Abstract
Evidence continues to emerge detailing the myriad of ways the gut microbiota influences host energy homeostasis. Among the potential mechanisms, short chain fatty acids (SCFAs), the byproducts of microbial fermentation of dietary fibers, exhibit correlative beneficial metabolic effects in humans and rodents, including improvements in glucose homeostasis. The underlying mechanisms, however, remain elusive. We here report that one of the main bacterially produced SCFAs, propionate, activates ileal mucosal free fatty acid receptor 2 to trigger a negative feedback pathway to lower hepatic glucose production in healthy rats in vivo. We further demonstrate that an ileal glucagon-like peptide-1 receptor-dependent neuronal network is necessary for ileal propionate and long chain fatty acid sensing to regulate glucose homeostasis. These findings highlight the potential to manipulate fatty acid sensing machinery in the ileum to regulate glucose homeostasis.
Keywords: fatty acid, glucose, glucose metabolism, intestine, lipid signaling
Introduction
Obesity and diabetes associate with shifts in the gut microbiome. Among the proposed mechanisms underlying the role of the microbiota in host energy homeostasis, production of short chain fatty acids (SCFAs)7 from non-digestible carbohydrates is hypothesized to be a main contributor (1). Alterations in SCFA production, either via prebiotic supplementation or surgical alteration, improve metabolic parameters (2–5). The three main SCFAs, acetate, propionate, and butyrate, differentially alter host metabolism (2, 3), and it appears that propionate exerts the most beneficial metabolic outcome as body weight changes via gastric bypass associate with rises in propionate (4), mice fed a high-fat diet supplemented with propionate improves glucose tolerance (5), and delivery of propionate to the colon of obese humans reduces weight gain (6).
SCFAs act on free fatty acid receptor-2 (FFAR2) and -3 (FFAR3), formerly termed GPR43 and GPR41, respectively (7, 8), which are expressed in intestinal epithelial cells (9, 10). Both receptors are localized in enteroendocrine cells (11) and SCFA-induced glucagon-like peptide-1 (GLP-1) release is impaired in primary intestinal cultures from FFAR2−/− and FFAR3−/− mice (12), whereas intestinal infusion of propionate fails to induce gut peptide release in FFAR2−/−, but not FFAR3−/− mice (13). Of note, these results are associative indications of intestinal FFAR2 regulating blood glucose levels via GLP-1, but to date, no one has addressed a direct causative role of intestinal FFAR2 activation in the regulation of glucose homeostasis in vivo.
To this end, we have characterized the role of the proximal small intestine in the regulation of glucose homeostasis, whereby duodenum and jejunum sensing mechanisms lower hepatic glucose production (GP) via a gut-brain-liver neuronal network involving gut peptide signaling (14–18). Despite a contribution in the control of food intake via activation of the ileal brake (19), much less is known about the glucoregulatory role of the ileal sensing (20). Given that FFAR2 is highly expressed in the ileum (9), and that conditions in the ileum are more favorable for microbial growth and SCFA production than the proximal small intestine (21, 22), we postulated that propionate triggers an ileal neuronal-dependent negative feedback pathway that requires ileal FFAR2 and is mediated via GLP-1 receptor (GLP-1R) signaling to lower GP and maintain whole body glucose homeostasis. Furthermore, ileal long chain fatty acids (LCFAs) stimulate GLP-1 secretion (23, 24) and vagal afferent firing (25). Thus, to further characterize the general ileal fatty acid-sensing pathways, we additionally investigated, for the first time to our knowledge, whether LCFA sensing in the ileum regulates glucose homeostasis and whether a similar GLP-1R neuronal network is required.
Experimental Procedures
Animals
Male Sprague-Dawley rats (250–300 g) were obtained from Charles River Laboratories (Montreal, QC) and were housed in individual cages maintained on a 12-h light/dark cycle. Rats had access to chow and water ad libitum, and were given 7 days to acclimatize before the designated surgeries were performed. Animals were randomly designated into experimental groups. The protocols were approved by the Institutional Animal Care Committee of UHN.
Ileal and Intravenous Cannulations
A gut catheter was inserted 2-cm proximal to the cecum to target the ileum and allow for infusion of various compounds (14). Intravenous catheters were placed into the jugular vein and carotid artery to allow for infusion of compounds and blood sampling. Rats were given 3–4 days to recover from the surgery before the infusion studies.
Viral Injection and Surgery
For experiments involving ileal FFAR2 knockdown, we utilized established protocols that target infection to a selective segment of the small intestine (16, 17, 26). Lentivirus (LV) expressing FFAR2 shRNA (1.0 × 106 infectious units of virus; Santa Cruz Biotechnology Inc., Dallas, TX) or mismatch (1.0 × 106 infectious units of virus; Santa Cruz Biotechnology Inc.) was diluted 1/10 into 0.2 ml of saline and injected into the ileal lumen and left for 20 min to optimize infection. An ileal catheter was subsequently inserted through the injection site, followed by vascular cannulation. Animals were allowed to recover for 3 days before the infusion studies.
Pancreatic (Basal Insulin)-Euglycemic Clamp with Steady-state Glucose Kinetics Assessment
This procedure was performed as described (15, 18). The night before the infusion-clamp studies, rats were fasted for 4–6 h. The pancreatic clamp technique (Fig. 1A) was performed in conjunction with tracer glucose-dilution methodology, allowing for the simultaneous calculation of GP and glucose uptake during intraileal fatty acid infusions. The experiments lasted a total of 200 min, with a continuous infusion of [3-3H]glucose (40 μCi bolus; 0.4 μCi min−1; PerkinElmer Life Sciences) initiated at the onset of the experiment (t = 0 min) and maintained throughout (t = 200 min). After reaching steady-state of the [3-3H]glucose infusion, the pancreatic clamp was initiated at t = 90 min (insulin at 1.2 milliunits kg−1 min−1 and somatostatin at 3 μg kg−1 min−1). Plasma samples were taken every 10 min and exogenous 25% glucose solution was infused accordingly to maintain euglycemia. In the final 50 min (t = 150–200 min), the intraileal infusions were initiated (0.01 ml min−1; treatments described below). Jejunum, ileum, and liver were removed from anesthetized rats immediately after the studies. The jejunal or ileal mucosal layer was separated from the smooth muscle layer. Tissue samples were frozen in liquid nitrogen and stored at −80 °C until use.
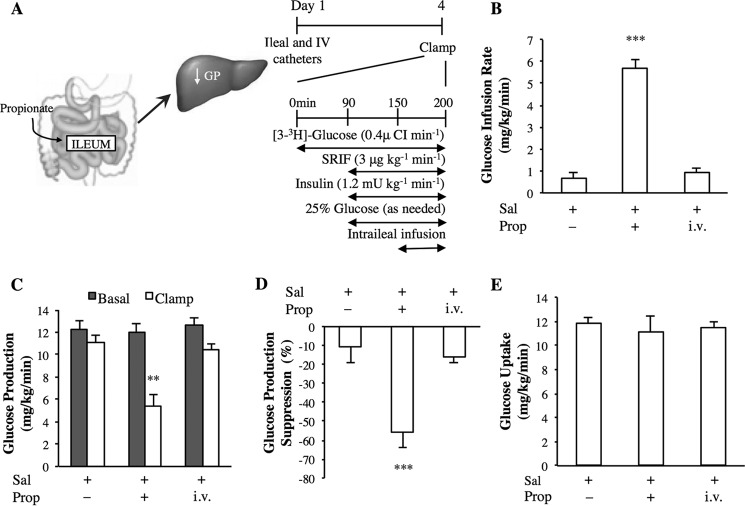
FIGURE 1.
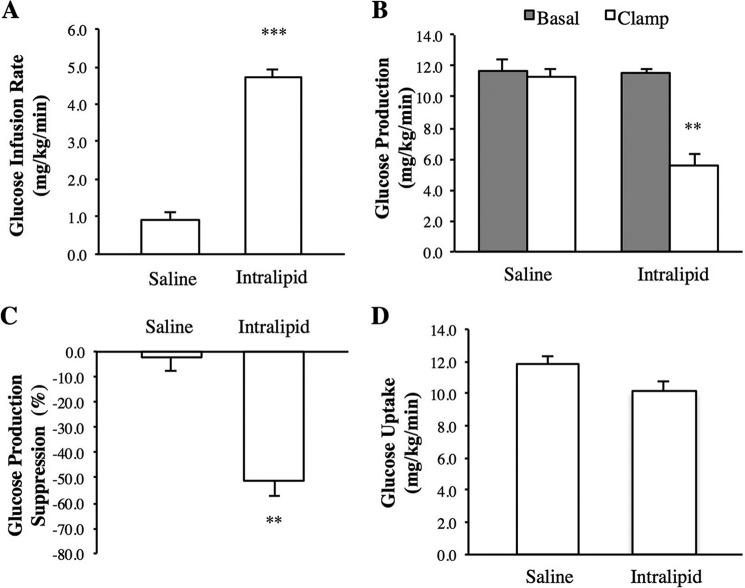
The effect of intraileal propionate infusion on glucose kinetics. A, schematic representation of the working hypothesis (left) and experimental procedure and pancreatic (basal insulin) euglycemic clamp protocol (right). SRIF, somatostatin. B–E, the glucose infusion rate (B), rate of GP (C), % suppression of GP (D), and glucose uptake (E) during the pancreatic clamp in rats with intraduodenal saline (Sal; n = 6) or propionate (Prop; n = 7) infusions, or intravenous (i.v.; n = 6) propionate infusion. Values are shown as mean ± S.E. **, p < 0.01; ***, p < 0.001, versus all other groups as determined by ANOVA with Tukey's post hoc test.
Treatments
The following treatments were given into the ileum (0.01 ml min−1 for 50 min): (a) saline, (b) 1% bile acid (phosphate-buffered saline (PBS) with lecithin and sodium taurocholate), (c) propionate (Prop; 5 mm; 4.8 μg min−1; Sigma), (d) 5% Intralipid (IL; 20% Intralipid diluted in saline; Baxter Corporation), (e) oleic acid (OA; 200 mm; 0.564 mg min−1; Sigma), (f) linoleic acid (LA; 200 mm; 0.56 mg min−1; Sigma), (g) triacsin-C (0.48 pmol min−1; Sigma), (h) exendin-9 (0.15 μg min−1; Tocris Bioscience, Ellisville, MO), and (i) tetracaine (0.01 mg min−1; Sigma; this dose selected based on a previous study that evaluated upper small intestinal fatty acid sensing (18)). Sodium propionate (>99% purity; Sigma) powder was dissolved in saline to make 5 mm propionate solution. This dose of propionate was chosen based on the fact that previous studies have mostly examined colonic SCFA mechanisms (13), and as colonic SCFA levels are likely to be supraphysiological as compared with the ileum (27), we chose a lower dose of SCFA (5 mm) than the concentration that was used in the colon. In addition, 5 mm SCFA has been documented to stimulate GLP-1 secretion in vitro (13) and more importantly, this dose of propionate is comparable with ileal propionate levels reported in humans (28). Intralipid was infused at one-fourth of the dose that was used in duodenal studies (18) because the amount of Intralipid detected in the ileum is lower than the duodenum following intragastric administration (29). Oleic or linoleic acids (99% purity; Sigma) were emulsified in PBS, 1% sodium taurocholate (Sigma), and 0.1% lecithin (l-α-phosphatidylcholine; Sigma) to make 200 mm solutions as described (30). A concentration of 200 mm was chosen for oleic or linoleic acids because this concentration is comparable with what was reported to stimulate gut peptide release when infused into the ileum (23, 31).
Protein Extraction and Western Blotting
Tissues were lysed on ice with a handheld homogenizer in a buffer containing: 50 mm Tris-hydrochloric acid (pH 7.5), 1 mm EGTA, 1 mm EDTA, 1% (w/v) Nonidet P-40, 1 mm sodium orthovanadate, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 0.27 m sucrose, 1 μm dithiothreitol (DTT) and protease inhibitor mixture (Roche Diagnostics, Laval, QC, Canada), and concentrations were determined with the Pierce 660 nm protein assay (Thermo Scientific, Rockford, IL). 30 μg of tissue lysates were subjected to electrophoresis in a mini-protean precast gel (4–15%; Bio-Rad) and transferred to nitrocellulose membranes. The membranes were incubated for 30 min with TBS-T containing 5% BSA. The membranes were then incubated with the indicated primary antibodies: anti-FFAR2 (1:200; sc-28424, Santa Cruz Biotechnology Inc.) or anti-β-actin (1:30,000, Sigma, catalog number A1978). The blots were then incubated with appropriate secondary antibodies (diluted 1:3000 for FFAR2 and 1:40,000 for actin) for 1 h in 5% skim milk at room temperature. After repeating the washing steps, the signal was detected with the enhanced chemiluminescence reagent. Western blots were developed with the Microchemi 2.0 DNR Bio-imaging system, and quantified protein levels with GelQuant software (DNR Bio-imaging system) and normalized to β-actin.
Biochemical Analysis
Plasma glucose levels were measured using the glucose oxidase method (GM9 Glucose Analyzer, Analox Instruments). Plasma insulin levels were determined by radioimmunoassay (Linco Research, St. Charles, MO). Plasma FFA levels were measured by an enzymatic assay (Wako Pure Chemical Industries, Osaka, Japan).
Statistical Analysis
Results were analyzed using unpaired Student's t test, or ANOVA (followed by Tukey's post hoc test), using GraphPad Prism 5.0d software. Differences were considered significant at p < 0.05. Data are presented as mean ± S.E. The time period of 60–90 min was averaged for the basal condition, and the time period of 180–200 min was averaged for the clamp condition.
Results
Intraileal Propionate Infusion Lowers GP via Preabsorptive Mechanisms
We first examined how whole body glucose metabolism changes in response to an acute rise of propionate in the ileum. Propionate was infused directly into the ileal lumen of awake, unrestrained healthy rats at a dose (5 mm) comparable with previous studies (13) and consistent with ileal propionate levels reported in humans (28). Insulin was maintained at plasma basal levels, which was achieved by the euglycemic-pancreatic clamp (Fig. 1A, Table 1). When propionate was infused into the ileal lumen for only 50 min, the exogenous glucose infusion rate required to maintain euglycemia was elevated compared with intraileal saline-infused rats (Fig. 1B), due to an inhibition of GP (Fig. 1, C and D) and not to an increase in the rate of glucose uptake, as peripheral glucose uptake did not change under these experimental conditions (Fig. 1E). Plasma FFA levels did not rise following intraileal propionate infusions (Table 1), suggesting that the effect of propionate was preabsorptive. Consistently, intravenous infusion of propionate administered at the same dose and duration as intraileal delivery (i.e. assuming 100% leakage into the circulation following ileal infusion) did not alter the glucose infusion rate, the rate of GP, or glucose uptake (Fig. 1, B–E). Therefore, although the physiological relevance of ileal SCFA sensing has yet to be tested, these data demonstrate that ileal propionate sensing triggers a negative feedback pathway to lower GP via preabsorptive mechanisms.
TABLE 1.
Plasma insulin, glucose, and free fatty acid concentrations of the groups receiving ileal infusions during the basal and clamp conditions
Data are mean ± S.E. (basal, 60–90 min; clamp, 180–200 min).
| Vehicle | Propionate | Oleic acid | Linoleic acid | |
|---|---|---|---|---|
| Basal | ||||
| Insulin (ng/ml) | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Glucose (mm) | 8.2 ± 0.4 | 6.2 ± 0.2 | 6.2 ± 0.2 | 7.0 ± 0.3 |
| Free fatty acids (mm) | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| Clamp | ||||
| Insulin (ng/ml) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 |
| Glucose (mm) | 7.5 ± 0.1 | 6.6 ± 0.2 | 6.6 ± 0.2 | 7.5 ± 0.3 |
| Free fatty acids (mm) | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
FFAR2 in the Ileum Is Necessary for Propionate Sensing
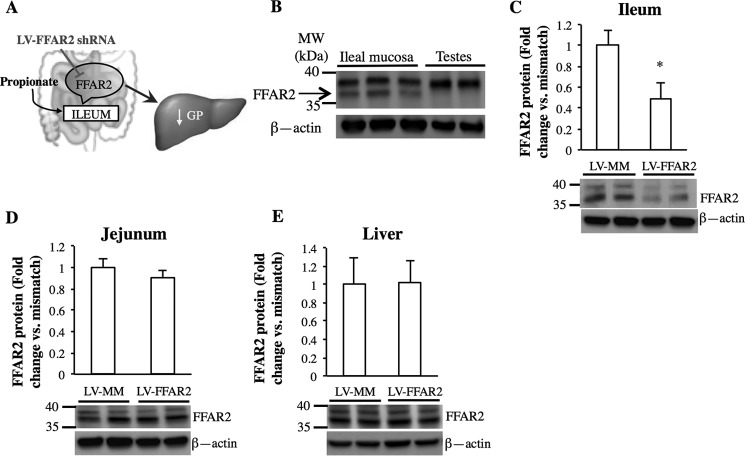
Given that propionate is a potent ligand of FFAR2 (7) and that FFAR2 is highly enriched in the ileal mucosa (9), we examined whether FFAR2 is required for ileal propionate sensing (Fig. 2A). First, Western blot analysis revealed that FFAR2 (37 kDa) was expressed in the ileal mucosa of rats, using the respective testes as a negative control (32) (Fig. 2B) and confirming previous findings obtained in humans and rodents (7, 8, 33), although the relative expression of FFAR2 in the ileal enteroendocrine cells versus other mucosal cells remains to be determined. To investigate the necessity of ileal FFAR2 in glucose control, a previously established intestinal targeted viral approach was employed to knockdown FFAR2 protein selectively in the ileum in vivo (16, 17, 26). Three days before the infusion experiments and prior to ileal cannulations, a lentivirus expressing either mismatched shRNA (LV-MM) or FFAR2 shRNA (LV-FFAR2) was injected into the ileal lumen. Western blot analysis validated the knockdown of FFAR2 protein in the ileal mucosa of LV-FFAR2 versus rats injected with LV-MM (∼51% reduction; Fig. 2C), with no changes in FFAR2 protein expression in the jejunal mucosa (Fig. 2D) or liver (Fig. 2E) of virus-injected rats, confirming the infection was localized to the ileum.
FIGURE 2.
Expression of FFAR2 is reduced in the ileal mucosa after infection of LV-FFAR2 shRNA. A, schematic representation of the working hypothesis. B, Western blot of the presence of FFAR2 in the ileal mucosa of rats. C–E, representative Western blots and quantitative analysis of FFAR2 protein expression normalized to β-actin in the ileum (C), jejunum (D), and liver (E) of rats with ileal infection of either LV-FFAR2 shRNA or LV-MM (*, p < 0.05; calculated by unpaired t test; n = 4 for each).
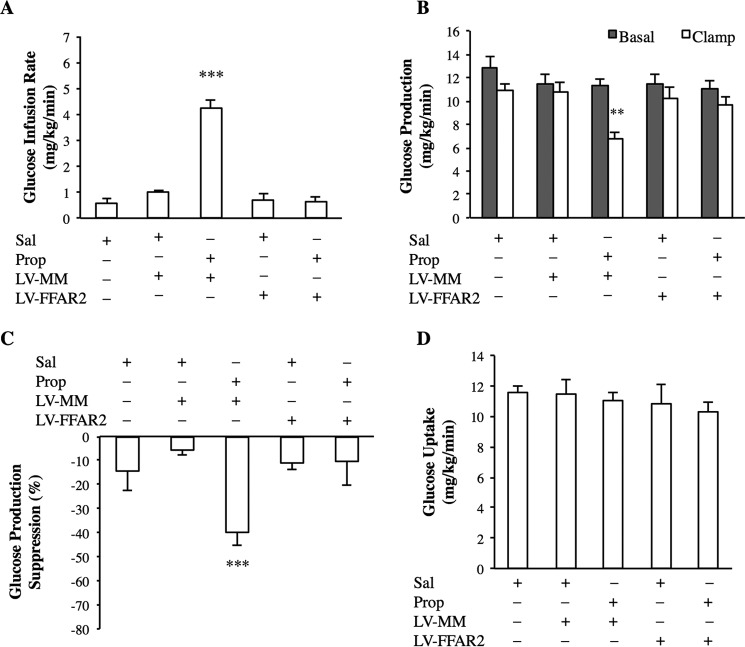
Rats injected with LV-MM or LV-FFAR2 had similar glucose kinetics in comparison to non-viral rats following a 50-min intraileal saline infusion (Fig. 3, A–D). Intraileal infusion of propionate for 50 min increased the glucose infusion rate (Fig. 3A) and lowered GP (Fig. 3, B and C), but did not alter the rate of glucose utilization (Fig. 3D) in LV-MM-injected control rats compared with intraileal saline infusion. Importantly, no difference in the glucose infusion rate, GP, or glucose uptake in LV-FFAR2 shRNA-injected rats infused with intraileal propionate was detected compared with intraileal saline-infused rats (Fig. 3, A–D).
FIGURE 3.
Intraileal propionate infusion activates ileal mucosal FFAR2 to lower glucose production. A–D, glucose infusion rate (A), rate of GP (B), % suppression of GP (C), and glucose uptake (D) during the pancreatic clamp in rats with either ileal LV-FFAR2 shRNA or LV-MM infection or no infection, infused with intraileal saline (n = 5 per group) or propionate (n = 6 per group). Values are shown as mean ± S.E. **, p < 0.01; ***, p < 0.001, versus all other groups as determined by ANOVA with Tukey's post hoc test.
To further establish site specificity and rule out the possibility that our ileal propionate infusion is activating colonic sensing mechanisms to lower GP, we performed an identical experiment to that above, except that LV-FFAR2 shRNA was injected into the proximal colon 3 days before clamp studies. Intraileal propionate infusion still resulted in a high glucose infusion rate (4.7 ± 0.4 mg kg−1 min−1) needed to maintain euglycemia, and lowered GP from basal (11.7 ± 0.3 versus 5.3 ± 0.3 mg kg−1 min−1; basal versus clamp) in rats with proximal colon LV-FFAR2 shRNA injection (n = 5). Taken together, intraileal propionate sensing activates ileal mucosa FFAR2 to lower GP in vivo.
An Ileal GLP-1 Receptor-dependent Neuronal Network Is Necessary for Propionate Sensing
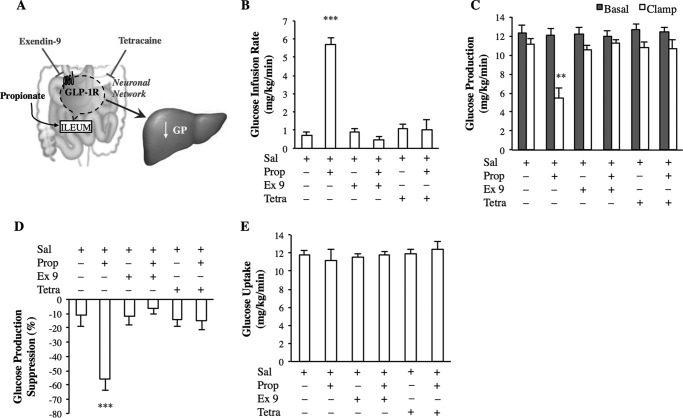
Given that intestinal FFAR2 activation increases GLP-1 secretion (12, 13), we investigated whether ileal GLP-1R signaling is necessary for the glucose-regulatory effect of propionate (Fig. 4A). Infusion of exendin-9, a GLP-1R antagonist, alone into the ileal lumen had no effect on glucose metabolism, but co-infusion with propionate abolished the ability of the intraileal 50-min infusion of propionate to increase the glucose infusion rate (Fig. 4B), and lower GP (Fig. 4C, D). There were no changes in the rate of glucose uptake detected among the groups (Fig. 4E). In light of the fact that both ileal FFAR2 knockdown and GLP-1R inhibition negated the ability of propionate to lower GP and FFAR2 activation increases GLP-1 release, it is likely that GLP-1R signaling (hence the increase of GLP-1 levels) in the gut is a downstream mediator of the ileal propionate-FFAR2 axis in lowering GP. Future studies are warranted to address this working hypothesis.
FIGURE 4.
GLP-1R signaling and a neuronal network are required for ileal propionate sensing. A, schematic representation of the working hypothesis. B–E, the glucose infusion rate (B), rate of GP (C), % suppression of GP (D), and glucose uptake (E) during the pancreatic clamp in rats with intraileal exendin-9 (Ex 9) and tetracaine (Tetra) administration with saline or in combination with propionate (n = 6 for all groups). Values are shown as mean ± S.E. **, p < 0.01; ***, p < 0.001, versus all other groups as determined by ANOVA with Tukey's post hoc test.
As gut vagal afferent innervation is necessary for the metabolic effect of intestinal GLP-1 action (34), we next assessed the involvement of a neuronal network (Fig. 4A). Although the intraileal 50-min infusion of tetracaine, a local anesthetic, alone had no effect on glucose homeostasis, tetracaine negated the ability of the intraileal 50-min infusion of propionate to increase the glucose infusion rate (Fig. 4B) and decrease GP (Fig. 4, C and D), independent of changes in the rate of glucose uptake (Fig. 4E). These results indicate that ileal propionate activates ileal FFAR2 signaling to trigger a GLP-1R-dependent neuronal network to lower GP in vivo.
Metabolism of LCFA in the Ileum and a GLP-1 Receptor-dependent Neuronal Network Are Necessary for Ileal LCFA Sensing
In addition to SCFAs, several studies suggest that the ileal mucosa comes in contact with a significant amount of ingested lipids (23, 29, 35). For example, 60 min following administration, ∼12% of a radiolabeled IL load is present in the ileum (29). Therefore, given this, and the fact that intra-duodenal and -jejunal IL infusion triggers a neuronal-dependent negative feedback pathway to lower GP (14, 18), we first infused 5% IL into the ileal lumen of healthy rats for 50 min to activate ileal lipid sensing and evaluate the impact it has on glucose regulation. IL was infused at a dose one-fourth of that of previous duodenal studies (18), as IL is detected in a lower quantity in the ileum when compared with the duodenum following intragastric administration (29). Intraileal 50-min infusion of IL increased the glucose infusion rate (Fig. 5A) and lowered GP (Fig. 5, B and C), compared with saline vehicle alone infusion, with no changes detected in glucose uptake (Fig. 5D).
FIGURE 5.
The effect of intraileal Intralipid infusion on glucose kinetics. A–D, the glucose infusion rate (A), rate of GP (B), % suppression of GP (C), and glucose uptake during the pancreatic clamp in rats with intraduodenal saline (Sal; n = 6) or Intralipid (IL; n = 6) infusions. Values are shown as mean ± S.E. **, p < 0.01; ***, p < 0.001, versus all other groups as determined by ANOVA with Tukey's post hoc test.
To further characterize ileal lipid sensing mechanisms, we next infused either the monounsaturated fatty acid, OA, or the polyunsaturated fatty acid, LA, solubilized in 1% bile acid (Fig. 6A), at a dose of 200 mm, which has previously been shown to stimulate gut peptide release in the ileum (23, 31). OA and LA were chosen based on the fact that IL is composed mainly of OA and LA, and that both LCFAs stimulate gut peptide release (36). Independent intraileal 50-min infusion of either OA or LA increased the glucose infusion rate (Fig. 6B) and lowered GP (Fig. 6, C and D), compared with 1% bile acid vehicle alone infusion, with no change in glucose utilization (Fig. 6E) or plasma insulin levels (Table 1). This GP-lowering effect of OA or LA was preabsorptive, as plasma FFA levels were not elevated following OA, LA, or control in the intraileal 50-min infusions (Table 1), and intravenous infusions of OA or LA at the same dose and duration as intraileal LCFA infusion did not alter whole body glucose metabolism (Fig. 6, B–E). Our findings illustrate a novel ileal LCFA sensing pathway that lowers GP in vivo.
FIGURE 6.
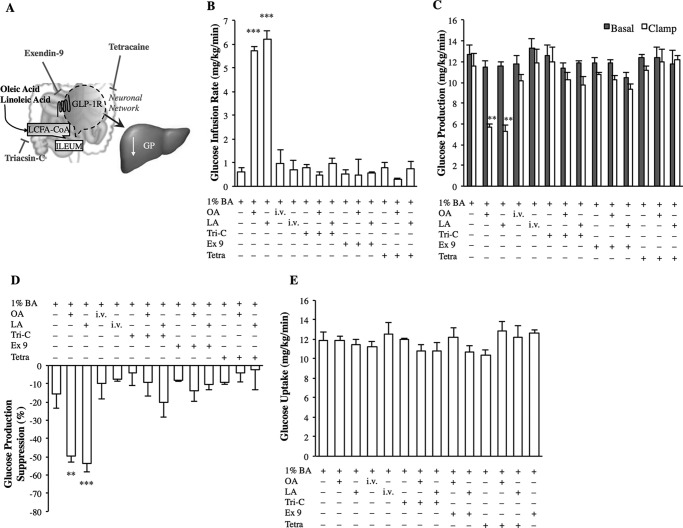
Ileal LCFA metabolism and a GLP-1R dependent network are necessary for OA and LA sensing to lower glucose production. A, schematic representation of the working hypothesis. B–E, the glucose infusion rate (B), rate of GP (C), % suppression of GP (D), and glucose uptake (E) during the pancreatic clamp in rats with intraileal or intravenous infusion of vehicle (1% bile acids), oleic acid (OA), or linoleic acid (LA), or intraileal infusion of triacsin-C (Tri-C), exendin-9 (Ex 9), or tetracaine (Tetra) alone or coinfused with either OA or LA (n = 6 for all groups). Values are shown as mean ± S.E. **, p < 0.01; ***, p < 0.001, versus all other groups except LA or OA, as determined by ANOVA with Tukey's post hoc test.
Esterification of duodenal and jejunal LCFAs into LCFA-CoA via acyl-C synthase is required to lower GP (14, 18). In the ileum, we here discovered that intraileal coinfusion of OA or LA with triacsin-C, an inhibitor of long-chain acyl-CoA synthase (Fig. 6A), abolished the ability of intraileal OA or LA 50 min infusion to increase the glucose infusion rate and lower GP, whereas triacsin-C + 1% bile acid vehicle alone into the ileum did not affect glucose metabolism (Fig. 6, B–E). These findings alternatively confirm that ileal LCFA preabsorptive sensing lowers GP and that metabolism of LCFAs in the ileum is a necessary step.
Last, we determined if an ileal GLP-1R-dependent neuronal network not only mediates the GP-lowering effect of ileal SCFA but also LCFA sensing (Fig. 6A). Intraileal infusion of exendin-9 + 1% bile acid vehicle alone had no effect on glucose metabolism (Fig. 6, B–E), but co-infusion with either OA or LA intraileally negated the ability of either intraileal infusion of OA or LA to increase the glucose infusion rate (Fig. 6B) and decrease GP (Fig. 6, C and D). Furthermore, whereas tetracaine + 1% bile acid vehicle alone had no effect on glucose homeostasis (Fig. 6, B–E), co-infusion of tetracaine with OA or LA abolished the ability of the intraileal 50-min infusion of OA or LA to increase the glucose infusion rate (Fig. 6B) and decrease GP (Fig. 6, C and D). Thus, although the physiological relevance of ileal fatty acid sensing remains to be investigated, the current study suggests a mechanistic pathway for ileal fatty acids to decrease GP through a GLP-1R-dependent neuronal network in vivo.
Discussion
We here demonstrate, for the first time to our knowledge, that propionate sensing in the ileum triggers an ileal FFAR2 negative feedback pathway to lower GP through a GLP-1R-dependent neuronal network in vivo. This is in line with the fact that FFAR2−/− (but not FFAR3−/−) mice have reduced circulating GLP-1 levels that are associated with an impaired response to an oral glucose tolerance test (12), whereas the metabolic improvements of SCFA supplementation is maintained in FFAR3−/− mice (5). Our current discoveries not only directly integrate these associative findings for the first time, but also demonstrate site-specificity as the propionate-FFAR2-GLP-1 signaling axis in the ileum potently lowers GP in vivo. These findings highlight ileal FFAR2 as a potential mediator for the improved glucose homeostasis observed following SCFA supplementation either via direct intake of a SCFA-enriched meal or prebiotic treatment that increases SCFA production (5, 38, 39). Furthermore, given that gastric bypass surgery in rodents associates with a change in gut microbiota (i.e. stimulating SCFA production) and decrease in the acetate:propionate ratio (4), the currently described ileal propionate-FFAR2 signaling axis may also mediate the glucose-lowering effect of gastric bypass surgery, although these hypotheses clearly remain to be directly tested.
Of note, the ileal GLP-1 signaling pathway not only mediates propionate but also LCFA oleate and linoleate sensing in the ileum to lower GP, consistent with the fact that both LCFAs and SCFAs stimulate GLP-1 release (13, 36). In addition, our discoveries illustrate that a neuronal network is necessary for ileal SCFA and LCFA sensing to lower GP independent of changes in plasma insulin levels. Thus, in addition to the well described incretin effect of GLP-1, our findings support an additional working hypothesis that suggest ileal fatty acids stimulate local GLP-1 release to act through an ileal GLP-1R-dependent paracrine neuronal axis to lower GP. This working hypothesis is supported by the fact that SCFAs, LCFAs, and GLP-1 trigger vagal firing (25, 40, 41), GLP-1Rs are expressed in the nodose ganglia that contains the cell bodies of vagal afferents (42), GLP-1 is rapidly degraded in circulation (43), and that the GLP-1R is in close proximity to L-cells (37). Although the direct site of vagal activation remains to be delineated (i.e. portal versus intestinal), our findings are in line with the notion that intestinal vagal afferent firing is necessary for nutrient-induced GLP-1 signaling to regulate glucose homeostasis (34). While the physiological relevance of ileal fatty acid sensing remains to be investigated, the current study suggests the presence of a mechanistic ileal fatty acid sensing pathway involved in glucose regulation. Interestingly, as more undigested LCFAs could reach the ileum after bariatric surgery, future studies are warranted to address the relevance of ileal LCFA sensing and assess the relative contribution of jejunal (14) versus ileal nutrient sensing in the anti-diabetic effect of bariatric surgery.
In summary, we unveil that ileal short and long chain fatty acid sensing machinery triggers a GLP-1R-dependent negative feedback neuronal network to lower GP and regulate glucose homeostasis in rodents. Furthermore, we highlight FFAR2 in the ileum as a mechanistic target for lowering GP. Specifically, we postulate that microbiota-derived propionate activates FFAR2 in the ileal enteroendocrine cells to release GLP-1 and triggers local vagal GLP-1R to lower GP (Fig. 7). We propose changes in the fatty acid sensing mechanisms in the ileum regulate glucose homeostasis.
FIGURE 7.
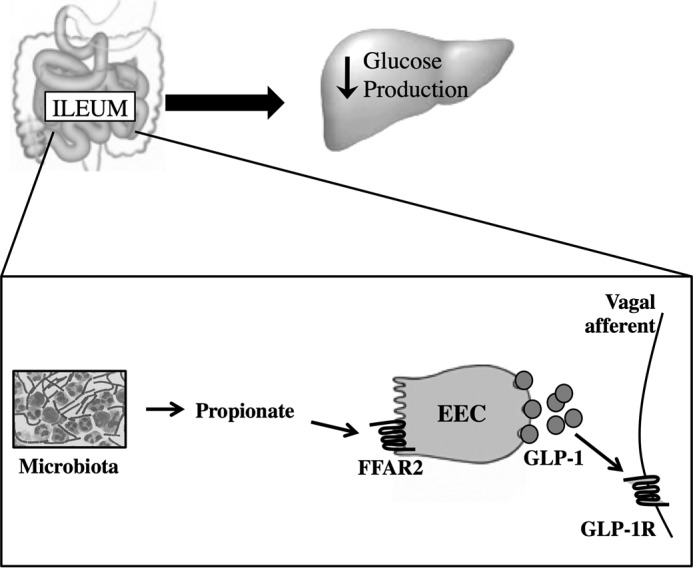
Proposed pathway for ileal propionate sensing to lower glucose production. In the ileum, microbiota-produced propionate activates FFAR2, localized in the enteroendocrine cells, and induces GLP-1 secretion. GLP-1 binds to GLP-1Rs located on the vagal afferent neurons, to ultimately reduce GP.
Author Contributions
M. Z-T. conducted and designed experiments, performed data analyses, and wrote the manuscript. F. A. D. edited the manuscript and conducted and assisted with experiments. B. A. R., P. V. B., C. D. C., and B. M. F. assisted with experiments. T. K. T. L. supervised the project, designed experiments, and edited the manuscript. T. K. T. L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by Canadian Institutes of Health Research Foundation Grant FDN-143204 (to T. K. T. L.). The authors declare that they have no conflicts of interest with the contents of this article.
- SCFA
- short chain fatty acid
- FFAR2
- fatty acid receptor 2
- FFAR3
- fatty acid receptor 3
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- glucagon-like peptide-1 receptor
- GP
- glucose production
- IL
- Intralipid
- LA
- linoleic acid
- LCFA
- long chain fatty acid
- OA
- oleic acid
- LV
- lentivirus
- ANOVA
- analysis of variance.
References
- 1. Tilg H., and Moschen A. R. (2014) Microbiota and diabetes: an evolving relationship. Gut 63, 1513–1521 [DOI] [PubMed] [Google Scholar]
- 2. Frost G., Sleeth M. L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L., Anastasovska J., Ghourab S., Hankir M., Zhang S., Carling D., Swann J. R., Gibson G., Viardot A., Morrison D., Louise Thomas E., and Bell J. D. (2014) The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5, 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao Z., Yin J., Zhang J., Ward R. E., Martin R. J., Lefevre M., Cefalu W. T., and Ye J. (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liou A. P., Paziuk M., Luevano J. M. Jr., Machineni S., Turnbaugh P. J., and Kaplan L. M. (2013) Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 5, 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin H. V., Frassetto A., Kowalik E. J. Jr., Nawrocki A. R., Lu M. F., Kosinski J. R., Hubert J. A., Szeto D., Yao X., Forrest G., and Marsh D. J. (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PloS One 7, e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chambers E. S., Viardot A., Psichas A., Morrison D. J., Murphy K. G., Zac-Varghese S. E., MacDougall K., Preston T., Tedford C., Finlayson G. S., Blundell J. E., Bell J. D., Thomas E. L., Mt-Isa S., Ashby D., et al. (2015) Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown A. J., Goldsworthy S. M., Barnes A. A., Eilert M. M., Tcheang L., Daniels D., Muir A. I., Wigglesworth M. J., Kinghorn I., Fraser N. J., Pike N. B., Strum J. C., Steplewski K. M., Murdock P. R., Holder J. C., Marshall F. H., Szekeres P. G., Wilson S., Ignar D. M., Foord S. M., Wise A., and Dowell S. J. (2003) The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 [DOI] [PubMed] [Google Scholar]
- 8. Le Poul E., Loison C., Struyf S., Springael J. Y., Lannoy V., Decobecq M. E., Brezillon S., Dupriez V., Vassart G., Van Damme J., Parmentier M., and Detheux M. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 [DOI] [PubMed] [Google Scholar]
- 9. Kaji I., Karaki S., Tanaka R., and Kuwahara A. (2011) Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J. Mol. Histol. 42, 27–38 [DOI] [PubMed] [Google Scholar]
- 10. Li G., Su H., Zhou Z., and Yao W. (2014) Identification of the porcine G protein-coupled receptor 41 and 43 genes and their expression pattern in different tissues and development stages. PLoS ONE 9, e97342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nøhr M. K., Pedersen M. H., Gille A., Egerod K. L., Engelstoft M. S., Husted A. S., Sichlau R. M., Grunddal K. V., Poulsen S. S., Han S., Jones R. M., Offermanns S., and Schwartz T. W. (2013) GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154, 3552–3564 [DOI] [PubMed] [Google Scholar]
- 12. Tolhurst G., Heffron H., Lam Y. S., Parker H. E., Habib A. M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., and Gribble F. M. (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Psichas A., Sleeth M. L., Murphy K. G., Brooks L., Bewick G. A., Hanyaloglu A. C., Ghatei M. A., Bloom S. R., and Frost G. (2015) The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. (Lond) 39, 424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breen D. M., Rasmussen B. A., Kokorovic A., Wang R., Cheung G. W., and Lam T. K. (2012) Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat. Med. 18, 950–955 [DOI] [PubMed] [Google Scholar]
- 15. Cheung G. W., Kokorovic A., Lam C. K., Chari M., and Lam T. K. (2009) Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 10, 99–109 [DOI] [PubMed] [Google Scholar]
- 16. Côté C. D., Rasmussen B. A., Duca F. A., Zadeh-Tahmasebi M., Baur J. A., Daljeet M., Breen D. M., Filippi B. M., and Lam T. K. (2015) Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat. Med. 21, 498–505 [DOI] [PubMed] [Google Scholar]
- 17. Duca F. A., Côté C. D., Rasmussen B. A., Zadeh-Tahmasebi M., Rutter G. A., Filippi B. M., and Lam T. K. (2015) Metformin activates a duodenal AMPK-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 21, 506–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang P. Y., Caspi L., Lam C. K., Chari M., Li X., Light P. E., Gutierrez-Juarez R., Ang M., Schwartz G. J., and Lam T. K. (2008) Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 452, 1012–1016 [DOI] [PubMed] [Google Scholar]
- 19. van Avesaat M., Troost F. J., Ripken D., Hendriks H. F., and Masclee A. A. (2015) Ileal brake activation: macronutrient-specific effects on eating behavior? Int. J. Obes. (Lond) 39, 235–243 [DOI] [PubMed] [Google Scholar]
- 20. Trung V. N., Yamamoto H., Murata S., Kuwahara A., and Tani T. (2014) Ileal glucose infusion leads to increased insulin sensitivity and decreased blood glucose levels in Wistar rats. J. Invest. Surg. 27, 332–337 [DOI] [PubMed] [Google Scholar]
- 21. Booijink C. C., Zoetendal E. G., Kleerebezem M., and de Vos W. M. (2007) Microbial communities in the human small intestine: coupling diversity to metagenomics. Future Microbiol. 2, 285–295 [DOI] [PubMed] [Google Scholar]
- 22. Wang M., Ahrné S., Jeppsson B., and Molin G. (2005) Comparison of bacterial diversity along the human intestinal tract by direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol. Ecol. 54, 219–231 [DOI] [PubMed] [Google Scholar]
- 23. Iakoubov R., Ahmed A., Lauffer L. M., Bazinet R. P., and Brubaker P. L. (2011) Essential role for protein kinase Czeta in oleic acid-induced glucagon-like peptide-1 secretion in vivo in the rat. Endocrinology 152, 1244–1252 [DOI] [PubMed] [Google Scholar]
- 24. Schwartz A., Ort T., Kajekar R., Wade P. R., and Hornby P. J. (2010) Electrical stimulation of the isolated rat intestine in the presence of nutrient stimulus enhances glucagon-like peptide-1 release. Physiol. Meas. 31, 1147–1159 [DOI] [PubMed] [Google Scholar]
- 25. Randich A., Tyler W. J., Cox J. E., Meller S. T., Kelm G. R., and Bharaj S. S. (2000) Responses of celiac and cervical vagal afferents to infusions of lipids in the jejunum or ileum of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R34–43 [DOI] [PubMed] [Google Scholar]
- 26. Kokorovic A., Cheung G. W., Breen D. M., Chari M., Lam C. K., and Lam T. K. (2011) Duodenal mucosal protein kinase C-δ regulates glucose production in rats. Gastroenterology 141, 1720–1727 [DOI] [PubMed] [Google Scholar]
- 27. Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., and Macfarlane G. T. (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zoetendal E. G., Raes J., van den Bogert B., Arumugam M., Booijink C. C., Troost F. J., Bork P., Wels M., de Vos W. M., and Kleerebezem M. (2012) The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 6, 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodriguez M. D., Kalogeris T. J., Wang X. L., Wolf R., and Tso P. (1997) Rapid synthesis and secretion of intestinal apolipoprotein A-IV after gastric fat loading in rats. Am. J. Physiol. 272, R1170–1177 [DOI] [PubMed] [Google Scholar]
- 30. Woltman T., Castellanos D., and Reidelberger R. (1995) Role of cholecystokinin in the anorexia produced by duodenal delivery of oleic acid in rats. Am. J. Physiol. 269, R1420–1433 [DOI] [PubMed] [Google Scholar]
- 31. Dumoulin V., Moro F., Barcelo A., Dakka T., and Cuber J. C. (1998) Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology 139, 3780–3786 [DOI] [PubMed] [Google Scholar]
- 32. Regard J. B., Sato I. T., and Coughlin S. R. (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karaki S., Mitsui R., Hayashi H., Kato I., Sugiya H., Iwanaga T., Furness J. B., and Kuwahara A. (2006) Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 324, 353–360 [DOI] [PubMed] [Google Scholar]
- 34. Hayes M. R., Kanoski S. E., De Jonghe B. C., Leichner T. M., Alhadeff A. L., Fortin S. M., Arnold M., Langhans W., and Grill H. J. (2011) The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am. J. Physiol. Regul. Integr Comp. Physiol. 301, R1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Booth C. C., Read A. E., and Jones E. (1961) Studies on the site of fat absorption: 1. the sites of absorption of increasing doses of I-labelled triolein in the rat. Gut 2, 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Côté C. D., Zadeh-Tahmasebi M., Rasmussen B. A., Duca F. A., Lam T. K. (2014) Hormonal signaling in the gut. J. Biol. Chem. 289, 11642–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richards P., Parker H. E., Adriaenssens A. E., Hodgson J. M., Cork S. C., Trapp S., Gribble F. M., and Reimann F. (2014) Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63, 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cani P. D., Daubioul C. A., Reusens B., Remacle C., Catillon G., and Delzenne N. M. (2005) Involvement of endogenous glucagon-like peptide-1(7–36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J. Endocrinol. 185, 457–465 [DOI] [PubMed] [Google Scholar]
- 39. Pan X. D., Chen F. Q., Wu T. X., Tang H. G., and Zhao Z. Y. (2009) Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J. Zhejiang Univ. Sci. B 10, 258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lal S., Kirkup A. J., Brunsden A. M., Thompson D. G., and Grundy D. (2001) Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G907–915 [DOI] [PubMed] [Google Scholar]
- 41. Gaisano G. G., Park S. J., Daly D. M., and Beyak M. J. (2010) Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol. Motil. 22, 470–479 [DOI] [PubMed] [Google Scholar]
- 42. Nakagawa A., Satake H., Nakabayashi H., Nishizawa M., Furuya K., Nakano S., Kigoshi T., Nakayama K., and Uchida K. (2004) Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton. Neurosci. 110, 36–43 [DOI] [PubMed] [Google Scholar]
- 43. Holst J. J. (2007) The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 [DOI] [PubMed] [Google Scholar]