Abstract
Aggregation of islet amyloid polypeptide (IAPP) contributes to beta cell dysfunction in type 2 diabetes and islet transplantation. Like other amyloidogenic peptides, human IAPP induces macrophage IL-1β secretion by stimulating both the synthesis and processing of proIL-1β, a pro-inflammatory cytokine that (when chronically elevated) impairs beta cell insulin secretion. We sought to determine the specific mechanism of IAPP-induced proIL-1β synthesis. Soluble IAPP species produced early during IAPP aggregation provided a Toll-like-receptor-2- (TLR2-) dependent stimulus for NF-κB activation in HEK 293 cells and bone marrow-derived macrophages (BMDMs). Non-amyloidogenic rodent IAPP and thioflavin-T-positive fibrillar amyloid produced by human IAPP aggregation failed to activate TLR2. Blockade of TLR6 but not TLR1 prevented hIAPP-induced TLR2 activation, consistent with stimulation of a TLR2/6 heterodimer. TLR2 and its downstream adaptor protein MyD88 were required for IAPP-induced cytokine production by BMDMs, a process that is partially dependent on autoinduction by IL-1. BMDMs treated with soluble but not fibrillar IAPP provided a TLR2-dependent priming stimulus for ATP-induced IL-1β secretion, whereas late IAPP aggregates induced NLRP3-dependent IL-1β secretion by LPS-primed macrophages. Moreover, inhibition of TLR2 and depletion of islet macrophages prevented up-regulation of Il1b and Tnf expression in human IAPP-expressing transgenic mouse islets. These data suggest participation by both soluble and fibrillar aggregates in IAPP-induced islet inflammation. IAPP-induced activation of TLR2 and secretion of IL-1 may be important therapeutic targets to prevent amyloid-associated beta cell dysfunction.
Keywords: amyloid, diabetes, innate immunity, protein aggregation, toll-like receptor (TLR)
Introduction
Islet amyloid deposition (1), macrophage infiltration (2, 3), and up-regulation of pro-inflammatory cytokines (4) are common pathological features of pancreatic islets from patients with type 2 diabetes. Amyloid deposits are comprised primarily of islet amyloid polypeptide (IAPP),5 a 37-amino acid peptide that is co-secreted with insulin by beta cells. Recent evidence suggests that IAPP aggregates trigger islet secretion of IL-1, which acts alone or in combination with other pro-inflammatory cytokines to impair beta cell insulin secretion (5, 6). Human but not non-amyloidogenic rodent IAPP induces IL-1β synthesis in bone marrow-derived and intra-islet macrophages (7, 8). IAPP also activates the NACHT, LRR and PYD domain-containing protein 3 (NLRP3) inflammasome, required for processing of proIL-1β into its mature form (9). Blockade of IL-1 signaling with IL-1 receptor (IL-1R) antagonist (IL-1Ra) limits IAPP-induced secretion of pro-inflammatory cytokines by macrophages and chemokines by islets (8), suggesting that this cytokine is a central mediator of islet inflammation in the setting of amyloid formation.
Resident macrophages are the major source of IAPP-induced Il1b gene expression in mouse islets (7, 8), but it is unclear how IAPP is sensed by islet macrophages to provide an initial stimulus for proIL-1β synthesis. Other amyloidogenic peptides of both mammalian and eukaryotic origin activate the transcription factor NF-κB by interacting with cell-surface TLRs, in particular TLR2 (10–12). During amyloid formation in vitro, protein monomers form soluble pre-fibrillar oligomers prior to generation of non-branching fibrils. Many amyloid-forming peptides appear to follow similar aggregation pathways and produce oligomeric species with common exposed epitopes (13). Soluble oligomers are thought to be the primary species that effect tissue damage (14), although amyloid fibrils are associated with beta cell apoptosis in islets from patients with type 2 diabetes (15). It is possible that (like other amyloidogenic peptides) IAPP activates multiple pattern recognition receptors, and moreover that different types of aggregates (e.g. soluble oligomers or amyloid fibrils) could be involved in distinct recognition events by islet macrophages. Such a finding would have important implications for the development of amyloid inhibitors, which have the potential to stabilize specific intermediate aggregates, as well as for strategies to limit IAPP-induced islet inflammation.
Increased expression of IL-1-related genes is a characteristic of islets from patients with type 2 diabetes (16). Remarkably, multiple clinical trials have now demonstrated improved insulin secretion in response to anti-IL-1 therapy with no detectable effects on insulin resistance (17). These data suggest that the islet may be particularly susceptible to IL-1-induced inflammation, perhaps due to high IL-1R expression on beta cells (18) and to an islet-localized stimulus for IL-1 such as IAPP aggregation. Here, we evaluated the mechanism of IAPP-induced IL-1β synthesis in macrophages and sought to determine the role of different species of IAPP aggregates on innate immune activation.
Experimental Procedures
Mice
Wild-type (WT) C57BL/6, Tlr2−/− (B6.129-Tlr2tm1Kir/J), Myd88−/− (B6.129P2(SJL)-Myd88tm1.1Defr/J), Nlrp3−/− (B6.129S6-Nlrp3tm1Bhk/J), and FVB/NJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Hemizygous FVB/N-Tg(Ins2-IAPP)RHFSoel/J mice were bred with WT FVB mice to produce hemizygous hIAPP transgenic mice (hIAPPTg/o). Mice were maintained in compliance with Canadian Council on Animal Care guidelines. Studies were approved by the University of British Columbia Committee on Animal Care.
Islet and Cell Culture
Mouse islets were isolated by ductal collagenase injection and filtration (19) and cultured as described previously (7). For macrophage depletion, islets were treated immediately following isolation for 48 h with 1 mg/ml clodronate delivered in liposomes (20) (ClodLip BV, Amsterdam, The Netherlands). Bone marrow-derived macrophages (BMDMs) were propagated from mouse femur marrow (8). RAW 264.7 macrophages (ATCC TIB-71) were maintained in high-glucose DMEM supplemented with 10% FBS, 2 mm GlutaMAXTM, and 1 mm sodium pyruvate. HEK 293 cells co-transfected with human TLR2 or TLR4 and an NF-κB/AP-1-secreted alkaline phosphatase (SEAP) reporter gene were obtained from InvivoGen and maintained according to the manufacturer's instructions (San Diego, CA).
Peptide Preparation
Human and rodent IAPP (Bachem, Torrance, CA) were dissolved in hexafluoro-2-propanol, lyophilized, and stored at −20°C. Immediately prior to each experiment, IAPP was dissolved in 0.1 m acetic acid and diluted in culture medium. To assess amyloid fibril formation, IAPP aggregation was monitored by thioflavin T fluorescence (21). For examination of fibril formation by transmission electron microscopy (TEM), 3-μl aliquots of IAPP prepared by dissolution at 100 μm in distilled water and incubation at room temperature for 0–16 h were adsorbed to glow-discharged carbon-coated collodion film on copper grids. Grids were blotted, negatively stained with uranyl acetate, and imaged with a FEI Tecnai Transmission Electron Microscope.
Cytokine Secretion
In experiments involving multiple incubations (e.g. priming followed by activation), the activating stimulus was added to medium without washing or removal of the priming stimulus. Levels of 12 cytokines and chemokines in cell supernatants were assessed by multiplex assay (Millipore, Bedford, MA) and analyzed on a Luminex-100 system according to the manufacturer's instructions (Luminex Corporation, Austin, TX). For single cytokine analysis in BMDM and islet supernatants, TNF-α and IL-1β levels were determined by ELISA (BioLegend, San Diego, CA; Abcam, Toronto, Canada).
Gene Expression Analysis
RNA was isolated with a PurelinkTM RNA Micro Kit (Invitrogen). cDNA was synthesized using a Superscript VILOTM cDNA Synthesis Kit (Invitrogen). RT-qPCR was performed with Fast SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK), and reactions were run on an ABI 7500 Fast Real-Time PCR System. Primer sequences were obtained from PrimerBank (22) as described previously (7).
Immunocytochemistry
Cells were seeded in black 96-well clear-bottom plates and cultured as indicated. Monolayers were stained with propidium iodide for 30 min at 37°C, fixed with 4% formaldehyde for 15 min at room temperature, permeabilized with 1% Triton X-100 for 15 min, and blocked using a commercial solution optimized for HCS Arrayscan assays (Thermo Fisher Scientific, Waltham, MA). Cells were stained with rabbit anti-mouse NF-κB (1:230; Thermo Fisher Scientific) for 1 h followed by Dylight 549 goat anti-rabbit IgG (1:400; Thermo Fisher Scientific) and Hoechst (1 μg/ml) for 1 h. Staining was quantified using an ArrayScan VTI HCS reader and Molecular Translocation BioApplication Software Module (Thermo Fisher Scientific). Eight-well chamber slides were prepared in parallel and imaged on a BX61 microscope (Olympus, Center Valley, PA).
Western Blot
WT and Nlrp3−/− BMDMs were lysed, protein was quantified, and lysates were separated on 4–20% polyacrylamide gels along with recombinant mature mouse IL-1β (eBioscience, San Diego, CA) and transferred to nitrocellulose membranes as previously described (7). Membranes were incubated in anti-mouse β-actin monoclonal antibody (1:5000 overnight at 4 °C; #NB600–501, Novus Biologicals, Littleton, CO) followed by infrared-dye-conjugated goat anti-mouse IgG (1:10,000 for 45 min at room temperature; #926–68070 LI-COR Biotechnology, Lincoln, NE) and imaged on a LI-COR Odyssey (Lincoln, NE). Blots were subsequently washed and incubated overnight with goat anti-mouse IL-1β (1:250 overnight at 4 °C; R&D Systems, Minneapolis, MN) followed by Alexa Fluor-conjugated donkey-anti goat IgG (1:5,000 for 45 min at room temperature; #A-11055, ThermoFisher, Waltham, MA) and imaged on a Typhoon Trio Plus (GE Healthcare Life Sciences, Mississauga, Canada).
Statistical Analyses
Data were analyzed with GraphPad Prism and are expressed as mean ± S.D. of the indicated number of replicates. Differences between two groups were evaluated with a two-tailed t test. Differences among three or more groups were evaluated with a one-way or two-way ANOVA and Bonferroni post-tests.
Results
Human IAPP Aggregation Intermediates Activate a TLR2/6 Heterodimer
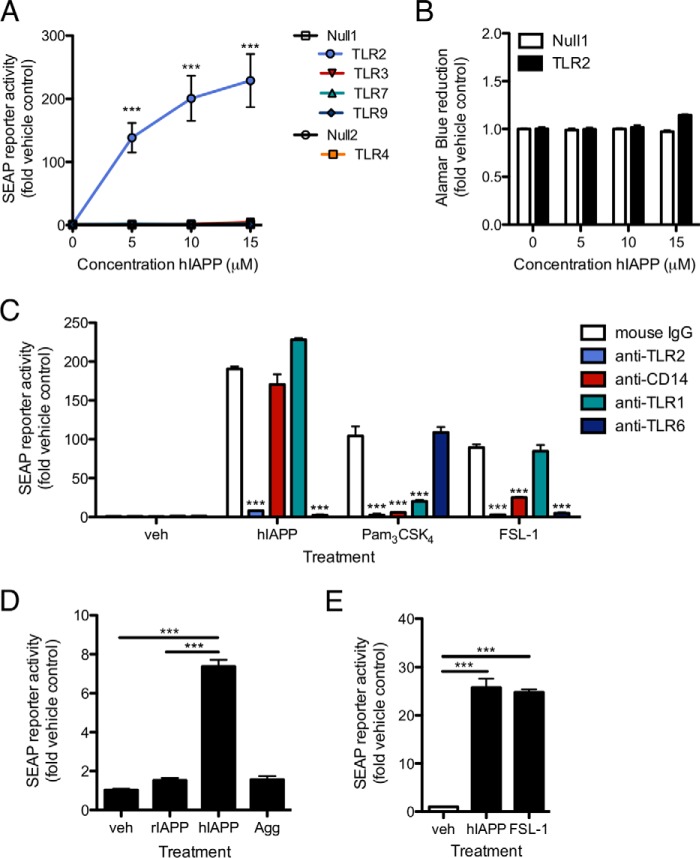
To determine whether the initial signal for induction of proIL-1β synthesis by human IAPP (hIAPP) is delivered by TLR activation, we screened a panel of human TLR-expressing HEK 293 cells co-transfected with an NF-κB/AP-1 SEAP reporter construct. hIAPP induced NF-κB/AP-1 activation in HEK 293 cells expressing TLR2 and CD14 but not in the parental cell line or in cells expressing TLR3, TLR4 and CD14, TLR7, or TLR9 (Fig. 1A). TLR2 expression had no effect on HEK 293 cell viability in the presence of hIAPP (Fig. 1B). Because TLR2 can form heterodimers with either TLR1 or TLR6, both expressed endogenously by HEK 293 cells, we evaluated the effect of neutralizing antibodies targeting TLR1, TLR2, and TLR6 on hIAPP-induced reporter activity. NF-κB/AP-1 activation was blocked in cells treated with anti-TLR2 or anti-TLR6 but not anti-TLR1 neutralizing antibody, suggesting that IAPP activates a TLR2/6 heterodimer (Fig. 1C). Blockade of CD14, required for a maximal response to both the TLR1/2 ligand Pam3CSK4 and the TLR2/6 ligand FSL-1 (23), did not significantly affect the response to hIAPP (Fig. 1C), suggesting that this co-receptor is not required for hIAPP-induced TLR2 activation. Freshly dissolved hIAPP but not non-amyloidogenic rodent IAPP (rIAPP) or pre-aggregated fibrillar hIAPP triggered TLR2 signaling (Fig. 1D), an effect also observed in HEK 293 cells expressing mouse TLR2 (Fig. 1E). Thus, early aggregates of hIAPP (but not fully aggregated peptide) trigger activation of TLR2.
FIGURE 1.
Early aggregates of hIAPP activate TLR2. HEK 293 cells co-transfected with the indicated human TLR, CD14 (TLR2- and TLR4-expressing cells), and a NF-κB/AP-1 SEAP reporter construct were treated with freshly dissolved hIAPP for 24 h. A, SEAP activity was measured by colorimetric assay. Null1 and Null2 represent the parental cell lines. B, viability was assessed by Alamar Blue fluorescence in TLR2-expressing and control HEK 293 cells. C, human TLR2/CD14-expressing HEK 293 cells (with endogenous TLR1 and TLR6 expression) were pre-treated with anti-TLR1, TLR2, TLR6, or CD14 neutralizing antibody (5 μg/ml) or mouse IgG isotype control for 30 min prior to addition of hIAPP (10 μm) or control ligands for TLR1/2 (Pam3CSK4) or TLR2/6 (FSL-1) at 10 ng/ml. D, human TLR2-expressing cells were treated with rIAPP or hIAPP (10 μm) or the mass equivalent of hIAPP that had pre-aggregated for 24 h (Agg). E, HEK 293 cells expressing mouse TLR2 were treated with freshly-dissolved hIAPP (10 μm) for 24 h to confirm activation of rodent TLR2. Data represent mean ± S.D. of 3 replicates per treatment and are representative of three independent experiments. veh: vehicle control. ***, p < 0.001 relative to parental cell line (A, B) or vehicle control (C–E).
TLR2 Is Required for hIAPP-induced NF-κB Activation and Cytokine Secretion by Macrophages
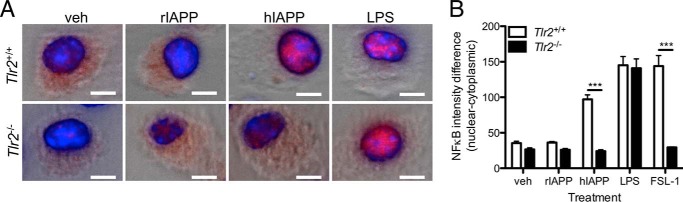
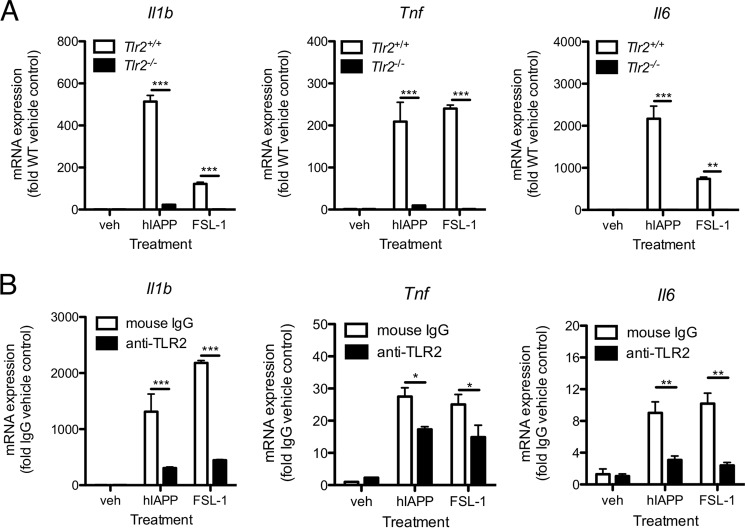
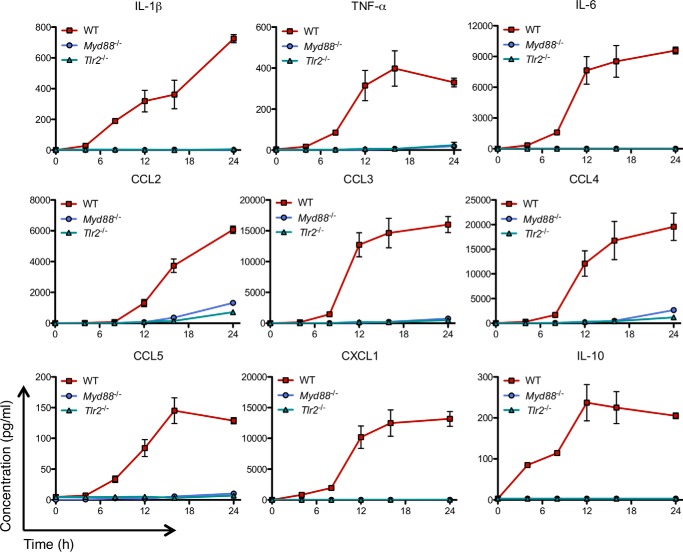
To determine whether TLR2 is required for hIAPP-induced pro-inflammatory cytokine secretion by macrophages, we assessed nuclear translocation of the NF-κB p65 subunit in BMDMs from WT and TLR2-deficient mice. p65 was observed in the nucleus in WT but not Tlr2−/− cells 1 h following treatment with hIAPP (Fig. 2, A and B). Moreover, hIAPP-induced Il1b, Tnf, and Il6 expression was significantly attenuated in BMDMs from Tlr2−/− mice (Fig. 3A) and in RAW 264.7 cells treated with an anti-TLR2 neutralizing antibody (Fig. 3B). We next evaluated the requirement for TLR2 and its downstream adaptor protein MyD88 in hIAPP-induced cytokine and chemokine secretion over a 24 h time course without a second distinct stimulus for NLRP3. Both TLR2 and MyD88 were required for hIAPP-induced secretion of IL-1β, TNF-α, IL-6, CCL2, CCL3, CCL4, CCL5, CXCL1, and IL-10 (Fig. 4). Thus, TLR2 is required for hIAPP-induced NF-κB activation and pro-inflammatory cytokine secretion by BMDMs.
FIGURE 2.
TLR2 is required for hIAPP-induced NF-κB activation in macrophages. BMDMs from WT (Tlr2+/+) or Tlr2−/− C57BL/6 mice were treated with hIAPP or rIAPP (10 μm) or LPS (10 ng/ml) for 1 h. A, representative staining for the NF-κB p65 subunit (red) and nuclei (DAPI, blue) overlaid on brightfield images. Scale bar: 5 μm. B, difference in staining intensity between nucleus and cytoplasm was quantified with a Thermo Fisher ArrayScan VTI HCS instrument. Data represent mean ± S.D. of 3 replicates per treatment and are representative of 3 independent experiments. veh: vehicle control. ***, p < 0.001 relative to WT.
FIGURE 3.
TLR2 deficiency or neutralization attenuates hIAPP-induced pro-inflammatory gene expression in macrophages. A, WT (Tlr2+/+) or Tlr2−/− BMDMs were treated with hIAPP (10 μm) or FSL-1 (10 ng/ml) for 4 h. mRNA expression was assessed by RT-qPCR and normalized to the housekeeping gene Rplp0. B, RAW 264.7 macrophages were pre-incubated with anti-TLR2 neutralizing antibody (5 μg/ml) or mouse IgG isotype control for 1 h prior to addition of hIAPP (10 μm) or FSL-1 (10 ng/ml) for 4 h. mRNA expression was assessed by RT-qPCR and normalized to the housekeeping gene Rplp0. veh: vehicle control. *, p < 0.05; **, p < 0.01; ***, p < 0.001 relative to WT (A) or isotype control (B). Data represent mean ± S.D. of 4 replicates per treatment and are representative of 3 independent experiments.
FIGURE 4.
Deficiency of TLR2 or MyD88 prevents hIAPP-induced macrophage cytokine secretion. BMDMs from WT, Myd88−/−, or Tlr2−/− C57BL/6 mice were treated with hIAPP (10 μm) for 0–24 h. Cytokine secretion was measured by Luminex assay. All cytokines/chemokines shown were induced by hIAPP after 8–12 h. Data represent mean ± S.D. of 3 replicates per treatment and are representative of 3 independent experiments.
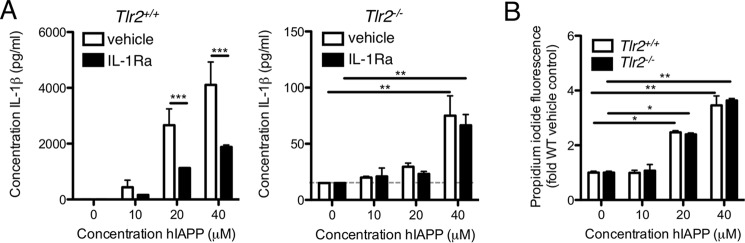
In apparent contrast to these results, our previous work implicated an IL-1R-dependent, TLR2-independent mechanism of hIAPP-induced pro-inflammatory cytokine secretion by BMDMs (8). In the present study, we first confirmed that hIAPP-induced IL-1β secretion is itself partially inhibited by IL-1Ra, consistent with autoinduction (Fig. 5A). This inhibitory effect of IL-1Ra was observed in WT but not Tlr2−/− BMDMs, which presumably lack the signal required for up-regulation of Il1b expression. However, concentrations of hIAPP high enough to impair cell membrane integrity as measured by propidium iodide uptake induced low but detectable levels of IL-1β even in Tlr2−/− BMDMs (Fig. 5, A and B), suggesting additional TLR2- and IL-1-independent mechanisms for IL-1β secretion. Variability among lots of IAPP, a phenomenon that has been well-documented (24), may account for differences in aggregation kinetics, IAPP/cell membrane interactions, and mechanisms of in vitro cytokine secretion among studies.
FIGURE 5.
IL-1 receptor activation amplifies hIAPP-induced IL-1β secretion. A, BMDMs from WT (Tlr2+/+) or Tlr2−/− C57BL/6 mice were pre-incubated with IL-1 receptor antagonist (IL-1Ra, 2 μg/ml) prior to treatment with hIAPP for 24 h. IL-1β in supernatants was measured by ELISA. B, loss of cell membrane integrity as a measure of cell death was assessed by propidium iodide incorporation. Data represent mean ± S.D. of 3 replicates per treatment and are representative of 3 independent experiments. Dotted line represents the lower limit of detection of the assay. veh: vehicle control. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Pre-fibrillar and Fibrillar hIAPP Aggregates Have Distinct Effects on the Synthesis and Secretion of IL-1β
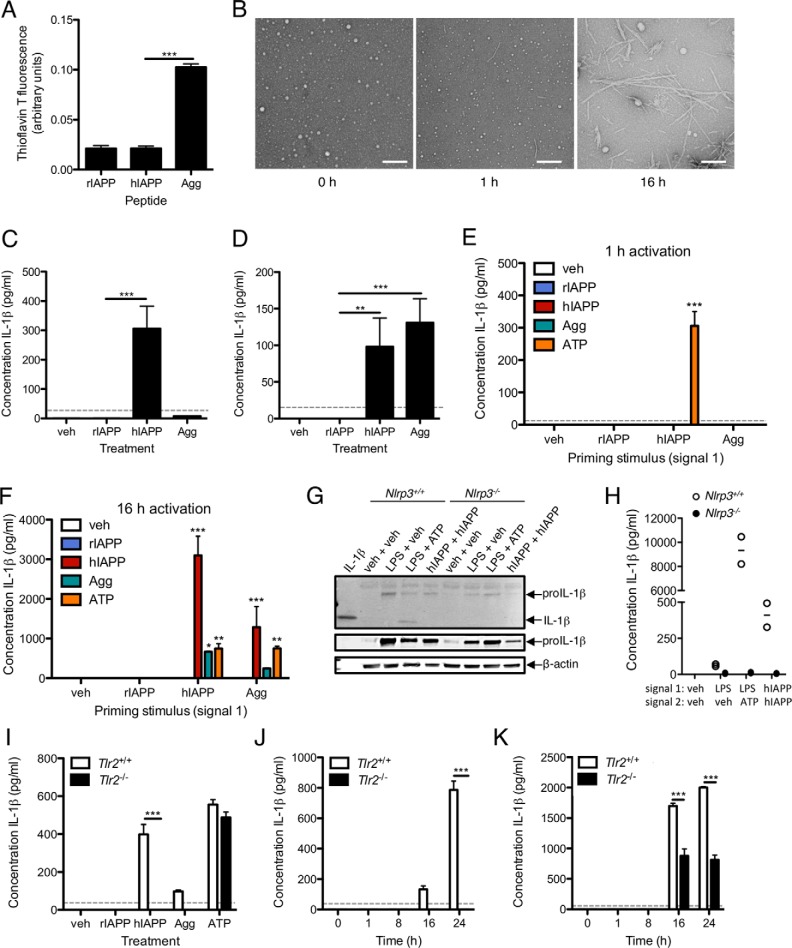
We next assessed the signals required for IL-1 secretion in response to hIAPP aggregation. Secretion of mature IL-1β requires induction of proIL-1β synthesis (signal 1, which can be provided by TLR2 activation) and cleavage of proIL-1β (signal 2, which can be provided by NLRP3-induced caspase-1 activation). Amyloid fibrils were prepared by pre-incubation of hIAPP at 37 °C for 24 h and confirmed to contain β-sheet structure by increased thioflavin T fluorescence (Fig. 6A), which reaches a maximum after ∼3 h of hIAPP aggregation in culture medium (8). Fibril formation was verified by electron microscopy (Fig. 6B). Freshly dissolved hIAPP but not amyloid fibrils induced IL-1β release after 3 h incubation (to induce proIL-1β synthesis) followed by 1 h treatment with ATP (to induce NLRP3 activation and IL-1β secretion; Fig. 6C). To determine whether amyloid fibrils could induce IL-1β secretion following delivery of a distinct stimulus for proIL-1β, BMDMs were treated with LPS for 3 h (signal 1) followed by rIAPP, soluble hIAPP, or pre-aggregated hIAPP (signal 2). BMDMs primed with LPS and then treated with freshly dissolved or pre-aggregated hIAPP produced similar levels of IL-1β after 16 h (Fig. 6D), suggesting that fibrillar hIAPP (also formed after ∼3 h in cultures treated with freshly-dissolved hIAPP) is the primary stimulus for signal 2, i.e. NLRP3 activation and proIL-1β cleavage. Of note, neither form of hIAPP was able to induce IL-1β secretion after only 1 h of treatment, consistent with a mechanism for NLRP3 activation that requires more time than the response to ATP and may involve phagocytosis with subsequent lysosomal disruption (8, 9).
FIGURE 6.
Pre-fibrillar and fibrillar hIAPP aggregates have distinct effects on the synthesis and secretion of IL-1β. A, amyloid fibril content was determined by measurement of thioflavin T fluorescence in freshly dissolved preparations of rIAPP or hIAPP (10 μm) or the mass equivalent of hIAPP that was allowed to pre-aggregate for 24 h at 37 °C (Agg). B, hIAPP (100 μm) was dissolved in water and imaged by TEM after the indicated period of aggregation. Scale bar = 100 nm. C, BMDMs were treated with the indicated stimulus for 3 h (signal 1, to induce proIL-1β synthesis) followed by ATP (5 mm) for 1 h (signal 2, to induce proIL-1β cleavage and mature IL-1β secretion). D, BMDMs were treated with LPS (10 ng/ml) for 3 h (signal 1) followed by the indicated activation stimulus for 16 h (signal 2). E, BMDMs were treated with the indicated priming stimulus (signal 1) for 3 h followed by 1 h or (F) 16 h activation (signal 2) with the indicated form of IAPP (10 μm) or ATP (5 mm). G and H, WT or Nlrp3−/− BMDMs were treated with the indicated signal 1 for 3 h, followed by the indicated signal 2 for 16 h. IL-1β was detected in cell lysates by Western blot (G) and in supernatants by ELISA (H). Immunoblot data show two different exposures of proIL-1β and are representative of 2 independent experiments; ELISA data show 2 biological replicates per genotype. I, WT or Tlr2−/− BMDMs were treated as in C to determine the effect of TLR2 on generation of signal 1. J, WT or Tlr2−/− BMDMs were treated with hIAPP (10 μm) for 0–24 h without priming or (K) following 3 h of pre-treatment with 10 ng/ml LPS to provide an independent source of signal 1. IL-1β in supernatants was measured by ELISA. Data represent mean ± S.D. of 3 replicates per treatment and are representative of 3 independent experiments unless otherwise noted. Dotted line represents the lower limit of detection of the assay. veh: vehicle control. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Next, we evaluated the capacity of rIAPP, hIAPP, and pre-aggregated hIAPP to induce IL-1β secretion when delivered in sequential combinations as both the priming and activation stimuli. BMDMs were treated with rIAPP, hIAPP, or pre-aggregated hIAPP as a priming stimulus (signal 1) for 3 h followed by 1 h (Fig. 6E) or 16 h (Fig. 6F) activation with one of the same three forms of IAPP to provide signal 2. BMDMs treated for 3 h with freshly-dissolved hIAPP but not rIAPP or pre-aggregated hIAPP provided a stimulus for subsequent ATP-induced IL-1β secretion (Fig. 6E), consistent with the requirement for pre-fibrillar species to activate TLR2. When the 1 h activation period was increased to 16 h, both freshly dissolved and pre-aggregated hIAPP stimulated IL-1β secretion in cells primed with hIAPP (Fig. 6F). However, cells exposed to additional freshly-dissolved hIAPP for 16 h produced 3.5-fold more IL-1β than those treated with pre-aggregated peptide (Fig. 6F). Thus, the contribution of soluble hIAPP (or the process of early hIAPP aggregation) to IL-1β secretion may be the limiting factor under these conditions. Delivery of soluble and fibrillar peptide in the opposite order (i.e. pre-aggregated hIAPP prior to freshly dissolved hIAPP) resulted in 2-fold less IL-1β, consistent with soluble aggregates being primarily responsible for priming and suggesting that the aggregation process generates stimuli in the optimal order for a maximal IL-1β response. The priming signal did not require NLRP3, as freshly-dissolved hIAPP induced proIL-1β protein expression in both WT and Nlrp3−/− BMDMs (Fig. 6G), but there was no secreted IL-1β detected from Nlrp3−/− cells after 16 h (Fig. 6H). We did not detect mature IL-1β in cell lysates at this time point, consistent with our previous data showing processing of proIL-1β into mature IL-1β with release of mature peptide into the medium between 16 and 24 h (7). TLR2 deficiency also prevented IL-1β release in response to 3 h incubation with hIAPP (to induce proIL-1β synthesis) followed by 1 h treatment with ATP (to induce NLRP3 activation and IL-1β secretion; Fig. 6I). Of note, no IL-1β was secreted by TLR2-deficient hIAPP-treated BMDMs over a 24-h time course (Fig. 6J) unless LPS was added to provide an alternate stimulus for proIL-1β expression (Fig. 6K).
TLR2 Is Required for Maximal hIAPP-induced Islet Macrophage Cytokine Expression
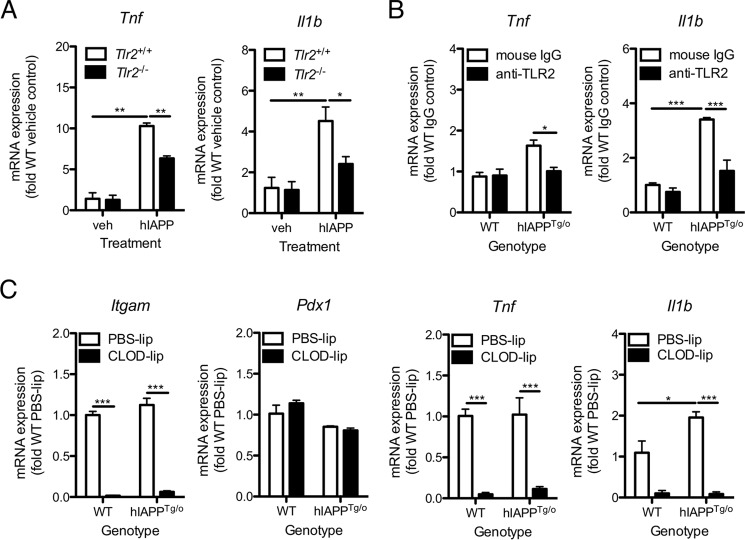
We next assessed the capacity of hIAPP to induce pro-inflammatory cytokine expression in cultured islets, which contain resident macrophages. Synthetic hIAPP caused up-regulation of mRNA encoding the pro-inflammatory cytokines IL-1β and TNF-α in isolated islets after 4 h (Fig. 7A). Expression of both Il1b and Tnf was reduced by ∼50% in islets from TLR2-deficient mice (Fig. 7A). Expression of Il1b but not Tnf was also elevated in hIAPPTg/o compared with WT islets after 7 days of culture at 22 mm glucose; both cytokines were significantly inhibited by anti-TLR2 neutralizing antibody (Fig. 7B). Clodronate-liposome-mediated islet macrophage depletion (as confirmed by a decrease in Itgam expression) significantly reduced islet Tnf and Il1b expression (Fig. 7C), suggesting that macrophages are the major source of these cytokines in both WT and hIAPP-expressing transgenic islets.
FIGURE 7.
Blockade of TLR2 prevents up-regulation of islet macrophage Il1b and Tnf expression in response to synthetic and endogenous hIAPP. A, islets were isolated from 12-week-old WT (Tlr2+/+) or Tlr2−/− C57BL/6 mice. After overnight recovery, islets were treated with hIAPP (10 μm) for 4 h. mRNA expression was assessed by RT-qPCR. B, islets from hIAPPTg/o mice and WT littermate controls were cultured with 10 μg/ml neutralizing antibody or isotype control for 7 days in 22 mm glucose to promote amyloid formation. mRNA expression was assessed by RT-qPCR. C, WT and hIAPPTg/o islets were treated with clodronate-containing liposomes (CLOD-lip, 1 mg/ml clodronate) or control liposomes (PBS-lip) for 48 h to deplete islet macrophages. Islets were subsequently cultured in 22 mm glucose for 4 days to promote amyloid formation and mRNA expression was assessed by RT-qPCR. Expression levels were normalized to the housekeeping gene Rplp0. Data represent mean ± S.E. of islets from 4 mice per treatment and are representative of 3 independent experiments. veh: vehicle control. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
Chronic exposure to elevated IL-1 causes beta cell death and dysfunction due to altered gene transcription, changes in protein activity, and induction of oxidative stress (25). Data from clinical studies suggest that anti-IL-1 agents can improve beta cell function without affecting insulin sensitivity (17). Recent work has demonstrated that IAPP (the principal component of islet amyloid) activates caspase-1 and NLRP3 in cultured macrophages (8, 9) and islet macrophages (7), induces expression of Il1b and proIL-1β (7, 8, 26), and triggers secretion of mature IL-1β by bone marrow-derived macrophages (7). Moreover, islet macrophages are the major source of hIAPP-induced IL-1β in islets; both IL-1Ra (26) and systemic macrophage depletion can improve hIAPP-induced islet dysfunction (7, 8). Our present data address two critical questions: (a) what is the mechanism by which IAPP is sensed by innate immune cells, and (b) which aggregation state of the peptide is required for proIL-1β synthesis and IL-1 secretion? These studies identify IAPP species generated during the early stages of aggregation as stimuli for TLR2-dependent proIL-1β expression, and species produced later during aggregation as stimuli for NLRP3-dependent IL-1β secretion. Using an ex vivo model of islet amyloid formation, they also support an important role for macrophage TLR2 in IAPP-induced islet inflammation.
Understanding the characteristics of different hIAPP species (toxic, TLR2-activating, NLRP3-activating, and inert) will be critical for the successful development of aggregation inhibitors as diabetes therapeutics. Our data suggest that the progression of IAPP aggregation from soluble oligomer through to amyloid fibril generates two sequential signals required for IL-1β secretion. First, early aggregates provide a TLR2-dependent stimulus leading to expression of proIL-1β, an observation consistent with the modest elevation of Il1b in lean hIAPP transgenic mice, which lack significant amyloid fibril deposition (26). Second, amyloid fibrils induce secretion of mature IL-1β, which then acts via the IL-1R to amplify expression of other cytokines such as TNF-α (8). Consistent with this, a significant increase in expression of Il1b as well as a panel of other pro-inflammatory cytokines was observed only when significant amyloid fibril deposition was present, as in obese hIAPP transgenic mice (26). Thus, two distinct species of aggregated hIAPP each provide one of the two signals necessary for IL-1β secretion.
Masters et al. (9) reported NLRP3 activation by prefibrillar hIAPP oligomers; however, these authors compared IL-1β release induced by both pre-aggregated fibrils and by soluble peptide after overnight culture. Since hIAPP rapidly aggregates to form fibrils during the first few hours of culture, it is likely that cells in those studies were exposed to fibrillar species formed during overnight culture and not only the intended smaller aggregates. Other reports have suggested that fibrillar aggregates of amyloidogenic peptides such as lysozyme are responsible for activation of TLR2 (12); whether fibrils exist in equilibrium with smaller species that interact with the receptor or whether indirect mechanisms for TLR2 activation are involved should be addressed by future work. Indeed, it is possible that hIAPP itself or soluble factors induced by hIAPP interact with TLR2, leading to the synthesis of proIL-1β and to post-translational modification of regulatory proteins required for inflammasome priming (27, 28). Several studies have suggested that amyloid-induced NLRP3 activation lies downstream of lysosomal disruption, mitochondrial dysfunction, or oxidative stress (29). While we cannot rule out the release of secreted factors that contribute to activation of both TLR2 and NLRP3, this work demonstrates that pre-fibrillar hIAPP species (typically the form implicated in disruption of beta cell membranes (14)) are not required for IL-1β secretion in cells primed with another stimulus. Moreover, additional studies are needed to evaluate the consequences of hIAPP-induced TLR2 activation in vivo. Small molecule inhibitors of TLR2 that are currently under development may be helpful in this regard (30, 31).
Mammalian TLRs are a family of membrane-bound pattern recognition receptors that recognize common microbial structural motifs as well as endogenous ligands; for example, TLR2 complexes with TLR1 or TLR6 to recognize bacterial lipoproteins. We found that activation of TLR2 serves as a critical trigger for hIAPP-induced Il1b expression in both BMDMs and cultured islets. Interestingly, diverse amyloidogenic peptides appear to act as endogenous ligands for TLR2: activation of TLR1/2 has been described for Aβ (32, 33), serum amyloid A (34), lysozyme fibrils (12), curli fibrils (11), and α-synuclein (35). Lysozyme may also activate TLR2/6 (12), and our data suggest that heterodimerization with TLR6 is required for sensing of IAPP by TLR2. Other membrane-bound co-receptors such as CD14 partner with the TLRs to enhance ligand recognition (36). CD14 interacts with Aβ (37) and curli fibrils (38) but is not required for NF-κB activation by lysozyme (12), serum amyloid A (10), or (as we have shown here) IAPP. Multiple diseases of protein aggregation are also associated with activation of NLRP3 (29). Masters et al. provided direct evidence for IAPP-induced NLRP3 inflammasome activation in dendritic cells (9); here, we have demonstrated that this effect is observed in macrophages, can be attributed to amyloid fibrils, and is accompanied by TLR2 activation by species formed at an earlier stage of hIAPP aggregation. Importantly, given increasing evidence for cross-talk between TLR and NLRP3 signaling pathways (27, 39), IAPP and other amyloidogenic peptides may act as particularly potent pro-inflammatory stimuli because of their capacity to induce both pathways. The activation of pattern recognition receptors by both microbial amyloids (e.g. curli fibrils) and endogenous amyloids (e.g. IAPP) suggests that inflammation in amyloid disease may be a consequence of the evolution of the innate immune response to foreign peptide aggregates with similar structural motifs.
Collectively, these findings provide a mechanistic link between two important islet pathologies in type 2 diabetes, amyloid, and inflammation. Distinct forms of IAPP, the principal component of islet amyloid, contribute to IL-1β release: early aggregates, which induce Il1b expression and proIL-1β synthesis via TLR2; and amyloid fibrils, which induce NLRP3-dependent IL-1β secretion. While no current therapies are available that specifically target islet amyloid formation, anti-TLR2, NLRP3, or IL-1 agents may provide an alternative approach for protecting the beta cell against hIAPP-induced dysfunction.
Author Contributions
C. W-R., J. A. E., and C. B. V. conceived and coordinated the study and wrote the paper. C. W-R. designed, performed, and analyzed experiments. H. C. D. designed, performed, and analyzed experiments shown in Fig. 6, B, G, and H. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank D. L. Dai (University of British Columbia) for assistance with islet isolation and K. Hu (University of British Columbia) for assistance with Arrayscan analysis. We thank G. Martens and B. Ross of the University of British Columbia BioImaging Facility for assistance with TEM.
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR; MOP-123338) and by core support to the Child & Family Research Institute (CFRI) from the BC Children's Hospital Foundation and Canucks for Kids Fund Childhood Diabetes Laboratories. The authors declare that they have no conflicts of interest with the contents of this article.
- IAPP
- islet amyloid polypeptide
- AP-1
- activator protein 1
- BMDM
- bone marrow-derived macrophage
- CCL
- chemokine (C-C motif) ligand
- CXCL1
- chemokine (C-X-C motif) ligand 1
- hIAPPTg/0
- human islet amyloid polypeptide transgenic (hemizygous)
- hIAPP
- human islet amyloid polypeptide
- IL-1R
- interleukin-1 receptor
- IL-1Ra
- interleukin-1 receptor antagonist
- LPS
- lipopolysaccharide
- MyD88
- myeloid differentiation primary response gene 88
- NF-κB
- nuclear factor κB
- NLRP3
- NACHT, LRR and PYD domain-containing protein 3
- rIAPP
- rodent islet amyloid polypeptide
- SEAP
- secreted alkaline phosphatase
- TLR
- Toll-like receptor
- TNF-α
- tumor necrosis factor-α.
References
- 1. Hull R. L., Westermark G. T., Westermark P., and Kahn S. E. (2004) Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 3629–3643 [DOI] [PubMed] [Google Scholar]
- 2. Ehses J. A., Perren A., Eppler E., Ribaux P., Pospisilik J. A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M. K., Biollaz G., Fontana A., Reinecke M., Homo-Delarche F., and Donath M. Y. (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 [DOI] [PubMed] [Google Scholar]
- 3. Richardson S. J., Willcox A., Bone A. J., Foulis A. K., and Morgan N. G. (2009) Islet-associated macrophages in type 2 diabetes. Diabetologia 52, 1686–1688 [DOI] [PubMed] [Google Scholar]
- 4. Donath M. Y., Böni-Schnetzler M., Ellingsgaard H., Halban P. A., and Ehses J. A. (2010) Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol. Metab. 21, 261–267 [DOI] [PubMed] [Google Scholar]
- 5. Bendtzen K., Mandrup-Poulsen T., Nerup J., Nielsen J. H., Dinarello C. A., and Svenson M. (1986) Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 232, 1545–1547 [DOI] [PubMed] [Google Scholar]
- 6. Corbett J. A., Wang J. L., Sweetland M. A., Lancaster J. R. Jr., and McDaniel M. L. (1992) Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans: evidence for the beta-cell as a source and site of action of nitric oxide. J. Clin. Invest. 90, 2384–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westwell-Roper C. Y., Ehses J. A., and Verchere C. B. (2014) Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and beta-cell dysfunction. Diabetes 63, 1698–1711 [DOI] [PubMed] [Google Scholar]
- 8. Westwell-Roper C., Dai D. L., Soukhatcheva G., Potter K. J., van Rooijen N., Ehses J. A., and Verchere C. B. (2011) IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J. Immunol. 187, 2755–2765 [DOI] [PubMed] [Google Scholar]
- 9. Masters S. L., Dunne A., Subramanian S. L., Hull R. L., Tannahill G. M., Sharp F. A., Becker C., Franchi L., Yoshihara E., Chen Z., Mullooly N., Mielke L. A., Harris J., Coll R. C., Mills K. H. G., et al. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu S., Liu Y., Hao W., Wolf L., Kiliaan A. J., Penke B., Rübe C. E., Walter J., Heneka M. T., Hartmann T., Menger M. D., and Fassbender K. (2012) TLR2 is a primary receptor for Alzheimer's amyloid beta peptide to trigger neuroinflammatory activation. J. Immunol. 188, 1098–1107 [DOI] [PubMed] [Google Scholar]
- 11. Tükel C., Wilson R. P., Nishimori J. H., Pezeshki M., Chromy B. A., and Bäumler A. J. (2009) Responses to amyloids of microbial and host origin are mediated through Toll-like receptor 2. Cell Host Microbe 6, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gustot A., Raussens V., Dehousse M., Dumoulin M., Bryant C. E., Ruysschaert J. M., and Lonez C. (2013) Activation of innate immunity by lysozyme fibrils is critically dependent on cross-beta sheet structure. Cell. Mol. Life Sci. 70, 2999–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., and Glabe C. G. (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 14. Zraika S., Hull R. L., Verchere C. B., Clark A., Potter K. J., Fraser P. E., Raleigh D. P., and Kahn S. E. (2010) Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia 53, 1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jurgens C. A., Toukatly M. N., Fligner C. L., Udayasankar J., Subramanian S. L., Zraika S., Aston-Mourney K., Carr D. B., Westermark P., Westermark G. T., Kahn S. E., and Hull R. L. (2011) β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am. J. Pathol 178, 2632–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahdi T., Hänzelmann S., Salehi A., Muhammed S. J., Reinbothe T. M., Tang Y., Axelsson A. S., Zhou Y., Jing X., Almgren P., Krus U., Taneera J., Blom A. M., Lyssenko V., Esguerra J. L., et al. (2012) Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab. 16, 625–633 [DOI] [PubMed] [Google Scholar]
- 17. Donath M. Y., Dalmas É., Sauter N. S., and Böni-Schnetzler M. (2013) Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 17, 860–872 [DOI] [PubMed] [Google Scholar]
- 18. Böni-Schnetzler M., Thorne J., Parnaud G., Marselli L., Ehses J. A., Kerr-Conte J., Pattou F., Halban P. A., Weir G. C., and Donath M. Y. (2008) Increased interleukin (IL)-1β messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J. Clin. Endocrinol Metab. 93, 4065–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salvalaggio P. R. O., Deng S., Ariyan C. E., Millet I., Zawalich W. S., Basadonna G. P., and Rothstein D. M. (2002) Islet filtration: a simple and rapid new purification procedure that avoids ficoll and improves islet mass and function. Transplantation 74, 877–879 [DOI] [PubMed] [Google Scholar]
- 20. van Rooijen N., and van Kesteren-Hendrikx E. (2003) “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 373, 3–16 [DOI] [PubMed] [Google Scholar]
- 21. Westwell-Roper C., Dunne A., Kim M. L., Verchere C. B., and Masters S. L. (2013) Activating the NLRP3 inflammasome using the amyloidogenic peptide IAPP. Methods Mol. Biol. 1040, 9–18 [DOI] [PubMed] [Google Scholar]
- 22. Spandidos A., Wang X., Wang H., and Seed B. (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38, D792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Bergenhenegouwen J., Plantinga T. S., Joosten L. A., Netea M. G., Folkerts G., Kraneveld A. D., Garssen J., and Vos A. P. (2013) TLR2 & Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J. Leukocyte Biol. 94, 885–902 [DOI] [PubMed] [Google Scholar]
- 24. Konarkowska B., Aitken J. F., Kistler J., Zhang S., and Cooper G. J. (2006) The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 273, 3614–3624 [DOI] [PubMed] [Google Scholar]
- 25. Novotny G. W., Lundh M., Backe M. B., Christensen D. P., Hansen J. B., Dahllöf M. S., Pallesen E. M., and Mandrup-Poulsen T. (2012) Transcriptional and translational regulation of cytokine signaling in inflammatory beta-cell dysfunction and apoptosis. Arch. Biochem. Biophys. 528, 171–184 [DOI] [PubMed] [Google Scholar]
- 26. Westwell-Roper C. Y., Chehroudi C. A., Denroche H. C., Courtade J. A., Ehses J. A., and Verchere C. B. (2015) IL-1 mediates amyloid-associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia 58, 575–585 [DOI] [PubMed] [Google Scholar]
- 27. Juliana C., Fernandes-Alnemri T., Kang S., Farias A., Qin F., and Alnemri E. S. (2012) Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghonime M. G., Shamaa O. R., Das S., Eldomany R. A., Fernandes-Alnemri T., Alnemri E. S., Gavrilin M. A., and Wewers M. D. (2014) Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J. Immunol. 192, 3881–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masters S. L., and O'Neill L. A. J. (2011) Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol. Med. 17, 276–282 [DOI] [PubMed] [Google Scholar]
- 30. Cheng K., Wang X., Zhang S., and Yin H. (2012) Discovery of small-molecule inhibitors of the TLR1/TLR2 complex. Angew. Chem. Int. Ed. Engl. 51, 12246–12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang Y. C., Kao W. C., Wang W. Y., Wang W. Y., Yang R. B., and Peck K. (2009) Identification and characterization of oligonucleotides that inhibit Toll-like receptor 2-associated immune responses. FASEB J. 23, 3078–3088 [DOI] [PubMed] [Google Scholar]
- 32. Reed-Geaghan E. G., Savage J. C., Hise A. G., and Landreth G. E. (2009) CD14 and toll-like receptors 2 and 4 are required for fibrillar A{β}-stimulated microglial activation. J. Neurosci. 29, 11982–11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., Lacy-Hulbert A., El Khoury J., Golenbock D. T., and Moore K. J. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He R. L., Zhou J., Hanson C. Z., Chen J., Cheng N., and Ye R. D. (2009) Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 113, 429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim C., Ho D. H., Suk J. E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H. J., and Lee S. J. (2013) Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 4, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akashi-Takamura S., and Miyake K. (2008) TLR accessory molecules. Curr. Opin Immunol. 20, 420–425 [DOI] [PubMed] [Google Scholar]
- 37. Fassbender K., Walter S., Kühl S., Landmann R., Ishii K., Bertsch T., Stalder A. K., Muehlhauser F., Liu Y., Ulmer A. J., Rivest S., Lentschat A., Gulbins E., Jucker M., Staufenbiel M., et al. (2004) The LPS receptor (CD14) links innate immunity with Alzheimer's disease. FASEB J. 18, 203–205 [DOI] [PubMed] [Google Scholar]
- 38. Rapsinski G. J., Newman T. N., Oppong G. O., van Putten J. P., and Tükel Ç. (2013) CD14 protein acts as an adaptor molecule for the immune recognition of Salmonella curli fibers. J. Biol. Chem. 288, 14178–14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hara H., Tsuchiya K., Kawamura I., Fang R., Hernandez-Cuellar E., Shen Y., Mizuguchi J., Schweighoffer E., Tybulewicz V., and Mitsuyama M. (2013) Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 14, 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]