Abstract
The Piezo2 channel is a newly identified mammalian mechanical transducer that confers rapidly adapting mechanically activated (RA-MA) currents in primary afferent neurons. The Piezo2 channels sense rapid membrane displacement, but it is not clear whether they are sensitive to osmotic swelling, which slowly increases static plasma membrane tension (SPMT). Here, we show that SPMT exerts a profound impact on the mechanical sensitivity of RA-MA channels in primary afferent neurons. RA-MA currents are greatly enhanced, and the mechanical threshold was reduced in both primary afferent neurons of rat dorsal root ganglia (DRG) and HEK293 cells heterologously expressing Piezo2 when these cells undergo osmotic swelling to increase SPMT. Osmotic swelling switches the kinetics of RA-MA currents to the slowly adapting type in both cultured DRG neurons and HEK293 cells heterologously expressing Piezo2. The potentiation of RA-MA currents is abolished when cultured DRG neurons are treated with cytochalasin D, an actin filament disruptor that prevents SPMT of cultured DRG neurons from an increase by osmotic swelling. Osmotic swelling significantly increases DRG neuron mechano-excitability such that a subthreshold mechanical stimulus can result in action potential firing. Behaviorally, the mechanical hind paw withdrawal threshold in rats is reduced following the injection of a hypotonic solution, but this osmotic effect is abolished when cytochalasin D or Gd3+ is co-administered with the hypo-osmotic solution. Taken together, our findings suggest that Piezo2-mediated mechanotransduction is regulated by SPMT in primary afferent neurons. Because SPMT can be changed by multiple biological factors, our findings may have broad implications in mechanical sensitivity under physiological and pathological conditions.
Keywords: dorsal root ganglia, ion channel, mechanotransduction, neuron, pain, plasma membrane, mechanically activated currents, membrane tension, piezo2 channels
Introduction
Sensing mechanical stimuli such as gentle touch and noxious pinch enables multiple important tasks in life such as social interaction and avoidance of harmful environments. One key step for sensing mechanical stimuli is mechanotransduction, the process by which mechanical energy is transduced into electrical signals at primary afferent endings. McCarter et al. (1) first showed that mechanical stimuli elicit depolarizing currents flowing through primary afferent neurons in the dorsal root ganglia (DRG),4 suggesting the presence of mechanically activated ion channels that may transduce mechanical stimuli into electrical signals in primary afferents. Mechanically activated currents in DRG neurons are subsequently characterized in more detail (2–5), and the most commonly observed whole-cell mechanically activated currents in DRG neurons are rapidly adapting mechanically activated (RA-MA) currents that usually have decay time constants of less than 10 ms (2, 3, 6). RA-MA currents are found to be mediated by nonselective cation channels (7), and they can be blocked by Gd3+ and ruthenium red (1, 2). RA-MA currents have been recorded from different DRG neurons, some of which feature non-nociceptive characteristics, whereas others show nociceptive properties (2), suggesting that they may be involved in both non-nociceptive and nociceptive mechanical transduction. The molecular identity conferring RA-MA currents in primary afferent neurons has recently been elucidated to be Piezo2, a large transmembrane protein that forms mechanically sensitive ion channels in mammalian primary afferents (6). Piezo2 channels are also expressed on Merkel cells of hair follicles and touch domes of the skin, where they are key molecules for the transduction of gentle touch (8–10). The role of Piezo2 channels in animal behavioral responses to mechanical stimuli has recently been demonstrated using Piezo2 knock-out mice (11).
In primary afferent neurons, studies have shown that RA-MA currents are up-regulated by a transcriptional mechanism by the proinflammatory neurotrophin nerve growth factor (12). Activators of PKC, given in vitro and in vivo, also cause increases in RA-MA currents and behavioral sensitization to mechanical stimulation, respectively (12). In a heterologous expression system, Piezo2 current amplitude is shown to be increased by bradykinin receptor β2 activation, and the up-regulation of Piezo2 function is shown to be mediated by protein kinase A and protein kinase C (13). A GTP-dependent run-up of Piezo2-type RA-MA currents is observed in cultured DRG neurons (14). In addition, a role for Piezo2 in EPAC1-dependent mechanical allodynia has been reported recently (15). Up-regulation of mechanotransduction at primary afferent nerve endings has been proposed to be a mechanism underlying mechanical allodynia and hyperalgesia, exaggerated pain states induced by gentle touch and mild noxious stimuli, respectively, seen under pathological conditions.
Previous studies on the regulation of Piezo2 channel functions have mainly focused on biochemical aspects such as modulation by second messengers. However, an emerging field of research on ion channel regulation in recent years has been to explore the impact of membrane biophysical properties on the activity of different ion channels (16, 17). It has been shown that membrane biophysical properties such as static plasma membrane tension (SPMT) have a significant impact on functions of some ion channels ranging from directly gating (e.g. bacterial MscL) to influencing voltage-gating (e.g. some voltage-gated K+ channels) and ligand-gating (e.g. NMDA receptors) (16–18). It is probably not totally unexpected that Piezo2 functions can be regulated by SPMT, but so far it has been unknown to what extent this biophysical factor may change the sensitivity of Piezo2 mechanotranduction in sensory neurons. In this study, we use osmotic swelling as a means to increase SPMT (19, 20), and we show that SPMT has a profound impact on Piezo2-mediated currents.
Experimental Procedures
Sprague-Dawley rats (250–350 g, both genders) were used. Animal care and use conformed to National Institutes of Health guidelines for the care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. DRG neuron cultures were prepared as described previously (21). In brief, rats were anesthetized with isoflurane and sacrificed by decapitation. DRGs were rapidly dissected out bilaterally and incubated with 0.2% collagenase and 0.5% dispase for 1 h at 37 °C in minimum essential medium for suspension culture (Invitrogen). After trituration to dissociate neurons, DRG neurons were plated on glass coverslips pre-coated with poly-d-lysine (12.5 μg/ml in distilled H2O) and laminin (20 μg/ml in Hanks' buffered salt solution, BD Biosciences). The cells were cultured in minimum essential medium (Invitrogen) that also contained nerve growth factor (2.5S NGF; 10 ng/ml; Roche Applied Science), 5% heat-inactivated horse serum (JRH Biosciences, Lenexa, KS), uridine/5-fluoro-2′-deoxyuridine (10 μm), 8 mg/ml glucose, and 1% vitamin solution (Invitrogen). The cultures were maintained in an incubator at 37 °C with a humidified atmosphere of 95% air and 5% CO2. Cells were used for patch clamp recordings after culturing for 3–7 days.
To perform patch clamp recordings, coverslips with cultured neurons were placed in a 0.5-ml microchamber, mounted on a stage of an Olympus IX70 inverted microscope (Olympus), and continuously perfused at 2 ml/min with a normal bath solution at a room temperature of 23 °C. The normal bath was an isotonic solution that contained (in mm) 145 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES, pH 7.3, and an osmolarity of 320 mosm. Normal patch clamp recording internal solution was an isotonic solution that contained (in mm) 70 Cs2SO4, 5 KCl, 2.4 MgCl2, 0.5 CaCl2, 5 EGTA, 10.0 HEPES, 5.0 Na2ATP, 0.33 GTP-Tris salt; pH was adjusted to 7.35 with CsOH, and osmolarity was adjusted with sucrose to 320 mosm. Recording electrodes were fabricated from thin wall capillaries using a Flaming/Brown P-97 puller (Sutter Instrument Co. Novato, CA). Resistances of electrodes were 4–6 megohms after filling recording internal solutions. Membrane access resistance after establishing the whole-cell configuration was ∼10 megohms and was not compensated. Junction potential between bath and electrode solution was calculated to be 11 mV and was corrected for the data analysis. Voltage clamp recordings were performed with cells held at −71 mV (command voltage of −60 mV minus 11 mV for junction potential correction). Signals were recorded with an Axopatch 200B amplifier, filtered at 2 kHz, and sampled at 5 kHz using pCLAMP 9.0 (Axon Instruments). Unless otherwise indicated, all recordings were performed on small-sized DRG neurons ranging from 20 to 30 μm in diameter.
To apply mechanical stimulation, the membranes of DRG cell bodies were displaced by a heat-polished glass pipette (membrane displacement probe). The tip of the probe was ∼4 μm in diameter. The probe was positioned at an angle of 45° to the surface of the dish, and the movement of the probe was controlled by a piezo-electric device (Physik Instruments, Auburn, MA). The tip of the probe was positioned in such a way that a 2-μm movement did not contact the cell; 3 μm had a visible contact but little membrane movement, and a 4-μm stimulus produced an observable membrane deflection. Therefore, tip forward steps of 2 and 3 μm were assigned as position −1 and 0 μm, respectively; a 4-μm forward step was recorded as an initial step of 1-μm membrane displacement. The probe was moved at a speed of 0.5 μm/ms. Membrane displacements by the probe were visualized as live images, which were continuously captured by a CCD camera through a 40× objective and displayed on an 11-inch monitor throughout each experiment. To ensure the consistency of membrane displacement over a long recording time, under the visual guidance we corrected any drift of the probe just before applying mechanical stimulation in each test, and we further corrected the relative distance between the tip of the probe and the cell membrane when osmotic swelling was introduced to the recorded cells. For most recordings, a fixed membrane displacement (6 or 7 μm, 500-ms duration) was applied immediately after breaking into the whole-cell mode and then continually applied at an interval of 2 min for up to 30 min. In some experiments, a series of graded membrane displacement steps were applied at 1-μm increments, and the duration of each step was 500 ms. The interval was 6 s when the displacement steps were applied. Assessment of RA-MA channel numbers and unitary current size was performed using the nonstationary fluctuation analysis described in our previous study (22). In brief, RA-MA currents were repeatedly evoked by the same membrane displacement of 7 μm for up to 30 times. Current variances during multiple stimulations were plotted against mean current values. The channel numbers and unitary current size were obtained by fitting data into the equation, σ2 = i·I − I2/N, where σ2 is current variance; i is unitary current size; I is mean value of currents, and N is channel numbers. Because membrane displacement might affect membrane seal to change access resistance, membrane properties were continually monitored during each recording. This was achieved by applying 5-mV test pulses. Data were discarded if membrane access resistance changed during recordings.
To determine the effects of osmotic swelling on RA-MA currents in cultured DRG neurons, osmotic swelling was produced by either intracellular hypertonicity (for most experiments) or extracellular hypotonicity. Osmotic swelling was determined by measuring the increases of cell diameters over time following the introduction of intracellular hypertonicity or extracellular hypotonicity. For intracellular hypertonicity, hypertonic recording internal solution was used, and the solution was similar to normal recording internal solution except the osmolarity was adjusted with sucrose to 420 mosm. To determine the effects of intracellular BAPTA on RA-MA currents, 20 mm BAPTA was included in the hypertonic recording internal solution. In some experiments to elicit neuronal action potentials while intracellular hypertonicity was introduced, the recording internal solution was similar to the normal recording internal solution except 70 mm Cs2SO4 was replaced by 135 mm potassium gluconate, and pH was adjusted with KOH to 7.3 and osmolarity adjusted with sucrose to 420 mosm. In experiments to introduce hypotonicity extracellularly, hypotonic solution with osmolarity of 220 mosm was used, and the solution was similar to normal bath solution except NaCl concentration was reduced from 145 to 90 mm. In one set of experiments, the TRPV4 activator GSK1016790A was tested for its effect on RA-MA currents. RA-MA currents were recorded using isotonic recording internal solution, and GSK1016790A (100 nm) was continuously applied through a bath for up to 30 min. In experiments to determine the role of actin filaments in RA-MA channel functions, cytochalasin D (CD, 10 μm), latrunculin A (LatA, 5 μm), or jasplakinolide (Jasp, 5 μm) was applied intracellularly by including each of them in the recording internal solution.
In some experiments, human embryonic kidney 293 (HEK293) cells that heterologously expressed mouse Piezo2 ion channels were tested. HEK293 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cells were plated onto 12-mm round glass coverslips placed in 12-well plates. Mouse Piezo2 plasmids (2 μg/well) were co-transfected with GFP plasmids (0.3 μg/ml) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. Transfected cells were identified and recorded 12–24 h following the transfection. Mechanically activated currents in Piezo2-expressing HEK293 cells were recorded under the conventional whole-cell mode either with normal patch clamp recording internal solution or hypertonic recording internal solution. Mechanical stimulation was applied in the same fashion as that in the recordings of RA-MA currents from cultured DRG neurons.
Immunostaining was performed to examine Piezo2 channel expression on cultured DRG neurons. Cells were incubated at room temperature for 30 min in a 4% paraformaldehyde solution and further incubated for 2 h in a mixture solution of 0.4% Triton X-100 and 4% paraformaldehyde. After three rinses with PBS, the cells were incubated for 2 h at room temperature with a 2% Triton X-100 solution. The cells were rinsed two times with a 1% goat serum PBS and then incubated for 1 h in a solution of 4.5% normal goat serum in PBS with 0.4% Triton X-100 to block nonspecific antibody binding. The cells were incubated with a polyclonal rabbit anti-Piezo2 antibody (1:500; Novus Biologicals, Littleton, CO) overnight at 4 °C. Following three rinses with 1% goat serum PBS solution, the cells were further incubated with a secondary antibody for 1 h at room temperature. The secondary antibody (1:1000 in 1% goat serum PBS solution) was a goat anti-rabbit IgG conjugated with Alexa-488 (Molecular Probes, Eugene, OR). The cells were rinsed three times with 1% goat serum PBS solution, coverslipped with a glycerol-based anti-photobleach medium. Cell images were taken under an epifluorescent or a confocal fluorescent microscope. Fluorescent intensity values were measured at the perimeters and also at the sites on cells ∼5 μm away from the perimeters. The ratio of fluorescent intensities between these two locations was then calculated.
Changes of membrane tension in cultured DRG neurons during osmotic swelling were determined by using the micropipette aspiration technique as described previously (23, 24). In brief, micropipette was pulled from thin wall borosilicate glass capillaries (∼1.5 mm external diameter and ∼1.4 mm internal diameter) using Flaming/Brown P-97 puller (Sutter Instrument Co., Novato, CA). The tips of the micropipettes ranged between5 and 8 μm internal diameter depending on cell sizes, and the shape of the tip of each micropipette was in a cylindrical tube at a length of about 10 μm. The tips of the micropipettes were smoothed, and the shank of the tip was bent into a 45° angle. Micropipettes were usually prepared on the day of the experiment. Micropipettes were filled with bath solution that contained 5% horse serum to allow the cell membrane to move smoothly into the micropipette during aspiration. The micropipette was affixed on a head stage of a high speed pressure clamp (HSPC) device, and the head stage was mounted on a holder of a micromanipulator to allow fine control of the position of the micropipette. At the beginning of each experiment, the pressure in the pipette was equilibrated to the atmospheric pressure. Before aspirating cultured DRG neurons, the micropipette approached the cells at a 45° angle so that the shank of the micropipette tip was actually aligned horizontally to the focal plane while approaching the cells. Then the micropipette was moved toward a cell until the tip of the micropipette was in full contact with the membrane of the cell. To apply aspiration, steps of negative pressures were generated by HSPC device, and the negative pressures were delivered to the micropipette tip via the head stage of the HSPC. The HSPC was controlled, and pressure steps were programmed using pCLAMP 9.0 (Axon Instruments). Each negative pressure step was at 0.005 or 0.01 mm Hg and was applied until membrane projection was stabilized at a certain length before advancing to the next pressure step. A test for a single pressure step usually took ∼2 min. The negative pressure steps were continually applied to the cell until obtaining the negative pressure at which the membrane projection reached the length of the radius of the micropipette tip. During each negative pressure step, real time images of membrane deformation were acquired by a CCD camera and displayed on a 17-inch video monitor to track the membrane projection that was aspirated into the pipette. Membrane tension was determined based on the law of LaPlace and Young-LaPlace Equation 1 (23),
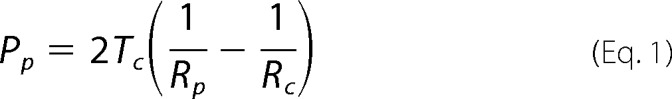 |
where Tc is the plasma membrane tension; Rc is the radius of the cell outside the pipette; Rp is the radius of the pipette; Pp is the pipette pressure to result in Lp = Rp; Lp is the length of membrane projection in the pipette, and Pp = Pc (intracellular pressure) when Lp = Rp.
The animal behavioral response to mechanical stimulation was examined using the von Frey test. In each test, a rat was placed in a transparent plastic chamber (length × width × height = 20 × 10 × 14 cm) suspended above a wire mesh platform (Ugo Basile, Italy). After habituation for 10 min, mechanical sensitivity of rat right hind paws was examined using the von Frey filaments (Ugo Basil, Italy). A series of von Frey filaments (force range from 0.16 to 26 g) were used sequentially in ascending order to push against the plantar surface of the hind paws. Each filament was applied to the plantar surface of the hind paw five times with a 10-s inter-trial interval. For each trial, the filament was applied until buckling of the filament occurred and was then held for 5 s. The mechanical hind paw withdrawal threshold was defined as the force value of a filament that produced ≥3 hind paw withdrawals in response to five stimuli. In each experiment, the baseline withdrawal threshold was first measured 15 min before the injection of testing compounds. The intraplantar hind paw of the rat was then injected with one of the following solutions in a volume of 100 μl: isotonic saline solution (310 mosm), 0.5% DMSO in isotonic saline solution; cytochalasin D (1 mm) in 0.5% DMSO isotonic saline solution; Gd3+ (0.3 mm) in isotonic saline solution; hypotonic saline solution (155 mosm); hypotonic saline solution plus cytochalasin D (1 mm). or hypotonic saline solution plus Gd3+ (0.3 mm). Withdrawal thresholds were then measured at 5, 15, 30, and 45 min after the injection.
Cell diameters were measured from images captured by a digital camera, and a cell diameter was calculated as the average of the longest and shortest widths of the cell. Whole-cell recording data were analyzed using Clampfit 9 software. The threshold for eliciting RA-MA currents was defined as the minimum membrane displacement that evoked a visible inward RA-MA current. The onset latency of an RA-MA current in response to a membrane displacement was the time delay from the contact of cell membranes by mechanical stimulation probe to the initiation of the RA-MA current. Decay time constant of an RA-MA current was obtained by fitting the current decay phase in the range of 10–90% with a single exponential equation. The value of SPMT at each time point represents averaged SPMT around that time point with the time bin of ±1 min. Unless otherwise indicated, all chemicals were purchased from Sigma. Data are reported as mean ± S.E. Statistical significance (p < 0.05) was assessed by one-way analysis of variance followed by multiple comparison using Student's t tests with no corrections (25, 26).
Results
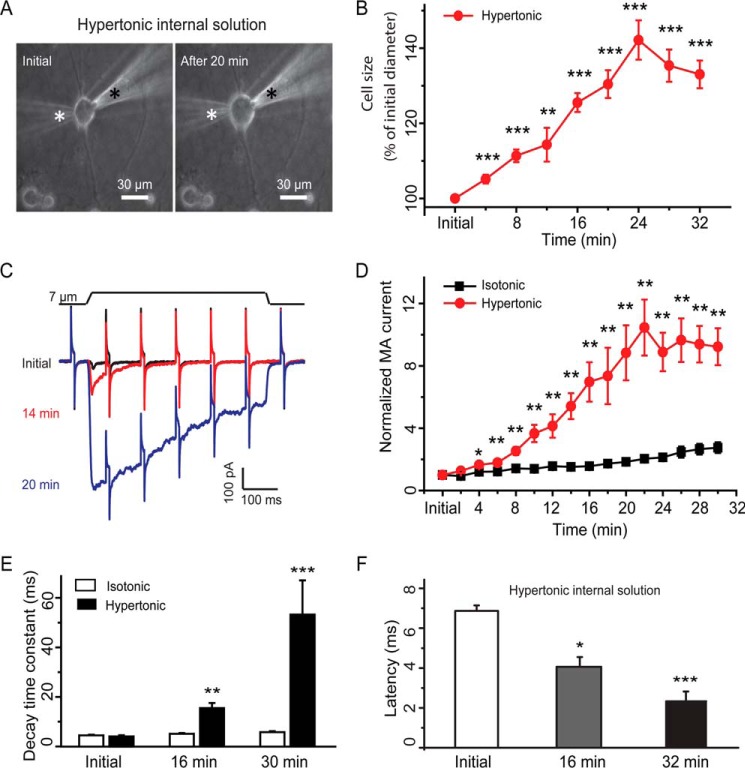
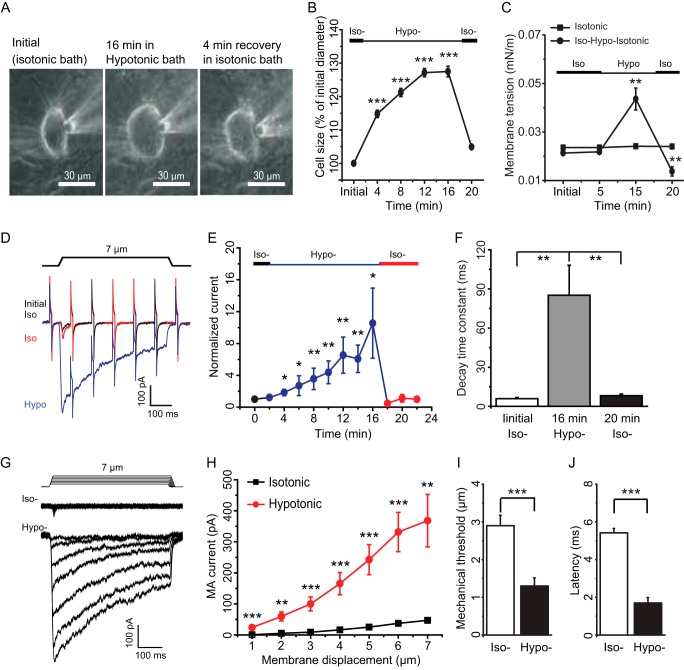
We examined the effects of osmotic swelling on RA-MA currents that were elicited by displacements of cultured DRG neuron membranes with a mechanical stimulation probe. In most experiments described in this study, osmotic swelling was induced by a hypertonic recording internal solution (420 mosm) filled in patch clamp recording electrodes. The reason for using hypertonic recording internal solution was to maintain a cation gradient equivalent to that of conventional whole-cell patch clamp recording condition, a desirable condition for studying RA-MA currents for this work. We measured cell sizes and RA-MA currents immediately after establishing the whole-cell mode (initial time) and then continued the measurements every 2 min for up to 30 min or longer. Fig. 1, A and B, shows cell sizes at initial time after establishing the whole-cell mode and at different time points afterward. All cultured DRG neurons swelled, and cell sizes increased over time for up to 24 min after establishing the whole-cell mode with hypertonic recording internal solution (Fig. 1B). For example, cell diameters were increased by 26% (n = 26) at 16 min, 30% (n = 24) at 20 min, and 42% (n = 22) at 24 min after establishing the whole-cell mode. However, there was usually no further increase in cell sizes afterward and some cells were lysed after 40 min.
FIGURE 1.
Osmotic swelling potentiates RA-MA currents in cultured DRG neurons. A, images show a DRG neuron at initial time (left) and 20 min (right) after establishing the whole-cell mode with a recording electrode that contained a hypertonic recording internal solution (420 mosm). The positions of the recording electrode and mechanical probe are indicated by a white and a black star, respectively. B, summary data of the osmotic swelling expressed as increases of cell diameters over time after establishing the whole-cell mode (n = 22). C, three sample traces show the mechanically activated currents evoked by a 7-μm membrane displacement step at the initial time (black), 14 min (red), and 20 min (blue) after establishing the whole-cell mode. The membrane displacement step is indicated above the current traces. The traces also include continual membrane tests by voltage steps of 5 mV at the interval of 100 ms. D, summary data of the increases of RA-MA currents over time after establishing the whole-cell mode with hypertonic (red circles, n = 18) or isotonic (black squares, n = 20) recording internal solution. E, decay time constants of RA-MA currents at initial time, 16 and 30 min after establishing the whole-cell mode with hypertonic (solid bars, n = 14) or isotonic (open bars, n = 30) recording internal solution. F, latency of RA-MA current onset at initial time, 16 and 30 min after establishing the whole-cell mode with hypertonic recording internal solution (n = 10). Data represent mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with the value at initial time.
We evoked RA-MA currents by a 7-μm membrane displacement. Only those cells exhibiting RA-MA currents at the initial time were further tested. Because of osmotic swelling, the distance between the tip of the stimulation probe and the cell membrane became shortened over time. Accordingly, we adjusted the distance right before each test. The osmotic swelling of cultured DRG neurons was accompanied by gradual increases of RA-MA current amplitudes (Fig. 1, C and D). For example, the amplitude of RA-MA currents was increased 2.5-fold (n = 23, p < 0.01) at 8 min, 7-fold (n = 19, p < 0.01) at 16 min, and 9-fold (n = 18, p < 0.01) at 20 min after establishing the whole-cell mode. As a control we used isotonic recording internal solution to record RA-MA currents over time after whole-cell mode. We only observed a small degree of increases in RA-MA current amplitude under the isotonic condition (Fig. 1D), consistent with RA-MA current run-up in DRG neurons observed previously (14). For experiments with hypertonic recording internal solution, the osmotic swelling was also accompanied by gradually slowing RA-MA current kinetics over time. RA-MA currents had a decay time constant of 4.0 ± 0.5 ms (n = 14, Fig. 1E) when recorded at initial time after establishing the whole-cell mode with hypertonic recording internal solution. RA-MA currents became the intermediately adapting type with a decay time constant prolonged to 15.2 ± 2.3 ms (n = 14, p < 0.01, Fig. 1E) at the 16-min time point, and the RA-MA currents became the slowly adapting type for many cells recorded at the 30-min time point, and overall the decay time constants were prolonged to 53.3 ± 13.8 ms (n = 14, p < 0.001, Fig. 1E). In contrast, with isotonic recording internal solution there was no significant change in decay time constants of RA-MA currents over time after establishing the whole-cell mode (Fig. 1E). The onset latency for eliciting RA-MA currents was shortened by osmotic swelling over time. With hypertonic recording internal solution, the latency was 6.87 ± 0.28 ms (n = 10) at initial time and reduced to 4.06 ± 0.49 ms (n = 10, p < 0.05) and 2.33 ± 0.50 ms (n = 10, p < 0.001) following osmotic swelling for 16 and 30 min, respectively. In all the above recordings before and following osmotic swelling with hypertonic recording internal solution, membrane tests were continually conducted to monitor membrane resistance of the recorded cells, and we did not observe obvious changes in membrane resistance except during the RA-MA current period (Fig. 1C).
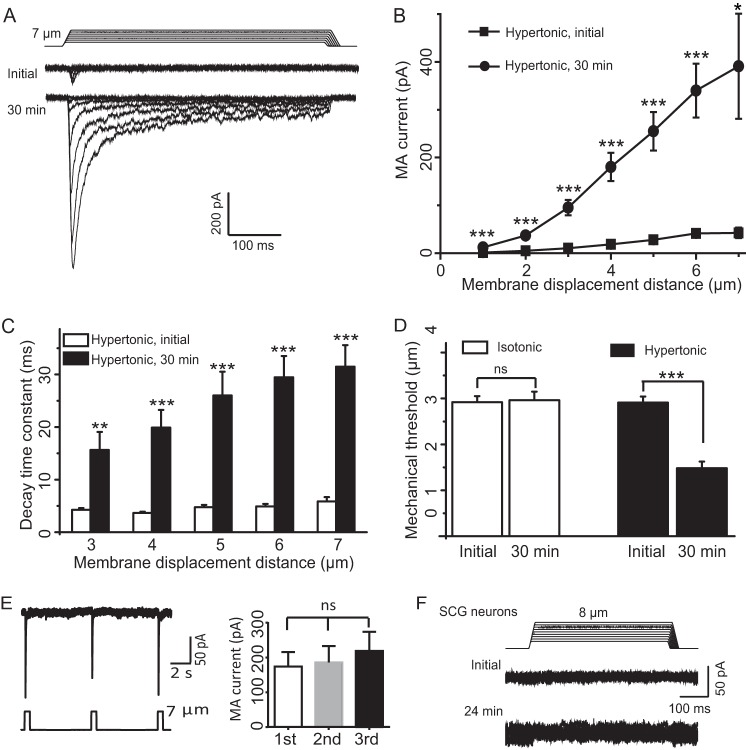
We next determined the effect of osmotic swelling on RA-MA currents evoked at different stimulation intensities. Different membrane displacements, i.e. different stimulation intensities, were applied to cells to elicit RA-MA currents at initial time and 30 min after establishing the whole-cell mode with hypertonic recording internal solution. As shown in Fig. 2, A and B, for membrane displacements from 1 to 7 μm in a 1-μm increment, RA-MA currents elicited following 30 min of osmotic swelling were significantly larger than initial RA-MA currents. For example, RA-MA currents increased from 41.3 ± 5.8 pA (n = 22) to 339.9 ± 56.1 pA (n = 19) at 6 μm of membrane displacements. RA-MA current kinetics were slowed significantly, and decay time constants of RA-MA currents following 30 min of osmotic swelling were significantly longer than those at initial time after establishing the whole-cell mode (Fig. 2C). The increases in the decay time constants occurred in all membrane displacement steps tested, and the degree of the increases was significantly higher with greater membrane displacement steps of 5, 6, and 7 μm than with the step of 3 μm (p < 0.05, Fig. 2C). Osmotic swelling resulted in a significant reduction of mechanical threshold. Mechanical thresholds were reduced by ∼50% following osmotic swelling with hypertonic recording internal solution for 30 min (n = 26, p < 0.001, Fig. 2D). In contrast, there was no significant difference in mechanical threshold between initial time and 30 min after establishing the whole-cell mode with isotonic recording internal solution (n = 33, Fig. 2D). The changes in mechanical thresholds were unlikely due to the repeated stimulation because no significant change in RA-MA currents was observed when the same displacement was repeatedly applied (Fig. 2E). Superior cervical ganglion (SCG) neurons were previously used as a negative control to show that RA-MA currents were expressed specifically in primary afferent neurons and not in SCG neurons (1). Using SCG as a control, we determined whether RA-MA currents could be elicited by membrane displacement in SCG neurons when these neurons underwent osmotic swelling. In contrast to cultured DRG neurons, there was no RA-MA current that could be elicited by membrane displacement in SCG neurons before and following osmotic swelling (n = 14, Fig. 2F).
FIGURE 2.
Osmotic swelling potentiates DRG neuron RA-MA currents at different mechanical stimulation intensities. A, two sets of sample traces of RA-MA currents elicited by different membrane displacement steps at the initial time (top) and 30 min (bottom) after establishing the whole-cell mode. The patch clamp electrode contained the hypertonic recording internal solution. Membrane displacement steps are at the increment of 1 μm and indicated above RA-MA current traces. B, summary data of the experiments represented in A. Squares and circles are the RA-MA currents evoked at initial time (n = 22) and 30 min (n = 19) after establishing the whole-cell mode, respectively. C, decay time constants for the experiments represented in A. Open bars and solid bars are decay time constant at initial time (n = 22) and 30 min (n = 15) after establishing the whole-cell mode, respectively. D, mechanical thresholds at initial time and 30 min after establishing the whole-cell mode. Open bars, recordings with isotonic recording internal solution (n = 33); solid bars, recordings with hypertonic recording internal solution (n = 26). E, sample trace (left) and summary data (right) of RA-MA currents evoked by repeated membrane displacements at the interval of 6 s (n = 9). F, sample traces show that membrane displacement did not elicit RA-MA currents in SCG neurons recorded with hypertonic recording internal solution either at the initial time or 24 min after establishing the whole-cell mode (n = 14). Data represent mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significantly different.
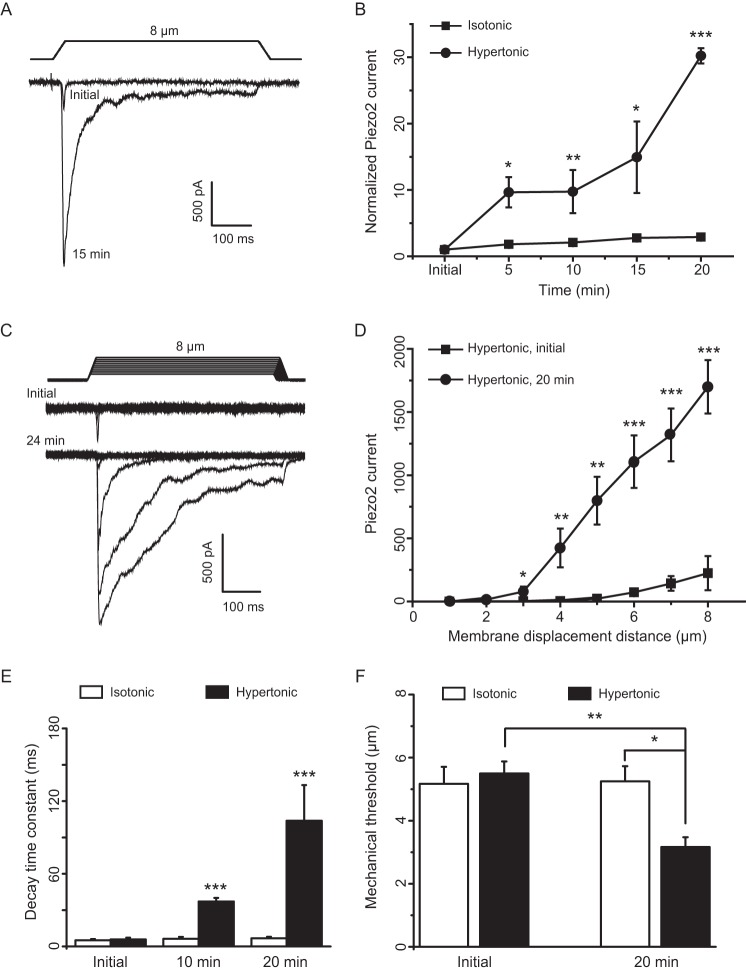
RA-MA currents in DRG neurons are previously shown to be mediated by Piezo2 channels (6), and osmotic swelling-induced potentiation of RA-MA currents in our cultured DRG neurons are most likely due to the increase of mechanical sensitivity of Piezo2 channels. To directly determine whether mechanical sensitivity of Piezo2 channels can be enhanced by osmotic swelling, we expressed Piezo2 channels heterologously in HEK293 cells, and we directly tested whether Piezo2-mediated RA-MA currents (Piezo2 currents) could be potentiated and the current kinetics shifted to slowly adapting type following osmotic swelling. Similar to the recordings from cultured DRG neurons, Piezo2 currents in HEK293 cells were significantly potentiated over time following osmotic swelling with hypertonic recording internal solution (Fig. 3, A and B). For example, when a membrane displacement distance of 8 μm was applied, Piezo2 current amplitudes increased 30-fold (n = 5, p < 0.001) at 20 min after establishing the whole-cell mode with hypertonic recording internal solution (Fig. 3B). The potentiation of Piezo2 currents by osmotic swelling was observed at different membrane displacement distances tested up to 8 μm (Fig. 3, C and D, n = 8). The potentiation was accompanied by significantly slowing Piezo2 current kinetics as was evidenced by the prolonged decay time constants after osmotic swelling (Fig. 3, C and E). The decay time constants were 5.8 ± 1.5 ms (n = 7) at initial time, prolonged to 37.1 ± 3.0 ms (n = 7, p < 0.001) at 10 min, and 103.8 ± 29.5 ms (n = 7, p < 0.001) at 20 min after establishing the whole-cell mode with hypertonic recording internal solution (Fig. 3E). In contrast, with isotonic recording internal solution, Piezo2 currents remained to be rapidly adapting over time with the time constant being kept ∼6 ms (n = 5) from initial time to 20 min after establishing the whole-cell mode (Fig. 3E). Mechanical threshold was substantially reduced from 5.5 ± 0.4 μm (n = 8) at initial time to 3.2 ± 0.3 μm (n = 8, p < 0.01) after establishing whole-cell mode for 20 min with hypertonic recording internal solution (Fig. 3F). In contrast, there was no significant change of mechanical threshold when isotonic recording internal solution was used (n = 6, Fig. 3F).
FIGURE 3.
Osmotic swelling potentiates RA-MA currents in HEK293 cells heterologously expressing Piezo2 channels. A, sample traces of Piezo2-mediated RA-MA currents (Piezo2 currents) recorded from a Piezo2-expressing HEK293 cell at initial time and 15 min after establishing the whole-cell mode with hypertonic recording internal solution. A membrane displacement step of 8 μm is indicated above the current traces. B, summary data of Piezo2 currents over time after establishing the whole-cell mode with hypertonic (circles, n = 5) or isotonic (squares, n = 6) recording internal solution. C, two sets of sample traces of Piezo2 currents elicited by different membrane displacement steps. The Piezo2 currents were recorded at the initial time (top) and 20 min (bottom) after establishing the whole-cell mode with hypertonic recording internal solution. Membrane displacement steps in the increment of 1 μm are indicated above the traces. D, summary data of the Piezo2 currents evoked by different membrane displacement steps. Piezo2 currents were recorded at initial time (squares, n = 8) and 20 min (circles, n = 8) after establishing the whole-cell mode with hypertonic recording internal solution. E, decay time constants of Piezo2 currents over time after establishing the whole-cell mode with hypertonic (closed bars, n = 7) or isotonic (open bars, n = 5) recording internal solution. F, mechanical thresholds for eliciting Piezo2 currents at initial time and 20 min after establishing the whole-cell mode; the recordings were made with hypertonic (closed bars, n = 8) or isotonic (open bars, n = 6) recording internal solution. Data represent mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001.
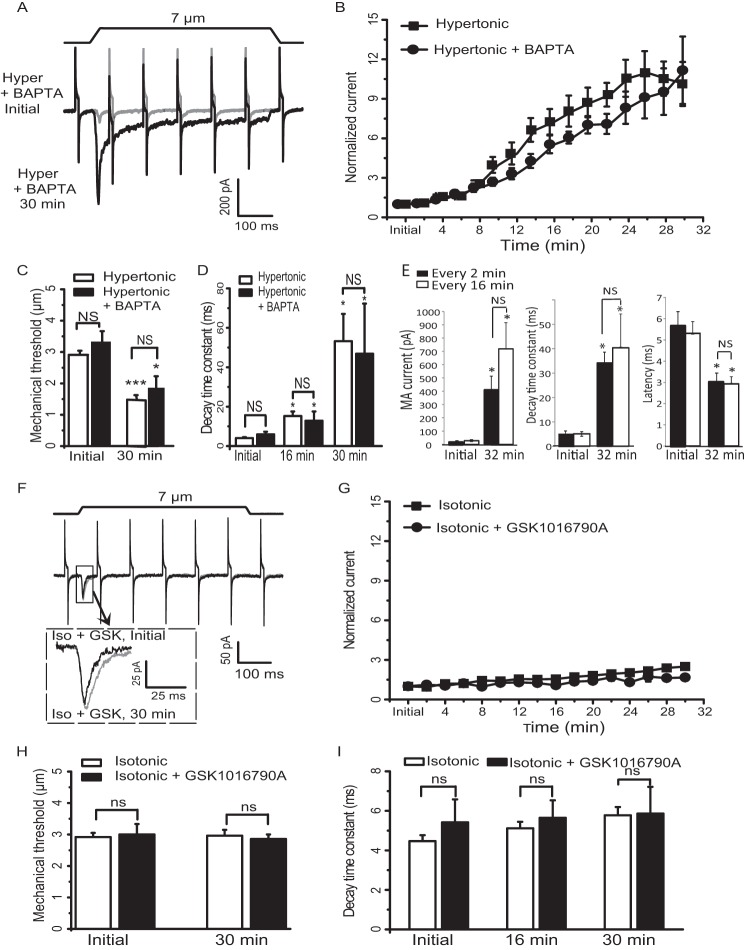
Osmotic swelling may activate TRPV4 channels to increase intracellular Ca2+ in cultured DRG neurons. To determine whether osmotic swelling-induced potentiation of RA-MA currents is dependent on the elevation of intracellular Ca2+ following TRPV4 activation, we included the rapid Ca2+ chelator BAPTA (20 mm) in our hypertonic recording internal solution and determined whether osmotic swelling-induced potentiation of RA-MA currents was affected. As shown in Fig. 4, A and B, RA-MA currents were still potentiated under this condition in a degree similar to the experiments using hypertonic recording internal solution that did not contain BAPTA. For example, 30 min after establishing the whole-cell mode, RA-MA currents were potentiated by ∼11-fold (n = 8) in experiments using hypertonic recording internal solution with BAPTA, which was not significantly different from the degree of potentiation (∼10-fold, n = 10) in experiments using hypertonic recording internal solution without BAPTA. Mechanical thresholds were significantly reduced by ∼50% 30 min after establishing the whole-cell mode using hypertonic internal recording solutions either with or without BAPTA, but there was no significant difference in mechanical thresholds between these two conditions (Fig. 4C). Similar to the results using hypertonic recording internal solution without BAPTA, the kinetics of RA-MA currents were also significantly slowed when BAPTA was included in the hypertonic recording internal solution. The decay time constants measured at the same time points after establishing the whole-cell mode were not significantly different between the experiments with and without BAPTA in recording internal solution (Fig. 4D). We determined whether osmotic swelling-induced potentiation is use-dependent. Two stimulation paradigms were applied, one was to apply stimulation every 2 min (n = 8), and the other was to apply the stimulation every 16 min (n = 8). Although osmotic swelling resulted in significant increases in RA-MA current amplitude and decay time constants as well as deceases in current latency 30 min after establishing whole-cell mode, these RA-MA current parameters at the 30-min time point did not show significant difference between the two stimulation paradigms (Fig. 4E). To further determine whether TRPV4 channels mediated osmotic swelling-induced potentiation of Piezo2 currents, we examined whether GSK1016790A, a highly potent TRPV4 activator, could directly potentiate RA-MA currents recorded from cultured DRG neurons using isotonic recording internal solution. Bath application of 100 nm GSK1016790A resulted in small and slow inward currents in cultured DRG neurons tested (n = 7, not shown), indicating the presence of TRPV4 channels in these cells. However, the amplitudes of RA-MA currents recorded with isotonic recording internal solution were not significantly different between the experiments in the absence (n = 26) and presence of 100 nm GSK1016790A (n = 7, Fig. 4, F and G). There was also no significant difference in mechanical threshold between the experiments in the absence (n = 26) and presence of GSK1016790A (n = 7, Fig. 4H). Furthermore, the kinetics of RA-MA currents was not affected by GSK1016790A as there was no significant difference in the decay time constants between the experiments in the absence (n = 26) and presence of GSK1016790A (n = 6, Fig. 4I).
FIGURE 4.
Osmotic swelling-induced RA-MA current potentiation in cultured DRG neurons is independent of intracellular Ca2+ and TRPV4 activation. A, two sample traces show RA-MA currents recorded at initial time (gray trace) and 30 min (black trace) after establishing the whole-cell mode with hypertonic recording internal solution that contained 20 mm BAPTA. Membrane displacement was 7 μm. B, summary data of the potentiation of RA-MA currents over time after establishing the whole-cell mode using hypertonic recording internal solution; the solution was either without (squares, n = 10) or with 20 mm BAPTA (circles, n = 8). C, mechanical thresholds of RA-MA currents recorded using hypertonic recording internal solution without (open bars, n = 26) or with 20 mm BAPTA (solid bars, n = 6). D, decay time constants of RA-MA currents recorded using isotonic recording internal solution without (open bars, n = 10) or with 20 mm BAPTA (solid bars, n = 6). E, RA-MA current amplitude (left), latency (middle), and decay time constant (right) obtained in either of the following two stimulation paradigms, stimuli are delivered every 2 min (n = 8) or delivered every 16 min (n = 8). F, sample traces show RA-MA currents recorded before (black) and following (gray) the bath application of 100 nm GSK1016790A for 30 min. The RA-MA currents were recorded using isotonic recording internal solution. Inset, expanded scale for the boxed region. Membrane displacement was 7 μm. G, summary data of the RA-MA currents recorded over time in the absence (squares, n = 26) and presence (circles, n = 7) of 100 nm GSK1016790A. H, mechanical thresholds of RA-MA currents recorded using isotonic recording internal solution without (open bars, n = 26) or with 100 nm GSK1016790A (closed bars, n = 7). I, decay time constants of RA-MA currents recorded using isotonic recording internal solution without (open bars, n = 26) or with GSK1016790A (closed bars, n = 6). RA-MA currents were at initial time and 30 min in C and H; 32 min in E, and at initial time and 16 and 30 min in D and I after establishing the whole-cell mode. Data represent mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with initial time; ns and NS, no significant difference, compared at the same time point.
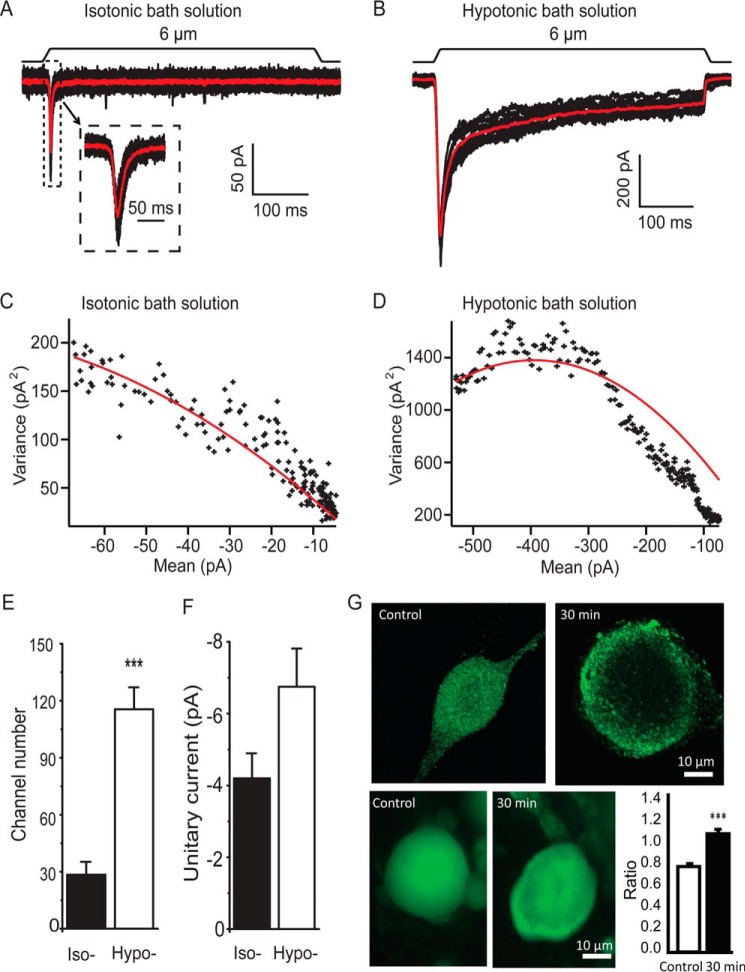
The potentiation of RA-MA currents by osmotic swelling could be due to an increase in the numbers and/or single channel conductance of RA-MA channels on cultured DRG membranes following osmotic swelling. To test this idea, we conducted nonstationary fluctuation analysis to assess the potential changes in RA-MA channel numbers and unitary currents (Fig. 5, A–F) following osmotic swelling. In this set of experiments, variances of RA-MA currents were obtained following repeated mechanical stimulation, and channel numbers and unitary current sizes were derived from the plots of the variances against mean values (22). The measured RA-MA channel numbers were 28.4 ± 6.8 (n = 9) in isotonic bath solution and were 4-fold higher (115.5 ± 11.6, n = 6) following the application of hypotonic bath solution for 30 min (Fig. 5E). However, there was no significant difference in RA-MA unitary current size between control (n = 9) and cells treated with hypotonic bath solution (n = 6, Fig. 5F). We performed immunostaining using an antibody against Piezo2 channels for the cells without or with osmotic swelling by hypotonic bath solution. We found that Piezo2 immunofluorescent intensity measured at the perimeters of cells was relatively higher than that measured at the sites away from the perimeters of cells after the cells were treated with hypotonic bath solution for 30 min (n = 39, Fig. 5G). In contrast, Piezo2 immunofluorescent intensity at the perimeters was relatively lower than that measured at the sites away from the perimeters in control without the hypotonic treatment (n = 46, Fig. 5G). The intensity ratios of the two regions were significantly higher for cells after osmotic swelling (n = 39) than for the control cells without osmotic swelling (n = 46, p < 0.001, Fig. 5G).
FIGURE 5.
Assessment of channel numbers and unitary current sizes of RA-MA channels on DRG membranes. A, set of RA-MA traces recorded from a cultured DRG neuron in response to repeated stimulation. The recording was conducted in isotonic bath solution. The red trace is the average of the multiple responses. Inset, at an expanded scale. B, similar to A except RA-MA currents were recorded 30 min after treating the cell in hypotonic bath solution (220 mosm). C and D, plots of the variance versus mean values of RA-MA currents with curve fitting (red) for the experiments in isotonic (C) and hypotonic bath solution (D). E, summary data of channel numbers in cells tested in isotonic bath solution (n = 9) and hypotonic bath solution (n = 6). F, summary data of unitary current size in cells tested in isotonic bath solution (n = 9) and hypotonic bath solution (n = 6). G, top two images show confocal imaging of Piezo2 immunoreactivity in a cell without (control, left) and a different cell with osmotic swelling (right); piezo2 immunoreactivity intensity is higher on plasma membranes than in cytosol compartment following osmotic swelling in hypotonic bath solution for 30 min (right). Bottom two images are similar to the top images except they were taken under epifluorescence illumination. Bar graph on bottom right panel shows summary data of immunofluorescent intensity ratio between the values measured at the perimeters and measured 5 μm away from the perimeters in the cells; epifluorescence images were used for the summary data. Open bar, control cells without osmotic swelling (n = 46); solid bar, cells treated with 220 mosm hypotonic bath solution for 30 min (n = 39). Data represent mean ± S.E., ***, p < 0.001.
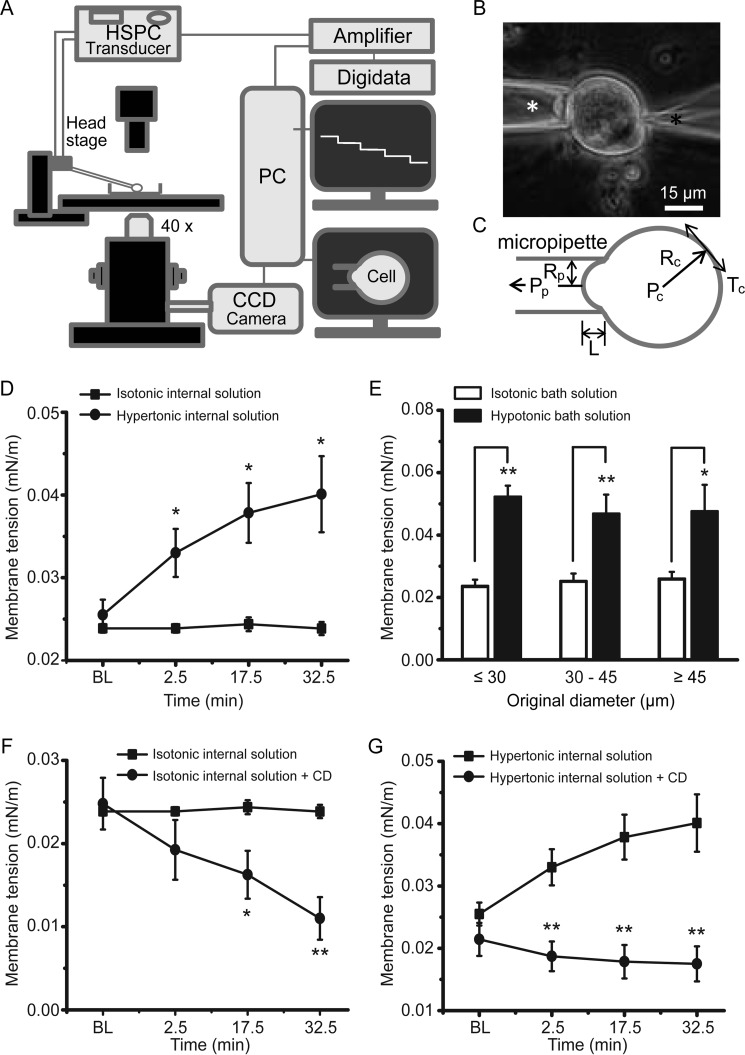
A main effect of osmotic swelling on a cell is increasing SPMT (19, 20, 27). We quantitatively measured SPMT in cultured DRG neurons under the isotonic condition and following osmotic swelling using the micropipette aspiration technique (Fig. 6, A–C). Under the isotonic condition, SPMT was 0.024 ± 0.0005 mN/m (n = 8) at baseline and was not significantly changed over time after establishing the whole-cell mode (Fig. 6D). In contrast, SPMT was significantly increased to 0.033 ± 0.003 mN/m at 2.5 min (n = 12), 0.038 ± 0.004 mN/m at 17.5 min (n = 12), and 0.040 ± 0.005 mN/m at 32.5 min (n = 12) after establishing the whole-cell mode using hypertonic recording internal solution (Fig. 6D). SPMT was also significantly increased following the bath application of a hypotonic solution (220 mosm) for 10–15 min (Fig. 6E), and the degree of SPMT changes was not dependent on original cell sizes (Fig. 6E). For example, for small sized neurons (original diameters ≤30 μm), SPMT was increased from 0.024 ± 0.002 mN/m (n = 11) under the isotonic condition to 0.052 ± 0.004 mN/m (n = 9) in hypotonic bath solution for 10 min (Fig. 6E). Although osmotic swelling increased SPMT, disrupting actin microfilaments using 10 μm CD significantly reduced SPMT under the isotonic condition (Fig. 6F). For example, the plasma membrane tension was decreased from 0.025 ± 0.003 mN/m (n = 10) under the isotonic condition to 0.011 ± 0.003 mN/m (n = 7) 32.5 min after establishing the whole-cell mode using isotonic internal solution that contained 10 μm CD (Fig. 6F). We further determined whether disrupting actin microfilaments by CD may prevent SPMT from increase by osmotic swelling. As shown in Fig. 6G, although hypertonic recording internal solution increased SPMT, SPMT was not increased over time when 10 μm CD was present in the internal solution (Fig. 6G). For example, SPMT was increased from 0.026 ± 0.002 mN/m (n = 12) at baseline to 0.040 ± 0.005 mN/m (n = 11) 32.5 min after establishing the whole-cell mode with the hypertonic recording internal solution that did not contain CD. In contrast, SPMT was at 0.018 ± 0.003 mN/m (n = 7) 32.5 min after establishing the whole-cell mode with the hypertonic recording internal solution that contained 10 μm CD, significantly lower than the control measured at the same time point (Fig. 6G).
FIGURE 6.
Static plasma membrane tension of primary afferent neurons is measured by the micropipette aspiration technique. A, schematic diagram illustrates the setup for measuring SPMT using a micropipette aspiration technique. In this setup, a micropipette is connected to an HSPC device via its head stage. Stepwise negative pressures are generated by HSPC and delivered to the micropipette to aspirate a cell. B, image shows a DRG neuron whose membranes are being aspirated into the micropipette (white asterisk indicated). The cell is also patched under the whole-cell mode by a recording electrode (black asterisk indicated) that contains a hypertonic recording internal solution. C, parameters needed for calculating membrane tension based on the Young-Laplace equation. Tc, membrane tension; Rc, cell radius; Pc, intracellular pressure; Pp, pipette aspiration pressure; Rp, pipette radius; L, length of membrane projection within pipette. D, SPMT measured from small-sized cultured DRG neurons (original diameter ≤30 μm) under the whole-cell mode with patch clamp recording electrode containing isotonic (solid squares, n = 8) and hypertonic (solid circles, n = 12) recording internal solution. BL, baseline before establishing the whole-cell mode; Time, time after establishing the whole-cell mode. E, SPMT measured from cells whose original sizes (in diameter) were small (≤30 μm), medium (30–45 μm), and large (≥45 μm). Cells were not patched by recording electrodes. SPMT measurements were performed on cells in isotonic bath solution (open bars, n = 11 for small cells; n = 12 for medium cells; n = 6 for larger cells). SPMT was also measured following the application of hypotonic bath solution (solid bars, n = 9 for small cells; n = 10 for medium cells; n = 6 for larger cells). F, SPMT measured from small-sized cells under the whole-cell patch clamp recording mode with patch clamp recording electrode containing isotonic recording internal solution without (solid squares, n = 10) and with 10 μm CD (solid circles, n = 7). G, SPMT measured from small-sized cells under the whole-cell patch clamp recording mode with electrode containing hypertonic recording internal solution without (solid squares, n = 12) and with 10 μm CD (solid circles, n = 7).
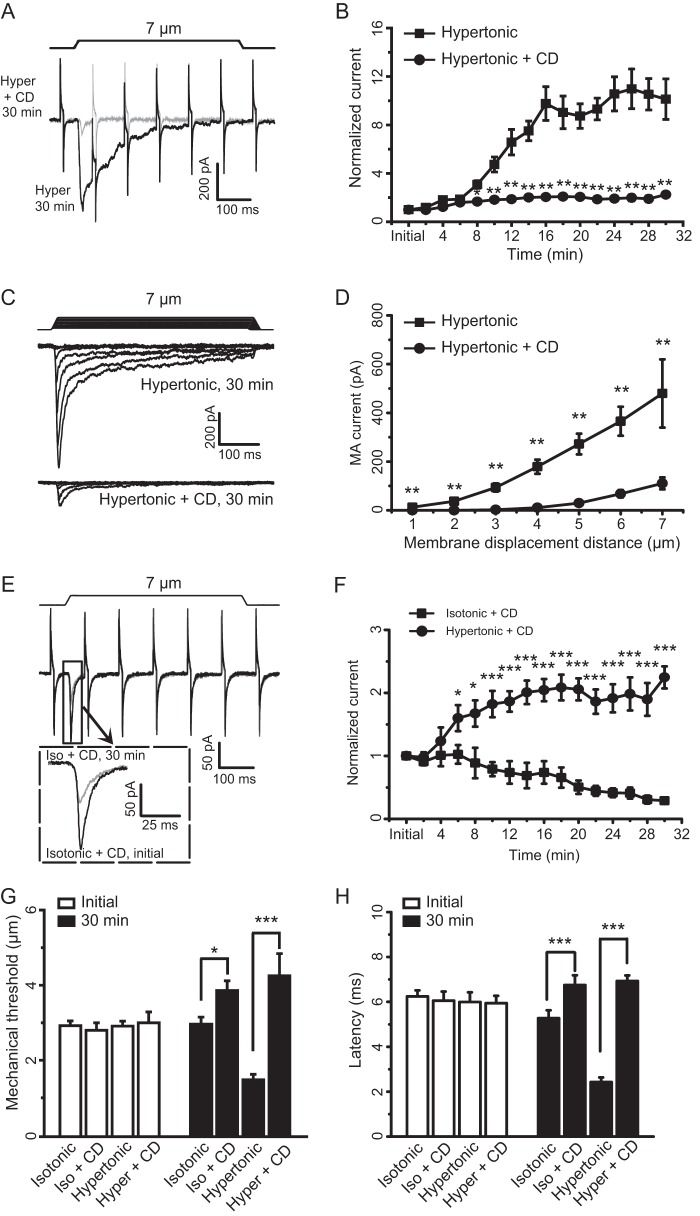
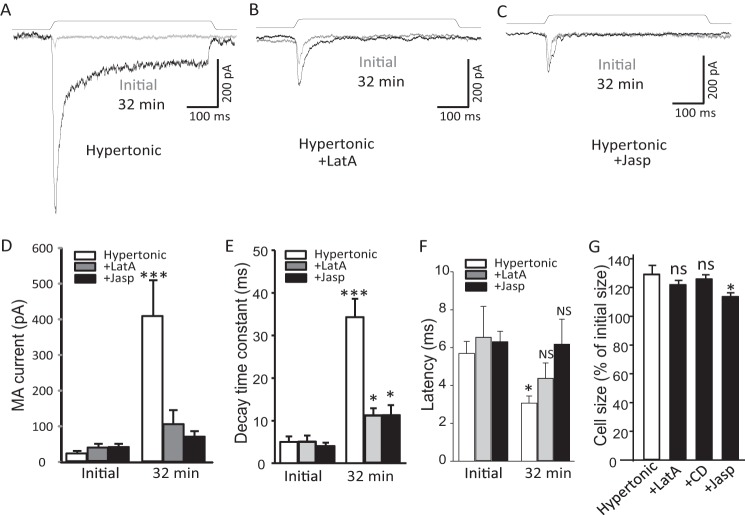
To further elucidate that the increases of SPMT by osmotic swelling are required for the mechanical sensitization, we included 10 μm CD in our hypertonic recording internal solution to prevent the increases of SPMT and examined RA-MA currents. Although RA-MA current amplitude increased more than 10-fold (n = 14) after 16 min with hypertonic recording internal solution alone, the potentiation was almost completely abolished when CD was included in the hypertonic recording internal solution (Fig. 7, A and B, n = 8). The effect of CD on osmotic swelling-induced RA-MA current potentiation was observed at different mechanical stimulation intensities tested from membrane displacement of 1–7 μm (n = 7, Fig. 7, C and D).We further determined whether RA-MA currents recorded using isotonic recording internal solution were also dependent on intact actin filaments in cultured DRG neurons. As shown in Fig. 7, E and F, when CD was included in the isotonic recording internal solution, RA-MA current amplitude was gradually reduced after establishing the whole-cell mode. For example, RA-MA currents were 80% of control at 10 min (n = 9), 50% of control at 20 min (n = 9), and 30% of control at 30 min (n = 9) after establishing the whole-cell mode (Fig. 7F). We analyzed the effects of CD on mechanical threshold for eliciting RA-MA currents. At the initial time after establishing the whole-cell mode, mechanical thresholds were not significantly different among the four experimental groups that used the following: (i) isotonic recording internal solution (n = 50); (ii) isotonic recording internal solution with CD (n = 10); (iii) hypertonic recording internal solution (n = 33); or (iv) hypertonic internal solution with CD (n = 9). Thirty minutes after establishing the whole-cell mode, mechanical threshold recorded with isotonic recording internal solution that contained CD (3.86 ± 0.26 μm, n = 7) was significantly higher than that recorded with isotonic recording internal solution that did not include CD (2.96 ± 0.19 μm, n = 26, p < 0.05) (Fig. 7G). Similarly, 30 min after establishing the whole-cell mode, mechanical threshold recorded using hypertonic recording internal solution with CD (4.25 ± 0.17 μm, n = 8) was significantly higher than that recorded using hypertonic recording internal solution without CD (1.48 ± 0.15 μm, n = 26, p < 0.001, Fig. 7G). We also analyzed the effect of CD on the onset latency of RA-MA currents. At the initial time after establishing the whole-cell mode, onset latencies were not significantly different among the four experimental groups using the following: (i) isotonic recording internal solution (n = 14); (ii) isotonic recording internal solution with CD (n = 7); (iii) hypertonic recording internal solution (n = 14); or (iv) hypertonic internal solution with CD (n = 7). Thirty minutes after establishing the whole-cell mode, onset latency recorded using isotonic recording internal solution with CD (6.75 ± 0.43 ms, n = 6) was significantly longer than that recorded using isotonic recording internal solution without CD (5.28 ± 0.35 ms, n = 14, p < 0.001) (Fig. 7H). Similarly, 30 min after establishing the whole-cell mode, onset latency recorded using hypertonic recording internal solution with CD (6.93 ± 0.25 ms, n = 6) was significantly higher than that recorded using hypertonic recording internal solution without CD (2.43 ± 0.21 ms, n = 14, p < 0.001) (Fig. 7H). We tested LatA, which disrupts actin filament by a mechanism different from that of CD, on RA-MA potentiation induced by osmotic swelling (Fig. 8). Although osmotic swelling significantly increased RA-MA current amplitude and decay time constant as well as shortened latency (n = 9, Fig. 8, A, B, and D–F), the effects of osmotic swelling on RA-MA currents were significantly diminished by LatA (n = 9, Fig. 8, A and B, D--F). We also tested Jasp, which affects cytoskeleton functions by stabilizing actin filaments, on RA-MA potentiation induced by osmotic swelling (Fig. 8). Interestingly, Jasp had similar effects as LatA on osmotic swelling-induced changes in RA-MA current amplitude, decay time constant, and latency (n = 12) (Fig. 8, A–C and D–F). We also examined effects of CD, LatA, and Jasp on osmotic swelling. The degree of osmotic swelling was significantly reduced by Jasp but not by LatA and CD (Fig. 8G).
FIGURE 7.
Disruption of actin filaments by cytochalasin D abolishes osmotic swelling-induced potentiation of RA-MA currents in cultured DRG neurons. A, two sample traces show RA-MA currents recorded 30 min after establishing the whole-cell mode using hypertonic recording internal solution without (black) or with 10 μm cytochalasin D (CD, gray). The membrane displacement step was 7 μm. B, summary data of RA-MA currents over time after establishing the whole-cell mode using hypertonic recording internal solution either without (squares, n = 14) or with 10 μm CD (circles, n = 8). C, two sets of sample traces of RA-MA currents elicited by different membrane displacement steps and recorded 30 min after establishing the whole-cell mode. The recordings were done by using hypertonic recording internal solution either without (top) or with 10 μm CD (bottom). Membrane displacement steps at 1-μm increments are indicated above RA-MA current traces. D, summary data of the experiments represented in C using hypertonic recording internal solution either without (squares, n = 7) or with 10 μm CD (circles, n = 7). E, two sample traces show RA-MA currents recorded at initial time (black) and 30 min (gray) after establishing the whole-cell mode using isotonic recording internal solution that also contained 10 μm CD. Inset, expanded scale for the boxed region. The membrane displacement step was 7 μm. F, summary data of RA-MA currents over time for the experiments represented in E (squares, n = 9). RA-MA currents that were recorded with hypertonic recording internal solution containing 10 μm CD (n = 9) are also presented in this graph for a comparison. G and H, summary data of mechanical thresholds (G) and onset latency (H) for eliciting RA-MA currents at initial time (open bars) and 30 min (closed bars) after establishing the whole-cell mode. Mechanical thresholds (G) and latency (H) were obtained by recordings made with the following recording internal solutions: isotonic internal solution (isotonic), isotonic internal solution that contained 10 μm CD (iso + CD), hypertonic internal solution (hypertonic), and hypertonic internal solution that contained 10 μm CD (hyper + CD). Data represent Mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FIGURE 8.
Osmotic swelling-induced potentiation of RA-MA currents is diminished by latrunculin A and jasplakinolide. A–C, three sets of sample traces show RA-MA currents evoked by membrane displacements at initial time (gray trace in each panel) and 32 min after establishing the whole-cell mode (black trace in each panel). The experiments were performed with hypertonic recording internal solution without (control, A), with 5 μm LatA (B), or with 5 μm Jasp (C). D–F, summary data of the experiments illustrated in A–C show the effects of LatA or Jasp on osmotic swelling-induced changes in RA-MA current amplitude (D), decay time constant (E), and latency (F). D–F, open bars, control (n = 9); gray bars, recording internal solution contained 5 μm latrunculin A (n = 9); black bars, recording internal solution contained 5 μm jasplakinolide (n = 12). The recordings at initial time and 32 min after establishing the whole-cell mode are indicated in each bar graph. G, changes in cell size 32 min after establishing the whole-cell mode with hypertonic recording internal solution without (n = 9), with the addition of Lat A (n = 9), or CD (n = 6), or Jasp (n = 12). Cell sizes are normalized to their original sizes measured at initial time after establishing the whole-cell mode. Data represent mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS, no significant different.
We determined the reversibility of both SPMT and RA-MA currents of DRG neurons. In this set of experiments, isotonic internal recording solution was used in patch clamp recording electrode, and we induced osmotic swelling in cultured DRG neurons by extracellular hypotonic bath solution and then performed recovery experiments by returning cells in isotonic bath solution after the osmotic swelling (Fig. 9, A–C). As shown in Fig. 9, A and B, hypotonic bath solution produced osmotic swelling over time (Fig. 9B), which was accompanied by a significant increase in SPMT (Fig. 9C). For example, cell diameters increased to ∼130% (n = 7, p < 0.001) of their initial sizes following the bath application of hypotonic solution for 16 min (Fig. 9B); SPMT increased from 0.022 ± 0.001 mN/m to 0.044 ± 0.005 mN/m (n = 6, p < 0.001, Fig. 9C). When cells were returned to isotonic bath solution, cell sizes were rapidly restored to near their initial sizes within 4 min (n = 7, Fig. 9, A and B), and SPMT was reduced to 0.014 ± 0.002 mN/m (n = 6, p < 0.01, Fig. 9C). Consistent with the increases in SPMT (Fig. 9C), bath application of hypotonic solution potentiated RA-MA currents (Fig. 9, D and E) and slowed RA-MA current kinetics (Fig. 9F) significantly over time. For example, RA-MA current amplitudes were increased 7-fold (n = 7, p < 0.01) following the application of hypotonic solution for 12 min (Fig. 9E). RA-MA current decay time constant was prolonged from ∼6 ms (n = 7) in isotonic bath solution to ∼90 ms (n = 7, p < 0.01) 16 min following the application of hypotonic solution (Fig. 9, D and F). The potentiated RA-MA currents in hypotonic extracellular solution were restored to near original amplitude (n = 7, Fig. 9, D and E) and kinetics (n = 5, Fig. 9, D and F) in 4 min after returning cells in isotonic bath solution, a condition that reduced SPMT (Fig. 9C).
FIGURE 9.
Osmotic swelling, static plasma membrane tension, and RA-MA currents can be rapidly reversed in isotonic condition. A, images show osmotic swelling of a DRG neuron by extracellular application of a hypotonic solution (220 mosm). Left, in isotonic bath; middle, following the application of hypotonic solution for 16 min; right, after a 4-min recovery in isotonic bath. B, summary data of the time course of osmotic swelling-induced changes of cell sizes following extracellular hypotonicity and recovery in isotonic solution (black circles, n = 7). C, summary data of the changes of SPMT induced by extracellular hypotonicity (Hypo) and recovery in isotonic solution (Iso) (black circles, n = 7). SPMT was also measured in isotonic solution over the same time period (black squares, n = 7). D, sample traces of RA-MA currents at initial time in isotonic bath (black), 16 min following the extracellular application of hypotonic solution (blue), and following a 4-min recovery in isotonic bath solution (red). E, summary of the increases of RA-MA currents over time from isotonic solution (black, n = 7) and following bath application of hypotonic solution (blue, n = 7) and restoration of RA-MA currents following the bath application of isotonic bath solution (red, n = 7). F, summary data of the decay time constants at initial time in isotonic bath (left, n = 7), 16 min following the application of the hypotonic solution (middle, n = 7), and following a 4-min recovery in isotonic bath solution (right, n = 5). The membrane displacement is 7 μm in E and F. G, sample traces of RA-MA currents evoked by different membrane displacement steps before and following the bath application of hypotonic solution for 16 min. H, summary data of the experiments illustrated in G in isotonic bath (black squares, n = 7) and hypotonic bath (red circles, n = 7). I, mechanical thresholds for evoking RA-MA currents in isotonic bath (open bars, n = 10) and following the application of hypotonic solution for 16 min (solid bars, n = 7). J, latency for RA-MA current onset in isotonic bath solution (open bars, n = 10) and following the application of hypotonic solution for 16 min (solid bars, n = 6). Data represent mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001.
We determined the effect of extracellular hypotonicity on RA-MA currents elicited by different mechanical stimulation intensity. Different membrane displacement steps were applied to cells to elicit RA-MA currents before (in isotonic solution) and 16 min following the application of hypotonic solution extracellularly. As shown in Fig. 9, G and H, for membrane displacement steps tested from 1 to 7 μm, RA-MA currents elicited following 16 min of osmotic swelling were significantly larger than initial RA-MA currents recorded in isotonic bath solution. For example, at 7-μm membrane displacement step, RA-MA currents were 47.1 ± 6.4 pA (n = 7) recorded in isotonic solution and increased to 368.3 ± 84.6 pA (n = 7, p < 0.01) after 16 min osmotic swelling in hypotonic extracellular solution. Osmotic swelling by extracellular hypotonicity resulted in a significant reduction of mechanical threshold. Mechanical threshold was 2.9 ± 0.3 μm (n = 10) in isotonic solution and was reduced by ∼50% (n = 7, p < 0.001) following osmotic swelling for 16 min (Fig. 9I). Osmotic swelling by extracellular hypotonicity also resulted in a significant reduction of RA-MA current onset latency from 5.4 ± 0.2 ms (n = 10) in isotonic solution to 1.7 ± 0.3 ms (n = 6, p < 0.001) following osmotic swelling for 16 min (Fig. 9J).
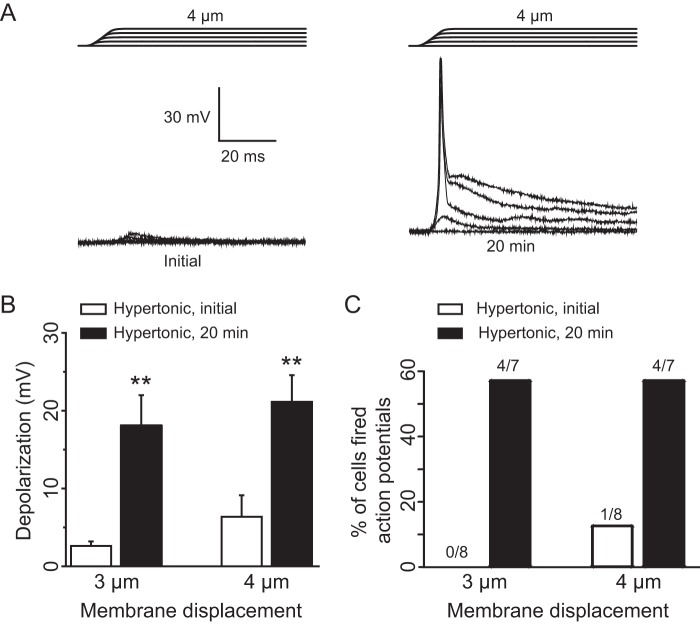
We determined whether osmotic swelling can increase mechano-excitability of primary afferent neurons such that these neurons can be excited to fire action potentials at lower mechanical stimulation intensity. As shown in Fig. 10, A–C, at the initial time after establishing the whole-cell mode, membrane displacement resulted in small membrane depolarization (2.6 ± 0.6 mV at 3 μm, n = 8, Fig. 10B; 6.4 ± 2.8 mV at 4 μm, n = 8, Fig. 10, A and B). Action potential firing was not elicited in all eight cells tested by 3 μm displacement and was elicited only in one cell out of eight cells tested by 4 μm displacement (Fig. 10C). In contrast, for the same cells 20 min after osmotic swelling using hypertonic recording internal solution, there was a large increase of membrane depolarization to 18.1 ± 3.9 mV by 3 μm membrane displacement (n = 7) and to 21.1 ± 3.4 mV by 4 μm membrane displacement (n = 7) (Fig. 10, A and B), and more than 50% of cells (4/7) fired action potentials in responses to membrane displacement at 3 or 4 μm (Fig. 10, A and C). Osmotic swelling slightly increased resting membrane potentials in some cells, which was unlikely to be a main factor contributing to the enhanced mechano-excitability.
FIGURE 10.
Osmotic swelling increases mechano-excitability of cultured DRG neurons. A, sample traces of membrane depolarization recorded from a DRG neuron following membrane displacements. The trace on the left is the membrane response at initial time after establishing the whole-cell mode, and the trace on the right is the membrane response recorded 20 min later. Membrane displacement distances were 1–4 μm in a 1-μm increment (indicated above the recording traces). B, summary data of the membrane depolarization at the membrane displacement of 3 (left) and 4 μm (right). Open bars are responses at initial time (n = 8) after establishing the whole-cell mode, and solid bars are responses at 20 min later (n = 7). C, percent of cells firing action potentials in response to membrane displacement at 3 and 4 μm. Open bars are responses at initial time after establishing the whole-cell mode and solid bars are at 20 min later. The fractions of cells that fired action potentials are indicated above each bar. **, p < 0.01.
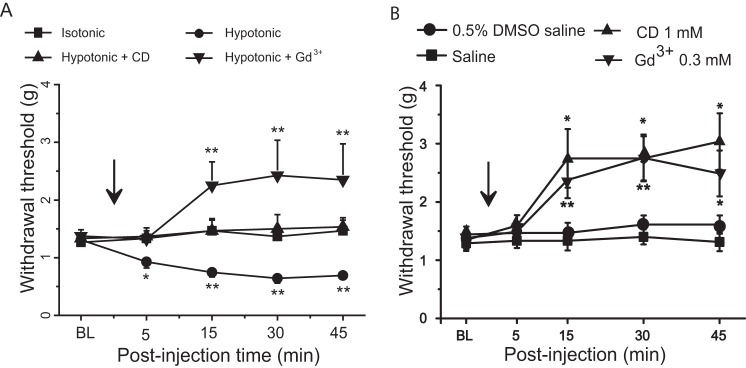
We determined whether osmotic disturbance may lead to behavioral mechanical hypersensitivity in vivo (Fig. 11A). The von Frey test was used to measure hind paw withdrawal threshold following a subcutaneous injection of a hypotonic saline solution (0.45% NaCl, 155 mosm, 100 μl) into the hind paws (Fig. 11A). The hind paw withdrawal thresholds were reduced several minutes after the injection of hypotonic saline solution and were kept at lower thresholds for up to 45 min, the longest time tested. In contrast, there was no significant change in the withdrawal thresholds when isotonic saline solution (0.9% NaCl, 310 mosm) was subcutaneously administered into the hind paws. We determined whether CD, which abolished osmotic swelling-induced potentiation of RA-MA currents in DRG neurons, might alleviate hypotonic saline-induced mechanical hypersensitivity in vivo. This was done by subcutaneous co-injection of CD and hypotonic solution into the hind paws prior to measurement of hind paw withdrawal threshold. As shown in Fig. 11A, the withdrawal threshold was not reduced by hypotonic solution when CD (1 mm) was co-administered. Furthermore, when hypotonic saline solution was injected together with Gd3+ (0.3 mm), a compound that blocks Piezo2 channels, hind paw withdrawal threshold was heightened rather than reduced (Fig. 11A). We also determined whether administration of CD or Gd3+ may directly increase mechanical thresholds (i.e. mechanical hyposensitivity) in animals when these compounds were made in isotonic solution. As shown in Fig. 11B, although injection of isotonic vehicles (0.5% DMSO in saline, n = 9; saline, n = 7) did not have significant effects on hind paw withdrawal threshold, injection of CD (1 mm, n = 9) or Gd3+ solutions (0.3 mm, n = 9) resulted in a significant increase in the hind paw withdrawal threshold (Fig. 11B).
FIGURE 11.
Extracellular hypotonicity induces behavioral mechanical hypersensitivity. A, mechanical withdrawal thresholds measured in rat hind paws using the von Frey test before and following hind paw injection of 100 μl of the following solutions: an isotonic saline solution (0. 9%, 310 mosm, squares, n = 8); a hypotonic saline solution (0.45%, 155 mosm, circles, n = 8); a hypotonic saline solution (155 mosm) containing 1 mm cytochalasin D (triangles, n = 8); and a hypotonic saline solution (155 mosm) containing 0.3 mm Gd3+ (reversed triangles, n = 8). B, mechanical withdrawal thresholds measured in rat hind paws using the von Frey test before and following hind paw injection of 100 μl of the following solutions: an isotonic saline solution (squares, n = 7); a 0.5% DMSO in saline (circles, n = 9); 1 mm CD in 0.5% DMSO saline (CD, triangles, n = 9); 0.3 mm Gd3+ in saline solution (Gd3+, reversed triangles, n = 9). BL, baseline measured before the injections. An arrow indicates the time when the solutions are injected into the hind paws. Data represent mean ± S.E., *, p < 0.05; **, p < 0.01.
Discussion
In this study, we show that RA-MA currents in primary afferent neurons are greatly potentiated, their kinetics slowed by osmotic swelling, and the effect of osmotic swelling is via the increase of SPMT. We further demonstrate that the sensitization of primary afferent mechanotransduction requires intact actin filaments in primary afferent neurons to maintain a high level of SPMT. RA-MA currents in cultured DRG neurons are mediated by Piezo2 channels (6, 14), and in our study RA-MA currents in HEK293 cells heterologously expressing Piezo2 channels are also greatly potentiated and their kinetics slowed by osmotic swelling, suggesting that Piezo2 channels in cultured DRG neurons are regulated by osmotic swelling via SPMT. Our behavioral outcomes suggest that the sensitization of mechanotransduction may account for behavioral mechanical hypersensitivity induced by subcutaneous injection of hypotonic solution.
In our study, osmotic swelling produces enormous enhancement of RA-MA current amplitude and switches its rapidly adapting kinetics to slowly adapting type. This potentiation is much greater than the enhancement of RA-MA currents shown previously following the treatment of primary afferent neurons with inflammatory mediators or protein kinase C activators (12, 13). Previous studies have shown that functions of Piezo2 channels can be up-regulated through intracellular signaling pathways, including protein kinase A, protein kinase C, and EPAC1 (12, 13, 15), raising the possibility that osmotic swelling-induced sensitization of RA-MA channels in our study may also be mediated by these intracellular signaling pathways. Osmotic swelling may activate TRPV4 channels (28) to increase intracellular Ca2+ concentrations, which may subsequently activate intracellular second messenger systems to up-regulate RA-MA channel functions. However, this possibility is discounted by our finding that potentiation of RA-MA currents by osmotic swelling is not affected when intracellular Ca2+ was chelated by the fast Ca2+ chelator BAPTA. Furthermore, RA-MA currents are not affected by the TRPV4 activator GSK1016790A.
We show that osmotic swelling-induced potentiation of RA-MA currents requires the increase of SPMT. To our knowledge, this study is the first to quantitatively determine the SPMT of mammalian somatosensory neurons and to directly relate this biophysical property to the functions of mechanically activated channels in cultured DRG neurons. SPMT of cultured DRG neurons is measured to be 0.024 mN/m under isotonic conditions and is increased to 0.040 mN/m following osmotic swelling for 30 min. These values are close to the SPMT of molluscan neurons under isotonic conditions and following osmotic swelling (20). Our results indicate that a small change of SPMT (less than 2-fold increase) can result in a large potentiation of RA-MA currents (10-fold increase) and also lead to a switch of RA-MA current kinetics from rapidly adapting to a slowly adapting type. Osmotic swelling-induced potentiation of RA-MA currents in our study is abolished when actin filaments are disrupted by CD and LatA. Actin filament is a major cytoskeleton component essential for maintaining SPMT of a cell (19), and disruption of actin filaments would substantially reduce SPMT of a cell. Indeed, CD not only reduces SPMT in our cultured DRG neurons under isotonic conditions, but also prevents the SPMT from increases by osmotic swelling. The results with CD in this study further support the idea that SPMT has a profound impact on mechanical sensitivity in primary afferent neurons. Our results with SPMT measurement suggest that the kinetics of RA-MA currents is also impacted by SPMT. The rapid recovery of both RA-MA current amplitude and kinetics when cells return to the isotonic condition further supports the idea that the effects of osmotic swelling on RA-MA currents are mainly due to the change of SPMT.
We show that cultured DRG neurons increase their sizes by about 40% under our osmotic swelling conditions. However, a flat plasma membrane can only tolerate an ∼3% increase in area before lysis (19). The 40% increase of DRG neuron size in our study suggests that primary afferent neurons may have membrane domains hidden from the cell surface. Hidden membrane domains have been observed in other cell types in the forms of caveolae, vacuole-like dilations, and membrane ruffles (19, 20, 27, 29). Studies have revealed that caveolae are specialized lipid rafts that are important for harboring ion channels and regulating their functions (30). There is a possibility that Piezo2 channels also mainly reside in these membrane domains and are kept in a configuration less sensitive to mechanical stimulation by a rapid membrane displacement. However, high SPMT may expose these hidden Piezo2 channels to the cell surface. This idea is supported by our evidence showing the increased channel numbers rather than channel conductance of RA-MA channels following osmotic swelling by using nonstationary fluctuation analysis. In addition, translocation of RA-MA channels to plasma membranes was also suggested by the changes of Piezo2 immunoreactivity on cultured DRG neurons following osmotic swelling. This translocation may prime RA-MA channels to have lower energy barriers for channel opening by a rapid membrane displacement.
We show that hypotonic solutions reduce mechanical threshold in hind paws, a result consistent with a previous study performed by application of hypotonic solution to the dura (31). An interpretation of our behavioral outcomes is that hypotonic solution leads to the potentiation of Piezo2 mechanotransduction in vivo as well. However, hypotonic solution may also activate other ion channels such as TRPV4 on primary afferent nerves to affect mechanical sensitivity (28, 31, 32). Therefore, the potentiation of Piezo2 mechanotransduction by osmotic swelling may only partially contribute to the behavioral mechanical hypersensitivity following the injection of hypotonic solution. In our study, we show that behavioral mechanical hypersensitivity induced by hypotonic solution is abolished by CD. This is consistent with our in vitro study showing that CD prevents osmotic swelling-induced potentiation of RA-MA currents. Interestingly, a previous study also shows that CD alleviates mechanical hyperalgesia in an animal model of inflammatory pain (33). In addition, we show that mechanical hypersensitivity induced by hypotonic solution is abolished by Gd3+, a blocker of Piezo2 channels (6, 8). These findings support the idea that sensitization of Piezo2 mechanotransduction at least partially contributes to the behavioral mechanical hypersensitivity induced by hypotonic solution in our in vivo experiments.
One question about our findings is whether the potentiation of mechanotransduction shown in our study may have any physiological and/or pathological implications. Unless under extreme conditions, such as exposure of wounded skin and ulcerated oral mucosa tissues directly to water, the osmotic environment surrounding primary afferents is unlikely to be disturbed to the degree similar to our experimental condition. Interestingly, two previous studies show primary afferent axon swelling to result in 18–21% increases in the axonal cross-sectional area in vincristine-induced neuropathy in rats (34, 35). It will be interesting to study in the future whether Piezo2-mediated mechanotransduction may be sensitized under these conditions. However, in addition to osmotic swelling, SPMT can be altered by biological factors that affect acting filament network, modify the interactions between acting filaments and molecules in plasma membranes (e.g. phosphoinositides), and change metabolic states of membrane lipids (36, 37). It will be interesting to study in the future whether the biological factors that change SPMT may significantly affect Piezo2-mediated mechanotransduction in primary afferents to contribute to the alternation of mechanical sensations under pathological conditions.
Author Contributions
Z. J. conducted most of the experiments and analyzed the results. R. I., J. L., and V. K. conducted some experiments. J. G. G. conceived the idea for the project and wrote the paper.
Acknowledgments
We thank Jennifer DeBerry for comments on an earlier version of this manuscript and A. Patapoutian for providing mouse Piezo2 plasmid.
This work was supported in whole or part by National Institute of Health Grants R01 DE018661 and R01 DE023090 (to J. G. G.), a scholarship from Nature Science Foundation of China 81571080 (to Z. J.), and Hebei Province of China Grant H2015206240 (to Z. J.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- DRG
- dorsal root ganglia
- CD
- cytochalasin d
- LatA
- latrunculin A
- Jasp
- jasplakinolide
- SPMT
- static plasma membrane tension
- RA-MA
- rapidly adapting mechanically activated currents
- HSPC
- high speed pressure clamp
- SCG
- superior cervical ganglion
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- N
- newton.
References
- 1. McCarter G. C., Reichling D. B., and Levine J. D. (1999) Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci. Lett. 273, 179–182 [DOI] [PubMed] [Google Scholar]
- 2. Drew L. J., Wood J. N., and Cesare P. (2002) Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci. 22, RC228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu J., and Lewin G. R. (2006) Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J. Physiol. 577, 815–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coste B., Crest M., and Delmas P. (2007) Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J. Gen. Physiol. 129, 57–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jia Z., Ling J., and Gu J. G. (2012) Temperature dependence of rapidly adapting mechanically activated currents in rat dorsal root ganglion neurons. Neurosci. Lett. 522, 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coste B., Mathur J., Schmidt M., Earley T. J., Ranade S., Petrus M. J., Dubin A. E., and Patapoutian A. (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCarter G. C., and Levine J. D. (2006) Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Mol. Pain 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikeda R., Cha M., Ling J., Jia Z., Coyle D., and Gu J. G. (2014) Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 157, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maksimovic S., Nakatani M., Baba Y., Nelson A. M., Marshall K. L., Wellnitz S. A., Firozi P., Woo S. H., Ranade S., Patapoutian A., and Lumpkin E. A. (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woo S. H., Ranade S., Weyer A. D., Dubin A. E., Baba Y., Qiu Z., Petrus M., Miyamoto T., Reddy K., Lumpkin E. A., Stucky C. L., and Patapoutian A. (2014) Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ranade S. S., Woo S. H., Dubin A. E., Moshourab R. A., Wetzel C., Petrus M., Mathur J., Bégay V., Coste B., Mainquist J., Wilson A. J., Francisco A. G., Reddy K., Qiu Z., Wood J. N., et al. (2014) Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Castro A., Drew L. J., Wood J. N., and Cesare P. (2006) Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc. Natl. Acad. Sci. U.S.A. 103, 4699–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubin A. E., Schmidt M., Mathur J., Petrus M. J., Xiao B., Coste B., and Patapoutian A. (2012) Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2, 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia Z., Ikeda R., Ling J., and Gu J. G. (2013) GTP-dependent run-up of Piezo2-type mechanically activated currents in rat dorsal root ganglion neurons. Mol. Brain 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eijkelkamp N., Linley J. E., Torres J. M., Bee L., Dickenson A. H., Gringhuis M., Minett M. S., Hong G. S., Lee E., Oh U., Ishikawa Y., Zwartkuis F. J., Cox J. J., and Wood J. N. (2013) A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 4, 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reeves D., Ursell T., Sens P., Kondev J., and Phillips R. (2008) Membrane mechanics as a probe of ion-channel gating mechanisms. Phys. Rev. E Stat. Nonlin. Soft. Matter Phys. 78, 041901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diz-Muñoz A., Fletcher D. A., and Weiner O. D. (2013) Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 23, 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paoletti P., and Ascher P. (1994) Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron 13, 645–655 [DOI] [PubMed] [Google Scholar]
- 19. Morris C. E., and Homann U. (2001) Cell surface area regulation and membrane tension. J. Membr. Biol. 179, 79–102 [DOI] [PubMed] [Google Scholar]
- 20. Dai J., Sheetz M. P., Wan X., and Morris C. E. (1998) Membrane tension in swelling and shrinking molluscan neurons. J. Neurosci. 18, 6681–6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsuzuki K., Xing H., Ling J., and Gu J. G. (2004) Menthol-induced Ca2+ release from presynaptic Ca2+ stores potentiates sensory synaptic transmission. J. Neurosci. 24, 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ikeda R., and Gu J. G. (2014) Piezo2 channel conductance and localization domains in Merkel cells of rat whisker hair follicles. Neurosci. Lett. 583, 210–215 [DOI] [PubMed] [Google Scholar]
- 23. Hochmuth R. M. (2000) Micropipette aspiration of living cells. J. Biomech. 33, 15–22 [DOI] [PubMed] [Google Scholar]
- 24. Oh M. J., Kuhr F., Byfield F., and Levitan I. (2012) Micropipette aspiration of substrate-attached cells to estimate cell stiffness. J. Vis. Exp. 67, 3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saville D. J. (2003) Basic statistics and the inconsistency of multiple comparison procedures. Can. J. Exp. Psychol. 57, 167–175 [DOI] [PubMed] [Google Scholar]
- 26. Saville D. J. (1990) Multiple comparison procedures: the practical solution. Am. Stat. 44, 174–180 [Google Scholar]
- 27. Pietuch A., Brückner B. R., and Janshoff A. (2013) Membrane tension homeostasis of epithelial cells through surface area regulation in response to osmotic stress. Biochim. Biophys. Acta 1833, 712–722 [DOI] [PubMed] [Google Scholar]
- 28. Liedtke W. (2005) TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J. Physiol. 567, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinha B., Köster D., Ruez R., Gonnord P., Bastiani M., Abankwa D., Stan R. V., Butler-Browne G., Vedie B., Johannes L., Morone N., Parton R. G., Raposo G., Sens P., Lamaze C., and Nassoy P. (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dart C. (2010) Lipid microdomains and the regulation of ion channel function. J. Physiol. 588, 3169–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei X., Edelmayer R. M., Yan J., and Dussor G. (2011) Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 31, 1595–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alessandri-Haber N., Dina O. A., Chen X., and Levine J. D. (2009) TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 29, 6217–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dina O. A., McCarter G. C., de Coupade C., and Levine J. D. (2003) Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron 39, 613–624 [DOI] [PubMed] [Google Scholar]
- 34. Tanner K. D., Levine J. D., and Topp K. S. (1998) Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J. Comp. Neurol. 395, 481–492 [PubMed] [Google Scholar]
- 35. Topp K. S., Tanner K. D., and Levine J. D. (2000) Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J. Comp. Neurol. 424, 563–576 [PubMed] [Google Scholar]
- 36. Gilden J., and Krummel M. F. (2010) Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton 67, 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsujita K., and Itoh T. (2015) Phosphoinositides in the regulation of actin cortex and cell migration. Biochim. Biophys. Acta 1851, 824–831 [DOI] [PubMed] [Google Scholar]