Abstract
Here we report a “configuration-dependent” mechanism of action for IL-15:IL-15Rα (heterodimeric IL-15 or hetIL-15) where the manner by which IL-15:IL-15Rα molecules are presented to target cells significantly affects its function as a vaccine adjuvant. Although the cellular mechanism of IL-15 trans-presentation via IL-15Rα and its importance for IL-15 function have been described, the full effect of the IL-15:IL-15Rα configuration on responding cells is not yet known. We found that trans-presenting IL-15:IL-15Rα in a multivalent fashion on the surface of antigen-encapsulating nanoparticles enhanced the ability of nanoparticle-treated dendritic cells (DCs) to stimulate antigen-specific CD8+ T cell responses. Localization of multivalent IL-15:IL-15Rα and encapsulated antigen to the same DC led to maximal T cell responses. Strikingly, DCs incubated with IL-15:IL-15Rα-coated nanoparticles displayed higher levels of functional IL-15 on the cell surface, implicating a mechanism for nanoparticle-mediated transfer of IL-15 to the DC surface. Using artificial antigen-presenting cells to highlight the effect of IL-15 configuration on DCs, we showed that artificial antigen-presenting cells presenting IL-15:IL-15Rα increased the sensitivity and magnitude of the T cell response, whereas IL-2 enhanced the T cell response only when delivered in a paracrine fashion. Therefore, the mode of cytokine presentation (configuration) is important for optimal immune responses. We tested the effect of configuration dependence in an aggressive model of murine melanoma and demonstrated significantly delayed tumor progression induced by IL-15:IL-15Rα-coated nanoparticles in comparison with monovalent IL-15:IL-15Rα. The novel mechanism of IL-15 transfer to the surface of antigen-processing DCs may explain the enhanced potency of IL-15:IL-15Rα-coated nanoparticles for antigen delivery.
Keywords: antigen presentation, dendritic cell, immunotherapy, nanotechnology, T cell, CD8+ T cell, IL-15, IL-15 receptor α, antigen delivery, nanoparticles
Introduction
It is well established that dendritic cells (DCs)2 play a key role as professional antigen-presenting cells in initiating and coordinating antigen-specific adaptive immunity (1, 2). By constitutively sampling the external microenvironment through receptor-mediated and non-receptor mediated endocytic pathways, DCs initiate, program, and regulate the effector response against a variety of antigens (3–7). Although much is known about the immunobiology of DC antigen processing and presentation and the effect of different cytokines on DC maturation and their subsequent effect on the immune response (8), little is known about how the configuration or geometry of cytokine transmission affects the DC response. For example, physiologically, although IL-2 and IL-15 belong to the same four a-helix family of cytokines, IL-2 is transmitted as a soluble paracrine or autocrine factor (9, 10), whereas IL-15 is trans-presented on the surface of DCs and other cell types through a stable complex (11) with IL-15Rα (12, 13) or secreted as a soluble paracrine complex (14, 15). The quality and manner by which cytokines are delivered to DCs can have a profound effect on the subsequent effector immune response (16, 17).
Nanosystems such as nanoparticles (18, 19), nanotubes (20), or even macromolecular systems (21) comprise an emerging strategy for controlling antigen and cytokine transmission to DCs (22–24). Perhaps one of the most explored carriers has been the polymeric poly(lactide-co-glycolide) (PLGA) system because of its significant promise and established effect in clinical settings (25–27) and well understood methods of formulation for delivery of small-molecule drugs, nucleic acids, and protein antigens (22, 28–31). Several studies have demonstrated the promise of antigen-loaded PLGA nanoparticles as nanoparticle-based vaccines and delivery systems targeting DCs (32–39).
IL-15 is a homeostatic cytokine for CD8+ T cells and natural killer cells and an important regulator of the cytotoxic immune response (40). IL-15 signals by binding to cells expressing the IL-2/IL-15 β/γ receptor and is found complexed to IL-15Rα (12–15). This IL-15:IL-15Rα complex is termed heterodimeric IL-15 (hetIL-15). IL-15Rα is expressed by several cell types in the body, most notably by activated monocytes and DCs. Co-expression of IL-15 and IL-15Rα in the same cell and intracellular complex formation is essential for the production and function of IL-15 (11, 41, 43). IL-15Rα has a very strong affinity to IL-15 (KD ∼10−11 m). Indeed, the main bioactive form of IL-15 is found exclusively in a complex with IL-15Rα in humans and mice (44) and is the main form of circulating IL-15 in the blood of lymphodepleted melanoma patients (45). This mechanism of IL-15 signaling has been shown to mediate the bulk of its immunological functions, including the differentiation, activation, and survival of CD8+ memory T cells and natural killer cells (46–49). IL-15 has already gained significant clinical interest as a highly promising immunotherapeutic agent (40, 50–52), and the anti-tumor efficacy of the IL-15:IL-15Rα complex has been demonstrated in a variety of preclinical tumor models (53–56).
IL-15 has also been investigated for its ability to augment antigen-specific responses stimulated by vaccines. Multiple studies using DNA vaccination models have reported that the inclusion of an IL-15 expression plasmid enhanced functional and protective immunity (57–59). The administration of the IL-15 protein as an adjuvant enhanced the antigen-specific T cell response to peptide-pulsed DCs (60) and, in a separate study, was able to increase antibody titers against a recombinant bacterial superantigen by a DC-specific mechanism (61). Some of these effects may be due to the action of IL-15 on DCs. Indeed, IL-15-transduced DCs are more activated than normal DCs and show improved survival and function (62). When given during DC differentiation, IL-15 potentiated stronger DC-mediated CD8 stimulation (63–65) and mediated reversal of the suppressive effects of the tumor microenvironment by restoring the antigen-processing machinery inhibited by tumor gangliosides (66). Therefore, IL-15 may interact with DCs and other antigen-presenting cells (APCs) to enhance their stimulation of other immune cells.
Because of the substantial ability of IL-15 to promote the activation and survival of CD8 effector and memory T cells and as a vaccine adjuvant in general, we reasoned that the inclusion of IL-15 on the surface of NPs would potentiate the response of APCs to encapsulated antigen. In previous work, our group and others have shown that the quality of antigen-specific memory CD8+ T cells after immunization is increased with the sustained delivery and increased availability of antigen afforded by PLGA-mediated prolonged antigen release (33, 34, 67, 68). The humoral and cellular immune responses can be potentiated further by the introduction of rationally selected ligands to the PLGA formulation. The addition of pathogen-associated molecular patterns to antigen delivery systems, for example, generated protection against West Nile strains (33, 34), lethal avian and flu strains in mice, and H1N1 in rhesus macaques (67). The use of IL-15 as an adjuvant on nanoparticulate PLGA delivery platforms for sustained release of antigen has not yet been explored.
Here we test this potential adjuvant by binding stable complexes of IL-15:IL-15Rα to the surface of PLGA nanoparticles encapsulating the model antigen ovalbumin (OVA). IL-15:IL-15Rα presented on nanoparticles acts as an adjuvant for the DC-mediated activation of OVA-specific immune responses, and this adjuvant activity (in terms of the cellular immune response) requires the delivery of antigen and heterodimeric IL-15 to the same DCs. The adjuvant activity is mediated through transfer of IL-15 from the nanoparticles to the surface of recipient DCs through a mechanism that involved internalization of the NP and export of IL-15 to the DC membrane. Our findings reveal new strategies for the enhancement of IL-15 function in vivo as an immunotherapeutic adjuvant.
Experimental Procedures
Nanoparticle Formulation
Endotoxin-free OVA (InvivoGen) was encapsulated in avidin-coated PLGA nanoparticles (NPs) using a water/oil/water double emulsion technique. 2 mg of ovalbumin protein was dissolved in 200 μl of sterile PBS and added dropwise to 50 mg of PLGA (50:50 monomer ratio, Durect Corp.) dissolved in 3 ml of chloroform while vortexing. The emulsion was then added dropwise to a mixture of 3 ml of 5% poly(vinyl alcohol) and 1 ml of an avidin-palmitic acid conjugate at 5 mg/ml in 2% sodium deoxycholate while vortexing. The avidin-palmitate partitions to the chloroform-water interface, immobilizing avidin at the NP surface upon solvent evaporation (69). The double emulsion was sonicated three times for 10 s each on ice and then transferred into a 200-ml volume of 0.2% PVA in distilled H2O to evaporate the solvent by magnetic stirring at room temperature for 90 min. Particles were then washed three times with distilled H2O and lyophilized for long-term storage at −20 °C. The encapsulation of IL-2 or class I peptides was performed in a similar manner. 200 μg of SIINFEKL or 1 mg of MART-115–40 peptide (KGHGHSYTTAEEAAGIGILTVILGVL) were dissolved in 200 μl of sterile PBS and added dropwise, while vortexing, to the PLGA/chloroform solution before emulsification in PVA as described above. For interleukin 2 (Chiron Corp.), 200 μl of a 1 mg/ml solution in PBS was used. The remaining nanoparticle formulation steps were followed after loading of the encapsulant. Nanoparticle size was analyzed using scanning electron microscopy as well as diffusion measurements in aqueous solution using a NanoSight NS500 instrument (NanoSight).
Cytokine Preparation
Optimized dual promoter plasmids expressing the two chains of the human heterodimeric cytokine IL-15:IL-15RαFc have been described previously. IL-15:IL-15RαFc is a fusion protein comprising the human soluble heterodimeric IL-15, in which the sIL-15Rα chain is fused to the Fc region of human IgG1 (70). Human IL-15:IL-15RαFc was synthesized and secreted by a stable, clonal HEK293 cell line as reported previously (15) and purified by protein A affinity chromatography. The dose of heterodimeric IL-15 in these experiments was calculated and expressed as the equivalent amount of single-chain IL-15 found within the heterodimer, as determined by amino acid analysis. The heterodimer was reacted at a 1:10 molar ratio with NHS-LC-LC-biotin (Thermo Fisher Scientific) for 1 h at 37 °C and then dialyzed for 48 h in PBS in a 3000 molecular weight cutoff dialysis bag (Thermo Fisher Scientific) to remove excess unreacted biotin.
Transmission Electron Microscopy (TEM)
IL-15-bearing NPs were treated with anti-human IL-15 (R&D Systems) and then treated with anti-mouse IgG labeled with 3-nm gold nanoparticles. The resulting NPs were mounted on copper grids, washed, and imaged on a FEI Tecnai Biotwin TEM instrument. NP-treated cells were fixed overnight in paraformaldehyde and then sectioned under liquid nitrogen before mounting and imaging by TEM.
Preparation of Murine Antigen-specific T Cells and Bone Marrow-derived Dendritic Cells
All mice used in these studies were purchased from Taconic Biosciences. Primary splenocytes were obtained from homogenized mouse spleens. CD8+ T cells were purified from the splenic homogenate of 8- 16-week-old female Rag2/OT-I transgenic mice by negative immunoselection according to the instructions of the manufacturer (StemCell Technologies). T cells were cultured in complete RPMI-10 medium consisting of RPMI 1640 medium, 10% FBS, 1% l-glutamine, 50 μm β-mercaptoethanol, 1% penicillin, 1% streptomycin, 0.05% minimum essential medium vitamin solution, 1% sodium pyruvate, and 1% non-essential amino acids (all cell culture supplements were purchased from Invitrogen). When necessary, the splenic homogenate was labeled with CFSE before purification.
DCs were generated from murine bone marrow according to established protocols (72). Bone marrow was flushed out from the femora and tibiae of 8- 16-week-old C57Bl/6 mice, and erythrocytes were depleted by hypotonic lysis (Lonza). The remaining bone marrow cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 1% HEPES buffer, 1% penicillin-streptomycin-glutamine, 100 μm β-mercaptoethanol, and 20 ng/ml murine GM-CSF. 5 days after the beginning of culture, non-adherent and semiadherent cells were collected and cultured in fresh medium for 2 more days before being used in experiments.
Preparation of Human Antigen-specific T Cells and HLA-A2+ DCs
The human CD8+ T cell receptor transgenic cell line DMF5, which is reactive to the MART-127–35 epitope, was provided by John R. Wunderlich (Surgery Branch, NCI/National Institutes of Health). Protocols for generating mature monocyte-derived DCs over a 48-h period (rather than a 7- to 10-day period) have been described previously (73–75). Peripheral blood was isolated from healthy human donors (HLA-A*0201+ and HLA-A*0201−) after informed consent was obtained, and mononuclear cells were purified by Ficoll-Hypaque gradient centrifugation. Purified cells were suspended in serum-free DC medium (Cell-Genix) with 1% human serum and plated at 15 × 106 cells/well in a 6-well tissue culture plate. Non-adherent cells and medium were removed and replaced with fresh DC medium 90 and 150 min after the start of incubation. The media was then replaced with DC medium supplemented with 800 IU/ml GM-CSF and 1000 IU/ml IL-4 for 24 h at 37 °C to generate immature DCs capable of internalizing and cross-presenting antigen.
For stimulation with DMF-5 T cells, human DCs were first incubated with nanoparticles or other treatments overnight. A maturation mixture of 10 ng/ml TNFα, 10 ng/ml IL-1β, 1000 IU/ml IL-6, and 1 μg/ml prostaglandin E2 was added to each sample for the final 4 h of culture. DCs were then harvested, washed, and co-cultured with DMF-5 T cells at a ratio of 1:2 DCs:T cells for 72 h at 37 °C. The efficiency of antigen-specific T cell activation was assessed using a human IFN-γ ELISA kit according to the instructions of the manufacturer.
T Cell and DC Co-culture Studies
DCs were seeded in 96-well U-bottomed plates at 25,000 cells/well. Nanoparticle constructs were added to DC cultures at 0.1 mg/ml and allowed to incubate for 8 h. When necessary, avidin-coated nanoparticles were allowed to bind with biotinylated IL-15:IL-15Rα (prepared as described previously) by incubating at room temperature for 15 min. DCs were then washed twice by centrifugation at 1500 rpm for 5 min and aspirated carefully, and then the culture medium was replaced. T cells were added at 50,000 cells/well and allowed to co-incubate for 72 h. At the end of the incubation, all cells were centrifuged, and supernatants were recovered for analysis by ELISA.
Flow Cytometric Analysis
CFSE-labeled CD8+ T cells were fixed in 2% paraformaldehyde and analyzed directly by flow cytometry. Before analysis, 5 × 104 6-μm polystyrene beads were added to each sample as an internal standard to determine absolute cell counts. For IL-15 retention studies, nanoparticle-treated bone marrow-derived dendritic cells were stained with phycoerythrin- or allophycocyanin-labeled anti-human IL-15 (R&D Systems) diluted 1:20 in 1% FBS in PBS. Staining was performed for 30 min at 4 °C, and then cells were washed once in 1× PBS and fixed in 2% paraformaldehyde. For detection of phosphorylated signaling molecules, cells were stimulated with IL-15 for 1 h and then fixed with 4% paraformaldehyde for 20 min. Cells were washed and then permeabilized overnight in 100% methanol at −20 °C. Finally, cells were washed and stained with Alexa Fluor 488-conjugated anti-phospho-STAT5 for 30 min at room temperature in the dark and then resuspended in 2% paraformaldehyde. All samples were run on an Accuri C6 flow cytometer (BD Biosciences), and data were analyzed using FlowJo (TreeStar) or Accuri C6 software.
Exosome Preparation
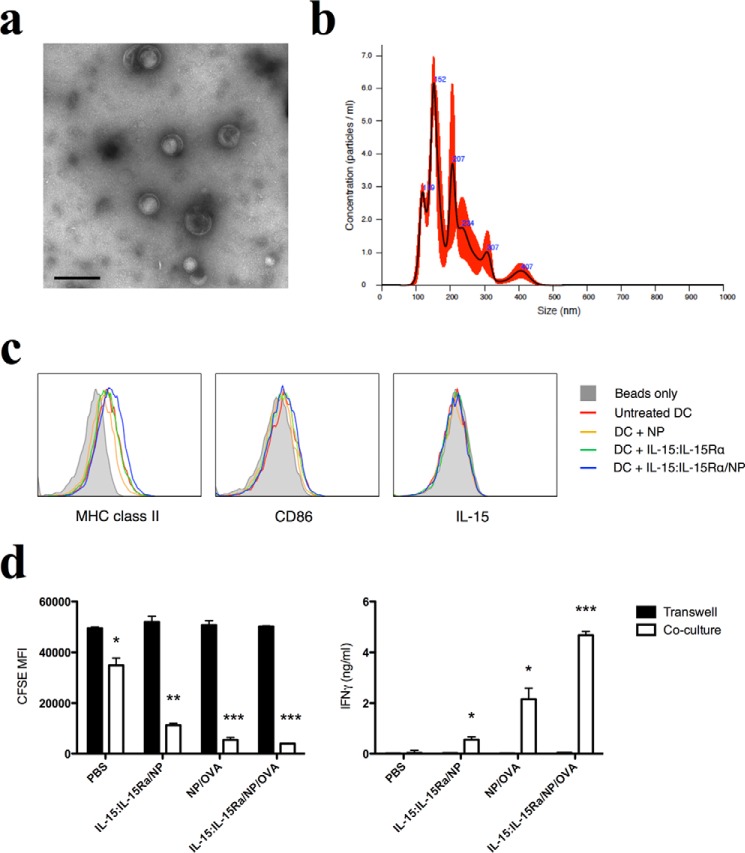
Exosomes were purified from DC culture supernatants by a sequential centrifugation protocol. DCs were incubated with NP treatments in complete medium for 24 h. DCs were then washed twice in serum-free media and replated in serum-free exosome production media containing 1% BSA for a further 24 h. Exosomes were purified from the resulting conditioned media by sequential centrifugation at 400 rcf, 2000 rcf, 10,000 rcf, and 100,000 rcf. Exosome preparations were adsorbed to 5 μm of aldehyde-sulfate beads (Life Technologies) that were then stained with antibodies and analyzed by flow cytometry.
Transwell Experiments
DCs were incubated overnight with NP constructs and then washed three times in media. 2.5 × 105 DCs/well were plated in a 24-well plate. CD8+ T cells were added at a 1:1 ratio either in cell-cell contact or separated by a 400-μm pore size transwell (Costar). Cells were stimulated for 3 days and then assayed for proliferation and cytokine secretion.
Artificial APC Studies
Artificial APCs (aAPCs) were constructed by incubating avidin-coated NPs with biotin-H2-Kb MHC dimers loaded with SIINFEKL (SIINFEKL:MHC) and equimolar anti-mouse CD28 antibody (BD Biosciences). When present, biotin-IL-15:IL-15Rα was added at a concentration of 1.5 μg of IL-15/mg of NP. To compare between NP-loaded DCs and aAPCs, 0.1 mg/ml of each NP construct was used to stimulate OT-I CD8+ T cells. DCs were incubated with NPs overnight and washed before adding T cells. aAPCs were added directly to T cells. T cells were stimulated for 3 days and then analyzed for cell counts and cytokine secretion.
In Vivo Subcutaneous Tumor Studies
OVA-expressing murine B16 melanoma cells (B16-OVA) were a gift from the laboratory of Dr. Lieping Chen. B16-OVA cells were cultured in DMEM (Gibco) with 10% FBS and suspended in 1× PBS on ice prior to injection. For metastatic studies, 8-week-old C57Bl/6 mice were challenged intravenously with 1 × 105 cells injected in 50 μl of PBS. Treatments began the day after B16-OVA challenge, and mice were euthanized by CO2 asphyxiation when symptoms of pulmonary distress were observed. Each treatment consisted of 1 μg of IL-15:IL-15Rα and 0.4 mg of PEG-modified PLGA nanoparticles.
For subcutaneous studies, 8-week-old C57Bl/6 mice were sedated with isoflurane (Baxter), and the right hind flank was shaved prior to a subcutaneous injection of 0.5 × 106 cells in 50 μl of PBS. The mice were monitored, and treatment began when the largest tumor reached 4 mm in diameter, 7–10 days after B16-OVA injection. Tumor sizes were normalized between groups after initial measurement. Systemic nanoparticle injections were performed intraperitoneally. When each component was given, each dose consisted of 1 μg of IL-15:IL-15Rα, 0.4 mg of PEG-modified PLGA nanoparticles, and 4 μg of endotoxin-free ovalbumin. Tumor size was monitored every other day by caliper measurements. Mice were euthanized by CO2 asphyxiation when tumors reached 15 mm in greatest dimension or when the mice exhibited signs of sickness.
Results
Characterization of Antigen-loaded Nanoparticles Presenting Multivalent IL-15:IL-15Rα
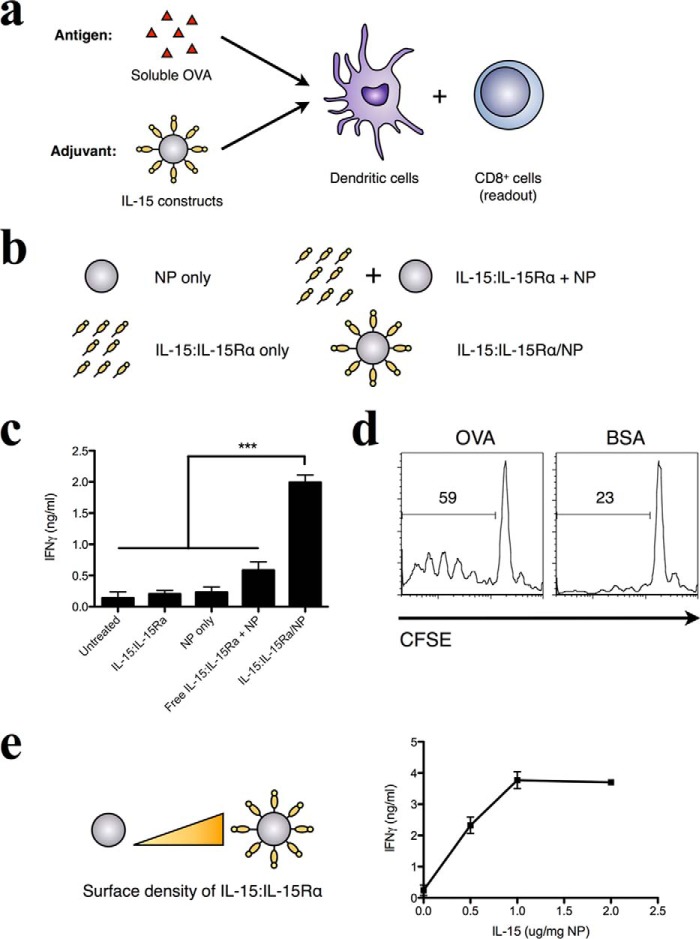
Biotinylated human IL-15:IL-15Rα was mixed with avidin-coated nanoparticles at a ratio of 2 μg/ml IL-15:1 mg/ml nanoparticles. Assuming maximal binding, this ratio yields ∼800 molecules of IL-15:IL-15Rα on the surface of each NP. To demonstrate complex exposure on the NP, we used phycoerythrin-labeled anti-human IL-15 and analyzed NP binding by flow cytometry. Bare nanoparticles were used as a negative control, and the dependence of IL-15:IL-15Rα on the avidin-biotin interaction was tested using nanoparticles treated with excess free biotin before binding with IL-15:IL-15Rα. Nanoparticles treated with biotinylated IL-15:IL-15Rα were stained uniformly with anti-IL-15, whereas pretreatment of nanoparticles with biotin completely inhibited IL-15 binding (Fig. 1a). The amount of avidin loaded onto NPs was determined by a total protein content assay to be 40 μg/mg of NP.
FIGURE 1.

IL-15:IL-15Rα is bound to the surface of fabricated PLGA nanoparticles. a, biotinylated IL-15:IL-15Rα was incubated with avidin-coated nanoparticles to create IL-15:IL-15Rα-coated nanoparticle constructs (IL-15:IL-15Rα/NP). IL-15:IL-15Rα binding was analyzed by flow cytometry after staining with anti-human IL-15 PE. The conditions tested were IL-15:IL-15Rα/NP (blue curve), bare nanoparticles (solid gray curve), and nanoparticles blocked with excess free biotin before IL-15:IL-15Rα binding (red curve). b, TEM images of nanoparticles. IL-15:IL-15Rα/NP were stained with anti-human IL-15 and 6 nm gold-linked anti-mouse IgG1 and then imaged by TEM. Arrows indicate regions where gold nanoparticles are associated with IL-15 clusters on the surface of nanoparticles. c, TEM images of NP-treated DCs. DCs were treated with IL-15:IL-15Rα/NP or uncoated NPs and then fixed overnight in paraformaldehyde and sectioned in liquid nitrogen. Sections were stained with anti-human IL-15 and 6 nm gold-linked anti-mouse IgG1 and then imaged by TEM. Arrows indicate IL-15 labeling with gold nanoparticle-linked antibodies.
To qualitatively demonstrate the presence of IL-15:IL-15Rα on nanoparticles, we examined the modified NPs using TEM. Anti-human IL-15 was labeled with gold nanoparticle-linked anti-IgG1. IL-15:IL-15Rα-bound nanoparticles were labeled with gold nanoparticles, whereas gold labeling was not observed on bare nanoparticles at similar staining concentrations (Fig. 1b).
To show that IL-15:IL-15Rα-coated NPs were internalized by DCs, we prepared sections of DCs incubated with either IL-15:IL-15Rα-coated NPs or uncoated NPs and stained the sections for IL-15. On TEM analysis, NPs could be seen in the endosomal compartments of both DC preparations, but only the IL-15:IL-15Rα-treated DCs showed IL-15 staining in the endosomal compartments (Fig. 1c). This confirmed that the modified NPs were internalized by DCs and that intracellular IL-15 can be detected after NP internalization.
IL-15:IL-15Rα on Nanoparticles Increases DC-mediated Stimulation of CD8+ T Cells
To assess the effect of IL-15:IL-15Rα NP (without encapsulated antigen) on DC-mediated stimulation of CD8+ T cells, NPs with and without the IL-15 complex were first incubated with murine bone marrow-derived DCs. Because human IL-15:IL-15Rα is cross-reactive with both mouse and human cells (15, 44), it is suitable for use in our murine experimental system. CD8 responses were monitored using purified splenic CD8+ T cells from OT-I transgenic mice specific for the Kb-restricted peptide SIINFEKL (OVA 257–264) (76). DCs were incubated with 50 μg/ml exogenous OVA in the presence of various IL-15:IL-15Rα-bearing nanoparticle constructs (Fig. 2a). Additionally, we compared the effect of free IL-15:IL-15Rα together with free antigen. To control for effects induced by the nanoparticle vehicle itself, two additional controls were included: nanoparticles alone and nanoparticles preincubated with biotin to block surface binding sites and then mixed with IL-15:IL-15Rα (Fig. 2b).
FIGURE 2.
IL-15:IL-15Rα-bearing nanoparticles stimulate cross-priming of antigen-specific CD8+ T cells by DCs. a, DCs were incubated with 50 μg/ml of soluble endotoxin-free OVA for 8 h and then washed and co-incubated with purified OVA-specific CD8+ T cells. 72 h later, cytokine levels in cell culture supernatants were evaluated by ELISA. b, during antigen uptake, DCs were co-incubated with various adjuvant formulations as depicted. Avidin-coated PLGA nanoparticles were given at 0.1 mg/ml, and IL-15:IL-15Rα was given at 0.2 μg/ml. Biotinylated IL-15:IL-15Rα was loaded onto nanoparticles by mixing the components for 15 min at room temperature before adding to DCs. For conditions where IL-15 is separated from the nanoparticle surface (IL-15:IL-15Rα + NP), nanoparticles were blocked with free biotin for 15 min before addition of IL-15. c, the adjuvant effect of these formulations was evaluated by IFN-γ production from OT-I CD8+ T cells. Data were analyzed by a one-way ANOVA and a Tukey post-hoc test. *** indicates a significance level of p ≤ 0.001. d, the antigen-specific nature of the T cell response was tested in the case of a specific antigen (OVA) and nonspecific antigen (BSA) using CFSE-labeled OT-I CD8+ T cells as responder cells. e, IL-15 was titrated on a fixed concentration of nanoparticles to determine the dose dependence of IFN-γ production on IL-15 concentration. These results are representative examples of multiple (n = 3) identical experiments.
When DCs were incubated with soluble OVA and nanoparticles bearing IL-15:IL-15Rα, a greater production of IFN-γ was observed compared with free IL-15:IL-15Rα at identical concentrations (Fig. 2c). In the absence of CD8+ T cells, little IFN-γ was observed (data not shown), implicating CD8+ T cells as the major producer of observed IFN-γ upon DC stimulation. The activation of CD8 T cells was antigen-specific because OT-I T cells failed to proliferate when an equal concentration of exogenous BSA was used in place of OVA (Fig. 2d). Interestingly, increasing the density of surface-bound IL-15:IL-15Rα correlated with increased levels of IFN-γ produced by CD8+ T cells, suggesting a dose-response effect on IFN-γ because of IL-15:IL-15Rα treatment (Fig. 2e). These results indicate that nanoparticles presenting IL-15:IL-15Rα act to enhance the antigen-specific cross-priming of CD8+ T cells by DCs.
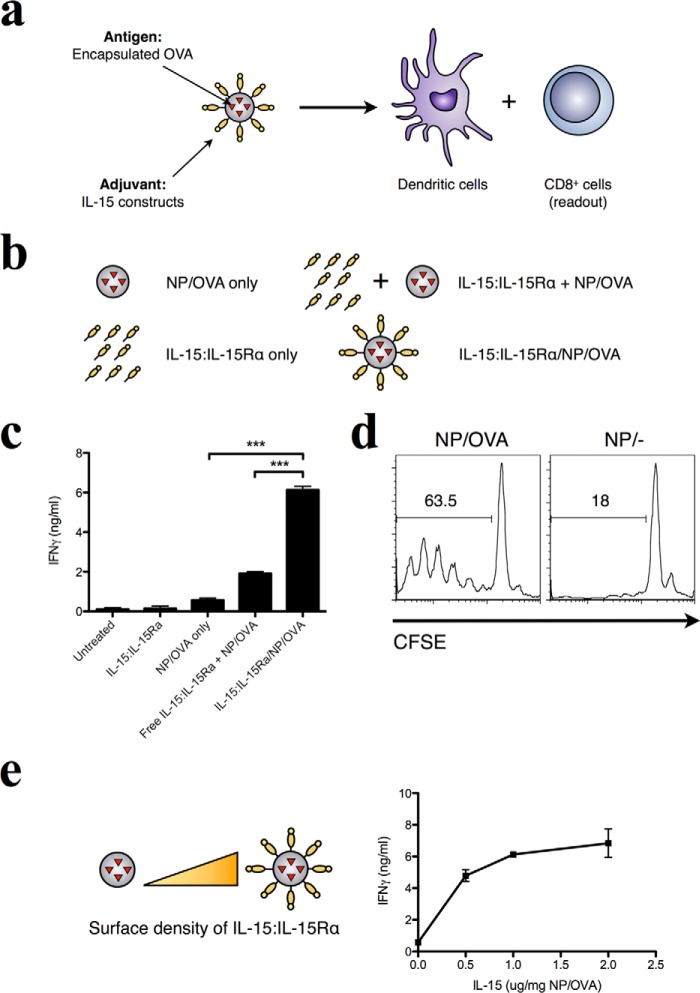
Previous work has shown that the rate of antigen delivery (persistence of antigen) affects the long-term memory immune response and vaccine efficacy in pathogen recall (67, 68). We therefore hypothesized that multivalent presentation of IL-15:IL-15Rα may synergize with antigen encapsulation in PLGA to enhance CD8+ T cell stimulation (Fig. 3a). PLGA nanoparticles encapsulating ovalbumin (NP/OVA) were prepared by a double emulsion method and used as the antigen source in place of exogenous OVA (Fig. 3b). NP/OVA particles were determined to have loaded 40 μg of OVA/mg of NP. When IL-15:IL-15Rα was trans-presented on nanoparticles that also encapsulated antigen, CD8+ T cell activation by DCs that internalized these particles was similarly enhanced above that of free IL-15:IL-15Rα alone, NP/OVA alone, or NP/OVA with IL-15:IL-15Rα separated from the particle surface (Fig. 3c). As before, when T cells were removed, cytokine production was abrogated (data not shown). The antigen specificity of the CD8+ response was confirmed when particles without antigen loading failed to elicit T cell proliferation (Fig. 3d). In addition, the IFN-γ response was dependent on IL-15:IL-15Rα surface density on the antigen-loaded nanoparticles (Fig. 3e), similar to what was observed with exogenous antigen. The resulting maximal IFN-γ responses in this experiment were much higher than those shown in Fig. 2 (6 ng/ml in Fig. 3c versus 2 ng/ml in Fig. 2c), indicating a stronger immune response elicited by the encapsulated antigen (38, 39, 77, 78). We confirmed this by comparing free OVA and NP/OVA particles at the same dose (data not shown).
FIGURE 3.
Surface-presented IL-15:IL-15Rα enhances the immunostimulatory capacity of antigen-loaded nanoparticles for the DC-mediated cross-priming of CD8+ T cells. a, the adjuvant nature of IL-15:IL-15Rα was confirmed in assays where endotoxin-free OVA was encapsulated within nanoparticles at 40 μg of OVA/mg of NP. b, the same adjuvant constructs were tested as in Fig. 1. c, the adjuvant effect of these formulations was evaluated by IFN-γ production from OT-I CD8+ T cells. Data were analyzed by a one-way ANOVA and a Tukey post-hoc test. *** indicates a significance level of p ≤ 0.001. d, the antigen-specific nature of the T cell response was tested with nanoparticles encapsulating OVA (NP/OVA) and unloaded nanoparticles (NP/−) using CFSE-labeled OT-I CD8+ T cells as responder cells. e, IL-15 was titrated on a fixed concentration of OVA-loaded nanoparticles to determine the dose dependence of IFN-γ production on IL-15 concentration.
Taken together, these results demonstrate that the surface presentation of IL-15:IL-15Rα on nanoparticles enhances the capacity of DCs to cross-prime CD8+ T cells in an antigen-specific manner. This effect was confirmed in cases where antigen was either internalized exogenously (Fig. 2) or present within the signaling nanoparticles (Fig. 3). We also verified that the DC-T cell interaction is critical for the cytokine response because the exposure of either cell type alone to IL-15:IL-15Rα nanoparticle constructs failed to produce significant levels of cytokines (data not shown). We therefore conclude that IL-15:IL-15Rα is able to act within the context of the DC:T cell interface to enhance the DC-mediated T cell response.
Synergy between Heterodimeric IL-15 and Encapsulated Antigen Requires Coordinated Delivery to DCs and T Cells
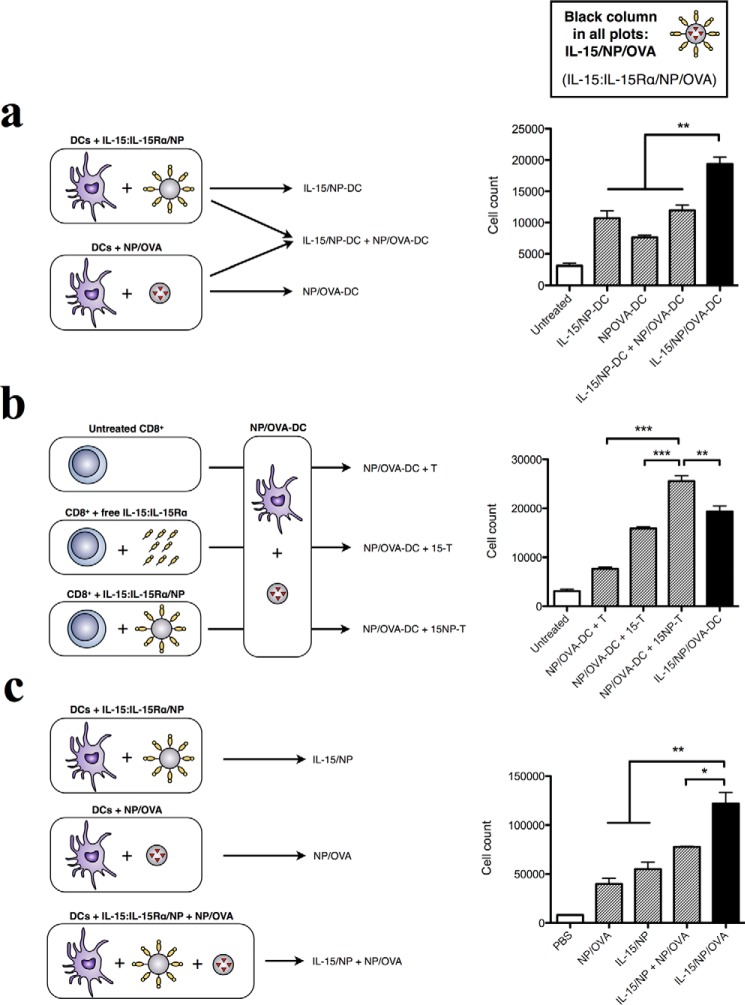
The antigen delivery system employed here has two components: IL-15:IL-15Rα trans-presented on NPs, which we have shown to be more effective than free IL-15:IL-15Rα in inducing CD8+ T cell responses, and encapsulated antigen (OVA), which is also more effective than free antigen for cross-presentation and induction of both CD4+ and CD8+ T cell responses (33, 39, 77). Given their separate functions, we asked whether the co-localization of both multivalent IL-15 and encapsulated antigen offer clear advantages compared with separate NPs delivering both elements. It is currently unknown whether the co-localized delivery of multivalent IL-15 and antigen to antigen-presenting cells has an effect on the effector response. We tested this hypothesis in three different ways, comparing our results each time to NPs presenting the IL-15 heterodimer and encapsulating OVA antigen (IL-15/NP/OVA, black columns to the right of each plot in Fig. 4).
FIGURE 4.
The effects of trans-presented IL-15:IL-15Rα and encapsulated antigen require coordinated delivery to DCs and T cells. To investigate the requirements for the adjuvant effect of IL-15:IL-15Rα, DCs were compared in their ability to stimulate OT-I CD8+ T cells under different conditions of IL-15:IL-15Rα and OVA delivery. a, the requirement for both particle types to go to the same cell was tested by mixing DCs that were separately given IL-15/NP or NP/OVA and comparing them with DCs given IL-15/NP/OVA. b, the effect of priming CD8+ T cells with IL-15 constructs was tested by priming CD8+ T cells overnight with free or NP-bound IL-15:IL-15Rα and stimulated with NP/OVA-DCs. c, to test the requirement for OVA or IL-15 to be delivered on the same particle, DCs were incubated with different particle constructs overnight and then used to activate OT-I CD8+ T cells. In all cases, the T cell response was compared against untreated DCs and IL-15/NP/OVA-treated DCs. OT-1 cell counts were determined as a measure of total stimulatory capacity of each group. Data were analyzed by a one-way ANOVA and a Tukey post-hoc test. * indicates a significance level of p ≤ 0.05, ** p ≤ 0.01, and *** indicates p ≤ 0.001.
First, we hypothesized that the delivery of multivalent IL-15 (IL-15-NP) and encapsulated OVA (NP/OVA) to the same cells would be more effective than if they were delivered to separate cells. DCs were incubated with empty IL-15:IL-15Rα-coated NPs (IL-15/NP-DCs). Separately, another population of DCs from the same batch were incubated with OVA-encapsulating NPs (NP/OVA-DCs). These cells were mixed together at a ratio equivalent to the ratio of IL-15:IL-15Rα and antigen associated with a single particle (IL-15/NP-DC + NP/OVA-DC). DCs incubated with separate or single particles (maintaining the same ratio of IL-15 to antigen) were then used to stimulate OT-I CD8+ T cells. We found that mixed cells (separately receiving either IL-15 or antigen) were less effective at inducing CD8 T cell proliferation than the combined particle system (IL-15/NP/OVA) (Fig. 4a). Notably, the mixed DCs produced IFN-γ responses on par with IL-15/NP-DCs and NP/OVA-DCs, suggesting that enhanced CD8+ T cell responses are stimulated by the localization and potential synergy of IL-15 and antigen at the same DC.
IL-15 can powerfully affect CD8+ T cell proliferation and activation and also enhance their persistence in vivo (17, 48, 79, 80). Indeed, tumor or virus-specific T cells cultivated with IL-15 have been shown to exhibit a high activity against their respective antigenic targets (81). Therefore, we hypothesized that NP-presented IL-15:IL-15Rα may also prime CD8+ T cells against NP/OVA-loaded DCs. To address this question, we incubated OT-I CD8+ T cells overnight with buffered saline as a control (T), IL-15:IL-15Rα alone (15-T), or nanoparticle-bound IL-15:IL-15Rα (15NP-T). We separately stimulated DCs with antigen by loading them with NP/OVA particles (Fig. 4b, left panel). After 12 h, T cells and DCs were washed, counted, plated together at the appropriate cell concentrations, and allowed to incubate for a further 72 h. The results are shown in Fig. 4b. We observed that CD8+ T cell proliferation in response to antigen-loaded DC was greater when T cells were preincubated with IL-15:IL-15Rα. Interestingly, NP-bound IL-15:IL-15Rα was a stronger priming stimulus compared with free IL-15:IL-15Rα (Fig. 4b, right panel). In addition, the proliferation of CD8+ T cells preactivated with IL-15:IL-15Rα/NP and incubated with NP/OVA-DCs (Fig. 4b, NP/OVA-DC + 15NP-T) was greater compared with the one obtained with DCs using the combined nanoparticle platform (Fig. 4b, IL-15/NP/OVA, black column). This result suggested that IL-15 trans-presented on NP and antigen-loaded NP may function on the separate axes of effector cells and antigen-presenting cells. IL-15 may act to prime and activate CD8s to respond to antigen, whereas nanoparticle-encapsulated antigen is internalized by DCs and cross-presented to CD8s.
Having tested how our nanoparticle components work when delivered to different cells (separate delivery to different DCs and separate delivery to CD8+ cells and DCs), we then tested a case where all nanoparticle components were delivered to the same DC. We asked whether the IL-15 and antigen components had to be presented on the same NP vehicle or whether trans-presented IL-15 and encapsulated antigen could still synergize when delivered on separate NP vehicles. We made separate nanoparticle constructs with trans-presented IL-15:IL-15Rα and encapsulated antigen, respectively, and prevented transfer of any biotinylated IL-15:IL-15Rα by blocking the avidin binding sites on NP/OVA using free biotin. We then tested these separated constructs (IL-15:IL-15Rα/NP + NP/OVA) alongside the combined construct (IL-15:IL-15Rα/NP/OVA) and along with the separate components in soluble form. We found that the combined construct was the most effective at stimulating CD8+ T cell proliferation (Fig. 4c), indicating that delivery on the same vehicle is needed for maximal stimulation. Again, both the separate and combined constructs performed significantly better than the soluble configurations where IL-15 and OVA were delivered apart from their optimal NP configurations (Figs. 2 and 3). Overall, these results demonstrate a need for the localized delivery of trans-presented IL-15:IL-15Rα and encapsulated antigen for synergy to occur.
Multivalent IL-15:IL-15Rα on NP Functions in the Same Manner with Human Cells
The translational potential of these findings depends on whether the same findings are observed with human effector and antigen-presenting cells. To validate our findings using a clinically relevant human antigen, NPs were loaded with a 26-mer peptide derived from the Melan-A/MART-1 melanoma-specific antigen (82). IL-15:IL-15Rα NPs loaded with the MART-1 antigen were incubated with human HLA-A2+ DCs and then used to stimulate DMF-5 responder cells (Fig. 5a). DMF-5 cells carry TCR receptors specific for the MART-127–35 sequence and respond to antigen-specific stimulation by producing IFN-γ (83). Consistent with observed findings in the mouse system with the encapsulated OVA antigen, the MART-1 antigen encapsulated in NPs trans-presenting multivalent IL-15:IL-15Rα led to a greater IFN-γ response from DMF5 cells compared with soluble IL-15:IL-15Rα (Fig. 5b). This result highlights the clinical potential of this mode of IL-15 delivery and possibly points to the promise of enhancing a cytokine response by changes in the contextual delivery aspect compared with, for example, genetic manipulation of the IL-15 protein sequence to affect the desired response (84, 85).
FIGURE 5.
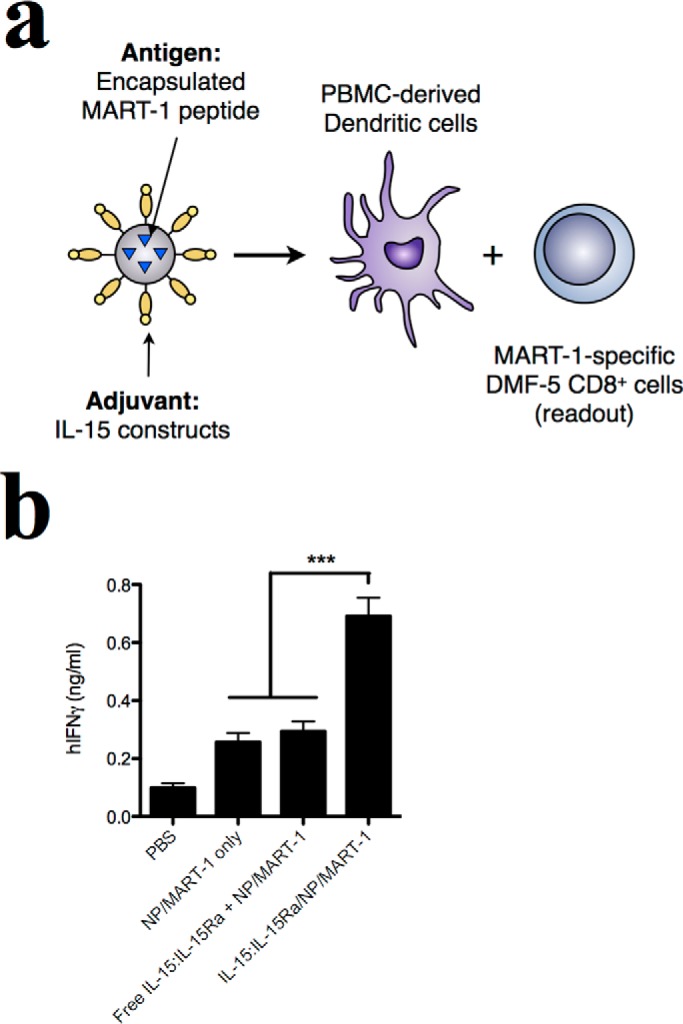
IL-15:IL-15Rα-bearing nanoparticles stimulate cross-priming of human antigen-specific CD8+ T cells by DCs. a, nanoparticles encapsulating a MART-1-derived 26-mer peptide were loaded into human HLA-A2+ PBMC-derived DCs and used to stimulate MART-1-specific DMF-5 T cells. b, the adjuvant effect of the nanoparticle formulations was evaluated by IFN-γ production from DMF-5 cells after 72 h in culture with DCs. Data were analyzed by a one-way ANOVA and a Tukey post-hoc test. *** indicates a significance level of p ≤ 0.001. PBMC, peripheral blood-derived mononuclear cells.
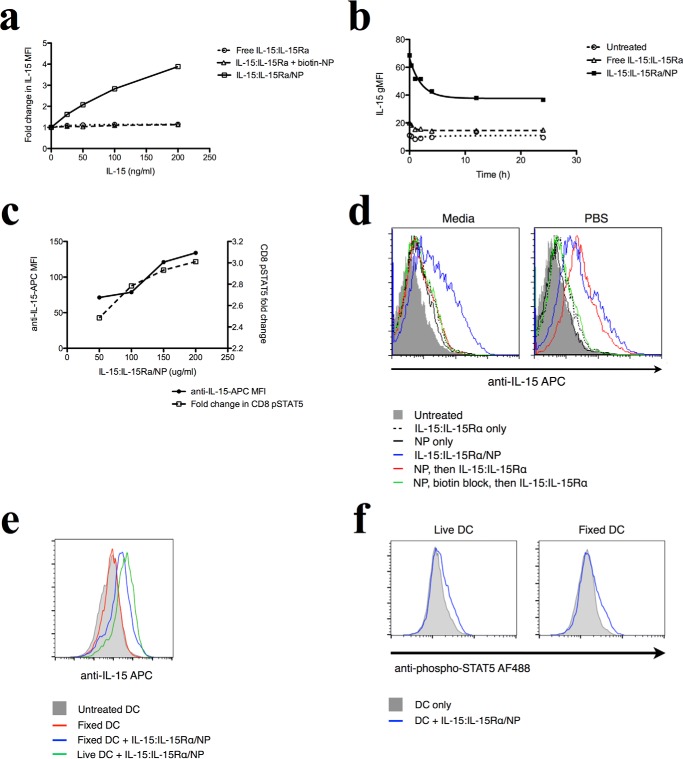
The Mechanism of Action of Multivalent NP-presented IL-15:IL-15Rα Involves Particle-mediated Transfer of IL-15 to the DC Surface
We set out to determine the molecular mechanism by which IL-15:IL-15Rα augments the stimulatory capacity of antigen-presenting DCs. Because IL-15 trans-presentation on DCs affects the activation of CD8 T cells, we asked whether delivered IL-15 may be retained on the surface of DCs for stimulation during T cell encounters. To address this question, we treated mouse DCs with free human IL-15:IL-15Rα, nanoparticle-bound IL-15:IL-15Rα, or IL-15:IL-15Rα mixed with biotin-blocked nanoparticles and stained DCs for human IL-15 2 h later. Staining for human IL-15 ensured that we would detect only the delivered molecules and not endogenous murine IL-15. We found that incubating DCs with nanoparticle-bound IL-15:IL-15Rα resulted in increased levels of detectable human IL-15 on the DC surface in a manner commensurate with the density of IL-15:IL-15Rα on the nanoparticle surface. In contrast, free human IL-15:IL-15Rα did not result in accumulation of human IL-15 on the DC surface (Fig. 6a).
FIGURE 6.
Delivered NP-bound IL-15 is retained on the surface of DCs and signals CD8+ T cells. a, DCs were incubated with different concentrations of free or NP-bound IL-15:IL-15Rα constructs for 2 h and then stained for human IL-15. b, to track the fate of surface-retained IL-15 on DCs over time, DCs were incubated with 150 ng/ml of free or NP-bound IL-15 for varying amounts of time and then fixed and stained for human IL-15. c, DCs were incubated with different levels of IL-15:IL-15Rα-bound nanoparticles for 1 h and then washed and incubated with purified CD8 T cells for 1 h. CD8 cells were fixed and stained for phosphorylated STAT5. d, to determine whether avidin binding sites are detectable on the surface of NP-treated cells, DCs were incubated with NPs in PBS for 1 h and then exposed to biotinylated IL-15. IL-15/NP was given as a positive control, and some cells were blocked with biotin after NP binding to saturate avidin binding sites. This experiment was performed in both media and PBS. e, fixed and live DCs were co-incubated with IL-15-coated NPs for 2 h and then washed extensively and used to stimulate purified CD8+ T cells. T cells were then stained for phospho-STAT5. Curves where IL-15 was present are shown in blue, and control cells are shown in gray. f, fixed and live DCs were co-incubated with IL-15-coated NPs for 2 h and then washed and stained for IL-15.
We next sought to characterize the persistence of this IL-15:IL-15Rα retention on the DC surface. DCs were treated with IL-15:IL-15Rα constructs for 2 h, washed, and cultured over time before staining with phycoerythrin-labeled anti-human IL-15. DCs treated with NP-bound IL-15:IL-15Rα showed an initial 200% increase in fluorescence because of IL-15 labeling compared with untreated controls (Fig. 6b). Although the fluorescence decayed steadily over 8 h, a strikingly high level of IL-15 was still retained on the surface of DCs, leading to a >100% increase in IL-15:IL-15Rα-associated fluorescence compared with untreated cells. This amount was steady after 8 h and remained despite wash steps. In contrast, when IL-15:IL-15Rα was given in soluble form, very little IL-15 accumulated at the DC surface, and what little did accumulate was cleared quickly from the membrane (Fig. 6b).
Next we asked whether the surface-retained IL-15:IL-15Rα was able to functionally stimulate CD8+ T cells. DCs were treated with different concentrations of nanoparticle-bound IL-15:IL-15Rα for 2 h, resulting in different levels of IL-15 retention on the DC surface, as revealed by flow cytometry. DCs were then washed and used to stimulate purified CD8+ T cells at a 1:1 DC:T cell ratio for 1 h. T cells were then stained for phosphorylated STAT5 to evaluate IL-15-specific signaling. We found that increasing levels of IL-15 on the DC surface correspondingly increased the level of phosphorylated STAT5 in CD8+ T cells (Fig. 6c), demonstrating that the IL-15 delivered to the surface of DCs remains functional and capable of stimulating CD8+ T cells.
Because IL-15 is present in high concentrations at the DC surface within a short time after NP delivery, it is possible that non-internalized NP nonspecifically associated with the cell membrane and displayed IL-15, leading to the observed high IL-15 levels at steady state. If this is the case, then the treatment of DCs with avidin-coated NPs (the base scaffold on which IL-15 is displayed) would result in the display of avidin binding sites on the DC surface, allowing for the binding of biotinylated ligands such as biotin-IL-15:IL-15Rα. To test this hypothesis, DCs were incubated with avidin-coated NPs in either media or PBS for 1 h at 37 °C. Biotin-IL-15:IL-15Rα was then added to the DCs for 15 min (Fig. 6d; NP, then IL-15:IL-15Rα; red line). All cells were washed and stained for IL-15 and analyzed by flow cytometry.
When the experiment was performed in media, avidin binding sites could not be detected via IL-15-associated fluorescence (Fig. 6d, left panel). However, when the experiment was performed in PBS, the “NP, then IL-15:IL-15Rα” group resulted in fluorescence similar to our positive control (Fig. 6d, IL-15:IL-15Rα/NP, blue line), suggesting the binding and detection of IL-15 at the DC surface (Fig. 6d, right panel). Biotin blockade prior to IL-15 binding (Fig. 6d; NP, biotin block, then IL-15:IL-15Rα, green line) abrogated the IL-15 signal, showing that the effect is due to biotin binding sites on the cell surface. IL-15 labeling was not detected after treating the cells with free IL-15:IL-15Rα alone, indicating that this effect is not due to any biotin binding proteins on the cell surface (Fig. 6d).
If IL-15-bearing NPs associate nonspecifically with the DC surface, then DCs may be able to retain IL-15 at their membranes in the absence of active cell processes. We therefore investigated the ability of fixed DCs to present IL-15 to CD8+ T cells. DCs were fixed with paraformaldehyde and then incubated with IL-15:IL-15Rα/NP for 2 h. We found that these fixed, IL-15-treated DCs stained positive for IL-15, indicating that fixed DCs can accumulate IL-15 on their surface (Fig. 6e). The IL-15 staining was at a lower level than live DCs (Fig. 6e, green line) exposed to IL-15:IL-15Rα/NP for the same duration, indicating that some active cellular mechanisms may aid in (but are not required for) IL-15 accumulation on the cell surface.
Finally, we asked whether the IL-15 presented on the surface of fixed DCs was bioactive. Live and fixed DCs were co-incubated with IL-15:IL-15Rα/NP for 2 h and then washed extensively and incubated with CD8+ T cells for 1 h. Phospho-STAT5 staining was used to assess IL-15 signaling in CD8+ cells, and live or fixed DCs unexposed to IL-15 were used as controls. We found that the IL-15 present on the surface of both live and fixed DCs was able to stimulate STAT5 phosphorylation in CD8+ T cells (Fig. 6f), demonstrating that fixed DCs are able to present bioactive IL-15.
Our data suggest that the incubation of DCs with NPs results in some of the NPs embedding in the cell surface, exposing their surface ligands (IL-15 or avidin) for interactions with neighboring cells. Such behavior could account for the retention of IL-15 on the DC surface. The fact that IL-15 binding (in the NP, then IL-15:IL-15Rα group) could not be detected in the presence of media suggests that media components were able to shield or block the interaction of biotin-IL-15 with embedded avidin-NPs. Indeed, under the right circumstances, the avidin-biotin interaction on a surface can be blocked or inhibited (86, 87). Furthermore, active cellular mechanisms are not required for the retention of bioactive IL-15 at the DC surface. Although fixed DCs cannot process and present antigen, they are able to act as physical scaffolds for NP-delivered multivalent IL-15. Overall, our findings demonstrate that nanoparticle-bound IL-15:IL-15Rα delivers functional IL-15:IL-15Rα to the surface of DCs in a manner dependent on the density of IL-15:IL-15Rα bound to the nanoparticles and persists on the DC surface for at least 24 h.
NP-delivered IL-15 Is Not Observed on DC-derived Exosomes
We investigated the possibility that IL-15 might be exported from the DC membrane via exosomes. Indeed, IL-15Rα has been detected previously on human DC-derived exosomes (88). We therefore purified exosomes from DCs by sequential ultracentrifugation (89). DCs were allowed to take up NPs overnight (untreated control, free IL-15:IL-15Rα, NP only, and IL-15:IL-15Rα/NP), and then exosomes were purified from the DC-conditioned media. Exosome preparations were examined by TEM, revealing circular and cup-shaped membrane vesicles about 100 nm in diameter (Fig. 7a). Exosome size distribution was also determined by particle tracking analysis (NanoSight) and found to be between 100 and 300 nm (Fig. 7b).
FIGURE 7.
IL-15 is not associated with DC-derived exosomes. a, exosome preparations were visualized by TEM. Scale bar = 200 nm. A representative image is shown. b, representative size distribution plot of exosomes sized using nanoparticle tracking analysis. Black line, mean; red, error bars. c, exosome-associated proteins were analyzed by flow cytometry. Exosomes were adsorbed to aldehyde/sulfate beads and stained for MHC class II, CD86, and IL-15. d, NP-loaded DCs were assayed for their ability to stimulate antigen-specific T cell activation via exosomes. DCs were used to stimulate CFSE-labeled T cells either separated by a transwell (Transwell, black columns) or in direct cell contact (Co-culture, white columns). T cells were assayed for CFSE dilution (left panel) and IFN-γ secretion. The differences in mean values between the co-culture and Transwell experiments for each condition were compared using an unpaired t-test. *** indicates a significance level of p ≤ 0.001, ** indicates p ≤ 0.01, and * indicates p ≤ 0.05.
We then examined exosome-associated proteins in our preparations by flow cytometry. All exosome preparations stained positive for class II MHC and slightly positive for CD86 (Fig. 7c), both of which are known to be present in DC-derived exosomes (90). However, none of the exosome preparations contained detectable levels of IL-15 (Fig. 7c), suggesting that IL-15 is not associated with DC-derived exosomes.
If IL-15 and antigen were being rereleased by exosomes, then DC-derived exosomes may be able to directly stimulate OT-I T cells in the absence of cell contact. We therefore tested the ability of our NP-treated DCs to stimulate T cell activation in a transwell experiment. DCs were incubated overnight with the following nanoparticle constructs: IL-15:IL-15Rα/NP, NP/OVA, and IL-15:IL-15Rα/NP/OVA. After extensive washing to remove excess NPs, DCs were used to stimulate CD8+ T cells either in cell-cell contact or separated by a 400-μm pore size transwell. The addition of the transwell completely abolished the proliferative (Fig. 7d, left panel) and cytokine response (Fig. 7d, right panel) of the CD8+ T cells for all NP groups, suggesting that exosomes do not carry IL-15 or antigen to T cells across the transwell membrane. We therefore conclude that IL-15 is not observed on DC-derived exosomes and that it does not contribute to T cell stimulation via an exosome-mediated mechanism.
IL-15 Enhancement of the Antigen-specific CD8+ Response Mediated by NP Multivalent Presentation Is Not due to Enhancement of Antigen Processing, NP Uptake, or DC-specific IL-15 Signaling
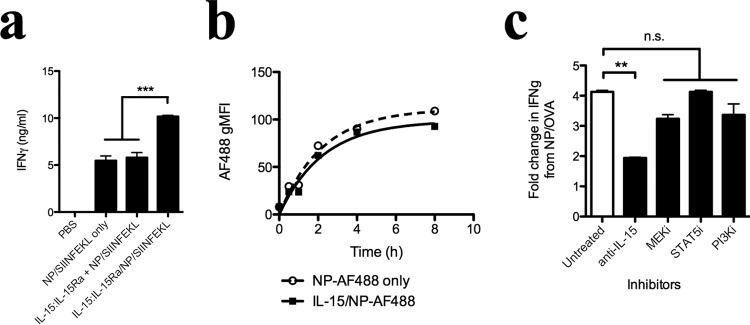
Given that NP delivered an encapsulated form of the whole OVA protein, we next asked whether IL-15:IL-15Rα was acting on the antigen-processing machinery in DCs. To answer this question, we encapsulated the SIINFEKL peptide within IL-15:IL-15Rα-coated nanoparticles. We observed that a stronger IFN-γ response was obtained than when either IL-15:IL-15Rα was not added or detached from the biotin-blocked nanoparticle surface (Fig. 8a). APCs incubated with IL-15:IL-15Rα NP therefore potentiate CD8+ T cell stimulation in a manner independent of antigen.
FIGURE 8.
The enhancement of CD8+ T cell activation by coordinated delivery of IL-15:IL-15Rα is independent of antigen processing, nanoparticle internalization, or signaling pathways downstream of the IL-2/15Rβγ receptor. a, To test the requirement for antigen processing, nanoparticles encapsulating the ovalbumin-derived SIINFEKL peptide were loaded into murine bone marrow-derived dendritic cells and used to stimulate OT-I CD8 T cells. b, to test the effect of IL-15 on nanoparticle uptake, Alexa Fluor 488-labeled nanoparticles were incubated with DCs in the presence or absence of surface-bound IL-15:IL-15Rα. DCs were fixed and analyzed by flow cytometry at various time points after addition of particles. c, DCs were incubated with signaling pathway inhibitors for 2 h prior to nanoparticle uptake, washed, and incubated overnight with OVA-encapsulating nanoparticles in the presence or absence of surface-presented IL-15:IL-15Rα. DCs were then washed and co-incubated with OT-I CD8+ T cells. Data are presented as the resulting -fold increase in IFN-γ when IL-15:IL-15Rα is added to the surface of OVA-encapsulating nanoparticles. Data were analyzed by a one-way ANOVA and a Tukey post-hoc test. *** indicates a significance level of p ≤ 0.001, ** indicates a significance level of p ≤ 0.01, and n.s. indicates that differences are not statistically significant.
Next we asked whether NP-presented IL-15:IL-15Rα affected the rate of NP internalization and, therefore, antigen uptake. Avidin-coated NPs were surface-labeled with Alexa Fluor 488 and then incubated with DCs in the presence or absence of surface-bound IL-15:IL-15Rα. DCs were fixed and analyzed for Alexa Fluor 488 fluorescence by flow cytometry. We found that IL-15:IL-15Rα had no discernable effect on the rate of fluorescence accumulation in DCs (Fig. 8b), indicating that the trans-presentation of IL-15:IL-15Rα does not affect the rate of NP internalization in our system.
Finally, we asked whether IL-15:IL-15Rα induces its effects by activating signaling pathways within DCs during NP uptake. 2 h prior to NP uptake, DCs were incubated with either 10 μg/ml anti-human IL-15, 50 μm STAT5 inhibitor (N′-((4-oxo-4H-chromen-3-yl)methyl-ene)nicotinohydrazide), 2 μm PI3K inhibitor (LY294002), or 2 μm MEK inhibitor (U0126). We assessed the effect of these inhibitors by evaluating the -fold increase in CD8-mediated IFN-γ secretion when surface-bound IL-15:IL-15Rα was added to NP/OVA during particle uptake by DCs. Although the addition of anti-human IL-15 did partially decrease IFN-γ production, none of the other inhibitors appreciably affected the activation of CD8+ T cells by the NP-loaded DCs (Fig. 8c). In addition, DCs did not show elevated levels of STAT5 phosphorylation upon stimulation with IL-15:IL-15Rα, lending support to our hypothesis that the primary effect of delivered IL-15:IL-15Rα to DCs is to stimulate neighboring T cells.
Artificial Antigen Presentation Together with IL-15:IL-15Rα Improves the Sensitivity and Magnitude of the Antigen-specific Response
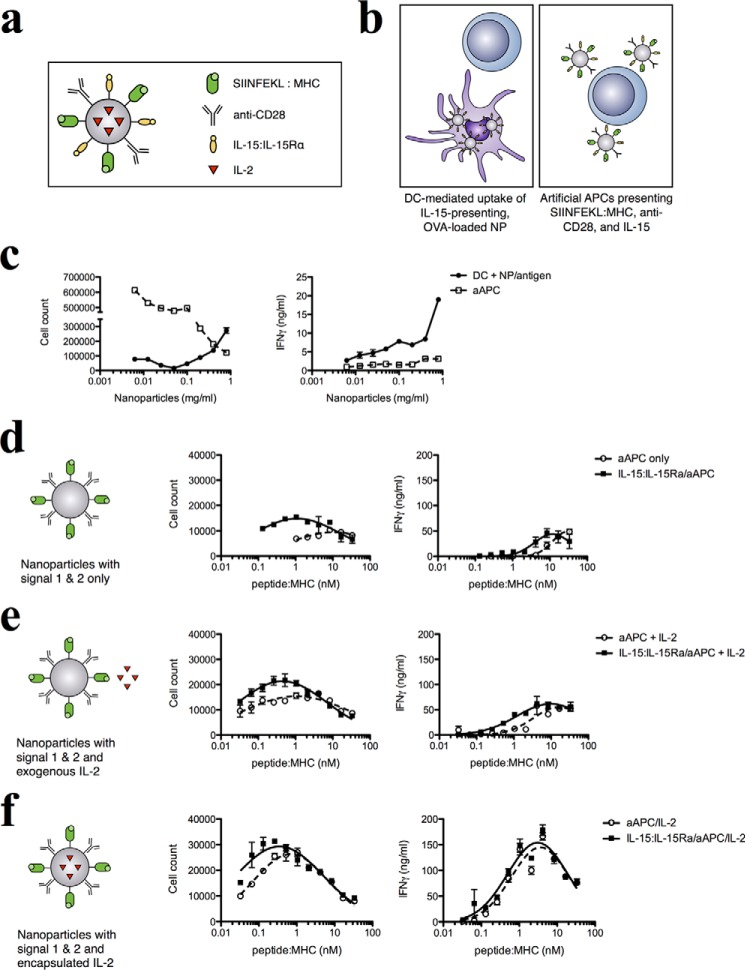
APCs provide at least three essential signals to activate T cells: antigen-specific stimulation by peptide-MHC complexes; costimulatory signals, most commonly via the CD28/B7 interaction; and the release of cytokines to modulate T cell expansion, survival, and function. Our data indicated that IL-15:IL-15Rα-bearing, antigen-loaded NPs are able to enhance specific T cell responses, transferring IL-15:IL-15Rα molecules to the DC surface in a manner dependent on the NP surface density of IL-15:IL-15Rα but independent of NP internalization, antigen processing, and DC signaling. This effect echoes the physiological mechanism of action of IL-15, which is trans-presented on the surface of activated monocytes and DCs via binding to membrane-bound IL-15Rα (13). Therefore, we wanted to understand what happens when IL-15 is presented in a physiological fashion in a fully functioning system with specified parameters. To do so, we designed a nanoparticle-based antigen-presenting cell that provides all of the essential signals for T cell stimulation: MHC-complexed SIINFEKL for antigen presentation, anti-CD28 antibodies for costimulation, and the presence or absence of trans-presented IL-15:IL-15Rα as the cytokine signal (Fig. 9a). We have demonstrated previously that an aAPC system consisting of antigen presentation, costimulation, and cytokine secretion is able to efficiently expand target T cell populations (16, 91).
FIGURE 9.
Trans-presented IL-15 improves the sensitivity and magnitude of the antigen-specific CD8+ response and synergizes with additional cytokine signals. a, a nanoparticle-based artificial APC was used to investigate the effect of surface-retained IL-15:IL-15Rα on antigen presentation. b, aAPCs were compared with DCs loaded with antigen-encapsulating NPs for their ability to stimulate CD8+ T cells. c, T cell stimulation was assessed by cell proliferation (left panel) and IFN-γ secretion (right panel). Next we assessed the effect of IL-15 trans-presentation on aAPCs. Biotinylated, SIINFEKL-loaded H2-Kb MHC I dimers were titrated on all nanoparticle groups along with equimolar concentrations of anti-CD28. This was done in the presence or absence of surface-bound IL-15:IL-15Rα. The resulting constructs were co-incubated with OT-I CD8+ T cells, and T cell proliferation was assessed. d—f, data for cell proliferation (left panels) and IFN-γ secretion (right panels) are shown for three different experiments: blank nanoparticles (d), blank nanoparticles with 5 ng/ml of exogenous IL-2 (e), and nanoparticles encapsulating 0.5 ng/mg of encapsulated IL-2 (f).
To assess the difference in levels of T cell stimulation by natural versus artificial APCs, we compared the ability of both systems to stimulate 1 × 105 OT-I CD8+ T cells under conditions of equivalent antigen presentation. To equalize antigen presentation between NP-loaded DCs and aAPCs, we loaded DCs with 0.1 mg/ml NP/OVA. Because DCs produce one SIINFEKL-presenting MHC for every ∼3000 OVA proteins processed (92), this level of NP/OVA loading produces peptide:MHC on the order of 1 nm. We therefore compared the following T cell stimulation systems: DCs that internalized hetIL-15-coated, antigen-loaded NPs and aAPCs coated with hetIL-15 and 1 nm each of SIINFEKL:MHC and anti-CD28 (Fig. 9b). The same mass of NP and amount of IL-15 were used for each system.
Both systems stimulated CD8+ T cell proliferation and led to IFN-γ secretion. However, aAPCs stimulated greater cell proliferation and lower IFN-γ production compared with NP-loaded DCs (Fig. 9c). These differences may reflect changes in the molecular composition and physical aspects of the natural and artificial APCs.
We then examined the effect of IL-15 presentation on our aAPC system. Equimolar concentrations of SIINFEKL-loaded MHC dimers and anti-CD28 were titrated onto a fixed concentration of nanoparticles that were then used to stimulate OT-I CD8+ T cells in the presence or absence of a fixed concentration of IL-15:IL-15Rα added to the nanoparticle surface. In the absence of IL-15:IL-15Rα, maximal T cell proliferation of 1 × 104 cells occurred at about 10 nm peptide:MHC. In the presence of IL-15:IL-15Rα, a maximal proliferation of 1.5 × 104 cells occurred at 1 nm peptide:MHC, representing a 10-fold increase in T cell sensitivity to antigen and a 50% increase in the magnitude of response (Fig. 9d, left panel). Similarly, the antigen concentration required for maximal IFN-γ secretion was reduced from 30 to 10 nm peptide:MHC with the addition of IL-15:IL-15Rα, although the highest amount of IFN-γ produced remained the same (Fig. 9d, right panel). This demonstrated the ability of trans-presented IL-15:IL-15Rα to improve the sensitivity and magnitude of the antigen-specific CD8+ T cell response.
Besides IL-15, another critical cytokine provided by APCs to T cells is IL-2. IL-2 and IL-15 share two of three receptor chains and identical signaling pathways but effect different responses in immunity through differential receptor distribution and modes of action (79, 93–95). IL-15 is trans-presented on the surface of activated monocytes and DCs, whereas IL-2 is secreted in a paracrine fashion from DCs or helper T cells. We hypothesized that the physiological presentation of both IL-15 and IL-2, through the trans-presentation of IL-15 and paracrine delivery of IL-2, can combine the effects of both cytokines to improve the T cell response in our aAPC system. We tested this by adding two different components to our system: exogenous IL-2 at a concentration of 0.5 ng/ml and encapsulating IL-2 within our PLGA nanoparticles at a concentration of 5 ng/mg PLGA. Without IL-15, exogenous IL-2 improves the CD8 response to approximately the same level as when IL-15:IL-15Rα was added, with a maximum proliferation of 1.5 × 104 cells at 1 nm peptide:MHC. Addition of IL-15:IL-15Rα on the surface of the aAPCs further improves this to 2.1 × 104 cells at 0.45 nm (Fig. 9e, left panel). A slight increase in the sensitivity of IFN-γ production (from 3.6 to 3.1 nm peptide:MHC) was also observed (Fig. 9e, right panel). Encapsulated IL-2 is a powerful stimulant for T cell proliferation (16, 96). Without IL-15:IL-15Rα, responding T cells peaked at 0.7 nm peptide:MHC for 2.6 × 104 cells, whereas the addition of IL-15:IL-15Rα increased the response still further to 2.9 × 104 cells at 0.35 nm peptide:MHC, effectively doubling the sensitivity of T cells to antigen compared with encapsulated IL-2 alone (Fig. 9f, left panel). The addition of encapsulated IL-2 appeared to saturate the IFN-γ response, resulting in greatly increased maximal cytokine production but no change in antigen sensitivity of IFN-γ production (Fig. 9f, right panel). Our results demonstrate that IL-15:IL-15Rα is able to synergize with paracrine delivered IL-2 to enhance T cell activation.
IL-15:IL-15Rα and Encapsulated Antigen Synergize to Delay Tumor Kinetics in an in Vivo Melanoma Model
We evaluated the in vivo effects of varying IL-15 trans-presentation during nanoparticle-mediated antigen delivery by testing various NP configurations in the treatment of an aggressive murine melanoma model. We first tested whether IL-15:IL-15Rα synergizes with antigen delivery to stimulate anti-tumor responses in vivo. C57Bl/6 mice were challenged intravenously with 1 × 105 B16 melanoma cells expressing ovalbumin (B16-OVA) and then treated systemically with three different IL-15:IL-15Rα configurations: free IL-15:IL-15Rα, IL-15:IL-15Rα/NP, and IL-15:IL-15Rα/NP/OVA. Only the third configuration co-delivered IL-15 together with antigen. Control groups were given either PBS or blank nanoparticles alone. Although survival was not improved significantly between free and NP-bound IL-15:IL-15Rα, the addition of encapsulated OVA led to a significant increase in survival (Fig. 10a). IL-15:IL-15Rα can therefore synergize with antigen delivery in the anti-tumor immune response.
FIGURE 10.
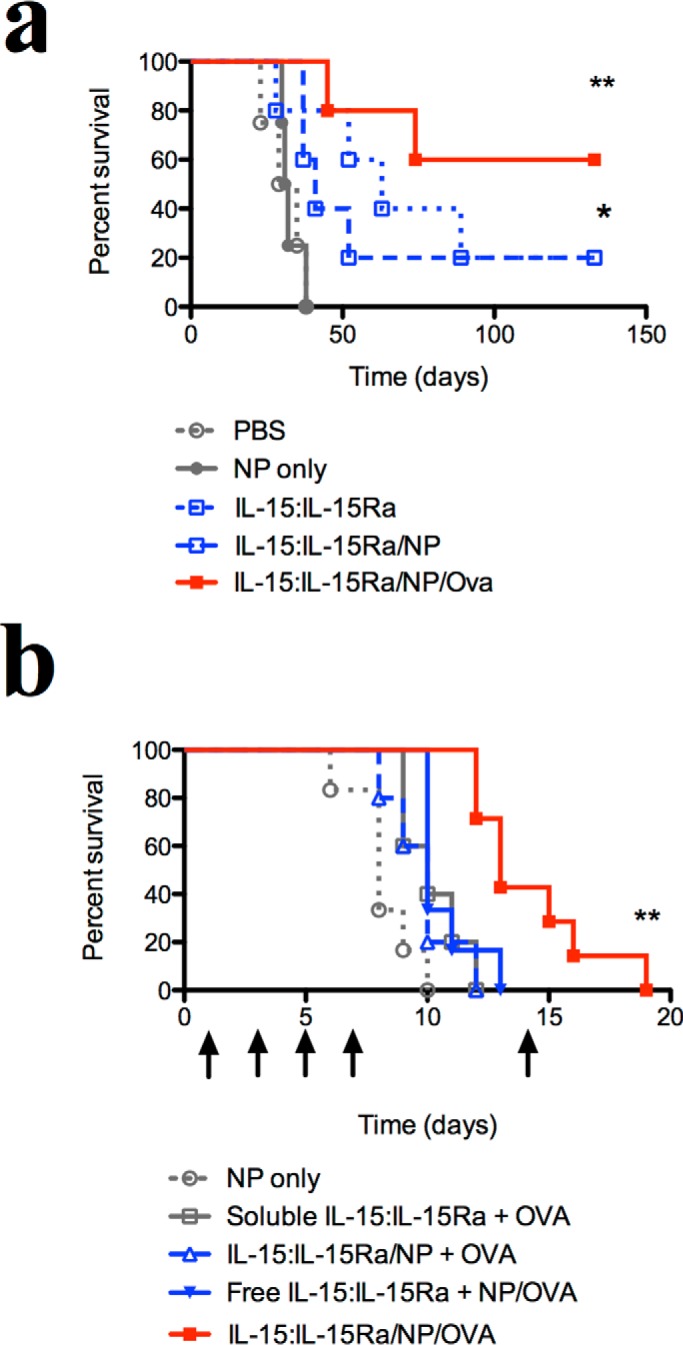
In vivo effect of trans-presented IL-15 and encapsulated antigen in a melanoma model. To test the adjuvant effects of surface-presented IL-15:IL-15Rα in vivo, we used a B16 melanoma model. a, C57Bl/6 mice were injected intravenously with 1 × 105 OVA-expressing B16 melanoma cells (B16-OVA) to establish pulmonary tumor nodules (n = 5 mice/group). 1 day, 2 days, and 10 days after B16-OVA inoculation, mice were injected intraperitoneally with different IL-15:IL-15Rα constructs. Mice were sacrificed upon signs of pulmonary distress. Survival curves were compared against the negative control (PBS) using the Mantel-Cox test. ** indicates a significance level of p ≤ 0.01, and * indicates p ≤ 0.05. b, to test the effect of IL-15 configuration, mice were inoculated subcutaneously with 0.5 × 106 OVA-expressing B16 melanoma cells. Doses of nanoparticle or IL-15 constructs were injected intraperitoneally on days 1, 3, 5, 7, and 14 after tumors reached a minimum diameter of 4 mm. Arrows indicate treatment times (n = 6 mice/group). Tumors were measured every other day, and mice were sacrificed when tumors exceeded 15 mm in greatest diameter. Survival curves were compared by Mantel-Cox and Gehan-Breslow-Wilcoxon analysis. Survival curves were compared against the negative control (NP only) using the Mantel-Cox test. ** indicates a significance level of p ≤ 0.01 for the IL-15:IL-15Ra/NP/OVA group.
We next tested the specific configuration of IL-15 and OVA necessary for anti-tumor responses. C57Bl/6 mice were inoculated subcutaneously with 0.5 × 106 B16-OVA cells and then treated systemically with IL-15:IL-15Rα and ovalbumin antigen in four different configurations: soluble IL-15:IL-15Rα and OVA protein only; trans-presented IL-15:IL-15Rα and soluble OVA (IL-15:IL-15Rα/NP + OVA); soluble IL-15:IL-15Rα and encapsulated OVA (IL-15:IL-15Rα + NP/OVA); and trans-presented IL-15:IL-15Rα on NPs encapsulating OVA (IL-15:IL-15Rα/NP/OVA). A control group was treated with blank nanoparticles only. The IL-15:IL-15Rα/NP/OVA particles significantly extended the survival of treated mice compared with other configurations of IL-15:IL-15Rα and ovalbumin (Fig. 10b). Notably, configurations where either IL-15:IL-15Rα or OVA was detached from the NPs did not enhance animal survival beyond the control group. Our findings align with recent literature showing that OVA vaccination requires an adjuvant to potentiate antigen-specific protective immunity (97–100) and further highlight the importance of adjuvant configuration in the case of IL-15. Overall, we show that nanoparticles trans-presenting IL-15:IL-15Rα and encapsulating antigen demonstrate in vivo efficacy in the treatment of an aggressive model of murine melanoma.
Discussion
We highlight in this work the effect of the mode of cytokine delivery on the immune response specifically with respect to IL-15 signaling. IL-15:IL-15Rα on nanoparticle scaffolds enhances the sensitivity and magnitude of the antigen-specific stimulation of CD8+ T cells upon stimulation by nanoparticle-treated DCs. Our experiments highlight, we believe for the first time, the configuration-dependent delivery of IL-15:IL-15Rα as a key factor for its biological action: that its presentation on the surface of a nanoparticle or cell leads to enhancement of its adjuvant effect. Several groups have previously compared the effects of soluble and trans-presented IL-15, but most compared the action of IL-15 in the presence or absence of cells or scaffolds presenting IL-15Rα (17, 48, 101). However, because IL-15Rα enhances activity when bound to IL-15 (11, 44, 55, 56), these studies could not differentiate between the topological effects of IL-15 from the agonist behavior of IL-15Rα. Mortier et al. (47) have compared the activation of natural killer cells by DC supernatants containing IL-15:IL-15Rα and DCs trans-presenting the same, although the membrane-bound concentration of IL-15:IL-15Rα was unknown. Giron-Michel et al. (102) have demonstrated differential signaling and functions between soluble and membrane-bound IL-15:IL-15Rα in human hematopoietic progenitor cells, although IL-15 was not controlled for concentration and the membrane-bound form of IL-15 investigated was a novel form complexed with the βγ receptors. By showing that the same quantity of IL-15:IL-15Rα delivered to DCs can differentially affect responding T cells, we show that the action of IL-15:IL-15Rα can be regulated by its configuration during delivery or presentation to cells. Because IL-15:IL-15Rα is physiologically present in both membrane-bound and soluble forms, our work suggests the increased efficacy of surface-presented IL-15:IL-15Rα as an explanation for why the major effects of IL-15 are dependent on its trans-presentation on cell membranes.
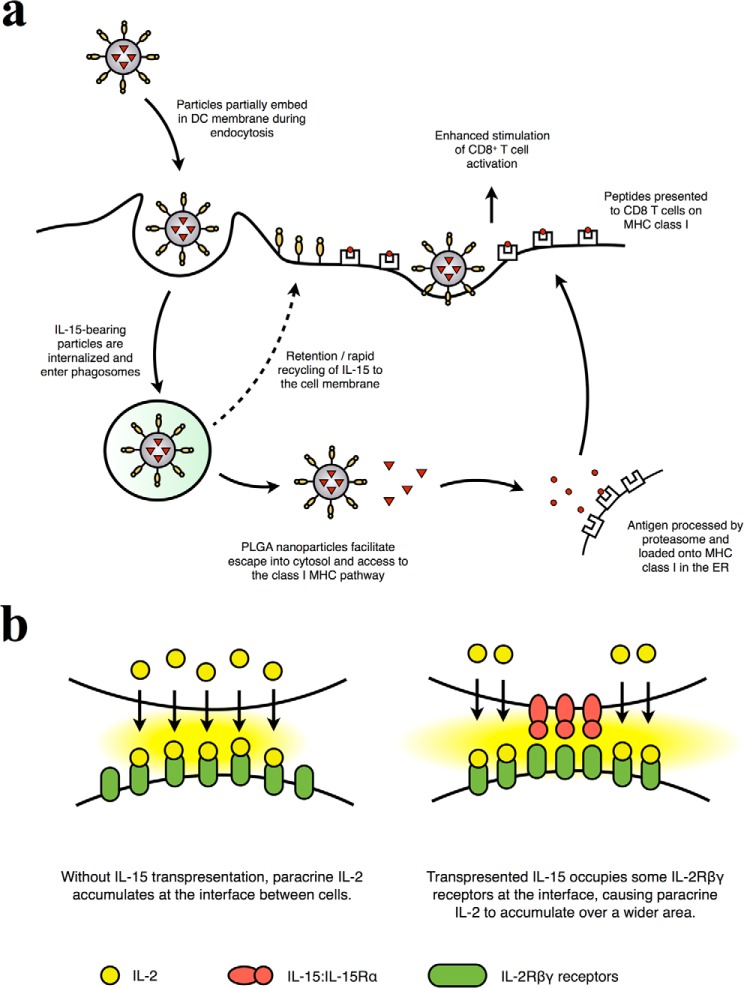
We observed that the retention of functional IL-15:IL-15Rα on the surface of DCs is a mechanism for the nano-adjuvant behavior seen with our IL-15:IL-15Rα constructs. The nanoparticle-bound IL-15:IL-15Rα is transferred to the DC cell surface, where it stimulates CD8+ T cells alongside peptide:MHC engagement (Fig. 11a). IL-15Rα and IL-2Rα have been observed previously to co-localize with class I and class II MHC in supramolecular receptor clusters, supporting our observation that the transfer of IL-15:IL-15Rα to the DC membrane could enhance the sensitivity and magnitude of T cell stimulation (103). Three mechanisms could account for this IL-15-mediated adjuvant behavior: the scaffolding of IL-15:IL-15Rα to the DC membrane by nanoparticles, the recycling of IL-15:IL-15Rα to the DC membrane after nanoparticle internalization, or export of IL-15 to T cells via DC-derived exosomes. The mechanism of NP-mediated scaffolding is supported by the observations that NPs accumulate at the cell membrane (104, 105). Indeed, avidin binding sites could be detected on the DC surface shortly after addition of untreated NPs (Fig. 6d), and the nonspecific interaction of NPs with the DC membrane is supported by our observation that fixed DCs can present bioactive IL-15 (Fig. 6, e and f). Alternatively, studies by Waldmann and co-workers (13, 48) have suggested that IL-15 and IL-15Rα are recycled and stored intracellularly after internalization, indicating that IL-15 may be transported to the cell membrane after NP uptake, which may explain the longevity of surface-retained IL-15 on DCs in our experiments. We showed that intraendosomally delivered IL-15 could be detected after internalization, where it may survive to be recycled to the cell membrane. Finally, previous work has shown that IL-15Rα associated with DC-derived exosomes can stimulate natural killer cells, leading to proliferation and IFN-γ production (88). However, in our system, we did not observe proliferation or IFN-γ being produced from CD8+ transwell cultures, nor did we observe exosome-associated IL-15 (Fig. 7).
FIGURE 11.
Proposed mechanism of adjuvant IL-15:IL-15Rα action. a, the IL-15:IL-15Rα on nanoparticles synergizes with encapsulated antigen to stimulate antigen-specific CD8+ T cells. As nanoparticles are internalized, they embed in the cell membrane, presenting IL-15:IL-15Rα on the cell surface. Alternatively, IL-15:IL-15Rα could also be recycled to the DC membrane after it enters the nedosome. The PLGA nanoparticles aid in endosomal escape and cross-presentation of antigen on MHC class I. At the DC surface, IL-15:IL-15Rα directly stimulates CD8+ T cells and enhances their sensitivity to DC-presented antigen. b, IL-15:IL-15Rα trans-presentation at the cell-NP interface may enhance interfacial IL-2 accumulation. The close apposition of an interacting cell and IL-2-releasing nanoparticle normally leads to accumulation of IL-2 at the cell-NP interface. However, the addition of trans-presented IL-15:IL-15Rα engages a portion of the IL-2Rβγ receptors at the interface, reducing the number of receptors available for IL-2 binding and forcing IL-2 to accumulate over a wider area. This effectively increases the total number of activated IL-2Rβγ receptors in the cell-NP interaction because the signaling induced by IL-15:IL-15Rα trans-presentation and IL-2 binding is identical.
In our use of the artificial APC system to elucidate the mechanism of action of our NP-loaded DCs, we noted that the two systems generated differential responses. Although we observed increased proliferation with aAPCs compared with NP-loaded DCs, that trend is reversed when we examined IFN-γ levels. Antigen-specific proliferation and IFN-γ release can be dissociated from each other in clonotypic activation of T cells in murine lines and in immune responses from splenic T cells (106, 107). In addition, the presence of low levels of IL-2 can enhance IFN-γ secretion independent of proliferation (107). We detected the presence of IL-2 produced in the case of NP-loaded DCs but not from aAPCs (data not shown), which may contribute to the higher levels of IFN-γ seen with NP-loaded DCs. aAPCs provide a very strong stimulus for T cell proliferation (16, 96, 108) and, indeed, outperform NP-loaded DCs in expanding T cells. We note that artificial APCs are by no means equivalent to natural DCs in terms of the breadth and intensity of immune responses triggered. However, by providing a basic approximation of antigen recognition, co-stimulation, and cytokine signaling, the utility of aAPCs for elucidating immune mechanisms is demonstrated in this work.
Our work affirms that the physiological presentation of IL-15 and IL-2 is important for their action on T cells. Use of the IL-15:IL-15Rα complex and paracrine delivery of IL-2 were shown to better activate T cells than if either cytokine were delivered alone and could stimulate even stronger responses if delivered in combination in our aAPC system. We have previously described this phenomenon for IL-2 (16, 96) and modeled the interaction of paracrine IL-2 with a receiving cell under a variety of conditions (71, 109–110), which provides a basis for understanding how these two cytokines would interact with one another. When an aAPC releases a cytokine such as IL-2, mathematical modeling indicates that IL-2 accumulates significantly at the particle:cell interface. Locally concentrated IL-2 would bind readily to available IL-2/15βγ receptors, thereby enhancing T cell activation. If trans-presented IL-15:IL-15Rα were present at the particle:cell interface, it would occupy a portion of the IL-2/15βγ receptors, allowing the local concentration of IL-2 to increase further. Such receptor occupancy would not hinder cytokine-induced T cell activation because the signaling ends of IL-2/15βγ are the same whether bound to IL-2 or IL-15:IL-15Rα (93); nor would the number of IL-2/15βγ receptors decrease because engagement by trans-presented IL-15:IL-15Rα does not trigger significant down-regulation of IL-2/15βγ (42). The combined action of physiologically presented IL-2 and IL-15 would therefore continue to increase T cell activation until available IL-2/15βγ receptors are saturated (Fig. 11b). These results shed more light on the interaction between IL-2 and IL-15 and show that the cytokines can be combined to stimulate robust T cell responses at levels below receptor saturation. These findings could have implications for the design of IL-2- and IL-15-based immunotherapies.
Multiple clinical trials for single-chain IL-15 and heterodimeric IL-15 are currently recruiting or underway. Initial results from the first-in-human phase I trial of single-chain IL-15 indicate a modest form of capillary leak that was less severe than that induced by IL-2 but also moderate toxicities, including hypotension and thrombocytopenia (52). In light of the significant clinical interest behind IL-15, our findings provide new strategies for optimizing its therapeutic delivery, aimed at maximizing its effectiveness while limiting its associated toxicities. Our results have particular significance in the application of IL-15 to therapeutic cancer vaccines where the nanoparticle-mediated combination delivery of IL-15 and antigen to target DCs may prove to be a useful strategy.
Author Contributions
E. H., T. M. F., and R. A. F. conceived the study. E. H. and T. M. F. designed the experiments, coordinated the study, and wrote the paper. E. H. performed the experiments and analyzed the data for Figs. 1 and 6–9. E. H. and I. M. U. performed the experiments and analyzed the data for Figs. 2–4 and 10. D. J. H. performed the experiment shown in Fig. 5. C. B. and G. N. P. provided the heterodimeric IL-15 used in all experiments. S. J. provided the SIINFEKL:MHC used in the artificial APC experiments. All authors critically reviewed and approved the final version of the manuscript.
Acknowledgments
We thank Drs. Lieping Chen and Frederick Sigworth for critical reading of the manuscript and many helpful discussions. We also thank Drs. Donoven Kim and Michael McHugh for assistance with the animal experiments, Ewa Menet and Geoffrey Lyon (Yale Immunobiology Flow Cytometry Core), and Dr. Xinran Liu (Yale School of Medicine Electron Microscopy Core) for technical assistance.
C. B. and G. N. P. are inventors on United States Government-owned patents and patent applications related to heterodimeric IL-15 and gene expression optimization. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- DC
- dendritic cell
- PLGA
- polymeric poly(lactide-co-glycolide)
- hetIL-15
- heteromeric IL-15
- APC
- antigen-presenting cell
- NP
- nanoparticle
- OVA
- ovalbumin
- TEM
- transmission electron microscopy
- aAPC
- artificial antigen-presenting cell
- CFSE
- carboxyfluorescein succinimidyl ester.
References
- 1. Banchereau J., and Steinman R. M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 2. Steinman R. M., and Banchereau J. (2007) Taking dendritic cells into medicine. Nature 449, 419–426 [DOI] [PubMed] [Google Scholar]
- 3. Théry C., and Amigorena S. (2001) The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13, 45–51 [DOI] [PubMed] [Google Scholar]
- 4. Mahnke K., Guo M., Lee S., Sepulveda H., Swain S. L., Nussenzweig M., and Steinman R. M. (2000) The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 151, 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sallusto F., Cella M., Danieli C., and Lanzavecchia A. (1995) Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savina A., and Amigorena S. (2007) Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 219, 143–156 [DOI] [PubMed] [Google Scholar]
- 7. Engering A. J., Cella M., Fluitsma D., Brockhaus M., Hoefsmit E. C., Lanzavecchia A., and Pieters J. (1997) The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur. J. Immunol. 27, 2417–2425 [DOI] [PubMed] [Google Scholar]
- 8. Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.-J., Pulendran B., and Palucka K. (2000) Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 [DOI] [PubMed] [Google Scholar]
- 9. Malek T. R. (2008) The Biology of Interleukin-2. Annu. Rev. Immunol. 26, 453–479 [DOI] [PubMed] [Google Scholar]
- 10. Huse M., Lillemeier B. F., Kuhns M. S., Chen D. S., and Davis M. M. (2006) T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol. 7, 247–255 [DOI] [PubMed] [Google Scholar]
- 11. Bergamaschi C., Rosati M., Jalah R., Valentin A., Kulkarni V., Alicea C., Zhang G. M., Patel V., Felber B. K., and Pavlakis G. N. (2008) Intracellular interaction of interleukin-15 with its receptor α during production leads to mutual stabilization and increased bioactivity. J. Biol. Chem. 283, 4189–4199 [DOI] [PubMed] [Google Scholar]
- 12. Stonier S. W., and Schluns K. S. (2010) Trans-presentation: A novel mechanism regulating IL-15 delivery and responses. Immunol. Lett. 127, 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubois S., Mariner J., Waldmann T. A., and Tagaya Y. (2002) IL-15Ra recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 [DOI] [PubMed] [Google Scholar]
- 14. Bergamaschi C., Bear J., Rosati M., Beach R. K., Alicea C., Sowder R., Chertova E., Rosenberg S. A., Felber B. K., and Pavlakis G. N. (2012) Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood 120, e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chertova E., Bergamaschi C., Chertov O., Sowder R., Bear J., Roser J. D., Beach R. K., Lifson J. D., Felber B. K., and Pavlakis G. N. (2013) Characterization and favorable in vivo properties of heterodimeric soluble IL-15: IL-15Rα cytokine compared with IL-15 monomer. J. Biol. Chem. 288, 18093–18103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steenblock E. R., Fadel T., Labowsky M., Pober J. S., and Fahmy T. M. (2011) An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. J. Biol. Chem. 286, 34883–34892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kokaji A. I., Hockley D. L., and Kane K. P. (2008) IL-15 Transpresentation augments CD8+ T cell activation and is required for optimal recall responses by central memory CD8+ T cells. J. Immunol. 180, 4391–4401 [DOI] [PubMed] [Google Scholar]
- 18. Elamanchili P., Diwan M., Cao M., and Samuel J. (2004) Characterization of poly(d,l-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine 22, 2406–2412 [DOI] [PubMed] [Google Scholar]
- 19. Cho N.-H., Cheong T.-C., Min J. H., Wu J. H., Lee S. J., Kim D., Yang J.-S., Kim S., Kim Y. K., and Seong S.-Y. (2011) A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nature Nantechnol. 6, 675–682 [DOI] [PubMed] [Google Scholar]
- 20. Villa C. H., Dao T., Ahearn I., Fehrenbacher N., Casey E., Rey D. A., Korontsvit T., Zakhaleva V., Batt C. A., Philips M. R., and Scheinberg D. A. (2011) Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano 5, 5300–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Koker S., De Geest B. G., Singh S. K., De Rycke R., Naessens T., van Kooyk Y., Demeester J., De Smedt S. C., and Grooten J. (2009) Polyelectrolyte microcapsules as antigen delivery vehicles to dendritic cells: uptake, processing, and cross-presentation of encapsulated antigens. Angew. Chem. Int. Ed. Engl. 48, 8485–8489 [DOI] [PubMed] [Google Scholar]
- 22. Hamdy S., Haddadi A., Hung R. W., and Lavasanifar A. (2011) Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Delivery Rev. 63, 943–955 [DOI] [PubMed] [Google Scholar]
- 23. Klippstein R., and Pozo D. (2010) Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. Nanomedicine 6, 523–529 [DOI] [PubMed] [Google Scholar]
- 24. Akagi T., Baba M., and Akashi M. (2012) in Polymers in Nanomedicine (Kunugi S., and Yamaoka T. eds.) pp 31–64, Springer, Berlin [Google Scholar]
- 25. Hrkach J., Von Hoff D., Mukkaram Ali M., Andrianova E., Auer J., Campbell T., De Witt D., Figa M., Figueiredo M., Horhota A., Low S., McDonnell K., Peeke E., Retnarajan B., Sabnis A., Schnipper E., Song J. J., Song Y. H., Summa J., Tompsett D., Troiano G., Van Geen Hoven T., Wright J., LoRusso P., Kantoff P. W., Bander N. H., Sweeney C., Farokhzad O. C., Langer R., and Zale S. (2012) Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 4, 128ra39. [DOI] [PubMed] [Google Scholar]
- 26. Park J., Fong P. M., Lu J., Russell K. S., Booth C. J., Saltzman W. M., and Fahmy T. M. (2009) PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine 5, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng J., Teply B. A., Sherifi I., Sung J., Luther G., Gu F. X., Levy-Nissenbaum E., Radovic-Moreno A. F., Langer R., and Farokhzad O. C. (2007) Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 28, 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sadat Tabatabaei Mirakabad F., Nejati-Koshki K., Akbarzadeh A., Yamchi M. R., Milani M., Zarghami N., Zeighamian V., Rahimzadeh A., Alimohammadi S., Hanifehpour Y., and Joo S. W. (2014) PLGA-based nanoparticles as cancer drug delivery systems. Asian Pacific Journal of Cancer Prevention 15, 517–535 [DOI] [PubMed] [Google Scholar]
- 29. Danhier F., Ansorena E., Silva J. M., Coco R., Le Breton A., and Préat V. (2012) PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release 161, 505–522 [DOI] [PubMed] [Google Scholar]
- 30. Lü J.-M., Wang X., Marin-Muller C., Wang H., Lin P. H., Yao Q., and Chen C. (2009) Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 9, 325–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walter E., Dreher D., Kok M., Thiele L., Kiama S. G., Gehr P., and Merkle H. P. (2001) Hydrophilic poly(DL-lactide-co-glycolide) microspheres for the delivery of DNA to human-derived macrophages and dendritic cells. J. Control. Release 76, 149–168 [DOI] [PubMed] [Google Scholar]
- 32. Bandyopadhyay A., Fine R. L., Demento S., Bockenstedt L. K., and Fahmy T. M. (2011) The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials 32, 3094–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Demento S. L., Bonafé N., Cui W., Kaech S. M., Caplan M. J., Fikrig E., Ledizet M., and Fahmy T. M. (2010) TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J. Immunol. 185, 2989–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Demento S. L., Eisenbarth S. C., Foellmer H. G., Platt C., Caplan M. J., Saltzman W. M., Mellman I., Ledizet M., Fikrig E., Flavell R. A., and Fahmy T. M. (2009) Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine 27, 3013–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Z., Tongchusak S., Mizukami Y., Kang Y. J., Ioji T., Touma M., Reinhold B., Keskin D. B., Reinherz E. L., and Sasada T. (2011) Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials 32, 3666–3678 [DOI] [PubMed] [Google Scholar]
- 36. Hamdy S., Molavi O., Ma Z., Haddadi A., Alshamsan A., Gobti Z., Elhasi S., Samuel J., and Lavasanifar A. (2008) Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 26, 5046–5057 [DOI] [PubMed] [Google Scholar]
- 37. Saluja S. S., Hanlon D. J., Sharp F. A., Hong E., Khalil D., Robinson E., Tigelaar R., Fahmy T. M., and Edelson R. L. (2014) Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen. Int. J. Nanomedicine 9, 5231–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solbrig C. M., Saucier-Sawyer J. K., Cody V., Saltzman W. M., and Hanlon D. J. (2007) Polymer nanoparticles for immunotherapy from encapsulated tumor-associated antigens and whole tumor cells. Mol. Pharm. 4, 47–57 [DOI] [PubMed] [Google Scholar]
- 39. Shen H., Ackerman A. L., Cody V., Giodini A., Hinson E. R., Cresswell P., Edelson R. L., Saltzman W. M., and Hanlon D. J. (2006) Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology 117, 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steel J. C., Waldmann T. A., and Morris J. C. (2012) Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 33, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bergamaschi C., Jalah R., Kulkarni V., Rosati M., Zhang G. M., Alicea C., Zolotukhin A. S., Felber B. K., and Pavlakis G. N. (2009) Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor α in vivo. J. Immunol. 183, 3064–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perdreau H., Mortier E., Bouchaud G., Solé V., Boublik Y., Plet A., and Jacques Y. (2010) Different dynamics of IL-15R activation following IL-15 cis- or trans-presentation. Eur. Cytokine Netw. 21, 297–307 [DOI] [PubMed] [Google Scholar]
- 43. Sandau M. M., Schluns K. S., Lefrancois L., and Jameson S. C. (2004) Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15Ra by the same cells. J. Immunol. 173, 6537–6541 [DOI] [PubMed] [Google Scholar]
- 44. Rubinstein M. P., Kovar M., Purton J. F., Cho J.-H., Boyman O., Surh C. D., and Sprent J. (2006) Converting IL-15 to a superagonist by binding to soluble IL-15Ra. Proc. Nat. Acad. Sci. U.S.A. 103, 9166–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bergamaschi C., Bear J., Rosati M., Beach R. K., Alicea C., Sowder R., Chertova E., Rosenberg S. A., Felber B. K., and Pavlakis G. N. (2012) Circulating interleukin-15 (IL-15) exists as heterodimeric complex with soluble IL-15 receptor α (IL-15R) in human serum. Blood 120, e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huntington N. D., Legrand N., Alves N. L., Jaron B., Weijer K., Plet A., Corcuff E., Mortier E., Jacques Y., Spits H., and Di Santo J. P. (2009) IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 206, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mortier E., Woo T., Advincula R., Gozalo S., and Ma A. (2008) IL-15R chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205, 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato N., Patel H. J., Waldmann T. A., and Tagaya Y. (2007) The IL-15/IL-15Ra on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc. Natl. Acad. Sci. U.S.A. 104, 588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rückert R., Brandt K., Bulanova E., Mirghomizadeh F., Paus R., and Bulfone-Paus S. (2003) Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur. J. Immunol. 33, 3493–3503 [DOI] [PubMed] [Google Scholar]
- 50. Jakobisiak M., Golab J., and Lasek W. (2011) Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev. 22, 99–108 [DOI] [PubMed] [Google Scholar]
- 51. Cheever M. A. (2008) Twelve immunotherapy drugs that could cure cancers. Immunol. Rev. 222, 357–368 [DOI] [PubMed] [Google Scholar]
- 52. Conlon K. C., Lugli E., Welles H. C., Rosenberg S. A., Fojo A. T., Morris J. C., Fleisher T. A., Dubois S. P., Perera L. P., Stewart D. M., Goldman C. K., Bryant B. R., Decker J. M., Chen J., Worthy T. A., Figg W. D. Sr., Peer C. J., Sneller M. C., Lane H. C., Yovandich J. L., Creekmore S. P., Roederer M., and Waldmann T. A. (2015) Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 33, 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bessard A., Solé V., Bouchaud G., Quéméner A., and Jacques Y. (2009) High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor α fusion protein, in metastatic melanoma and colorectal cancer. Mol. Cancer Ther. 8, 2736–2745 [DOI] [PubMed] [Google Scholar]
- 54. Epardaud M., Elpek K. G., Rubinstein M. P., Yonekura A. R., Bellemare-Pelletier A., Bronson R., Hamerman J. A., Goldrath A. W., and Turley S. J. (2008) Interleukin-15/interleukin-15R complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 68, 2972–2983 [DOI] [PubMed] [Google Scholar]
- 55. Dubois S., Patel H. J., Zhang M., Waldmann T. A., and Müller J. R. (2008) Preassociation of IL-15 with IL-15Ra-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44 high T cells and its antitumor action. J. Immunol. 180, 2099–2106 [DOI] [PubMed] [Google Scholar]
- 56. Stoklasek T. A., Schluns K. S., and Lefrançois L. (2006) Combined IL-15/IL-15Ra immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177, 6072–6080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boyer J. D., Robinson T. M., Kutzler M. A., Vansant G., Hokey D. A., Kumar S., Parkinson R., Wu L., Sidhu M. K., Pavlakis G. N., Felber B. K., Brown C., Silvera P., Lewis M. G., Monforte J., Waldmann T. A., Eldridge J., and Weiner D. B. (2007) Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc. Natl. Acad. Sci. U.S.A. 104, 18648–18653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kutzler M. A., Robinson T. M., Chattergoon M. A., Choo D. K., Choo A. Y., Choe P. Y., Ramanathan M. P., Parkinson R., Kudchodkar S., Tamura Y., Sidhu M., Roopchand V., Kim J. J., Pavlakis G. N., Felber B. K., Waldmann T. A., Boyer J. D., and Weiner D. B. (2005) Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol. 175, 112–123 [DOI] [PubMed] [Google Scholar]
- 59. Xin K.-Q., Hamajima K., Sasaki S., Tsuji T., Watabe S., Okada E., and Okuda K. (1999) IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine 17, 858–866 [DOI] [PubMed] [Google Scholar]
- 60. Rubinstein M. P., Kadima A. N., Salem M. L., Nguyen C. L., Gillanders W. E., and Cole D. J. (2002) Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J. Immunol. 169, 4928–4935 [DOI] [PubMed] [Google Scholar]
- 61. Saikh K. U., Kissner T. L., Nystrom S., Ruthel G., and Ulrich R. G. (2008) Interleukin-15 increases vaccine efficacy through a mechanism linked to dendritic cell maturation and enhanced antibody titers. Clin. Vaccine Immunol. 15, 131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tourkova I. L., Yurkovetsky Z. R., Gambotto A., Makarenkova V. P., Perez L., Balkir L., Robbins P. D., Shurin M. R., and Shurin G. V. (2002) Increased function and survival of IL-15-transduced human dendritic cells are mediated by up-regulation of IL-15Ra and Bcl-2. J. Leukocyte Biol. 72, 1037–1045 [PubMed] [Google Scholar]
- 63. Pulendran B., Dillon S., Joseph C., Curiel T., Banchereau J., and Mohamadzadeh M. (2004) Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur. J. Immunol. 34, 66–73 [DOI] [PubMed] [Google Scholar]
- 64. Saikh K. U., Khan A. S., Kissner T., and Ulrich R. G. (2001) IL-15-induced conversion of monocytes to mature dendritic cells. Clin. Exp. Immunol. 126, 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dubsky P., Saito H., Leogier M., Dantin C., Connolly J. E., Banchereau J., and Palucka A. K. (2007) IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur. J. Immunol. 37, 1678–1690 [DOI] [PubMed] [Google Scholar]
- 66. Tourkova I. L., Shurin G. V., Chatta G. S., Perez L., Finke J., Whiteside T. L., Ferrone S., and Shurin M. R. (2005) Restoration by IL-15 of MHC class I Antigen-processing machinery in human dendritic cells inhibited by tumor-derived gangliosides. J. Immunol. 175, 3045–3052 [DOI] [PubMed] [Google Scholar]
- 67. Kasturi S. P., Skountzou I., Albrecht R. A., Koutsonanos D., Hua T., Nakaya H. I., Ravindran R., Stewart S., Alam M., Kwissa M., Villinger F., Murthy N., Steel J., Jacob J., Hogan R. J., García-Sastre A., Compans R., and Pulendran B. (2011) Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Demento S. L., Cui W., Criscione J. M., Stern E., Tulipan J., Kaech S. M., and Fahmy T. M. (2012) Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 33, 4957–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fahmy T. M., Samstein R. M., Harness C. C., and Mark Saltzman W. (2005) Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials 26, 5727–5736 [DOI] [PubMed] [Google Scholar]
- 70. Bergamaschi C., Kulkarni V., Rosati M., Alicea C., Jalah R., Chen S., Bear J., Sardesai N. Y., Valentin A., Felber B. K., and Pavlakis G. N. (2015) Intramuscular delivery of heterodimeric IL-15 DNA in macaques produces systemic levels of bioactive cytokine inducing proliferation of NK and T cells. Gene Ther. 22, 76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Labowsky M., and Fahmy T. M. (2012) Diffusive transfer between two intensely interacting cells with limited surface kinetics. Chem. Eng. Sci. 74, 114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Inaba K., Swiggard W. J., Steinman R. M., Romani N., and Schuler G. (2001) Isolation of dendritic cells. Curr. Protoc. Immunol. 10.1002/0471142735.im0307s25 [DOI] [PubMed] [Google Scholar]
- 73. Ho W. Y., Nguyen H. N., Wolfl M., Kuball J., and Greenberg P. D. (2006) In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naïve repertoire. J. Immunol. Methods 310, 40–52 [DOI] [PubMed] [Google Scholar]
- 74. Obermaier B., Dauer M., Herten J., Schad K., Endres S., and Eigler A. (2003) Development of a new protocol for 2-day generation of mature dendritic cells from human monocytes. Biol. Proced. Online 5, 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dauer M., Obermaier B., Herten J., Haerle C., Pohl K., Rothenfusser S., Schnurr M., Endres S., and Eigler A. (2003) Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J. Immunol. 170, 4069–4076 [DOI] [PubMed] [Google Scholar]
- 76. Kelly J. M., Sterry S. J., Cose S., Turner S. J., Fecondo J., Rodda S., Fink P. J., and Carbone F. R. (1993) Identification of conserved T cell receptor CDR3 residues contacting known exposed peptide side chains from a major histocompatibility complex class I-bound determinant. Eur. J. Immunol. 23, 3318–3326 [DOI] [PubMed] [Google Scholar]
- 77. Hirosue S., Kourtis I. C., van der Vlies A. J., Hubbell J. A., and Swartz M. A. (2010) Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: cross-presentation and T cell activation. Vaccine 28, 7897–7906 [DOI] [PubMed] [Google Scholar]
- 78. Fifis T., Gamvrellis A., Crimeen-Irwin B., Pietersz G. A., Li J., Mottram P. L., McKenzie I. F., and Plebanski M. (2004) Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 173, 3148–3154 [DOI] [PubMed] [Google Scholar]
- 79. Waldmann T. A. (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 [DOI] [PubMed] [Google Scholar]
- 80. Teague R. M., Sather B. D., Sacks J. A., Huang M. Z., Dossett M. L., Morimoto J., Tan X., Sutton S. E., Cooke M. P., Ohlén C., and Greenberg P. D. (2006) Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat. Med. 12, 335–341 [DOI] [PubMed] [Google Scholar]
- 81. Mueller K., Schweier O., and Pircher H. (2008) Efficacy of IL-2- versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur. J. Immunol. 38, 2874–2885 [DOI] [PubMed] [Google Scholar]
- 82. Kawakami Y., Eliyahu S., Sakaguchi K., Robbins P. F., Rivoltini L., Yannelli J. R., Appella E., and Rosenberg S. A. (1994) Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 180, 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Johnson L. A., Heemskerk B., Powell D. J. Jr., Cohen C. J., Morgan R. A., Dudley M. E., Robbins P. F., and Rosenberg S. A. (2006) Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 177, 6548–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhu X., Marcus W. D., Xu W., Lee H. I., Han K., Egan J. O., Yovandich J. L., Rhode P. R., and Wong H. C. (2009) Novel human interleukin-15 agonists. J. Immunol. 183, 3598–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim Y. S., Maslinski W., Zheng X. X., Stevens A. C., Li X. C., Tesch G. H., Kelley V. R., and Strom T. B. (1998) Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fcγ2a protein blocks delayed-type hypersensitivity. J. Immunol. 160, 5742–5748 [PMC free article] [PubMed] [Google Scholar]
- 86. Ding Z., Fong R. B., Long C. J., Stayton P. S., and Hoffman A. S. (2001) Size-dependent control of the binding of biotinylated proteins to streptavidin using a polymer shield. Nature 411, 59–62 [DOI] [PubMed] [Google Scholar]
- 87. Noppl-Simson D. A., and Needham D. (1996) Avidin-biotin interactions at vesicle surfaces: adsorption and binding, cross-bridge formation, and lateral interactions. Biophys. J. 70, 1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Viaud S., Terme M., Flament C., Taieb J., André F., Novault S., Escudier B., Robert C., Caillat-Zucman S., Tursz T., Zitvogel L., and Chaput N. (2009) Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Rα. PLoS ONE 4, e4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Théry C., Amigorena S., Raposo G., and Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 10.1002/0471143030.cb0322s30 [DOI] [PubMed] [Google Scholar]
- 90. Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., and Amigorena S. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat. Med. 4, 594–600 [DOI] [PubMed] [Google Scholar]
- 91. Steenblock E. R., Wrzesinski S. H., Flavell R. A., and Fahmy T. M. (2009) Antigen presentation on artificial acellular substrates: modular systems for flexible, adaptable immunotherapy. Expert Opin. Biol. Ther 9, 451–464 [DOI] [PubMed] [Google Scholar]
- 92. Princiotta M. F., Finzi D., Qian S.-B., Gibbs J., Schuchmann S., Buttgereit F., Bennink J. R., and Yewdell J. W. (2003) Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity 18, 343–354 [DOI] [PubMed] [Google Scholar]
- 93. Ring A. M., Lin J.-X., Feng D., Mitra S., Rickert M., Bowman G. R., Pande V. S., Li P., Moraga I., Spolski R., Ozkan E., Leonard W. J., and Garcia K. C. (2012) Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat. Immunol. 13, 1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Arneja A., Johnson H., Gabrovsek L., Lauffenburger D. A., and White F. M. (2014) Qualitatively different T cell phenotypic responses to IL-2 versus IL-15 are unified by identical dependences on receptor signal strength and duration. J. Immunol. 192, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fehniger T. A., Cooper M. A., and Caligiuri M. A. (2002) Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 13, 169–183 [DOI] [PubMed] [Google Scholar]
- 96. Steenblock E. R., and Fahmy T. M. (2008) A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol. Ther. 16, 765–772 [DOI] [PubMed] [Google Scholar]
- 97. Almeida J. P., Lin A. Y., Figueroa E. R., Foster A. E., and Drezek R. A. (2015) In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small 11, 1453–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Håkerud M., Selbo P. K., Waeckerle-Men Y., Contassot E., Dziunycz P., Kündig T. M., Høgset A., and Johansen P. (2015) Photosensitisation facilitates cross-priming of adjuvant-free protein vaccines and stimulation of tumour-suppressing CD8 T cells. J. Control. Release 198, 10–17 [DOI] [PubMed] [Google Scholar]
- 99. Hansen J., Lindenstrøm T., Lindberg-Levin J., Aagaard C., Andersen P., and Agger E. M. (2012) CAF05: cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunol. Immunother. 61, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jin J.-O., Zhang W., Du J.-Y., Wong K.-W., Oda T., and Yu Q. (2014) Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 9, e99396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kobayashi H., Dubois S., Sato N., Sabzevari H., Sakai Y., Waldmann T. A., and Tagaya Y. (2005) Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 105, 721–727 [DOI] [PubMed] [Google Scholar]
- 102. Giron-Michel J., Giuliani M., Fogli M., Brouty-Boyé D., Ferrini S., Baychelier F., Eid P., Lebousse-Kerdilès C., Durali D., Biassoni R., Charpentier B., Vasquez A., Chouaib S., Caignard A., Moretta L., and Azzarone B. (2005) Membrane-bound and soluble IL-15/IL-15R complexes display differential signaling and functions on human hematopoietic progenitors. Blood 106, 2302–2310 [DOI] [PubMed] [Google Scholar]
- 103. Vámosi G., Bodnár A., Vereb G., Jenei A., Goldman C. K., Langowski J., Tóth K., Mátyus L., Szöllösi J., Waldmann T. A., and Damjanovich S. (2004) IL-2 and IL-15 receptor α-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. Proc. Natl. Acad. Sci. U.S.A. 101, 11082–11087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stearns R. C., Paulauskis J. D., and Godleski J. J. (2001) Endocytosis of ultrafine particles by A549 cells. Am. J. Respir. Cell Mol. Biol. 24, 108–115 [DOI] [PubMed] [Google Scholar]
- 105. Zhao Y., Sun X., Zhang G., Trewyn B. G., Slowing I. I., and Lin V. S. (2011) Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano 5, 1366–1375 [DOI] [PubMed] [Google Scholar]
- 106. Hecht T. T., Longo D. L., and Matis L. A. (1983) The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. J. Immunol. 131, 1049–1055 [PubMed] [Google Scholar]
- 107. Sa Q., Woodward J., and Suzuki Y. (2013) IL-2 Produced by CD8+ immune T cells can augment their IFN-γ production independently from their proliferation in the secondary response to an intracellular pathogen. J. Immunol. 190, 2199–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Perica K., Bieler J. G., Schütz C., Varela J. C., Douglass J., Skora A., Chiu Y. L., Oelke M., Kinzler K., Zhou S., Vogelstein B., and Schneck J. P. (2015) Enrichment and Expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano 9, 6861–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Labowsky M., Lowenthal J., and Fahmy T. M. (2015) An in silico analysis of nanoparticle/cell diffusive transfer: application to nano-artificial antigen-presenting cell:T-cell interaction. Nanomedicine 11, 1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Labowsky M., and Fahmy T. M. (2013) Effect of cell surface deformation on synaptic factor accumulation during the early stages of T cell activation. Chem. Eng. Sci. 90, 275–283 [Google Scholar]