Abstract
The Rho family small GTPase Cdc42 has been implicated in a wide range of cellular functions including the establishment of cell polarity and the remodeling of the actin cytoskeletal architecture, resulting in the tight regulation of cell growth and survival during developmental processes. The complete knock-out of Cdc42 in the mouse is embryonic-lethal, and its targeted deletion in various tissues has been shown to disrupt tissue homeostasis. Thus far, in most studies, the targeted deletion of Cdc42 occurred during embryogenesis. Here, we have used a conditional gene deletion strategy in mice to probe the specific role of Cdc42 during adult mammary gland function. Cdc42 conditional-knock-out females were unable to adequately nourish their pups, due to a disorganized epithelial compartment within their mammary glands. A closer examination showed that their mammary epithelial cells were not able to maintain functional alveolar lumens, due to an inability to establish normal apical/basal epithelial polarity, as well as proper cell-cell contacts. Loss of these essential epithelial characteristics led to a premature sloughing off of the Cdc42-null epithelial cells. Overall our findings demonstrate that Cdc42 plays essential roles in mammary gland function post pregnancy, where it helps to establish proper epithelial cell polarity and tissue homeostasis during lactation.
Keywords: CDC42, cell polarity, mammary gland, signal transduction, signaling
Introduction
The Rho family GTPase Cdc42 has been shown to function as a key regulator in establishing and maintaining epithelial structures in a broad spectrum of multicellular organisms, ranging from Caenorhabditis elegans to Homo sapiens (1–3). In vitro two-dimensional and three-dimensional cell culture systems have provided molecular insights into how Cdc42 regulates the establishment and maintenance of epithelial cell polarity and morphology. In two-dimensional cultures of Madin-Darby canine kidney (MDCK)3 cells, Cdc42 was shown to be involved in the maintenance of epithelial cell morphology, by regulating polarized membrane transport, cell-cell adhesion, and cytoskeletal remodeling (4). Studies using a three-dimensional cell culture system comprised of MDCK cells suggested that Cdc42 is necessary for proper apical membrane specification (5, 6), while an analogous system using colorectal carcinoma Caco-2 cells showed that Cdc42 regulates directional vesicular trafficking and mitotic spindle orientation, but not apical-basal cell polarity during cyst formation (7). Together, these findings demonstrate that Cdc42 regulates epithelial cell morphology at multiple levels, and that its specific functional roles are dependent on the cellular context.
Those actions of Cdc42 that ensure the proper maintenance of epithelial structures served as a forecast of critically important functions for this GTPase in various developmental processes. Indeed, a number of studies using conditional knock-out (CKO) mice have shown that the deletion of Cdc42 in epithelial stem/progenitor cells from a variety of tissues results in the disruption of intact epithelial structures, leading to severe and even lethal defects in embryonic organogenesis and tissue homeostasis (8, 9). Defects in epithelial structure that accompany the deletion of Cdc42 have been suggested to affect cell fate determination, proliferation, survival, and differentiation during embryonic development (10–14).
The importance of Cdc42 in the development of the mammary gland, prior to pregnancy, has been suggested from studies using transgenic mice, as well as from experiments performed with primary epithelial cells derived from conditional knock-out mice. The virgin mouse mammary gland contains sparse ductal networks that extend into the mammary fat pad and culminate in terminal end bud units. Upon pregnancy, the mammary gland becomes a high-output secretory tissue, largely through the signaling cues of prolactin and progesterone, which instruct the virgin epithelial bed to undergo rapid proliferation to fill the entire mammary fat pad with alveoli that are capable of milk secretion (15). At the time of parturition, luminal epithelial differentiation and lactogenesis begin within the alveoli, and continue through the early stages of newborn life, providing sufficient nutrients to sustain the significant and rapid growth rates of the suckling neonates. After weaning, the expanded milk-producing alveoli undergo massive-scale apoptosis, termed involution, with the entire mammary gland regressing back to a pre-pregnancy-like state, awaiting the next round of pregnancy and lactation (15).
The inducible expression of Cdc42 in pre-pregnant mammary ductal epithelial cells caused hyper-branching of mammary ducts and deformation of terminal end bud units (16). A modest increase in Cdc42 expression (1.5-fold) was sufficient for pre-pregnant mammary ductal epithelial cells to exhibit more invasive phenotypes, thus suggesting that the tight regulation of Cdc42 function is essential for this stage of mammary gland development (16). Studies in three-dimensional cell culture model systems, using pre-pregnant stage primary mammary epithelial cells, showed that the deletion of Cdc42 inhibited acinar formation by causing defects in apical-basal cell polarity, cell-cell contact, mitotic spindle orientation, cell proliferation, and cell survival (17). Additionally, the expression of the dominant negative Cdc42(T17N) mutant also inhibited the establishment of acinar structures and, consequently, prolactin-dependent synthesis of milk proteins in mammary epithelial cells (18). However, introducing the Cdc42(T17N) mutant into established acini did not affect prolactin-induced milk production, suggesting that Cdc42 is essential for establishing acinar structures but not for milk production in mammary acini (18).
While the studies described above show that Cdc42 plays an important part in normal mammary development before pregnancy, thus far, it has not been demonstrated whether Cdc42 exerts additional in vivo functions during the entire process of lactation. Therefore, to investigate the importance of Cdc42 in mammary alveolar epithelial cells during lactation, we generated conditional knock-out mice in which Cdc42 is deleted specifically in milk-producing alveolar epithelial cells. This was achieved by crossing floxed Cdc42 (Cdc42flox/flox) mice with transgenic mice expressing Cre recombinase under the control of the whey acidic protein (WAP) promoter (19). We found that the deletion of Cdc42 in the lactating alveolar cells of female mice prevented them from being able to sufficiently nourish their pups. This was due to a severe impairment of mammary alveoli formation, resulting from the loss of intact luminal epithelial structures and premature cell sloughing. Interestingly, alveolar cells in Cdc42 conditional knock-out (CCKO) lactating mammary glands still maintained their function as milk-producing cells, and did not exhibit significant defects in cell proliferation and survival during lactogenesis, nor did they show any sign of cellular apoptosis even within the time window when control glands underwent the early stages of involution. When taken together, our current findings show that the ability of Cdc42 to establish cell polarity and cell-cell contacts plays an essential role in ensuring the proper homeostasis of lactating mammary glands. Moreover, they demonstrate that Cdc42 is a key regulator of mammary gland development and function, not only during the pre-pregnancy developmental stage, but also during the post-pregnancy period of lactogenesis.
Experimental Procedures
Mouse Strains and Husbandry
Mice used in this study were housed and handled in accordance with the Cornell University Institutional Animal Care and Use Committee (IACUC). Cdc42flox/flox mice (controls) were crossed to WAP-Cre(+) Cdc42wt/flox mice to generate conditional deletion females genotyped as Cdc42flox/flox;WAP-Cre(+). These females were then mated at the age of 8 weeks, and removed from their breeding partner male after a mating plug was observed. For fostering experiments, litters were fostered to a timed mating-matched mother on day one post-partum.
For pup growth experiments, litter size was normalized to 6 pups by fostering, and then each pup was tail marked for identification. The mass of each pup was recorded daily until the age of 21 days, at which point they were weaned from their mother and placed on standard rodent chow. Pup growth measurement was repeated for five litters, resulting in the measurement of 30 pups total for each strain of mouse. For tissue collection, mice were euthanized by carbon dioxide inhalation, and then the fourth pair of mammary glands was harvested.
Antibodies
Antibodies used for Western blotting and immunohistochemistry or immunofluorescence were purchased from the following companies: anti-Cdc42 (ab64533), anti-Ki67 (ab15580), anti-aPKCζ (ab4139), anti-N-Cadherin (ab12221), anti-Twist (ab50581), anti-Cytokeratin 8 (ab59400), anti-TGFβ1 (ab92486), and anti-Cytokeratin 14 (ab53115) antibodies were from Abcam Inc; anti-phospho-Stat 5 (CS9359), anti-Snail (CS3879), anti-vinculin (CS 4650), anti-phospho-SMAD 2/3 (CS8828) and anti-E-Cadherin (CS3195) antibodies were from Cell Signaling Technology. Anti-cleaved caspase-3 (AB3623) antibody was purchased from EMD Millipore, anti-β-actin (MA5–15739) was purchased from ThermoFisher Scientific, and anti-Par 6 (25525) antibody was obtained from Santa Cruz Biotechnology.
SDS-PAGE and Western Blot Analyses
Immediately following euthanasia, the fourth pair of mammary glands was harvested from mice, frozen in liquid nitrogen, and ground using a mortar and pestle. The tissue powder was then suspended in triple detergent buffer (150 mm NaCl, 50 mm Tris-HCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 0.02% sodium azide, 1 mm sodium vanadate, pH 8.0) with protease inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin) and incubated on ice for 20 min. The extracted proteins were centrifuged at 13,000 rpm for 15 min at 4 °C, after which the supernatant was removed and used for Bio-Rad protein assays. Samples were subjected to polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Perkin Elmer). The membranes were blocked using 5% milk, and incubated overnight on a rocker at 4 °C with the primary antibodies at a dilution of 1:1000. Membranes were then washed three times for 5 min in TBST, and incubated with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) for 1 h, after which an ECL reagent (Perkin Elmer) was used to obtain chemiluminescence of the signal onto x-ray film.
Whole Mount Staining
Whole mount staining of the entire mammary gland was performed by spreading the gland onto a Superfrost Plus slide (VWR) and then placing another slide on top. These two slides were compressed together for 15 min, with the mammary gland between them, using two 2-inch binder clips. The top slide was then removed and the mammary glands were fixed in Carnoy's fixative (6 parts 100% ethanol, 3 parts chloroform, 1 part glacial acetic acid) for 6 h, after which they were rehydrated using a succession of 20-min ethanol washes (70%, 50%, 25%), and finally, distilled water. Epithelial staining was achieved by submerging the mammary glands (still mounted on one slide) in carmine aluminum staining solution, containing 0.2% carmine aluminum, 0.5% aluminum potassium sulfate, and a thymol crystal, overnight at room temperature. After staining, the tissues were dehydrated by performing 20-min ethanol washes (70%, 95%, 100%), followed by clearing of the tissue through two 20-min washes in mixed xylenes, and mounted and coverslipped using Permount. Imaging was performed using an Olympus SZ-11-CTV dissecting microscope fitted with an Olympus Camedia C-5050 digital camera.
Immunohistochemistry and Immunofluorescence
The fourth pair of mammary glands were harvested from pregnant or lactating females, rinsed briefly in PBS, and fixed in 4% paraformaldehyde for 15 h at 4 °C. These glands were embedded in paraffin, sectioned at 5-μm thickness, and placed onto VWR Superfrost slides.
For immunohistochemistry staining, the tissue sections were deparaffinized and rehydrated, and subjected to heat-induced epitope retrieval (HIER) using either an EDTA buffer (10 mm Tris base, 1 mm EDTA, 0.05% Tween 20, pH 9.0) or a sodium citrate buffer (10 mm sodium citrate, 0.05% Tween 20, pH 6.0). Antigen retrieval buffers were heated to 95 °C, at which point sample slides were added, and then kept at 95 °C for 15 min to induce antigen retrieval. Slides were left in the heated antigen retrieval buffer to cool at room temperature for 15 min, after which they were rinsed using three 5-min PBS washes. Samples were processed using an ImmunoCruz ABC staining kit (Santa Cruz) along with the primary antibodies, dehydrated by 5-min washes with varying amounts of ethanol (70%, 95%, 100%), cleared by two 5-min washes of mixed xylenes, and mounted with Permount (Fisher Scientific). Imaging was performed using an Olympus AX70 compound microscope equipped with a MicroFire camera and PictureFrame image processor (Optronics).
For immunofluorescence staining, tissue sections were deparaffinized, rehydrated, and subjected to HIER as above, and then blocked in 10% bovine serum albumin in PBS for one hour at room temperature in a moisture chamber. Primary antibody dilutions were added to the samples, which were replaced in the moisture chamber and incubated for 15 h at 4 °C. Samples were then removed from the primary antibody dilutions, and rinsed in four 5-min PBS washes, followed by incubation in Alexa Fluor 594-conjugated antibody (Life Technologies) for one hour at room temperature in a moisture chamber. Samples were rinsed in three 5-min PBS washes, and incubated in 4,6-diaminodino-2-phenylindole (Sigma Aldrich) at a 1:5000 dilution in double distilled water (ddH2O) for 5 min at room temperature. Samples were then rinsed using three 5-min ddH2O washes, and sealed with KPL Fluorescent Mounting Media. Epifluorescence imaging was performed on a Zeiss inverted Axioskop equipped with a COOKE Axiocam/Sensicam camera.
For Fast Green/Sirius Red staining, the tissue sections were deparaffinized by two 10-min washes of mixed xylenes (Fisher Scientific), and rehydrated by successive 5-min washes with different amounts of ethanol (100%, 95%, 70%), followed by two 5-min washes with ddH2O. The tissue sections were then immersed in a solution of 0.1% Fast Green, 0.1% Sirius Red, and 1.2% picric acid for 30 min at room temperature, dehydrated by 5-min washes with 95 and 100% ethanol, cleared with two 5-min washes of mixed xylenes, and mounted with Permount (Fisher Scientific).
Primary Mammary Epithelial Cell Isolation and Three-dimensional Culture
The fourth pair of mammary glands were immediately harvested from euthanized 12-week-old virgin Cdc42flox/flox female mice. Following the protocol from Lo et al. (20), the glands were minced briefly with two sterile razor blades, then digested at 37 °C with 100 rpm rotation in digestion buffer (2 g/liter trypsin (Invitrogen), 2 g/liter collagenase type-iv (Invitrogen), 5% v/v FBS (Invitrogen), and 5 μg/ml insulin (Sigma) in DMEM/F12 medium (Invitrogen)). Digested mammary glands were then centrifuged at 400 × g for 15 min at room temperature. The top 15 ml of fat cell-containing medium were pipetted off and resuspended in DMEM/F12, and then centrifuged again at 400 × g for 15 min at room temperature, along with the original resuspended pellet of organoids. The resulting pellets were then resuspended and combined in DMEM/F12 and centrifuged at 400 × g for 15 min at room temperature. This pellet was resuspended in DMEM/F12 containing 2 units/μl DNase 1 (Sigma) for 5 min at 37 °C with gentle swirling. The suspension was centrifuged at 400 × g for 15 min at room temperature, after which the pellet was resuspended in DMEM/F12 and pulse-centrifuged twice at 260 × g at room temperature. The pellet was resuspended in DMEM/F12 containing 1× insulin-transferrin-sodium selenite (ITS, Sigma) and 1× penicillin/streptomycin (PS, Invitrogen), and organoids were counted. The solutions were centrifuged at 260 × g for 15 min at room temperature, after which the pelleted organoids were resuspended in Growth Factor Reduced Matrigel (BD Biosciences), and plated in 8-well chamber slides at ∼200 organoids per well. DMEM/F12/ITS/PS (300 μl) were added to the top of the matrigel, and the organoids were cultured at 37 °C and 5% CO2 in an incubator overnight. After 24 h, the medium was exchanged with DMEM/F12/ITS/PS containing 9 nm TGFɑ (Sigma). After 6 days of culture, exchanging the DMEM/F12/ITS/PS/TGFɑ medium every second day, the organoids formed hollowed alveoli. Adenovirus was then added to the medium: control cultures were given Ad-CMV-GFP virus (Vector Biolabs) at approximately MOI 80, while experimental cultures were given Ad-Cre-GFP virus (Vector Biolabs) at approximately MOI 80. Cells were imaged on an Olympus CK2 inverted microscope with an Olympus Camedia C-5050 digital camera.
RNA Sequencing
The fourth pair of mammary glands was harvested from euthanized mice at lactation day 5, and flash frozen in liquid nitrogen and crushed with a mortar and pestle. The pulverized tissue was then homogenized using a Qiagen RNA Shredder kit, and RNA was immediately extracted using a Qiagen RNeasy kit. The extracted total RNA was then sequenced at the Cornell University Institute of Biotechnology Genomics Facility. RNA sequences were then analyzed using the TopHat Alignment and Cufflinks Assembly software on Illumina's BaseSpace website, and further analyzed using the iPathway Guide.
Results
Cdc42 Knock-out Mothers Are Unable to Sufficiently Nourish Pups
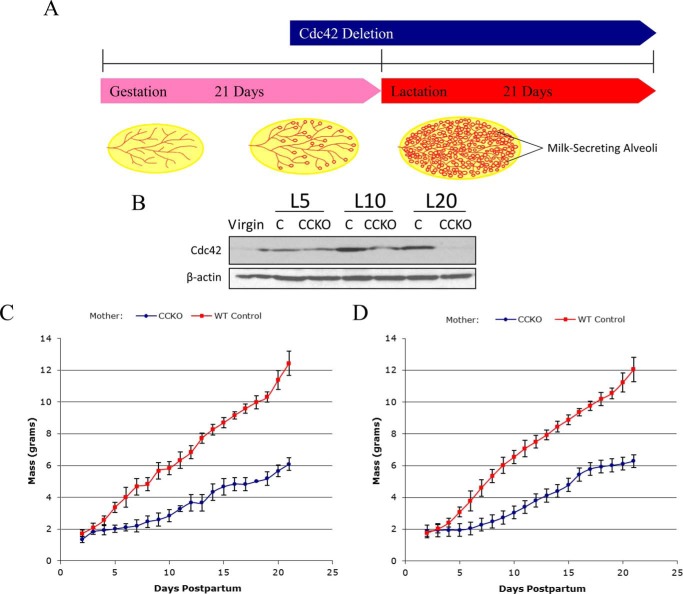
To knock-out Cdc42 in milk-producing alveolar epithelial cells, we mated Cdc42flox/flox mice (21) with transgenic mice carrying a Cre recombinase gene under the promoter region for Whey acidic protein (WAP), in which Cre recombinase is expressed specifically in mammary alveolar epithelial cells during lactation (Fig. 1A). Females that were genotyped either as Cdc42flox/flox; WAP-Cre(−) (control), or as Cdc42flox/flox; WAP-Cre(+) (CCKO), were then mated to induce pregnancy. Western blotting of lysates from the fourth pair of mammary glands showed a reduction in Cdc42 protein levels in CCKO mothers, compared with controls, as lactation progressed (Fig. 1B).
FIGURE 1.
The conditional deletion of Cdc42 in the lactating mammary gland stunts nursing pup growth. A, schematic timeline describing the conditional gene deletion strategy used in this study, with illustrations depicting the development of the mammary gland in each developmental stage. B, Western blot of tissue lysates showing whole-gland Cdc42 expression from the stages of virgin to lactation day 20 (L20), with β-actin serving as a loading control. C, growth plot depicting average weight of individual pups (n = 30) feeding from a CCKO mother (blue), or from a control mother (red), throughout lactation. Error bars represent the standard deviation to the mean pup mass at each given time point. D, growth plot depicting average weight of individual pups (n = 30) that were fostered from a control mother to a CCKO mother (blue), or from a CCKO mother to a control mother (red). Error bars represent the standard deviation to the mean pup mass at each given time point.
Pups nursing from CCKO females displayed severely stunted growth rates (Fig. 1C). To ensure that this phenotype was due to mammary gland defects in the CCKO mothers, and not a result of potential developmental defects in their pups, we fostered pups born to CCKO mothers to control mothers at day 1 post-partum, and vice-versa. Pups born to CCKO mothers had normal growth rates when feeding from a control mother (Fig. 1D), while pups born to the control mothers showed growth defects when feeding from a CCKO mother. After weaning, pups that had nursed from CCKO mothers were able to attain near-normal weights on standard rodent chow, and this growth was accelerated when a milk substitute was added to the food (data not shown). As an added control, pups born to a Cdc42flox/wt; WAP-Cre(+) mother were analyzed, and followed the same growth rates as pups nursing from the Cdc42flox/flox; WAP-Cre(−) control mothers (data not shown). Thus, the growth defects exhibited by pups were apparently due to an inhibited secretory output of the CCKO mothers, and not from developmental defects of the pups themselves, nor from Cre expression alone. The pups nursed from CCKO mothers did not exhibit increased mortality, thereby suggesting that CCKO mothers can produce a sufficient amount of milk for pup survival, but not enough to support their normal growth. The same growth defects of pups nursing from CCKO mothers were observed in a second round of lactation of CCKO mothers, after a complete period of involution (data not shown). Given these findings, we set out to perform a more detailed examination of mammary gland development in CCKO mice versus their control counterparts.
Cdc42 Knock-out Causes an Impaired Development of Lactating Mammary Glands
We first examined and compared the overall structures of the mammary glands from CCKO and control females. In whole mount images using carmine red staining, both control and CCKO mothers showed normal ductal epithelial development before pregnancy, and we did not detect any significant difference in ductal elongation, ductal branching, nor in the size and shape of terminal end buds (Fig. 2, panels a and b). However, as lactation proceeded, especially at the end of the lactation phase (lactation day 20), the alveolar units of CCKO females appeared significantly underdeveloped, compared with control females (Fig. 2, panels c and d). The mammary glands of control mothers showed mature, expanded alveoli, whereas the alveoli of CCKO mothers showed a significantly latent morphology (Fig. 2, arrowheads in panels e and f).
FIGURE 2.
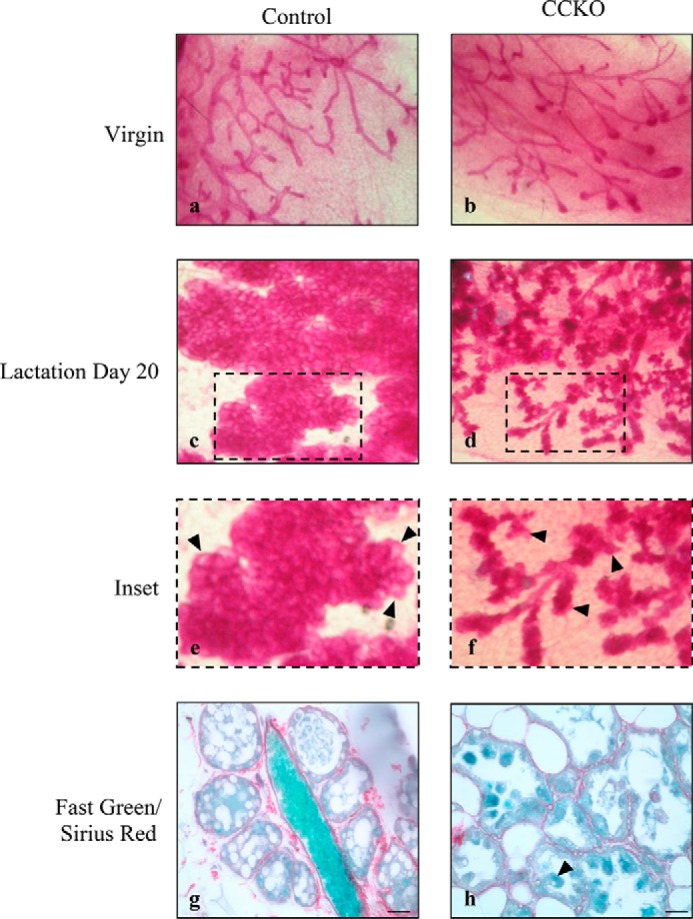
Conditional deletion of Cdc42 in mammary glands inhibits normal alveologenesis during lactation. Whole mount images of mammary glands using Carmine Red staining to visualize the epithelial network in control and CCKO mice in the virgin stage (a, b), and at lactation day 20 (c, d). e, f: insets from c, d with arrowheads to indicate fully expanded alveoli in control mice, or latent alveoli in CCKO mice. g, h: Fast Green and Sirius Red staining depicting the epithelial and collagen basement membrane structures in alveoli sections, respectively. Arrowhead in h indicates abnormally sloughing off of an epithelial cell. Scale bars represent 50 μm. The results are representative of the analysis of >3 mice each from control and CCKO groups.
A closer examination showed that the luminal epithelial cells of CCKO mothers exhibited a single-layered structure surrounded by collagen basement membrane, as displayed by staining with Fast Green and Sirius Red, respectively (Fig. 2, panel h). Thus, apparently the deletion of Cdc42 in mammary alveolar cells did not affect the transition of a multi-layered terminal bud unit into a single-layered alveolar epithelium. However, CCKO mammary glands lacked the normal epithelial organization found in control mothers. Alveolar cells of control mammary glands formed a smooth inner luminal surface within each acinus and exhibited a flattened squamous epithelial shape (Fig. 2, panel g). In contrast, many of the inner luminal surfaces of CCKO mammary glands were deformed, and the cells facing alveolar lumens commonly showed cuboidal epithelial structures (Fig. 2, panel h). We also noticed that several cells in the CCKO glands appeared to have sloughed off from the alveolar structures into the inner luminal cavity (Fig. 2, panel h, indicated by arrowhead).
Because the deletion or loss of Cdc42 function has been shown to cause impairments in cell proliferation and increased apoptosis in pre-pregnant mammary ductal epithelial cells (17, 18), we examined whether the deletion of Cdc42 in mammary luminal alveolar cells resulted in similar defects, thereby leading to latent alveologenesis. Prolactin-dependent signaling is critical for the proper morphogenesis and secretory capability of mammary gland cells during pregnancy and lactation (15). Activation of the prolactin receptor results in the phosphorylation and activation of STAT5 in lactating mammary glands (22, 23). We found that the phosphorylation levels of STAT5 appeared to be maintained and even elevated in CCKO mammary glands in the later stage of lactation (e.g. lactation day 20), compared with controls (Fig. 3A). These results were corroborated with immunofluorescence staining for Ki67, a marker for mitotic cells, of 5 μm-thick sections excised from control and CCKO mammary glands. At an earlier time point in lactation (lactation day 13), CCKO mammary glands showed only a modest increase in Ki67-positive nuclei, compared with control glands (Fig. 3B, top two panels). However, at a later time point (lactation day 20), CCKO mammary glands continued to display mitotic activity, while control glands ceased proliferating as they entered into the early stage of involution, such that there was no appreciable Ki67 staining (Fig. 3B, bottom two panels, and quantification in Fig. 3C).
FIGURE 3.
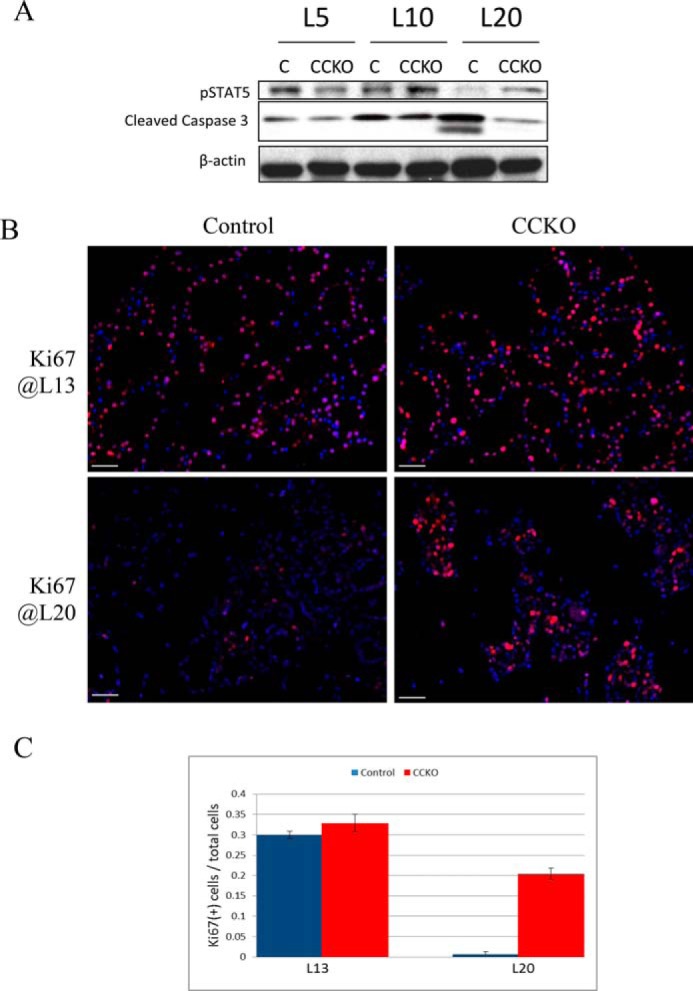
Changes in proliferation and apoptosis in CCKO mammary glands. A, Western blot of whole-gland tissue lysates depicting phosphorylated Stat5 (pStat5) and cleaved caspase 3 expression throughout lactation in the mammary gland, with β-actin serving as a loading control. L5, lactation day 5; L10, lactation day 10; L20, lactation day 20. B, immunofluorescence staining depicting Ki67 expression (red) in 5 μm-thick sections from the mammary glands of CCKO and control mice at lactation day 13 (L13, top row) and lactation day 20 (L20, bottom row). Nuclei are stained blue. Scale bars represent 50 μm. The results are representative of the analysis of >3 mice each from control and CCKO groups. C, quantification of Ki67(+) cells relative to total cells in control and CCKO samples from Fig. 3B. Error bars represent standard deviation to the mean across samples from 3 mice of each genotype group.
We then analyzed the apoptotic activities of control and CCKO mammary glands, using cleaved caspase 3 as a read-out. During lactation (i.e. lactation days 5 and 10), there was little or no difference in cleaved caspase 3 levels, when comparing lysates from control and CCKO mammary glands (Fig. 3A). However, at the end of lactation (L20), which is the early stage of involution, control glands showed an up-regulation of cleaved caspase 3 levels, whereas CCKO mammary glands did not (Fig. 3A, third panel from top). Taken together, these results indicate that the underdevelopment of the CCKO mammary glands is neither due to a reduction in cell proliferation nor to an increase in cellular apoptosis.
Deletion of Cdc42 Disrupts Epithelial Cell Polarity in Lactating Mammary Alveolar Cells
We next examined whether the defects in mammary gland development exhibited by the CCKO mice were related to alterations in the epithelial structure of the alveoli. At lactation day 13, most alveolar epithelial cells in CCKO mammary glands had much lower levels of Cdc42 expression, compared with control glands (Fig. 4, red arrows in panels a and b).
FIGURE 4.
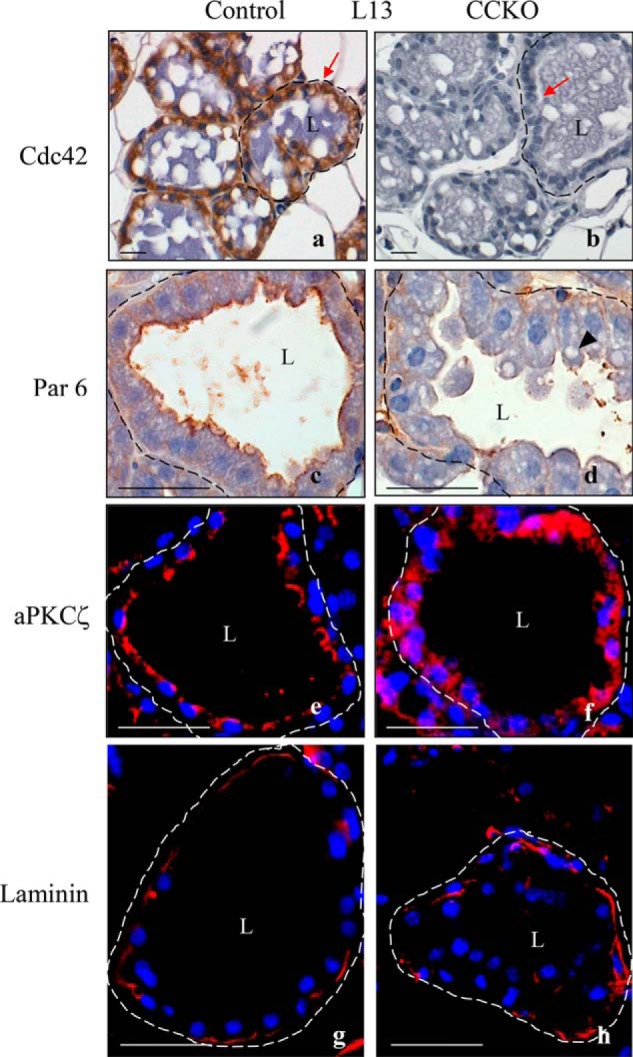
Cdc42 deletion disrupts apical/basal polarity in mammary alveolar epithelial cells. a, b, immunohistochemistry images of 5 μm-thick sections from lactation day 13 depicting Cdc42 expression (brown) in alveolar luminal epithelial cells of the mammary gland from a control mouse (red arrow in a), as compared with a CCKO mouse (red arrow in b). c, d: immunohistochemistry images depicting the localization of Par6 at lactation day 13. Arrowhead in d indicates lipid accumulations. e and f, immunofluorescence images showing the localization of aPKCζ (red) at lactation day 13. Nuclei are stained blue. g and h, immunofluorescence images depicting laminin deposition (red) surrounding an alveolus from control and CCKO mammary glands. Nuclei are stained blue. Dashed lines outline representative alveoli. L marks the central lumen of an alveolus. Scale bars represent 50 μm. The results are representative of the analysis of >3 mice each from control and CCKO groups.
The deformation of epithelial structures in CCKO alveolar tissue (Fig. 2, panels g and h) was more evident when cells were immunostained with the apical cell surface markers, Par6 and atypical PKCζ (aPKCζ). These two proteins form a complex with Cdc42 at the apical plasma membrane, controlling the establishment and maintenance of apical-basal cell polarity (24). In control mammary alveolar tissues, Par6 and aPKCζ were localized at the apical surface of the epithelial cells forming alveolar structures, as shown by immunohistochemistry and immunofluorescence images of 5-μm thick sections (Fig. 4, panels c and e, respectively). However, in CCKO mammary glands, the deformed epithelial cells exhibited only a partial apical membrane localization of Par6, whereas, aPKCζ lost its apical distribution and was diffusely distributed within the cell bodies (Fig. 4, panels d and f, respectively).
Since the de-regulation of Cdc42 has been shown to alter the composition of the extracellular matrix, as well as stromal-epithelial cell interactions in pre-pregnant mammary glands (16, 25), we examined whether Cdc42 deletion in mammary alveolar cells affected extracellular basement membrane structures. However, both control and CCKO alveolar tissues retained intact laminin deposition (Fig. 4, panels g and h), and we did not detect significant alterations in the presence of myoepithelial cells, when comparing cytokeratin 14-immunostaining between control and CCKO mammary alveoli (data not shown).
Deletion of Cdc42 Disrupts Epithelial Cell Morphology in Lactating Mammary Alveolar Cells
We next examined cell-cell adhesion structures in control and CCKO lactating mammary alveolar tissues. Immunofluorescence images revealed that control alveolar tissues at lactation day 13 expressed Cdc42 and E-cadherin, and that E-cadherin was localized at basolateral membranes and especially at the cell-cell contacts of epithelial cells (Fig. 5A, panels a and c). In CCKO alveolar tissues, Cdc42 deletion was accompanied by E-cadherin being diffusely dispersed within the cell bodies and no longer congregated at cell-cell contacts (Fig. 5A, panels b and d). Collectively, these results suggest that Cdc42 is necessary for the maintenance of cell-cell adhesion as well as apical-basal cell polarity in established mammary epithelial cells, such that its loss leads to disorganized epithelial structures in mammary alveolar glands.
FIGURE 5.
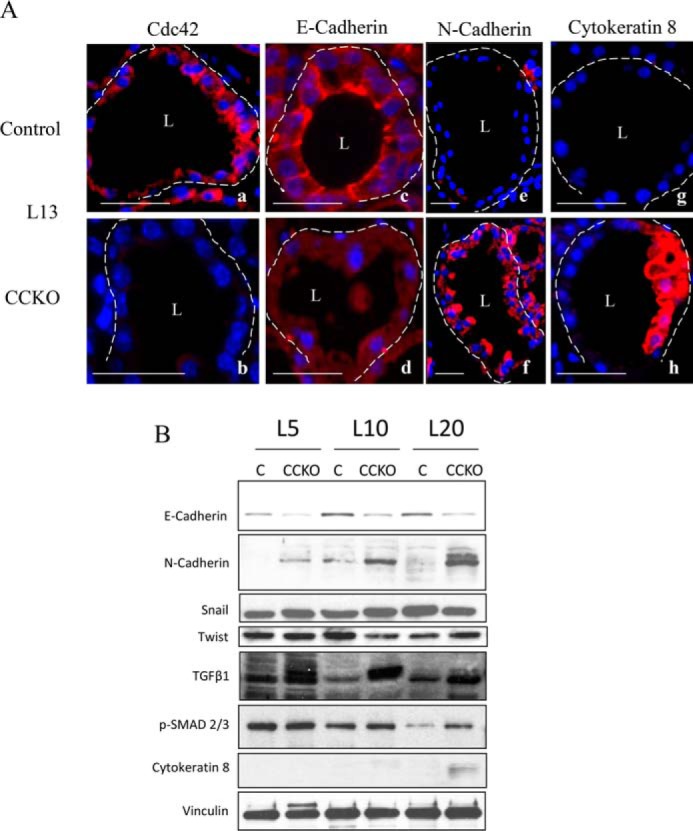
Epithelial identity changes in CCKO mammary alveolar epithelial cells. A, immunofluorescence staining (red) in 5 μm-thick sections from representative control and CCKO mammary alveoli at lactation day 13 depicting the expression and cellular localization of Cdc42 (a and b), E-cadherin (c and d), N-cadherin (e and f), and cytokeratin 8 (g and h). Nuclei are blue. Dashed lines outline representative alveoli. L marks the central lumen of an alveolus. Scale bars represent 50 μm. The results are representative of the analysis of >3 mice each from control and CCKO groups. B, Western blot of whole mammary gland tissue lysates probed for E-cadherin, N-Cadherin, Snail, Twist, TGFβ1, phospho-SMAD 2/3, and cytokeratin 8 throughout lactation, with vinculin serving as a loading control.
E-cadherin expression was down-regulated in CCKO mammary glands, beginning as early as lactation day 5 and persisting throughout the entire lactation phase, accompanied by an increased expression of N-cadherin (Fig. 5B). This alternation in the cadherin expression profiles was observed in luminal mammary alveolar cells (Fig. 5A, panels c–f). An overall shift in the expression of E-cadherin to N-cadherin often indicates an epithelial to mesenchymal transition (EMT) (26–28). We also found that TGFβ1 signaling through SMAD 2/3 appears to be increased in CCKO glands (Fig. 5B). However, the expression of Snail, which represses E-Cadherin expression and thereby promotes EMT in several tissues during development (26–28), was not changed in control versus CCKO glands, nor was Twist, another marker of EMT (Fig. 5B). Thus, these findings suggest that a full EMT has not occurred.
To identify the differentiation status of the cells comprising the CCKO mammary glands, we examined several markers for mammary epithelial cells by immunofluorescence and found that the expression of cytokeratin 8 (CK8) was up-regulated in CCKO alveoli throughout lactation, as compared with controls (Fig. 5A, panels g and h, Fig. 5B). Importantly, although general epithelial structures were disrupted in CCKO glands, lipid accumulation and casein micelles were still apparent in CCKO glands (Fig. 4, panel d, indicated by arrowhead), suggesting that the deformed epithelial cells retained the ability to produce milk.
Deletion of Cdc42 Causes Premature Cell Exfoliation in Lactating Mammary Alveoli
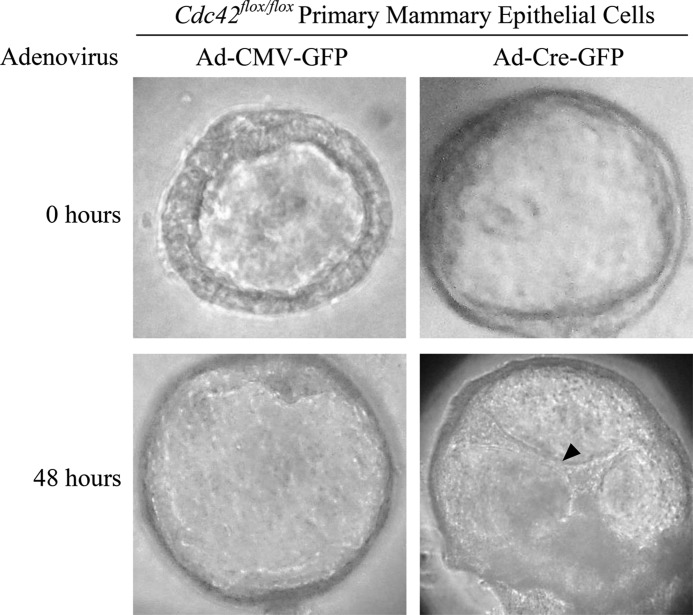
In the preceding sections, we showed that CCKO mammary glands failed to exhibit properly intact epithelial structures, leading to an abnormal alveolar architecture that contained cells which were detached from the epithelial planes and found within the luminal cavities (Fig. 2, panel h, indicated by arrowhead). Studies performed using the three-dimensional culture of primary mammary epithelial cells isolated from Cdc42flox/flox mice showed that the deletion of Cdc42 (via adenovirus-mediated Cre expression) in antecedently-formed mammary acini caused the epithelial cells to slough off or migrate into the alveolar lumen (indicated by an arrowhead, Fig. 6). This provided us with a clue regarding the reason for the apparent under-development of the alveoli in CCKO mice (Fig. 2), and led us to examine whether this phenotype was the result of the mammary alveolar epithelial cells sloughing off into the lumen and being removed by nursing pups. To examine this possibility, we removed the nursing pups from control and CCKO mothers for five hours before harvesting the mammary glands at lactation day 19, thereby allowing any content within the mammary alveolar lumens to accumulate, instead of being removed from the ductal network and ingested by the nursing pups. Upon this treatment, CCKO mammary glands showed an accumulation of Cdc42-negative cells collected within the luminal cavities, while control glands did not (Fig. 7).
FIGURE 6.
Cdc42 deletion causes luminal filling in primary mammary epithelial cells. Phase contrast images depicting primary mammary epithelial cells forming acini with hollowed lumens at the time of adenovirus transduction (top panels). Forty-eight hours after transduction, control (Ad-CMV-GFP) acini retain hollowed lumens (bottom left panel), while Cdc42-deletion (Ad-Cre-GFP) acini display cells that have migrated into the lumen (arrowhead, bottom right panel).
FIGURE 7.
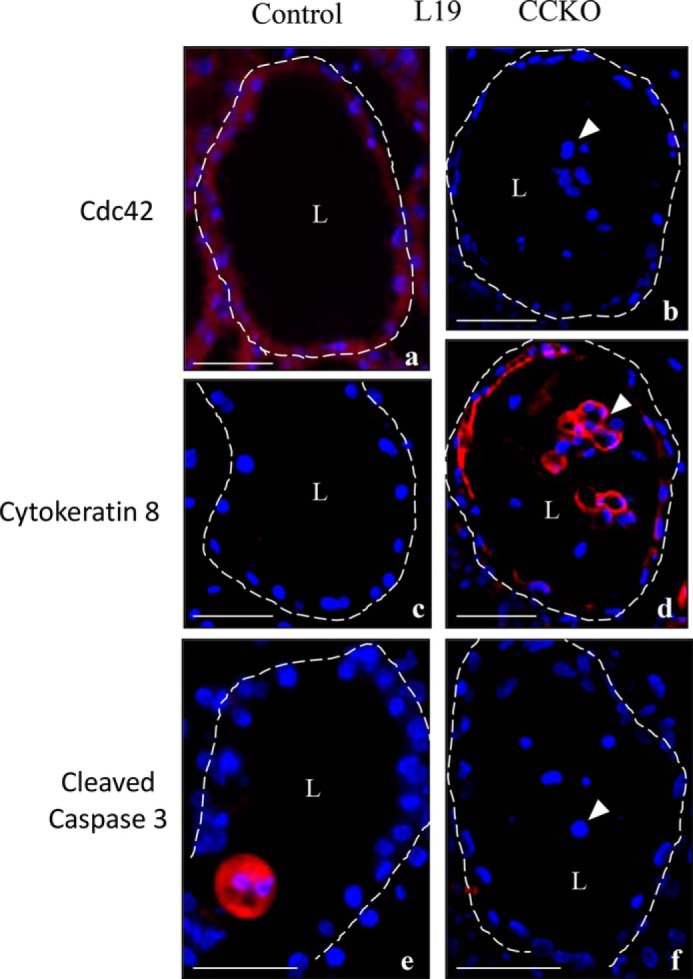
Epithelial cell loss in CCKO mammary glands is due to premature sloughing. Immunofluorescence staining (red) in 5-μm thick sections from representative control and CCKO mammary alveoli at lactation day 13, depicting the expression and cellular localization of Cdc42 (a and b), cytokeratin 8 (c and d), and cleaved caspase 3(e and f). Arrowheads point to prematurely sloughed cells. Nuclei are stained blue. Dashed lines outline representative alveoli. L marks the central lumen of an alveolus. Scale bars represent 50 μm. The results are representative of the analysis of >3 mice each from control and CCKO groups.
Immunofluorescence staining of serial sections showed that the Cdc42-negative cells, whether floating in the luminal space or still embedded within the alveoli in CCKO mammary glands, were indeed responsible for the up-regulated expression of CK8 (indicated by arrowheads, Fig. 7, panels a–d). The floating cells in the CCKO mammary glands did not show cleaved caspase 3 staining (indicated by arrowhead, Fig. 7, panel f), suggesting that these cells were not removed from the epithelial planes due to apoptosis, which we occasionally detected within alveolar lumens in control mammary glands (Fig. 7, panel e). When combined with the findings from the preceding section, these results suggest that CCKO mammary alveolar cells lose their communication with neighboring cells, and thereby gain aberrant migratory capability. This results in their detachment from mammary epithelial planes, thereby resulting in an overall impairment in the development and function of CCKO mammary glands.
Discussion
In this study, we have examined the roles played by Cdc42 in adult mammary gland function during lactation. We show that the conditional deletion of Cdc42 results in a marked under-development of mammary alveoli, thus significantly impairing the ability of CCKO mothers to provide the proper nourishment to their pups. This impairment in the development of lactating mammary alveoli is not caused by a reduction in cell proliferation nor through an increase in cellular apoptosis. Rather, our studies show that CCKO mammary alveoli exhibit a latent and under-developed morphology because their alveolar cells prematurely exfoliate from epithelial planes during lactation, which is the result of structural defects and alterations in cell status caused by the deletion of Cdc42.
At least some of the structural defects are likely the outcome of the inability of CCKO mammary alveolar epithelial cells to maintain proper cell polarity after the onset of lactogenesis. Indeed, the deletion of Cdc42 in pre-pregnant mammary epithelial cells has been reported to disrupt intact epithelial structures during acinar formation, due to defects in apical-basal cell polarity, cell-cell adherens structures, and tight junctions (17, 18). While Cdc42 has also been suggested to be important for establishing basement membrane structures by controlling stromal-epithelial interactions, as well as for the deposition and processing of extracellular matrix proteins in pre-pregnant mammary glands (16, 25), we did not detect any disruption of basement membrane structures, nor histological differences in stromal layers, in lactating CCKO mammary alveoli.
One of the most obvious changes accompanying Cdc42 deletion in mammary glands during lactogenesis was a marked reduction in E-cadherin expression at the whole gland-level, as well as the disruption of E-cadherin-dependent cell-cell adhesion structures. At earlier lactation periods (lactation day 5), these changes were observed in a subset of the epithelial cell population within the mammary gland as the cells began to lose Cdc42 expression. However, as lactation proceeded (lactation day 13), the diffuse localization of E-Cadherin was more readily apparent throughout the luminal alveolar epithelia. We attribute this to the non-synchronous deletion of Cdc42 throughout the mammary gland at earlier time-points, leading to more thorough gene deletion as lactation proceeded. While the changes observed at the earlier time periods were more subtle than those at later time points, even these subtle differences in the secretory capacity of the mammary gland can have a large effect on the newborn pups' high demand for nutrients (29). These data are consistent with reports implicating Cdc42 in the maintenance of E-cadherin homeostasis through its ability to promote the endocytosis and lysosomal degradation of non-trans-interacting E-cadherin, as mediated by the Par protein complex, WASP, and CIP4 (30, 31), and by preventing trans-interacting E-cadherin from being endocytosed, as promoted by IQGAP-1 (32, 33). Interestingly, the changes in E-cadherin levels observed in CCKO mammary alveoli were accompanied by a significant up-regulation of N-cadherin expression. This led us to examine whether the Cdc42 deletion promoted EMT, whose hallmark phenotype is the shift from the up-regulated expression of E-cadherin to N-cadherin (26–28). However, while we did see elevated TGFβ1 signaling through SMAD 2/3, we did not detect the up-regulation of other marker proteins for EMT, including Snail and Twist (26–28). Taken together, these data suggest that the deletion of Cdc42 caused a shift in mammary epithelial cell status, but did not induce EMT.
As lactation proceeded, CCKO mammary alveolar cells also exhibited a striking up-regulation of CK8, which has been shown to be expressed in the mammary ductal epithelial cells of virgin female mice, but absent in luminal epithelial cells of the lactating mouse mammary gland (34). Since the alterations in cell status occurred over an extended period of time following the deletion of Cdc42, these changes might be indirect effects caused by Cdc42 deletion. Apparently, the epithelial cell morphology of mammary alveolar cells, as controlled by Cdc42, has an essential role in maintaining their cell identity. Considering that both N-cadherin and CK8 are suggested to be up-regulated in invasive breast cancer (35, 36), CCKO mammary alveolar cells, upon losing cell-to-cell contacts and proper tissue architecture, might acquire a shift in epithelial cell identity. This may also explain the ability of the alveolar cells to exfoliate and undergo a premature sloughing off into the lumens.
The lactating CCKO mammary glands exhibited sustained cell proliferation even within the time window when control glands were undergoing the beginning stages of involution (lactation day 20). We also observed a sustained activation of STAT5, which is a key regulator of maturation and cell proliferation in mammary alveologenesis. However, studies using the in vitro three-dimensional cell culture model have shown that the introduction of the dominant-negative Cdc42(T17N) mutant into established acini did not affect the prolactin-induced phosphorylation of STAT5 (18). Collectively, these results would suggest that it is unlikely Cdc42 directly regulates prolactin-dependent STAT5 activation. Instead, the loss of Cdc42 may give rise to an overall delay in the steps that control the balance between the cessation of cell proliferation and the onset of programmed cell death necessary for proper adult mammary gland function. Indeed, we found the levels of activated caspase-3 to be significantly reduced at lactation day 20 in CCKO mammary glands, compared with controls.
Structural deformities in the CCKO mammary tissue might also explain some of the phenotypes accompanying Cdc42 deletion. In the normal development of the mammary gland after weaning, continued milk production increases intra-mammary pressure, which inhibits the secretion of hormones promoting milk production, while inducing the secretion of hormones promoting involution (15). Since CCKO mammary glands are underdeveloped and lose the cell-cell barriers that normally maintain intra-mammary pressure, CCKO mammary glands might fail to secrete hormones that promote involution.
We also analyzed global gene expression changes between the control and CCKO mice at lactation day 5, by using RNA Seq analysis. Of 23,352 genes and 30,608 transcripts analyzed, only 67 genes and 57 transcripts were found to be differentially expressed with significance between the two samples (data not shown). These differentially expressed genes mapped mostly to stress response biological programs, as evaluated using iPathway Guide software, which might be an indirect consequence of the abnormal epithelial cell status and premature sloughing of cells in CCKO mammary glands.
In conclusion, our work and that of others suggest that the physiological functions of Cdc42 can be quite diverse and specific to different cell types and stages of development (16–18, 25). Here we have shown that the loss of Cdc42 and the inability of CCKO cells to establish proper cell polarity and cell-cell contacts have severe consequences for the functioning of adult mammary glands. Certainly, an important question for the future concerns how the physiological functions of Cdc42 in normal mammary cells correspond to its aberrant behavior in some forms of breast cancer, in which it is overexpressed (37). This could give rise to de-regulated Cdc42-signaling activities that interfere with the establishment or maintenance of proper cell polarity. Thus, it is easy to imagine how on the one hand, the impact of altered mammary cell polarity and glandular structure that accompanies the loss or de-regulation of Cdc42 can result in improper mammary gland function and lactogenesis, while on the other hand, the overexpression of Cdc42 and its excessive signaling disrupts normal cell polarity and contributes to the transformed state and the malignant phenotypes that accompany breast cancer progression. A better understanding of these Cdc42 functions during the development of different forms of breast cancer could lead to new strategies regarding how and when to target Cdc42-signaling events as possible anti-cancer therapies.
Author Contributions
All authors participated in the conception and design of the experiments. J. E. D., M. J. L., X. P., and S. M. performed the experiments and critiqued the manuscript. J. E. D., M. E., M. J. L., M. A. A., and R. A. C. wrote the manuscript.
Acknowledgments
We thank Cindy Westmiller for excellent secretarial assistance. We also thank the Immunopathology Research and Development Laboratory within Cornell University's Department of Biomedical Sciences, and the Cornell University Institute of Biotechnology's Genomics Facility for their expert technical assistance and equipment use.
This work was supported by National Institutes of Health Grants GM047458 and GM040654 (to R. A. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- MDCK
- Madin-Darby canine kidney
- aPKCζ
- atypical PKCζ
- CC3
- cleaved caspase 3
- CK8
- cytokeratin 8
- CK14
- cytokeratin 14
- CCKO
- Cdc42 conditional knock-out
- EMT
- epithelial to mesenchymal transition
- HIER
- heat-induced epitope retrieval
- WAP
- whey acidic protein.
References
- 1. Nelson W. J. (2003) Adaptation of core mechanisms to generate cell polarity. Nature 422, 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Etienne-Manneville S. (2004) Cdc42–the centre of polarity. J. Cell Sci. 117, 1291–1300 [DOI] [PubMed] [Google Scholar]
- 3. Drubin D. G., and Nelson W. J. (1996) Origins of cell polarity. Cell 84, 335–344 [DOI] [PubMed] [Google Scholar]
- 4. Rojas R., Ruiz W. G., Leung S.-M., Jou T.-S., and Apodaca G. (2001) Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 12, 2257–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martín-Belmonte F., Yu W., Rodríguez-Fraticelli A. E., Ewald A. J., Werb Z., Alonso M. A., and Mostov K. (2008) Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr. Biol. 18, 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., and Mostov K. (2007) PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaffe A. B., Kaji N., Durgan J., and Hall A. (2008) Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell Biol. 183, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pedersen E., and Brakebusch C. (2012) Rho GTPase function in development: how in vivo models change our view. Exp. Cell Res. 318, 1779–1787 [DOI] [PubMed] [Google Scholar]
- 9. Melendez J., Grogg M., and Zheng Y. (2011) Signaling role of Cdc42 in regulating mammalian physiology. J. Biol. Chem. 286, 2375–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kesavan G., Sand F. W., Greiner T. U., Johansson J. K., Kobberup S., Wu X., Brakebusch C., and Semb H. (2009) Cdc42-mediated tubulogenesis controls cell specification. Cell 139, 791–801 [DOI] [PubMed] [Google Scholar]
- 11. Yuan H., Zhang H., Wu X., Zhang Z., Du D., Zhou W., Zhou S., Brakebusch C., and Chen Z. (2009) Hepatocyte-specific deletion of Cdc42 results in delayed liver regeneration after partial hepatectomy in mice. Hepatology 49, 240–249 [DOI] [PubMed] [Google Scholar]
- 12. Wu X., Li S., Chrostek-Grashoff A., Czuchra A., Meyer H., Yurchenco P. D., and Brakebusch C. (2007) Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev. Dyn. 236, 2767–2778 [DOI] [PubMed] [Google Scholar]
- 13. Wu X., Quondamatteo F., and Brakebusch C. (2006) Cdc42 expression in keratinocytes is required for the maintenance of the basement membrane in skin. Matrix Biol. 25, 466–474 [DOI] [PubMed] [Google Scholar]
- 14. Wu X., Quondamatteo F., Lefever T., Czuchra A., Meyer H., Chrostek A., Paus R., Langbein L., and Brakebusch C. (2006) Cdc42 controls progenitor cell differentiation and β-catenin turnover in skin. Genes Dev. 20, 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neville M. C., McFadden T. B., and Forsyth I. (2002) Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia 7, 49–66 [DOI] [PubMed] [Google Scholar]
- 16. Bray K., Gillette M., Young J., Loughran E., Hwang M., Sears J. C., and Vargo-Gogola T. (2013) Cdc42 overexpression induces hyperbranching in the developing mammary gland by enhancing cell migration. Breast Cancer Res. 15, R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bray K., Brakebusch C., and Vargo-Gogola T. (2011) The Rho GTPase Cdc42 is required for primary mammary epithelial cell morphogenesis in vitro. Small GTPases 2, 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akhtar N., and Streuli C. H. (2006) Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J. Cell Biol. 173, 781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wagner K. U., Wall R. J., St-Onge L., Gruss P., Wynshaw-Boris A., Garrett L., Li M., Furth P. A., and Hennighausen L. (1997) Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25, 4323–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo A., Mori H., Mott J., and Bissell M. (2012) Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J. Mammary Gland Biol. Neoplasia 17, 103–110 [DOI] [PubMed] [Google Scholar]
- 21. Peng X., Lin Q., Liu Y., Jin Y., Druso J. E., Antonyak M. A., Guan J. L., and Cerione R. A. (2013) Inactivation of Cdc42 in embryonic brain results in hydrocephalus with ependymal cell defects in mice. Protein Cell 4, 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallego M. I., Binart N., Robinson G. W., Okagaki R., Coschigano K. T., Perry J., Kopchick J. J., Oka T., Kelly P. A., and Hennighausen L. (2001) Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev. Biol. 229, 163–175 [DOI] [PubMed] [Google Scholar]
- 23. Liu X., Robinson G. W., Gouilleux F., Groner B., and Hennighausen L. (1995) Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl. Acad. Sci. U.S.A. 92, 8831–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin D., Edwards A. S., Fawcett J. P., Mbamalu G., Scott J. D., and Pawson T. (2000) A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2, 540–547 [DOI] [PubMed] [Google Scholar]
- 25. McHenry P. R., Sears J. C., Herrick M. P., Chang P., Heckman-Stoddard B. M., Rybarczyk M., Chodosh L. A., Gunther E. J., Hilsenbeck S. G., Rosen J. M., and Vargo-Gogola T. (2010) P190B RhoGAP has pro-tumorigenic functions during MMTV-Neu mammary tumorigenesis and metastasis. Breast Cancer Res. 12, R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalluri R., and Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J. M., Dedhar S., Kalluri R., and Thompson E. W. (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 172, 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thiery J. P. (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15, 740–746 [DOI] [PubMed] [Google Scholar]
- 29. Long W., Wagner K.-U., Lloyd K. C. K., Binart N., Shillingford J. M., Hennighausen L., and Jones F. E. (2003) Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development 130, 5257–5268 [DOI] [PubMed] [Google Scholar]
- 30. Leibfried A., Fricke R., Morgan M. J., Bogdan S., and Bellaiche Y. (2008) Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr. Biol. 18, 1639–1648 [DOI] [PubMed] [Google Scholar]
- 31. Georgiou M., Marinari E., Burden J., and Baum B. (2008) Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. 18, 1631–1638 [DOI] [PubMed] [Google Scholar]
- 32. Izumi G., Sakisaka T., Baba T., Tanaka S., Morimoto K., and Takai Y. (2004) Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J. Cell Biol. 166, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuroda S., Fukata M., Nakagawa M., Fujii K., Nakamura T., Ookubo T., Izawa I., Nagase T., Nomura N., Tani H., Shoji I., Matsuura Y., Yonehara S., and Kaibuchi K. (1998) Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science 281, 832–835 [DOI] [PubMed] [Google Scholar]
- 34. Taylor-Papadimitriou J., Stampfer M., Bartek J., Lewis A., Boshell M., Lane E. B., and Leigh I. M. (1989) Keratin expression in human mammary epithelial cells cultured frm normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J. Cell Sci. 94, 403–413 [DOI] [PubMed] [Google Scholar]
- 35. Hazan R. B., Phillips G. R., Qiao R. F., Norton L., and Aaronson S. A. (2000) Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 148, 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Livasy C. A., Karaca G., Nanda R., Tretiakova M. S., Olopade O. I., Moore D. T., and Perou C. M. (2006) Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod. Pathol. 19, 264–271 [DOI] [PubMed] [Google Scholar]
- 37. Fritz G., Just I., and Kaina B. (1999) Rho GTPases are over-expressed in human tumors. Int. J. Cancer 81, 682–687 [DOI] [PubMed] [Google Scholar]