon sur Yvette, France).
The CatC activity in cell lysates, BALF, and sputum supernatants was measured spectrofluorometrically (Spectra Max Gemini EM) at 460 nm with or without the nitrile inhibitors l-Thi-l-Phe-CN, l-Thi-d-Phe-CN, or d-Thi-l-Phe-CN using Gly-Phe-AMC (30 μm final) as the substrate in 50 mm sodium acetate, 30 mm NaCl, 1 mm EDTA, 2 mm DTT, pH 5.5, at 37 °C.
The PR3 activity in cell lysates was measured at 420 nm with or without the PR3 inhibitor Ac-PYDAP(O-C6H6-4-Cl)2 (31) (50 μm in 50 mm HEPES buffer, 750 mm NaCl, 0.05% Nonidet P-40, pH 7.4, at 37 °C) using Abz-VADnVADYQ-EDDnp (20 μm) as a substrate. Cell lysates were first incubated with 5 mm EDTA for 10 min at 37 °C to measure CatG activity at 420 nm in 50 mm HEPES buffer, 100 mm NaCl, 0.05% Nonidet P-40, pH 7.4, at 37 °C in the presence or not of 2 μm antichymotrypsin using Abz-TPFSGQ-EDDnp (32) (20 μm) as a substrate.
Analysis of Cleavage Products by Reverse-phase Chromatography
The intramolecularly quenched fluorogenic substrate Abz-VADnVADYQ-EDDnp (20 μm final) (where Abz is 2-aminobenzoic acid and EDDnp stands for N-(2,4-dinitrophenyl)ethylendiamine) was incubated with PLB-985 cell lysate for 2 h, and proteins were precipitated with absolute ethanol (4 volumes). The supernatants were dried under a vacuum, dissolved in 200 μl of 0.01% TFA (v/v), and fractionated by reverse-phase chromatography (C18 column, flow rate 0.3 ml/min, linear gradient (0–60%, v/v) of acetonitrile in 0.01% trifluoroacetic acid for 30 min). Eluted peaks were monitored at 220 nm.
Extravidin-peroxidase Detection of Active PR3
Active PR3 in PLB-985, HL-60, and neutrophil lysates was detected using selective PR3 activity-based probe Bt-[PEG]66-PYDAP(O-C6H4-4-Cl)2 (31). The volumes of lysates were adjusted so that the final concentration of PR3 was 20 nm and incubated with Bt-[PEG]66-PYDAP(O-C6H4-4-Cl)2 (250 nm final) for 30 min at 37 °C in PBS. The samples were separated by SDS-PAGE under native conditions and after transfer membranes were incubated overnight at 4 °C with extravidin horseradish peroxidase (HRP) (diluted 1/4000 in PBS Tween 0.1%, 3% BSA). The membranes were then washed 3 times (10 min each time) with PBS-Tween 1%, and the proteins were revealed by chemiluminescence (ECL Plus Western blotting kit detection reagents, GE Healthcare).
Animal Experiments
BALF were collected from mice (C57BL/6, Balb/cRj, n = 5) and rats (Sprague-Dawley, n = 2) (33). Those for each species were pooled (mouse or rat), centrifuged at 500 × g for 5 min at 4 °C, and concentrated ∼10-fold by ultrafiltration. Their CatC activity/content was then determined.
Lipopolysaccharide (LPS) was isolated from Pseudomonas aeruginosa serotype 10 (Sigma) suspended in sterile water. A group of 5 cynomolgus macaques was fasted overnight before sedation with ketamine (0.35 mg/kg, intramuscular; corresponding to ∼0.3 ml of Imalgène® 1000). They were anesthetized by isoflurane inhalation (5% vaporizer output). Each monkey was given 100% O2 for 10 min, its throat was sprayed with 5% xylocaine, and it was intubated with a pediatric endotracheal tube. LPS (0.4 mg/kg) was aerosolized with a microsprayer® IA1B (Aerosolizer® IA1B, PennCentury, Philadelphia, PA) connected to a luer-lock 10-ml plastic syringe. BALF were collected 24 h post-LPS administration and centrifuged at 500 × g for 5 min at 4 °C. The cell-free supernatants were concentrated ∼20-fold by ultrafiltration. Their CatC activity/content was then determined. The cells from the pellet were counted.
Macaque blood samples (10 ml) were collected from their femoral veins into lithium-heparin tubes. Blood neutrophils were purified (>98%) on a Ficoll gradient. All animal experiments and procedures were approved by the local animal experimentation ethics committee (Comité d'Ethique de Val de Loire (CEEA VdL, no. 2013-01-2)).
Western Blotting
The samples were separated by SDS-PAGE under denaturing/reducing conditions and transferred to Hybond-ECL membranes. Free sites were saturated by incubation in PBS, 0.1% Tween, 5% fat-free milk for 1 h, and the membranes were then incubated with anti-CatC antibody (diluted 1:800), anti-His6-tag antibody (diluted 1:1000), or anti-PR3 (diluted 1:1000) in PBS, 0.1% Tween, 5% nonfat milk overnight at 4 °C. The membranes were washed 3 times in PBS, 0.1% Tween and incubated for 1 h with a peroxidase-coupled secondary antibody diluted 1:10,000 in PBS, 0.1% Tween, and 5% nonfat milk.
Three anti-CatC antibodies were used; two recognizing the heavy chain (Ab1 and Ab3) and one (Ab2) recognizing the propeptide. None but one of the anti-human CatC antibodies tested cross-reacted with macaque CatC. The antibody (Ab3) was produced by Everest Biotech Ltd, EB11824). The immunogen was the C-terminal peptide sequence of the CatC heavy chain 214RNVHGINFVSPVRNQ228, which is common to both human and macaque CatC.
Results
CatC in Human Myelomonoblastic PLB-985 and Promyelocytic HL-60 Precursor Cells
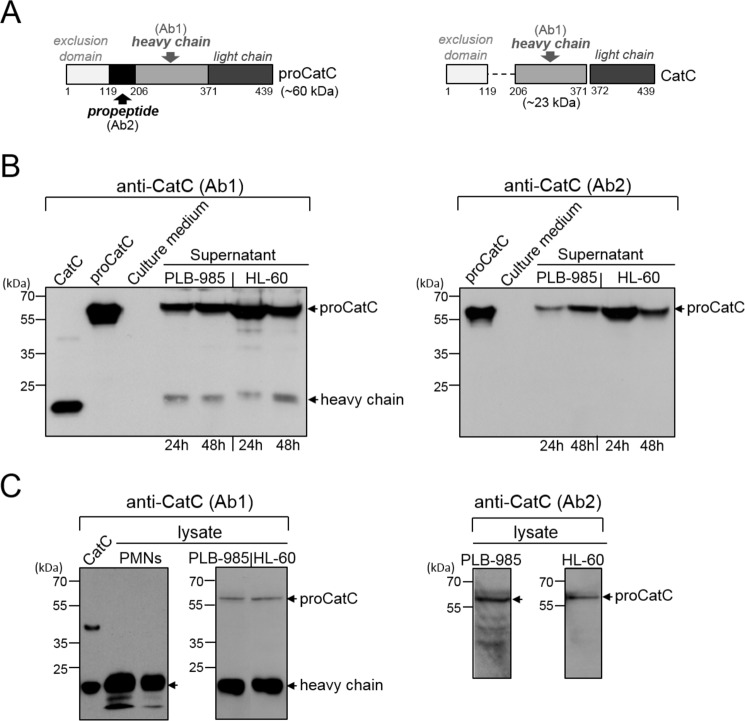
We analyzed the supernatants and cell lysates of PLB-985/HL-60 by WB using anti-CatC antibodies that recognized either the mature molecule and its proform (Ab1) or only the proform (Ab2). PLB-985 and HL-60 cells mainly secreted the ∼60-kDa proCatC, whereas the cell lysate contained large amounts of mature immunoreactive CatC as revealed by the ∼20-kDa heavy chain (Fig. 1, A–C). The proteolytic activity recorded using the CatC substrate Gly-Phe-AMC in the precursor cell lysates was completely inhibited by the CatC inhibitor Gly-Phe-CHN2 (data not shown).
FIGURE 1.
CatC in PLB-985 and HL-60 cells. A, structures of proCatC (left) and mature CatC (right). Arrows indicate the recognition domains of antiCatC antibodies (Ab1, Ab2). B, Western blot analysis of 30-fold concentrated PLB-985 and HL-60 cell supernatants (50 μg of protein/lane) using anti-CatC antibodies Ab1 (left) and Ab2 (right). The cells were cultured for 24 h or 48 h. C, Western blot analysis of PLB-985 cell lysates (50 and 200 μg of protein/lane) and HL-60 cell lysates (50 and 200 μg of protein/lane) using anti-CatC antibodies Ab1 (left) and Ab2 (right). Similar results were obtained in three independent experiments. Recombinant CatC, proCatC, and neutrophil lysates (50 and 20 μg of protein/lane) were used as controls. PMNs, polymorphonuclear neutrophils.
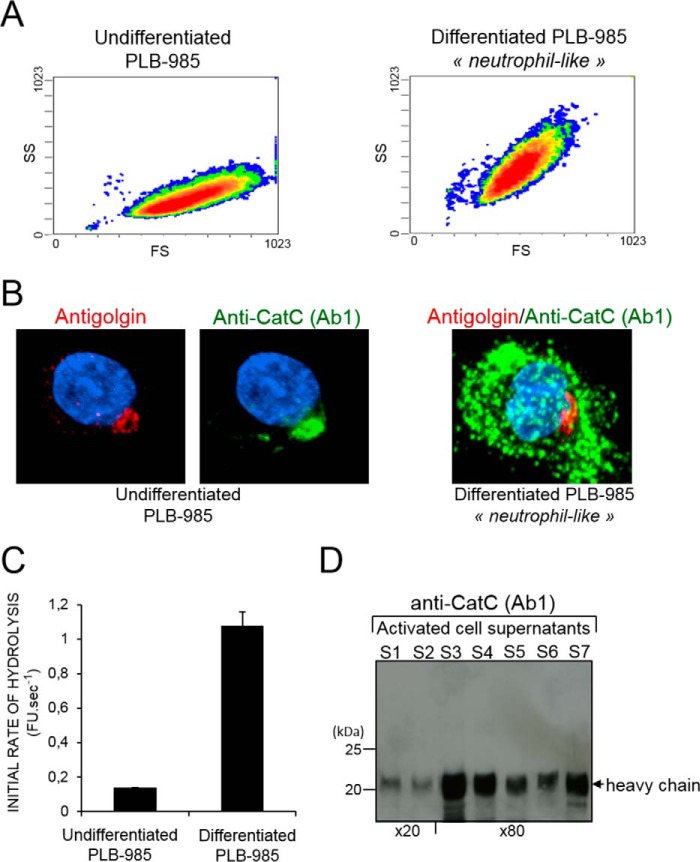
CatC is mainly located in the Golgi of granule-free undifferentiated PLB-985 cells, whereas HL-60 and differentiated neutrophil-like cells store active CatC in cytoplasmic granules (Fig. 2, A and B). However, we observed a similar rate of hydrolysis of the CatC substrate Gly-Phe-AMC in lysates of undifferentiated cells, differentiated cells, and purified blood neutrophils. Proteolytically active CatC was not detected in the cell-free supernatant of undifferentiated PLB-985 activated by A23187, but it was found in supernatants after these cells had been differentiated into neutrophil-like cells and in mature neutrophils (Fig. 2, C and D).
FIGURE 2.
Intracellular localization and secretion of mature CatC by activated cells. A, sideward (SS) versus forward (FS) scatter plot of undifferentiated (left panel) and differentiated PLB-985 cells (right). B, confocal microscopy of undifferentiated (left) and differentiated (right) PLB-985 cells immunostained with anti-CatC (Ab1) antibodies (green) and anti-golgin-84 antibodies (red) showing the initial localization of CatC in the Golgi of immature cells and its distribution throughout the cell after differentiation. C, CatC activity in cell-free supernatants of undifferentiated and differentiated PLB-985 cells after treatment with the calcium ionophore A23187. FU, fluorescence unit. D, immunoblot analysis of 20- or 80-fold concentrated supernatants of neutrophils (S1 to S7) after their activation with A23187 using the anti-CatC antibody Ab1. Similar results were found in three independent experiments.
Because CatC is proteolytically active in PLB-985 cells and localized in the Golgi, we wondered whether the proforms of elastase-like proteases were activated before their storage into primary granules. Indeed we found active PR3 in a PLB-985 cell lysate incubated with the specific biotinylated PR3 activity-based probe Bt-[PEG]66-PYDAP(O-C6H4-4-Cl)2 (Fig. 3A) and by recording the cleavage of the PR3 fluorogenic substrate Abz-VADnVADYQ-EDDnp (Fig. 3B). Active CatG was also identified using its selective fluorogenic substrate Abz-TPFSGQ-EDDnp. This demonstrates that PR3 and CatG and most probably the related proteases are activated independently of the formation of storage granules, as PLB-985 cells do not contain dense granules in their cytoplasm. It must be noted, however, that HL-60 cells and neutrophils contain ∼100-fold more proteolytically active elastase-like proteases than PLB-985 cells. We then examined how proCatC was activated in neutrophilic precursor cells.
FIGURE 3.
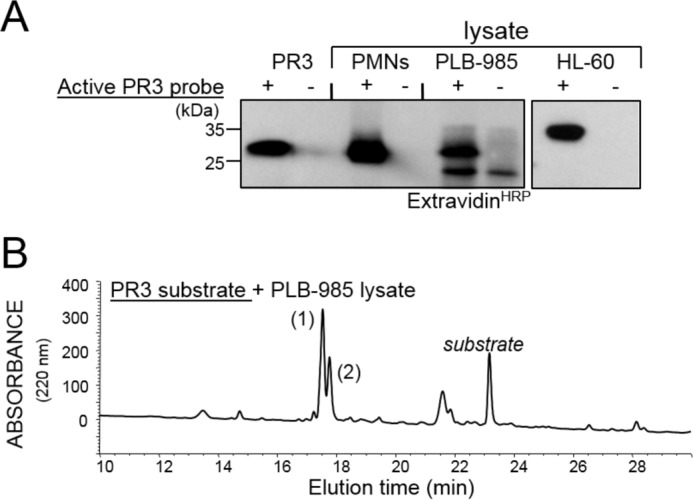
Active PR3 in PLB-985. A, immunodetection of purified PR3, PR3 in PLB-985, HL-60, and neutrophil lysates using Bt-[PEG]66-PYDAP(O-C6H4-4-Cl)2, a selective biotinylated probe of proteolytically active PR3. PMNs, polymorphonuclear neutrophils. B, RP-HPLC fractionation of cleavage products on a C18 cartridge after incubating the PR3 substrate Abz-VADnVADYQ-EDDnp with a PLB-985 cell lysate. Peak 1 is Abz-VADnV, and 2 is ADYQ-EDDnp. Similar results were observed in three independent experiments.
Involvement of CatS in the Maturation of ProCatC in PLB-985 and HL-60 Cells
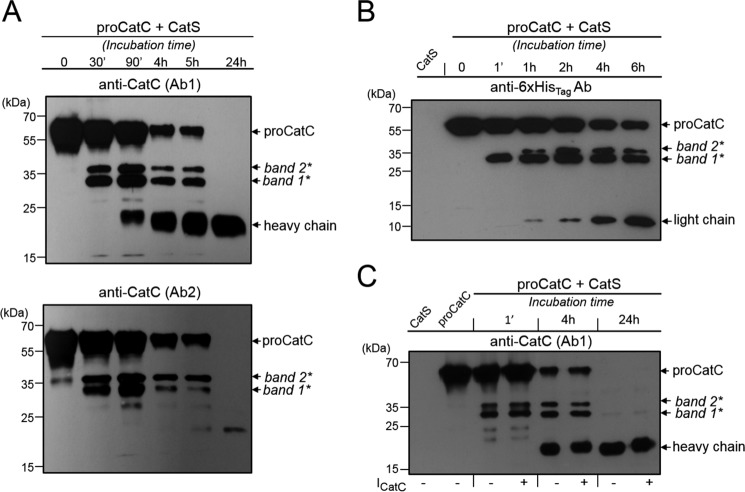
We first looked for the presence of CatL and CatS in PLB-985 and HL-60 cells because both have previously been reported as proCatC activators (6). We detected intracellular CatS but not CatL by WB, and we, therefore, investigated the in vitro processing and activation of recombinant proCatC-His6 by recombinant CatS using the anti-CatC antibodies Ab1 and Ab2 (Fig. 4A) and an anti-His6-tag Ab (Fig. 4B). ProCatC fragmentation by CatS was initiated by the accumulation of a 36-kDa fragment (band2*) and a 33-kDa fragment (band1*), both composed of the truncated propeptide, the heavy chain, and the light chain. The two peptides were then further processed to release the heavy chain and the light chain. The same 36- and 33-kDa fragments were visualized when proCatC was incubated with CatS in the presence of a CatC nitrile inhibitor (ICatC, compound 1) (Figs. 4C and 5A). This means that CatC does not process the 36-kDa fragment into the 33-kDa peptide. Incubating CatS with a selective cell-permeable CatS nitrile inhibitor (29) (ICatS, compound 2, Fig. 5B), E64c or E64d resulted in a total inhibition of proCatC processing (data not shown).
FIGURE 4.
In vitro processing of proCatC by CatS. A, human recombinant proCatC-His6 (1 μm) was incubated with recombinant CatS (0.2 μm) for different incubation times at 37 °C, and its fragmentation was investigated by Western blotting using anti-CatC antibodies Ab1 (upper panel) and Ab2 (lower panel). B, ProCatC-His6 (5 μm) was incubated at 37 °C with recombinant CatS (0.2 μm), and its fragmentation was analyzed by Western blotting using an anti-His6-tag antibody. C, ProCatC-His6 (1 μm) was incubated at 37 °C with recombinant CatS (0.2 μm) in the presence or absence of the CatC inhibitor ICatC (2 μm). CatC was analyzed by Western blotting using anti-CatC antibody (Ab1). Similar results were observed in three independent experiments.
FIGURE 5.
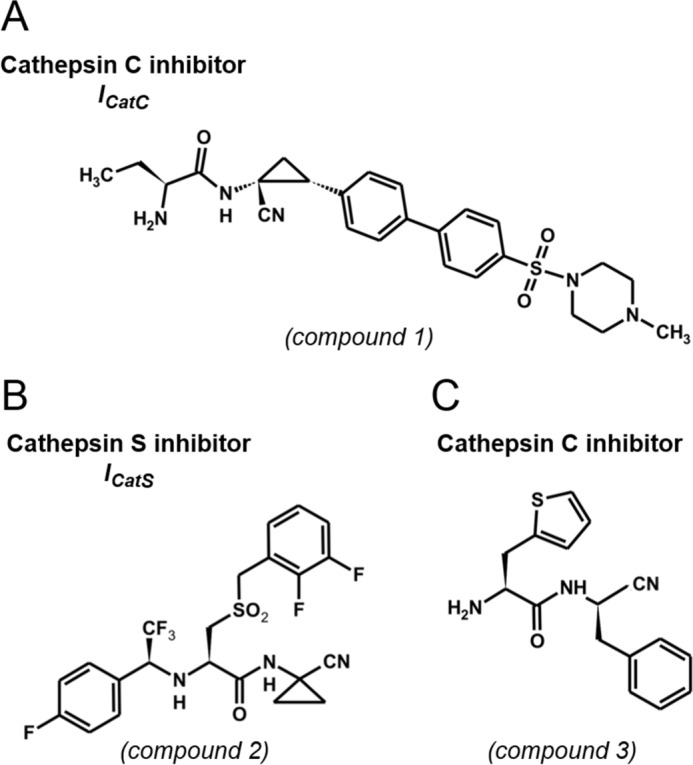
Structure of reversible CatS and CatC nitrile inhibitors used in this study. A, compound 1, (S)-2-amino-N-((1R, 2R)-1-cyano-2-(4′-(4-methylpiperazin-1-ylsulfonyl) biphenyl-4-yl)cyclopropyl)butanamide. B, compound 2, N-(1-cyanocyclopropyl)-3-((2,3-difluorobenzyl)sulfonyl)-2-((2,2,2-trifluoro-1-(4-fluorophenyl)ethyl)amino)propanamide. C, compound 3, β-(2-thienyl)-l-alanyl-d-phenylalanine nitrile.
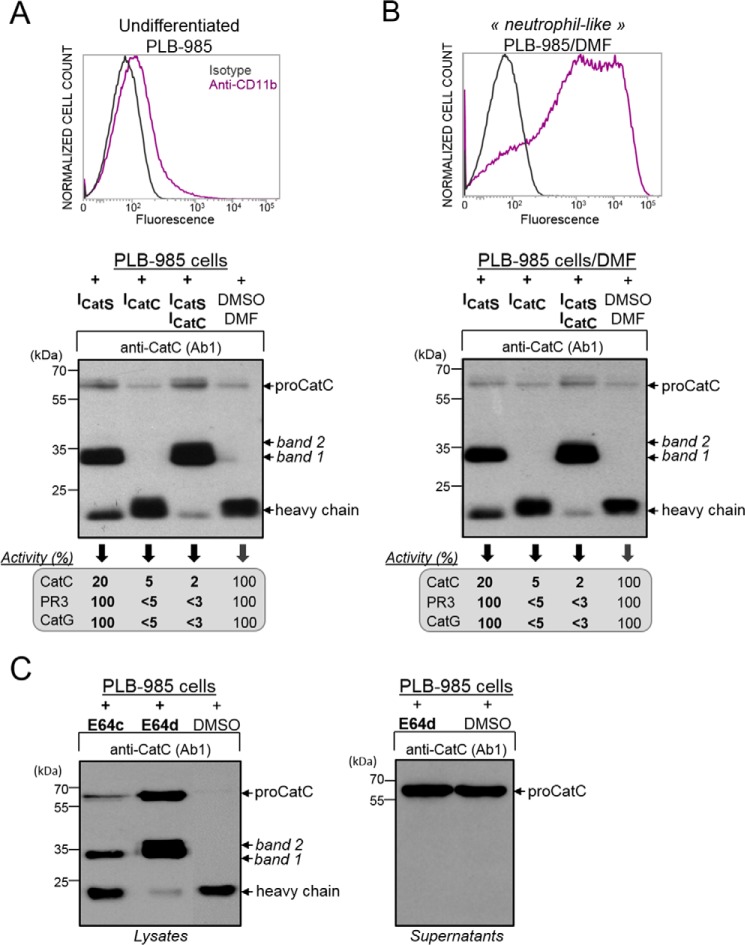
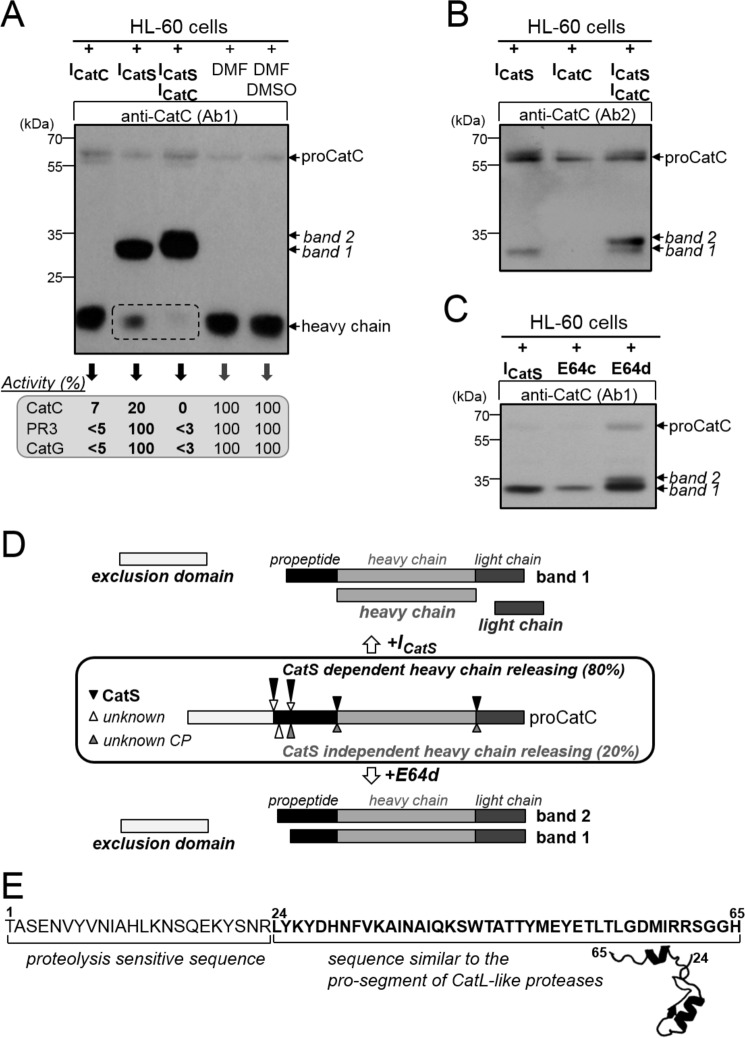
However, the addition of ICatS in the PLB-985 cell medium for 1 week did not fully prevent proCatC activation (Fig. 6A). But only the 33-kDa fragment was present under these conditions (Fig. 6A). This 33-kDa peptide was revealed by both antiCatC antibodies, Ab1 and Ab2, showing that it contains the truncated propeptide, the heavy chain, and the light chain. ∼80% of the CatC activity was inhibited under these conditions, and we obtained the same result incubating ICatS with PLB-985 cells during their differentiation into neutrophil-like cells (Fig. 6B). We then incubated PLB-985 cells with the broad spectrum cysteine protease inhibitors E64c or E64d to check whether another cysteine protease is involved in proCatC processing. Using ICatS, only the 35-kDa band was generated after 48 h of incubation with E64c, and a large fraction of mature CatC was observed (Fig. 6C). However, the two bands of 33 and 35 kDa appeared when we used E64d, the cell permeability of which is thought to be higher than that of E64c, and almost no heavy chain of mature CatC was observed (Fig. 6C). As previously reported, the two bands of 33 and 35 kDa were revealed by both Ab1 and Ab2 anti-CatC antibodies. Neither of these two fragments was secreted by the cells, unlike proCatC, which was constitutively secreted (Fig. 6C). The same result was observed when HL-60 cells were incubated with ICatS, E64c, or E64d (Fig. 7, A–C). This finding strongly suggests that at least one other cysteine protease that differs from CatS, but is also blocked by E64d, is involved in this process that impairs the release of the heavy chain (Fig. 7D).
FIGURE 6.
Processing of proCatC in PLB-985 cells cultured in the presence or absence of synthetic inhibitors. A, cell surface CD11b expression was analyzed by flow cytometry. Undifferentiated PLB-985 were cultured for 1 week in the presence of ICatS (10 μm), ICatC (2 μm), or after adding the DMSO/DMF containing buffer alone. Cell lysates were analyzed by Western blotting using anti-CatC antibody (Ab1). The percentage of CatC, PR3, and CatG activity was determined using the respective selective substrates for each protease and is given in the box. B, PLB-985 cells were differentiated into neutrophil-like in the presence of DMF and with or without ICatS (10 μm) or ICatC (2 μm) or DMSO/DMF. Cell lysates were analyzed by Western blotting using anti-CatC antibody (Ab1). The % of CatC, PR3, and CatG activity measured using their respective selective substrates were shown in the box. Cell surface CD11b expression was analyzed by flow cytometry as in A. C, undifferentiated PLB-985 cells cultured in the presence of E64c (100 μm), E64d (100 μm), or DMSO alone for 48 h were lysed. Cell lysates and supernatants of PLB-985 cells were analyzed by Western blotting using anti-CatC antibody Ab1 (left and right panels, respectively). Similar results were observed in five independent experiments.
FIGURE 7.
Processing of proCatC in HL-60 cells cultured with or without synthetic inhibitors. A, HL-60 cells were cultured for 1 week in medium containing ICatS (10 μm), ICatC (2 μm), ICatS/ICatC (10 μm/2 μm), DMF, or DMSO/DMF, and then total cell lysates were analyzed by Western blotting using anti-CatC antibody (Ab1). The percentages of residual CatC, PR3, and CatG activity toward their respective selective substrates were given in the box. B, HL-60 cells were cultured for 1 week in medium containing ICatS (10 μm), ICatC (2 μm), and ICatS/ICatC inhibitors (10 μm/2 μm), and their lysates were analyzed by Western blotting using anti-CatC antibody (Ab2). C, HL-60 cells were cultured for 48 h in medium containing ICatS (10 μm), E64c (100 μm), or E64d (100 μm), and then total cell extracts were analyzed by Western blotting using anti-CatC antibody (Ab1). Similar results were observed in five independent experiments. D, diagram summary showing the processing of proCatC in HL-60 cells cultured in the presence of ICatS or E64d. Arrows indicate the position of proteolytic cleavages in proCatC. CP, cysteine protease. E, partial sequence of the CatC propeptide (residues 1–65) showing the N-terminal proteolysis-sensitive extension (residues 1–24) and the structurally conserved sequence found in CatL-like cysteine proteases (residues 24–65). Model structure was obtained using cysteine peptidase C (PDB code 4HWY; Ref. 37) as a template.
Formation of two intermediates, a 33- and 35-kDa band, suggests that the CatC propeptide contains an extended proteolysis-sensitive region at its N-terminal end. This is in keeping with our observation by molecular modeling of a structurally undefined sequence at the N-terminal end (Fig. 7E). We next raised the question as to whether CatS inhibition affects the activity of elastase-like proteases in human neutrophilic precursors.
Influence of CatS and CatC Inhibition on the Maturation of Elastase-like Proteases in PLB-985 and HL-60 Cells
We showed above that after 1 week of culture in the presence of ICatS, PLB-985 and HL-60 cells still retained ∼20% of the CatC activity observed in control cells in the absence of inhibitor. This was enough to fully activate PR3 and CatG whatever the cell line (Figs. 6 and 7), although HL-60 cells contained ∼100-fold more proteolytically active elastase-like proteases than PLB-985. An almost complete inactivation of the elastase-like activity in precursor cells was obtained using ICatC but was slightly more efficient by combining ICatS and ICatC in the cell culture (Figs. 6 and 7). We checked that ICatC alone did not impair the correct processing of proCatC after a 1-week incubation time with precursor cells, but the combination of ICatS and ICatC led to the appearance of both the 33-kDa and the 35-kDa fragments as we observed incubating the cells with E64d (Figs. 6 and 7). This means that ICatC most probably inhibits the cysteine protease activity responsible of the remaining CatC activity measured in ICatS-treated cells (Figs. 6 and 7). Only the 33-kDa peptide was formed when we replaced ICatC by Gly-Phe-CHN2, an irreversible, cell-permeable CatC inhibitor confirming this hypothesis. Indeed we observed a lower amount of unprocessed PR3 zymogen and no PR3 activity in HL-60 cells after a 1-week treatment with a combination of ICatS and ICatC (Fig. 8). The next step was to look at the fate of CatC in lung secretions of healthy and diseased individuals.
FIGURE 8.
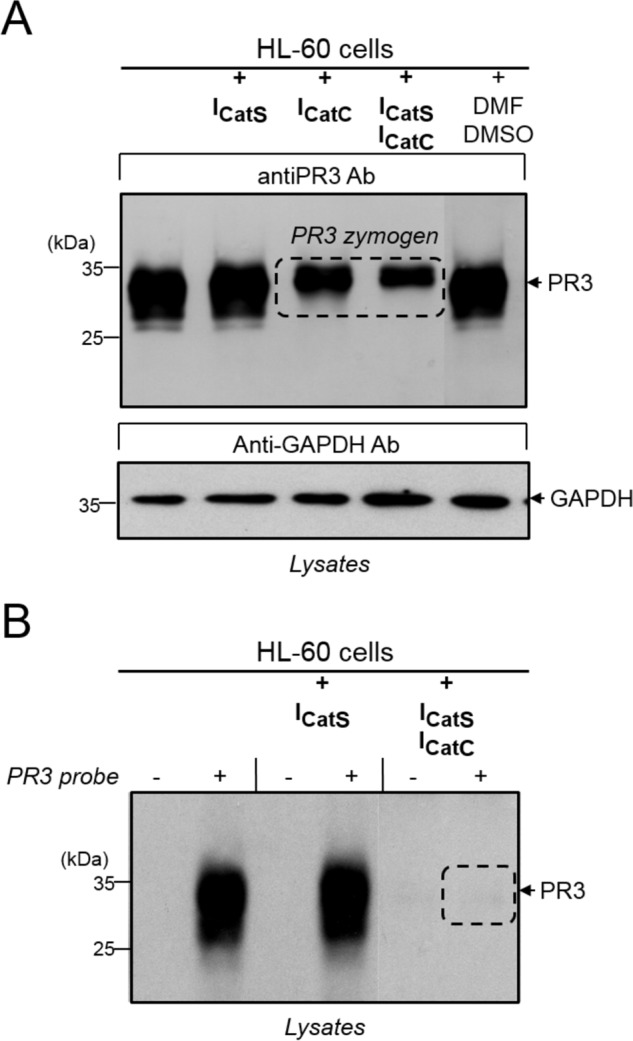
The effect of CatS and CatC inhibitors on the levels and activity of PR3 in HL-60 cells. A, HL-60 cells exposed to ICatS (10 μm), ICatC (2 μm), ICatS/ICatC (10 μm/2 μm), or DMSO/DMF for 1 week were lysed, and immune-reactive PR3 was analyzed by Western blotting using an antiPR3 antibody (Ab). GAPDH was used as the loading control. B, the same lysates were used to label proteolytically active PR3 using a selective biotinylated PR3 activity based-probe Bt-[PEG]66-PYDAP(O-C6H6-4-Cl)2. Lysates incubated with or without the biotinylated PR3 activity-based probe was examined by Western blotting after SDS-PAGE using extravidin peroxidase. Similar results were observed in three independent experiments.
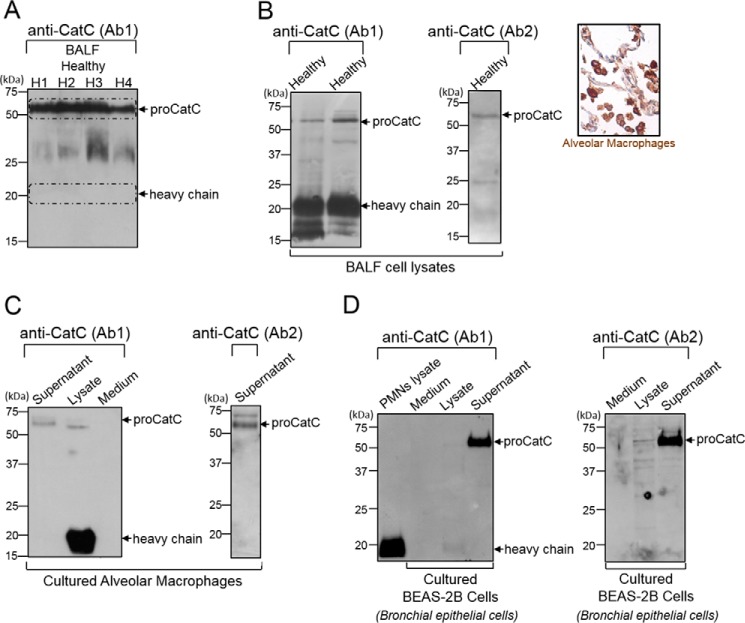
ProCatC in BALF from Healthy Individuals
We first looked for the presence of CatC in BALF of healthy controls. BALF from healthy smokers and non-smokers contained ∼80–90% macrophages. BALF were centrifuged, and their CatC content and enzymatic activity were determined by WB and by kinetic analysis, respectively. There was proCatC but no mature CatC in the cell-free supernatants of BALF from healthy individuals (Fig. 9A). Conversely, the cell pellets obtained by centrifugation of BALF and their corresponding lysates after Nonidet P-40 treatment contained large amounts of mature CatC (Fig. 9B) despite the almost complete lack of neutrophils. The macrophage origin of mature CatC was confirmed using cultured alveolar macrophages (AMs). They contained large amounts of mature intracellular CatC that was detected after lysis with Nonidet P-40, whereas intact cultured AMs secreted small amounts of proCatC (Fig. 9C).
FIGURE 9.
CatC in BALF from healthy subjects and in BALF cell lysates, alveolar macrophages, and BEAS-2B bronchial epithelial cells. A, anti-CatC (Ab1) immunoblots of 30-fold concentrated BALF (H1 to H4) from healthy subjects. Shown are immunoblots with anti-CatC antibodies Ab1 and Ab2 of BALF cell lysates (inset, immunostaining of healthy human lung tissue with Anti-CatC (Ab1) showing the CatC in the cytoplasm of alveolar macrophages) (B), cultured alveolar macrophage lysates and supernatants (C), and cultured BEAS-2B cell lysates and supernatants (D). PMNs, polymorphonuclear neutrophils. Similar results were observed in three independent experiments.
We determined whether lung epithelial cells also secreted proCatC because resident AMs may not be the only source of secreted proCatC in healthy lungs. BEAS-2B human bronchial epithelial cells and A549 alveolar epithelial cells were analyzed for their total protein content, CatC, and enzymatic activity using the CatC substrate Gly-Phe-AMC. BEAS-2B cells secreted significant amounts of proCatC (Fig. 9D) but had no significant CatC activity when compared with alveolar or cultured macrophages (BEAS-2B/AM activity ratio ∼1/200). Unlike bronchial epithelial cells, A549 alveolar epithelial cells secreted a low amount of proCatC but had significant CatC activity. Lysates of A549 cells, however, contained ∼50 times less CatC activity than the AM lysates (data not shown). Thus, resident AMs in the airways produce active CatC but do not secrete it. However they together with bronchial and alveolar epithelial cells release constitutive proCatC into lung secretions. Immunostaining of control human lung tissue with anti-CatC (Ab1) showed the enzyme predominantly in the cytoplasm of AMs, which confirmed our results (Fig. 9B).
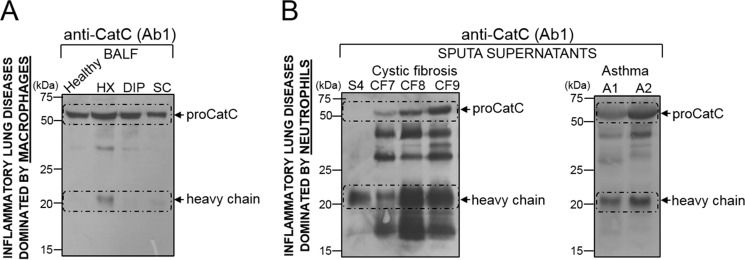
Mature CatC in Pulmonary Secretions from Patients with Chronic Lung Disease Characterized by Neutrophilic Inflammation
We analyzed the CatC content of BALF from patients with chronic inflammatory respiratory diseases in which the predominant inflammatory cells were macrophages (histiocytosis X, desquamative interstitial pneumonia, sarcoidosis), or neutrophils (CF, neutrophilic asthma). Mature CatC was detected in the supernatants of all the sputa from patients with CF (n = 10) and asthma (n = 5) but little or none in samples from patients with histiocytosis X (HX; n = 5), desquamative interstitial pneumonia (DIP; n = 4), and sarcoidosis (SC; n = 4) (Fig. 10 and Table 1). Gly-Phe-AMC was also rapidly hydrolyzed by neutrophil-containing fluids. Incubating the mixtures with the CatC nitrile inhibitor l-Thi-l-Phe-CN (25) completely inhibited substrate hydrolysis in most samples, except in those from CF patients, confirming that this was due to CatC. RP-HPLC analysis showed that the lack of inhibition in CF sputa was due to the rapid degradation of the inhibitor by a so-far unidentified peptidase (data not shown). Replacing either the P1 or the P2 residue in the inhibitor l-Thi-l-Phe-CN (IC50 = 14 nm) with its d stereoisomer l-Thi-d-Phe-CN (compound 3, Fig. 5C; IC50 = 139 nm) and d-Thi-l-Phe-CN (IC50 = 249 nm)) resulted in a proteolytically resistant, cell-impermeable compound that inhibited purified recombinant CatC and CatC in CF sputa. We estimated the concentration of active CatC in CF sputa to be in the nanomolar range from the rate of substrate hydrolysis by recombinant CatC (Table 1). The presence of mature CatC was correlated with the total number of neutrophils in the samples.
FIGURE 10.
CatC in lung secretions from patients with chronic inflammatory lung diseases dominated either by macrophages or by neutrophils. A, immunoblots of 30-fold concentrates of BALF from patients with histiocytosis X (HX), desquamative interstitial pneumonia (DIP), or sarcoidosis (SC) using anti-CatC antibody Ab1. B, immunoblots of 20-fold concentrates of sputum supernatants from patients with cystic fibrosis (CF7 to CF9) and from patients with neutrophilic asthma (A1 and A2). The S4 control was a supernatant of ionophore-activated neutrophils.
TABLE 1.
CatC in homogenized cystic fibrosis sputa
| Sputum number | Sputum mass | PBS | CatC | CatC | Amount of CatC |
|---|---|---|---|---|---|
| g | ml | ng/ml | nm | ng/g sputum | |
| CF1 | 0.34 | 0.5 | 80 | 0.4 | 118 |
| CF2 | 0.7 | 1 | 88 | 0.45 | 126 |
| CF3 | 0.7 | 0.8 | 30 | 0.15 | 43 |
| CF4 | 2.1 | 3 | 146 | 0.73 | 208 |
| CF5 | 1.3 | 2 | 293 | 1.5 | 450 |
| CF6 | 1 | 3.5 | 60 | 0.3 | 210 |
CatC: a Putative Marker of Neutrophilic Lung Inflammation in a Macaque Model
We used BALF from animals with LPS-induced neutrophilic inflammation to further correlate CatC activity and the type of lung inflammation. Our attempts to measure CatC activity in rodents with lung inflammation were unsuccessful because active CatC was constitutively present in their BALF (Fig. 11). We then induced neutrophilic inflammation in five macaques (Macaca fascicularis) with LPS. The lung secretions of these primates contained low concentrations of proCatC and no active CatC, as in humans (Fig. 11). The BALF obtained 24 h after LPS aerosolization contained large amounts of activated neutrophils, as indicated by the CD11b on their surface (data not shown) and contained potent Gly-Phe-AMC cleaving activity that was >90% inhibited by the CatC inhibitor Gly-Phe-CHN2 (Fig. 11A). Analysis of these post-LPS BALF by WB confirmed the presence of mature CatC (Fig. 11B). We concluded that CatC activity is strongly correlated with the neutrophil count in the inflamed macaque lungs.
FIGURE 11.
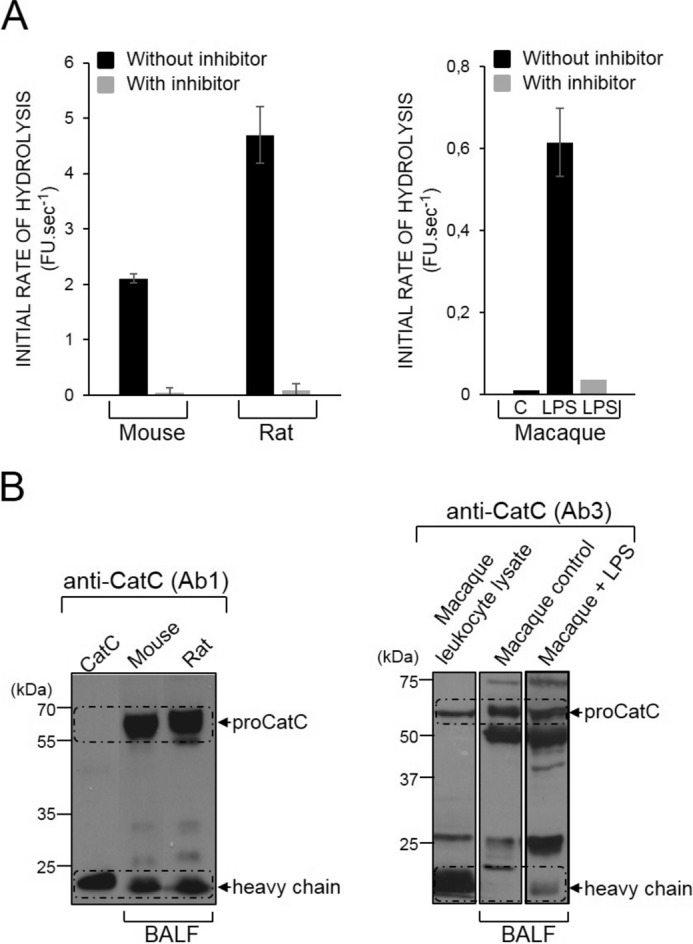
CatC in the BALF of rodents and macaques. A, initial rates of hydrolysis of the CatC substrate Gly-Phe-AMC (50 μm) by BALF from mice, rats, and macaques before and after incubation with the CatC diazomethyl ketone inhibitor Gly-Phe-CHN2 (25 μm). CatC activity was detected in the control BALF of rodents but not in that of macaques (n = 5) before LPS treatment. FU, fluorescence unit. B, immunoblotting of BALF from rodents and macaques showing the active CatC heavy chain in the BALF of rodents and that of LPS-treated macaques. Similar results were observed in three independent experiments.
Discussion
Most of CatL-like cysteine cathepsins (CatL, CatS, CatK, CatV) retain an endopeptidase activity with an active-site cleft that extends along the whole length of the two-domain interface (3). CatC, CatH, or CatB possess additional structural features that restrict access to their active site and reduce the number of binding sites so that they act as exopeptidases (3). The best characterized targets of the cysteine protease CatC are the zymogens of serine proteases in hematopoietic cell lineage of the bone marrow (1, 2, 15). The biosynthetically generated, active proteases are stored in the granules of cytotoxic T lymphocytes and natural killer cells (granzyme A and B), mast cells (chymase), and neutrophils (human neutrophil elastase, CatG, PR3, NSP4) (15, 34–36). CatC thus represents an attractive pharmacological target for several inflammatory diseases in which these multiple serine proteases are involved.
In this work we set out to determine the fate and function of CatC and of its precursor proCatC in promyelocytic cells, then in more differentiated cells, and finally in neutrophils in peripheral tissues. For this purpose we chose PLB-985 and HL-60 cells, which mimic neutrophilic precursors at different stages of neutrophil maturation in addition to in vitro differentiated neutrophil-like cells and mature blood neutrophils. Mature CatC was present in the Golgi of PLB-985 myelomonoblastic precursors and in the cytoplasmic granules of neutrophil-like cells resulting from the differentiation of immature cells. The proform of NSPs, exemplified here by PR3 and CatG, are converted into their active form concomitantly to synthesis and activation of CatC in PLB-985 cells. This strongly suggests that NSPs are activated before they are packaged into primary granules in more differentiated neutrophil-like cells and mature neutrophils. Precursor cells (PLB-985/HL-60), neutrophil-like differentiated cells, and purified blood neutrophils all contained similar amounts of mature CatC, but only precursor cells contained small amounts of proCatC.
The in vitro processing of proCatC by CatL and CatS has been previously reported by Dahl et al. (6), but these cysteine proteases are not required for the activation of proCatC in mice under in vivo conditions (7). We found CatS, but not CatL, in human neutrophilic precursors (PLB-985/HL-60). CatS processed proCatC in vitro in two consecutive steps; step 1 released the exclusion domain and two peptides of 36 and 33 kDa composed of the truncated propeptide, the heavy chain, and the light chain. Step 2 resulted in the release of the heavy chain from each peptide. The presence of a proteolysis-sensitive segment at the N terminus of the propeptide as shown here by the appearance of two peptides of 35 and 33 kDa has been previously observed by Dahl et al., (6), who identified a CatL-cleavage site at the Lys-14–Asn-15 bond of the propeptide. Molecular modeling studies showed that the propeptide sequence downstream the proteolysis-sensitive sequence retained a secondary structure with two crossed α-helices, as in related cysteine cathepsins (3, 37), that confers resistance to proteolysis.
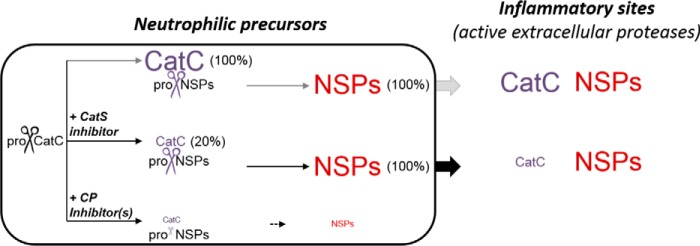
Inhibition of CatS using a cell-permeable nitrile inhibitor altered the processing of proCatC but did not fully prevent activation of proCatC in PLB-985/HL-60 cells. The low amount of active CatC (∼20%) was, however, enough to fully activate elastase-like proteases in neutrophilic precursor cells. We found that at least one additional, E64d-sensitive, cysteine protease that differed from CatS also participated in the release of the heavy and light chains in precursor cells. This additional protease was inhibited by a CatC inhibitor (ICatC). The combination of both ICatS and ICatC almost fully prevented proCatC processing and the activation of elastase-like proteases in a human cell culture.
Our experimental data indicate that activation of proCatC in precursor cells is a multistep process that can be completed by CatS alone in vitro. Nevertheless, CatS can also be partially replaced by an unidentified protease(s) displaying some functional similarity with CatS activity. This is consistent with previous results showing that in vivo activation of proCatC can even occur in the absence of CatS and/or CatL (7). We also showed here that all proteases involved in the proCatC processing operate in a post-RE compartment because brefeldin A does not impair proCatC level (data not shown).
Our data also highlight the challenge of eliminating NSP activities with CatC inhibitors. For this therapeutic application, CatC activity must be reduced by >95%. Our data support the conclusions of Méthot et al. (25, 26) who reported that very high fractional inhibition of the CatC activity by chronic administration of a CatC inhibitor is required to prevent the activation of NSPs in vivo. CatH has been shown to serve as a backup for CatC-mediated granzyme B activation in cytotoxic lymphocytes (38). In human neutrophil precursor cell lines we did not observe any additional convertase activity when CatC is inhibited in keeping with the results of Pham et al. (19). Using a mixture of inhibitors that prevent both the activation of proCatC and the activity of its mature form should offer a better in vivo control of the CatC activity resulting in a reduction of the proteolytic activity of NSPs at inflammatory sites (Fig. 12). Whether a decrease in CatC resulting from CatS inhibition would have additional biological effects is currently not clear. Other so far nonidentified target substrates of CatC may exist in cells and tissues with beneficial or detrimental effects. Here we noticed that peripheral white blood cells still transport and externalize active CatC, although it is no longer needed for zymogen conversion; it is packaged together with the active NSPs in primary granules of neutrophils. Hence active NSPs together with CatC can be secreted to fulfill additional extracellular functions in peripheral tissues. Our view is consistent with previous observations on neutrophils (20, 39) and monoblastic U937 cells (1) and with ongoing clinical trials that evaluate the potential of CatS inhibitors in patients with psoriasis or rheumatoid arthritis (40).
FIGURE 12.
Summary of the activation of neutrophil serine proteases in neutrophilic precursors showing that almost complete inhibition of CatC is required to prevent the production of active NSPs at inflammatory sites. CP, cysteine protease.
Although CatC activity was not detected in the lung secretions of healthy individuals, we found it in the lung secretions of patients with neutrophilic inflammation like CF and asthma. In contrast, we found little or no mature CatC in the BALF of patients suffering from inflammatory lung diseases dominated by macrophages (histiocytosis X, desquamative interstitial pneumonia, sarcoidosis). The BALF with a low neutrophil content obtained from patients with non-small lung cell cancer (n = 4) also contained little or no CatC (data not shown). CatC can thus be considered as a useful biomarker of active pulmonary neutrophilic inflammation because its concentration in the soluble fraction of lung secretions is correlated with the number of neutrophils. This was confirmed by measuring the CatC activity in the bronchial airway lavage fluid of macaques after LPS instillation. The main sources of proCatC in lung secretions were epithelial cells and residential AM. Epithelial cells did not contain significant amounts of active CatC, unlike AM.
The functional significance of the active CatC released by activated neutrophils at inflammatory sites remains to be explored. Increases in the extracellular CatC activity have only been observed in pathophysiological situations, in squamous carcinogenesis (41), and in central nervous system inflammation (42). A lack or absence of CatC is reported to reduce IL-1-dependent sterile inflammatory responses, e.g. in response to dying cells and silica crystals (43), and is associated with the increased replication of viruses during acute viral infection (44) of CatC-deficient mice.
The CatC gene of patients suffering from Papillon-Lefèvre syndrome displays loss-of-function mutations in both alleles. This genetic defect causes severe periodontal destruction, one of the hallmarks of the syndrome (16, 45). The resulting clinical phenotype is currently explained by reduced neutrophil recruitment to inflammatory sites and the lack of elastase-like activity. The results of experiments in CatC-null mice are consistent with this explanation. CatC-deficient mice are resistant to experimentally induced arthritis, and fewer mice die in sepsis experiments (15, 46). Again, these findings can be explained by a lack of the active neutrophil proteases necessary for the migration of neutrophils to inflammatory sites. CatC is generally regarded as a dipeptidyl-aminopeptidase, and it has been reported that extracellular CatC secreted into the lung tissue of dogs could play a role in matrix remodeling (47). In support of this idea, we recently observed an extracellular aminopeptidase activity in the sputa from patients with neutrophilic asthma (48). A convincing demonstration of its direct involvement in a pathophysiological process comes from a study showing that CatC may proteolytically down-regulate the antibacterial activity of surfactant protein D (49). This action of CatC reduces the clearance of microorganisms during bacterial lung infections. As a result, CatC−/− mice were more resistant to Klebsiella pneumoniae than control mice. From this result it is tempting to speculate that an increased CatC activity in inflamed lungs favors infection and has a negative influence on these conditions. Thus, a CatC inhibitor should help clear bacteria during severe neutrophilic lung infections.
Author Contributions
B. K., Y. H., M. L., D. E. J., and F. G. planned the experiments. Y. H., M. L., V. H., S. D.-C., S. M.-A., L. V., M. D., and N. H.-V. performed the experiments. M. L., A. L., R. W., and C. J. S. synthetized the inhibitors. B. K., F. G., D. E. J., Y. H., M. S. T., G. L., A. L., R. W., and C. J. S. analyzed the data. G. L., M. S. T., N. H.-V., R. W., and C. J. S. revised the paper. B. K., F. G., D. E. J., and Y. H. wrote the paper. B. K. supervised the project.
Acknowledgments
We thank Christophe Epinette, Yasser K. Mehdi, Christelle Parent (INSERM U-1100), Jérôme Montharu, Georges Roseau (Laboratory Animal Facilities, Université François Rabelais), and Julien Burlaud-Gaillard (Département des Microscopies, Université François Rabelais) for technical assistance. We thank Dr. Jan Voskuil of Everest Biotechnology (Oxfordshire, UK) for the anti-CatC antibody (Ab3, antiCatC EB11824) and Virginie Malak, of Santa Cruz Biotechnology, for the anti-CatC antibody (Ab1, antiCatC D-6/immunostaining of lung tissue). The English text was edited by Dr. Owen Parkes. We thank Heike Kittel for excellent technical assistance.
This work was supported by the “Association Vaincre la Mucoviscidose (VLM)” (RF20130500913), INSERM (ITMO Immunology, Hematology, Pneumology), “Région Centre-Val de Loire (Project BPCOlyse),” and the “National Science Centre” in Poland (UMO-2012/07/N/ST5/00128). In addition, this project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement 668036 (RELENT). The authors declare that they have no conflicts of interest in the research.
This work is dedicated to the memory of Heike Kittel who died on Sept. 21, 2015 in an accident.
- Cat
- cathepsin
- AM
- alveolar macrophages
- BALF
- broncho-alveolar lavage fluids
- CF
- cystic fibrosis
- NSP
- neutrophil serine protease
- PR3
- proteinase 3
- AMC
- aminomethylcoumarin
- DMF
- dimethylformamide
- WB
- Western blotting
- Boc
- t-butoxycarbonyl
- Bt
- biotin
- CHN2
- diazomethane
- Thi
- β-(2-thienyl)-alanine
- Abz
- 2-aminobenzoic acid, EDDnp, N-(2,4-dinitrophenyl)ethylendiamine.
References
- 1. McGuire M. J., Lipsky P. E., and Thiele D. L. (1993) Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J. Biol. Chem. 268, 2458–2467 [PubMed] [Google Scholar]
- 2. Turk D., Janjić V., Stern I., Podobnik M., Lamba D., Dahl S. W., Lauritzen C., Pedersen J., Turk V., and Turk B. (2001) Structure of human dipeptidyl peptidase I (cathepsin C): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J. 20, 6570–6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turk V., Stoka V., Vasiljeva O., Renko M., Sun T., Turk B., and Turk D. (2012) Cysteine cathepsins: from structure, function, and regulation to new frontiers. Biochim. Biophys. Acta 1824, 68–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mach L., Mort J. S., and Glössl J. (1994) Maturation of human procathepsin B: proenzyme activation and proteolytic processing of the precursor to the mature proteinase in vitro are primarily unimolecular processes. J. Biol. Chem. 269, 13030–13035 [PubMed] [Google Scholar]
- 5. Maubach G., Schilling K., Rommerskirch W., Wenz I., Schultz J. E., Weber E., and Wiederanders B. (1997) The inhibition of cathepsin S by its propeptide: specificity and mechanism of action. Eur. J. Biochem. 250, 745–750 [DOI] [PubMed] [Google Scholar]
- 6. Dahl S. W., Halkier T., Lauritzen C., Dolenc I., Pedersen J., Turk V., and Turk B. (2001) Human recombinant pro-dipeptidyl peptidase I (cathepsin C) can be activated by cathepsins L and S but not by autocatalytic processing. Biochemistry 40, 1671–1678 [DOI] [PubMed] [Google Scholar]
- 7. Mallen-St Clair J., Shi G. P., Sutherland R. E., Chapman H. A., Caughey G. H., and Wolters P. J. (2006) Cathepsins L and S are not required for activation of dipeptidyl peptidase I (cathepsin C) in mice. Biol. Chem. 387, 1143–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tran T. V., Ellis K. A., Kam C. M., Hudig D., and Powers J. C. (2002) Dipeptidyl peptidase I: importance of progranzyme activation sequences, other dipeptide sequences, and the N-terminal amino group of synthetic substrates for enzyme activity. Arch. Biochem. Biophys. 403, 160–170 [DOI] [PubMed] [Google Scholar]
- 9. Dolenc I., Turk B., Pungercic G., Ritonja A., and Turk V. (1995) Oligomeric structure and substrate induced inhibition of human cathepsin C. J. Biol. Chem. 270, 21626–21631 [DOI] [PubMed] [Google Scholar]
- 10. Cigic B., and Pain R. H. (1999) Location of the binding site for chloride ion activation of cathepsin C. Eur. J. Biochem. 264, 944–951 [DOI] [PubMed] [Google Scholar]
- 11. Pham C. T., Armstrong R. J., Zimonjic D. B., Popescu N. C., Payan D. G., and Ley T. J. (1997) Molecular cloning, chromosomal localization, and expression of murine dipeptidyl peptidase I. J. Biol. Chem. 272, 10695–10703 [DOI] [PubMed] [Google Scholar]
- 12. Rao N. V., Rao G. V., and Hoidal J. R. (1997) Human dipeptidyl-peptidase I: gene characterization, localization, and expression. J. Biol. Chem. 272, 10260–10265 [DOI] [PubMed] [Google Scholar]
- 13. Brown G. R., McGuire M. J., and Thiele D. L. (1993) Dipeptidyl peptidase I is enriched in granules of in vitro and in vivo activated cytotoxic T lymphocytes. J. Immunol. 150, 4733–4742 [PubMed] [Google Scholar]
- 14. Wolters P. J., Raymond W. W., Blount J. L., and Caughey G. H. (1998) Regulated expression, processing, and secretion of dog mast cell dipeptidyl peptidase I. J. Biol. Chem. 273, 15514–15520 [DOI] [PubMed] [Google Scholar]
- 15. Adkison A. M., Raptis S. Z., Kelley D. G., and Pham C. T. (2002) Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J. Clin. Invest. 109, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toomes C., James J., Wood A. J., Wu C. L., McCormick D., Lench N., Hewitt C., Moynihan L., Roberts E., Woods C. G., Markham A., Wong M., Widmer R., Ghaffar K. A., Pemberton M., Hussein I. R., Temtamy S. A., Davies R., Read A. P., Sloan P., Dixon M. J., and Thakker N. S. (1999) Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat. Genet. 23, 421–424 [DOI] [PubMed] [Google Scholar]
- 17. Haneke E. (1979) The Papillon-Lefevre syndrome: keratosis palmoplantaris with periodontopathy: report of a case and review of the cases in the literature. Hum. Genet. 51, 1–35 [DOI] [PubMed] [Google Scholar]
- 18. Papillon M., and Lefevre P. (1924) Two cases of symmetrically familial palmar and plantar hyperkeratosis (Meleda disease) within brother and sister combined with severe dental alterations in both cases. Bull. Soc. Fr. Dermatol. Syphiligr. 31, 82–87 [Google Scholar]
- 19. Pham C. T., Ivanovich J. L., Raptis S. Z., Zehnbauer B., and Ley T. J. (2004) Papillon-Lefevre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J. Immunol. 173, 7277–7281 [DOI] [PubMed] [Google Scholar]
- 20. Sørensen O. E., Clemmensen S. N., Dahl S. L., Østergaard O., Heegaard N. H., Glenthøj A., Nielsen F. C., and Borregaard N. (2014) Papillon-Lefevre syndrome patient reveals species-dependent requirements for neutrophil defenses. J. Clin. Invest. 124, 4539–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Firatli E., Tüzün B., and Efeoǧlu A. (1996) Papillon-Lefevre syndrome: analysis of neutrophil chemotaxis. J. Periodontol. 67, 617–620 [DOI] [PubMed] [Google Scholar]
- 22. Liu R., Cao C., Meng H., and Tang Z. (2000) Leukocyte functions in 2 cases of Papillon-Lefevre syndrome. J. Clin. Periodontol. 27, 69–73 [DOI] [PubMed] [Google Scholar]
- 23. Guay D., Beaulieu C., Truchon J. F., Jagadeeswar Reddy T., Zamboni R., Bayly C. I., Methot N., Rubin J., Ethier D., and David Percival M. (2009) Design and synthesis of dipeptidyl nitriles as potent, selective, and reversible inhibitors of cathepsin C. Bioorg. Med. Chem. Lett. 19, 5392–5396 [DOI] [PubMed] [Google Scholar]
- 24. Laine D. I., and Busch-Petersen J. (2010) Inhibitors of cathepsin C (dipeptidyl peptidase I). Expert Opin. Ther. Pat. 20, 497–506 [DOI] [PubMed] [Google Scholar]
- 25. Méthot N., Guay D., Rubin J., Ethier D., Ortega K., Wong S., Normandin D., Beaulieu C., Reddy T. J., Riendeau D., and Percival M. D. (2008) In vivo inhibition of serine protease processing requires a high fractional inhibition of cathepsin C. Mol. Pharmacol. 73, 1857–1865 [DOI] [PubMed] [Google Scholar]
- 26. Méthot N., Rubin J., Guay D., Beaulieu C., Ethier D., Reddy T. J., Riendeau D., and Percival M. D. (2007) Inhibition of the activation of multiple serine proteases with a cathepsin C inhibitor requires sustained exposure to prevent pro-enzyme processing. J. Biol. Chem. 282, 20836–20846 [DOI] [PubMed] [Google Scholar]
- 27. Korkmaz B., Lesner A., Letast S., Mahdi Y. K., Jourdan M. L., Dallet-Choisy S., Marchand-Adam S., Kellenberger C., Viaud-Massuard M. C., Jenne D. E., and Gauthier F. (2013) Neutrophil proteinase 3 and dipeptidyl peptidase I (cathepsin C) as pharmacological targets in granulomatosis with polyangiitis (Wegener granulomatosis). Semin. Immunopathol. 35, 411–421 [DOI] [PubMed] [Google Scholar]
- 28. Faurschou M., and Borregaard N. (2003) Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 5, 1317–1327 [DOI] [PubMed] [Google Scholar]
- 29. Gauthier J. Y., Black W. C., Courchesne I., Cromlish W., Desmarais S., Houle R., Lamontagne S., Li C. S., Massé F., McKay D. J., Ouellet M., Robichaud J., Truchon J. F., Truong V. L., Wang Q., and Percival M. D. (2007) The identification of potent, selective, and bioavailable cathepsin S inhibitors. Bioorg. Med. Chem. Lett. 17, 4929–4933 [DOI] [PubMed] [Google Scholar]
- 30. Korkmaz B., Attucci S., Juliano M. A., Kalupov T., Jourdan M. L., Juliano L., and Gauthier F. (2008) Measuring elastase, proteinase 3, and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrates. Nat. Protoc. 3, 991–1000 [DOI] [PubMed] [Google Scholar]
- 31. Guarino C., Legowska M., Epinette C., Kellenberger C., Dallet-Choisy S., Sieńczyk M., Gabant G., Cadene M., Zoidakis J., Vlahou A., Wysocka M., Marchand-Adam S., Jenne D. E., Lesner A., Gauthier F., and Korkmaz B. (2014) New selective peptidyl di(chlorophenyl)-phosphonate esters to visualize and block neutrophil proteinase 3 in human diseases. J. Biol. Chem. 289, 31777–31791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Attucci S., Korkmaz B., Juliano L., Hazouard E., Girardin C., Brillard-Bourdet M., Réhault S., Anthonioz P., and Gauthier F. (2002) Measurement of free and membrane-bound cathepsin G in human neutrophils using new sensitive fluorogenic substrates. Biochem. J. 366, 965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dubois A. V., Midoux P., Gras D., Si-Tahar M., Bréa D., Attucci S., Khelloufi M. K., Ramphal R., Diot P., Gauthier F., and Hervé V. (2013) Poly-l-lysine compacts DNA, kills bacteria, and improves protease inhibition in cystic fibrosis sputum. Am. J. Respir. Crit. Care Med. 188, 703–709 [DOI] [PubMed] [Google Scholar]
- 34. Pham C. T., and Ley T. J. (1999) Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc. Natl. Acad. Sci. U.S.A. 96, 8627–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolters P. J., Pham C. T., Muilenburg D. J., Ley T. J., and Caughey G. H. (2001) Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J. Biol. Chem. 276, 18551–18556 [DOI] [PubMed] [Google Scholar]
- 36. Perera N. C., Wiesmüller K. H., Larsen M. T., Schacher B., Eickholz P., Borregaard N., and Jenne D. E. (2013) NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity. J. Immunol. 191, 2700–2707 [DOI] [PubMed] [Google Scholar]
- 37. Redecke L., Nass K., DePonte D. P., White T. A., Rehders D., Barty A., Stellato F., Liang M., Barends T. R., Boutet S., Williams G. J., Messerschmidt M., Seibert M. M., Aquila A., Arnlund D., Bajt S., Barth T., Bogan M. J., Caleman C., Chao T. C., Doak R. B., Fleckenstein H., Frank M., Fromme R., Galli L., Grotjohann I., Hunter M. S., Johansson L. C., Kassemeyer S., Katona G., Kirian R. A., Koopmann R., Kupitz C., Lomb L., Martin A. V., Mogk S., Neutze R., Shoeman R. L., Steinbrener J., Timneanu N., Wang D., Weierstall U., Zatsepin N. A., Spence J. C., Fromme P., Schlichting I., Duszenko M., Betzel C., and Chapman H. N. (2013) Natively inhibited Trypanosoma brucei cathepsin B structure determined by using an x-ray laser. Science 339, 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D'Angelo M. E., Bird P. I., Peters C., Reinheckel T., Trapani J. A., and Sutton V. R. (2010) Cathepsin H is an additional convertase of pro-granzyme B. J. Biol. Chem. 285, 20514–20519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lominadze G., Powell D. W., Luerman G. C., Link A. J., Ward R. A., and McLeish K. R. (2005) Proteomic analysis of human neutrophil granules. Mol. Cell Proteomics 4, 1503–1521 [DOI] [PubMed] [Google Scholar]
- 40. Wilkinson R. D., Williams R., Scott C. J., and Burden R. E. (2015) Cathepsin S: therapeutic, diagnostic, and prognostic potential. Biol. Chem. 396, 867–882 [DOI] [PubMed] [Google Scholar]
- 41. Ruffell B., Affara N. I., Cottone L., Junankar S., Johansson M., DeNardo D. G., Korets L., Reinheckel T., Sloane B. F., Bogyo M., and Coussens L. M. (2013) Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes Dev. 27, 2086–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koike M., Shibata M., Ezaki J., Peters C., Saftig P., Kominami E., and Uchiyama Y. (2013) Differences in expression patterns of cathepsin C/dipeptidyl peptidase I in normal, pathological and aged mouse central nervous system. Eur. J. Neurosci. 37, 816–830 [DOI] [PubMed] [Google Scholar]
- 43. Kono H., Orlowski G. M., Patel Z., and Rock K. L. (2012) The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J. Immunol. 189, 3734–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andoniou C. E., Fleming P., Sutton V. R., Trapani J. A., and Degli-Esposti M. A. (2011) Cathepsin C limits acute viral infection independently of NK cell and CD8+ T-cell cytolytic function. Immunol. Cell Biol. 89, 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hart T. C., Hart P. S., Bowden D. W., Michalec M. D., Callison S. A., Walker S. J., Zhang Y., and Firatli E. (1999) Mutations of the cathepsin C gene are responsible for Papillon-Lefevre syndrome. J. Med. Genet. 36, 881–887 [PMC free article] [PubMed] [Google Scholar]
- 46. Mallen-St Clair J., Pham C. T., Villalta S. A., Caughey G. H., and Wolters P. J. (2004) Mast cell dipeptidyl peptidase I mediates survival from sepsis. J. Clin. Invest. 113, 628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolters P. J., Laig-Webster M., and Caughey G. H. (2000) Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am. J. Respir. Cell Mol. Biol. 22, 183–190 [DOI] [PubMed] [Google Scholar]
- 48. Korkmaz B., Jégot G., Lau L. C., Thorpe M., Pitois E., Juliano L., Walls A. F., Hellman L., and Gauthier F. (2011) Discriminating between the activities of human cathepsin G and chymase using fluorogenic substrates. FEBS J. 278, 2635–2646 [DOI] [PubMed] [Google Scholar]
- 49. Sutherland R. E., Barry S. S., Olsen J. S., Salantes D. B., Caughey G. H., and Wolters P. J. (2014) Dipeptidyl peptidase I controls survival from Klebsiella pneumoniae lung infection by processing surfactant protein D. Biochem. Biophys. Res. Commun. 450, 818–823 [DOI] [PMC free article] [PubMed] [Google Scholar]