Abstract
The target of rapamycin (TOR) kinase is found at the core of two evolutionarily conserved complexes known as TOR complexes 1 and 2 (TORC1 and TORC2). In fission yeast, TORC2 is dispensable for proliferation under optimal growth conditions but is required for starvation and stress responses. We have previously reported that loss of function of TORC2 renders cells highly sensitive to DNA replication stress; however, the mechanism underlying this sensitivity is unknown. TORC2 has one known direct substrate, the kinase Gad8, which is related to AKT in human cells. Here we show that both TORC2 and its substrate Gad8 are found in the nucleus and are bound to the chromatin. We also demonstrate that Gad8 physically interacts with the MluI cell cycle box-binding factor (MBF) transcription complex that regulates the G1/S progression and the response to DNA stress. In mutant cells lacking TORC2 or Gad8, the binding of the MBF complex to its cognate promoters is compromised, and the induction of MBF target genes in response to DNA replication stress is reduced. Consistently, the protein levels of Cdt2 and Cig2, two MBF target genes, are reduced in the absence of TORC2-Gad8 signaling. Taken together, our findings highlight critical functions of TORC2 in the nucleus and suggest a role in surviving DNA replication stress via transcriptional regulation of MBF target genes.
Keywords: cell cycle, DNA binding protein, DNA damage response, target of rapamycin (TOR), yeast, Gad8, MBF, S. pombe
Introduction
Target of rapamycin (TOR)2 is an atypical protein kinase that was isolated as the target of the immunosuppressive and anticancer drug rapamycin. TOR proteins play a central role in growth, proliferation, and survival and can be found in two distinct and evolutionarily conserved complexes, TORC1 and TORC2 (1–3). The two TOR complexes are key regulators of cellular growth; however, they respond to different stimuli and phosphorylate distinct sets of protein substrates (1–3). Human cells contain a single TOR protein, known as mTOR, which functions as the catalytic subunit of both mTORC1 and mTORC2. In the fission yeast, Schizosaccharomyces pombe, there are two genes encoding for TOR proteins. Tor1 interacts with Ste20 (Rictor in human cells) and Sin1 (mSin1 in human cells) to form TORC2, whereas Tor2 interacts with Mip1 (Raptor in human cells) to form TORC1 (4). TORC1 is essential for growth in S. pombe and plays a critical role in regulating the switch between growth and sexual development in response to nitrogen starvation. Consistently, disruption of S. pombe TORC1 results in many features characteristic of nitrogen-starved cells (5–8), and TORC1-dependent phosphorylation of the small ribosomal protein Rps6 is diminished under nitrogen starvation (9).
S. pombe TORC2 is not essential for growth but is required for cell survival under a wide variety of stress conditions, including high or low temperatures, oxidative or osmotic stress, and DNA damage or replication stresses (10, 11). TORC2 is also essential for entrance into a stationary (G0-like) phase and sexual development, two processes that occur in response to nutritional starvation. Under normal growth conditions, TORC2 plays a role in regulating the timing of mitosis (12), amino acid uptake (13), gene silencing, and telomere length maintenance (11). Interestingly, most if not all of the cellular functions of S. pombe TORC2 are mediated via phosphorylation and activation of its downstream substrate Gad8, an AGC (PKA, PKG, and PKC kinases)-like kinase with structural and functional similarities to AKT, the most well established substrate kinase of mTORC2 (11, 14, 15). Although S. pombe TORC1 is responsive to the availability of nitrogen or amino acids (5–8, 16), we (17) and the Shiozaki and co-workers (18) have recently shown that S. pombe TORC2 responds to the availability of glucose. Upon glucose withdrawal or shift from glucose to a less favorable carbon source such as glycerol, there is a reduction of TORC2-dependent phosphorylation of Gad8 at serine 546 (equivalent to serine 473 in AKT) and concomitant reduced Gad8 kinase activity (17, 18). Thus, S. pombe TORC1 and TORC2 play essential roles in regulating growth by responding to two distinct key nutritional cues.
As mentioned above, our functional analysis of cells lacking TORC2 or Gad8 indicated a role for TORC2-Gad8 in the survival under DNA damage and replication stresses (10, 11). In the absence of TORC2 or Gad8, cells are hypersensitive to hydroxyurea (HU), methyl methanesulfonate (MMS), or camptothecin. Disruption of S. pombe TORC2 signaling does not affect Rad3 (ATR)-dependent Chk1 phosphorylation and activation, and cells lacking TORC2 or Gad8 transiently arrest cell cycle progression in response to DNA damage (10, 11). However, TORC2-Gad8 mutants accumulate high levels of DNA damage sites (Rad52 foci), suggesting a defect in DNA damage repair or elevated levels of DNA fork replication stalling (10). Interestingly, accumulation of DNA damage sites and sensitivity to DNA-damaging conditions were also observed in TORC2 mutant cells in the distantly related yeast Saccharomyces cerevisiae, suggesting that a TORC2 function in DNA damage tolerance is conserved in evolution (19, 20).
One of the hallmarks of the DNA replication checkpoint is the transcriptional up-regulation of genes that allow cells to adapt to replication stress. In S. pombe, these genes are composed of the same set of genes that are normally required for DNA synthesis and the G1/S transition (21). This set includes ∼20 genes that are subjected to transcriptional regulation by the MluI cell cycle box-binding factor (MBF) complex (22). The MBF complex contains the essential core subunit, Cdc10, and at least two other proteins, Res1 and Res2 (23). S. pombe MBF has a functional homologue in S. cerevisiae that is also referred to as MBF and contains the Swi6 and Mbp1 proteins (24). A mammalian functional homologue also exists and is named E2F (25). The MBF complex is subjected to positive and negative regulation that is crucial for restricting transcriptional activation at the G1/S transition. The fission yeast MBF complex is bound to its target promoters throughout the cell cycle (26). Activation during replication stress is achieved by down-regulation of the co-repressor Nrm1 (27) and the repressor Yox1 (28–30). In response to replicative stress, such as the presence of HU, Rad3 phosphorylates and activates Cds1 (Chk2 in human cells), which in turn phosphorylates the MBF transcriptional repressor Yox1, thereby inhibiting the binding of Yox1 to the MBF complex and allowing up-regulation of genes required for DNA replication (31). More recently, it was demonstrated that upon DNA damage, such as high levels of MMS, Chk1 is activated and phosphorylates Cdc10, leading to its release from targeted promoters and resulting in down-regulation of DNA replication genes, possibly to prevent progression into S phase until the DNA damage is repaired (31).
Here we show that the catalytic subunit of TORC2 (Tor1) and the major substrate of TORC2, Gad8, are found in the nucleus and are bound to the chromatin. Gad8 physically interacts with the MBF complex and is required for its full activation in response to replication stress. These results unravel a novel mode of regulation of the MBF-mediated transcriptional activation and highlight another aspect of TORC2-dependent regulation that may contribute to the role of TORC2 in surviving DNA replication stress.
Experimental Procedures
Yeast Strains, Growth Conditions, and Chemicals
S. pombe strains are described in Table 1. All experiments were performed by using standard genetic and molecular yeast techniques as described (32). Yeast cells were cultured in rich YE medium (0.5% yeast extract, 3% glucose) supplemented with adenine and uracil at 30 °C as described previously (33) or in Edinburgh minimal medium (5 g/liter NH4Cl) as described before (32). Gene deletions and tagging were performed by standard PCR-based methods (34).
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| TA1126 | gad8::ura4+≪kanMX-gad8-6HA leu1–32 ura4-D18 ade6-M210 h90 | YGRCa |
| TA2534 | gad8::ura4≪hph-gad8-6HA ade6-M210 leu1–32 ura4 h90 | This study |
| TA1125 | tor1::ura4+gad8::ura4+≪kanMX-gad8-6HA leu1–32 ura4-D18 ade6-M210 h90 | YGRC |
| TA1368 | gad8::ura4≪kanMX-gad8K259R-6HA ade6-M210 leu1 ura4-D18, h90 | YGRC |
| TA2322 | 3FLAG-Tor1[hph] leu1–32 h− | This study |
| TA2524 | 3FLAG-Tor2[hph] leu1–32 h− | This study |
| TA2 | leu1–32 ura4-D18 ade6-M210 h− | Laboratory stock |
| TA2672 | kanMX6-res1-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2679 | gad8::ura4≪hph-gad8-6HA kanMX6-res1-13MYC leu1–32 ura4 ade6 h90 | This study |
| TA2465 | kanMX6-res2-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2665 | gad8::ura4≪hph-gad8-6HA kanMX6-res2-13MYC leu1–32 ade6 h90 | This study |
| TA2678 | kanMX6-cdc10-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2687 | gad8::ura4≪hph-gad8-6HA kanMX6-cdc10-13MYC leu1–32 ade6 h90 | This study |
| TA2681 | gad8::ura4≪HPH-gad8-K259R-6HA ade6 leu1–32 RES1-13MYC::kanMX6 h90 | This study |
| TA2797 | gad8::ura4≪HPH-gad8-K259R-6HA ade6 leu1–32 RES2-13MYC::kanMX6 h90 | This study |
| TA2690 | gad8::ura4≪HPH-gad8-K259R-6HA ade6 leu1–32 CDC10-13MYC::kanMX6 h90 | This study |
| TA743 | leu1–32 cds1::ura4+ ura4-D18 h− | A. Carr (53) |
| TA751 | leu1–32 cds1::ura4+tor1::his1+ ura4-D18 | Laboratory stock |
| TA2766 | leu1–32 ura4-D18 ade6-M216 h90 YOX1-13Myc<kanMX6 | This study |
| TA2780 | gad8::ura ade6-M216 leu1–32 ura4-D18 YOX1-13MYC::kanMX6 h90 | This study |
| TA2791 | h90 leu1–32 ura4-D18 ade6–216 tor1::ura4+ YOX1-13Myc<kanMX6 | This study |
| TA742 | ade6–704 leu1–32 chk1::ura4+ ura4-D18 h− | A. Carr (54) |
| TA749 | Ade- leu1–32 chk1::ura4+tor1::his1+ ura4-D18 | Laboratory stock |
| TA2899 | h + leu1–32 ura4-D18 cdc10-2E:kanMX6 | N. Rhind (22) |
| TA2915 | tor1::ura4+ leu1–32 ura4-D18 ade6 leu1–32 cdc10-2E:kanMX6 | This study |
| TA1029 | gad8::ura4+ ade6-M216 leu1–32 ura4-D18, h− | M. Yamamoto (14) |
| TA101 | tor1::ura4+ leu1–32 ura4-D18 ade6–216 h- | Laboratory stock |
| TA2725 | kanMX6-cdt2-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2745 | gad8::ura4+kanMX6-cdt2-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2771 | tor1::ura4+kanMX6-cdt2-13MYC leu1–32 ura4-D18 ade6–216 h90 | This study |
| TA2872 | gad8::ura kanMX6-res1-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2873 | tor1::ura4+ kanMX6-res1-13MYC leu1–32 ura4-D18 ade6–216 h90 | This study |
| TA2886 | gad8::ura kanMX6-res2-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2874 | tor1::ura4+ kanMX6-res2-13MYC leu1–32 ura4-D18 ade6–216 h90 | This study |
| TA2882 | gad8::ura kanMX6-cdc10-13MYC leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2881 | tor1::ura4+ kanMX6-cdc10-13MYC leu1–32 ura4-D18 ade6–216 h90 | This study |
| TA2769 | yox1::kanMX6 leu1–32 ura4-D18 ade6-M216 h90 | This study |
| TA2965 | tor1::ura4+ yox1::kanMX6 leu1–32 ura4D-18 ade6 | This study |
a Yeast Genetic Resource Center, Japan.
Protein Extraction and Immunoprecipitation Assays
Cells were grown to midlogarithmic phase, washed once with water, and resuspended in lysis buffer (PBS, pH 7.0, 200 mm NaCl, 0.5 mm EGTA, 0.5 mm EDTA, 0.1% Triton X-100, protease inhibitor mixture, and 1 mm phenylmethylsulfonyl fluoride). Cells were broken by bead beating (45 min at 4 °C) with glass beads and centrifuged for 5 min at 1000 × g, and the supernatant was collected. 20 μg of total protein extract was resolved by SDS-PAGE using 10% acrylamide gels. For immunoprecipitations, 500 μg of proteins were prepared and precleared with a 20-μl protein A-Sepharose and protein G-Sepharose bead mixture (GE Healthcare). 2 μl of MYC antibody were added to the cleared extract and incubated overnight at 4 °C. The beads were washed five times with lysis buffer at a NaCl concentration of 220 mm. The resulting immunoprecipitates were loaded for SDS-PAGE using 10% acrylamide gels. Trichloroacetic acid protein extraction was performed as described in Foiani et al. (35).
Western Blotting
Proteins were resolved by SDS-PAGE in 10–15% acrylamide gels, transferred to nitrocellulose membranes, blocked with 5% milk in TBS with Tween 20, and immunoblotted with the indicated antibodies. Detection was carried out using the ECL SuperSignal detection system (Thermo Scientific).
Chromatin Isolation
Yeast fractionations were performed as described in Keogh et al. (36). 25 ml of each strain were grown to an A600 of ≈1 in YE medium. The cells were collected by centrifugation, washed with SB (1 m sorbitol, 10 mm dl-dithiothreitol (DTT), and 20 mm Tris·Cl at pH 7.4), and transferred to a 2-ml test tube. Cells were suspended in 1 ml SB, 125 μl of Zymolyase 20T (Seigagaku Corp.; 10 mg/ml in SB) were added, and samples were incubated at 30 °C with rotation for 60 min. The resulting spheroplasts were collected, washed twice with SB, and suspended in 500 μl of EBX (20 mm Tris·Cl at pH 7.4, 100 mm NaCl, 10 mm DTT, and protease inhibitors). Triton X-100 was added to a final concentration of 0.5%, and the samples were kept on ice for 10 min with gentle mixing. An aliquot was taken for immunoblotting (whole cell extracts), and the remainder of the lysate was layered over 1 ml of NIB (20 mm Tris·Cl at pH 7.4, 100 mm NaCl, 1.2 m sucrose, 10 mm DTT, and protease inhibitors) and centrifuged (13,000 × g, 15 min, 4 °C). A sample of the upper layer of the cytoplasmic fraction was taken. The glassy white nuclear pellet was suspended in 500 μl of EBX, and Triton X-100 was added to a final concentration of 1% to lyse the nuclear membrane. Samples were kept on ice for 10 min with gentle mixing, and an aliquot was taken (nuclear fraction). The chromatin fraction was collected by centrifugation (16,000 × g, 10 min, 4 °C). For the disruption of the chromatin, the chromatin fraction was resuspended in EBX except that the concentration of the NaCl was 250 mm or in EBX supplemented with 2 mm MgCl2, 1 mm DTT, and a 1:360 dilution of Benzonase (Sigma, E1014; 250 units/μl) and treated for 30 min at 37 °C with mixing every 10 min. The suspension was centrifuged (16,000 × g, 10 min, 4 °C), and the supernatant was collected.
Indirect Immunofluorescence
Indirect immunofluorescencewas performed as described in Schmelzle et al. (37). Yeast cells with a genomic tagged Gad8–6HA were grown to midlogarithmic phase and fixed for 2 h in the growth medium containing formaldehyde (3.7% final) and potassium phosphate buffer (100 mm final, pH 6.5). Cells were washed and resuspended in sorbitol buffer (1.2 m sorbitol and 100 mm potassium phosphate, pH 6.5). Cell walls were digested for 1 h at 37 °C in sorbitol buffer supplemented with DTT (10 mm) and Zymolyase 20T (12.5 mg/ml). Spheroplasts were fixed on poly-l-lysine-coated glass slides and permeabilized with PBT (53 mm Na2HPO4, 13 mm NaH2PO4, 75 mm NaCl, 1% BSA, and 0.1% Triton X-100). Immunofluorescence directed against the HA epitope was performed by application of monoclonal anti-HA (F7; Santa Cruz Biotechnology) at a dilution of 1:1000 in PBT for 2 h and subsequently Cy3-conjugated rabbit anti-mouse IgG (Molecular Probes) diluted 1:1000 in PBT for an additional 60 min. DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) at a concentration of 1 μg/ml. Cells were visualized with an Evos microscope (60× objective). Antibodies were checked for specificity in each experiment using wild-type cells lacking the corresponding epitope.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed as described in Aligianni et al. (29). 50 ml of each strain were grown to an A600 of ≈1 in YE medium. 1.5 ml of formaldehyde (37% solution) were added for 15 min, and then the formaldehyde was quenched with 2.5 ml of 2.5 m glycine for 5 min. Cells were then harvested and washed once with 15 ml of cold PBS. Cells were broken for 10 min with glass beads in 600 μl of lysis buffer (50 mm HEPES-KOH, pH 7.5, 140 mm NaCl, 1 mm EDTA, 1% Triton X-100, and 0.1% sodium deoxycholic acid). The supernatant was removed to a new tube (the lysate); the glass beads were washed once with 500 μl of lysis buffer and added to the lysate. The protein extract was sonicated six times for 10 s at 80% amplitude with 1 min on ice between each time. The sonicated material was centrifuged for 30 min at 2500 rpm. The supernatant was used for immunoprecipitation as described above, and 10% of the extract was saved as the input material. The beads after the immunoprecipitation were washed once with lysis buffer, once with lysis buffer with 360 mm NaCl, once with washing buffer (10 mm Tris/HCl, pH 8, 0.25 m LiCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholic acid, and 1 mm EDTA), and once with TE (10 mm Tris/HCl, pH 8, and 10 mm EDTA). The washed beads and the input were treated with elution buffer (50 mm Tris/HCl, pH 8, 10 mm EDTA, and 1% SDS) overnight at 65 °C. The DNA was precipitated, resuspended in water, and used for PCR real time analysis. All experiments are plotted as the average of at least three independent biological repeats (each biological repeat is the average of three technical PCR repeats). All measurements were normalized to the level of PCR amplification using the actin ORF.
Real Time Quantitative PCR (qRT-PCR)
RNA extractions and qRT-PCR analysis were performed as described in Laor et al. (38). 50 ml of each strain were grown to an A600 of ≈1 in YE medium before further processing.
Results
TORC2-Gad8 Is Found in the Nucleus and Is Bound to the Chromatin
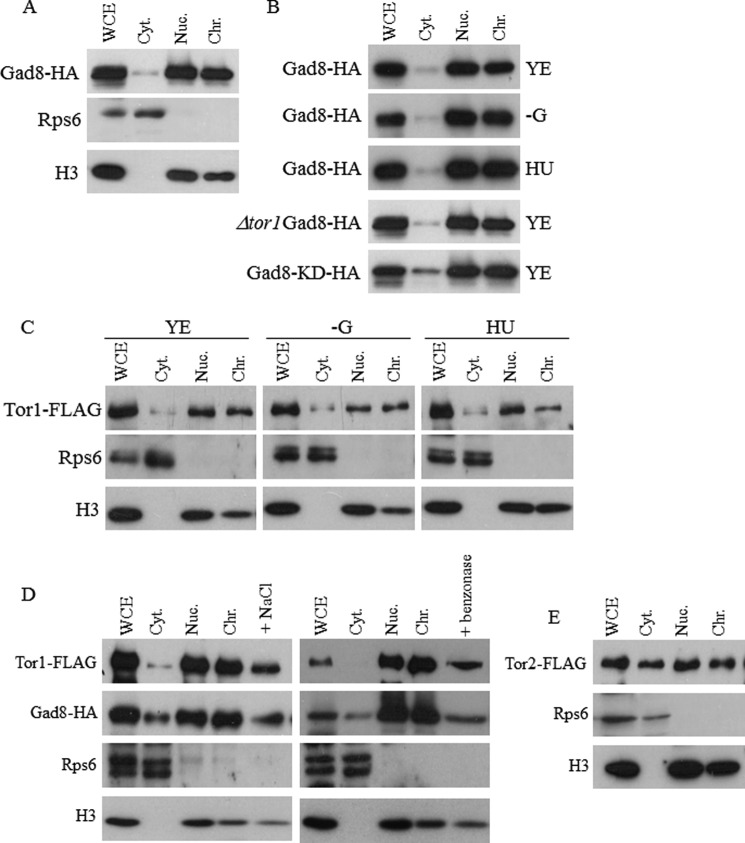
TOR complexes were originally thought to act predominantly in the cytoplasm or in association with cellular membranes. TORC1 is closely associated with the vacuolar membrane in many different organisms, whereas TORC2 is associated with the plasma membrane (39–42). More recently, many studies have shown that TORC1 (39, 41–45) and TORC2 (39–42) are also found in the nucleus and are required for several nuclear functions. AKT, the kinase acting downstream of mTORC2, was also originally located to the plasma membrane but is currently known to have additional functions in the nucleus (28, 32, 34, 36, 46). Because we have identified several nuclear functions for TORC2-Gad8, such as control of gene expression, telomere length maintenance, and gene silencing (11), we examined the cellular localization of Gad8 using biochemical fractionations. We isolated proteins from cells expressing chromosomally tagged Gad8-HA, a fully active version of the Gad8 protein (14, 17), and separated proteins that are localized to the nucleus from those in the non-nucleus fractions. Detection of histone H3 and Rps6 was used as markers for nuclear and cytoplasmic proteins, respectively (Fig. 1A). We found that the majority of Gad8 is localized to the nucleus, whereas only a minor fraction of the protein is in the cytoplasmic fraction. Biochemical separations of the nuclear fraction into chromatin-bound and soluble proteins suggest that Gad8 is bound to the chromatin (Fig. 1A). The association of Gad8 to the chromatin is independent of Gad8 activity. Accordingly, a kinase-inactive mutant of Gad8 (Gad8-KD-HA (14)) remained mainly in the chromatin fraction. Also, inactive Gad8-HA isolated from cells lacking the catalytic subunit of TORC1 (Δtor1) localized to the nucleus (Fig. 1B), and Gad8-HA remained bound to the chromatin fraction under glucose starvation, a condition under which Gad8 is inactive (17, 18) (Fig. 1B). Because cells mutated in TORC2 or gad8+ are sensitive to DNA replication stress (10, 11), we examined the cellular distribution of Gad8-HA in the presence of HU but did not detect a significant effect on the pattern of Gad8-HA localization (Fig. 1B).
FIGURE 1.
Cytoplasmic and nuclear distribution of Tor1 (TORC2), Gad8, and Tor2 (TORC1). A, subcellular fractionations of cells suggest that a large proportion of Gad8 is in the nucleus in association with the chromatin fraction. Whole cell extracts (WCE) of chromosomally tagged gad8-HA were separated into cytoplasmic (Cyt), nuclear (Nuc), and chromatin (Chr) fractions and immunoblotted with the indicated antibodies. Antibodies against Rps6 and histone 3 (H3) were used to confirm purity of cytosol and nuclear fractions, respectively. B, the association of Gad8 to the chromatin is independent of its activity. Wild-type, Δtor1, or gad8-KD (gad8K259D; a kinase-dead allele) cells carrying the gad8-HA allele were grown in rich YE medium, treated for 1 h with 12 mm HU, or transferred for 1 h to Edinburgh minimal medium containing no carbon source (−G). C, subcellular fractionations of cells suggest that Tor1 is mainly nuclear and is associated with the chromatin. Cells containing chromosomally FLAG-tagged Tor1 were left untreated in rich YE medium, treated for 1 h with 12 mm HU, or transferred for 1 h to Edinburgh minimal medium containing no carbon source (−G). D, release of Gad8 or Tor1 from the chromatin whole cell extracts (WCE) of chromosomally tagged gad8-HA or tor1-FLAG fractionated as described above. The chromatin fraction was treated either with 250 mm NaCl or Benzonase, and the supernatant was analyzed. E, Tor2 is equally distributed between the cytoplasmic and non-nuclear fractions. Cells containing chromosomally FLAG-tagged Tor2 were biochemically fractionated as above and immunoblotted with the indicated antibodies.
Because Gad8 is a substrate for Tor1, the catalytic subunit of TORC2, we next analyzed the localization of a chromosomally tagged Tor1 (Tor1-FLAG). Similar to Gad8, Tor1-FLAG was found in association with the chromatin fraction (Fig. 1C), and its nuclear localization was not affected by glucose starvation or treatment with HU (Fig. 1C). When we attempted to disrupt the chromatin fraction either by treatment of the chromatin pellets with 250 mm NaCl or by addition of the nuclease Benzonase, we partially released the H3 histone from the chromatin pellets and similarly partially released Gad8 or Tor1 from the chromatin fraction (Fig. 1D). These findings support the association of Tor1 or Gad8 with the chromatin fraction.
Although Tor1-FLAG is mainly associated with the nucleus, Tor2-FLAG, the catalytic subunit of TORC1, is evenly distributed between the nuclear fraction and the non-nuclear fraction (Fig. 1E). These results suggest that both TORC1 and TORC2 are found in the nucleus, but TORC2-Gad8 can be more readily detected in the nucleus.
To further explore the subcellular localization of Gad8, we used in situ immunofluorescence. We found that Gad8-HA is dispersed throughout the cytoplasm but is also found in the nucleus (Fig. 2). Quantitative differences in cytoplasmic to nuclear distribution probably reflect differences in the methods of detection. However, both methods of detection indicate that Gad8 is found both in the cytoplasm and in the nucleus.
FIGURE 2.
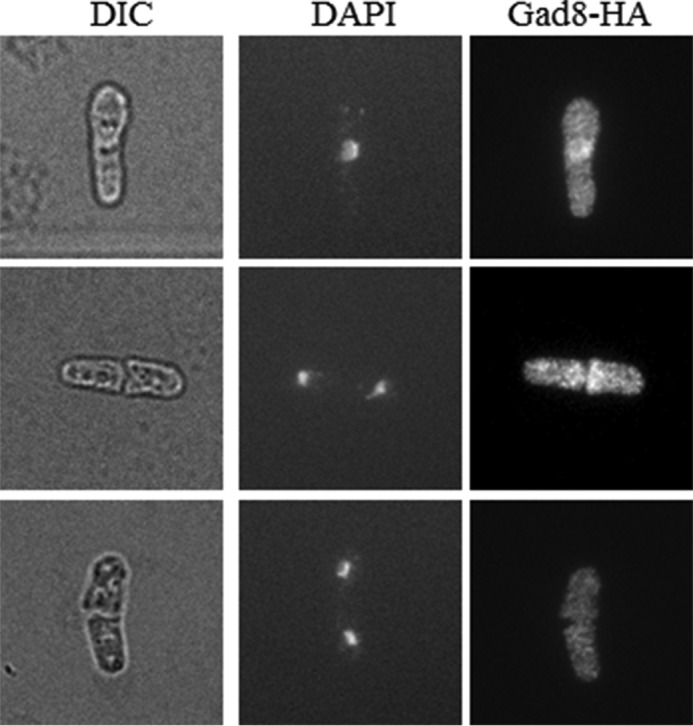
Immunostaining suggests that Gad8 is dispersed throughout the cell. Cells carrying chromosomally tagged gad8-HA were fixed and subjected to indirect immunofluorescence using a monoclonal anti-HA antibody. DIC, differential interference contrast.
Gad8 Physically Interacts with the MBF Complex
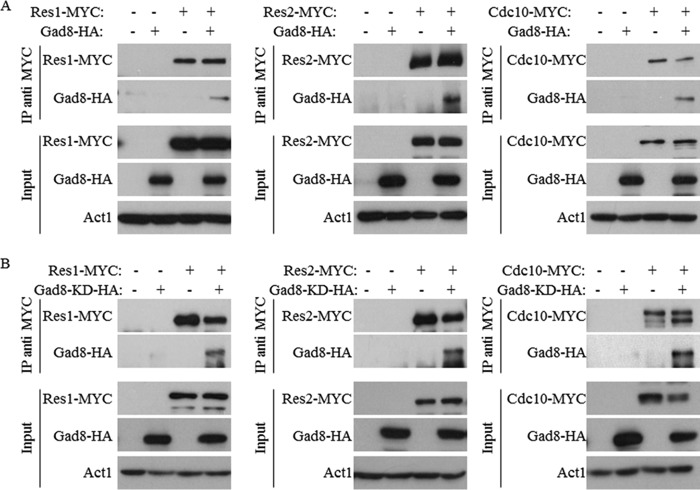
We have previously shown that mutants lacking TORC2 or gad8+ are sensitive to DNA replication stress induced by HU (10, 11). One of the major transcription factors activated in response to DNA replication stress is the MBF complex (22, 27, 31). We therefore speculated that TORC2-Gad8 may directly interact with the MBF complex. To address this possibility, we examined physical interactions between Gad8 and the main subunits of the MBF complex, Cdc10, Res1, and Res2. We created double tagged strains that expressed genomic tagged Gad8-HA together with Cdc10-MYC, Res1-MYC, or Res2-MYC. Gad8 readily co-immunoprecipitated with each of the tested MBF subunits (Fig. 3A). The interaction between Gad8 and the MBF complex is independent of Gad8 activity as the kinase-dead version of Gad8 also readily interacted with the different subunits of the MBF complex (Fig. 3B). Because the MBF complex is regulated in response to DNA stresses (31), we analyzed the interaction between Gad8 and the MBF complex under DNA replication stress (HU) or in the presence of the DNA-damaging agent MMS. In both cases, the interaction between Gad8 and the MBF complex is preserved (Fig. 4). These results demonstrate a physical interaction between Gad8 and the MBF complex that is independent of the activity of Gad8 or DNA replication or -damaging conditions.
FIGURE 3.
Gad8 physically interacts with the MBF complex. A, detection of physical interactions between Res1, Res2, or Cdc10 and Gad8. Protein extracts from wild-type cells expressing the indicated chromosomally tagged proteins were immunoprecipitated with anti-MYC antibody. Western blotting was performed using either anti-HA or anti-MYC to detect the presence of tagged proteins within the immune complexes. The expression levels of the indicated proteins before immunoprecipitations is shown (Input). Act1 was used as a loading control. B, the kinase activity of Gad8 is not required for its interaction with Res1, Res2, or Cdc10. Physical interactions between Res1, Res2, or Cdc10 and gad8-KD (gad8K259D; a kinase-dead allele) were detected as above. IP, immunoprecipitation.
FIGURE 4.
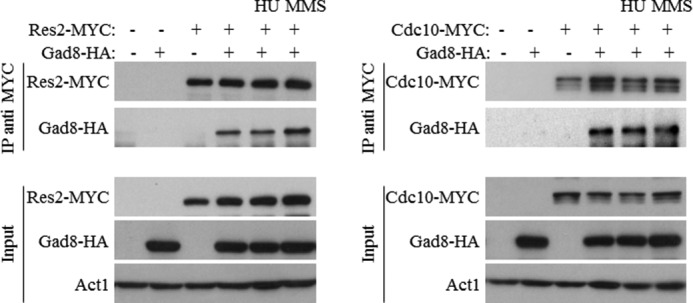
Gad8 physically interacts with the MBF complex under DNA stress conditions. Cells carrying the indicated chromosomally tagged proteins were grown in rich medium and left untreated or exposed for 1 h to 12 mm HU or 0.03% MMS. Western blotting was performed using either anti-HA or anti-MYC as described in Fig. 2. IP, immunoprecipitation.
TORC2-Gad8 Affects the Efficiency of DNA Binding Activity of the MBF Complex
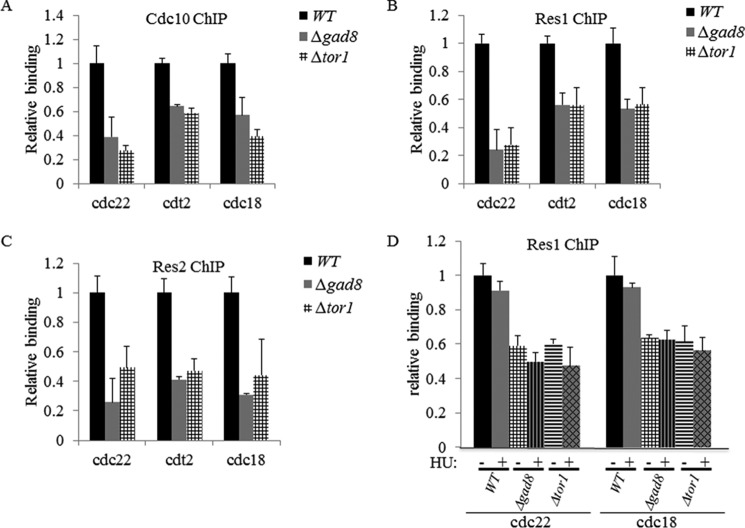
Gad8 interacted with the subunits of the MBF complex (Figs. 3 and 4), and our previous functional analysis of Tor1 indicated a reduced transcriptional activation in response to hydroxyurea of cdt2+ and cdc18+, two MBF-targeted genes (11). Thus, we examined the effect of Δtor1 and Δgad8 mutations on the binding efficiency of the MBF complex to its promoters. We selected three well established targets of the MBF complex: cdt2+, encoding the adaptor subunit of an E3 ubiquitin ligase that is essential for initiating DNA replication; cdc18+, encoding a minichromosome maintenance complex loader; and cdc22+, encoding for the large subunit of ribonucleotide reductase. We found that the binding of the MBF subunits Cdc10, Res1, and Res2 to the promoters of cdc22+, cdt2+, and cdc18+was reduced to 20–60% in Δtor1 or Δgad8 mutant cells compared with wild type under normal growth conditions or under DNA replication stress (Fig. 5). These results suggest that TORC2-Gad8 is required for efficient binding of the MBF complex and may thus affect transcriptional regulation of MBF-targeted genes.
FIGURE 5.
Tor1 and Gad8 are required for efficient binding of the MBF complex to its target promoters. A, loading of Cdc10 on cdc22, cdt2, and cdc18 promoters was measured by ChIP analysis of chromatin extracts isolated from wild-type (WT), Δgad8, or Δtor1 cells carrying chromosomally tagged Cdc10-MYC. The level of binding is quantified on anti-MYC-immunoprecipitated DNA. Each value is the mean of at least three independent assays, and the error bars indicate S.D. B, loading of Res1 on cdc22, cdt2, and cdc18 promoters was measured by ChIP analysis of cells carrying chromosomally tagged Res1-MYC. C, loading of Res2 on cdc22, cdt2, and cdc18 promoters was measured by ChIP analysis of cells carrying chromosomally tagged Res2-MYC. D, Tor1 and Gad8 are required for efficient binding of the MBF complex under DNA replication stress. Loading of Res1 on cdc22 and cdc18 promoters was analyzed by ChIP analysis of untreated cells (−) or cells treated with 12 mm HU for 3 h (+).
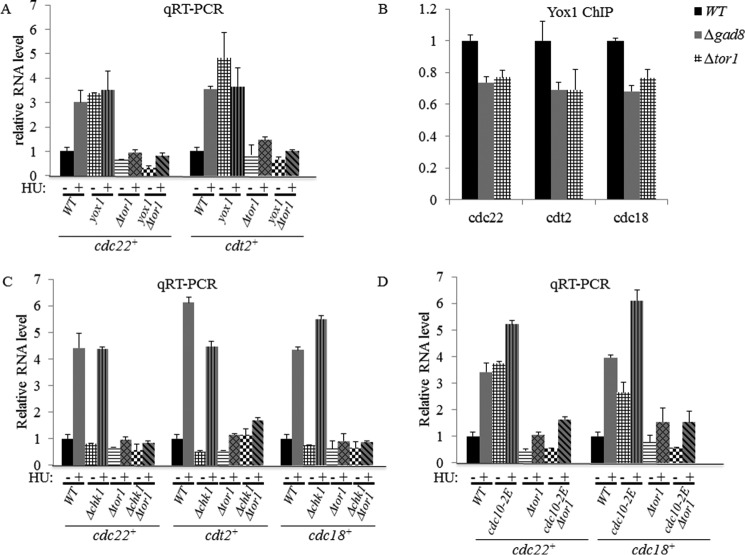
TORC2-Gad8 Increased Expression of MBF Target Genes in Response to DNA Replication Stress
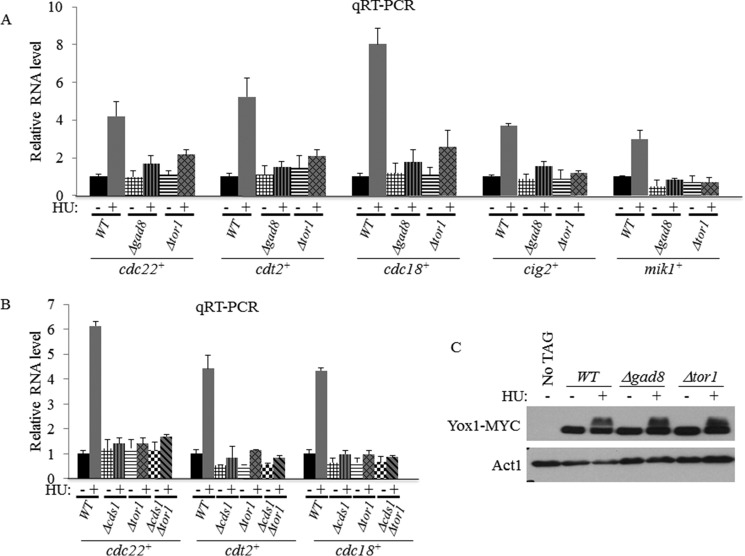
To further examine the effect of TORC2-Gad8 on MBF-mediated transcription, we used qRT-PCR to examine the transcript levels of five different MBF target genes, cdt2+, cdc18+, cdc22+, cig2+, and mik1+, in Δtor1 or Δgad8 mutant cells. We did not observe a significant difference between the wild-type and mutant cells in transcript levels under normal growth conditions (Fig. 6, A and B). However, although wild-type cells showed a 4–8-fold induction of gene expression of the MBF target genes in response to HU, there was only a 1.5–2.6-fold induction in Δtor1 or Δgad8 mutant cells (Fig. 6A). Thus, TORC2-Gad8 is required for activation of MBF in response to HU.
FIGURE 6.
Tor1 and Gad8 are required for induction of MBF-targeted genes independent of Yox1 phosphorylation. A, TORC2-Gad8 is required for induction of MBF-targeted genes in response to HU. Expression levels of cdc22+, cdt2+, cdc18+, cig2+, and mik1+ in WT, Δgad8, or Δtor1 cells were determined by qRT-PCR. Total RNA was prepared from untreated cells (−) or cells treated with 12 mm HU for 3 h (+). The level of act1+ mRNA was used as a reference. Each value is the mean of at least three independent assays, and the error bars indicate S.D. B, the expression level of MBF-targeted genes in Δcds1Δtor1 double mutant cells is similar to that in Δcds1 or Δtor1 single mutant cells. C, Yox1 is normally phosphorylated in TORC2 or Gad8 mutant cells. Total protein extracts were prepared by trichloroacetic acid extractions from WT, Δgad8, or Δtor1 strains expressing genomic Yox1-MYC. Cells were grown in rich medium (−) or treated with 12 mm HU for 1 h (+). Antibody against Act1 was used as a loading control.
When cells are exposed to DNA replication stress, the checkpoint kinase Rad3 phosphorylates and activates the kinase Cds1, which in turn phosphorylates and inactivates the MBF repressor Yox1, allowing induction of MBF-dependent transcription (31). We previously showed that Δtor1 mutant cells are sensitive to HU in a manner that is additive with the sensitivity of Δcds1 mutant cells to the drug (10, 11), suggesting that Cds1 and Tor1 work on two separate pathways. Here, we examined the level of MBF-dependent transcription in Δtor1 Δcds1 double mutant cells. Upon exposure to HU, the double mutant cells show no induction of MBF transcription, similar to the single parental strains. We detected no additive effects with respect to the level of expression of cdc22+, cdt2+, or cdc18+ under normal growth conditions (Fig. 6B). To further explore the relationship between TORC2- and Cds1-dependent pathways, we examined the phosphorylation status of Yox1, a downstream effector of Cds1 (31), in Δtor1 or Δgad8 cells. We found that Yox1 is normally phosphorylated in response to HU in Δtor1 or Δgad8 mutant cells (Fig. 6C). In agreement with Yox1 being a negative regulator of the MBF complex (47), deletion of yox1+ caused induction in MBF-dependent transcription under normal growth conditions. However, the double mutant Δtor1 Δyox1 failed to induce MBF-dependent transcription under normal conditions or in the presence of HU (Fig. 7A). Thus, TORC2-Gad8 is required for MBF gene induction independently of Yox1 status of phosphorylation. Consistently, deletion of yox1+ in a tor1+ background did not repress the HU or camptothecin sensitivity of tor1+ mutants (data not shown). We also examined the possibility that TORC2-Ga8 may affect the binding of Yox1 to its cognate promoters. Yox1 binding to the MBF target genes is weakly reduced in Δtor1 or Δgad8 mutant cells (Fig. 7B) and cannot explain the inability of TORC2-Gad8 mutant cells to induce MBF transcription in response to HU. Taken together, our results suggest that TORC2-Gad8 does not regulate MBF transcription via the Cds1-Yox1 pathway.
FIGURE 7.
Functional TORC2 is required for the elevated level of MBF-dependent transcription. A, deletion of yox1+ in the background of Δtor1 cells does not affect MBF-dependent transcription. Expression levels of cdc22+ and cdt2+ were determined by qRT-PCR. Total RNA from WT, Δyox1, Δtor1, or Δyox1 Δtor1 double mutant cells was analyzed by qRT-PCR. Total RNA was prepared from untreated cells (−) or cells treated with 12 mm HU for 3 h (+). Each value is the mean of at least three independent assays, and the error bars indicate S.D. B, Tor1 and Gad8 have only a minor effect on the loading of Yox1 to MBF promoters. ChIP of Yox1 on cdc22, cdt2, and cdc18 promoters was analyzed. C, deletion of chk1+ in the background of Δtor1 cells does not affect MBF-dependent transcription. Total RNA from WT, Δchk1, Δtor1, or Δchk1Δtor1 double mutant cells was analyzed by qRT-PCR as described above. D, total RNA from WT, cdc10-2E, Δtor1, or cdc10-2E Δtor1 double mutant cells was analyzed by qRT-PCR as described above.
We also examined the effect of a cdc10-2E mutant allele on MBF-mediated transcriptional activation in the absence of TORC2-Gad8. cdc10-2E is a cdc10 allele in which serine 720 and threonine 723 are substituted with glutamate, mimicking constitutive phosphorylation of these sites and leading to constitutive activation of Cdc10 and elevated levels of MBF-mediated transcription in the absence of DNA replication stress. The cdc10-2E mutation suppressed the HU sensitivity of Δcds1 cells (22), supporting a scenario in which Cds1 activation in response to HU leads to phosphorylation and activation of Cdc10. More recently it was suggested that Chk1 is the kinase responsible for phosphorylation of Cdc10 in response to exposure to MMS (31). We confirmed that Δchk1 did not affect the levels of cdc22+, cdt2+, or cdc18+ transcripts in response to HU, whereas the response was abolished in single Δtor1 or double Δtor1 Δchk1 mutant cells (Fig. 7C). Importantly, the cdc10-2E mutation did not rescue the defect in activation of MBF-mediated transcription in Δtor1 or Δgad8 mutant cells (Fig. 7D) or the sensitivity of Δtor1 mutant cells to HU (data not shown). These results support a model in which the TORC2-Gad8 pathway regulates MBF activity in a manner that is independent of both Cds1 and Chk1.
TORC2-Gad8 Affects the Expression Level of Cdt2 or Cig2 at the Protein Level
One of the MBF-targeted genes is cdt2+, encoding the alternating partner of the E3 ubiquitin ligase Cul4-Ddb1-Cdt2, which is involved in the degradation of the licensing factor Cdt1 (48). Because we showed reduced binding of the MBF complex to the promoter of cdt2+ in Δtor1 or Δgad8 mutant cells (Fig. 5) as well as a reduced level of transcription in response to HU (Figs. 6 and 7), we examined the protein levels of Cdt2 in the mutant cells. We observed a reduction of Cdt2 protein levels under normal conditions as well as slower and weaker induction of Cdt2 in response to HU (Fig. 8A). Thus, TORC2-Gad8 affects regulation of Cdt2 protein level under normal and replication stress conditions. However, overexpression of cdt2+ in Δtor1 mutant cells failed to suppress their sensitivity to HU or camptothecin (data not show). Notably, we found that the level of Cdt2 is reduced in Δtor1 or Δgad8 even under non-stressed conditions (Fig. 8A), conditions under which we do not detect a decrease in transcript level of cdt2+ (Fig. 6A). These findings may suggest a post-transcriptional mode of regulation of TORC2-Gad8 that affects the Cdt2 level.
FIGURE 8.
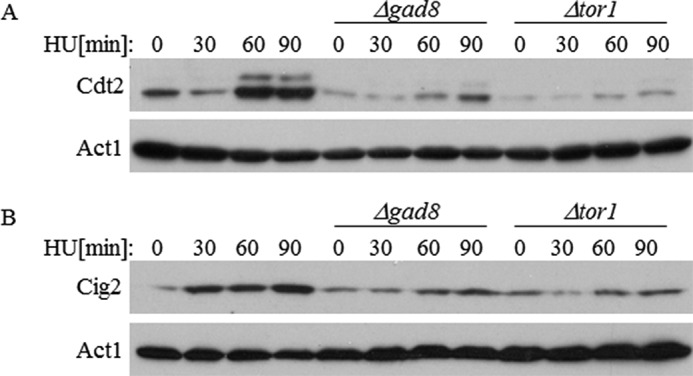
Reduced levels of Cdt2 and Cig2 in TORC2-Gad8 mutant cells. A, extracts isolated from WT, Δgad8, or Δtor1 cells expressing chromosomally tagged Cdt2-MYC were analyzed at the indicated time points after HU treatment (12 mm). Act1 was used as a loading control. B, protein extracts were prepared as described above. Anti-Cig2 antibody was used.
cig2+ is another well known target gene of the MBF complex (23). Cig2 is a B-type G1/S cyclin. Similar to Cdt2, the protein level of Cig2 is increased in response to HU in wild-type cells but not in Δtor1 or Δgad8 mutant cells (Fig. 8B). The reduction of Cdt2 and Cig2 protein levels in TORC2 or Δgad8 mutant cells further demonstrates the significant role that TORC2-Gad8 plays in controlling MBF target gene expression.
Discussion
A major question in the TOR field is where the TOR complexes localize within the cell. It has already been shown that TOR phosphorylates distinct substrates at different cellular locations, and there are varying results on the cellular localization of TOR complexes (39–42). In particular, relatively little is known about the roles that TOR complexes play in the nucleus. In this report, we demonstrate that the catalytic subunits of S. pombe TORC1 (Tor2) and TORC2 (Tor1) are found in the nucleus and are bound to the chromatin fraction (Fig. 1). Using biochemical fractionations, we found that Tor2 (TORC1) is evenly distributed between the nucleus and non-nucleus fraction, whereas Tor1 (TORC2) and its substrate Gad8 are mainly localized to the nucleus. In this respect, it is interesting to note that using a similar approach it has been shown that human mTORC2 is more readily detected in the nucleus compared with mTORC1 (49, 50). A large body of evidence demonstrates that AKT, the mammalian homologue of Gad8, is found in the nucleus where it functions to prevent apoptosis and control cell cycle progression, cell differentiation, mRNA export, and DNA repair (for a review, see Ref. 46). Although our biochemical approach indicates that the majority of Tor1 and Gad8 resides in the nucleus, our immunostaining approach indicates a more even distribution between the cytoplasm and the nucleus. Quantitative differences in cytoplasmic to nuclear distribution may reflect the method of detection. Although the indirect immunofluorescence procedure fixes growing cells, the biochemical fractionation procedure requires the preparation of spheroplasts, which is stressful for the cells and may thus induce localization of Tor1 or Gad8 into the nucleus. However, our data suggest that Tor1 and Gad8 can be found in the nucleus where they may interact with specific targets, such as the MBF complex. Differences in the techniques used may also explain the discrepancy between our findings and previous results showing by fluorescence microscopy that Gad8-GFP and Ste20-GFP are mainly localized to the cytoplasm (51). Because the Gad8-GFP and Gad8-HA constructs are both functional, the observed differences are not due to compromised activity of the tagged protein but could result from the use of different protein tags.
Our finding that TORC2-Gad8 is found in the nucleus is supported by the readiness by which Gad8 co-immunoprecipitated with the nuclear MBF complex (Figs. 3 and 4). The interaction of Gad8 with Cdc10, Res1, and Res2, the three main subunits of the MBF complex, is independent of Gad8 activity and does not respond to DNA replication stress. The MBF complex is important for transcription of DNA replication genes and genes required for the response to DNA damage (31). We have previously shown that TORC2 is essential for the survival of S. pombe cells under DNA replication stress conditions (10, 11). In this study, we show that in the absence of TORC2 or Gad8 the MBF complex is loaded less efficiently to its targeted promoters, cdc18+, cdc22+, and cdt2+ (Fig. 5), and there is a significant decrease of transcriptional induction of the MBF-dependent genes in response to DNA replication stress (Fig. 6). Although we detected a reduction in binding of the MBF complex to its cognate promoters in TORC2-Gad8 mutant cells under normal growth conditions, there is no reduction in gene expression under normal growth conditions (Figs. 6 and 7). Thus, the reduced binding of the MBF complex in TORC2-Gad8 mutant cells is insufficient only for transcriptional induction.
Consistent with the inability of TORC2-Gad8 mutant cells to induce MBF-mediated transcription in response to HU, we detected reduced protein levels of Cdt2 and Cig2, two MBF target genes, in Δtor1 or Δgad8 mutant cells. Interestingly, we observed a decrease in the protein level of Cdt2 also under normal growth conditions (Fig. 8), suggesting that TORC2-Gad8 may also affect the protein level of Cdt2 post-transcriptionally.
The activation of the MBF complex is regulated by Cds1 and Chk1, the two main checkpoint kinases that lie downstream of Rad3 (ATR). Cds1 regulates the phosphorylation of Yox1, thereby inhibiting the repressive effect of Yox1 on MBF activity. We did not detect any effect on Yox1 phosphorylation in Δtor1 or Δgad8 mutant cells. Furthermore, disruption of yox1+ did not suppress the inability of TORC2-Gad8 mutant cells to induce MBF transcription in response to HU (Fig. 7), suggesting that TORC2-Gad8 does not affect MBF activity via the Cds1-Yox1 module. Also, TORC2-Gad8 does not appear to affect MBF activity via Chk1. We detected no activation of Chk1 in the absence of TORC2 or Gad8 (10, 11), and disruption of chk1+ did not affect the defect in MBF activation in TORC2-Gad8 mutant cells. Our observation that the cdc10-2E allele cannot rescue the defect in MBF activation in TORC2-Gad8 mutant cells or their HU sensitivity further implies that TORC2 regulates the MBF independently of the DNA damage or replication checkpoint.
In mammalian cells, E2F, the homologue of the MBF complex, interacts with DNA damage proteins to function as a sequence-specific transcriptional activator of cellular genes, including those associated with growth, proliferation, and DNA damage response (52). The strong conservation in the DNA damage response between yeast and mammalian cells suggests that TORC2 may be involved in similar processes in humans too and may be relevant to DNA damage-related diseases, among them cancer.
Author Contributions
A. C. contributed to study design, researched,analyzed the data, and wrote the manuscript. M. K. reviewed and edited the manuscript. R. W. contributed to study design and wrote, reviewed, and edited the manuscript.
Acknowledgments
We thank M. Yamamoto, A. Carr, and N. Rhind for strains and members of the Kupiec laboratory for encouragement and support.
This work was supported by Israel Science Foundation Grant 688/14 and Open University of Israel Grant 31040) (to R. W.). The authors declare that they have no conflicts of interest with the contents of this article.
- TOR
- target of rapamycin
- TORC1 and -2
- TOR complex 1 and 2, respectively
- HU
- hydroxyurea
- MMS
- methyl methanesulfonate
- MBF
- MluI cell cycle box-binding factor
- mTOR
- mechanistic target of rapamycin
- mTORC
- mTOR complex
- ATR
- ataxia telangiectasia and Rad3-related protein
- qRT-PCR
- real time quantitative PCR.
References
- 1. Wullschleger S., Loewith R., and Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 2. Loewith R. (2011) A brief history of TOR. Biochem. Soc. Trans. 39, 437–442 [DOI] [PubMed] [Google Scholar]
- 3. Cybulski N., and Hall M. N. (2009) TOR complex 2: a signaling pathway of its own. Trends Biochem. Sci. 34, 620–627 [DOI] [PubMed] [Google Scholar]
- 4. Hayashi T., Hatanaka M., Nagao K., Nakaseko Y., Kanoh J., Kokubu A., Ebe M., and Yanagida M. (2007) Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12, 1357–1370 [DOI] [PubMed] [Google Scholar]
- 5. Matsuo T., Otsubo Y., Urano J., Tamanoi F., and Yamamoto M. (2007) Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol. Cell. Biol. 27, 3154–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisman R., Roitburg I., Schonbrun M., Harari R., and Kupiec M. (2007) Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 175, 1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez B., and Moreno S. (2006) Fission yeast Tor2 promotes cell growth and represses cell differentiation. J. Cell Sci. 119, 4475–4485 [DOI] [PubMed] [Google Scholar]
- 8. Davie E., Forte G. M., and Petersen J. (2015) Nitrogen regulates AMPK to control TORC1 signaling. Curr. Biol. 25, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakashima A., Sato T., and Tamanoi F. (2010) Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J. Cell Sci. 123, 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schonbrun M., Kolesnikov M., Kupiec M., and Weisman R. (2013) TORC2 is required to maintain genome stability during S phase in fission yeast. J. Biol. Chem. 288, 19649–19660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schonbrun M., Laor D., López-Maury L., Bähler J., Kupiec M., and Weisman R. (2009) TOR complex 2 controls gene silencing, telomere length maintenance, and survival under DNA-damaging conditions. Mol. Cell. Biol. 29, 4584–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petersen J., and Nurse P. (2007) TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat. Cell Biol. 9, 1263–1272 [DOI] [PubMed] [Google Scholar]
- 13. Weisman R., Roitburg I., Nahari T., and Kupiec M. (2005) Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics 169, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsuo T., Kubo Y., Watanabe Y., and Yamamoto M. (2003) Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 22, 3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikeda K., Morigasaki S., Tatebe H., Tamanoi F., and Shiozaki K. (2008) Fission yeast TOR complex 2 activates the AGC-family Gad8 kinase essential for stress resistance and cell cycle control. Cell Cycle 7, 358–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawai M., Nakashima A., Ueno M., Ushimaru T., Aiba K., Doi H., and Uritani M. (2001) Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr. Genet. 39, 166–174 [DOI] [PubMed] [Google Scholar]
- 17. Cohen A., Kupiec M., and Weisman R. (2014) Glucose activates TORC2-Gad8 protein via positive regulation of the cAMP/cAMP-dependent protein kinase A (PKA) pathway and negative regulation of the Pmk1 protein-mitogen-activated protein kinase pathway. J. Biol. Chem. 289, 21727–21737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hatano T., Morigasaki S., Tatebe H., Ikeda K., and Shiozaki K. (2015) Fission yeast Ryh1 GTPase activates TOR complex 2 in response to glucose. Cell Cycle 14, 848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimada K., Filipuzzi I., Stahl M., Helliwell S. B., Studer C., Hoepfner D., Seeber A., Loewith R., Movva N. R., and Gasser S. M. (2013) TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol. Cell 51, 829–839 [DOI] [PubMed] [Google Scholar]
- 20. Weisman R., Cohen A., and Gasser S. M. (2014) TORC2—a new player in genome stability. EMBO Mol. Med. 6, 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bähler J. (2005) Cell-cycle control of gene expression in budding and fission yeast. Annu. Rev. Genet. 39, 69–94 [DOI] [PubMed] [Google Scholar]
- 22. Dutta C., Patel P. K., Rosebrock A., Oliva A., Leatherwood J., and Rhind N. (2008) The DNA replication checkpoint directly regulates MBF-dependent G1/S transcription. Mol. Cell. Biol. 28, 5977–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Bruin R. A., and Wittenberg C. (2009) All eukaryotes: before turning off G1-S transcription, please check your DNA. Cell Cycle 8, 214–217 [DOI] [PubMed] [Google Scholar]
- 24. Koch C., Moll T., Neuberg M., Ahorn H., and Nasmyth K. (1993) A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase. Science 261, 1551–1557 [DOI] [PubMed] [Google Scholar]
- 25. Guan X. L., Souza C. M., Pichler H., Dewhurst G., Schaad O., Kajiwara K., Wakabayashi H., Ivanova T., Castillon G. A., Piccolis M., Abe F., Loewith R., Funato K., Wenk M. R., and Riezman H. (2009) Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol. Biol. Cell 20, 2083–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wuarin J., Buck V., Nurse P., and Millar J. B. (2002) Stable association of mitotic cyclin B/Cdc2 to replication origins prevents endoreduplication. Cell 111, 419–431 [DOI] [PubMed] [Google Scholar]
- 27. de Bruin R. A., Kalashnikova T. I., Aslanian A., Wohlschlegel J., Chahwan C., Yates J. R. 3rd, Russell P., and Wittenberg C. (2008) DNA replication checkpoint promotes G1-S transcription by inactivating the MBF repressor Nrm1. Proc. Natl. Acad. Sci. U.S.A. 105, 11230–11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ivanova T., Gómez-Escoda B., Hidalgo E., and Ayté J. (2011) G1/S transcription and the DNA synthesis checkpoint: common regulatory mechanisms. Cell Cycle 10, 912–915 [DOI] [PubMed] [Google Scholar]
- 29. Aligianni S., Lackner D. H., Klier S., Rustici G., Wilhelm B. T., Marguerat S., Codlin S., Brazma A., de Bruin R. A., and Bähler J. (2009) The fission yeast homeodomain protein Yox1p binds to MBF and confines MBF-dependent cell-cycle transcription to G1-S via negative feedback. PLoS Genet. 5, e1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Purtill F. S., Whitehall S. K., Williams E. S., McInerny C. J., Sharrocks A. D., and Morgan B. A. (2011) A homeodomain transcription factor regulates the DNA replication checkpoint in yeast. Cell Cycle 10, 664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ivanova T., Alves-Rodrigues I., Gómez-Escoda B., Dutta C., DeCaprio J. A., Rhind N., Hidalgo E., and Ayté J. (2013) The DNA damage and the DNA replication checkpoints converge at the MBF transcription factor. Mol. Biol. Cell 24, 3350–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreno S., Klar A., and Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 33. Weisman R., and Choder M. (2001) The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 276, 7027–7032 [DOI] [PubMed] [Google Scholar]
- 34. Longtine M. S., McKenzie A. 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., and Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 35. Foiani M., Marini F., Gamba D., Lucchini G., and Plevani P. (1994) The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol. Cell. Biol. 14, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keogh M. C., Mennella T. A., Sawa C., Berthelet S., Krogan N. J., Wolek A., Podolny V., Carpenter L. R., Greenblatt J. F., Baetz K., and Buratowski S. (2006) The Saccharomyces cerevisiae histone H2A variant Htz1 is acetylated by NuA4. Genes Dev. 20, 660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmelzle T., Beck T., Martin D. E., and Hall M. N. (2004) Activation of the RAS/cyclic AMP pathway suppresses a TOR deficiency in yeast. Mol. Cell. Biol. 24, 338–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laor D., Cohen A., Kupiec M., and Weisman R. (2015) TORC1 regulates developmental responses to nitrogen stress via regulation of the GATA transcription factor Gaf1. mBio 6, e00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Betz C., and Hall M. N. (2013) Where is mTOR and what is it doing there? J. Cell Biol. 203, 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malik A. R., Urbanska M., Macias M., Skalecka A., and Jaworski J. (2013) Beyond control of protein translation: what we have learned about the non-canonical regulation and function of mammalian target of rapamycin (mTOR). Biochim. Biophys. Acta 1834, 1434–1448 [DOI] [PubMed] [Google Scholar]
- 41. Li H., Tsang C. K., Watkins M., Bertram P. G., and Zheng X. F. (2006) Nutrient regulates Tor1 nuclear localization and association with rDNA promoter. Nature 442, 1058–1061 [DOI] [PubMed] [Google Scholar]
- 42. Kim J. E., and Chen J. (2000) Cytoplasmic-nuclear shuttling of FKBP12-rapamycin-associated protein is involved in rapamycin-sensitive signaling and translation initiation. Proc. Natl. Acad. Sci. U.S.A. 97, 14340–14345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Workman J. J., Chen H., and Laribee R. N. (2014) Environmental signaling through the mechanistic target of rapamycin complex 1: mTORC1 goes nuclear. Cell Cycle 13, 714–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bachmann R. A., Kim J. H., Wu A. L., Park I. H., and Chen J. (2006) A nuclear transport signal in mammalian target of rapamycin is critical for its cytoplasmic signaling to S6 kinase 1. J. Biol. Chem. 281, 7357–7363 [DOI] [PubMed] [Google Scholar]
- 45. Vazquez-Martin A., Cufí S., Oliveras-Ferraros C., and Menendez J. A. (2011) Raptor, a positive regulatory subunit of mTOR complex 1, is a novel phosphoprotein of the rDNA transcription machinery in nucleoli and chromosomal nucleolus organizer regions (NORs). Cell Cycle 10, 3140–3152 [DOI] [PubMed] [Google Scholar]
- 46. Martelli A. M., Tabellini G., Bressanin D., Ognibene A., Goto K., Cocco L., and Evangelisti C. (2012) The emerging multiple roles of nuclear Akt. Biochim. Biophys. Acta 1823, 2168–2178 [DOI] [PubMed] [Google Scholar]
- 47. Caetano C., Klier S., and de Bruin R. A. (2011) Phosphorylation of the MBF repressor Yox1p by the DNA replication checkpoint keeps the G1/S cell-cycle transcriptional program active. PLoS One 6, e17211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ralph E., Boye E., and Kearsey S. E. (2006) DNA damage induces Cdt1 proteolysis in fission yeast through a pathway dependent on Cdt2 and Ddb1. EMBO Rep. 7, 1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosner M., and Hengstschläger M. (2012) Detection of cytoplasmic and nuclear functions of mTOR by fractionation. Methods Mol. Biol. 821, 105–124 [DOI] [PubMed] [Google Scholar]
- 50. Rosner M., and Hengstschläger M. (2008) Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum. Mol. Genet. 17, 2934–2948 [DOI] [PubMed] [Google Scholar]
- 51. Tatebe H., Morigasaki S., Murayama S., Zeng C. T., and Shiozaki K. (2010) Rab-family GTPase regulates TOR complex 2 signaling in fission yeast. Curr. Biol. 20, 1975–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stevens C., and La Thangue N. B. (2004) The emerging role of E2F-1 in the DNA damage response and checkpoint control. DNA Repair 3, 1071–1079 [DOI] [PubMed] [Google Scholar]
- 53. Lindsay H. D., Griffiths D. J., Edwards R. J., Christensen P. U., Murray J. M., Osman F., Walworth N., and Carr A. M. (1998) S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 12, 382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Furuya K., Poitelea M., Guo L., Caspari T., and Carr A. M. (2004) Chk1 activation requires Rad9 S/TQ-site phosphorylation to promote association with C-terminal BRCT domains of Rad4TOPBP1. Genes Dev. 18, 1154–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]